Abstract
N-linked glycoproteins play important roles in biological processes, including cell-to-cell recognition, growth, differentiation, and programmed cell death. Specific N-linked glycoprotein changes are associated with disease progression and identification of these N-linked glycoproteins has potential for use in disease diagnosis, prognosis, and prediction of treatments. In this review, we summarize common strategies for N-linked glycoprotein characterization and applications of these strategies to identification of glycoprotein changes associated with disease states. We also review the N-linked glycoproteins altered in diseases such as breast cancer, lung cancer, and prostate cancer. Although assays for these glycoproteins have potential clinical utility, research is needed to translate these glycoproteins to clinical biomarkers.
Keywords: Disease association, Glycoproteomics, N-linked glycoproteins
1 Introduction
Protein glycosylation is one of the most common modifications made to proteins [1]. Glycans can be attached to proteins either via an amide group (N-linked glycosylation) or a hydroxyl group (O-linked glycosylation). N- and O-linked glycosylations are distinct protein modifications; they occur through different biosynthetic pathways, and potentially have independent functions [2]. N-linked glycosylation plays fundamental roles in many biological processes such as cell adhesion, cell migration, and signal transduction [3]. Abnormal expression of N-linked glycoproteins has been observed in various diseases, and previous studies have shown that glycoprotein changes can be used as biomarkers for disease diagnosis [4, 5]. The majority of the biomarkers used in diagnosis, prognosis, and prediction are N-linked glycosylated proteins [6]. Examples include carbohydrate antigen CA-19–9 used for diagnosis of colon cancer [7], prostate-specific antigen (PSA) for prostate cancer [8], α-fetoprotein for liver cancer [9], and β-human chorionic gonadotropin for germ cell tumors [10]. In addition, since N-linked glycoproteins are most membrane-bound proteins or extracellular proteins [11], they are accessible for therapeutic purposes, such as Her2 receptor for breast cancer therapy [12].
For in-depth characterization of N-linked glycoproteins to identify disease-associated glycoprotein changes, glycoproteins must be efficiently separated from other cellular components before further characterization. Several methodologies have been developed to achieve this.
2 Methodologies used to identify N-linked glycoproteins associated with disease
2.1 Lectin-affinity chromatography
Glycoproteins or glycopeptides can be affinity isolated with an immobilized glycan-binding protein such as lectin [13]. Certain lectins possess affinity for particular oligosaccharide moieties, and thus various lectins bind to different structures of glycans on glycoconjugates [14–16]. Various lectins have been used in glycoprotein isolation: examples include Con A for high mannose type N-glycans, Lens culinaris agglutinin for core-fucosylated N-glycans, Sambucus nigra for sialylated N-glycans (Table 1). Although lectins do not possess absolute specificity, subtle differences in glycoprotein profiles can be detected [17]. Advantages of this selection approach are reversible binding, multiple affinity selectors [18], and retrievability of glycans for characterization and quantification [19]. For instance, isotopic glycosidase elution, labeling on lectin-column chromatography, and iTRAQ 8-plex isobaric tags have been used to identify and quantify N-glycosylation sites in lung cancer sera [20].
Table 1.
Specificity of selected lectins used to capture specific glycoproteins
| Name/abbreviation | Origin | Binding preferencea) |
|---|---|---|
| LCA | Lens culinaris | Fuca1-6GlcNAc and a-Man, a-Glc |
| PSA | Pisum sativum | 1,6-Fucosylation of the trimannosyl cor e and a-Man |
| AAL | Aleuria aurantia | Fuc al,6-GlcNAc4Fuc al3/l,4-GlcNAc, Fuc al,2-Gal |
| AAA | Anguilla anguilla | Fuc linked to the GlcNAc |
| LTA | Lotus tetragonolobus | Fuc a1,3/1,4-GlcNAc, Fuc a1,2-Gal, Lex |
| Con A | Concanavalin | Two nonsubstituted or C2-substituted a-mannopyranosyl residues in one molecule |
| Man unsubstituted a t C3, C4, C6 | ||
| RCA | Ricinus communis | Terminal Galb1,4GlcNAc, Gal |
| WGA | Wheat germ | Bisected hybrid type sugar chains, terminal a-GlcNAc or chitobiose, glycoprotein with clustered NeuAc |
| SNA | Sambucus nigra | NeuAc(a-2,6)Gal(b-1,4)GlcNAc4(a-2,3)NeuAc, no interaction with terminal b-Gal, b-GalNAc or NeuAc-GalNAc |
| MAL | Maackia amurensis | NeuAc a23Gal bl,4GlcNAc |
Fuc, fucose; Glc, glucose; Man, mannose.
2.2 Hydrazide chemistry
Zhang et al. reported a method for selective isolation, identification, and quantification of N-linked glycoproteins via hydrazide chemistry [21]. This method involves the conjugation of glycoproteins or glycopeptides to a solid support after oxidization of the carbohydrates on the glycoproteins/glycopeptides and specific release of formerly N-linked glycopeptides by peptide-N-glycosidase F [21]. This method has been applied to the identification of glycoprotein changes associated with different histological subtypes of ovarian cancer [22], aggressive prostate cancer [23], lung cancer [24], and skin cancer [25].
The hydrazide chemistry methods can be modified to analyze cell-surface glycoproteins [26,27], sialylation-specific glycopeptides [28,29], and glycopeptides containing glycans [30]. Rather than using N-linked glycopeptides for quantification, Chen et al. quantified nonglycosylated peptides derived from the glycoproteins immobilized on hydrazide beads and identified glycoproteins associated with hepatocellular carcinoma (HCC) [31].
2.3 Boronic acid
Affinity ligands based on boronic acid derivatives have been used to capture carbohydrates, nucleosides, glycolipids, RNA, and glycoproteins [32, 33]. The principle of boronate-affinity chromatography is that boronic acids can form covalent ester bonds with cis-diols under basic conditions so that glycopeptides can be separated from other molecules [34, 35]. The boronate ester bond can be reversibly hydrolyzed under neutral or acidic conditions. Suksrichavalit et al. reported synthesis of a “clickable” boronic acid ligand by introduction of a terminal acetylene group into commercially available 3-aminophenyl boronic acid [36]. Compared to other boronic acid methods, the new clickable boronic acid approach showed superior effectiveness in separating model glycoproteins (ovalbumin and RNase B) from BSA and RNase A in the presence of crude E. coli proteins.
2.4. Metabolic incorporation of sugar analogs for glycoprotein isolation
Metabolic oligosaccharide engineering is an emerging strategy for glycoprotein profiling. Synthetic monosaccharides containing azide [37–40], ketone [41], and thiol [42] functional groups have been metabolically incorporated onto glycoproteins in cells and living organisms, thereby arming them for covalent reaction with affinity probes. Azido monosaccharides are useful analogs due to the small size of the azide, absence in biological systems, and orthogonality to cellular functional groups [43]. Azide-labeled glycoproteins can be detected through the reaction with phosphines using Staudinger ligation [44] or alkynes using click chemistry [45,46].
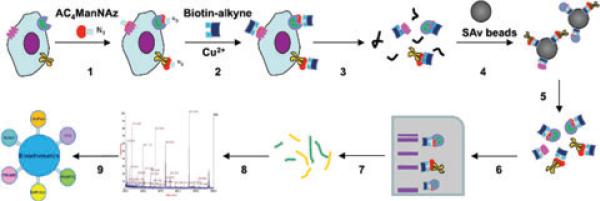
Yang et al. used metabolic oligosaccharide engineering to identify metastasis-associated cell-surface sialoglycoproteins in prostate cancer via the metabolic incorporation of AC4ManNAz [47]. The experimental workflow of this study is illustrated in Fig. 1 [47]. First, metabolic labeling of non-metastatic and highly metastatic prostate cancer cell lines was conducted using an azide-containing mannose analog. Second, the azide-labeled glycoproteins were chemoselectively conjugated to biotinylated alkyne. Third, the biotinylated proteins were enriched by streptavidin capture. Finally, the enriched proteins were separated by 1D gel electrophoresis, digested to peptides, and identified by LC-MS/MS. Using this method, a number of glycoproteins are identified with overexpression in highly metastatic prostate cancer cell lines. Bertozzi et al. successfully profiled the cell-surface glycoproteins in a prostate cancer cell line (PC-3 cells) and primary human prostate cancer tissue treated with peracetylated N-azidoacetylgalactosamine [48]. Over 70 cell- surface glycoproteins were identified, and CD146 and integrin β-4 were biochemically validated in this study.
Figure 1.
Experimental workflow of analysis of cell-surface sialoglycoproteins using click chemistry. (1) Metabolic labeling of cells with peracetylated azidomannose (AC4ManNAz). (2) Chemoselective conjugation of azido sugars with a biotinylated alkyne capture reagent via Cu (I)-catalyzed click chemistry. (3) Lysis of labeled cells. (4) Affinity purification using streptavidin (SAv) resins. (5) Elution of captured sialoglycoproteins. (6) SDS-PAGE separation of sialoglycoproteins. (7) Isolation of gel slices and subsequent digestion and release of peptides. (8) Analysis of peptides by LC-MS/MS. (9) Bioinformatic analysis.
2.5 Other methods
Other methodologies have also been used to analyze glycoproteins. SEC can be used to isolate glycopeptides as glycopeptides have increased mass compared to nonglycopeptides [49]. Hydrophilic interaction LC followed by partial deglycosylation [50] and an online combination of RP/RP and porous graphitic carbon LC [51] are chromatographic methods for glycoprotein isolation. An innovative fluorescence-based multiplexed proteomics technology was also reported for identification and differential analysis of both glycosylation patterns and protein expression levels in a single experiment using gel electrophoresis and serial staining with Pro-Q Emerald 488 glycoprotein stain and SYPRO Ruby protein stain for glycosylation and protein, respectively [52].
3 Disease-associated N-linked glycoproteins identified by glycoproteomics
A number of N-linked glycoprotein changes have been identified of association with different diseases using glycoproteomic approaches (Table 2). Studies have focused on common cancers including lung cancer, HCC, skin cancer, prostate cancer, ovarian cancer, and breast cancer. The cancer-associated glycoproteins were identified by different methodologies including lectin-affinity chromatography, hydrazide chemistry, and metabolic labeling. Many of these cancer-associated glycoproteins are extracellular proteins, such as cathepsin-L, tenascin-C, and versican [53].
Table 2.
Disease-associated glycoproteins identified by glycoproteomics
| Protein name | Alternation | Diseases | Reference | Method used |
|---|---|---|---|---|
| Alpha-1-antichymotrypsin (ACT) | Upregulated | Nonsmall cell lung cancer (NSCLC) | [24] | Hydrazide chemistry |
| Alpha-1-antichymotrypsin (ACT) | Upregulated | Hepatocellular carcinoma (HCC) | [81] | Hydrazide chemistry |
| Alpha-1-antitrypsin, 40 kDa variant | Upregulated | HIV | [82] | 2DE analysis |
| Arylsulfatase B | Upregulated | Skin cancer | [25] | Hydrazide chemistry |
| Cathepsin L | Upregulated | Aggressive prostate cancer | [53] | Hydrazide chemistry |
| CEA5 | Upregulated | Mucinous ovarian carcinoma | [54] | Hydrazide chemistry |
| CEA6 | Upregulated | Mucinous ovarian carcinoma | [54] | Hydrazide chemistry |
| CUB domain containing protein 1 | Upregulated | Metastatasic prostate cancer | [47] | Metabolic labeling |
| ER-associated DNAJ (ERdj3) | Upregulated | Paclitaxel-resistant oviarian cancer cells | [83] | Fluorescence-based multiplexed proteomics and multilectin affinity chromatography |
| Fucosylated GP73 | Upregulated | Hepatocellular carcinoma (HCC) | [69] | Lectin |
| Fucosylated Haptoglobin | Upregulated | Lung cancer | [70] | 2DE analysis |
| Galectin-3-binding protein (Gal3BP) (Mac-2 BP, S90K) | Upregulated | Most ovarian cancer subtypes | [54] | Hydrazide chemistry |
| Galectin-3-binding protein (Gal3BP)(Mac-2 BP, S90K) | Upregulated | Hepatocellular carcinoma (HCC) | [31] | Hydrazide chemistry |
| Insulin-like growth factor binding protein 3 (IGFBP-3) | Downregulated | Hepatocellular carcinoma (HCC) | [31] | Hydrazide chemistry |
| Insulin-like growth factor binding protein 3 (IGFBP-3) | Downregulated | Nonsmall cell lung cancer (NSCLC) | [24] | Hydrazide chemistry |
| Mesothelin | Upregulated | High-grade serous, low-grade serous, and transitional-cell ovarian carcinoma | [54] | Hydrazide chemistry |
| Metalloproteinase inhibitor 1 (TIMP1), glycosylated form | Upregulated | Lung cancer | [84] | Lectin |
| Microfibrillar-associated protein 4 | Upregulated | Aggressive prostate cancer | [53] | Hydrazide chemistry |
| Palmitoyl-protein thioesterase 1 (PPT1) | Upregulated | Paclitaxel-resistant ovarian cancer cells | [83] | Fluorescence-based multiplexed proteomics and multilectin affinity chromatography |
| Periostin | Upregulated | Aggressive prostate cancer | [53] | Hydrazide chemistry |
| Periostin | Upregulated | Most ovarian cancer subtypes | [54] | Hydrazide chemistry |
| Prohibitin 1 (PHB) | Upregulated | Liver cancer | [85] | Lectin |
| Prostaglandin D synthase (lipocalin-type) (L-PGDS) | Downregulated | Nonsmall cell lung cancer (NSCLC) | [24] | Hydrazide chemistry |
| Tenascin-C | Upregulated | Skin cancer | [25] | Hydrazide chemistry |
| Thrombospondin 1 (TSP-1) | Downregulated | Hepatocellular carcinoma (HCC) | [31] | Hydrazide chemistry |
| Triose phosphate isomerase (TPI) | Upregulated | Paclitaxel-resistant oviarian cancer cells | [83] | Fluorescence-based multiplexed proteomics and multilectin affinity chromatography |
| Tumor rejection anatigen (gp96) | Upregulated | Paclitaxel resistant oviarian cancer cells | [83] | Fluorescence-based multiplexed proteomics and multilectin affinity chromatography |
| Versican | Upregulated | Breast cancer | [28] | Hydrazide chemistry |
| Versican | Upregulated | Most ovarian cancer subtypes | [54] | Hydrazide chemistry |
Interestingly, abnormal expressions of certain glycoproteins are associated with more than one type of cancer. For example, elevated alpha-1-antichymotrypsin is associated with both nonsmall cell lung carcinoma and HCC. Upregulated galectin-3-binding protein (Gal3BP or Mac-2 BP) is associated with both HCC and ovarian cancer, whereas downregulated expression of insulin-like growth factor binding protein 3 is associated with both HCC and nonsmall cell lung carcinoma. Elevated periostin levels are associated with both aggressive prostate cancer and ovarian cancer, and elevations of versican level are associated with both breast cancer [28] and ovarian cancer [54]. Most of these proteins are extracellular matrix (ECM) proteins or interact with ECM proteins. Galectin-3 binding protein is involved in tumor cell adhesion to the ECM [55] and can enhance extracellular level of protease in HT-29 cells [56]. Periostin is an important ECM protein involved in development and adhesion [57]. Periostin interacts with many other ECM proteins, such as fibronectin, collagen V, and tenascin-C [58, 59]. Epithelialmesenchymal transition (EMT), a process of morphologic transdifferentiation, is one of the critical steps of tumor metastasis [60–62]. EMT of cancer cells can enhance invasion into the surrounding desmoplastic stroma [63]. Periostin is recently reported as a member of the EMT program and periostin expression was found to correlate closest with progression variables in nonsmall cell lung cancer [63]. The opposite mechanism, mesenchymal-epithelial transition (MET), has been recently reported for the ECM protein versican in vitro [63]. Versican may play a critical role in intercellular signaling, connecting cellular reaction with the ECM and regulation of cell motility, growth, and differentiation [64]. Versican, as a putative indicator of MET, did not behave conversely to EMT proteins. Instead, versican behaved concordant to periostin in both stroma and epithelia of nonsmall cell lung cancer [63]. Although, the function and the subcellular compartment of the epithelial protein remain unclear, expression alteration of EMT-MET proteins has been documented in both desmolastic stroma and carcinoma cells [65,66].
Changes of many glycoproteins have been identified in multiple cancer types. The question is raised whether protein changes are a general biophysical effect of cancer or whether they are specific to certain cancer types. PSA is the current screening marker for prostate cancer; however, PSA is organ specific but not disease specific [67]. Development of cancer does not actually result in higher levels of PSA while the enlarged glands in men with benign prostatic hyperplasia secrete more PSA. The prostate gland leaks PSA into the bloodstream resulting in a higher blood level of PSA in men with prostatic hyperplasia and in those with cancer. Therefore, considering the size of prostate and adjusting for the value of PSA improves the accuracy of PSA as a prostate cancer biomarker [68]. Most disease-associated glycoproteins may not be organ specific like PSA, but might be cancer specific. For specific cancer diagnosis, cancer-specific glycoproteins can be combined with other markers and additional medical approaches to increase the accuracy of tests.
4 Quantification of glycosylated isoforms may improve biomarker performance
It is worthwhile to note that particular glycosylation forms of a glycoprotein are associated with particular cancers (Table 2). Advances in proteomic technologies have stimulated a great interest not only in glycoprotein identification, but also in comprehensive analysis of glycosylation of each glycoprotein. These studies have revealed that specific glycoforms of a glycoprotein may be associated with diseases. For example, fucosylated GP73 is overexpressed in HCC [69], and fucosylated haptoglobin is associated with lung cancer [70]. Differential glycosylation of complex glycans in membrane-bound and/or extracellular glycoproteins have clinical relevance [71–73]. Using antibodies against glycoproteins and glycans, Lim et al. found that serum levels of certain glycoforms of soluble CD44v increased in particular cancers [74]. They used polyclonal anti-CD44v antibody as an immobilized capture antibody and antiglycosylation antibodies as detection antibodies. Sera from patients with cancers had significantly higher levels of soluble CD44v molecules carrying cancer-associated glycotopes—sialy Lewis x and sialy Lewis a—compared to normal individuals, whereas the levels of CD44v molecules carrying nonmalignant glycotopes—sialyl 6-sulfo Lewis x and disialyl Lewis a—were higher in the sera of patients with benign diseases than those in patients with cancers.
Lectin immunosorbent assay was used to analyze the different glycosylation patterns of serum PSA and PSA from prostate cancer tissue [75,76]. In these studies, PSA was first captured with a PSA monoclonal antibody and then detected by a biotinylated lectin. Recently, our group performed simultaneous analysis of total, glycosylated, and sialylated PSA from prostate cancer and noncancerous tissues [77]. Selected reaction monitoring (SRM) was used to quantify total glycopeptides from PSA and sialylated PSA glycopeptide isolated from prostate cancer and noncancerous tissues. The abundance of glycosylated PSA and sialylated PSA was different relative to total PSA in prostate cancer and noncancerous tissues. These data showed that analysis of glycosylated PSA may improve the cancer specificity of this biomarker. Other reports have shown that specific glycoforms are associated with diseases [70, 78] and suggest that the quantification of different glycosylated isoforms of glycoproteins may provide unique information with clinical relevance.
5 Future directions
It is challenging to identify protein markers for disease diagnosis. With implementation of glycoproteomic methods, however, great progress has been made in identification of glycoproteins associated with various diseases. To increase the accuracy of diagnosis and predication of prognosis, associations of specific glycoforms with particular diseases will need to be determined. Use of a panel of proteins and glycoforms as well as other medical approaches may be combined to enhance accuracy. To identify particular cancer-specific proteins, organ-specific proteins may be identified and monitored in diseased tissues and body fluids [79]. Compared to global proteomics, glycoproteomics provide advantages of organ specificity and a focus on a subproteome to reduce sample complexity [80]. Organ-specific glycoproteins like PSA are potentially useful in disease diagnosis and should be explored as drug targets as the limited organ access will reduce the risk of side effects.
Acknowledgments
This work was supported by National Institutes of Health, National Cancer Institute, grant U01CA152813 and U24CA160036, and by National Heart Lung and Blood Institute contract N01-HV-00240 and grant P01HL107153.
Abbreviations
- ECM
extracellular matrix
- EMT
epithelial to mesenchymal transition
- HCC
hepatocellular carcinoma
- MET
mesenchymal-epithelial transition
- PSA
prostate-specific antigen
Footnotes
The authors have declared no conflict of interest.
References
- 1.Furukawa K, Kobata A. Protein glycosylation. Curr. Opin. Biotechnol. 1992;3:554–559. doi: 10.1016/0958-1669(92)90085-w. [DOI] [PubMed] [Google Scholar]
- 2.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian Y, Zhang H. Glycoproteomics and clinical applications. Proteomics Clin. Appl. 2010;4:124–132. doi: 10.1002/prca.200900161. [DOI] [PubMed] [Google Scholar]
- 4.Kay Li Q, Gabrielson E, Zhang H. Application of glycoproteomics for the discovery of biomarkers in lung cancer. Proteomics Clin. Appl. 2012;6:244–256. doi: 10.1002/prca.201100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peracaula R, Barrabes S, Sarrats A, Rudd PM, de Llorens R. Altered glycosylation in tumours focused to cancer diagnosis. Dis. Markers. 2008;25:207–218. doi: 10.1155/2008/797629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drake RR, Schwegler EE, Malik G, Diaz J, et al. Lectin capture strategies combined with mass spectrometry for the discovery of serum glycoprotein biomarkers. Mol. Cell. Proteomics. 2006;5:1957–1967. doi: 10.1074/mcp.M600176-MCP200. [DOI] [PubMed] [Google Scholar]
- 7.Gebauer G, Muller-Ruchholtz W. Tumor marker concentrations in normal and malignant tissues of colorectal cancer patients and their prognostic relevance. Anticancer Res. 1997;17:2939–2942. [PubMed] [Google Scholar]
- 8.Catalona WJ, Smith DS, Ratliff TL, Dodds KM, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. New Engl. J. Med. 1991;324:1156–1161. doi: 10.1056/NEJM199104253241702. [DOI] [PubMed] [Google Scholar]
- 9.Zhou L, Liu J, Luo F. Serum tumor markers for detection of hepatocellular carcinoma. World J. Gastroentero. 2006;12:1175–1181. doi: 10.3748/wjg.v12.i8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seregni E, Massimino M, Nerini Molteni S, Pallotti F, et al. Serum and cerebrospinal fluid human chorionic gonadotropin (hCG) and alpha-fetoprotein (AFP) in intracranial germ cell tumors. Int. J. Biol. Marker. 2002;17:112–118. doi: 10.1177/172460080201700206. [DOI] [PubMed] [Google Scholar]
- 11.Grigorian A, Torossian S, Demetriou M. T-cell growth, cell surface organization, and the galectin-glycoprotein lattice. Immunol. Rev. 2009;230:232–246. doi: 10.1111/j.1600-065X.2009.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simonds HM, Miles D. Adjuvant treatment of breast cancer: impact of monoclonal antibody therapy directed against the HER2 receptor. Expert Opin. Biol. Ther. 2007;7:487–491. doi: 10.1517/14712598.7.4.487. [DOI] [PubMed] [Google Scholar]
- 13.Cummings RD, Kornfeld S. Fractionation of asparagine-linked oligosaccharides by serial lectin-Agarose affinity chromatography. A rapid, sensitive, and specific technique. J. Biol. Chem. 1982;257:11235–11240. [PubMed] [Google Scholar]
- 14.Wu AM, Song SC, Tsai MS, Herp A. A guide to the carbohydrate specificities of applied lectins-2 (updated in 2000). Adv. Exp. Med. Biol. 2001;491:551–585. doi: 10.1007/978-1-4615-1267-7_37. [DOI] [PubMed] [Google Scholar]
- 15.Rudiger H, Gabius HJ. Plant lectins: occurrence, biochemistry, functions and applications. Glycoconj. J. 2001;18:589–613. doi: 10.1023/a:1020687518999. [DOI] [PubMed] [Google Scholar]
- 16.Hirabayashi J. Lectin-based structural glycomics: glycoproteomics and glycan profiling. Glycoconj. J. 2004;21:35–40. doi: 10.1023/B:GLYC.0000043745.18988.a1. [DOI] [PubMed] [Google Scholar]
- 17.Dai Z, Zhou J, Qiu SJ, Liu YK, et al. Lectin-based glycoproteomics to explore and analyze hepatocellular carcinoma-related glycoprotein markers. Electrophoresis. 2009;30:2957–2966. doi: 10.1002/elps.200900064. [DOI] [PubMed] [Google Scholar]
- 18.Jung K, Cho W, Regnier FE. Glycoproteomics of plasma based on narrow selectivity lectin affinity chromatography. J. Proteome. Res. 2009;8:643–650. doi: 10.1021/pr8007495. [DOI] [PubMed] [Google Scholar]
- 19.Madera M, Mechref Y, Klouckova I, Novotny MV. High-sensitivity profiling of glycoproteins from human blood serum through multiple-lectin affinity chromatography and liquid chromatography/tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007;845:121–137. doi: 10.1016/j.jchromb.2006.07.067. [DOI] [PubMed] [Google Scholar]
- 20.Ueda K, Takami S, Saichi N, Daigo Y, et al. Development of serum glycoproteomic profiling technique; simultaneous identification of glycosylation sites and site-specific quantification of glycan structure changes. Mol. Cell. Proteomics. 2010;9:1819–1828. doi: 10.1074/mcp.2010/000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Li XJ, Martin DB, Aebersold R. Identification and quantification of N-linked glycoproteins using hydrazide chemistry, stable isotope labeling and mass spectrometry. Nat. Biotechnol. 2003;21:660–666. doi: 10.1038/nbt827. [DOI] [PubMed] [Google Scholar]
- 22.Tian Y, Yao Z, Roden RB, Zhang H. Identification of glycoproteins associated with different histological subtypes of ovarian tumors using quantitative glycoproteomics. Proteomics. 2011;11:4677–4687. doi: 10.1002/pmic.201000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian Y, Bova GS, Zhang H. Quantitative glycoproteomic analysis of optimal cutting temperature-embedded frozen tissues identifying glycoproteins associated with aggressive prostate cancer. Anal. Chem. 2011;83:7013–7019. doi: 10.1021/ac200815q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng X, Hood BL, Sun M, Conrads TP, et al. Lung cancer serum biomarker discovery using glycoprotein capture and liquid chromatography mass spectrometry. J. Proteome Res. 2010;9:6440–6449. doi: 10.1021/pr100696n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian Y, Kelly-Spratt KS, Kemp CJ, Zhang H. Identification of glycoproteins from mouse skin tumors and plasma. Clin. Proteomics. 2008;4:117–136. doi: 10.1007/s12014-008-9014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wollscheid B, Bausch-Fluck D, Henderson C, O'Brien R, et al. Mass-spectrometric identification and relative quantification of N-linked cell surface glycoproteins. Nat. Biotechnol. 2009;27:378–386. doi: 10.1038/nbt.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gundry RL, Raginski K, Tarasova Y, Tchernyshyov I, et al. The mouse C2C12 myoblast cell surface N-linked glycoproteome: identification, glycosite occupancy, and membrane orientation. Mol. Cell. Proteomics. 2009;8:2555–2569. doi: 10.1074/mcp.M900195-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian Y, Esteva FJ, Song J, Zhang H. Altered expression of sialylated glycoproteins in breast cancer using hydrazide chemistry and mass spectrometry. Mol. Cell. Proteomics. 2012;11:M111 011403. doi: 10.1074/mcp.M111.011403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almaraz RT, Tian Y, Bhattarcharya R, Tan E, et al. Metabolic flux increases glycoprotein sialylation: implications for cell adhesion and cancer metastasis. Mol. Cell. Proteomics. 2012;11:M112 017558. doi: 10.1074/mcp.M112.017558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nilsson J, Ruetschi U, Halim A, Hesse C, et al. Enrichment of glycopeptides for glycan structure and attachment site identification. Nat. Methods. 2009;6:809–811. doi: 10.1038/nmeth.1392. [DOI] [PubMed] [Google Scholar]
- 31.Chen R, Tan Y, Wang M, Wang F, et al. Development of glycoprotein capture-based label-free method for the high-throughput screening of differential glycoproteins in hepatocellular carcinoma. Mol. Cell. Proteomics. 2011;10:M110 006445. doi: 10.1074/mcp.M110.006445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawkins CJ, Lavin MF, Parry DL, Ross IL. Isolation of 3,4-dihydroxyphenylalanine-containing proteins using boronate affinity chromatography. Anal. Biochem. 1986;159:187–190. doi: 10.1016/0003-2697(86)90326-x. [DOI] [PubMed] [Google Scholar]
- 33.Liu XC, Scouten WH. Boronate affinity chromatography. Methods Mol. Biol. 2000;147:119–128. doi: 10.1007/978-1-60327-261-2_12. [DOI] [PubMed] [Google Scholar]
- 34.Preinerstorfer B, Lammerhofer M, Lindner W. Synthesis and application of novel phenylboronate affinity materials based on organic polymer particles for selective trapping of glycoproteins. J. Sep. Sci. 2009;32:1673–1685. doi: 10.1002/jssc.200800679. [DOI] [PubMed] [Google Scholar]
- 35.Ren L, Liu Y, Dong M, Liu Z. Synthesis of hydrophilic boronate affinity monolithic capillary for specific capture of glycoproteins by capillary liquid chromatography. J. Chromatogr. A. 2009;1216:8421–8425. doi: 10.1016/j.chroma.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 36.Suksrichavalit T, Yoshimatsu K, Prachayasittikul V, Bulow L, Ye L. “Clickable” affinity ligands for effective separation of glycoproteins. J. Chromatogr. A. 2010;1217:3635–3641. doi: 10.1016/j.chroma.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 37.Saxon E, Bertozzi CR. Cell surface engineering by a modified Staudinger reaction. Science. 2000;287:2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- 38.Vocadlo DJ, Hang HC, Kim EJ, Hanover JA, et al. A chemical approach for identifying O-GlcNAc-modified proteins in cells. Proc. Natl. Acad. Sci. USA. 2003;100:9116–9121. doi: 10.1073/pnas.1632821100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hang HC, Yu C, Kato DL, Bertozzi CR. A metabolic labeling approach toward proteomic analysis of mucin-type O-linked glycosylation. Proc. Natl. Acad. Sci. USA. 2003;100:14846–14851. doi: 10.1073/pnas.2335201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawa M, Hsu TL, Itoh T, Sugiyama M, et al. Glycoproteomic probes for fluorescent imaging of fucosylated glycans in vivo. Proc. Natl. Acad. Sci. USA. 2006;103:12371–12376. doi: 10.1073/pnas.0605418103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahal LK, Yarema KJ, Bertozzi CR. Engineering chemical reactivity on cell surfaces through oligosaccharide biosynthesis. Science. 1997;276:1125–1128. doi: 10.1126/science.276.5315.1125. [DOI] [PubMed] [Google Scholar]
- 42.Sampathkumar SG, Li AV, Jones MB, Sun Z, Yarema KJ. Metabolic installation of thiols into sialic acid modulates adhesion and stem cell biology. Nat. Chem. Biol. 2006;2:149–152. doi: 10.1038/nchembio770. [DOI] [PubMed] [Google Scholar]
- 43.Bond MR, Kohler JJ. Chemical methods for glycoprotein discovery. Curr. Opin. Chem. Biol. 2007;11:52–58. doi: 10.1016/j.cbpa.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 44.Nilsson BL, Kiessling LL, Raines RT. Staudinger ligation: a peptide from a thioester and azide. Org. Lett. 2000;2:1939–1941. doi: 10.1021/ol0060174. [DOI] [PubMed] [Google Scholar]
- 45.Speers AE, Cravatt BF. Profiling enzyme activities in vivo using click chemistry methods. Chem. Biol. 2004;11:535–546. doi: 10.1016/j.chembiol.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 46.Agard NJ, Prescher JA, Bertozzi CR. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 2004;126:15046–15047. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- 47.Yang L, Nyalwidhe JO, Guo S, Drake RR, Semmes OJ. Targeted identification of metastasis-associated cell-surface sialoglycoproteins in prostate cancer. Mol. Cell. Proteomics. 2011;10:M110 007294. doi: 10.1074/mcp.M110.007294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hubbard SC, Boyce M, McVaugh CT, Peehl DM, et al. Cell surface glycoproteomic analysis of prostate cancer-derived PC-3 cells. Bioorg. Med. Chem. Lett. 2011;21:4945–4950. doi: 10.1016/j.bmcl.2011.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alvarez-Manilla G, Atwood J, 3rd, Guo Y, Warren NL, et al. Tools for glycoproteomic analysis: size exclusion chromatography facilitates identification of tryptic glycopeptides with N-linked glycosylation sites. J. Proteome. Res. 2006;5:701–708. doi: 10.1021/pr050275j. [DOI] [PubMed] [Google Scholar]
- 50.Hagglund P, Bunkenborg J, Elortza F, Jensen ON, et al. A new strategy for identification of N-glycosylated proteins and unambiguous assignment of their glycosylation sites using HILIC enrichment and partial deglycosylation. J. Proteome. Res. 2004;3:556–566. doi: 10.1021/pr034112b. [DOI] [PubMed] [Google Scholar]
- 51.Lam MP, Lau E, Siu SO, Ng DC, et al. Online combination of reversed-phase/reversed-phase and porous graphitic carbon liquid chromatography for multicomponent separation of proteomics and glycoproteomics samples. Electrophoresis. 2011;32:2930–2940. doi: 10.1002/elps.201100092. [DOI] [PubMed] [Google Scholar]
- 52.Ge Y, Rajkumar L, Guzman RC, Nandi S, et al. Multiplexed fluorescence detection of phosphorylation, glycosylation, and total protein in the proteomic analysis of breast cancer refractoriness. Proteomics. 2004;4:3464–3467. doi: 10.1002/pmic.200400957. [DOI] [PubMed] [Google Scholar]
- 53.Tian Y, Bova GS, Zhang H. Quantitative glycoproteomic analysis of optimal cutting temperature-embedded frozen tissues identifying glycoproteins associated with aggressive prostate cancer. Anal. Chem. 2011;83:7013–7019. doi: 10.1021/ac200815q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian Y, Yao Z, Roden RB, Zhang H. Identification of glycoproteins associated with different histological subtypes of ovarian tumors using quantitative glycoproteomics. Proteomics. 2011;11:4677–4687. doi: 10.1002/pmic.201000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sasaki T, Brakebusch C, Engel J, Timpl R. Mac-2 binding protein is a cell-adhesive protein of the extracellular matrix which self-assembles into ring-like structures and binds beta1 integrins, collagens and fibronectin. EMBO J. 1998;17:1606–1613. doi: 10.1093/emboj/17.6.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ulmer TA, Keeler V, Andre S, Gabius HJ, et al. The tumor-associated antigen 90K/Mac-2-binding protein secreted by human colon carcinoma cells enhances extracellular levels of promatrilysin and is a novel substrate of matrix metalloproteinases-2, -7 (matrilysin) and -9: Implications of proteolytic cleavage. Biochim. Biophys. Acta. 2010;1800:336–343. doi: 10.1016/j.bbagen.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 57.Ruan K, Bao S, Ouyang G. The multifaceted role of periostin in tumorigenesis. Cell. Mol. Life Sci. 2009;66:2219–2230. doi: 10.1007/s00018-009-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Norris RA, Damon B, Mironov V, Kasyanov V, et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J. Cell. Biochem. 2007;101:695–711. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takayama G, Arima K, Kanaji T, Toda S, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J. Allergy Clin. Immunol. 2006;118:98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 60.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat. Rev. Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 61.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 62.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 63.Soltermann A, Tischler V, Arbogast S, Braun J, et al. Prognostic significance of epithelial-mesenchymal and mesenchymal-epithelial transition protein expression in non-small cell lung cancer. Clin. Cancer Res. 2008;14:7430–7437. doi: 10.1158/1078-0432.CCR-08-0935. [DOI] [PubMed] [Google Scholar]
- 64.Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr. Opin. Cell. Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- 65.Kodama J, Hasengaowa, Kusumoto T, Seki N, et al. Prognostic significance of stromal versican expression in human endometrial cancer. Ann. Oncol. 2007;18:269–274. doi: 10.1093/annonc/mdl370. [DOI] [PubMed] [Google Scholar]
- 66.Sasaki H, Dai M, Auclair D, Fukai I, et al. Serum level of the periostin, a homologue of an insect cell adhesion molecule, as a prognostic marker in nonsmall cell lung carcinomas. Cancer. 2001;92:843–848. doi: 10.1002/1097-0142(20010815)92:4<843::aid-cncr1391>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 67.Caplan A, Kratz A. Prostate-specific antigen and the early diagnosis of prostate cancer. Am. J. Clin. Pathol. 2002;117:S104–S108. doi: 10.1309/C4UN-12LK-43HP-JXY3. [DOI] [PubMed] [Google Scholar]
- 68.Borgermann C, Kliner S, Swoboda A, Luboldt HJ, et al. Parameters to improve the specificity of the prostate-specific antigen. Early detection of prostate cancer. Urologe A. 2011;50:1095–1100. doi: 10.1007/s00120-011-2577-8. [DOI] [PubMed] [Google Scholar]
- 69.Block TM, Comunale MA, Lowman M, Steel LF, et al. Use of targeted glycoproteomics to identify serum glycoproteins that correlate with liver cancer in woodchucks and humans. Proc. Natl. Acad. Sci. USA. 2005;102:779–784. doi: 10.1073/pnas.0408928102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsai HY, Boonyapranai K, Sriyam S, Yu CJ, et al. Glycoproteomics analysis to identify a glycoform on haptoglobin associated with lung cancer. Proteomics. 2011;11:2162–2170. doi: 10.1002/pmic.201000319. [DOI] [PubMed] [Google Scholar]
- 71.Durand G, Seta N. Protein glycosylation and diseases: blood and urinary oligosaccharides as markers for diagnosis and therapeutic monitoring. Clin. Chem. 2000;46:795–805. [PubMed] [Google Scholar]
- 72.Drake PM, Cho W, Li B, Prakobphol A, et al. Sweetening the pot: adding glycosylation to the biomarker discovery equation. Clin. Chem. 2010;56:223–236. doi: 10.1373/clinchem.2009.136333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Galonic DP, Gin DY. Chemical glycosylation in the synthesis of glycoconjugate antitumour vaccines. Nature. 2007;446:1000–1007. doi: 10.1038/nature05813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lim KT, Miyazaki K, Kimura N, Izawa M, et al. Clinical application of functional glycoproteomics – dissection of glycotopes carried by soluble CD44 variants in sera of patients with cancers. Proteomics. 2008;8:3263–3273. doi: 10.1002/pmic.200800147. [DOI] [PubMed] [Google Scholar]
- 75.Meany DL, Zhang Z, Sokoll LJ, Zhang H, et al. Glycoproteomics for prostate cancer detection: changes in serum PSA glycosylation patterns. J. Proteome. Res. 2009;8:613–619. doi: 10.1021/pr8007539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Y, Tao SC, Bova GS, Liu AY, et al. Detection and verification of glycosylation patterns of glycoproteins from clinical specimens using lectin microarrays and lectin-based immunosorbent assays. Anal. Chem. 2011;83:8509–8516. doi: 10.1021/ac201452f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Y, Tian Y, Rezai T, Prakash A, et al. Simultaneous analysis of glycosylated and sialylated prostate-specific antigen revealing differential distribution of glycosylated prostate-specific antigen isoforms in prostate cancer tissues. Anal. Chem. 2011;83:240–245. doi: 10.1021/ac102319g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peracaula R, Tabares G, Royle L, Harvey DJ, et al. Altered glycosylation pattern allows the distinction between prostate-specific antigen (PSA) from normal and tumor origins. Glycobiology. 2003;13:457–470. doi: 10.1093/glycob/cwg041. [DOI] [PubMed] [Google Scholar]
- 79.Zhang H, Chan DW. Cancer biomarker discovery in plasma using a tissue-targeted proteomic approach. Cancer Epidem Biomar. 2007;16:1915–1917. doi: 10.1158/1055-9965.EPI-07-0420. [DOI] [PubMed] [Google Scholar]
- 80.Tian Y, Kelly-Spratt KS, Kemp CJ, Zhang H. Mapping tissue-specific expression of extracellular proteins using systematic glycoproteomic analysis of different mouse tissues. J. Proteome. Res. 2010;9:5837–5847. doi: 10.1021/pr1006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ishihara T, Fukuda I, Morita A, Takinami Y, et al. Development of quantitative plasma N-glycoproteomics using label-free 2D LC-MALDI MS and its applicability for biomarker discovery in hepatocellular carcinoma. J. Proteomics. 2011;74:2159–2168. doi: 10.1016/j.jprot.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 82.Zhang L, Jia X, Zhang X, Cao J, et al. Alpha-1 antitrypsin variants in plasma from HIV-infected patients revealed by proteomic and glycoproteomic analysis. Electrophoresis. 2010;31:3437–3445. doi: 10.1002/elps.201000153. [DOI] [PubMed] [Google Scholar]
- 83.Di Michele M, Marcone S, Cicchillitti L, Della Corte A, et al. Glycoproteomics of paclitaxel resistance in human epithelial ovarian cancer cell lines: towards the identification of putative biomarkers. J. Proteomics. 2010;73:879–898. doi: 10.1016/j.jprot.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 84.Ueda K, Takami S, Saichi N, Daigo Y, et al. Development of serum glycoproteomic profiling technique; simultaneous identification of glycosylation sites and site-specific quantification of glycan structure changes. Mol. Cell. Proteomics. 2010 doi: 10.1074/mcp.2010/000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu Z, Wu J, Zha X. Up-regulation of prohibitin 1 is involved in the proliferation and migration of liver cancer cells. Sci. China Life Sci. 2011;54:121–127. doi: 10.1007/s11427-010-4130-1. [DOI] [PubMed] [Google Scholar]