Abstract
In the present study, we compared the therapeutic effect of tumor-selective retroviral replicating vectors (RRV) expressing the yeast cytosine deaminase (CD) delivered by CED or simple injection, followed by systemic administration of the pro-drug, 5-fluorocytosine (5-FC). Treatment with RRV-CD and systemic 5-FC significantly increased survival in rodent U87MG glioma model in comparison to controls (p<0.01). Interestingly, CED of RRV-CD followed by 5-FC further enhanced survival in this animal model in comparison to intra-tumoral injection of RRV-CD followed by systemic 5-FC (p<0.05). High expression levels of Ki-67 were found in untreated tumors compared to treated. Untreated tumors were also much larger than treated. CED resulted in excellent distribution of RRV while only partial distribution of RRV was obtained after injection. Furthermore, RRV-CD and cytosine deaminase were also found in tumors from treated rats at study end-points. These results demonstrated that RRV vectors may efficiently transduce and stably propagate in malignant human glioma, thereby achieving a significant in-situ amplification effect after initial administration. We conclude that delivery of RRV into the glioma by CED provides much wider vector distribution than simple, injection, and this correlated with better therapeutic outcomes.
Keywords: Glioma, RRV, 5-fluorouracil, convection-enhanced delivery
Introduction
The prognosis for patients with malignant glioma, such as glioblastoma multiforme (GBM), is very poor despite combined therapeutic modalities that include surgery, radiation, and chemotherapy1, 2. Because these tumors grow rapidly and invade the surrounding brain parenchyma, it is often impossible to achieve complete surgical resection without causing severe neurologic damage3. Although it is an important therapy for GBM, chemotherapy has not consistently achieved clinical benefits, and significant response is seen in only 10 – 30% of patients4, 5. Outcomes for patients with gliomas remain dismal, and, therefore, there is an obvious need for the development of novel therapeutic strategies for the treatment of GBM, such as gene therapy.
Gene therapy offers tremendous potential advantages for the future of cancer treatment, and has been exploited to develop new strategies for selectively killing cancer cells or arresting their growth6. The most commonly used strategy in cancer gene therapy has been prodrug activator gene delivery. This approach allows the administration of a well -tolerated pro-drug that is then converted to an active anti-cancer metabolite by a specific enzyme introduced into the target cells by a prodrug activator gene6,7. Unique among replicating viruses being developed as oncolytic agents, murine leukemia virus (MLV)-based retroviral replicating vectors (RRV) replicate without immediate lysis of the host cell, and maintain viral persistence through stable integration6, 7. Furthermore, MLV does not infect quiescent cells, so RRV-mediated gene transfer is selective for dividing cells such as cancer cells6,7, 8, and further tumor specificity may accrue from tumor associated defects in the innate immune system9, CD converts the 5-FC into the potent chemotherapeutic agent 5-fluorouracil (5-FU)directly and selectively in the infected tumor cells6,7. The 5-FU can exit from the transduced cells and enter neighboring dividing cells, resulting in a bystander effect to achieve improved malignant cell killing10, 11. The CD/5-FC combination has been proven effective at controlling tumor growth in animals6–8, and is currently being evaluated in several clinical trials including trials for high grade glioma brain tumors (clinicaltrials.gov. NCT01470794, NCT01156584) However, in earlier applications of neurological gene therapy with non-replicating vectors, poor distribution of vector in the target tissue is invariably associated with poor efficacy, and it still remains an under-estimated, yet potentially critical, factor in gene therapy12, 13. In human tumors, strategies for improving RRV distribution, and hence efficacy, are potentially important goals.
Convection-enhanced delivery (CED) is an interstitial central nervous system (CNS) delivery technique14 that circumvents the blood–brain barrier in delivering agents directly into the brain. Traditional local delivery, such as injection of most therapeutic agents into the brain, has relied on diffusion that depends on a concentration gradient to overcome biological barriers. Thus, diffusion results in limited distribution of most delivered agents, and drug penetrates only a few millimeters from the source. Injection of therapeutics into non-malignant brain also has been associated with reflux and leakage near the injection site in human studies. In contrast, CED uses a fluid pressure gradient established at the tip of an infusion catheter and bulk flow to propagate substances within the extracellular fluid space14. CED also allows the extracellularly infused material to further propagate via the perivascular spaces and the rhythmic contractions of blood vessels act as an efficient motive force for the infusate15. As a result, a higher concentration of certain drugs can be distributed more evenly over a larger area of targeted tissue than would be seen with a simple injection. Currently, CED has been clinically tested in the fields of neurodegenerative diseases, such as Parkinson's disease16, 17, and neuro-oncology18, 19. Laboratory investigations with CED cover a broad field of application, including the delivery of viral particles20.
Although RRV vectors are capable of natural spread within the brain tumor, optimal methods for initial brain delivery have not been established. In this study, we compared the therapeutic effect of RRV-CD delivered by CED or manual injection followed by systemic administration of pro-drug 5-FC. We found CED significantly improves efficacy of therapeutic RRV-CD by maximizing vector spread and distribution, and tumor cell transduction in a rodent brain tumor model as compared to a injection technique. Our findings showed that CED should be considered when local therapies such as gene transfer with RRV are being translated into clinical therapy.
Materials and Methods
Cell line and RRV vector
The human GBM cell line, U87MG, was obtained from the Brain Tumor Research Center Tissue Bank at the University of California San Francisco. Cells were maintained as a monolayer in Eagle’s minimum essential medium supplemented with 10% fetal bovine serum, nonessential amino acids, 0.1 mg/ml streptomycin sulfate, and 100 U/ml penicillin G. Cells were cultured at 37°C in a humidified atmosphere consisting of 95% air and 5% CO2.
RRV AC3-emd vector encoding the GFP cDNA (RRV-GFP) was obtained as a gift from Dr. Noriyuki Kasahara (University of California, Los Angeles, CA) with permission of Tocagen, and the titer of vector used was 1000 TU/µl. RRV vector expressing the yeast cytosine deaminase prodrug activator gene, Toca 511(vocimagene amiretrorepvec)21, was obtained from Tocagen Inc. (San Diego, CA), and the titer of vector used was 6.3 × 105 TU/µl. We used GFP vectors at a low titer to allow easy visualization of vector spread. In the efficacy experiments, the goal was to maximize vector dose in order to reveal differences in efficacy. The prodrug 5-fluorocytosine (5-FC) was also obtained from Tocagen Inc. (San Diego, CA), and used at a dose of 500 mg/kg body weight (twice daily, half the dose at each time).
Animal Brain Tumor Model
Congenitally athymic, male, nude rats weighing 150 to 200 g (National Cancer Institute Animal Production Program, Frederick, MD) were housed under aseptic conditions that included filtered air, and sterilized food, water, bedding, and cages. For the intracranial xenograft tumor model, U87MG glioblastoma cells were harvested by trypsinization, washed once with phosphate-buffered saline (PBS) without Ca2+ and Mg2+, and re-suspended in PBS for implantation. Cells (2 × 105) in 10 µL PBS were implanted into the striatal region of brains as follows. Under deep isoflurane anesthesia, rats were placed in a small-animal stereotactic frame (David Kopf Instrument, Tujunga, CA). A sagittal incision was made to expose the cranium and this was followed by creation of a burr-hole in the skull 0.5 mm anterior and 3 mm lateral from the bregma with a small dental drill. The cell suspension (10 µL) was injected over 2 min at a depth of 4.5 mm from the brain surface; after two minutes, the needle was removed and the wound was closed. Experimentation was performed according to the National Institutes of Health guidelines and protocols approved by the Institutional Animal Care and Use Committee at the University of California San Francisco (San Francisco, CA).
For the RRV distribution study, 10 rats with U87MG striatal xenografts were randomly divided into 2 groups: group 1, injection of RRV-GFP vectors (n=5) and group 2, CED of RRV-GFP vectors (n=5). Ten days after tumor implantation, animals received either a manual injection, in which a hand-held Hamilton syringe was used to deliver a designated volume of vector, or CED of RRV vectors into the brain tumor. The animals were then euthanized 7 days after administration of the vectors, and perfused with PBS and 4% paraformaldehyde (PFA). For the efficacy study, 32 rats with U87MG xenografts were randomly divided into 3 groups (see below).
Delivery of RRV vectors by CED and injection
Twenty microliters of RRV vector was infused by CED into tumor as described22,23,24. Briefly, the infusion system consisted of a cannula with a 1-mm step25 connected to a 100-µl syringe (filled with RRV) that was in turn mounted onto stereotactic holder. The holder was connected to a micro-infusion pump (BeeHive, Bioanalytical System, West Lafayette, IN) to regulate the flow of fluid through the system. Based on the chosen coordinates, the stepped cannula was guided to the targeted region of the implanted brain tumor through burr-holes made in the skull. An infusion rate of 1 µL/min for 20 min was applied to achieve the 20 µl total infusion volume. Alternatively RRV, in a volume of 20 µl, was manually injected as a bolus into brain tumor via a syringe and a 26-gauge needle fitted with a cut pipet tip as a depth-stop to compare the RRV distribution between the two delivery methods.
Anti-tumor effect of RRV-CD and 5-FC in U87MG xenograft model
Thirty-two nude rats with U87MG xenografts were randomly divided into 3 groups: group 1, control (n=10); group 2, injection of RRV-CD (n=11); and group 3, CED of RRV-CD (n=11). Five days after tumor implantation, rats in groups 2 and 3 received either injection or CED of RRV-CD into the brain tumor, whereas control animals received CED of 0.9% normal saline. Five days after RRV administration, all the animals received intraperitoneal (IP) injections of 5-FC (500 mg/kg) daily for 7 days. Three rats in each group were euthanized 3 days after 5-FC treatment for evaluation of tumor size and expression levels of Ki-67. Antitumor efficacy was estimated by increase in median survival time as compared to control. Results are shown as Kaplan-Meier plots.
Immunohistochemistry
Animals were perfused with PBS, followed by 4% paraformaldehyde. Brains were post-fixed overnight in 4% paraformaldehyde, followed by 30% sucrose, and then were cut into 40-µm sections on a MICROM HM450 sliding microtome (Fisher Scientific, Philadelphia, PA). Sections were blocked first in 1% H2O2 and then in Biocare Sniper® (Biocare, Concord, CA) followed by incubation overnight at room temperature (RT) with primary rabbit anti-hrGFP polyclonal antibody (Millipore, Chemicon, Billerica, MA) diluted 1:500 with Da Vinci (Biocare, Concord, CA), or rabbit anti-gag polyclonal antibody (Abcam, Cambridge, MA) diluted 1:5,000 with Da Vinci (Biocare, Concord, CA), or at 4°C with rabbit anti-Ki67 polyclonal antibody (Novocastro, Buffalo Grove, IL) diluted to 1:5000 with Da Vinci®. The MACH 2 conjugated goat anti-rabbit polymer-horseradish peroxidase secondary antibody (Biocare, Concord, CA) was used to detect the primary antibodies. For cytosine deaminase immunohistochemistry, sections were incubated overnight at RT with primary sheep anti-cytosine deaminase polyclonal antibody (Abcam, Cambridge, MA) diluted to 1:2,000 with Da Vinci® (Biocare, Concord, CA). Biotinylated rabbit anti-sheep IgG was used to detect the primary antibody. Immunoreactivity was visualized with DAB working solution according to the supplier’s recommendations (Vector Laboratories, Inc., Burlingame, CA).
Volumetric quantification of RRV distribution
Brain sections with GFP immunohistochemistry were used for volumetric quantification of distribution of RRV. The distribution volume (Vd) of RRV in the brain tumor of each subject was quantified on an Apple Macintosh G4 computer with the Image J program (Image Processing and Analysis in Java). Regions-of-interest (ROI) derived in the distribution of RRV and brain tumor were manually defined, and the volume of the ROI was then calculated as area per brain section defined multiplied by slice thickness. The boundaries of each distribution and brain tumor were defined in the same manner in a series of brain sections.
Statistical Analysis
Results are expressed as mean ± SD, where applicable. The statistical analyses of distribution of RRV were performed by Student’s t-test. Confidence intervals are indicated by p values where appropriate.
Results
Prolonged survival in U87MG brain tumor xenograft model with CED of RRV-CD followed by systemic 5-FC
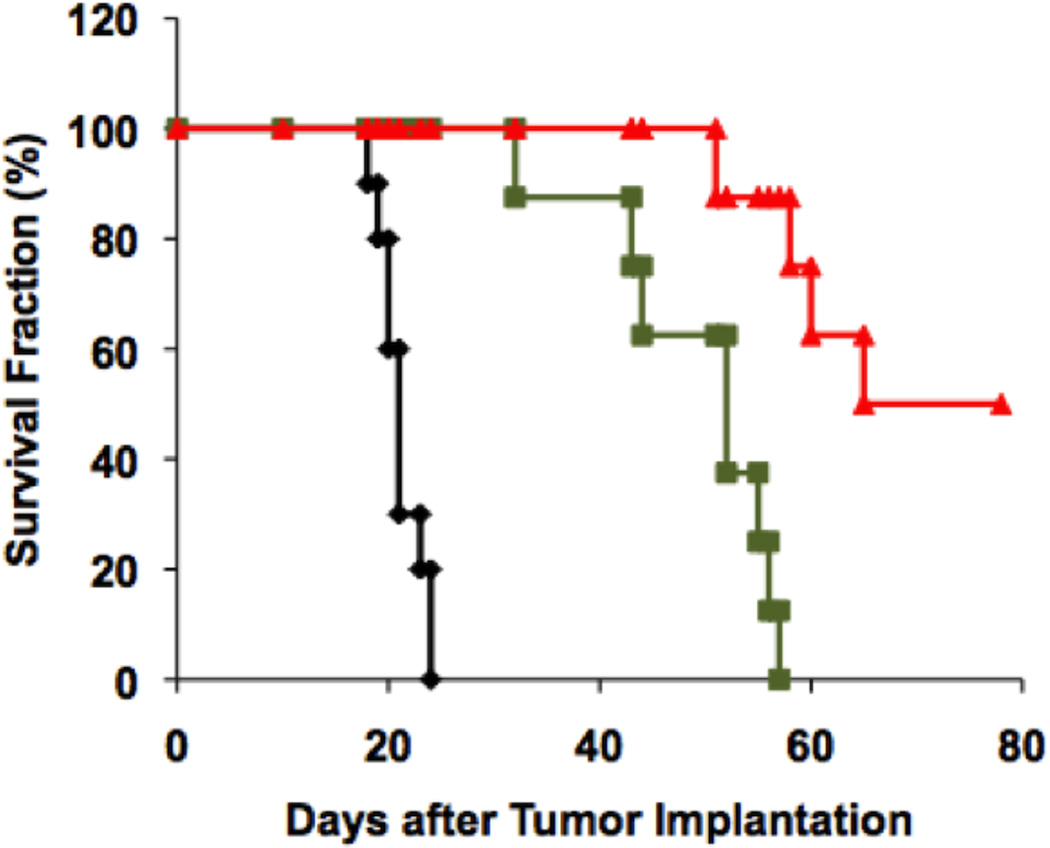
After CED or manual injection of RRV-CD into pre-established U87MG gliomas, we performed a single cycle of systemic 5-FC administration for 7 days. All rats from the control group, which only received IP injections of 5-FC, developed neurological symptoms due to large tumors and were euthanized between 18 to 24 days with median survival time (MST) of 21.1 ± 0.6 days after tumor implantation (Fig. 1). In contrast, RRV-CD and 5-FC-treated groups survived significantly longer (p < 0.01). Furthermore, CED of RRV-CD significantly improved the survival in animal with brain tumor as compared with injection of RRV-CD. In animals treated with injection of RRV-CD and IP 5-FC, the MST was 49.3 ± 3.1 days (Fig. 1). In the group that received CED of RRV-CD and IP 5-FC, 4 out of 8 rats were euthanized 51, 58, 59 and 65 days after tumor implantation, and the other 4 survived until the end-point of the efficacy study (77 days) (Fig. 1). Histopathologic evaluation was performed and brain tumors were found in all animals. Systemic injection of 5-FC caused weight loss of less than 10%, and no other side effects were found in this study.
Fig. 1. Survival study in nude rats with U87MG xenografts.
Thirty-two nude rats with implanted U87MG tumors were randomly divided into 3 groups: control (Black, n=10); manual injection of RRV-CD (Green, n=11); and CED of RRV-CD (Red, n=11). Five days after tumor implantation, rats received either a simple injection or CED of RRV-CD into the brain tumor whereas control animals received CED of 0.9% normal saline. Five days after RRV administration, all the animals received intraperitoneal injections of 5-FC (500 mg/kg) daily for 7 days. Antitumor efficacy was determined as increase in median survival compared to control. Results are shown by Kaplan-Meier plots.
To evaluate tumor size, 9 rats (3 in each group) were euthanized 3 days after 5-FC treatment and their brains were subjected to histological examination. Representative tumor sizes are shown in Figure 2. All 3 rats in control group developed large U87MG tumors throughout the hemisphere (Fig. 2A). In contrast, animals that received either a intra-tumoral injection (Fig. 2B) or CED (Fig. 2C) of RRV-CD, followed by one cycle of 5-FC administration, showed much smaller brain tumor compared to controls. The tumors in rats that received CED of RRV-CD and IP 5-FC tended to be smaller compared to those that were injected with RRV-CD and IP 5-FC. The brain sections from treated and untreated rats with intracranial gliomas were also processed for Ki-67 IHC. Treatment with RRV-CD and 5-FC led to dramatic reduction in Ki-67 index (Fig. 2D–F).
Fig. 2. Representative tumor sizes and Ki-67 immunohistochemistry.
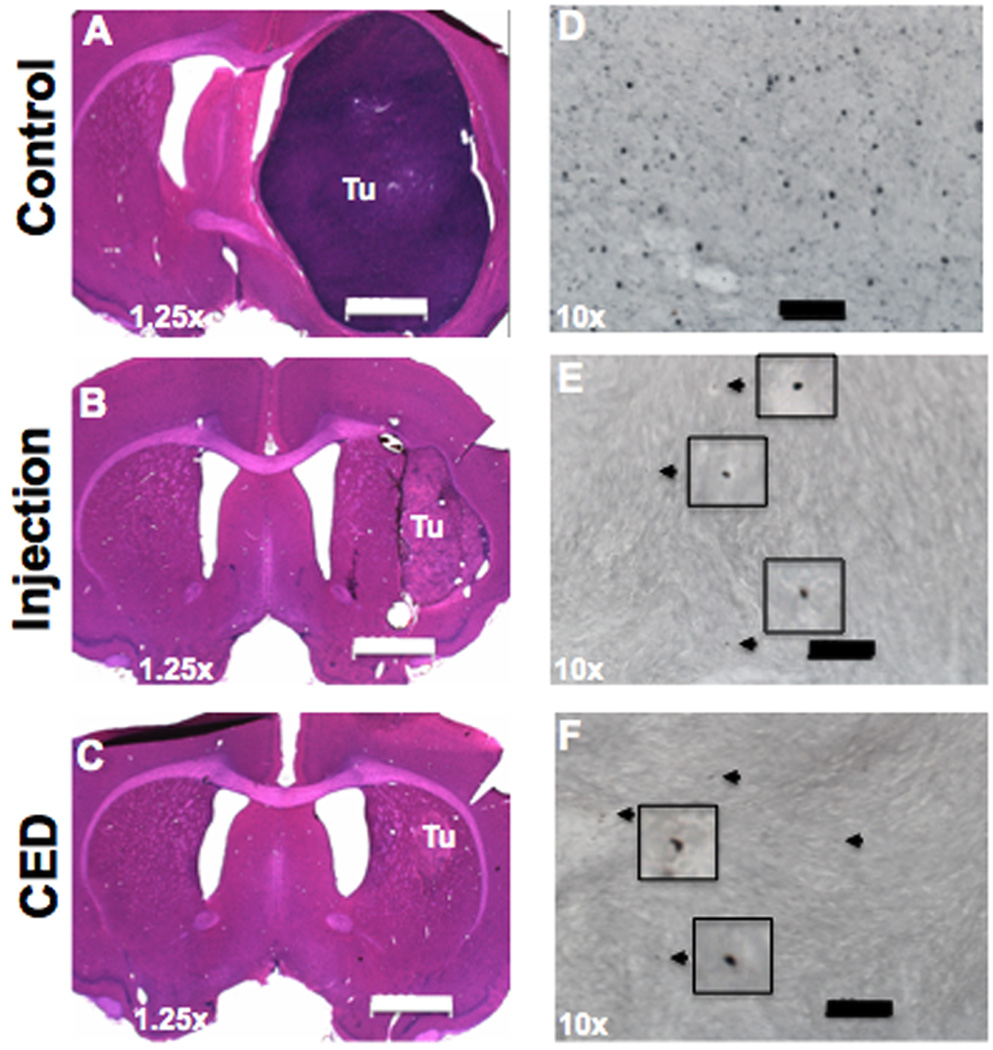
Shown are three representative sections of brains from control, injection and CED treatment groups 5 days after 5-FC treatment (20 days after tumor implantation). Scale bar is 2,000 µm in A–C, and 100 µm in D–F.
Distribution of RRV in brain tumor
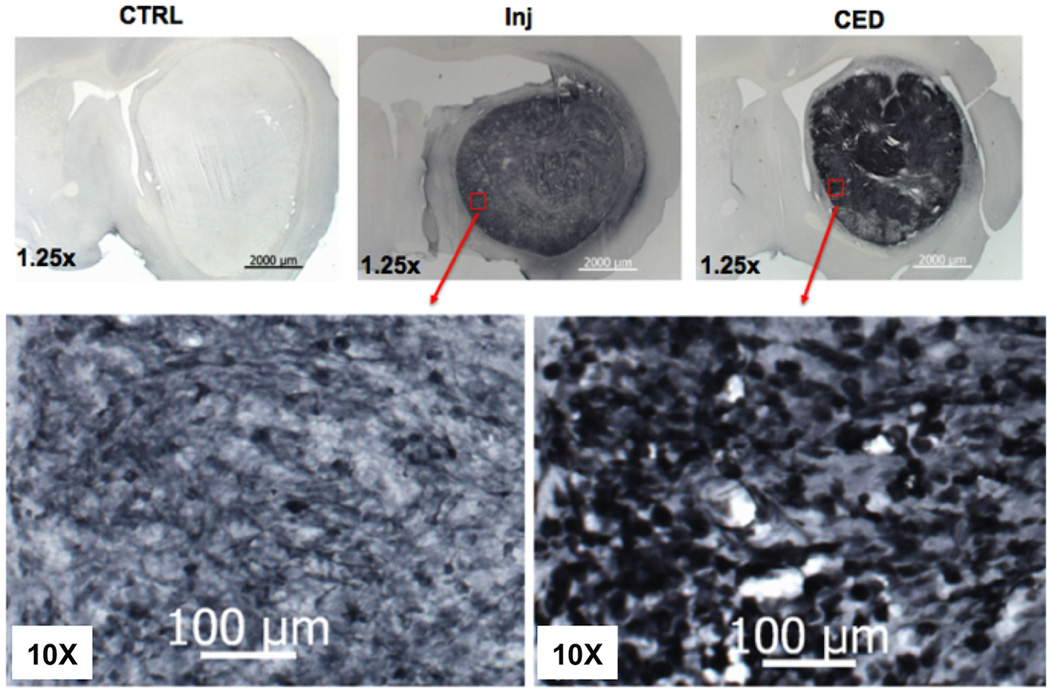
The therapeutic effect of any agent is largely dependent on its distribution in the target. To investigate why CED of RRV-CD significantly increased survival time relative to simple injection of RRV-CD, we studied the distribution of RRV vectors in the brain tumor. Ten nude rats with U87MG xenografts were randomly divided into two groups that both received RRV-GFP vector (20 µl), delivered into the brain tumor by CED (N=5) or injection (n=5). Animals were then euthanized 7 days after administration of vector. During CED, a stepped cannula was used that has been shown to prevent reflux and leakage in the rat brain25. The delivery time and infusion rate for CED were 20 min at 1 µl/min respectively. Excellent distribution of RRV-GFP was obtained in brain tumors 7 days after delivery by CED, ranging from 188.4 to 289.7 mm3 with mean volume of 242.6 ± 29.4 mm3. On the other hand, in rats that received injection of RRV-GFP vectors, relatively poor distribution of vector in the brain tumor was obtained, ranging from 77.6 to 129.8 mm3 with mean volume of 99.3 ± 11.7 mm3. No expression of RRV-GFP vector was found in normal brain by either delivery method.
When CED was used, the mean coverage was 79.3 ± 8% (range 64.3 – 97.5%). However, even with correct placement of the needle (post-mortem visualization of needle track), injection resulted in a much lower (p<0.05) mean volume of distribution (Vd) percentage of RRV-GFP in the tumor of 48.7 ± 6% (range: 38.0 – 64.8%). Figure 3 shows a comparison of representative GFP staining indicating RRV distribution in the brain tumors from animals administered RRV-GFP by CED (Fig. 3A) or manual injection (Fig. 3B). This is consistent with the results of the survival study. Better distribution of RRV in the tumor delivered by CED likely resulted in more efficient conversion of 5-FC to 5-FU and thereby antitumor effect.
Fig. 3. Representative GFP staining of distribution of RRV vectors in brain tumor.
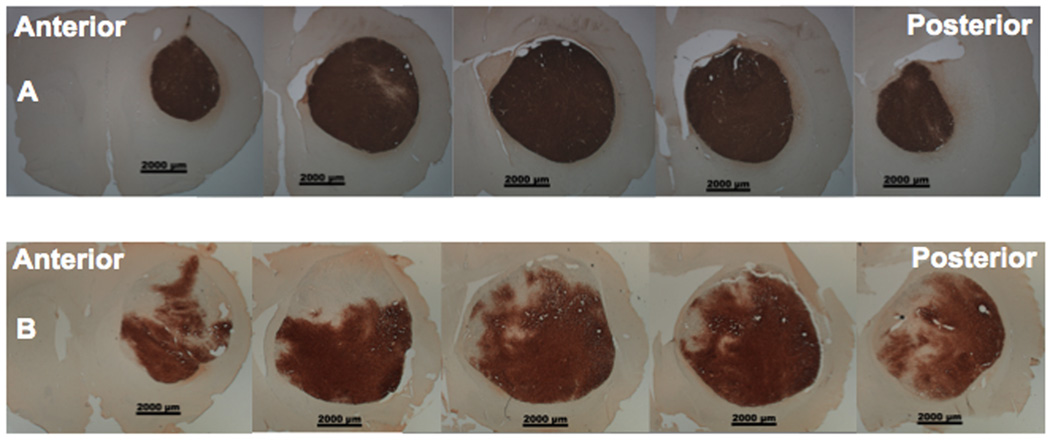
Panels indicate RRV-GFP delivered by CED (A) or manual injection (B).
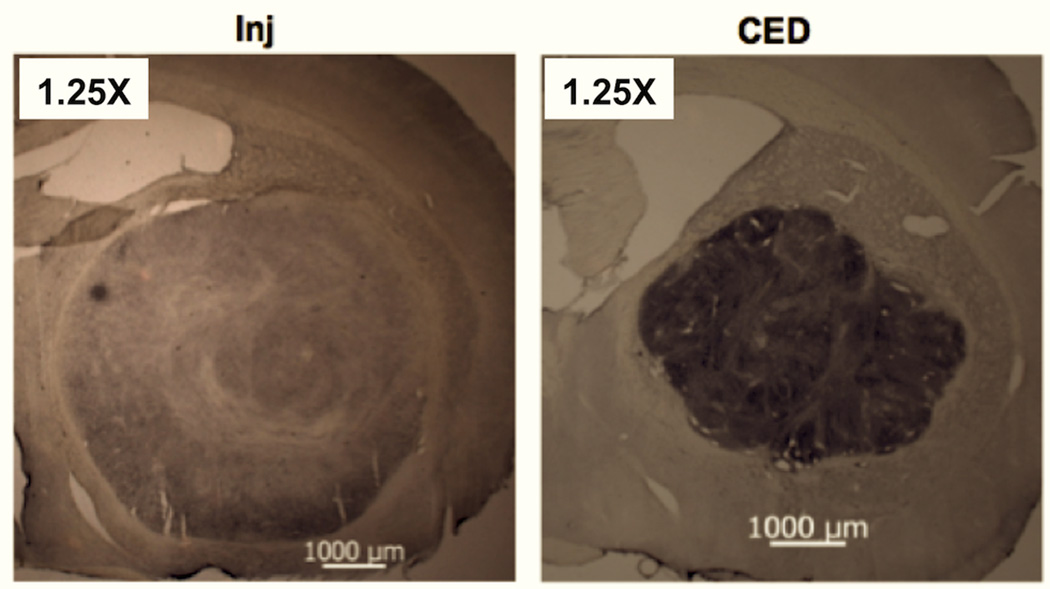
We also studied RRV-CD distribution with gag staining and by measurement of expression levels of cytosine deaminase in the tumor tissues of rats used in the efficacy study. RRV-CD efficiently transduced almost all the tumor cells and covered the entire brain tumor of rats at the efficacy study end-points (Fig. 4). Interestingly, higher expression of gag and cytosine deaminase was noted in tumors after CED of RRV-CD compared to manual injection (Fig. 5). These data indicate that multiple courses of treatment with 5-FC may have a more effective anti-tumor effect after a single CED delivery of RRV-CD into the brain tumor.
Fig. 4. Representative gag staining of RRV vectors in brain tumor at the end-point of the efficacy study.
Shown are representative sections from control (CTRL), injected (Inj) and CED rats stained with anti-gag antibodies (Methods). Lower panels are higher magnification images of injected (left) and CED (right) sections.
Fig. 5. Representative cytosine deaminase staining in the brain tumor at end-point of efficacy study.
Shown are representative low-magnification images of tumors after either simple injection or CED of RRV-CD at the efficacy study end-point.
Discussion
Systemic administration of 5-FU, one of the most active antineoplastic agents in conventional cancer chemotherapy, is ineffective against brain tumors, due to its relatively low diffusion across the blood–brain barrier at safe doses26. Direct intra-tumoral administration of 5-FU has been explored for local chemotherapy in patients with malignant gliomas27. However, due to its short half-life and cell cycle phase-specific activity, this approach requires a sustained-release polymer carrier system to achieve any significant therapeutic effect28, 29. Moreover, therapeutic efficacy is then restricted by limited diffusion of drug released from the polymer implantation site, consistent with the poor clinical efficacy of this approach28. Retroviral replicating vectors (RRV) expressing CD can achieve highly efficient gene transfer to tumors because each transduced tumor cell itself becomes a virus-producing cell, sustaining further transduction events after only an initial inoculation6, 7. RRV can achieve efficient delivery of pro-drug activator genes that permanently integrate into the target cell genome, stably expressing pro-drug-converting enzymes, such as CD that enables killing of the infected cell through production of chemotherapeutic drug, 5-FU, upon systemic administration of a well-tolerated pro-drug, 5-FC6,7, 8. Since pro-drug conversion is intracellular and confined to tumor cells and 5-FU has a short half-life26, the adverse side effects associated with systemic administration of toxic chemotherapeutic agent 5-FU can be avoided. Although RRV are capable of natural spread within the brain tumor, an optimal method for initial brain delivery has not been established. As observed in current and previous studies6, 7, due to an absolute requirement for cell mitosis to achieve productive infection, RRV showed an inherent tumor selectivity, selectively transducing glioma cells [3].
The relationship between RRV administration, virus spread, tumor growth and timing of 5-FC administration is not completely characterized. We decided to test the ability of CED to maximize RRV distribution. Our current results confirm and extend the findings of previous studies of RRV6, 7. In the present study, we compared brain tumor distribution and therapeutic effect of a single dose of RRV delivered by CED or direct manual injection followed by one cycle of systemic administration of 5-FC in rodent intracranial glioma xenograft model. Combination treatment with RRV-CD and 5-FC was able to achieve profound inhibition of pre-established U87MG gliomas, resulting in more than a doubling of the median survival time compared to controls. These findings are consistent with previous RRV studies6, 7. The immunohistochemical studies presented here also represent the first direct correlation between therapeutic effect of RRV and expression levels of Ki-67. In particular, we observed large tumors and high expression levels of Ki-67 only in controls without administration of vectors. Furthermore, we found that CED resulted in excellent early distribution of RRV, with only partial early distribution of RRV after simple injection. Therefore, CED of RRV-CD followed by 5-FC resulted in even more significant survival benefit relative to intra-tumoral manual injection of RRV-CD in our studies. The enhanced therapeutic effectiveness of the CED approach is a novel finding, and the treatment advantage provided by CED was largely attributable to the improved spread and distribution of RRV, potentially enabling more wide-spread production of enzyme and conversion of 5-FC to 5-FU. After initial administration of RRV within tumor, transduced tumor cells become virus-producing cells that sustain further transduction for a prolonged period, and this appears to enhance the 5-FU chemotherapeutic effect. More extensive initial delivery may allow more rapid complete transduction and/or higher vector copy number per tumor cell. This may be important given the limited time for vector spread in the rat tumor model. Notably, we observed complete RRV transduction of the entire U87MG tumor in some cases within 7 days after single CED delivery of vectors (Fig. 3), and 50% of the animals treated with only one cycle of 5-FC survived until termination of the study. These results demonstrated the improved effect of RRV for gene therapy when delivered by CED in this model. Furthermore, our data also showed that RRV-CD provides stable integration and persistence in dividing tumor cells even at the end-point of the efficacy study (Figs. 4 & 5), supporting the idea that repeated cycles of 5-FC treatment may achieve extended therapeutic benefit.
Our data demonstrate the advantage of good distribution of the vector after administration and spread to observe therapeutic effects in a rat model. Poorer distribution of RRV after simple manual injection may be due to reflux and leakage of vectors out of the target caused by injection force. Reflux and leakage during injection may decrease the effective dose of RRV in the target tumor. It is possible that slow infusion with stereotaxis or infusions into larger tumors could improve the injection results. It has been reported that after injection of RRV into glioma xenografts in nude mice, more than 98% transduction could be achieved throughout the entire tumor mass over a period of several weeks6. In contrast, our data showed that it took only one week for RRV vectors to cover the whole brain tumor after delivery by CED. Therefore, the CED approach may provide an earlier window for treatment of glioma with 5-FC, and gain more time for treatment with multiple courses of 5-FC.
CED has been developed as a drug delivery strategy and represents a powerful methodology for targeted therapy in the brain17, 18. Delivery of therapeutic agents by CED within the human brain is becoming a more frequent experimental treatment option in the management of brain tumors, and more recently in Phase 1 trials for gene therapy in Parkinson’s disease (PD)30. This technology potentially offers the clinician a more specific option in delivering therapeutic vectors to a larger and more consistent treatment volume than the standard diffusion-based injection. As shown by our animal experiments, eradication of tumors is a realistic possibility when extensive coverage of the glioma with RRV is achieved with CED and multiple cycles of 5-FC treatment are employed. Nevertheless, it remains to be determined whether the advantage of CED over injection observed in these experiments of RRV for the nude rat xenograft model, translates to naturally occurring tumors that have known heterogeneity and a mixture of live and necrotic areas. We have started to investigate this question in canine and human patients with advanced primary brain cancer.
In order to further improve the clinical prospects for CED-based therapy, we have introduced a number of innovations to CED for both current and future clinical applications. We have developed a fully integrated, FDA-approved brain tumor delivery system that consists of an MR-compatible aiming device, reflux-resistant cannula and predictive software to maximize delivery of therapeutic agents31. Real-time imaging allows us to visualize direct therapeutic delivery into the CNS32, 33 and, when used in combination with several infusion catheters, may permit extensive RRV coverage of larger tumors in human brain. In the human setting, malignant tumors are usually more than 2–3 cm in diameter at time of diagnosis, and may have various shapes. Our previous studies on naive primate brains have clearly shown that CED, when combined with our stepped cannula design, allows delivery of a therapeutic agent into many CNS structures at different depths34–36. This delivery platform permits monitoring distribution of RRV-CD fluid through using gadolinium additive and MRI monitoring during CED with a high level of precision, predictability and safety, and may have important implications in ensuring effective delivery of therapeutics into brain targets. Such an approach may improve the success rate for clinical trials involving direct brain drug delivery. Therefore, CED and these delivery innovations should be considered when localized therapeutic delivery, such as gene transfer, are being translated into clinical treatments.
In conclusion, we have shown that infusion of RRV-CD into human glioblastoma xenograft brain tumors in a rat model by CED directs wider vector distribution relative to simple manual injection of vector, thereby resulting in a substantial improvement in the therapeutic outcome. The CED approach used in this study offers a potentially effective method to deliver novel RRV-CD for gene therapy of malignant glioma. Furthermore, investigation into optimal spacing of RRV administration, virus spread, tumor growth and timing of 5-FC administration may yield further improvements in long-term survival.
Acknowledgments
This study was supported with funds from Tocagen Inc., San Diego, CA, and by a translational NIH grant to N.K. (U01-NS059821).
Financial Conflict-of-Interest
Harry Gruber, Carlos Ibanez, Joan Robbins and Douglas Jolly are employees of Tocagen Inc., which funded this study. Noriyuki Kasahara is a consultant and co-founder of Tocagen and has received research support from the Company.
References
- 1.Davis FG, Freels S, Grutsch J, Barlas S, Brem S. Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: an analysis based on Surveillance, Epidemiology, and End Results (SEER) data, 1973–1991. J Neurosurg. 1998;88(1):1–10. doi: 10.3171/jns.1998.88.1.0001. [DOI] [PubMed] [Google Scholar]
- 2.DeAngelis LM. Brain tumors. N Engl J Med. 2001;344(2):114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 3.Holland EC. Glioblastoma multiforme: the terminator. Proc Natl Acad Sci U S A. 2000;97(12):6242–6244. doi: 10.1073/pnas.97.12.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandes AA, Fiorentino MV. The role of chemotherapy in recurrent malignant gliomas: an overview. Cancer Invest. 1996;14(6):551–559. doi: 10.3109/07357909609076900. [DOI] [PubMed] [Google Scholar]
- 5.Hau P, Fabel K, Baumgart U, Rummele P, Grauer O, Bock A, et al. Pegylated liposomal doxorubicin-efficacy in patients with recurrent high-grade glioma. Cancer. 2004;100(6):1199–1207. doi: 10.1002/cncr.20073. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Tai CK, Kershaw AD, Solly SK, Klatzmann D, Kasahara N, et al. Use of replication-competent retroviral vectors in an immunocompetent intracranial glioma model. Neurosurg Focus. 2006;20(4):E25. doi: 10.3171/foc.2006.20.4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tai CK, Wang WJ, Chen TC, Kasahara N. Single-shot, multicycle suicide gene therapy by replication-competent retrovirus vectors achieves long-term survival benefit in experimental glioma. Mol Ther. 2005;12(5):842–851. doi: 10.1016/j.ymthe.2005.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostertag D, Amundson KK, Lopez Espinoza F, Martin B, Buckley T, Galvao da Silva AP, et al. Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector. Neuro Oncol. 2012;14(2):145–159. doi: 10.1093/neuonc/nor199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sgorbissa A, Tomasella A, Potu H, Manini I, Brancolini C. Type I IFNs signaling and apoptosis resistance in glioblastoma cells. Apoptosis. 2011;16(12):1229–1244. doi: 10.1007/s10495-011-0639-4. [DOI] [PubMed] [Google Scholar]
- 10.Amano S, Gu C, Koizumi S, Tokuyama T, Namba H. Tumoricidal bystander effect in the suicide gene therapy using mesenchymal stem cells does not injure normal brain tissues. Cancer letters. 2011;306(1):99–105. doi: 10.1016/j.canlet.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 11.Huber MH, Shirinian M, Lippman SM, Dimery IW, Frankenthaler RA, Hong WK. Phase I/II study of cisplatin, 5-fluorouracil and alpha-interferon for recurrent carcinoma of the head and neck. Invest New Drugs. 1994;12(3):223–229. doi: 10.1007/BF00873963. [DOI] [PubMed] [Google Scholar]
- 12.Lang FF, Bruner JM, Fuller GN, Aldape K, Prados MD, Chang S, et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol. 2003;21(13):2508–2518. doi: 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- 13.Walther W, Stein U. Viral vectors for gene transfer: a review of their use in the treatment of human diseases. Drugs. 2000;60(2):249–271. doi: 10.2165/00003495-200060020-00002. [DOI] [PubMed] [Google Scholar]
- 14.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91(6):2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadaczek P, Yamashita Y, Mirek H, Tamas L, Bohn MC, Noble C, et al. The "perivascular pump" driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol Ther. 2006;14(1):69–78. doi: 10.1016/j.ymthe.2006.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eberling JL, Jagust WJ, Christine CW, Starr P, Larson P, Bankiewicz KS, et al. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology. 2008;70(21):1980–1983. doi: 10.1212/01.wnl.0000312381.29287.ff. [DOI] [PubMed] [Google Scholar]
- 17.Gill SS, Patel NK, Hotton GR, O'Sullivan K, McCarter R, Bunnage M, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9(5):589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- 18.Kunwar S. Convection enhanced delivery of IL13-PE38QQR for treatment of recurrent malignant glioma: presentation of interim findings from ongoing phase 1 studies. Acta Neurochir Suppl. 2003;88:105–111. doi: 10.1007/978-3-7091-6090-9_16. [DOI] [PubMed] [Google Scholar]
- 19.Mardor Y, Roth Y, Lidar Z, Jonas T, Pfeffer R, Maier SE, et al. Monitoring response to convection-enhanced taxol delivery in brain tumor patients using diffusion-weighted magnetic resonance imaging. Cancer Res. 2001;61(13):4971–4973. [PubMed] [Google Scholar]
- 20.Richardson RM, Larson PS, Bankiewicz KS. Gene and cell delivery to the degenerated striatum: status of preclinical efforts in primate models. Neurosurgery. 2008;63(4):629–442. doi: 10.1227/01.NEU.0000325491.89984.CE. dicussion 642–4. [DOI] [PubMed] [Google Scholar]
- 21.Perez OD, Logg CR, Hiraoka K, Diago O, Burnett R, Inagaki A, et al. Design and selection of toca 511 for clinical use: modified retroviral replicating vector with improved stability and gene expression. Mol Ther. 2012;20(9):1689–1698. doi: 10.1038/mt.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol. 2000;164(1):2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- 23.Saito R, Bringas JR, Panner A, Tamas M, Pieper RO, Berger MS, et al. Convection-enhanced delivery of tumor necrosis factor-related apoptosis-inducing ligand with systemic administration of temozolomide prolongs survival in an intracranial glioblastoma xenograft model. Cancer Res. 2004;64(19):6858–6862. doi: 10.1158/0008-5472.CAN-04-1683. [DOI] [PubMed] [Google Scholar]
- 24.Saito R, Bringas JR, McKnight TR, Wendland MF, Mamot C, Drummond DC, et al. Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Res. 2004;64(7):2572–2579. doi: 10.1158/0008-5472.can-03-3631. [DOI] [PubMed] [Google Scholar]
- 25.Yin D, Forsayeth J, Bankiewicz KS. Optimized cannula design and placement for convection-enhanced delivery in rat striatum. J Neurosci Methods. 2010;187(1):46–51. doi: 10.1016/j.jneumeth.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wigmore PM, Mustafa S, El-Beltagy M, Lyons L, Umka J, Bennett G. Effects of 5-FU. Adv Exp Med Biol. 2010;678:157–164. doi: 10.1007/978-1-4419-6306-2_20. [DOI] [PubMed] [Google Scholar]
- 27.Menei P, Boisdron-Celle M, Croue A, Guy G, Benoit JP. Effect of stereotactic implantation of biodegradable 5-fluorouracil-loaded microspheres in healthy and C6 glioma-bearing rats. Neurosurgery. 1996;39(1):117–123. doi: 10.1097/00006123-199607000-00023. discussion 123–4. [DOI] [PubMed] [Google Scholar]
- 28.Fournier E, Passirani C, Montero-Menei C, Colin N, Breton P, Sagodira S, et al. Therapeutic effectiveness of novel 5-fluorouracil-loaded poly(methylidene malonate 2.1.2)-based microspheres on F98 glioma-bearing rats. Cancer. 2003;97(11):2822–2829. doi: 10.1002/cncr.11388. [DOI] [PubMed] [Google Scholar]
- 29.Benoit JP, Faisant N, Venier-Julienne MC, Menei P. Development of microspheres for neurological disorders: from basics to clinical applications. J Control Release. 2000;65(1–2):285–296. doi: 10.1016/s0168-3659(99)00250-3. [DOI] [PubMed] [Google Scholar]
- 30.Mittermeyer G, Christine CW, Rosenbluth KH, Baker SL, Starr P, Larson P, et al. Long-Term Evaluation of a Phase 1 Study of AADC Gene Therapy for Parkinson's Disease. Hum Gene Ther. 2012;23(4):377–381. doi: 10.1089/hum.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richardson RM, Kells AP, Martin AJ, Larson PS, Starr PA, Piferi PG, et al. Novel platform for MRI-guided convection-enhanced delivery of therapeutics: preclinical validation in nonhuman primate brain. Stereotact Funct Neurosurg. 2011;89(3):141–151. doi: 10.1159/000323544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito R, Krauze MT, Bringas JR, Noble C, McKnight TR, Jackson P, et al. Gadolinium-loaded liposomes allow for real-time magnetic resonance imaging of convection-enhanced delivery in the primate brain. Exp Neurol. 2005;196(2):381–389. doi: 10.1016/j.expneurol.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Krauze MT, McKnight TR, Yamashita Y, Bringas J, Noble CO, Saito R, et al. Real-time visualization and characterization of liposomal delivery into the monkey brain by magnetic resonance imaging. Brain Res Brain Res Protoc. 2005;16(1–3):20–26. doi: 10.1016/j.brainresprot.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Fiandaca MS, Forsayeth JR, Dickinson PJ, Bankiewicz KS. Image-guided convection-enhanced delivery platform in the treatment of neurological diseases. Neurotherapeutics. 2008;5(1):123–127. doi: 10.1016/j.nurt.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin D, Valles FE, Fiandaca MS, Bringas J, Gimenez F, Berger MS, et al. Optimal region of the putamen for image-guided convection-enhanced delivery of therapeutics in human and non-human primates. Neuroimage. 2009;187(1):46–51. doi: 10.1016/j.neuroimage.2009.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yin D, Richardson RM, Fiandaca MS, Bringas J, Forsayeth J, Berger MS, et al. Cannula placement for effective convection-enhanced delivery in the non-human primate thalamus and brainstem: Implications for clinical delivery of therapeutics. J Neurosurg. 2010;113(2):240–248. doi: 10.3171/2010.2.JNS091744. [DOI] [PubMed] [Google Scholar]