Abstract
There is a need to improve treatments for metastatic breast cancer. Here we show activation of the phosphoinositide 3-kinase (PI3K) and MAP kinase (MAPK) pathways in a MMTV-CreBRCA1f/fp53+/− mouse model of breast cancer. When treated with the pan-Class IA PI3K-inhibitor NVP-BKM120, tumor doubling was delayed from 5 to 26 days. NVP-BKM120 reduced AKT phosphorylation, tumor cell proliferation and angiogenesis. Resistant tumors maintained suppression of AKT phosphorylation but exhibited activation of the MAPK-pathway at the “pushing margin”. Surprisingly, PI3K-inhibition increased indicators of DNA damage, poly-ADP-ribosylation and γH2AX, but decreased Rad51 focus formation, suggesting a critical role of PI3K activity for Rad51 recruitment. PARP-inhibitor Olaparib alone attenuated tumor growth modestly; however, the combination of NVP-BKM120 and Olaparib delayed tumor doubling to more than 70 days in the mouse model and over 50 days in xenotransplants from human BRCA1-related tumors, suggesting that combined PI3K- and PARP-inhibition might be effective treatment for BRCA1-related tumors.
Keywords: PI3 Kinase inhibitor, NVP-BKM120, PARP-Inhibitor, Olaparib, BRCA1-related breast cancer
Introduction
Unresectable triple-negative breast cancer (TNBC) remains an incurable illness that invariably relapses after treatments considered standard of care, leading to death, often within months of diagnosis. Current chemotherapeutic regimens induce not only incomplete remissions that are short, but also result in toxicity that severely impacts a patient’s quality of life. These shortcomings have led to an extensive search for more effective treatments.
Female BRCA1 mutation carriers have an ~85% life-time risk of developing breast cancer. These cancers generally are negative for estrogen receptor, progesterone receptor and HER2 (e.g. triple negative), making them non-responsive to therapies that target these pathways. Sporadic triple negative breast cancers that emerge in patients without germline BRCA1 or BRCA2 mutations frequently show evidence for epigenetic silencing of BRCA1. Truncating mutations disrupting the C-terminal end of the BRCA1 protein predispose to breast cancer, whereas mutations in the N-terminal two-thirds result in elevated susceptibility to both breast and ovarian cancer (1).
Loss of BRCA1 in breast epithelial cells disables DNA damage repair via homologous recombination (HR). This defect leads to genomic instability but also sensitizes cells to the deleterious effects of other DNA-damaging agents such as Cisplatin or inhibitors of poly-ADP-ribosylation. Poly-ADP-ribose -polymerase (PARP) is a nuclear enzyme that senses DNA single strand breaks and is essential for base excision repair (BER). Once BER is disabled, cells rely on HR for DNA damage repair. Dysfunction of HR (such as in BRCA1-deficient cells) presents a context in which inhibition of BER (e.g., by treating with PARP-inhibitors) is synthetically lethal. Clinically, PARP-inhibitors have emerged as promising agents, inducing objective responses in 41% of patients with BRCA1-related breast cancer (2, 3) and 33% of patients with BRCA1-related ovarian cancer (4, 5). However, the remissions achieved with PARP-inhibitors have not been durable, and benefit in the subset of triple negative breast cancers (TNBCs) that are not BRCA1-related is currently uncertain.
Multiple lines of evidence suggest that growth factor signaling may be a sensible target for treatment of TNBC: Epidermal Growth Factor (EGFR) overexpression appears to correlate with the basaloid phenotype and is found in 60–70% of TNBC, including BRCA1-related cancers (6). We have previously shown that up-regulation of EGFR and the EGF-pathway is an early event in BRCA1-related tumorigenesis (7). IGF-1R levels are increased in BRCA1-related breast cancers (8) and genetic variants in the IGF pathway are associated with BRCA1-related tumorigenesis (9, 10). However, VEGFR (Vascular Endothelial Growth Factor Receptor) and EGFR inhibitors, alone or in combination with traditional chemotherapy, have not improved survival for patients with TNBC. One explanation for this lack of efficacy is that resistant tumor cells signal through alternate RTKs, turning the search for new therapeutic angles to nodal points of intracellular signal transduction such as MAPK and PI3K, whose inhibition may be harder for tumor cells to evade. Here we examine the mechanism and the efficacy of a PI3K inhibitor, NVP-BKM120, for the treatment of BRCA1-related breast cancer in a mouse model and report on a surprising in vivo synergy with PARP inhibition.
Results
Activation of the PI3K pathway in BRCA1-related breast cancer
We and others have previously shown that the MMTV-CreBRCA1f/fp53+/− mouse model faithfully recapitulates many aspects of human BRCA1-related breast cancer, including emergence on a background of multiple synchronous hyperproliferative lesions, high proliferative activity, absence of estrogen receptor expression and presence of EGFR-overexpression (11–14), although exon 11 deletion in this model results in the residual expression of a hypomorphic BRCA1 protein, rather than complete absence of the BRCA1 protein shown in other models (15).
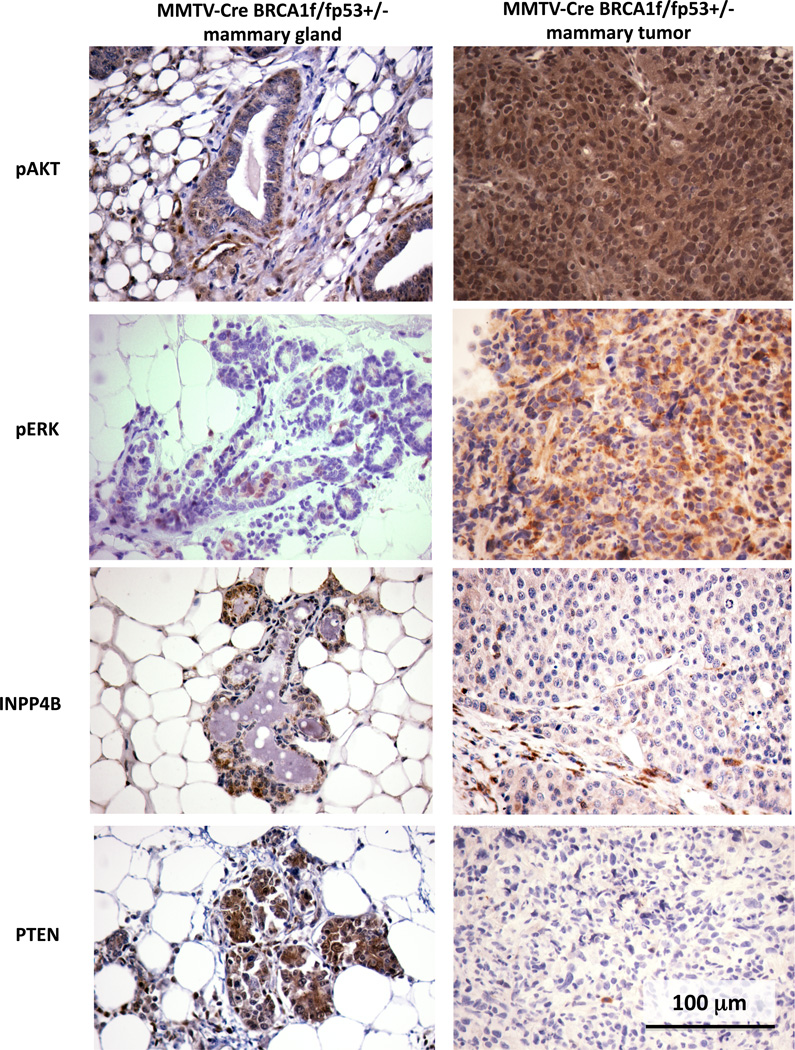
BRCA1 has been shown to suppress AKT (16) and ERK-activation in response to estrogen or EGF stimulation (17, 18) in cell based studies, suggesting that tumors with defects in BRCA1 might have an increase in AKT and/or ERK-phosphorylation. Consistently, we found that phosphorylation of AKT at Serine 473 was strongly positive in both the cytoplasm and the nucleus in these tumor cells (Fig. 1 upper right and Fig. S1), while in the normal adjacent tissue cytoplasmic AKT phosphorylation was only seen in the basal layer of cells, not in luminal cells (Fig. 1 upper left). Similarly, ERK-phosphorylation was absent in normal mammary epithelial cells, while cytoplasmic ERK-phosphorylation was seen in a majority, but not in all tumor cells (Fig. 1 second panel).
Fig. 1.
PI3K pathway activation in BRCA1-related breast cancer in MMTV-CreBRCA1f/fp53+/−. Tumor-bearing females were euthanized, tissues harvested and processed for immunohistochemistry. Displayed are representative images of immunohistochemistry for phospho-AKT (S473), pospho-(Thr202/Tyr204)-ERK, and the tumor-suppressor phosphatases INPP4B and PTEN. Adjacent normal mammary gland tissue is on the left, tumor tissue on the right. 400 x magnification.
Loss of function of PTEN, either through epigenetic silencing or through gross genomic loss, correlates with loss of function of BRCA1 in TNBC (19). Recently, Gewinner et al. (20) as well as Fedele et al. (21) showed that, similar to PTEN, the tumor suppressor phosphatase INPP4B is lost in approximately 60% of TNBC, including BRCA1-related breast cancers. Consistent with these data in human disease, INPP4B and PTEN expression were strong in normal glands of MMTV-CreBRCA1f/fp53+/− females, but lost in tumor tissues (Fig. 1 third and lower panel).
To examine whether activating PIK3CA mutations are responsible for the strong and uniform activation of AKT, we sequenced the PIK3CA gene of 11 murine BRCA1-deleted breast tumors. Consistent with the rarity of mutations in human TNBC, we found no activating hotspot mutations in exons 9 or 20 of PI3K. In human TNBC, activating mutations in PIK3CA are relatively rare and seen in only 8% of TNBC, confirming that the activation of the PI3K pathway in TNBC is mostly driven by regulatory mechanisms such as loss of PTEN and INPP4B, rather than by activating mutations in PIK3CA.
Collectively, these observations suggest that the MMTV-CreBRCA1f/fp53+/− mouse model accurately recapitulates the activation of growth factor signaling seen in human BRCA1-related breast cancer, including activation of the PI3K and MAPK pathways and the absence of activating PI3K mutations. Based on this data, we decided to study whether inhibition of PI3K would be an effective treatment for BRCA1-related breast cancer.
Pharmacodynamics of PI3K inhibition in BRCA1-related breast cancer
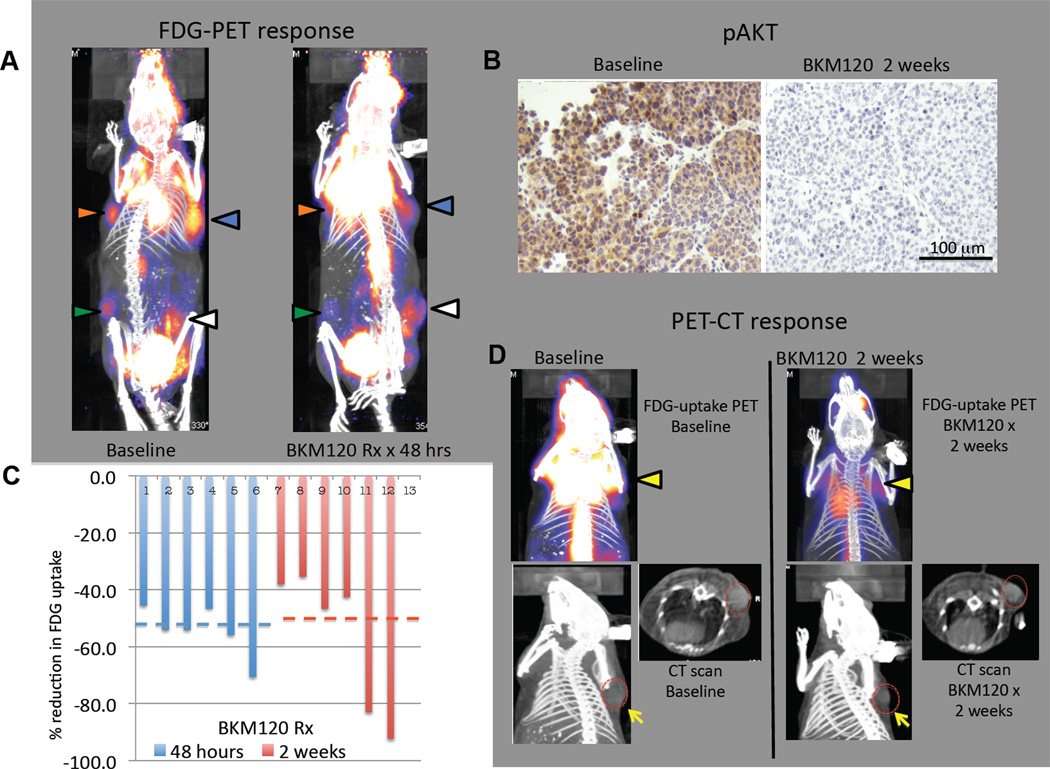
TNBCs, including the BRCA1 related subtype, exhibit high rates of glucose uptake, as judged by positron emission tomography (PET) using the radioactive glucose analog, 18F-fluorodeoxyglucose (FDG) (22, 23). Consistent with these observations in humans, we found that BRCA1-deleted tumors in our mouse model were highly avid for FDG. Tumors of sub-centimeter size were easily visualized using this technique (Fig. 2, S2, S3). In a previous study (24) mouse lung tumors that resulted from transgenic expression of the H1047R mutant of PIK3CA were found to have high rates of glucose uptake as judged by FDG-PET, and the PI3K/mTOR inhibitor BEZ235 caused a reduction in the FDG-PET signal within two days, consistent with the known role of PI3K in regulating glucose uptake and glycolysis (25–27). We found that within 48 hours of instituting treatment with NVP-BKM120, tumors in all treated animals showed a median decrease in FDG-uptake by 46.7 % (range 38.1 – 92.3), which was sustained after 2 weeks of continued treatment with NVP-BKM120 (median decrease by 54%, range 45.5 – 70.5%) and corresponded to inhibition of akt phosphorylation (Fig. 2 A–D, Fig. S2, S3). These results indicate that activation of the PI3K pathway contributes to the upregulation of glucose metabolism in BRCA1-related breast cancers and that oral delivery of NVP-BKM120 results in inhibition of this response. Further evidence that NVP-BKM120 inhibits PI3K signaling in the BRCA1 defective tumors was provided by the observation that phosphorylation of the downstream protein kinase, AKT at Ser-473 was strongly decreased in tumors treated with NVP-BKM120 (Fig. 2 B and S2, S3). It was remarkable that all BRCA1-related tumors examined showed a decrease in FDG-uptake and a decrease in AKT-phosphorylation in response to NVP-BKM120 (Fig. 2, S2 and S3), suggesting that a high level of PI3K signaling and the consequent enhanced glucose metabolism is a common event in tumors that result from loss of BRCA-1 function. In addition, our data suggest that inhibition of FDG-uptake may be an early and predictive pharmacodynamic marker for response to treatments with PI3K-inhibitors.
Fig. 2.
Pharmacodynamic effects of PI3K inhibitor NVP-BKM120 on breast carcinomas in MMTV-CreBRCA1f/fp53+/− mice. Female virgin mice developed spontaneous breast cancers at ages 8–12 months. A. Representative 18FDG PET-CT scan images of a tumor-bearing mouse at baseline (image on the left) and within 48 hours of after start of treatments with the PI3K-inhibitor NVP-BKM120 (50 mg/kg/day by gavage, image on the right). This mouse had developed 4 simultaneous tumors. Arrows in red, green, yellow and white are used to identify different tumors upon baseline (left) and post-treatment (right) imaging. The color palette for uptake ranges from dark blue to bright yellow with increasing count intensity. The changes in 18FDG-uptake were determined as described in Materials and Methods. They were a decrease by 45% (tumor with red arrow), 64 % (tumor green arrow), 64% (tumor yellow arrow), 56% (tumor white arrow). B. Suppression of AKT-phosphorylation on S473 as a result of treatments with NVP-BKM120 in vivo. Tumor tissue was obtained via core needle biopsy before and after two weeks of treatments with NVP-BKM120, fixed and processed for IHC with anti-pAKT (S473) antibodies. For additional IHC images see Fig. S1. C. Decrease in FDG-uptake in 6 mammary carcinomas. Relative decrease in FDG-uptake was determined by the ratio of uptake at 48 hours (blue bars) or 2 weeks (red bars) to baseline. Tumor-specific FDG-uptake was determined as described in Materials and Methods. For additional PET-CT images see Fig. S2. D. Concordance of decrease in FDG-uptake and tumor shrinkage during a 2-week treatment with PI3K inhibitor NVP-BKM120. The tumor-bearing animal was imaged with FDG-PET (upper panel, tumor indicated with a yellow arrow before and after treatment, decrease in uptake 93%) and concomitant CT scan (lower panel) before (left) and while on treatment (right). The tumor is again marked in the CT scan with a yellow arrow in the axial and sagittal plane. The red outline indicates the tumor circumference before treatment to visualize treatment effect on tumor size.
The PI3K inhibitor NVP-BKM120 exerts anti-angiogenic activity
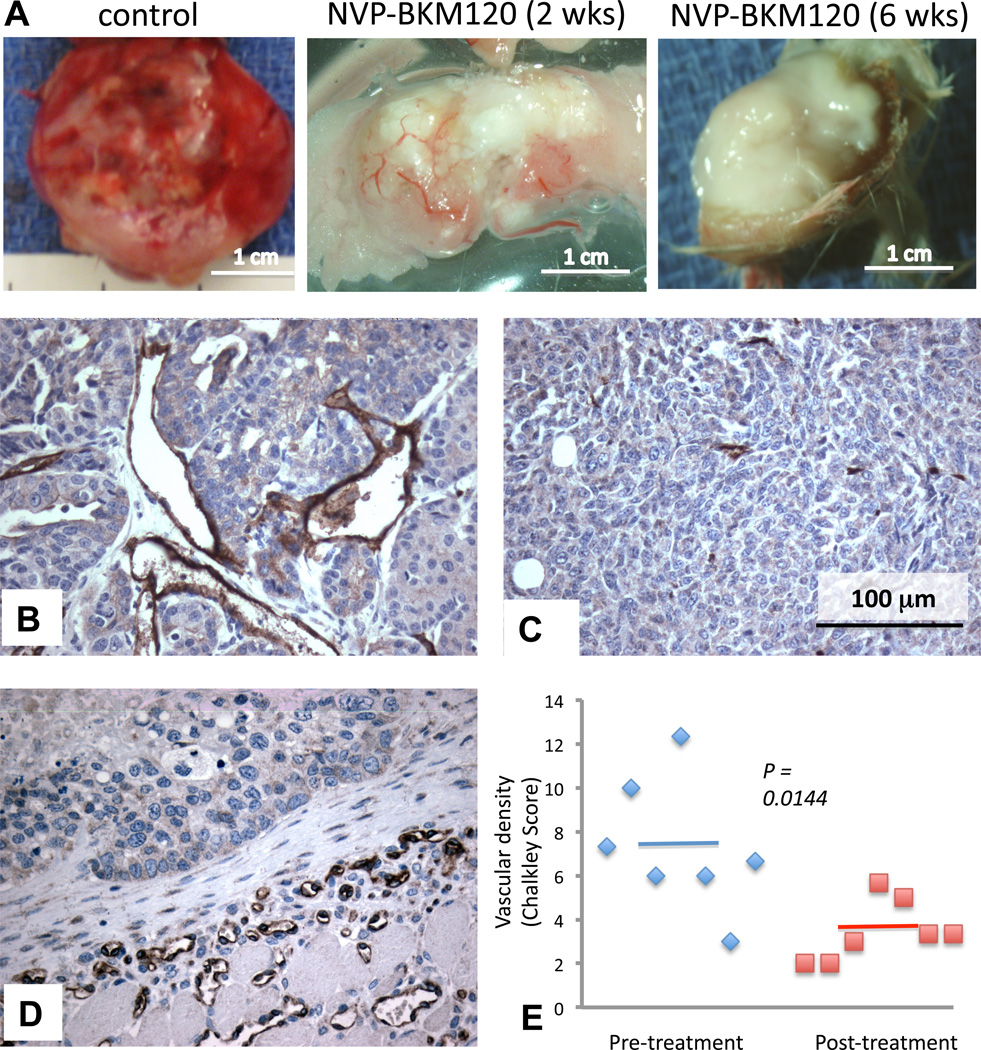
Tumor growth requires neo-vascularization of the expanding neoplastic tissue.. It was previously shown that NVP-BEZ235, a PI3K-inhibitor with activity against PI3Kα and mTOR, inhibits the sprouting of new blood vessels in tumors, and disrupts the integrity of existing blood vessels (27, 28). Spontaneous tumors in MMTV-CreBRCA1f/fp53+/− mice grow rapidly, and are highly vascular (Fig. 3A, left, Fig. 3B). However after treatment with NVP-BKM120, the gross pathology of tumors was notable for central pallor and, eventually, central necrosis (Fig. 3A, middle). In contrast, blood vessels in the tumor capsule remained initially intact, or became ectatic (Fig. 3A). Consistently, the tumor microvasculature, as visualized with an anti-CD31 stain, was diminished in response to NVP-BKM120 (Fig. 3C) while it was maintained in the tumor capsule (Fig. 3 D). The necrotic center of treated tumors was frequently hemorrhagic (data not shown), indicating disorganized collapse of the tumor vasculature. We used the Chalkley count of CD31-positive microvessels (29) to compare the vascularization before and after treatment with NVP-BKM120 and found that both the size and number of blood vessels were starkly reduced in treated tumors (Fig. 3 E). Thus, consistent with prior observations with BEZ235 (28) and recent data with NVP-BKM120 (30), our data confirm that NVP-BKM120’s anti-tumor activity is, in part, due to its anti-angiogenic activity, and thus this drug may have preferential activity in rapidly growing, endocrine-resistant tumors with a high degree of tumor angiogenesis.
Fig. 3.
Anti-angiogenic effects of PI3K inhibitor NVP-BKM120. A. Gross pathologic images of an untreated tumor (left), a tumor treated for 2 weeks (middle) and 6 weeks (right) with NVP-BKM120 at 50 mg/kg/day via gavage. B, C, D Immunohistochemistry to detect CD31 in an untreated tumor (B), the center of a tumor treated for 6 weeks (C) and the tumor capsule of a mammary tumor treated for 6 weeks (D). E: Determination of the Chalkley score to quantify CD31 staining. IHCs with anti-CD31 antibodies were performed in pre-treatment biopsies and in tumor specimen from mice at the time of tumor progression.
Effects of PI3K inhibition on compensatory pathways in tumor cells
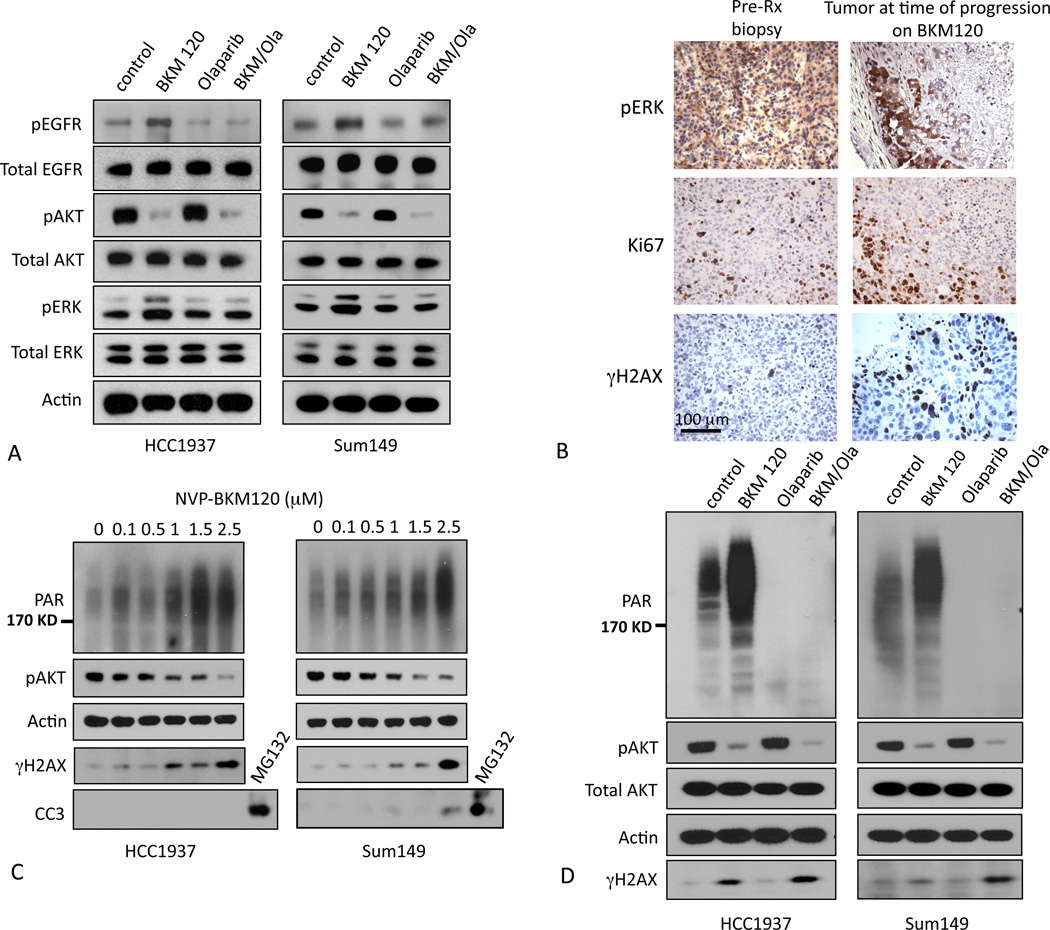
The upregulation of compensatory pathways in response to tumor cell treatments with inhibitors of mitogenic signaling is now a well-known phenomenon (31). Consistent with these prior observations, we found that NVP-BKM120 induced a compensatory activation of the EGFR/MAPK pathways in the human BRCA1 mutant breast cancer cell lines, HCC1937 (BRCA1 5382C mutation and homozygous deletion of PTEN and p53)(32), and SUM149 (BRCA1 2288delT, PTEN WT, p53 mutant) (33, 34) (Fig. 4 A, second lane for each cell line). As expected, treatments with the PARP-inhibitor Olaparib alone did not have a discernible effect on the activation status of EGFR, AKT or MAPK (Fig. 4 A, third lane for each cell line). However, with the combination treatment (Fig. 4 A, last lane), we found that compensatory activation of EGFR and MAPK could be blocked by the addition of Olaparib. These data suggest that PARP-inhibition in tumor cells either restricts mitogenic signaling to PI3K-mediated signaling, or disables mechanisms that would re-route mitogenic signaling via EGFR/ERK when PI3K is inhibited.
Fig. 4.
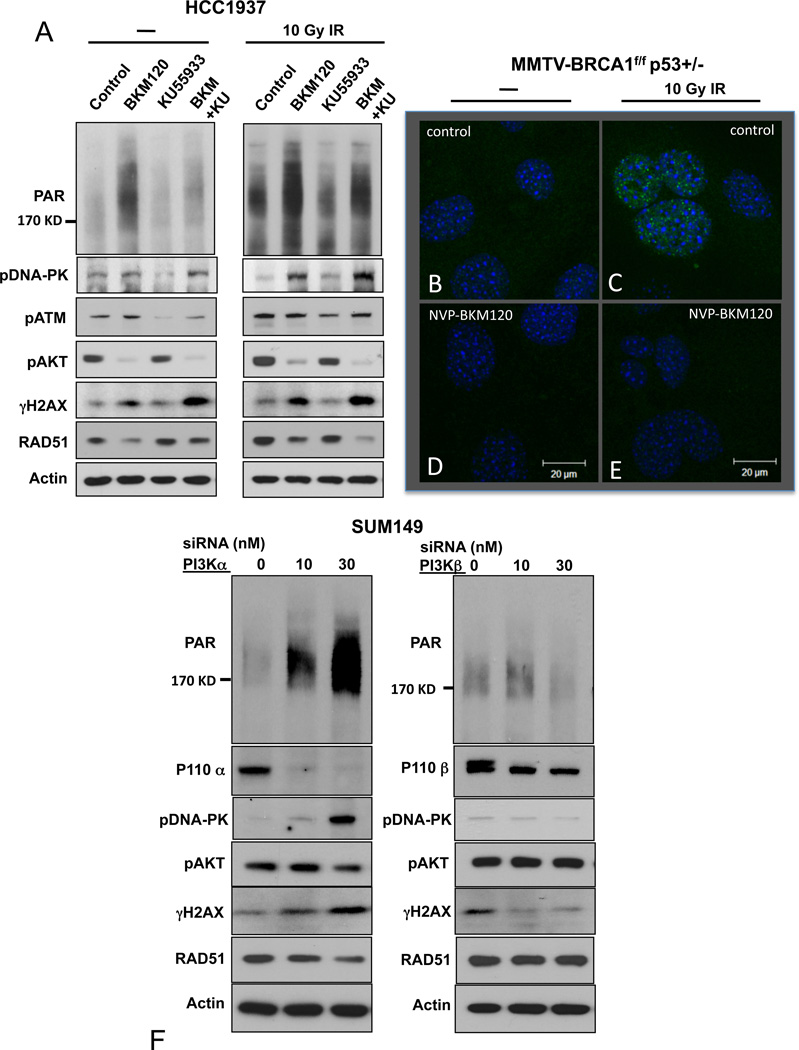
PI3K-inhibition increases poly-ADP-ribosylation and H2AX phosphorylation. A. Compensatory pathway activation induced by treatments with NVP-BKM120. HCC1937 or SUM149 cells were treated with NVP-BKM120, Olaparib or its combination as indicated for 72 hours, lysed and subjected to immunoblotting with antibodies against total AKT, EGFR, ERK and their phospho-specific epitopes. B. In vivo increase of γH2AX-positive cells after treatment with NVP-BKM120 and proliferative activity at the “pushing margin”. Tumor-bearing mice were subjected to a pre-treatment biopsy and then treated with NVP-BKM120 at 50 mg/kg/day. IHCs of pre-treatment biopsies and post-treatment tumor tissues were performed with antibodies as indicated. C. Effects of combined PI3K- and PARP-inhibition on BRCA1-mutant cells. Cells were treated with NVP-BKM120 at 1 µM and Olaparib 10 µM or their combination for 24 hours, lysed and subjected to immunoblotting with antibodies against PAR, pAKT, total AKT and γH2AX and Actin as indicated. D. BRCA1 mutant human HCC1937 or SUM149 cells were treated with vehicle control or NVP-BKM120 at the indicated concentrations for 24 hours, lysed and subjected to immunoblotting with antibodies against PAR, p-AKT (S473), γH2AX, Cleaved Caspase 3(CC3) as an apoptosis marker and Actin as a loading control.
Treatments with NVP-BKM120 increase indicators of DNA damage but decrease Rad51 recruitment to repair foci
Loss of BRCA1 function results in genome instability due to defects in DNA repair by homologous recombination. As a consequence, BRCA1−/− cells have high rates of DNA damage and are sensitized to the inhibition of alternative DNA repair mechanisms involving PARP-dependent poly (ADP) ribosylation (35). We examined the possibility that the high sensitivity of BRCA1 mutant tumors to PI3K pathway inhibitors is a consequence of a role for the PI3K pathway in maintaining cell survival during DNA repair or in facilitating DNA repair mechanisms. These experiments were carried out in vivo (Fig. 4B) and with the human BRCA1-mutant cell lines, HCC1937 and SUM149. We first examined the effect of NVP-BKM120 on DNA repair responses in cells grown on plastic. Surprisingly, we found that in both cell lines H2AX phosphorylation on Serine 139 (γH2AX, a marker for DNA ds damage) increased with increasing concentrations of NVP-BKM120 and that this correlated with diminishing phosphorylation of AKT (Fig. 4C). Similarly, tumors treated with NVP-BKM120 in vivo showed a substantial increase in the percentage of cells that express γH2AX (Fig. 4 B).
Tumors with loss of BRCA1 rely on PARP-dependent poly-ADP-ribosylation (PAR) of key proteins involved in DNA damage repair (35). Given the surprising increase in H2AX phosphorylation, we examined if treatment with NVP-BKM120 would also affect PARP activity. Treatment with NVP-BKM120 caused a dose dependent increase in overall poly-ADP-ribosylation (PAR) that paralleled the increase in H2AX phosphorylation and the decrease in AKT phosphorylation (Fig. 4 C). Importantly, this increase in poly-ADP-ribosylation was initially not accompanied by apoptotic cell death, as cells remained negative for cleaved caspase 3 (CC3) (Fig. 4 C). The basal and NVP-BKM120 enhanced poly-ADP-ribosylation could be completely blocked by treatment with the PARP inhibitor, Olaparib (Fig. 4 D), while γH2AX accumulation was enhanced with the combination of NVP-BKM120 and Olaparib (Fig. 4 D).
Thus, we observed that PI3K-inhibition caused a significant increase in activities indicative of both types of DNA damage: PARP activity, which is required for base excision (BER) and single strand break (SSB) repair, as well as H2AX phosphorylation, indicative of the presence of DNA double strand breaks (DSB). As H2AX is a substrate for the PI3Kinase-related kinases (PIKKs) ATM and DNA-PK, we asked if NVP-BKM120 had an effect on these kinases that would explain our findings. We examined PAR and γH2AX accumulation in HCC1937 cells in the absence and presence of the ATM-inhibitor KU-55933 (36) and monitored the response to ionizing radiation. As expected, KU-55933 led to a decrease in auto-phosphorylation of ATM (Fig. 5 A, third and fourth lane of each panel), and prevented the increase in H2AX phosphorylation seen in response to ionizing radiation. However, KU-55933 did not prevent the NVP-BKM120-induced induction of γH2AX, which was robust both at baseline and in response to ionizing radiation (Fig. 5 A, last lane of each panel), suggesting that an alternative kinase, such as DNA-PK is phosphorylating H2AX in response to PI3K inhibition. As shown in Fig. 5 A, we found a strong increase in auto-phosphorylation of DNA-PK in response to addition of NVP-BKM120 that corresponds to H2AX phosphorylation. Consistent with prior reports (30) these results clearly show that NVP-BKM120 is not acting through an off-target inhibition of ATM or DNA-PK and suggest that inhibition of PI3K by NVP-BKM120 leads to activation of DNA-PK through a yet unknown mechanism.
Fig. 5.
Effects of NVP-BKM120, KU-55933 and their combination on the DNA damage response. A. HCC1937 were treated for 18 hours with NVP-BKM120 at 2.5 µM, KU55933 at 10 µM or their combination, subjected to ionizing irradiation with 10 Gy or mock, lysed 6 hours later and subjected to immunoblotting with antibodies as indicated. B-E Loss of Rad51 focus formation in response to ionizing radiation in the presence of NVP-BKM120. Breast cancer cells were isolated from primary tumors from MMTV-Cre MMTV-CreBRCA1f/fp53+/− mice and either treated with vehicle control (B, C) or NVP-BKM120 (D, E) for 18 hours, followed by irradiation with 10 Gy. 6 hours later cells were fixed and processed for immunofluorescence with antibodies against Rad51 and counterstained with DAPI. F. Induction of DNA-PK and H2AX phosphorylation and loss of RAD51 occur in response to PI3Kα, not PI3Kβ-inhibition. SUM149 cells were transfected with siRNA pools depleting PI3Kα (left panel) or PI3Kβ (right panel). Cells were lysed after 48 hrs and subjected to immunoblotting with antibodies as indicated.
Consistent with the results in Fig. 4 C, we found that the PAR accumulation in the presence of NVP-BKM120 alone increased (Fig. 5 A, left panel, second lane). In the presence of the combination of NVP-BKM120 and KU-55933 PAR accumulation was attenuated but still greater than in the control, suggesting that the NVP-BKM120-induced increase in PAR was only partially offset by inhibition of ATM, again consistent with an ATM-independent mechanism for PAR-accumulation and its induction by PI3K-inhibition.
To determine if PI3K-inhibition affected the assembly of DNA damage repair foci, we examined the ability of tumor cells from our mouse model to recruit Rad51 to DNA damage repair foci (Fig. 5 B–E), following a protocol established previously (37). We generated cell cultures from tumors of MMTV-CreBRCA1f/fp53+/− mice and examined their ability to form DNA repair foci 6 hours after exposure to ionizing radiation (10 Gy). We found that there was residual double-strand repair activity as shown by the formation of Rad51 foci in this mouse model with a hypomorphic exon 11 deletion (Fig. 5 C). Surprisingly, the formation of Rad51 foci in response to ionizing radiation was completely blocked by pre-treatment of these cells with NVP-BKM120 (Fig. 5 D). A similar phenomenon was observed in HCC1937 cells: While ionizing radiation induced accumulation of Rad51 and H2AX phosphorylation as reported previously (38) (Fig. 5 A, control lanes), pre-treatment with the PI3K-inhibitor NVP-BKM120 led to a dissociation of this radiation response as we saw a failure to increase Rad51, but a prominent augmentation of radiation-induced H2AX phosphorylation in the presence of NVP-BKM120 (Fig. 5 A, second lane of each panel). The mechanism by which NVP-BKM120 decreases Rad51 recruitment to repair foci is yet unknown. However, this observation of a defective DSB repair response may, at least in part, provide an additional explanation for the in vivo synergy of PARP- and PI3K-inhibition.
Effects of NVP-BKM120 are specific for PI3Kα inhibition
Given the un-anticipated and striking effects of the pan-Class IA PI3K inhibitor, NVP-BKM120 on the DNA damage response, we asked if these effects were specific to a single Class IA PI3K isoform or required inhibition of multiple PI3Ks or could be an off-target effect of NVP-BKM120. In the BRCA1-mutant cell line SUM149 down-regulation of PI3Kα, but not PI3Kβ, with siRNA led to a stark increase in phosphorylation of DNA-PK, H2AX (γH2AX) and poly-(ADP)ribosylation (PAR) and a stark decrease in Rad51 accumulation (Fig. 5 F). These data confirm that it is the inhibition of PI3Kα that is decisive for the disruption of the DNA damage response in these cells.
Therapeutic efficacy of PI3K inhibitor NVP-BKM120 alone and in combination with the PARP-Inhibitor Olaparib
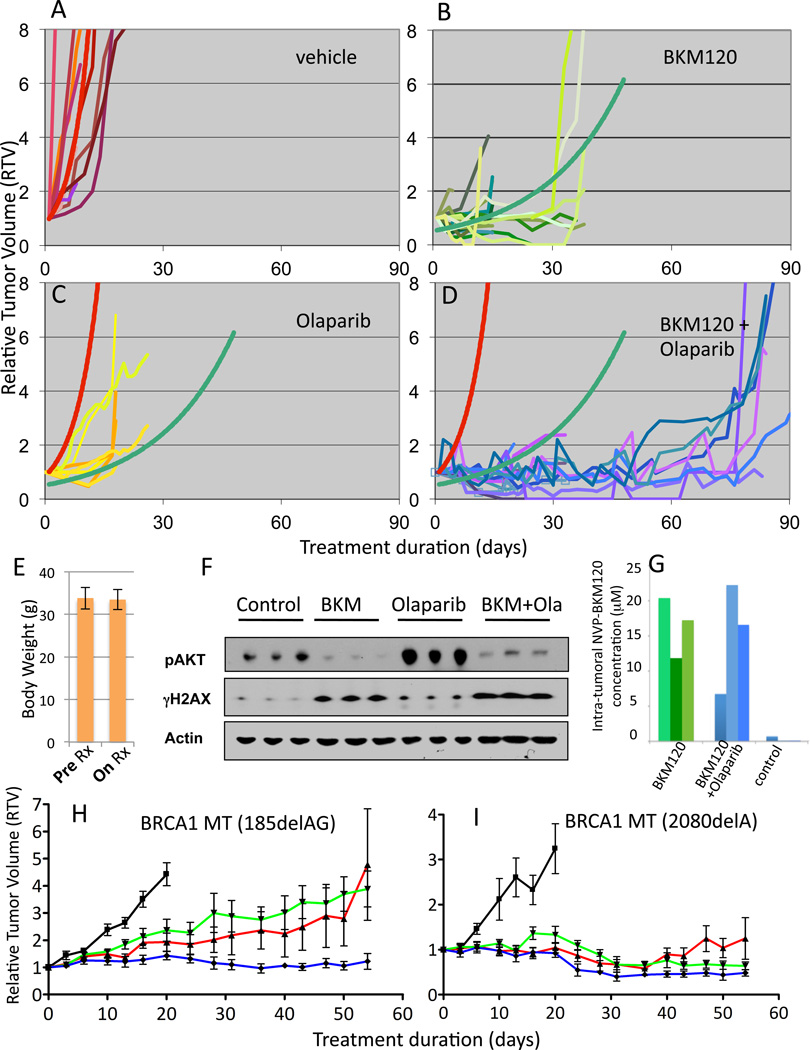
We first examined the effect of NVP-BKM120 and Olaparib on the growth on plastic of the two BRCA1 mutant cell lines. HCC1937 cells, with a genetic loss of PTEN, showed greater sensitivity to NVP-BKM120 than SUM149 cells, which have wild type PTEN (Fig. S 4A). SUM149, on the other hand, showed greater sensitivity to Olaparib (Fig. S 4B). The drug combination did not have much benefit beyond that of the most effective single agent in either cell line (Fig. S4) and isogenic reconstitution of PTEN in HCC1937 did not significantly alter drug sensitivities (Fig. S4 C, D), indicating that under the artificial conditions of growth on plastic with high levels of nutrients and oxygen, and in the absence of the native tumor microenvironment, this drug combination does not result in synergy. We next addressed whether NVP-BKM120 and Olaparib might have a more dramatic effect in vivo, on endogenous BRCA1-deleted tumors. We first showed that, consistent with the observations with the human BRCA1-mutant cell lines, NVP-BKM120 treatment of mice with BRCA1-deleted breast tumors (MMTV-CreBRCA1f/fp53+/−) resulted in an increase in phosphorylated H2AX in the recurrent tumors (Fig.4 B).
We next compared the effects of NVP-BKM120 and Olaparib as single agents and the combination of both drugs on tumor growth. Female virgin MMTV-CreBRCA1f/fp53+/− mice were observed for the development of spontaneous tumors, which typically occurs at age 8–12 months. Once tumors reached a diameter of 5–7 mm, mice were randomized to either vehicle control treatments, treatments with NVP-BKM120 via oral gavage, Olaparib intraperitoneally, or the combination of NVP-BKM120 with Olaparib, all once a day continuously. An initial set of mice was treated with NVP-BKM120 at 50 mg/kg/day, alone or in combination with Olaparib (50 mg/kg/day) and a second set at NVP-BKM120 30 mg/kg/day alone or in combination with Olaparib (50 mg/kg/day). No significant difference was seen with regard to efficacy or p-AKT-suppression between the two dose levels of NVP-BKM120 and data were pooled (Fig. 6 A–D). Tumors were measured at least 3 times a week, and relative tumor volume, as a ratio to baseline tumor volume, was calculated for each treatment modality (Fig. 6 A–D). Trendlines were determined based on the best fit to the data in vehicle control (red line) and NVP-BKM120 only (green line). Once tumors were established, their doubling time was rapid if treated with vehicle only, on average 5 days (Fig. 6 A). Treatments with NVP-BKM120 alone significantly prolonged tumor doubling time by a factor of 5 (26 days versus 5 days, Fig. 6 B), however, tumors eventually grew. (Fig. 6 B). In this mouse model, tumor growth was delayed threefold with the use of Olaparib (tumor doubling time 16 days versus 5 days, Fig. 6 C). When Olaparib and NVP-BKM120 were combined, we found a surprising in vivo synergistic activity, with a tumor doubling time of over 70 days, a 140-fold increase over control (red trendline). The dual combination of NVP-BKM120 and Olaparib did not result in measurable toxicity, such as weight loss (Fig. 6 E), even in mice that were treated for over 3 months. To ensure target inhibition, we obtained pre-treatment biopsies and matched tumor specimens within 2 hours of the last dose of NVP-BKM120 and found that NVP-BKM120 potently reduced AKT phosphorylation (Fig. 6 F and S1). In tumor tissue lysates from the combination treatment, we observed inhibition of p-AKT with the combination treatment and induction of γH2AX (Fig. 6 F), consistent with results observed in the in vitro studies with cell lines (Fig. 4). Interestingly, Olaparib alone led to an induction of AKT phosphorylation in vivo (Fig. 6 F), an observation consistent with an increased FDG-uptake in Olaparib-treated tumors (Fig. S3) as opposed to NVP-BKM120 or the combination, both of which strongly reduced FDG-uptake (Fig. 2, S2 and S3).
Fig. 6.
Anti-tumor efficacy of PI3K inhibitor NVP-BKM120 alone and in combination with Olaparib. A–D Tumor-bearing MMTV-CreBRCA1 p53+/− were treated with either vehicle control (A), NVP-BKM120 (B, 50 mg/kg/day (n=11) or 30 mg/kg/day (n=10)), Olaparib (C, 50 mg/kg/day (n=8)) or the combination of NVP-BKM120 and Olaparib (D, NVP-BKM 50 mg/kg/day+Olaparib 50 mg/kg/day (n=8) or NVP-BKM 30 mg/kg/day+Olaparib 50 mg/kg/day (n=7)) and tumor volumes were measured every 2–3 days using calipers. Trendlines for vehicle control (red curve) and NVP-BKM120 treatments (green curve) were calculated using all data points to determine best fit. The functions of the best-fit curves were used to determine tumor doubling times for all three treatment modalities and controls. E, Stable body mass with PI3K-inhibitor and PARP-inhibitor treatments Mice were weighed before and after treatments. F, G. Target inhibition and pharmacokinetics in vivo. Tumor tissues harvested from animals treated with NVP-BKM120 (30 mg/kg/day) alone or in combination with Olaparib (50 mg/kg/day) as indicated were harvested 3 hours after the last treatment and subjected to immunoblotting with antibodies against Actin, p-AKT and γH2AX (F) or lysed and subjected to Mass Spectrometry (G). For standards used see Materials and Methods and Fig. S5. H, I. Responses of human BRCA1 -related breast cancers implanted as xenotransplants into nude mice to NVP-BKM120, Olaparib or their combination. Breast cancer tissues from two patients, one with a 185delAG germline mutation (H) and the other one with a 2080delA germline mutation (I) were propagated as subcutaneous implants in nude mice. Tumors were allowed to grow to a size of 5 mm when mice were randomized to treatments with either vehicle control (black, —), NVP-BKM120 (red,  ), Olaparib (green,
), Olaparib (green,  )or their combination (blue,
)or their combination (blue,  ) (n=6 for each cohort, same dosing as in F). Tumor assessment with electronic calipers was done as described in Materials and Methods.
) (n=6 for each cohort, same dosing as in F). Tumor assessment with electronic calipers was done as described in Materials and Methods.
In order to examine if there was a pharmacokinetic interaction between NVP-BKM120 and Olaparib we examined NVP-BKM120 levels in animals treated with NVP-BKM120 at 30 mg/kg/day and the combination of NVP-BKM120 and Olaparib (30 mg/kg/day and 50 mg/kg/day, respectively). For these studies, tissue extracts were processed for Mass Spectrometry 3 hours after the last dose (Figs. 6G, S5). We found that while NVP-BKM120 levels in tumor tissues were variable, they were consistently in the micro-molar range and were not affected by concurrent administration of Olaparib. The mouse model used here for BRCA1-related breast cancer MMTV-CreBRCA1f/fp53+/−, results in the residual expression of a hypomorphic BRCA1 protein, and we did find residual Rad51 recruitment to repair foci (Fig. 5 C). This residual HR activity may also explain the incomplete responses of the BRCA1-del11 expressing mammary tumors to olaparib monotherapy (Fig. 6C).
To test the applicability of our results to human BRCA1-related breast cancer, we treated xenograft tumors established from patients with BRCA1-related breast cancer (Fig. 6 H, I). The first patient-derived tumor (Fig. 6 H) was derived from a patient with an N-terminal germline mutation in BRCA1 (185 del AG). At the time of tissue acquisition, this tumor had developed resistance to standard chemotherapy as well as Olaparib, which had been administered in the context of a clinical trial. Growth of this tumor was modestly attenuated by either NVP-BKM120 or Olaparib alone in NOD/SCID mice. However, the combination induced stability over a period of 8 weeks (Fig. 6 H), confirming the in vivo synergy that we observed in our genetically engineered mouse model of BRCA1-related breast cancer. The second human tumor (Fig. 6 I) was derived from a patient with a C-terminal BRCA1 germline mutation (2080 del A). The patient who donated this tumor specimen had not yet been treated, and the tumor showed exquisite sensitivity to the PARP-inhibitor, NVP-BKM120, and the combination of both drugs. These human ex vivo data confirm the sensitivity of BRCA1-related breast cancer to NVP-BKM120, Olaparib and their combination, and, taken together, justify the exploration of this combination in an early phase clinical trial.
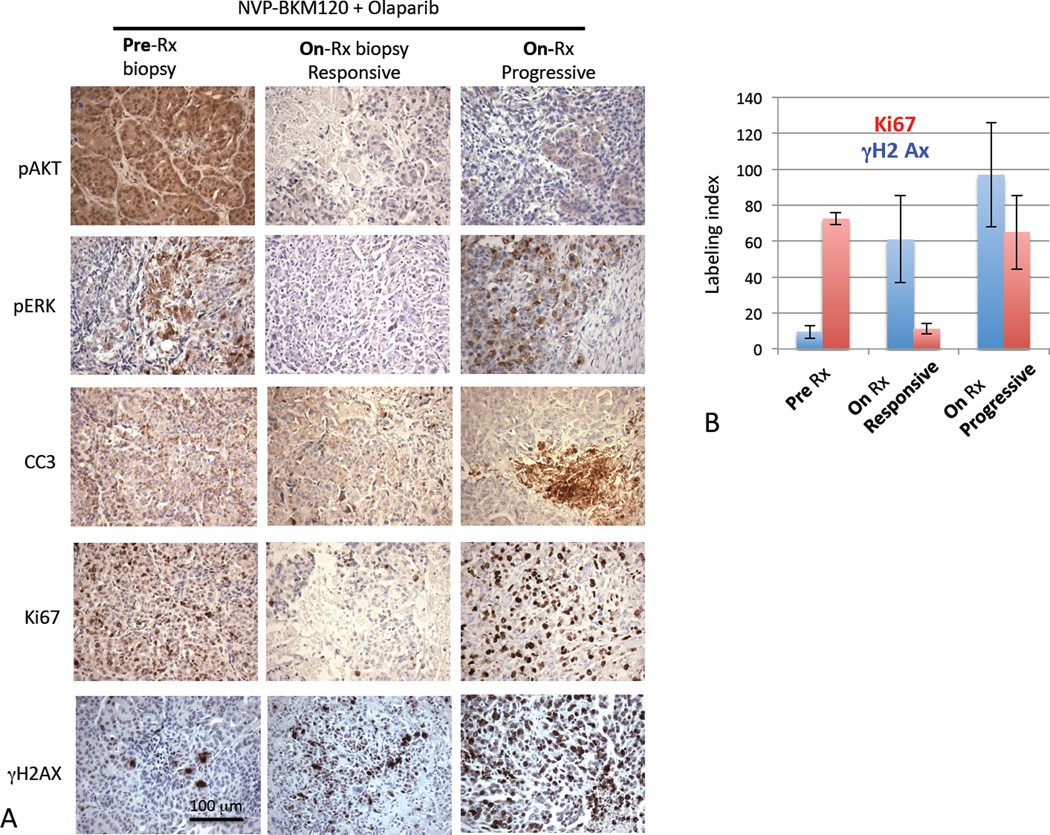
Resistance to treatments that include PI3K inhibitors occurs at the “pushing margin” and is associated with ERK-phosphorylation
Eventually, even in tumors that received dual treatments, resistance was observed and at that point, tumors re-grew rapidly (Fig. 6 A–D). To determine the nature of resistance to the NVP-BKM120 and Olaparib combination, we examined pre-treatment biopsies, on-treatment biopsies at the time of response on day 10 and post-treatment tissue at the time of progression (Fig. 7 A). Target inhibition, i.e. suppression of AKT phosphorylation, was maintained even in resistant tumors (Fig. 7 A, panel 1), suggesting that resistance to NVP-BKM120 is not due to PI3K p+thway activation but to relief of feedback inhibition of alternative pathways, including MAPK-activation as suggested earlier (31). The “pushing margin”, i.e. a highly proliferative rim of tumor cells that rarely infiltrate the surrounding tissue is a hallmark of BRCA1-related tumors (39), yet its biological basis is not understood. Interestingly, we found an increase in the number of cells with high phospho-ERK levels especially at the “pushing margin” of the tumor, paralleled by an increase in proliferating, i.e. Ki67-positive cells (Fig. 7 A, B, Fig. S6). This phenomenon, the concentration of p-ERK positive cells at the “pushing margin” was seen in tumors prior to treatment (Fig. 4 B, Fig. 7 A), at the time of progression on NVP-BKM120 alone (Fig. 4 B) or at the time of progression on the combination of the PARP-inhibitor with NVP-BKM120, while in responding tumors (day 10 biopsy) p-ERK positive cells were conspicuously absent (Fig. 7 A). As expected with PI3K inhibition and consistent with the p-ERK status of tumor cells, we found that tumors initially showed a stark decrease in proliferative activity (Ki67, Fig. 7 A, B), and that resistant tumors were characterized by high mitotic activity (Fig. 4 B, 7 A, B). Thus, activation of pro-proliferative MAPK-signaling may be a major driver for the resistance of tumors treated with PI3K-inhibitors.
Fig 7.
Signal transduction and proliferative activity of responsive and resistant tumors after treatments with NVP-BKM120 and Olaparib. A. Tumor-bearing MMTV-CreBRCA1f/fp53+/− mice were treated as indicated, and tumor tissues obtained as pre-treatment biopsies and at the time of progression. For tumor-bearing mice that were treated with the combination of NVP-BKM120 and Olaparib we also obtained a day 10-of-treatment biopsy (middle panel), at which point all tumors were responsive. Immunohistochemistry was performed with antibodies against antigens as indicated. B. Ki67 and γ-H2AX were scored by counting and averaging the number of positive nuclei per high-power field (HPF) in pre- and on-treatment biopsies and at the time of progression.
Discussion
We report here on a surprising in vivo synergy of NVP-BKM120 in combination with Olaparib for the treatment of BRCA1 mutant breast tumors, that suggests an important role of PI3Kα in the DNA damage response. Kumar et al. (40) showed that PI3K-β is required for the recruitment of NBS1 to DNA double-strand breaks (DSBs) and for the assembly of repair foci in response to ionizing radiation. It was shown previously that loss of PTEN, frequently seen in TNBC, leads not only to activation of the PI3K pathway, but also to an accumulation of DNA DSBs (41). In addition NVP-BKM120 enhances production of poly-ADP-ribose and phosphorylation of H2AX, suggesting increased DNA damage when the PI3K pathway is inhibited in the context of a BRCA1 mutation. In vivo H2AX phosphorylation in tumors increased when mice were treated with the combination of NVP-BKM120 and Olaparib during the period of response (day 10), and was highest at the time of treatment failure (Fig. 7), suggestive of a progressive accumulation of unrepaired DNA DSBs, which would contribute to the reliance on PARP activity for DNA damage repair and would explain the sensitivity to combined PARP and PI3K-inhibtion.
Of particular interest was our observation that, in spite of the increase in phosphorylation of H2AX in response to NVP-BKM120, both, NVP-BKM120 and depletion of PI3Kα, greatly reduced Rad51 incorporation into foci in cells treated with radiation. These results suggest that Class IA PI3K catalytic activity is required for recruitment of Rad51 into sites of DNA damage and raise the possibility that the increase in DNA-PK phosphorylation is a feedback response to this failure to form proper DNA damage repair complexes. BRCA1 is known to play a role in recruitment of Rad51 to sites of DNA damage (42) and thus it is possible that in BRCA1 defective cells, a PI3K dependent pathway becomes more critical for this recruitment. Clearly additional studies will be needed to understand the interactions between PI3K, Rad51 and DNA-PK in DNA repair processes.
Regulated PARP activity allows for DNA damage repair required for the maintenance of genomic stability. However, massive PARP-activation leads to depletion of its substrate NAD+ and consecutively depletion of ATP in an effort to replenish NAD+, resulting in energy loss and eventually cell death. Activation of PI3Kα leads to increased energy production via glycolysis. Glycolysis and poly (ADP) ribosylation both consume NAD+, and may compete for NAD+ available in the cytosol. Such metabolic competition makes sense for decisions on the fate of cells: If energy supply and glycolysis are high, the amount of NAD+ diverted into poly (ADP) ribosylation is limited, and cell death as a consequence of massive PARP-activation is avoided. Conversely, if glucose supply and glycolytic activity are low, NAD is consumed by PARP and the ensuing massive poly (ADP) ribosylation may lead to cell death (43). PARP-inhibition spares NAD+ which becomes available for glycoloysis and can protect cells from death, such as myocardial or CNS ischemia (44, 45), sepsis (46), or pancreatic islet cell damage (47). Consistent with this model we saw in vivo enhancement of glucose uptake (Fig. S3) and phosphorylation of AKT in response to Parp-inhibition, which was reversed by addition of the PI3K-inhibitor (Fig. 6E). Thus, a possible explanation for the in vivo synergy of PI3K and Parp-inhibitors is that PI3K-inhibition reverses the pro-survival effect of PARP-inhibition and thereby makes these drugs more effective, a combination that one would predict to be particularly effective in cancers with defects in homologous recombination (HR) such as BRCA1/2-related breast and ovarian cancers.
Finally, it is noteworthy that the in vivo approach allowed us to make several observations that could not be made in vitro: Much greater efficacy of the NVP-BKM120/Olaparib combination was observed in vivo than in vitro, suggesting that tumor microenvironment and metabolism may be important. Sequential tumor biopsies allowed us to monitor target inhibition in combination with tumormetrics allowed us to discover a potent synergy of PI3K inhibitor NVP-BKM120 with PARP inhibitor Olaparib to treat BRCA1-related breast cancer that may warrant exploration in an early phase clinical trial.
Materials and Methods
Materials
The PI3K inhibitor NVP-BKM120 was obtained through a Material Transfer Agreement with Novartis Pharmaceuticals. Olaparib was purchased from LC Laboratories (Woburn, MA) and KU-55933 was purchased from Selleck (Houston, TX). BRCA1-mutant human breast cancer cell line HCC1937 was from American Type Culture Collection; # CRL-2336, and maintained in DMEM/10% FBS and SUM149 a gift from Dr. Christina Gewinner, Division of Signal Transduction, BIDMC, maintained in Ham's F-12 with 5% fetal bovine serum (FBS), 5 µg/ml insulin, 2 µg/ml hydrocortisone, 5 µg/ml gentamicin and 2.5 µg/ml fungizone. Cell lines were authenticated by immunoblotting for BRCA1 and PTEN and tested for absence of mycoplasma.
Animal Experimentation
Animal experiments were conducted in accordance with IACUC-approved protocols at Beth Israel Deaconess Medical Center, Boston, and at the University of Vall d’Hebron, Barcelona, Spain. Female MMTV-CreBRCA1f/fp53+/− mice were obtained by breeding BRCA1 conditional knockout mice (01XC8,strain C57BL/6), originally generated by Drs. Xiaoling Xu and Chu-Xia Deng (12), who made these mice available to us via the NCI repository with MMTV-Cre (Jackson Laboratory B6129-TgN(MMTV-Cre)4Mam) (48) and p53 knockout (Taconic Farms, P53N12-M, C57BL/6) (49). At the time of the study mice had been inbred for 4 years (>7 generations). The floxed or wild type status of Brca1, the presence of the MMTV-Cre transgene and the p53 heterozygosity were determined by PCR as previously described (12). Mice were examined for the occurrence of tumors twice weekly. When tumormetrics were performed, the length and width of the tumor was determined using calipers, and the tumor volume was determined (width2 × length/2). Tumor volume was used as a measure of growth and was recorded as ratio to tumor volume at diagnosis. Tumor doubling times were calculated using the functions of the best fit curves for all data points in each treatment modality. NVP-BKM120 was resuspended in 5% Methylcellulose solution (Fluka) and administered via oral gavage at 50 mg/kg/day or 30 mg/kg/day. Olaparib was resuspended for intraperitoneal administration as described (15) and dosed at 50 mg/kg/day. For patient-derived tumor grafts consent for tumor use was obtained from patients under a protocol approved by the Vall d’Hebron Hospital Clinical Investigation Ethical Committee. Tumors were subcutaneously implanted in 6 week old female HsdCpb:NMRI-Foxn1nu mice (Harlan Laboratories, Italy). Animals were supplemented with 1µM estradiol (Sigma) in the drinking water. After tumor graft growth, tumor tissue was re-implanted into recipient mice, which were randomized upon implant growth.
FDG-PET-Scanning
0.3 to 0.4 mCi of fluorine-18-deoxyglucose were injected intravenously through the retroorbital vein of the anesthetized mouse. After a “washout” period of 1 hour the mouse was imaged on a NanoPET/CT (Bioscan/Mediso) scanner. The NanoPET/CT is a high-resolution small-animal multimodality scanner consisting of 12 lutetium yttrium oxyorthosilicate (LYSO) detector blocks. The blocks comprise a total of 39,780 crystals each with a dimension of 1.2×1.2×13 mm3.
Images were acquired in three dimensions. The mice remained supine and maintained their position throughout the procedure. First, a CT scan was performed and second, a whole-body 18F-FDG PET emission scan was acquired covering the same area as the CT scan. Counts per minute (cpm) were obtained, converted to mCi, and values were normalized for ROI volume and injected dose. In order to correct for metabolic variability between exams and to determine tumor-specific uptake changes, FDG-uptake rates were corrected for cardiac FDG-uptake (µCi/µCi injected/voxelstumor) / (µCi/µCi injected/voxelsheart). For studies involving repeat scanning, the change in tumor-specific FDG-uptake was determined in percent (1- (FDG-uptakepost / FDG-uptakepre)*100). Animals were housed in the Longwood SAIF satellite animal facility between scans.
Immunohistochemistry
For immunohistochemistry we used anti-cleaved caspase 3 (CC3 ) (9661S, Cell Signalling, Rabbit polyclonal Asp175), anti-Ki67 (9106-S; Thermo Scientific, Rabbit monoclonal SP6). All other antibodies used are described in the immunoblotting section below. All immunohistochemistries were done as described previously(11) including antigen retrieval with a citrate-buffer.
Immunoblotting
Cells were treated with mock, NVP-BKM120, Olaparib, KU-55933 or the combination and lysed in cell lysis buffer (9803, Cell Signaling) as per the manufacturer’s instructions. Immunoblots were performed using the Nupage System (Invitrogen). A total of 20 µg of protein were loaded, except for PAR, Phospho ATM and Phospho DNA-PK/PRKDC western blots, where 40 µg were loaded. Tumor tissue lysates were prepared similarly with the exception of tissue homogenization by using an electric homogenizer for 30 secs after addition of the lysis buffer. Primary antibodies used for western blotting were total AKT (9272), Cleaved Caspace 3 (9661), total ERK (4695), Phospho AKT Ser473 (4058), Phospho ERK Thr202/Tyr204 (9106), Phospho-Histone H2AX Ser139 (2577), PTEN (9559) from Cell Signaling. Phospho ATM Ser1981 (2152-1), Phospho DNA-PK/PRKDC Ser2056 (3892-1) from Epitomics, Inc. CD31 (ab28364), Actin (ab6276), INPP4B (ab81269) from Abcam; pADPr (sc56198) from Santacruz Biotechnology; and Ki-67 (RM-9106) was purchased from Thermo Scientific. Rad51 antibody was a gift from Dr. Ralph Scully.
Immunoflorescence
Cells were plated on coverslips in a 6-well plates and incubated overnight at 37°C with 5% CO2 before drug treatment. Cells were exposed to NVP-BKM-120 for 24 hrs followed by irradiation (10 Gy). Cells were fixed with 3% paraformaldehyde and 2% sucrose diluted in PBS 6 h post-irradiation and subsequently permeabilized with 0.5% TritonX-100 buffer (20 mM HEPES pH7.4, 50 mM NaCl, 3 mM MgCl, 300 mM sucrose) for 3 minutes on ice. Cells were incubated with a primary rabbit anti human Rad-51 antiserum at 1: 500 dilution in hybridization buffer (5% goat serum, 0.5% NaN3, 1× PBS) for 30 min at 37°C. Secondary antibody used was a donkey anti-rabbit Alexafluor 488 conjugated (Invitrogen) at a concentration of 1: 50. Images were acquired using a Zeiss 710 NLO laser scanning confocal microscope.
siRNA Transfections
The siRNAs were obtained from Dharmacon, Lafayette. SUM149 cells were transfected with either 10 or 30 nM pool of 4 siRNA sequences targeting PIK3CA (cat#L-003018-00-0005) or PIK3CB (cat#L-003019-00-0005) siRNA using HiPerFect Transfection Reagent (QIAGEN) according to the manufacturer's protocol. Control cells were treated with HiPerFect alone. Cells were grown and harvested 48 h after the transfection using cell lysis buffer (9803, Cell Signaling) as per the manufacturer’s instructions and analyzed by Immunoblotting.
Cell viability assay
For cell viability assays, breast cancer cells were seeded at a density of 250 cells/well in 96-well plates in the absence or presence of drugs, and cell viability was determined using the CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI, USA) according to the manufacturer's instructions, using a Wallac 3 plate reader.
Sequencing
Genomic DNA was isolated and PCR amplification performed for regions in the murine PI3K gene that are homologous to the regions frequently mutated in human breast cancer, i.e. E542K and E545K in the helical domain and H1047R in the kinase domain. Primers used were for exon 9: Forward CGCATACCTGCATCTGTTCTA; Reverse AAATGATGTGTGTGCTGGGT Exon 20: Forward AGCAGCTCACTGACCAGATGT; Reverse ACTCACTGCCATGCAGTGGA. PCR products were subjected to direct sequencing at Genewiz (Cambridge MA, USA).
Data Analysis
Determination of the Chalkley score was done as described (29, 50). Briefly, the three most vascular areas (hot spots) with the highest number of microvessel profiles in each tumor were photographed under an Olympus light microscope at 200 x; a digital mask representing the Chalkley grid area, 0.196 mm2, was used to count the CD31-positive spots in a blind fashion and the mean value of the three grid counts obtained. A two-sided t-Test was used to determine significance.
Targeted mass spectrometry (LC/MS/MS) for BKM-120 PK Study
Metabolites from 100 mg of mouse tumor samples were extracted using 80% methanol according to Yuan et al.(51) 10 µL were injected and analyzed using a 5500 QTRAP hybrid triple quadrupole mass spectrometer (AB/SCIEX) coupled to a Prominence UFLC HPLC system (Shimadzu) via selected reaction monitoring (SRM) for the Q1/Q3 transition of 410.8/367.0 for NVP-BKM120. ESI voltage was +4900V in positive ion mode using a dwell time of 4 msec and collision energy of 45. Approximately 15 data points were obtained for NVP-BKM120 per LC/MS/MS experiment. Samples were delivered to the MS via hydrophilic interaction chromatography (HILIC) using a 4.6 mm i.d x 10 cm Amide Xbridge column (Waters) at 350 µL/min. Gradients were run starting from 85% buffer B (HPLC grade acetonitrile) to 42% B from 0–5 minutes; 42% B to 0% B from 5–16 minutes; 0% B was held from 16–24 minutes; 0% B to 85% B from 24–25 minutes; 85% B was held for 7 minutes to re-equilibrate the column. NVP-BKM120 eluted at approximately 3.50 min. Buffer A was comprised of 20 mM ammonium hydroxide/20 mM ammonium acetate (pH=9.0) in 95:5 water:acetonitrile. Peak areas from the total ion current for the NVP-BKM120 metabolite SRM transition was integrated using MultiQuant v2.0 software (AB/SCIEX). For the concentration curve data, NVP-BKM120 was prepared at concentrations of 1 nM, 10 nM, 100 nM, 500 nM, 1 µM and 10 µM in 40% methanol. 5 µL of each sample were injected using the parameters described above.
Supplementary Material
Significance.
Current treatment options for triple-negative breast cancer are limited to chemotherapeutic regimens that have considerable toxicity and are not curative. We report here that the combination of a PI3K inhibitor with a PARP inhibitor provides in vivo synergy for treatment of an endogenous mouse model for BRCA1-related breast cancers, making this a candidate combination to be tested in human clinical trials.
Acknowledgments
The authors are grateful to the women who allowed us to study their tumor tissues. We are grateful to Ms. Min Yuan for help with mass spectrometry experiments. The authors wish to acknowledge Novartis Pharmaceuticals for providing NVP-BKM120, Dr. Celina Garcia-Garcia, University Hospital Vall d'Hebron for her work in the tissue collection study, and Drs. Ursula Matulonis and Eric Winer, Dana Farber Cancer Institute, for their support and discussion of the data.
Grant Support
L Cantley and G. Wulf are supported by a Stand Up to Cancer Dream Team Translational Research Grant, a Program of the Entertainment Industry Foundation (SU2C-AACR-DT0209) and by the Breast Cancer Research Foundation (BCRF). G. Wulf was supported by a Susan Komen Foundation grant BCTR0601030, J. Asara is supported by by NIH NCI grants 5P01CA120964-05 and 5P30CA006516-46; L. Cantley is supported by NIH grant GM41890 and NCI grant P01CA089021.
Abbreviations
- PARP
Poly (ADP-ribose) polymerase
- PI3K
Phosphatidylinositol 3-kinase
- PAR
Poly (ADP-ribose)
- MAPK
Mitogen-activated protein (MAP) kinase
- BRCA1
Breast cancer 1, early onset gene
Footnotes
Conflicts of interest:
LCC and JB have consulted for Novartis Pharmaceuticals, which is developing NVP-BKM120 for cancer treatment; JB has consulted for Astra Zeneca, which is developing Olaparib for cancer treatment.
References
- 1.Gayther SA, Warren W, Mazoyer S, Russell PA, Harrington PA, Chiano M, et al. Germline mutations of the BRCA1 gene in breast and ovarian cancer families provide evidence for a genotype-phenotype correlation. Nat Genet. 1995;11:428–433. doi: 10.1038/ng1295-428. [DOI] [PubMed] [Google Scholar]
- 2.Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 3.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 4.Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 5.Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, Gourley C, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28:2512–2519. doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- 6.Collins LC, Martyniak A, Kandel MJ, Stadler ZK, Masciari S, Miron A, et al. Basal cytokeratin and epidermal growth factor receptor expression are not predictive of BRCA1 mutation status in women with triple-negative breast cancers. Am J Surg Pathol. 2009;33:1093–1097. doi: 10.1097/PAS.0b013e31819c1c93. [DOI] [PubMed] [Google Scholar]
- 7.van der Groep P, Bouter A, van der Zanden R, Siccama I, Menko FH, Gille JJ, et al. Distinction between hereditary and sporadic breast cancer on the basis of clinicopathological data. J Clin Pathol. 2006;59:611–617. doi: 10.1136/jcp.2005.032151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maor S, Yosepovich A, Papa MZ, Yarden RI, Mayer D, Friedman E, et al. Elevated insulin-like growth factor-I receptor (IGF-IR) levels in primary breast tumors associated with BRCA1 mutations. Cancer Lett. 2007;257:236–243. doi: 10.1016/j.canlet.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Neuhausen SL, Brummel S, Ding YC, Steele L, Nathanson KL, Domchek S, et al. Genetic variation in IGF2 and HTRA1 and breast cancer risk among BRCA1 and BRCA2 carriers. Cancer Epidemiol Biomarkers Prev. 2011;20:1690–1702. doi: 10.1158/1055-9965.EPI-10-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuhausen SL, Brummel S, Ding YC, Singer CF, Pfeiler G, Lynch HT, et al. Genetic variation in insulin-like growth factor signaling genes and breast cancer risk among BRCA1 and BRCA2 carriers. Breast Cancer Res. 2009;11:R76. doi: 10.1186/bcr2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burga LN, Hu H, Juvekar A, Tung NM, Troyan SL, Hofstatter EW, et al. Loss of BRCA1 leads to an increase in epidermal growth factor receptor expression in mammary epithelial cells, and epidermal growth factor receptor inhibition prevents estrogen receptor-negative cancers in BRCA1-mutant mice. Breast Cancer Res. 2011;13:R30. doi: 10.1186/bcr2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X, Wagner KU, Larson D, Weaver Z, Li C, Ried T, et al. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet. 1999;22:37–43. doi: 10.1038/8743. [DOI] [PubMed] [Google Scholar]
- 13.Brodie SG, Xu X, Qiao W, Li WM, Cao L, Deng CX. Multiple genetic changes are associated with mammary tumorigenesis in Brca1 conditional knockout mice. Oncogene. 2001;20:7514–7523. doi: 10.1038/sj.onc.1204929. [DOI] [PubMed] [Google Scholar]
- 14.Shukla V, Coumoul X, Cao L, Wang RH, Xiao C, Xu X, et al. Absence of the full-length breast cancer-associated gene-1 leads to increased expression of insulin-like growth factor signaling axis members. Cancer Res. 2006;66:7151–7157. doi: 10.1158/0008-5472.CAN-05-4570. [DOI] [PubMed] [Google Scholar]
- 15.Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17079–1084. doi: 10.1073/pnas.0806092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang T, Ohashi A, Huang Y, Pandita TK, Ludwig T, Powell SN, et al. Negative Regulation of AKT Activation by BRCA1. Cancer Res. 2008;68:10040–10044. doi: 10.1158/0008-5472.CAN-08-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Razandi M, Pedram A, Rosen EM, Levin ER. BRCA1 inhibits membrane estrogen and growth factor receptor signaling to cell proliferation in breast cancer. Mol Cell Biol. 2004;24:5900–5913. doi: 10.1128/MCB.24.13.5900-5913.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan Y, Haas JP, Kim M, Sgagias MK, Cowan KH. BRCA1-induced apoptosis involves inactivation of ERK1/2 activities. The Journal of biological chemistry. 2002;277:33422–33430. doi: 10.1074/jbc.M201147200. [DOI] [PubMed] [Google Scholar]
- 19.Saal LH, Gruvberger-Saal SK, Persson C, Lovgren K, Jumppanen M, Staaf J, et al. Recurrent gross mutations of the PTEN tumor suppressor gene in breast cancers with deficient DSB repair. Nat Genet. 2008;40:102–107. doi: 10.1038/ng.2007.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gewinner C, Wang ZC, Richardson A, Teruya-Feldstein J, Etemadmoghadam D, Bowtell D, et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer cell. 2009;16:115–125. doi: 10.1016/j.ccr.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fedele CG, Ooms LM, Ho M, Vieusseux J, O'Toole SA, Millar EK, et al. Inositol polyphosphate 4-phosphatase II regulates PI3K/Akt signaling and is lost in human basal-like breast cancers. Proc Natl Acad Sci U S A. 107:22231–22236. doi: 10.1073/pnas.1015245107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Specht JM, Kurland BF, Montgomery SK, Dunnwald LK, Doot RK, Gralow JR, et al. Tumor metabolism and blood flow as assessed by positron emission tomography varies by tumor subtype in locally advanced breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:2803–2810. doi: 10.1158/1078-0432.CCR-10-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tafreshi NK, Kumar V, Morse DL, Gatenby RA. Molecular and functional imaging of breast cancer. Cancer Control. 2010;17:143–155. doi: 10.1177/107327481001700302. [DOI] [PubMed] [Google Scholar]
- 24.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 26.Vander Heiden MG. Targeting cell metabolism in cancer patients. Sci Transl Med. 2010;2:31ed1. doi: 10.1126/scitranslmed.3001210. [DOI] [PubMed] [Google Scholar]
- 27.Schnell CR, Stauffer F, Allegrini PR, O'Reilly T, McSheehy PM, Dartois C, et al. Effects of the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 on the tumor vasculature: implications for clinical imaging. Cancer Res. 2008;68:6598–6607. doi: 10.1158/0008-5472.CAN-08-1044. [DOI] [PubMed] [Google Scholar]
- 28.Yuan TL, Choi HS, Matsui A, Benes C, Lifshits E, Luo J, et al. Class 1A PI3K regulates vessel integrity during development and tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9739–9744. doi: 10.1073/pnas.0804123105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox SB, Leek RD, Weekes MP, Whitehouse RM, Gatter KC, Harris AL. Quantitation and prognostic value of breast cancer angiogenesis: comparison of microvessel density, Chalkley count, and computer image analysis. J Pathol. 1995;177:275–283. doi: 10.1002/path.1711770310. [DOI] [PubMed] [Google Scholar]
- 30.Maira SM, Pecchi S, Huang A, Burger M, Knapp M, Sterker D, et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther. 2012;11:317–328. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- 31.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomlinson GE, Chen TT, Stastny VA, Virmani AK, Spillman MA, Tonk V, et al. Characterization of a breast cancer cell line derived from a germ-line BRCA1 mutation carrier. Cancer Res. 1998;58:3237–3242. [PubMed] [Google Scholar]
- 33.Forozan F, Veldman R, Ammerman CA, Parsa NZ, Kallioniemi A, Kallioniemi OP, et al. Molecular cytogenetic analysis of 11 new breast cancer cell lines. Br J Cancer. 1999;81:1328–1334. doi: 10.1038/sj.bjc.6695007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saal LH, Gruvberger-Saal SK, Persson C, Lovgren K, Jumppanen M, Staaf J, et al. Recurrent gross mutations of the PTEN tumor suppressor gene in breast cancers with deficient DSB repair. Nat Genet. 2008;40:102–107. doi: 10.1038/ng.2007.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 36.Hickson I, Zhao Y, Richardson CJ, Green SJ, Martin NM, Orr AI, et al. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 2004;64:9152–9159. doi: 10.1158/0008-5472.CAN-04-2727. [DOI] [PubMed] [Google Scholar]
- 37.Drost R, Bouwman P, Rottenberg S, Boon U, Schut E, Klarenbeek S, et al. BRCA1 RING function is essential for tumor suppression but dispensable for therapy resistance. Cancer cell. 2011;20:797–809. doi: 10.1016/j.ccr.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 38.Yuan SS, Lee SY, Chen G, Song M, Tomlinson GE, Lee EY. BRCA2 is required for ionizing radiation-induced assembly of Rad51 complex in vivo. Cancer Res. 1999;59:3547–3551. [PubMed] [Google Scholar]
- 39.Lakhani SR, Jacquemier J, Sloane JP, Gusterson BA, Anderson TJ, van de Vijver MJ, et al. Multifactorial analysis of differences between sporadic breast cancers and cancers involving BRCA1 and BRCA2 mutations. J Natl Cancer Inst. 1998;90:1138–1145. doi: 10.1093/jnci/90.15.1138. [DOI] [PubMed] [Google Scholar]
- 40.Kumar A, Fernandez-Capetillo O, Carrera AC. Nuclear phosphoinositide 3-kinase beta controls double-strand break DNA repair. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7491–7496. doi: 10.1073/pnas.0914242107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128:157–170. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 42.Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. The Journal of biological chemistry. 2000;275:23899–23903. doi: 10.1074/jbc.C000276200. [DOI] [PubMed] [Google Scholar]
- 43.Ha HC, Snyder SH. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13978–13982. doi: 10.1073/pnas.96.24.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zingarelli B, Salzman AL, Szabo C. Genetic disruption of poly (ADP-ribose) synthetase inhibits the expression of P-selectin and intercellular adhesion molecule-1 in myocardial ischemia/reperfusion injury. Circ Res. 1998;83:85–94. doi: 10.1161/01.res.83.1.85. [DOI] [PubMed] [Google Scholar]
- 45.Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, et al. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- 46.Soriano FG, Liaudet L, Szabo E, Virag L, Mabley JG, Pacher P, et al. Resistance to acute septic peritonitis in poly(ADP-ribose) polymerase-1-deficient mice. Shock. 2002;17:286–292. doi: 10.1097/00024382-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Masutani M, Suzuki H, Kamada N, Watanabe M, Ueda O, Nozaki T, et al. Poly(ADP-ribose) polymerase gene disruption conferred mice resistant to streptozotocin-induced diabetes. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:2301–2304. doi: 10.1073/pnas.96.5.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner KU, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L, et al. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 1997;25:4323–4330. doi: 10.1093/nar/25.21.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr., Butel JS, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 50.Fox SB, Turner GD, Leek RD, Whitehouse RM, Gatter KC, Harris AL. The prognostic value of quantitative angiogenesis in breast cancer and role of adhesion molecule expression in tumor endothelium. Breast Cancer Res Treat. 1995;36:219–226. doi: 10.1007/BF00666042. [DOI] [PubMed] [Google Scholar]
- 51.Yuan M, Breitkopf SB, Yang X, Asara JM. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat Protoc. 2012;7:872–881. doi: 10.1038/nprot.2012.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.