Abstract
SJL mice represent a mouse model in which young adult females are susceptible to autoimmune disease while age matched male are relatively resistant. T cells primed in female SJL mice secrete cytokines associated with a Th1 phenotype. By contrast, T cells primed in males secrete cytokines associated with a Th2 phenotype. Activation of Th2-type T cells in males versus Th1 cells in females correlates with increased CD4+CD25+ T regulatory cells (Treg) in males. T cells primed in males depleted of CD4+CD25+ T cells preferentially secrete IFN-γ and decreased IL-4 and IL-10 compared to CD4+CD25+ T cells sufficient males suggesting that Treg influence subsequent antigen specific cytokine secretion. Treg from males and females exhibit equivalent in vitro T cell suppression. Treg from males expressed increased CTLA-4 and CD62L and preferentially secrete IL-10. These data suggest that an increased frequency of IL-10 secreting Treg in male SJL mice may contribute to resistance to autoimmune disease by favoring development of Th2 immune responses.
Keywords: autoimmunity, EAE, regulatory T cells, cytokines, mice
1. Introduction
T regulatory cells (Treg) are critical for the maintenance of peripheral tolerance and play pivotal roles in autoimmunity, allergy, tumor progression, transplantation and infectious diseases [1–4]. They also appear to be a major influence on the balance between protective and destructive immune responses [3], playing a critical down regulatory role by creating an anti-inflammatory environment. They inhibit both the expression of co-stimulatory molecules on antigen presenting cells (APC) [5–7] and secretion of pro-inflammatory cytokines in an IL-10-dependent manner [5, 8, 9]. Numerical or functional Treg deficiency has been linked to numerous Th1-associated autoimmune diseases [1, 2] including type 1 diabetes (T1D) [2, 10], arthritis [1, 2, 11–13], and multiple sclerosis [2, 14, 15]. Treg deficiency exacerbates T1D onset in nonobese diabetic mice [16] and increases severity of clinical disease in mice with experimental autoimmune encephalomyelitis (EAE) [17]. By contrast, adoptive transfer of Treg protects NOD mice from T1D development [16] and decreases EAE clinical severity [17–19]. These data highlight the important protective role of Treg in autoimmunity.
SJL mice exhibit normal protective immune responses to many viral and bacterial infections despite a variety of potential immune defects. These include deficiencies in CD5+ B cells, NK cells, a limited subset of T cell receptor genes, reduced IgE responses and a bias in the activation of Th1 responses in females and older males versus Th2 responses in young adult males [20]. Female SJL mice efficiently mount Th1-type response and are susceptible to both active and passive EAE at all ages [21–23]. By contrast, young male SJL mice (<8 weeks of age) are deficient in Th1-mediated delayed type hypersensitivity (DTH) response following immunization with a variety of protein antigens and are resistant to active EAE induction [21–23]. Th1 non-responsiveness in males, due to defective APC maturation [24], leads to the preferential induction of T cells secreting Th2 cytokines. This can be overcome by castration [25] or inhibition of IL-10 [26, 27]. Blocking IL-10 in males before and during T cell activation results in DTH responsiveness [26, 27] and treatment of females with recombinant IL-10 suppresses DTH response [27] suggesting that IL-10 plays a key role in the preferential induction of antigen specific Th2 cells and EAE resistance. The importance of APC in Treg proliferation and homeostasis [28–30], IL-10 secretion by Treg [1, 2, 17] and induction of Th1 responses in males following IL-10 neutralization [26, 27] prompted a comparison of the phenotype and function of Treg in young adult male and female SJL mice.
The data demonstrate an increase in Treg in male SJL mice compared to females with only minor differences in phenotype. Although the decreased Treg in females appears to be due to a maturational defect in thymus, no differences were found in suppressive capacity of these cells. However, depletion of Treg from males facilitates induction of T cells secreting Th1 type cytokines. Furthermore, males contain more Treg capable of IL-10 secretion, suggesting that both the increased Treg in males and their preferential secretion of IL-10 may contribute preferential Th2 activation and EAE resistance.
2. Materials and Methods
2.1. Mice
Male SJL mice were obtained from the Jackson Laboratory (Bar Harbor, ME) at 4–6 wk of age. Female SJL mice and C57BL/6 mice of both sexes were obtained from the National Cancer Institute (Frederick, MD) at 5–7 wk of age. Mice were housed at either the University of Southern California animal facility or the Biological Resources Unit of the Cleveland Clinic Foundation. All procedures were performed in compliance with the protocols approved by the Institutional Animal Care and Use Committees of the Keck School of Medicine, University of Southern California and the Cleveland Clinic Foundation.
2.2. Cell preparation, staining and flow cytometry
Single cell suspensions were prepared by pressing spleens and lymph nodes through 40μ nylon screens. For surface staining, cells were incubated for 15 min on ice with 1% mouse serum and 1% rat anti-mouse FcγIII/IIR in PBS containing 0.5% BSA (FACS buffer) to eliminate nonspecific binding. Cells were then stained on ice for 30 min with anti-CD4-PerCP (L3T4), anti-CD25-allophycocyanin and -PE (PC61), anti-GITR-FITC (DTA-1), anti-CD62L-PE (MEL-14), anti-CD103-FITC (M290), anti-TNFR2-PE (TR75-89); and rat IgG2b-FITC, rat IgG2a-PE and -FITC, and hamster IgG1-PE isotype control monoclonal antibodies (mAbs) (all from BD Biosciences, San Diego, CA) and washed 3× with FACS buffer. Intracellular CTLA-4 staining was performed using BD Biosciences reagents and protocol with slight modifications. Briefly, after staining with anti-CD4 and anti-CD25 mAbs, cells were fixed and permeabilized with cytofix/cytoperm for 20 min at 4 °C. The cells were then washed 2× with permeabilization/wash buffer and stained with anti-CTLA-4-PE (UC10-4F10-11) and hamster IgG2-PE (isotype control) for 40 min at 4 °C. The cells were then washed 2× with permeabilization/wash buffer and re-suspended in FACS buffer. Intracellular Foxp3 staining was performed following eBioscience, Inc. (San Diego, CA) protocol. Briefly, after surface staining, cells were re-suspended in fixation/permeabilization buffer for 16 h at 4 °C. The cells were then washed 3× with permeabilization buffer and stained with anti-Foxp3-PE (FJK-16s, eBioscience) and rat IgG2a-PE (isotype control) for 30 min at 4 °C. The cells were then washed 2× with permeabilization buffer and re-suspended in FACS buffer. Samples were acquired on a FACSCalibur (Becton Dickinson, San Jose, CA) and analyzed by FlowJo software (Tree Star, Inc. Ashland, OR).
2.3. Cell purification and activation
CD4+CD25+ T cells were purified by FACS (BD FACSAria™) after staining spleen cells with anti-CD4 (RM4-5) and anti-CD25 (PC61) mAbs. Purified CD4+CD25+ T cells were stimulated with plate-bound anti-CD3 (2μg/ml) in 96-well plates (4 × 105 cells/well) in complete RPMI 1640 supplemented with 10% FCS for 48 h. Supernatant were assayed for IL-10 by ELISA as previously described [23, 26].
2.4. In vitro suppression assay
CD4+ T cells obtained from spleen and lymph nodes were positively selected by magnetic beads following the manufacturer’s instructions (Miltenyi Biotec, Inc., Auburn CA), stained with anti-CD25 mAb (7D4, BD Biosciences) and separated into CD25+ and CD25− populations by FACS. Purified CD4+CD25− T cells were labeled by incubation in 5 μM CFSE (Molecular probes, Eugene, OR) for 5 min at 37 °C. CD4+CD25− cells (5×104 cells/well) were cultured in 96-well U-bottom plates in the presence of 1 μg/ml anti-CD3 mAb, 2×105 irradiated (2500 rad) spleen cells from naïve SJL donors in the presence or absence of CD4+CD25+ T cells at ratios of 1:1, 1:2, 1:4 and 1:8. Plates were incubated at 37 °C for 72 h and CFSE-labeled cells were analyzed for proliferation by flow cytometry using FlowJo software (Tree Star).
2.5. Antigen-specific cytokine secretion
Mice were immunized i.p. with 100 μg of fowl gamma globulin (FGG, Sigma) in 500 μl of DPBS and challenged with 150 μg of FGG in 25 μl PBS in the footpad 5 days later. Anti-CD25 mAb (PC61) or control GL113 mAb were administered i.p. at 500 μg/injection on days -2, 0, and 2 relative to immunization. Draining lymph nodes were removed 24 h post-challenge and cells cultured at 1 × 106 cells/ml in complete RPMI 1640 supplemented with 10% FCS containing 80 μg/ml of FGG. Supernatants were collected at 72 h and assayed for IFN-γ, IL-4, and IL-10 by ELISA as previously described [23, 26].
2.6. Statistical analysis
Statistical analysis was performed by Student’s t-test. Values of p ≤ 0.05 are considered significant. Results are expressed as mean ± SEM.
3. Results
3.1. Female SJL mice are deficient in CD4+CD25+ T cells
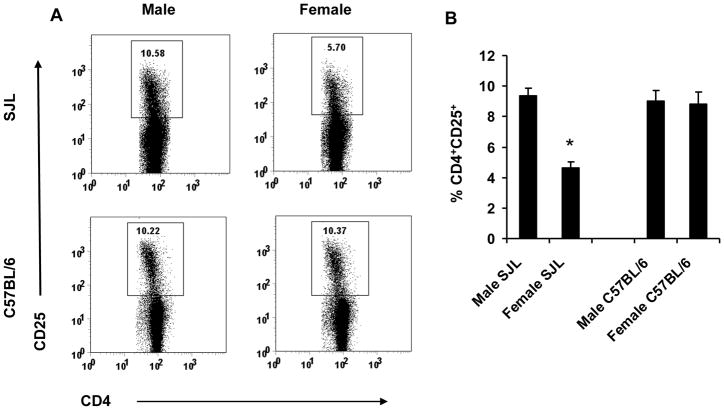
Autoimmunity has been linked to both numerical deficiencies and functional alterations of Treg [2], as well as gender [31]. In contrast to young adult males, age matched female SJL mice preferentially develop Th1 immune response and are susceptible to active EAE. Therefore we compared the frequency and phenotype of Treg from spleen and thymus of naïve male SJL to their female counterparts. Male SJL mice have approximately 2-fold more CD4+CD25+ T cells compared to EAE susceptible females (Fig. 1). However, the frequency of Treg in male SJL mice was equivalent to the frequency found in both male and female C57BL/6 mice which exhibit no sex-dependent susceptibility to EAE (Fig. 1). Foxp3 expression within the CD4+CD25+ T cell populations in male and female SJL were compared to C57BL/6 mice to insure that the increased frequency of CD4+CD25+ T cells in SJL males was not due to an abnormality in homeostasis or an indication of recently activated T cells. Male and female SJL, as well as C57BL/6 mice of both sexes, express equivalent frequencies of Foxp3+ T cells within the CD4+CD25+ T cell population (Table I, Fig. 2). These data indicate that male SJL mice and C57BL/6 mice of both sexes have ~2-fold more Treg compared to EAE susceptible female SJL mice.
Figure 1. Female SJL mice are deficient in CD4+CD25+ T cells.
Spleen cells from age-matched male and female SJL and C57BL/6 mice were analyzed by flow cytometry as described in Materials and Methods. Male and female SJL and C57BL/6 mice were compared for CD25 expression on CD4+ T cells. Data are representative of 4–8 independent experiments with similar results (A) and mean ± SEM (B). *p < 0.001 compared to SJL males and C57BL/6 males and females.
Table 1.
Phenotypic analysis of CD4+CD25+ T cells from spleens of male and female SJL mice.
| Phenotype | Sex | % § | MFI¶ |
|---|---|---|---|
| FoxP3+ | M | 92.4 ± 0.6 (n=4) | 386.5 ± 9.0 (n=4) |
| F | 90.2 ± 0.6 (n=4) | 370.8 ± 15.0 (n=4) | |
| CTLA-4+ | M | 32.9 ± 1.6 (n=8)* | 54.4 ± 4.0 (n=8)* |
| F | 24.4 ± 1.8 (n=8) | 43.2 ± 1.9 (n=8) | |
| CD62Lhi | M | 47.1 ± 2.8 (n=6)* | 403.2 ± 23.3 (n=6)* |
| F | 34.9 ± 4.2 (n=6) | 326.7 ± 17.7 (n=6) | |
| GITR+ | M | 94.7 ± 0.4 (n=5) | 99.8 ± 1.7 (n=5) |
| F | 94.2 ± 0.5 (n=5) | 103.8 ± 2.7 (n=5) | |
| CD103+ | M | 8.9 ± 1.2 (n=8) | 59.0 ± 3.0 (n=8) |
| F | 11.4 ± 0.4 (n=8) | 55.8 ± 2.9 (n=8) | |
| TNFR2+ | M | 50.8 ± 2.0 (n=8) | 90.7 ± 6.8 (n=8) |
| F | 47.7 ± 1.3 (n=8) | 81.3 ± 7.0 (n=8) |
Percentages of different subpopulations within CD4+CD25+ T cells.
MFI, mean fluorescence intensity of regulatory cell markers on the surface of CD4+CD25+ T cells (CD62Lhi, GITR, CD103 and TNFR2 expression). MFI of FoxP3 and CTLA-4 represent intracellular expression.
Data expressed as means ± SEM;
P<0.05, (M, males Vs F, females).
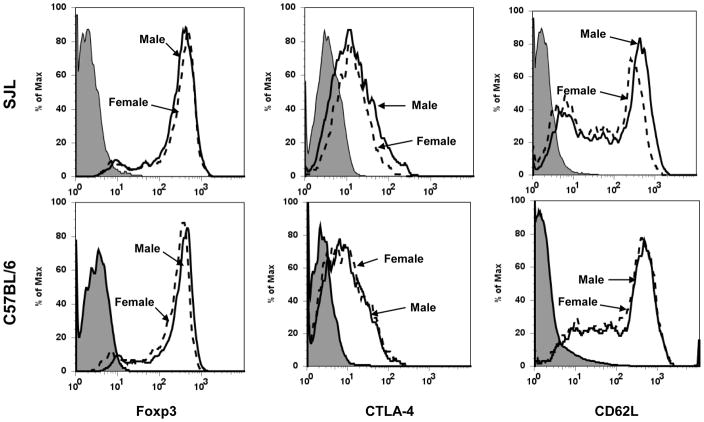
Figure 2. Treg in female SJL mice express reduced CTLA-4 and CD62L.
CD4+CD25+ T cells from age-matched male and female SJL and C57BL/6 mice were compared for the expression of Foxp3, CTLA-4 and CD62L by flow cytometry. Filled histograms represent isotype control, dashed lines represent data from females and continuous lines represent data from males. Data are representative of 4-8 independent experiments with similar results.
To determine if the reduced frequency of Treg in female SJL mice reflects an abnormality in peripheral homeostasis, Foxp3 expression was compared within thymic single positive CD4 T cells of male and female SJL and C57BL/6 mice. In female SJL mice the percentage of Foxp3+ cells in thymus is strikingly reduced compared to both male SJL and C57BL/6 mice of both sexes (Fig. 3). These data indicate that the reduced peripheral Treg population in female SJL mice is due to limited thymic maturation. Equivalent frequencies of CD4+CD25+ T cells from SJL males and females express GITR, CD103 and TNFR2 (Table 1). The levels of expression (mean fluorescence intensity) of these molecules are also similar in both males and females (Table 1). However, male derived CD4+CD25+ T cells exhibit a slightly increased frequency of CTLA-4+ and CD62Lhi cells compared to females (Table 1). Expression of CTLA-4 and CD62L are also slightly increased on male Treg compared to female derived cells (Fig. 2, Table 1). Altered expression of CTLA-4 and CD62L was confirmed by analysis of Foxp3+ cells in male and female SJL mice. Expression patterns of Treg associated molecules is similar to those expressed on CD4+CD25+ T cells (Fig. 4). By contrast, no differences were detected in frequencies or expression of CTLA-4 and CD62L by Treg derived from male and female C57BL/6 and their expression were similar to the expression by SJL male derived Treg (Fig. 2).
Figure 3. Reduced thymic maturation of CD4+Foxp3+ cells in female SJL mice.
Male and female SJL and C57BL/6 mice were compared for the expression of thymic Foxp3 within single positive CD4 T cells. Numbers represent percentages of Foxp3+ cells. Data presented are representative of 3 independent experiments with similar results (A) and mean ± SEM (B). *p < 0.05 compared to SJL males and C57BL/6 males and females.
Figure 4. Phenotypic characterization of Foxp3+ T cells in spleens of male and female SJL mice.
Spleen cells from age-matched male and female SJL mice were analyzed by flow cytometry as described in Materials and Methods. (A) Foxp3 expression was compared between male and female SJL CD4+ T cells. (B) Expression of CD25, CTLA-4, CD62L, GITR, CD103 and TNFR2 was compared on Foxp3+CD4+ T cells from male and female SJL spleens. Filled histograms represent isotype control, dotted lines represent data from females and continuous lines represent data from males. Data are representative of spleens from 4 mice per group processed individually.
3.2. Increased IL-10 secretion by male Treg
The functional capability of Treg from male and female SJL mice was initially compared by examining their ability to suppress T cell activation. Treg purified from males and females were tested for suppression of naïve CD4+CD25− T cells derived from both males and females. Suppression is dose-dependent and independent of the source of either the Treg or the effector T cell populations (Fig. 5). Therefore, although the frequency of Treg is increased in males, the data indicate that the differential ability to support Th1 vs. Th2 activation in vivo is independent of their ability to regulate T cell activation.
Figure 5. Equivalent suppression by Treg from male and female SJL mice.
CD4+CD25+ Treg and CD4+CD25− T cells were purified from spleens and lymph nodes of naïve male and female SJL mice. CD4+CD25− T cells (T effector) were labeled with CFSE and activated with anti-CD3 in presence of irradiated spleen cells. Treg were added at the indicated ratios and proliferation analyzed by flow cytometry at 72 h. Data are representative of 3 experiments.
The male sex hormone, testosterone, increases IL-10 production by CD4+ T cells [32] and IL-10 is critical for the preferential activation of Th2 cells in immunized male SJL mice [26, 27]. To determine if a differential capacity to secrete IL-10 correlated with the preferential activation of Th2 cells, CD4+CD25+ and CD4+CD25− T cells were purified from age-matched males and females and activated with anti-CD3. IL-10 secretion by the CD4+CD25− populations purified from both males and females was at the limit of detection (data not shown). By contrast, CD4+CD25+ T cells purified from both males and females secreted IL-10 (Fig. 6). However, Treg derived from males secreted significantly more IL-10 compared to Treg derived from age-matched females (Fig. 6). These results suggest that the increased frequency of CD4+CD25+ Treg in males, coupled with an increased ability to secrete IL-10, contributes to the preferential activation of T cells secreting Th2 associated cytokines following antigen exposure.
Figure 6. Treg from male SJL mice secrete increased IL-10.
CD4+CD25+ Treg from spleens of naïve 6 wk-old males and females were stimulated with anti-CD3 for 48 h. IL-10 concentrations determined by ELISA. Data are representative of 3 experiments performed in triplicate. Error bars = SEM. *p < 0.05 compared to females.
3.3. CD4+CD25+ T regulatory cells inhibit Th1 responses in male SJL mice
To demonstrate a relationship between Treg and preferential Th2 priming, males were depleted of CD25+ T cells prior to immunization. Control male and female mice were treated with an irrelevant antibody prior to immunization and compared to Treg depleted males. Depletion of Treg was confirmed by flow analysis (data not shown). Following antigen challenge lymph node derived T cells were stimulated with antigen and the supernatants tested for cytokines as previously described [23, 26]. T cells from immunized females stimulated with antigen secreted IFN-γ and only minimal levels of IL-4 and IL-10 (Fig. 7). By contrast, T cells derived from immunized males secreted both IL-4 and IL-10, but only minimal amounts of IFN-γ (Fig. 7), consistent with previous results [26]. By contrast, T cells derived from Treg depleted immunized male mice secreted IFN-γ and low levels of IL-4, a cytokine pattern similar to T cells derived from females (Fig. 7). Although Treg depletion did not reduce IL-10 secretion to the levels secreted by T cells derived from female mice, IL-10 secretion was reduced ~40% compared to the amount secreted by T cells derived from Treg sufficient males (Fig. 7). These data suggest that during T cell priming in males the Treg contribute to preferential activation of T cells secreting Th2 cytokines.
Figure 7. Treg regulate T cell priming in male SJL mice.
Male SLJ mice were depleted of CD4+CD25+ T cells prior to immunization and challenged with antigen 5 days post-immunization. Draining lymph nodes removed 24 h later. Cytokines secretion at 48 h after antigen stimulation were determined by ELISA. Results are the average of at least 3 experiments with 2 mice per group. Error bars = SEM. *p ≤ 0.01 compared to controls.
4. Discussion
Diminished Th1 induction following antigenic challenge of male SJL mice is not due to T cell anergy, but rather due to a skewing of the immune response towards a Th2 pathway [26, 27]. The mechanism of the preferential Th2 immune responses which develop in young adult males compared to age matched females following challenge with protein antigen is not precisely known except that antigen presenting cells from males produce reduced levels of IL-12 [27, 33, 34]. In this study we compared the frequency, function and phenotype of CD4+CD25+ Treg in male and female SJL mice and investigated the role of these cells in the development of a preferential Th2 immune response in males. Preferential Th2 response contributes to active EAE resistance in males and antigen specific Th2 cells from these mice also protect females from EAE following adoptive co-transfer with encephalitogenic T cells [23, 35, 36].
No differences were found comparing a variety of surface markers on CD4+ T cells in male and female SJL mice, with the exception of an increased frequency of CD4+CD25+ T cells in males. In contrast to the reduced levels of CD4+CD25+ T cells in females, associated with their susceptibility to autoimmune disease, the frequency in males is equivalent to strains of mice exhibiting no sex-dependent difference in susceptibility to autoimmune disease [37, 38]. CD4+CD25+ T cells in males and females both contain a similar high percentage of Foxp3+ cells, indicating that the vast majority of the CD4+CD25+ T cells in both sexes are Treg. In contrast to a decreased percentage of Foxp3+ T cells in the thymus of female SJL mice compared to male SJL mice and C57BL/6 mice of both sexes (Fig. 3), Romagnoli et al. (39) reported an increased percentage in the thymus of female SJL mice. The reason for this discrepancy is not obvious but may reflect different sources of SJL mice. Nonetheless, a decreased thymic frequency of Foxp3+ T cells in female SJL mice coupled with a decreased in the periphery suggests reduced thymic maturation. Thus, male SJL mice have approximately twice the frequency of Treg compared to females. Although APC contribute to Treg proliferation and homeostasis [28–30], the increased Treg frequency in males having defective APC maturation compared to females [24] suggests that Treg deficiency in females may not be due to APC dependent Treg expansion. Treg influence both peripheral tolerance and play a significant role in limiting adaptive immunity [1–4]. No difference was found comparing the potential of Treg from males and females to suppress T cell activation; however, depletion of Treg from males prior to immunization resulted in the priming of antigen-specific T cells that secrete increased IFN-γ and decreased IL-4 and IL-10 compared to T cells derived from immunized Treg sufficient males. These data support a role for Treg in skewing the immune response towards Th2 phenotype.
Treg derived from males secrete increased IL-10 compared to females, suggesting the possibility that Treg derived IL-10 contributes to the preferential priming of T cells secreting a Th2 cytokine profile. Indeed, previous data demonstrated that neutralization of IL-10 in males results in an antigen presenting cell population which preferentially supports priming of T cells secreting a Th1 type cytokine pattern [26, 27]. However, the antigen presenting cells derived from males express less IL-10 mRNA than female derived antigen presenting cells and the frequency of IL-10 secreting antigen presenting cells was slightly decreased compared to females [27]. Interactions between IL-10 and antigen presenting cells results in the down-regulation of surface expression of MHC class I, class II, co-stimulatory molecules and secretion of pro-inflammatory cytokines [40]. However, expression of MHC molecules, co-stimulatory molecules and adhesion molecules were identical on antigen presenting cells derived from males and females [27]. These data suggested that IL-10 from a cell type other than the antigen presenting cells, possibly Treg, plays a role in preferential activation of Th2 cells. However, the physiological level of IL-10 present in males appears to be insufficient to induce a detectable phenotypic change in expression of MHC or co-stimulatory molecules on the antigen presenting cells population [27].
GITR, CD103, TNFR2, CD62L and CTLA-4 are all associated with Treg function [1, 2, 41]. No difference in either the frequency or surface expression of GITR, CD103 or TNFR2 was found comparing Treg from males and females. By contrast, CTLA-4 and CD62L expression on Treg from males is slightly increased compared to the expression on Treg derived from females. As male SJL mice mature they acquire both the ability to mount a DTH response and exhibit increased susceptibility to EAE (21). Preliminary data indicate that the frequency, suppressive capability and secretion of IL-10 by Treg derived from males >12 weeks of age are unchanged by increasing age. Surface molecule expression on Treg is identical to young males with the exception of CD62L which decreases to the levels detected on female SJL mice (data not shown). Our results are consistent with a previous study showing comparable functional capability of natural Treg isolated from young and aged mice [38]. However, phenotypic and functional changes appear in CD4+CD25− T cell population in aged mice [38] which may be a contributing factor to similar EAE susceptibility in aged male and female SJL mice. The role of increased expression of the lymph node homing receptor CD62L on Treg derived from young males is unclear. CD62L has been associated with increased Treg-mediated suppression [42] and thus, it may function to increase the homing of IL-10 secreting Treg to area of T cell priming and contribute to the preferential activation of Th2 cells. Thus, decreased CD62L expression on Treg of aged SJL male may reduce their capability to migrate to draining LN and suppress development of self-reactive T cells following immunization with self antigens to induce EAE. CTLA-4 expression has been shown to regulate Treg-mediated suppression [43–45]; however, suppression did not correlate with Foxp3 expression [46]. Although the role of CTLA-4 in Treg regulation is controversial [44], the data suggest the possibility of a nonessential, but influential role.
The data demonstrate that CD4+CD25+ Treg influence the preferential priming of T cells secreting cytokines associated with a Th2 phenotype in male SJL mice. The increased frequency of Treg in EAE resistant young adult male SJL mice is consistent with the suggestion that a relative decrease in Treg contributes to the autoimmune disease susceptibility of female SJL mice. Importantly, transfer of Treg from naïve mice reduced clinical EAE in female SJL mice [17]. Furthermore, development of a dominant Th1 phenotype in female SJL mice may be attributed to both a deficiency of Treg and reduced production of immunosuppressive cytokine IL-10 by Treg.
Acknowledgments
The authors thank Ni Feng, Nermina Covic and Jeffery Tarcy for technical assistance and Jennifer Powers for cell sorting. This work was supported by National Institutes of Health grant NS 53824.
References
- 1.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–44. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–90. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 3.Belkaid Y. Role of Foxp3-positive regulatory T cells during infection. Eur J Immunol. 2008;38:918–21. doi: 10.1002/eji.200738120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Wijk F, Roord ST, Vastert B, De Kleer I, Wulffraat N, Prakken BJ. Regulatory T cells in autologous stem cell transplantation for autoimmune disease. Autoimmunity. 2008;41:585–91. doi: 10.1080/08916930802200182. [DOI] [PubMed] [Google Scholar]
- 5.Houot R, Perrot I, Garcia E, Durand I, Lebecque S. Human CD4+CD25high regulatory T cells modulate myeloid but not plasmacytoid dendritic cells activation. J Immunol. 2006;176:5293–8. doi: 10.4049/jimmunol.176.9.5293. [DOI] [PubMed] [Google Scholar]
- 6.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–80. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 7.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538–43. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 8.McKee S, Pearce EJ. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J Immunol. 2004;173:1224–31. doi: 10.4049/jimmunol.173.2.1224. [DOI] [PubMed] [Google Scholar]
- 9.Veldhoen M, Moncrieffe H, Hocking RJ, Atkins CJ, Stockinger B. Modulation of dendritic cell function by naive and regulatory CD4+ T cells. J Immunol. 2006;176:6202–10. doi: 10.4049/jimmunol.176.10.6202. [DOI] [PubMed] [Google Scholar]
- 10.Wu AJ, Hua H, Munson SH, McDevitt HO. Tumor necrosis factor-alpha regulation of CD4+CD25+ T cell levels in NOD mice. Proc Natl Acad Sci USA. 2002;99:12287–92. doi: 10.1073/pnas.172382999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–85. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esensten JH, Wofsy D, Bluestone JA. Regulatory T cells as therapeutic targets in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:560–5. doi: 10.1038/nrrheum.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sempere-Ortells JM, Perez-Garcia V, Marin-Alberca G, Peris-Pertusa A, Benito JM, Marco FM, Zubcoff JJ, Navarro-Blasco FJ. Quantification and phenotype of regulatory T cells in rheumatoid arthritis according to disease activity score-28. Autoimmunity. 2009;42:636–45. doi: 10.3109/08916930903061491. [DOI] [PubMed] [Google Scholar]
- 14.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venken K, Hellings N, Thewissen M, Somers V, Hensen K, Rummens JL, Medaer R, Hupperts R, Stinissen P. Compromised CD4+CD25high regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology. 2008;123:79–89. doi: 10.1111/j.1365-2567.2007.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Koldzic DN, Izikson L, Reddy J, Nazareno RF, Sakaguchi S, Kuchroo VK, Weiner HL. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:249–56. doi: 10.1093/intimm/dxh029. [DOI] [PubMed] [Google Scholar]
- 18.Yu P, Gregg RK, Bell JJ, Ellis JS, Divekar R, Lee HH, Jain R, Waldner H, Hardaway JC, Collins M, et al. Specific T regulatory cells display broad suppressive functions against experimental allergic encephalomyelitis upon activation with cognate antigen. J Immunol. 2005;174:6772–80. doi: 10.4049/jimmunol.174.11.6772. [DOI] [PubMed] [Google Scholar]
- 19.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–6. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 20.Matsushima GK, Stohlman SA. Immunological disorders of SJL mice. In: Rohova B, Vetvicka V, editors. Immunological disorders of mice. Boca Raton: CRC press; 1990. pp. 77–94. [Google Scholar]
- 21.Cua DJ, Hinton DR, Kirkman L, Stohlman SA. Macrophages regulate induction of delayed-type hypersensitivity and experimental allergic encephalomyelitis in SJL mice. Eur J Immunol. 1995;25:2318–24. doi: 10.1002/eji.1830250830. [DOI] [PubMed] [Google Scholar]
- 22.Matsushima GK, Stohlman SA. Maturation of the delayed-type hypersensitivity response in SJL mice: absence of effector cell induction. Eur J Immunol. 1988;18:1411–6. doi: 10.1002/eji.1830180917. [DOI] [PubMed] [Google Scholar]
- 23.Cua DJ, Hinton DR, Stohlman SA. Self-antigen-induced Th2 responses in experimental allergic encephalomyelitis (EAE)-resistant mice. Th2-mediated suppression of autoimmune disease. J Immunol. 1995;155:4052–9. [PubMed] [Google Scholar]
- 24.Stohlman SA, Matsushima GK, Casteel N, Frelinger JA. The defect in delayed-type hypersensitivity of young adult SJL mice is due to lack of functional antigen-presenting cells. Eur J Immunol. 1985;15:913–17. doi: 10.1002/eji.1830150909. [DOI] [PubMed] [Google Scholar]
- 25.Bebo BF, Jr, Zelinka-Vincent E, Adamus G, Amundson D, Vandenbark AA, Offner H. Gonadal hormones influence the immune response to PLP 139-151 and the clinical course of relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 1998;84:122–30. doi: 10.1016/s0165-5728(97)00214-2. [DOI] [PubMed] [Google Scholar]
- 26.Cua DJ, Coffman RL, Stohlman SA. Exposure to T helper 2 cytokines in vivo before encounter with antigen selects for T helper subsets via alterations in antigen-presenting cell function. J Immunol. 1996;157:2830–6. [PubMed] [Google Scholar]
- 27.Cua DJ, Stohlman SA. In vivo effects of T helper cell type 2 cytokines on macrophage antigen-presenting cell induction of T helper subsets. J Immunol. 1997;159:5834–40. [PubMed] [Google Scholar]
- 28.Tarbell KV, Petit L, Zuo X, Toy P, Luo X, Mquadmi A, Yang H, Suthanthiran M, Mojsov S, Steinman RM. Dendritic cell-expanded, islet-specific CD4+CD25+CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J Exp Med. 2007;204:191–201. doi: 10.1084/jem.20061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahnke K, Johnson TS, Ring S, Enk AH. Tolerogenic dendritic cells and regulatory T cells: A two-way relationship. J Dermatological Sci. 2007;46:159–67. doi: 10.1016/j.jdermsci.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Tamura C, Nakazawa M, Kasahara M, Hotta C, Yoshinari M, Sato F, Minami M. Impaired function of dendritic cells in alymphoplasia (aly/aly) mice for expansion of CD4+CD25+ regulatory T cells. Autoimmunity. 2006;39:445–53. doi: 10.1080/08916930600833390. [DOI] [PubMed] [Google Scholar]
- 31.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–80. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 32.Liva SM, Voskuhl RR. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol. 2001;167:2060–7. doi: 10.4049/jimmunol.167.4.2060. [DOI] [PubMed] [Google Scholar]
- 33.Wilcoxen SC, Kirkman E, Dowdell KC, Stohlman SA. Gender-dependent IL-12 secretion by APC is regulated by IL-10. J Immunol. 2000;164:6237–43. doi: 10.4049/jimmunol.164.12.6237. [DOI] [PubMed] [Google Scholar]
- 34.Kim S, Voskuhl RR. Decreased IL-12 production underlies the decreased ability of male lymph node cells to induce experimental autoimmune encephalomyelitis. J Immunol. 1999;162:5561–8. [PubMed] [Google Scholar]
- 35.Kirwin SJ, Dowdell KC, Hindinger C, Feng N, Bergmann CC, Hinton DR, Stohlman SA. Altered neuroantigen-specific cytokine secretion in a Th2 environment reduces experimental autoimmune encephalomyelitis. J Neuroimmunol. 2006;178:30–9. doi: 10.1016/j.jneuroim.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 36.Stohlman SA, Pei L, Cua DJ, Li Z, Hinton DR. Activation of regulatory cells suppresses experimental allergic encephalomyelitis via secretion of IL-10. J Immunol. 1999;163:6338–44. [PubMed] [Google Scholar]
- 37.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–78. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 38.Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25−Foxp3+ T cells in aged mice. J Immunol. 2006;176:6586–93. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- 39.Romagnoli P, Tellier J, Meerwiijk JPV. Genetic control of thymic development of CD4+CD25+Foxp3+ regulatory T lymphocytes. Eur J Immunol. 2005;35:3525–32. doi: 10.1002/eji.200535225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Subleski JJ, Kopf H, Howard OM, Mannel DN, Oppenheim JJ. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J Immunol. 2008;180:6467–71. doi: 10.4049/jimmunol.180.10.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu S, Yopp AC, Mao X, Chen D, Zhang N, Mao M, Ding Y, Bromberg JS. CD4+CD25+CD62+ T-regulatory cell subset has optimal suppressive and proliferative potential. Am J Transplant. 2004;4:65–78. doi: 10.1046/j.1600-6143.2003.00293.x. [DOI] [PubMed] [Google Scholar]
- 43.Holmberg D, Cilio CM, Lundholm M, Motta V. CTLA-4 (CD152) and its involvement in autoimmune disease. Autoimmunity. 2005;38:225–33. doi: 10.1080/08916930500050210. [DOI] [PubMed] [Google Scholar]
- 44.Sansom DM, Walker LS. The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology. Immunol Rev. 2006;212:131–48. doi: 10.1111/j.0105-2896.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 45.Ise W, Kohyama M, Nutsch KM, Lee HM, Suri A, Unanue ER, Murphy TL, Murphy KM. CTLA-4 suppresses the pathogenicity of self antigen-specific T cells by cell-intrinsic and cell-extrinsic mechanisms. Nat Immunol. 2010;11:129–35. doi: 10.1038/ni.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]