Abstract
Immunostimulatory sequences (ISS) are short DNA sequences containing unmethylated CpG dimers that have multiple effects on the host immune system, including the ability to stimulate antigen-specific cytotoxic T lymphocytes (CTLs) and drive Th1-type immune responses. Listeriolysin O (LLO)-containing pH-sensitive liposomes have been shown to efficiently deliver macromolecules to the cytosol of APCs and efficiently stimulate CTLs. We hypothesized that encapsulating ISS-oligodeoxyribonucleotides (ODNs) in this delivery system would enhance the cell-mediated immune response and skew Th1-type responses in protein antigen-based vaccination utilizing LLO-liposomes. In vitro studies indicated that co-encapsulation of ISS in LLO-liposomes engendered activation of the NF-κB pathway while maintaining the efficient cytosolic delivery of antigen mediated by the co-encapsulated LLO. Antigen-specific CTL responses monitored by using the model antigen ovalbumin (OVA) in mice were enhanced when mice were immunized with OVA and ISS-ODN-containing LLO-liposomes compared with those immunized with either OVA-containing LLO-liposomes or OVA-ISS conjugates. The enhanced immune responses were of the Th1-type as monitored by the robust OVA-specific IgG2a induction and the OVA CD8 peptide-stimulated IFN-γ secretion. Our study suggests that including ISS-ODN in LLO-containing pH-sensitive liposomes yields a vaccine delivery system that enhances the cell-mediated immune response and skews this response toward the Th1-type.
Keywords: liposome, listeriolysin O, ISS-ODN
Introduction
In traditional vaccination, live-attenuated viruses or inactivated pathogens have been widely utilized for the treatment and prevention of disease. Although these strategies have generated successful results for a large number of diseases, safety concerns associated with conventional vaccines have led to the development of modern vaccines such as protein antigen-based subunit vaccines.1 Typical subunit vaccines, without optimized delivery strategy and without adjuvants, weakly stimulate the immune system and induce both limited antibody response and minimal cellular immunity, particularly antigen-specific cytotoxic T lymphocyte (CTL) responses, while adjuvants such as alum and oil/water emulsions have been used to address these problems, albeit primarily to enhance the antibody-based humoral immunity.1, 2 In many cases, however, effective vaccines against tumors and viral infections need to efficiently stimulate both arms of the immune system: humoral and cell-mediated.3, 4 Increased efficacy may be partly achieved through the addition of molecular adjuvants, which act through an innate immune receptor to exert immunopotentiating properties that modulate the strength and type of immune response.1, 5 Selecting a suitable delivery system for molecular adjuvants and antigens is frequently necessary for achieving a robust immune response, and one way to accomplish this is to mimic the natural immune-stimulating properties of pathogens. Our approach tested in this study is to combine a nucleotide-based molecular adjuvant and protein antigen with LLO-containing pH-sensitive liposomes to simultaneously activate the innate immune system, as well as deliver the antigen to the cytosol of antigen-presenting cells (APCs) to stimulate the cellular arm of immunity.
Liposomes have the propensity to be taken up by APCs via the endocytic pathway; therefore, in order to facilitate the likelihood of interaction and enhance the adjuvanticity, we have chosen in this study a molecular adjuvant with its primary receptor that has been investigated to be located in the endosomal compartment.6 Immunostimulatory sequence-containing oligodeoxyribonucleotides (ISS-ODNs), are short, synthetic, single-stranded ODNs containing unmethylated cytosine phosphate guanine (CpG) sequences that mimic motifs primarily found in bacterial DNA.7 CpG motifs act as danger signals detected by a pattern recognition receptor (PRR), mainly Toll-like receptor 9 (TLR9), in the endosome alerting the vertebrate innate immune system of the invasion of intracellular pathogens.8 Upon recognition of CpG-containing DNA by TLR9, an immunostimulatory cascade induces the differentiation, maturation and proliferation of B cells, T cells, natural killer cells and monocytes/macrophages resulting in the production of various proinflammatory cytokines such as interleukin (IL)- 1, 6, 12 and 18 and interferon (IFN)- α, β, γ.5, 7 The antigen-specific effects of ISS-ODNs have been shown to be improved when the antigen and ISS-ODN are co-delivered to the same APC.9, 10
One approach to achieve co-delivery is to chemically conjugate protein antigens to ISS-ODNs, which have been tested and shown to increase antigen-specific IFN-γ secretion, IgG2a titers, and CTL activity compared with antigen and ISS-ODN mixture in solution.9, 10 Although antigen-ISS-ODN conjugates stimulate antigen-specific CTL activity in some cases of model antigen,11 there are limitations associated with antigen-ISS-ODN conjugates as a vaccine formulation: (i) the chemical characteristics of the antigen may limit the synthesis of the conjugates, (ii) the antigenic epitopes may be altered during conjugation, and (iii) the antigen dose and antigen-to-ISS ratio used in a vaccine may be non-optimal or restricted.12, 13 To overcome these problems, many types of delivery systems have been used to co-deliver antigens and ISS-ODNs, including, but not limited to, biodegradable microparticles, nanorods, and liposomes (reviewed by Krishnamachari et al.14). Most of the tested delivery systems enter the APCs via the endocytic pathway and potentiate MHC Class II-dependent humoral responses, but do not possess the capacity to activate MHC Class I-dependent cellular responses directly. Many systems rely on the alternative mechanism, i.e., less efficient cross-presentation; that is, the ability of APCs to process and present extracellular antigens to CD8+ T cells in order to engender CTL responses.15, 16 We previously demonstrated the utility of LLO-containing liposomes in actively potentiating CTL responses via enhanced cytosolic delivery of protein antigen directly into the cytosolic pathway of MHC I-dependent antigen presentation: in delivering whole protein antigen to the cytosol of macrophages in vitro and enhancing antigen-specific CTL activity in vivo in a murine model.6, 17, 18 LLO, the pore-forming hemolysin of a facultative intracellular bacteria, Listeria monocytogenes (Lm), exhibits optimal endosome-disrupting activity at pH 5.5 and promotes Lm escape from the phagolysosome for invasion into the cytosol.19
In the current study, we hypothesized that incorporating ISS-ODN in the LLO-containing liposome formulations would skew the immune response to the Th1-type and further improve the CTL activity compared with LLO-liposomes. We demonstrate that co-encapsulation of ISS-ODN in the LLO-containing liposomes activates the Th1-type cytokine pathway in vitro. Furthermore, using the model antigen ovalbumin (OVA), we show in vivo that the lip{LLO,OVA,ISS} (lip{} indicates liposomes encapsulating LLO, OVA and ISS-ODN) formulation stimulates a robust CTL response. Addition of ISS-ODN to the lip{LLO,OVA} formulations results in an enhanced the number of CD4+ and CD8+ IFN-γ-secreting T cells, as well as an increased Th1-type antibody response. The results from these studies indicate that the LLO and ISS-ODN-containing liposome formulation is capable of stimulating a robust adaptive immune response harnessing the mechanism and benefits of both LLO and ISS-ODN.
Materials and Methods
Mice
C57BL/6 (female, 8-12 weeks old; Charles River Laboratories, Portage, MI) and C57BL/10ScNJ (Tlr4Lps-del, H-2Kb, female, 6-12 weeks old; Jackson Laboratories, Bar Harbor, ME) were used in this study and were handled according to Institutional Guidelines.
Cell lines and tissue culture
All tissue culture media and reagents were purchased from Invitrogen (Carlsbad, CA), and all cells were maintained and experimental incubations were conducted in a humidified incubator at 37 °C and 5% CO2, unless otherwise noted. B3Z cells, an OVA SIINFEKL peptide-specific CD8+ T-cell hybridoma (CD8 OVA T1.3, H-2Kb-restricted), were maintained in RPMI-1640 media supplemented with 10% heat-inactivated fetal bovine serum (HI-FBS), 2 mM glutamine, 1 mM sodium pyruvate, 100 μg/mL streptomycin, 100 U/mL penicillin, 50 μM 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO), and 25 mM HEPES. Bone marrow was harvested from femurs and tibia of mice and differentiated into bone marrow-derived macrophages (BMM) in BMM media (DMEM supplemented with 20% HI-FBS, 30% L-cell conditioned media, 2 mM glutamine, 100 μg/mL streptomycin, 100 U/mL penicillin and 55 μM 2-mercaptoethanol) as described previously by Stier et al.20 BMM were harvested on day six of culture and frozen in liquid nitrogen until the experiment. For experiments, BMM were cultured in either BMM media or complete DMEM (DMEM + 10% HI-FBS, 100 μg/mL streptomycin and 100 U/mL penicillin) as described below.
Purification of LLO and preparation of liposomes
The hly gene (which encodes for LLO) was inserted into pET29b with a polyhistidine tag. Recombinant LLO was purified from E. coli, analyzed for purity and monitored for hemolytic activity as previously described.17 ISS-ODNs used in these studies were provided by Dynavax Technologies Corporation. ISS 1018 (5′-TGA CTG TGA ACG TTC GAG ATG A-3′), unmodified and 5′-disulfide-containing were synthesized with a nuclease-resistant phosphorothioate-modified backbone. The 5′-disulfide ISS was synthesized with a hexaethylene glycol linker disulfide bonded to a pyridyl leaving group that was removed upon reduction with TCEP as described below. Lipid films were made from a 2:1 (mol:mol) mixture of egg phosphatidylethanolamine: cholesteryl hemisuccinate (ePE:CHEMS; Avanti Polar Lipids, Alabaster, AL and Sigma-Aldrich, respectively) by removing chloroform and methanol using a rotary evaporator at < 10 mm Hg vacuum at RT. The lipid films were hydrated by vortexing with HBS, pH 8.4 containing LLO (100 μg), OVA (2 mg, Sigma-Aldrich, Grade VI) and/or ISS (0.625 mg). The optimal concentration of liposome-encapsulated LLO required for efficient cytosolic OVA delivery was previously determined by Mandal et al. and used in these studies.17 The liposomes were freeze-thawed and sonicated ten times, and unencapsulated proteins and ODNs were separated from liposomes by size-exclusion chromatography using a 1 × 25 cm Sepharose CL-4B column (GE Healthcare, Picastaway, NJ). Encapsulated OVA and LLO was analyzed by resolving the proteins in SDS-PAGE and measuring band intensities using densitometry. Protein concentrations were determined by staining with either Coomassie Blue and digitally recorded using a KODAK Digital Sciences Electrophoresis Documentation and Analysis System, or with SYPRO Red (Invitrogen) and visualized with a Molecular Dynamics Typhoon 9200 (GE Healthcare), and calculations were based on known concentrations of the proteins run in the same gel. ODNs were resolved in a 20% Tris-Borate-EDTA (TBE) polyacrylamide gel, and the concentrations were determined by staining with SYBR Green I (Invitrogen) and calculated from known ODN concentrations. The size of the liposomes was determined as previously described and yielded similar results.21
Preparation of conjugate {OVA-ISS}
Conjugate {OVA-ISS} were made as described by Cho et al. with the following modifications.9 Briefly, OVA was activated with sulfosuccinimidyl-4-(N-maleimidomethyl) cyclohexane-1-carboxylate (sulfo-SMCC; Pierce, Rockford, IL) at RT for 1 h, and residual sulfo-SMCC was removed by G-25 desalting column (GE Healthcare). A five molar excess of tris-(2-carboxyethyl) phosphine (TCEP, Pierce) was incubated with 5′-disulfide-linked phosphorothioate ODN at 40 °C for 2 h, and residual TCEP was removed using G25-Hi Prep column. Reduced 5′-thio-ISS ODN was mixed with modified OVA at a 5:1molar ratio in 150 mM sodium chloride, 100 mM sodium phosphate, pH 7.5 overnight at RT. Free ODN was removed from the conjugate using a Sephacryl S-200 column, and the conjugates were analyzed by SDS gel electrophoresis using NuPage 4-12% Bis/Tris gels (Invitrogen) and MOPS/SDS buffer. The ISS concentration, as determined by absorbance at 260nm (A260), and the OVA concentration, determined using the BCA assay, were used to calculate the ISS/OVA ratio in the conjugate{OVA-ISS}: the conjugate{OVA-ISS} used in the experiments on average consisted of ~ 2.4 ISS molecules per OVA.
In vitro IL-12 secretion analysis by ELISA
BMM (2 × 105/well) from C57BL/10ScNJ mice were plated in 96-well tissue culture plates in BMM media one day prior to liposome treatment. Cells were pulsed with OVA-containing LLO-liposomes diluted in complete DMEM for 2 h, washed and incubated with complete DMEM for 22 h. Cell culture supernatants were collected and stored at −80 °C until analyzed for IL-12 secretion using IL-12/IL-23p40 ELISA (R&D Systems, Minneapolis, MN) as per the manufacturer’s instructions.
In vitro antigen presentation assay
In vitro antigen presentation experiments were performed as described previously with the following modifications.6 BMM (2 × 105/well) from C57BL/10ScNJ mice were plated in 96-well tissue culture plates in complete DMEM one day prior to liposome treatment. BMM were incubated in serum-free DMEM for 1 h and then pulsed with liposomes in serum-free DMEM for 2 h. Cells were washed and incubated in complete DMEM for 3 h, fixed with 1% paraformaldehyde for 15 min at RT, and residual aldehydes were quenched with 0.2 M lysine for 20 min at RT. B3Z cells (2 × 105 cells/well) were added to the plate and incubated for 15 h. Cells were washed with PBS and the β-galactosidase substrate [0.15 mM chlorophenol red-β-D-galactopyranoside (CPRG; Calbiochem, Gibbstown, NJ), 100 μM 2-mercaptoethanol, 9 mM MgCl2, 0.125% NP40 in PBS] was added to the plate and incubated for 4 h, after which the absorbance was measured at 570 nm using a Molecular Devices Emax plate reader.22
Immunization protocol
C57BL/6 mice were primed with formulations containing 8 μg of OVA in 50 μL on week 0 and boosted with the same formulation on week 2 via the subcutaneous route at the base of the tail. Sera were collected on week 4 for antibody analyses. Mice were euthanized on week 6 and spleens were harvested for assays.
Ex vivo cytotoxic T lymphocyte assay
CTL activity was assessed using the standard 51Cr release assay with splenocytes isolated six weeks after priming. Briefly, the splenocytes were cultured in mouse media containing 2% Rat T-Stim (BD Biosciences) with 1 μg/mL SIINFEKL-peptide, then incubated for four days. Splenocytes were washed and re-plated in mouse media containing 2% Rat T-Stim for two additional days. On day six, the splenocytes (effector cells) were counted and plated in triplicate to the 96-well plates. Five thousand 51Cr-labeled target cells, either SIINFEKL peptide-pulsed EL-4 cells or no peptide-pulsed EL-4 cells, were added to the effector cells to achieve various effector:target ratios. MHC-mismatched 51Cr-labeled, peptide pulsed SV-BALB cells were also plated as targets to confirm MHC specificity (data not shown). The effector and target cells were incubated for 4 h, and the supernatants were analyzed for 51Cr release. The percent cytotoxicity was calculated based on the amount of 51Cr released into the supernatant using the equation: % specific lysis = [(mean killing-spontaneous release)/(maximum release-spontaneous release)] × 100, where maximal release was achieved by complete lysis of 51Cr-labeled targets in 1% Triton X-100, spontaneous release was determined by incubating 51Cr-labeled targets in media, and mean killing was determined by the cytotoxicity of 51Cr-labeled SIINFEKL-pulsed EL-4 cells.
ELISPOT assay for IFN-γ
ELISPOT was used to quantify the number of OVA-specific IFN-γ secreting cells. Nunc Maxisorb plates were coated with rat anti-murine IFN-γ (5 μg/mL, U-CyTech, Utrecht) in PBS, overnight at 4 °C followed by blocking with PBS + 1% BSA. Serial dilutions of splenocytes (starting at 2 × 105/well) were added to the plates in mouse media and stimulated with OVA (20 mg/mL), OVA CD8 peptide (1 μg/mL) or OVA CD4 peptide (1 μg/mL) overnight. Biotinylated rabbit anti-murine IFN-γ (U-CyTech) was detected using murine anti-biotin HRP (Sigma-Aldrich), which was developed with TMB for membranes (Sigma-Aldrich). Spots were analyzed using the BioReader 3000 immunospot image analyzer (BioSys, Karben, Germany).
Anti-OVA Ig ELISA
Sera were collected on week four and analyzed by ELISA to quantify the antibody isotypes induced by the immunization formulation. ELISA plates were coated with OVA (10 μg/mL) in 0.1 M sodium phosphate, pH 9.0. Serial dilutions of sera samples were added in duplicate, and anti-OVA isotype-specific secondary antibodies (goat anti-mouse IgG2a-biotin conjugated or goat anti-mouse IgG1-biotin conjugated; Southern Biotechnology Associates, Birmingham, AL) were detected with streptavidin-HRP (Pierce; Rockford, IL). Color was developed using TMB substrate (KPL), stopped with 2 N sulfuric acid, and the absorbance was read at 450 nm with a 650 nm background correction using a Molecular Devices plate reader. The titer was defined as the reciprocal of the serum dilution that gave an ELISA absorbance of 0.5 OD using a 4-parameter analysis (Softmax Pro97, Molecular Devices, Sunnyvale, CA).
Antigen-specific cytokine secretion
Splenocytes (5 × 105/well) were added to 96-well tissue culture plates in duplicate in 200 μL mouse media (RPMI 1640 (Lonza, Walkersville, MD), 10% HI-FBS (Gemini Bioproducts, West Sacramento, CA), 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin (Lonza), 2-mercaptoethanol (55 μM) supplemented with either OVA (25 μg/mL), MHC class I OVA peptide SIINFEKL, and MHC class II OVA peptide ISQAVHAAHAEINEAGR (1 μg/mL, Research Genetics, Huntsville, AL), or concanavalin A (10 μg/mL, Sigma-Aldrich). Splenocyte supernatants from a given mouse were combined when harvested on day 4 and stored at −80 °C until analyzed for IFN-γ or IL-5 secretion by ELISA. Samples were assayed in duplicate using ELISA plates coated with purified rat anti-mouse IFN-γ capture antibody (2 μg/mL; BD Biosciences, San Jose, CA) or purified rat anti-mouse IL-5 capture antibody (1 μg/mL; BD Biosciences) in 0.1 M sodium phosphate, pH 9.0 overnight at 4 °C. Plates were washed with PBST (PBS (R&D Systems) + 0.05% Tween 20), and then blocked with 1% BSA in PBS. Dilutions of the cell culture supernatants were added, and cytokines were detected with biotinylated rat anti-mouse IFN-γ or biotinylated rat anti-mouse IL-5 (BD Biosciences). Streptavidin-horse radish peroxidase (HRP, Pierce) was detected with TMB substrate (KPL, Gaithersburg, MD) and stopped with 2 N sulfuric acid. Absorbance values were read at 450 nm with a 650 nm correction using a Molecular Devices plate reader.
Statistical analyses
The in vivo data are from one experiment (n = 10 individual mice/group). The in vivo experiments were performed at 5 μg and 8 μg both yielding similar enhancements in the immune response between groups; the representative results from the experiments using the 8 μg dose are reported. Data from liposome treated animals were compared by t-tests and the p-value is given for significant differences. A p-value < 0.05 was considered significant.
Results
ISS-ODN-containing LLO-liposomes activate the NF-κB pathway for IL-12 secretion
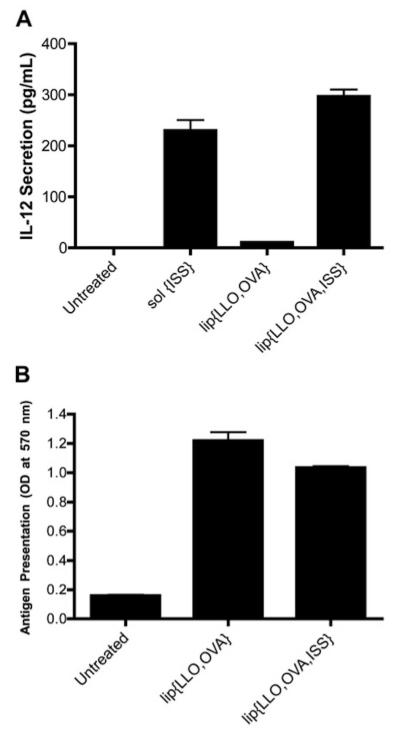
The immunomodulatory capabilities of ISS-ODNs arise as a result of their stimulating the production of specific cytokines and the downstream physiological changes they induce. We first investigated the effect of adding ISS-ODN to LLO-containing liposomes by monitoring IL-12, a Th1-type cytokine, in BMM culture. To confirm that the IL-12 secretion was not due to TLR4 activation, BMM from C57BL/10ScNJ mice, which are TLR4 (-/-) were used. BMM were treated with the various liposome formulations and secreted IL-12 was analyzed by ELISA. The cells treated with sol{ISS} (ISS in solution) resulted in 229.8 ± 35.6 pg/mL. The presence of ISS-ODN encapsulated in the liposomes significantly increased the amount of IL-12 secretion from 10.7 ± 0.8 pg/mL to 296.9 ± 22.7 pg/mL for lip{LLO,OVA} (lip{} indicates liposome encapsulating LLO and OVA) and lip{LLO, OVA, ISS}, respectively (Figure 1A). Sol{ISS} treatment was used in these experiments as a positive control, and thus was incubated with cells for 24 h which resulted in IL-12 secretion at a level similar to that induced by a 2 h incubation with liposome-encapsulated ISS. As expected based on the results from sol{ISS} and lip{LLO,OVA,ISS}, no difference was observed in IL-12 secretion when cells were treated with lip{LLO,OVA,ISS} or lip{LLO,OVA}sol{ISS} (data not shown). Taken together, these data suggested that encapsulating ISS in LLO-liposomes did not impair their ability to stimulate IL-12 secretion. These results were confirmed by monitoring translocation of NF-κB to the nucleus where lip{LLO,OVA}, lip{LLO, OVA,ISS} or free ISS-ODN stimulated nuclear NF-κB translation of 16 ± 12%, 75 ± 9% and 90 ± 5%, respectively (data not shown), while LPS alone was not capable of stimulating NF-κB translocation in BMM to levels beyond those of untreated cells (data not shown). Taken together, these results confirm that the co-encapsulation of ISS-ODN inside LLO-liposomes significantly enhances the activation of the NF-κB pathway resulting in the nuclear translocation of NF-κB and IL-12 secretion independent of TLR4 in BMM.
Figure 1.
Lip{LLO,OVA,ISS} induces IL-12 secretion and MHC Class I-restricted presentation of OVA-specific peptides by BMM. (A) BMM were treated with sol{ISS} (4.7 μg/mL) for 24 h or lip{LLO,OVA} (10 μg/mL OVA; 0 μg/mL ISS) or lip{LLO,OVA,ISS} (10 μg/mL OVA; 4.7 μg/mL ISS) for 2 h. After 2 h incubation, the cells were washed and further incubated for 22 h. Cell culture supernatants were analyzed for IL-12 secretion by ELISA. (B) BMM were pulsed with lip{LLO,OVA} or lip{LLO,OVA,ISS} (10 μg/mL OVA) for 2 h. Cells were washed, further incubated for 3 h and fixed; B3Z cells were added. After 15 h incubation with B3Z cells, the level of presentation of SIINFEKL OVA-CD8 peptide by BMM to B3Z cells as monitored by the conversion of CPRG substrate to chlorophenol red in the activated B3Z cells. Cells were pulsed with SIINFEKL peptide (90 nM) as a positive control, and OD values were 1.28. Data represent the average of triplicates ± S.D and are representative of results from multiple independent experiments that were repeated.
Encapsulation of ISS-ODN in LLO-liposomes does not alter the cytosolic delivery of antigen to APCs in vitro
We previously showed that LLO in LLO-containing liposomes carrying the model antigen OVA, denoted as lip{LLO,OVA} in the current study, significantly enhances the efficiency of cytosolic delivery of OVA.6, 17 Despite the well-known advantages of liposomes as a delivery vehicle such as carrying multiple copies and several types of macromolecules and drugs, it is critical to ensure that additional cargo of ISS-ODN in lip{LLO,OVA} does not drastically change the cytosolic delivery properties of LLO-liposomes. To test this, we monitored the amount of OVA delivered to the cytosol of APCs upon ISS-ODN co-encapsulation using an in vitro OVA antigen presentation assay. BMM were treated with LLO and OVA-containing pH-sensitive liposomes with and without ISS-ODN, and the levels of OVA peptide (SIINFEKL; OVA258-265) presented on MHC I, as a measure of the amount of OVA delivered to the cytosol, was monitored using B3Z-based assay. B3Z, which is a lacZ-inducible CD8+ T cell hybridoma cell line, expresses β-galactosidase upon binding the CD8 SIINFEKL peptide in context of H-2Kb MHC class I molecules.22 Hence, the amount of β-galactosidase substrate (CPRG) converted to product (chlorophenol red) is an indirect measure of the number of binding events.22 The amount of SIINFEKL peptide presented to B3Z cells was similar when BMMs were treated with lip{LLO,OVA} or lip{LLO,OVA,ISS} (Figure 1B); a slight reduction in antigen presentation, however, was observed when ISS-ODN was included. The slight decrease was attributed to a lower relative encapsulation efficiency of OVA when ISS-ODN was co-encapsulated; the amount of OVA delivery to the cytosolic pathway for antigen presentation, monitored by the B3Z-based assay, was dependent on the amount of LLO and OVA co-encapsulated inside the liposomes (data not shown), in agreement with our previous report.17 The in vitro studies in BMM suggest that lip{LLO,OVA,ISS} formulation is capable of eliciting adjuvant-specific cytokine secretion in APCs, while retaining the efficiency of cytosolic delivery of antigen for which LLO in pH-sensitive liposomes is noted.
Adding ISS-ODN to lip{LLO, OVA} enhances the OVA-specific CTL response in vivo
Based on the results from the in vitro studies which confirmed that lip{LLO,OVA,ISS} are capable of delivering antigen to the cytosol of BMMs and exerting an ISS effect, we immunized C57BL/6 mice to test the critical antigen-specific CTL effector function in order to assess the efficacy of our lip{LLO,OVA,ISS} vaccine carrier. The standard 51Cr release-based CTL assay was used to monitor the strength of CTL function in the context of our immunization regimen. As a way to evaluate the LLO-liposomal ISS-ODN data in the context of other ISS-ODN formulations reported in the literature, mice were immunized with sol{OVA} (OVA in solution), sol{OVA,ISS} (OVA and ISS-ODN mixed in solution) or conjugate{OVA-ISS} (OVA-ISS-ODN conjugates). In agreement with previously published studies, vaccination with lip{LLO,OVA} liposomes induced enhanced CTL activity as compared with sol{OVA}-immunized mice (15 ± 19 % and 1 ± 1%, respectively).17 Compared with sol{OVA}, the addition of ISS-ODN in solution with OVA improved CTL activity (21 ± 29%) which could be further enhanced when OVA and ISS were co-delivered to the same APC as was the case for conjugate{OVA-ISS} (36 ± 29%). Of all the formulations tested, splenocytes from the lip{LLO,OVA,ISS}-immunized mice exhibited the strongest CTL response (53 ± 34%; Figure 2). Co-encapsulating ISS-ODN in pH-sensitive LLO-containing liposomes enhanced the antigen-specific CTL activity compared with lip{LLO,OVA}-immunized at all effector:target ratios tested. The fact that the antigen-specific CTL activity in lip{LLO,OVA,ISS} is comparable to conjugate{OVA-ISS}-immunized mice is a significant finding, as antigen-ISS-ODN conjugates have heretofore been regarded as the gold standard for eliciting CTL responses that are as robust as “live” vaccines in mice.11 Given the difficulties associated with producing some antigen-ISS-ODN conjugates, co-encapsulating ISS-ODN and antigen in the LLO-containing pH-sensitive liposomes may be an attractive and effective alternative as a vaccine delivery system.
Figure 2.
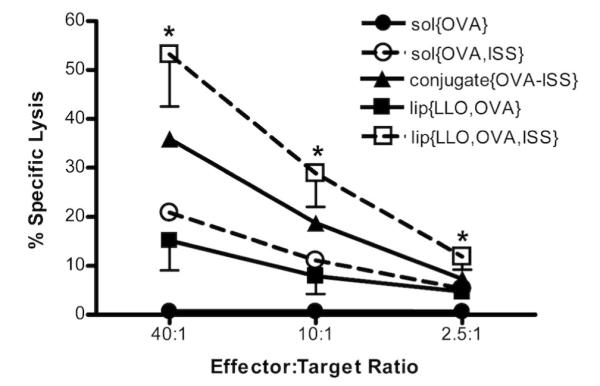
Enhanced OVA-specific cytotoxic activity by lip{LLO,OVA,ISS}. Mice were immunized subcutaneously at week zero and boosted at week two with the same formulations containing OVA (8 μg). OVA-specific CTL activity was monitored using 51Cr release assay. Each point represents the mean specific lysis of individual splenocytes cultures (n=10) for each group. Standard deviation is shown for liposome groups. * Statistical significance was observed when comparing lip{LLO,OVA} and lip{LLO,OVA,ISS} at all effector:target ratios, p=0.006, p=0.02, p=0.03 at 40:1, 10:1 and 2.5:1, respectively.
OVA-specific IFN-γ secreting cells
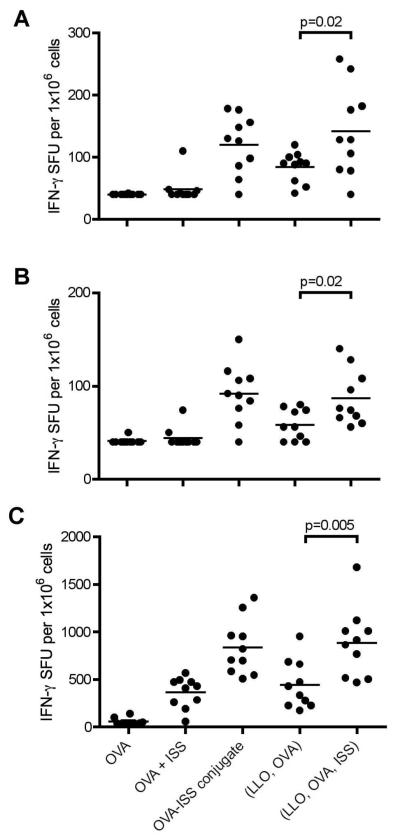
To further assess the types of cells activated in the cellular immune response after immunization, splenocytes isolated from C57BL/6 mice four weeks post-immunization were stimulated ex vivo with either (i) OVA protein (Figure 3A), (ii) the OVA-derived peptide specifically recognized by CD4+ T cells ISQAVHAAHAEINEAGR (OVA323-339) (Figure 3B), or (iii) the OVA-derived peptide specifically recognized by CD8+ T cells, SIINFEKL (OVA258-265) (Figure 3C) and monitored for the number of IFN-γ-secreting cells by ELISPOT assay. Vaccination with lip{LLO,OVA,ISS} significantly increased the number of functional IFN-γ secreting CD4+ T cells and CD8+ T cells compared with non-ISS vaccination with lip{LLO,OVA}. Vaccination with conjugate{OVA-ISS} or lip{LLO,OVA,ISS} resulted in relative mean fold-increase of at least twice as many CD4+ and CD8+ IFN-γ secreting cells in response to all stimulants compared to sol{OVA,ISS}, consistent with reports that co-delivery of antigen and ISS-ODN to the same APC is important for stimulating an optimal immune response.11, 23 Relatively low numbers of IFN-γ secreting cells were observed when the splenocytes were cultured in the absence of antigen or antigenic peptide, indicating that the induced immune responses monitored by the cytokine ELISA and ELISPOT assays are antigen-specific (data not shown). ELISPOT data indicated both CD4+ and CD8+ T cells were activated by lip{LLO,OVA,ISS} to secrete IFN-γ.
Figure 3.
Mice were immunized subcutaneously on week 0 and week 2 with formulations containing 8 μg OVA. Splenocytes were isolated 4 weeks after the final immunization and stimulated with (A) OVA (20 μg/mL), (B) CD4 peptide (1 μg/mL) or (C) CD8 peptide (1 μg/mL) for 24 h. Each data point represents an individual animal and the line represent the mean IFN-γ spot forming units (SFU) per 1×106 cells for each group (n=10). Values at the limit of detection of the assay were reported as 40. Statistical significance was observed when comparing lip{LLO,OVA} and lip{LLO, OVA, ISS} for all stimulants; p=0.02, p=0.02, p=0.005 for OVA, CD4, and CD8, respectively.
ISS-ODN-containing lip{OVA, LLO} drive Th1-type OVA-specific Ig responses
To investigate the type of antigen-specific humoral responses stimulated by the vaccination, sera from immunized mice were analyzed for OVA-specific IgG2a (Th1-type) and IgG1 (Th2-type) antibodies. Mice immunized with lip{LLO,OVA} produced more IgG1 antibodies than any of the other formulations (Figure 4); however, co-encapsulating ISS-ODN in the LLO-OVA-liposomes, i.e., lip{LLO,OVA,ISS} formulation, resulted in a significant reduction in IgG1 antibodies (p=0.005) and a significant enhancement in IgG2a antibodies (p=0.007); lip{LLO,OVA,ISS} thus generated more anti-OVA IgG2a antibodies than any other formulation. The Th1 immune skewing capabilities of the lip{LLO,OVA,ISS}-formulation was best demonstrated by comparing IgG2a:IgG1 ratios obtained by immunizing mice with different formulations. The lip{LLO,OVA,ISS}-immunized group showed the highest IgG2a:IgG1 anti-OVA antibody response, indicative of a stronger Th1-type response (Figure 4B).
Figure 4.
ISS-ODN enhances the Th1-type antibody response in conjugate{OVA-ISS} or lip{LLO,OVA,ISS}-immunized mice. Two weeks after the second immunization, sera were collected and analyzed for anti-OVA IgG1 or IgG2a antibodies using ELISA. (A) Each bar represents geometric mean titers ± standard error of the mean (GMT ± SEM) for each group (n=10). (B) Each bar represents the average of the IgG2a:IgG1 ratio ± S.D. for each group, which was calculated from the ratio of each individual animal (n=10). If no titer was observed, the lowest dilution tested, 600, was reported.
ISS-ODN incorporation in lip{OVA,LLO} enhances OVA-specific Th1-type cytokine secretion
To further support the ELISPOT assay and anti-OVA IgG isotype data for Th1-type immune responses potentiated by ISS-ODN co-delivery, splenocytes from immunized C57BL/6 mice were analyzed for OVA-specific Th1- or Th2-type cytokine responses (IFN-γ or IL-5 secretion, respectively) upon antigen stimulation. The sol{OVA} immunized group demonstrated low levels of OVA-specific IFN-γ secretion that were not above detectable background levels (843 pg/mL; Table 1), and this response was increased to detectable levels (4784 ± 3518 pg/mL) when ISS-ODN was included as mixture, i.e., sol{OVA,ISS}. Bulk IFN-γ secretion from splenocytes from mice immunized with conjugate{OVA-ISS} or lip{LLO,OVA,ISS} was the highest. Addition of ISS-ODN to the lip{LLO,OVA} formulation resulted in significantly (p=0.03) more IFN-γ secretion from those splenocytes compared with splenocytes from lip{LLO,OVA}-immunized mice. The strong response from conjugate{OVA-ISS}-immunized mice were in agreement with previously published data showing antigen-ISS-ODN conjugate formulations generate a strong IFN-γ (Th1)-promoting vaccine.9, 10 Our data indicate that similar levels of IFN-γ secretion can be achieved when co-delivering antigen and ISS-ODN using the conjugate{OVA-ISS} formulation or the lip{LLO,OVA,ISS} formulation. IFN-γ secretion results showed that ISS-ODN increased the production of Th1-type cytokines, which in turn are known to decrease the production of Th2-type cytokines.24, 25 This hypothesis was confirmed by monitoring the OVA-specific IL-5 secretion from the splenocytes of immunized mice which showed that splenocytes from groups with the highest IFN-γ secretion resulted in the lowest IL-5 secretion (Table 1).
Table 1.
Induction of OVA-specific IFN-γ and IL-5 secretion from splenocytes of immunized mice as determined by ELISA. Mice were immunized subcutaneously at week zero and boosted at week two with the same formulations. Mice were immunized with sol{OVA} (8 μg), sol{OVA,ISS} (8 μg OVA; 4 μg ISS) and conjugate{OVA-ISS} (8 μg OVA; 3.3 μg ISS), lip{LLO,OVA} (8 μg OVA), or lip{LLO,OVA,ISS} (8 μg OVA; 3.5 μg ISS). At week 6, splenocytes were isolated from mice and stimulated ex vivo with OVA (25 μg/mL). On day 4, supernatants were collected and analyzed for cytokine secretion using ELISA. Values below the limit of detection (LOD) were reported as the LOD × dilution factor, <843 pg/mL for IFN-γ and <37 pg/mL for IL-5. Data shown represent the mean ± S.D. for each group (n=10). Statistically significant differences (*) were observed when comparing lip{LLO,OVA} and lip{LLO,OVA,ISS} for both cytokines.
| Immunization Formulation |
Cytokines (pg/mL) |
|
|---|---|---|
| IFN-γ | IL-5 | |
| sol{OVA} | <843 | 2,665 ± 4,061 |
| sol{OVA,ISS} | 4,784 ± 3,518 | 230 ± 292 |
| conjugate{OVA-ISS} | 22,510 ± 11,280 | 148 ± 251 |
| lip{LLO,OVA} | 10,052 ± 4,608 | 460 ± 371 |
| lip{LLO,OVA,ISS} | 17,579 ± 8,786* | <37* |
Discussion
For the generation of an optimal immune response, APCs must receive antigens in the context of the appropriate activation and stimulation signals. During an infection, APCs are stimulated through the innate immune system, which recognizes a diverse array of pathogen-associated molecular patterns (PAMPs) such as unmethylated CpG motifs in DNA, lipopolysaccharides, flagellin, and double-stranded RNA. Recognition of one or more PAMPs results in APC maturation, which is characterized by cytokine secretion, upregulation of costimulatory molecules and increased antigen presentation. However, without concomitant immunostimulation along with delivery of antigen to the appropriate subcellular compartment, as is commonly the case with most subunit vaccines, the desired immune responses are difficult to be induced. Simple mixing of adjuvants with protein antigens has been shown to improve the antigen-specific immune response,26 but it is not nearly an optimal scenario for efficient induction of antigen-specific responses of desired types; antigen and adjuvant may not be co-delivered to the same APC in many cases or the APC may get exposed to the adjuvant first resulting in activation/maturation that prevents efficient uptake of antigen by the APC. Co-delivery achieved utilizing antigen ISS-ODN conjugation, may be practically restricted by the chemical limitations of some antigens, as well as the potential for altering the antigenic epitopes as a result of the conjugation process, which calls for alternative strategies of efficient and effective co-delivery of antigen and ISS-ODN.
We previously focused on developing a vaccine delivery system that efficiently delivers antigen to the cytosol of APCs to enhance MHC Class I processing and presentation as a means to augment CTL activity.17, 18 In this study, we hypothesized that incorporating the molecular adjuvant ISS-ODN in the LLO-containing liposome-based vaccine delivery system would enhance the cell-mediated immune response, and that the cellular immune response against a model antigen would be skewed toward the Th1-type. This delivery system exerts its effects after entering the endocytic pathway of APCs where the pH-sensitive liposomes are destabilized by the acidic pH and release their contents.6 Hence, ISS-ODN was an obvious molecular adjuvant to be incorporated and tested for its potential use in our LLO-containing pH-sensitive liposomal delivery platform since its main target receptor, TLR9, resides in endosomes.27 The rationale behind this approach is that ISS-ODN would be delivered to endosomes to interact with TLR9 and stimulate an innate immune response, while LLO would breach the endosomal membranes and promote the release of antigen into the cytosol for processing and presentation through the classical MHC I pathway. While Campbell et al.28 have shown both CpG-sequence dependence and TLR9 dependence for cytokine production in a murine model and Chikh et al.29 have demonstrated TLR9 dependence for cell activation from liposomal CpG-containing ODNs, it should be noted that there may be as yet unidentified cytosolic receptor(s) for ISS-ODNs that are activated upon the release and delivery of ISS-ODNs into the cytosol in our delivery systems, especially when one considers the existence of various PAMP receptors that have been identified in the cytosol. The in vitro and in vivo data presented in this report clearly demonstrate that enhanced cellular immunity and Th1-skewing are achieved and the use of ISS-ODN and antigen co-encapsulated in LLO-liposomes provides an efficient and effective approach, particularly when cellular immunity is required. These liposome formulations stimulate a strong cellular immune response while stimulating a robust humoral immune response at the same time. Testing of this class of formulation for protection against a significant health risk is surely justified at this point.
In conclusion, results in this study show that LLO-containing liposomes are capable of efficiently delivering both antigen and adjuvant to stimulate: (i) a robust CTL response, (ii) IFN-γ-secreting CD4+ and CD8+ T cells, and (iii) the production of Th1-type antibodies. The safety and efficacy of ISS-ODN have been demonstrated in clinical trials.7, 30 Because of their uptake by APCs and their proven safety in currently marketed products such as Doxil, liposomes are a promising delivery system. As demonstrated here, these components may be combined with LLO for an effective vaccine delivery system to target antigens to the cytosol.
ACKNOWLEDGEMENTS
The authors would like to thank Chester Provoda, Ph.D. for critical reading of this manuscript and helpful discussions. This work was supported by grants R01 AI047173 to KDL. CDA was a supported by GM008353 and the American Foundation for Pharmaceutical Education.
REFERENCES
- 1.Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5:505–17. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- 2.Ott G, Barchfeld GL, Van Nest G. Enhancement of humoral response against human influenza vaccine with the simple submicron oil/water emulsion adjuvant MF59. Vaccine. 1995;13:1557–62. doi: 10.1016/0264-410x(95)00089-j. [DOI] [PubMed] [Google Scholar]
- 3.Salerno-Goncalves R, Sztein MB. Cell-mediated immunity and the challenges for vaccine development. Trends Microbiol. 2006;14:536–42. doi: 10.1016/j.tim.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Datta SK, Cho HJ, Takabayashi K, Horner AA, Raz E. Antigen-immunostimulatory oligonucleotide conjugates: mechanisms and applications. Immunol Rev. 2004;199:217–26. doi: 10.1111/j.0105-2896.2004.00149.x. [DOI] [PubMed] [Google Scholar]
- 5.Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat Med. 2005;11:S63–8. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- 6.Lee KD, Oh YK, Portnoy DA, Swanson JA. Delivery of macromolecules into cytosol using liposomes containing hemolysin from Listeria monocytogenes. J Biol Chem. 1996;271:7249–52. [PubMed] [Google Scholar]
- 7.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 8.Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–8. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 9.Cho HJ, Takabayashi K, Cheng PM, Nguyen MD, Corr M, Tuck S, Raz E. Immunostimulatory DNA-based vaccines induce cytotoxic lymphocyte activity by a T-helper cell-independent mechanism. Nat Biotechnol. 2000;18:509–14. doi: 10.1038/75365. [DOI] [PubMed] [Google Scholar]
- 10.Tighe H, Takabayashi K, Schwartz D, Marsden R, Beck L, Corbeil J, Richman DD, Eiden JJ, Jr., Spiegelberg HL, Raz E. Conjugation of protein to immunostimulatory DNA results in a rapid, long-lasting and potent induction of cell-mediated and humoral immunity. Eur J Immunol. 2000;30:1939–47. doi: 10.1002/1521-4141(200007)30:7<1939::AID-IMMU1939>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Heit A, Schmitz F, O’Keeffe M, Staib C, Busch DH, Wagner H, Huster KM. Protective CD8 T cell immunity triggered by CpG-protein conjugates competes with the efficacy of live vaccines. J Immunol. 2005;174:4373–80. doi: 10.4049/jimmunol.174.7.4373. [DOI] [PubMed] [Google Scholar]
- 12.Wagner H. The immunogenicity of CpG-antigen conjugates. Adv Drug Deliv Rev. 2009;61:243–7. doi: 10.1016/j.addr.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Heit A, Schmitz F, Haas T, Busch DH, Wagner H. Antigen co-encapsulated with adjuvants efficiently drive protective T cell immunity. Eur J Immunol. 2007;37:2063–74. doi: 10.1002/eji.200737169. [DOI] [PubMed] [Google Scholar]
- 14.Krishnamachari Y, Salem AK. Innovative strategies for co-delivering antigens and CpG oligonucleotides. Adv Drug Deliv Rev. 2009;61:205–17. doi: 10.1016/j.addr.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heit A, Huster KM, Schmitz F, Schiemann M, Busch DH, Wagner H. CpG-DNA aided cross-priming by cross-presenting B cells. J Immunol. 2004;172:1501–7. doi: 10.4049/jimmunol.172.3.1501. [DOI] [PubMed] [Google Scholar]
- 16.Maurer T, Heit A, Hochrein H, Ampenberger F, O’Keeffe M, Bauer S, Lipford GB, Vabulas RM, Wagner H. CpG-DNA aided cross-presentation of soluble antigens by dendritic cells. Eur J Immunol. 2002;32:2356–64. doi: 10.1002/1521-4141(200208)32:8<2356::AID-IMMU2356>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 17.Mandal M, Lee KD. Listeriolysin O-liposome-mediated cytosolic delivery of macromolecule antigen in vivo: enhancement of antigen-specific cytotoxic T lymphocyte frequency, activity, and tumor protection. Biochim Biophys Acta. 2002;1563:7–17. doi: 10.1016/s0005-2736(02)00368-1. [DOI] [PubMed] [Google Scholar]
- 18.Mandal M, Kawamura KS, Wherry EJ, Ahmed R, Lee KD. Cytosolic delivery of viral nucleoprotein by listeriolysin O-liposome induces enhanced specific cytotoxic T lymphocyte response and protective immunity. Mol Pharm. 2004;1:2–8. doi: 10.1021/mp034021m. [DOI] [PubMed] [Google Scholar]
- 19.Portnoy DA, Jones S. The cell biology of Listeria monocytogenes infection (escape from a vacuole) Ann N Y Acad Sci. 1994;730:15–25. doi: 10.1111/j.1749-6632.1994.tb44235.x. [DOI] [PubMed] [Google Scholar]
- 20.Stier EM, Mandal M, Lee KD. Differential cytosolic delivery and presentation of antigen by listeriolysin O-liposomes to macrophages and dendritic cells. Mol Pharm. 2005;2:74–82. doi: 10.1021/mp049896v. [DOI] [PubMed] [Google Scholar]
- 21.Mathew E, Hardee GE, Bennett CF, Lee KD. Cytosolic delivery of antisense oligonucleotides by listeriolysin O-containing liposomes. Gene Ther. 2003;10:1105–15. doi: 10.1038/sj.gt.3301966. [DOI] [PubMed] [Google Scholar]
- 22.Sanderson S, Shastri N. LacZ inducible, antigen/MHC-specific T cell hybrids. Int Immunol. 1994;6:369–76. doi: 10.1093/intimm/6.3.369. [DOI] [PubMed] [Google Scholar]
- 23.Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol. 2004;4:249–58. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 24.van Duin D, Medzhitov R, Shaw AC. Triggering TLR signaling in vaccination. Trends Immunol. 2006;27:49–55. doi: 10.1016/j.it.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Krieg A. M. a. D., H. L. CpG ODN As a Th1 Immune Enhancer for Prophylactic and Therapeutic Vaccines. In: Hackett C. J. a. H., D. A., editors. Vaccine Adjuvants. Humana Press Inc.; Totowa: 2006. pp. 87–110. [Google Scholar]
- 26.Klinman DM, Klaschik S, Sato T, Tross D. CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases. Adv Drug Deliv Rev. 2009;61:248–55. doi: 10.1016/j.addr.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol. 2006;7:49–56. doi: 10.1038/ni1280. [DOI] [PubMed] [Google Scholar]
- 28.Campbell JD, Cho Y, Foster ML, Kanzler H, Kachura MA, Lum JA, Ratcliffe MJ, Sathe A, Leishman AJ, Bahl A, McHale M, Coffman RL, Hessel EM. CpG-containing immunostimulatory DNA sequences elicit TNF-alpha-dependent toxicity in rodents but not in humans. J Clin Invest. 2009;119:2564–76. doi: 10.1172/JCI38294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chikh G, de Jong SD, Sekirov L, Raney SG, Kazem M, Wilson KD, Cullis PR, Dutz JP, Tam YK. Synthetic methylated CpG ODNs are potent in vivo adjuvants when delivered in liposomal nanoparticles. Int Immunol. 2009;21:757–67. doi: 10.1093/intimm/dxp044. [DOI] [PubMed] [Google Scholar]
- 30.Barry M, Cooper C. Review of hepatitis B surface antigen-1018 ISS adjuvant-containing vaccine safety and efficacy. Expert Opin Biol Ther. 2007;7:1731–7. doi: 10.1517/14712598.7.11.1731. [DOI] [PubMed] [Google Scholar]