Abstract
The mesolimbic dopamine system is an essential participant in the initiation and modulation of various forms of goal-directed behavior, including drug reinforcement and addiction processes. Dopamine neurotransmission is increased by acute administration of all drugs of abuse, including the stimulants cocaine and amphetamine. Chronic exposure to these drugs via voluntary self-administration provides a model of stimulant abuse that is useful in evaluating potential behavioral and neurochemical adaptations that occur during addiction. This review describes commonly used methodologies to measure dopamine and baseline parameters of presynaptic dopamine regulation, including exocytotic release and reuptake through the dopamine transporter in the nucleus accumbens core, as well as dramatic adaptations in dopamine neurotransmission and drug sensitivity that occur with acute non-contingent and chronic, contingent self-administration of cocaine and amphetamine.
Keywords: Voltammetry, Cocaine, Amphetamine, Dopamine transporter, Dopamine, Review
2. INTRODUCTION
The mesolimbic dopamine system is an essential component of reinforcement and addiction processes (1–3). Firing rates of dopamine neurons in the ventral midbrain that project to limbic areas such as the nucleus accumbens, prefrontal cortex, hippocampus and amygdala, are sensitive to salient environmental stimuli. For example, dopamine neurons in the ventral tegmental area (VTA) and/or substantia nigra (SN) increase their firing in a phasic manner in response to stressors, during learning, and to salient conditioned and unconditioned stimuli, including cues that predict reward or the absence of reward (4–8).
Despite the important observations of these electrophysiological studies, such approaches have not provided information on the relationship between firing rate and changes in dopamine signaling. Thus, it is necessary to look to other methodologies to characterize additional forms of dopamine neurotransmission, particularly as they relate to extracellular levels of dopamine within terminal regions. The two most common techniques used to measure extracellular levels of dopamine are microdialysis and voltammetry, which will be explored in some detail below.
Elevation of extracellular dopamine levels is a common consequence of acute administration of nearly all drugs of abuse, including stimulants such as cocaine, amphetamines, PCP, methylphenidate, caffeine and nicotine, as well as non-stimulants such as alcohol, heroin and other opiates, marijuana, ketamine and inhalants (9–15). The ability of abused drugs to increase dopamine is thought to be an essential factor in the initiation of reward/reinforcement, although the exact cellular mechanisms leading to increased dopamine are different for each drug class, and the brain structures involved are not entirely overlapping. Stimulant drugs that bind to the dopamine transporter and reduce its ability to clear dopamine from the extracellular space can be categorized as either uptake inhibitors like cocaine or releasers like amphetamine. These distinctions will be discussed in later sections.
Pharmacological manipulations support a primary role for dopamine in reinforcement and reward. For example, D1 and D2-type dopamine receptor agonists are self-administered (16–19) and have cocaine-like discriminative stimulus effects (20–22). In addition, D1 and D2-type dopamine receptor antagonists attenuate self-administration of cocaine (23). Although considerable effort has been devoted to documenting the various neurobiological changes produced in response to chronic cocaine exposure in animals (24), the mechanisms through which cocaine engenders dependence-inducing brain adaptations are not fully understood. This review will describe the findings of studies which attempt to shed light on the most relevant adaptations of the dopamine system following stimulant self-administration.
3. NUCLEUS ACCUMBENS
Among the multiple dopaminergic terminal regions examined, the nucleus accumbens (NAc) stands out as a particularly important participant in reward-related motivated behavior, although other structures have also been implicated. In terms of circuitry, the NAc is well positioned to integrate limbic inputs associated with memory, affect, motivation and goal-directed motor activity (25–27). The NAc receives heavy afferent projections from the VTA (28), as well as a variety of cortical and subcortical structures including the prefrontal cortex (28,29), the hippocampus (28,30,31), and the basolateral amygdala (32,33). In turn, the NAc sends efferent projections to several structures including the ventral pallidum, caudate putamen (CP), globus pallidus, lateral hypothalamus, SN and VTA (34–36). These interconnections are believed to be critical in supporting motivated behavior associated with both natural and drug rewards.
Extensive research using electrophysiological, pharmacological, neurochemical, and lesion methodologies offers further support for NAc involvement in reward processing. For example, NAc neurons display time-locked discharge activity in response to cocaine-reinforced responding during self-administration (37,38), similar to that observed with dopamine neurons of the VTA. Additionally, animals will readily self-administer psychostimulants or co-infusions of D1 and D2 agonists into the NAc, indicating that these drugs have reinforcing actions within this region (39–42). In comparison, infusions of D1 receptor antagonists within the NAc increase cocaine self-administration rates, suggesting the importance of dopamine for the rewarding properties of this drug (43,44). The importance of the NAc is not limited to the actions of cocaine, given that amphetamine injections into this area are self-administered and have been shown to induce conditioned place-preference (45–47). Further, lesions of the NAc impair intravenous cocaine and amphetamine intake, indicating that stimulant self-administration requires an intact NAc (43,48,49).
The NAc can be anatomically divided into two distinct, yet functionally related components, the core and shell. Although these regions have unique patterns of afferent and efferent connections (28,32,34,50), they both receive heavy inputs from dopaminergic cells of the VTA (51). Importantly, although each region has been identified to participate in reward processing associated with drugs of abuse, a variety of observations demonstrate that the two regions often participate in distinct, albeit complementary, aspects of drug reward. For example, voltammetry studies suggest that the NAc core displays the greatest dopamine response following stimulant drug application (see below) and has been shown to participate in both reward and locomotor stimulant effects (40,52,53). Further, the NAc core appears to be subject to the bulk of morphological changes associated with sensitization (54), acquisition of opiate self-administration (55,56), and relapse to cocaine use (57). Nevertheless, several studies also suggest that the NAc shell is particularly involved in reward and reinforcement of stimulants, given that animals will self-administer these drugs preferentially into the NAc shell (39,40,58,59).
In studies of reward learning where cues predicting future rewards take on incentive value of their own, blockade of dopamine receptors in the NAc core strongly reduces incentive-cue responding (60), whereas dopamine receptor blockade in the shell has minimal effects (61), indicating that NAc core and shell have distinct roles in task performance. Indeed, lesions of the core but not shell reduce accuracy in several different incentive-cue responding tasks (62–64). While the core appears to drive goal-directed cue responding in reward paradigms and dopamine signaling magnitude is sensitive to the motivational value of the stimulus (65–67), in the NAc shell DA transmission appears to be critically dependent on other properties of the stimulus, namely its novelty and motivational valence, positive or negative (68,69). Thus, in general, the NAc core is involved in directing cue-driven, goal-directed behavior and the shell is involved in encoding the hedonic or emotional aspects of stimuli.
Addiction is thought to involve aberrant stimulus-response conditioning such that drug-taking in response to cues becomes a fixed, inflexible habit-like behavior which is resistant to modification by the availability of other rewards or the negative consequences of the drug-taking behavior (for review see (70)). Although the dorsal striatum plays a dominant role in directing habitual behaviors (71), there is a great deal of interest in the extent of participation of dopamine-dependent NAc core processes in cue-response associations. The following examination of the effects of stimulant administration on dopamine signaling in the NAc core is focused on this question.
4. STIMULANT ACTIONS
A great deal of research has been aimed at understanding the mechanisms of dopamine alterations with abused drugs as well as the adaptations that occur with chronic administration of those drugs. As mentioned above, drug-induced elevations in dopamine in the NAc brain region are an obligatory element of reinforcement and reward learning in animal models of addiction and although it is no longer generally believed that dopamine is itself the sole mediator of reward, it is accepted that initial rewarding effects of stimulant drugs require dopamine involvement (43,72–74). Extensive research has demonstrated unequivocally that cocaine, amphetamine and other stimulants target the dopamine system directly, although their exact effects can differ substantially. Cocaine is a competitive uptake inhibitor and increases extracellular levels of dopamine by inhibiting its uptake back into the presynaptic terminal after exocytotic release. Amphetamine, on the other hand, exerts its effects by functioning as a substrate at both the dopamine and vesicular monoamine transporters, ultimately causing transporter-mediated release of dopamine. The two mechanisms can be differentiated with rapid voltammetry measurements while the magnitude of effect on extracellular dopamine levels can be determined with microdialysis. These and other observations will be detailed below.
4.1. Cocaine and amphetamine
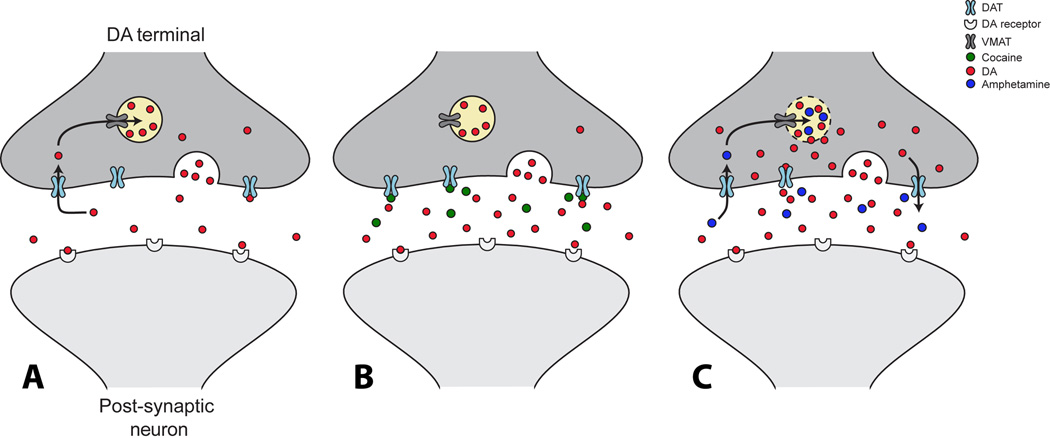
Under normal conditions, dopamine cell firing results in release of dopamine into the synaptic cleft where it typically diffuses for some distance and binds to both post-synaptic and extra-synaptic dopamine receptors. Dopamine is then removed from the extracellular space by the dopamine reuptake transporter (DAT) and is repackaged into synaptic vesicles for future release. Uptake inhibitors such as cocaine act as competitive agonists at the DAT which results in blockade of dopamine reuptake and accumulation of dopamine in the synaptic cleft. These alterations in dopamine levels allow for increased dopamine interactions with receptors and concomitant elevations in behavioral activity (Figure 1, A & B).
Figure 1. Effects of cocaine and amphetamine on the dopamine transporter.
(A) Under normal conditions, dopamine neuron firing results in dopamine-filled vesicles to fuse with the presynaptic membrane and subsequently dopamine is released into the synaptic cleft. Once in the synapse dopamine can bind to post-synaptic dopamine receptors and then is removed from extracellular space by the dopamine transporter (DAT). Once dopamine is back inside the presynaptic terminal, it is repackaged into synaptic vesicles for future release via the vesicular monoamine transporter (VMAT). (B) Cocaine increases levels of dopamine by binding to the DAT and thereby inhibiting dopamine uptake back into the terminal. As dopamine uptake is reduced, levels accumulate in the synapse and dopamine has a greater opportunity to bind to dopamine receptors. (C) Amphetamines increase the synaptic concentration of dopamine through two main mechanisms. Amphetamines interfere with the reuptake of dopamine through the DAT, and they disrupt vesicular packaging of dopamine which increases cytosolic levels of dopamine which can then leak out through the DAT via reverse transport.
By comparison, the actions of amphetamine involve dual actions at the transporter, due to the fact that amphetamine can act as a substrate and travel through the transporter into the terminal (Figure 1, A & C). At low concentrations the primary effect of amphetamine is to compete with dopamine for the substrate site on the transporter (75). In this capacity amphetamine acts much like cocaine and other classic uptake inhibitors. At higher concentrations, however, amphetamine can be transported into the terminal and subsequently travel into the synaptic vesicles via the vesicular monoamine transporter (VMAT). It is hypothesized that once levels of amphetamine accumulate to sufficient levels in the vesicle, the proton gradient across the vesicular membrane is disrupted and dopamine leaks out into the cytoplasm of the terminal (76,77). Accumulation in the terminal leads to binding to the cytoplasmic side of an inward-facing transporter, which subsequently transports dopamine in the reverse direction from inside the cell back out to the extracellular space (78,79). In contrast to that observed with cocaine and other uptake inhibitors, the net result of high doses of amphetamine in brain slices is vastly reduced dopamine release and ultimately an abolished dopamine response due to dopamine depletion in the synaptic vesicles. This however, is not the case in the whole animal, where non-lethal doses of amphetamine are incapable of producing complete depletion of available dopamine and may potentiate release in addition to inhibiting uptake and promoting reverse transport (80,81).
4.2. In vitro brain slice voltammetry
Voltammetry is a versatile technique that can be performed both in vivo in anesthetized or freely moving animals, and in vitro in brain slices containing the VTA, the frontal cortex, or the striatum. Voltammetry detects dopamine and various other species of interest by oxidation and reduction at the surface of a carbon fiber microelectrode. The oxidation/reduction reaction results in an exchange of electrons which produces a unique substance-specific change in current flow that is detected by a recording electrode - see (82). Fast scan cyclic voltammetry offers several advantages for characterization of dopamine neurotransmission. First, voltammetry can detect changes in extracellular dopamine concentrations with subsecond resolution, allowing for both release and uptake (Vmax and Km) to be measured in real time. Second, the unique oxidation/reduction signatures obtained in the measured cyclic voltammograms allows for accurate identification of dopamine. Thus, it is possible to positively identify whether the oxidized substance is dopamine, rather than another substance, by referring to the specific cyclic voltammogram observed. Despite this specificity, the oxidative current for norepinephrine and dopamine are similar and thus differentiation between these is difficult in regions containing both neurotransmitters. Finally, voltammetry techniques can be used to effectively detect exogenously applied dopamine as well as electrically stimulated and spontaneous release of endogenous dopamine. This allows researchers to address a multitude of questions in a variety of experimental preparations.
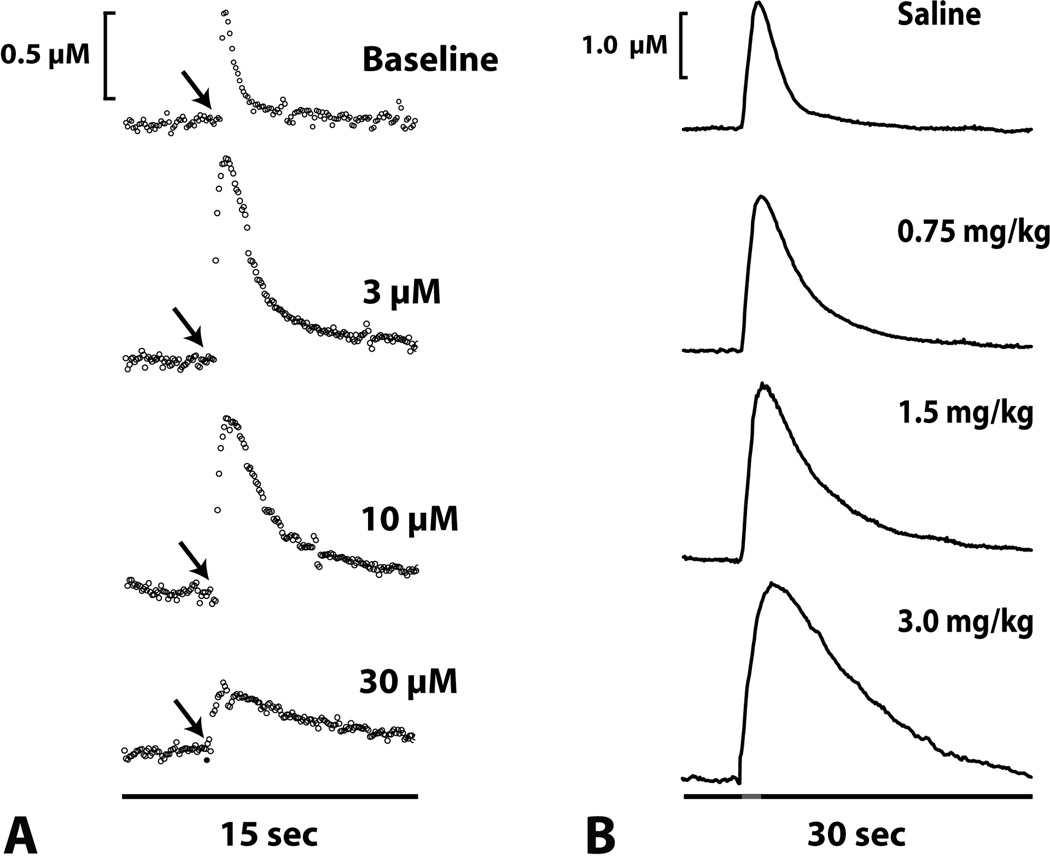
Using voltammetry, a variety of studies have investigated the effects of cocaine on dopamine signaling. Both in vitro and in vivo work indicates that low to moderate doses of cocaine increase levels of dopamine and increase Km, indicating reduced uptake by the DAT. Work from this laboratory demonstrates that cocaine reduces dopamine uptake in the NAc core and shell (83–85) as well as the CP (86). This reduction in dopamine uptake is characterized as a decreased rate of clearance back to baseline and reflects the competition between cocaine and dopamine for a binding site on the DAT. This apparent change in the affinity of dopamine for the transporter is used as a measure of uptake inhibition, and is commonly referred to as a change in “apparent Km”. As shown in Figure 2A, in the NAc, low concentrations of cocaine increase the peak height of stimulated dopamine release elicited by a single stimulation pulse (3 and 10 microM). Furthermore, these concentrations of cocaine also produce dramatic decreases in dopamine uptake, or in other words, increases in dopamine uptake inhibition. At higher concentrations of cocaine (30 microM), however, dopamine release is reduced, largely due to D2 autoreceptor activation. Similar effects of cocaine can also be observed in an in vivo model. As shown in Figure 2B, several doses of intravenous cocaine (0.75, 1.5, and 3.0 mg/kg) significantly increase dopamine release and uptake inhibition in the NAc core of an anesthetized rat. The maximal cocaine effect was reached 30 sec after intravenous delivery and returned to baseline levels after ~1 hr.
Figure 2. Cocaine dose-dependently reduces dopamine uptake in the NAc core.
(A) Shown are representative concentration-time plots of dopamine responses in the presence of increasing concentrations (3 µM, 10 µM, and 30 µM) of cocaine applied to a brain slice containing the NAc core. Cocaine concentrations were increased in a cumulative fashion, with new concentrations added every 30 minutes. Note that low concentrations of cocaine increased dopamine release and significantly inhibited dopamine uptake, as shown by a slower rate of decay back to baseline. At progressively higher concentrations, however, cocaine continues to inhibit the dopamine transporter and begins to decrease dopamine release. Arrows indicate the time of electrical stimulation (single pulse, 300 microA). (B) Shown are representative concentration-time plots of dopamine responses following varying doses of intravenous cocaine (0.75, 1.5, and 3.0 mg/kg) in an anesthetized rat. Pre-drug saline injections did not produce changes in dopamine uptake. In contrast, dopamine uptake inhibition was significantly increased following intravenous cocaine treatment. Red bar denotes the stimulation time (60Hz, 1sec, 300 microA).
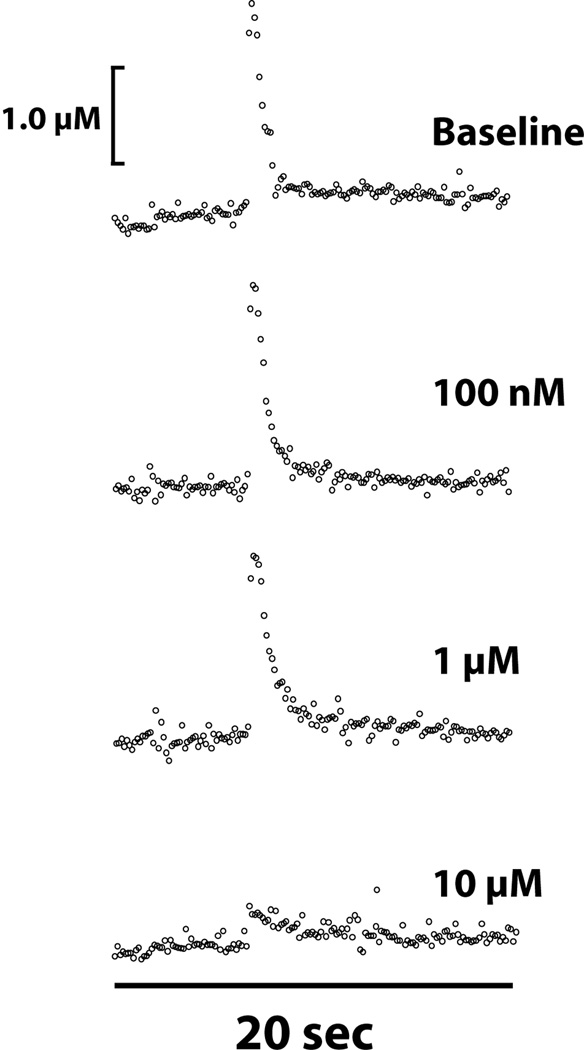
Voltammetry studies have also been used extensively to examine the effects of amphetamine on dopamine uptake in the striatum. Using mouse slices containing the NAc core, single pulse stimulation resulted in amphetamine-induced (1 µM) potentiation of dopamine release (87). This is consistent with the observation that extracellular levels of dopamine are gradually increased following 10 µM amphetamine when no stimulation is used (88). Further, work from this lab shows that the effects of amphetamine on uptake inhibition and decreased dopamine release change as a result of amphetamine concentration. Specifically, dopamine peak height gradually decreases with sequentially higher concentrations of amphetamine, indicating that the terminals are being depleted of dopamine (Figure 3). By comparison, other studies have found the opposite effect of amphetamine. In those studies, in which 1 and 10 µM amphetamine were shown to reduce dopamine uptake and decrease dopamine release in response to single pulse stimulation, but not with multiple stimulation pulses which resulted in increased dopamine release (85,88,89).
Figure 3. Amphetamine dose-dependently reduces dopamine uptake in the NAc core in vitro.
Shown are representative concentration-time plots of dopamine responses in the presence of increasing concentrations of amphetamine applied to a brain slice containing the NAc core. Amphetamine applied cumulatively to a NAc core slice produced increasing amounts of uptake inhibition. At higher amphetamine concentrations, dopamine peak height was reduced due to a depletion of vesicular stores of dopamine. Arrows indicate time of electrical stimulation (single pulse, 300 microA).
4.3. Microdialysis
In vivo microdialysis measures changes in extracellular dopamine levels of brain tissue which reflect the balance between release and uptake activity in large populations of dopamine neurons over time. Microdialysis takes advantage of the high sensitivity and excellent chemical resolution of high performance liquid chromatography (HPLC) to evaluate the concentrations of dopamine and other monoamines in small samples of extracellular fluid. Unlike voltammetry, microdialysis suffers from relatively low temporal resolution; however, several benefits of microdialysis also exist. First, analysis of dialysate samples using HPLC offers a high degree of chemical selectivity and sensitivity, beyond what is obtained with voltammetry. Microdialysis detection is capable of resolving the difference between dopamine and norepinephrine within regions rich in each of these neurotransmitters. Second, given that microdialysis typically utilizes sampling times that range from minutes to hours, it is useful to examine changes in behavior or physiology that involves slow processes such as sleep/wake cycles and the time course of drug effects that may reflect the temporal integration of transient changes in dopamine efflux. Third, microdialysis is more versatile and offers a degree of simplicity that makes it the most common method for measuring changes in neurotransmission in the awake, behaving animal.
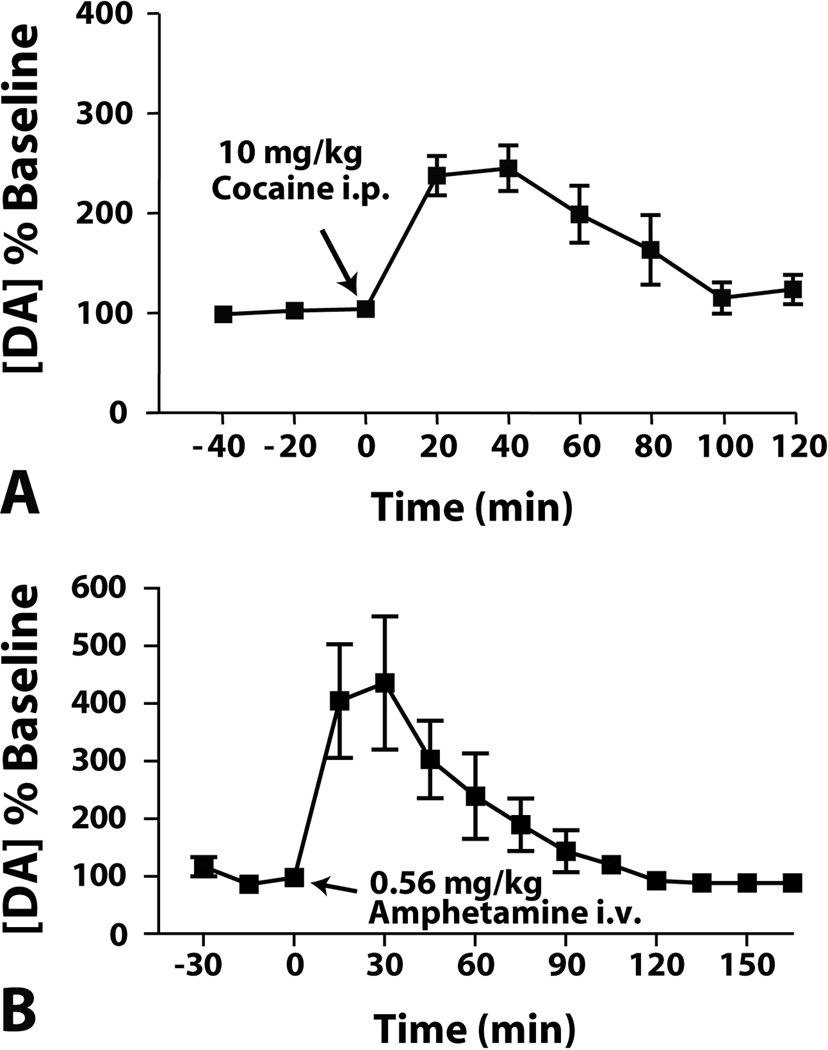
An extensive literature exists describing the effects of cocaine on mesolimbic dopamine function. Some of the first evidence that cocaine elevated extracellular dopamine levels in a variety of brain regions, particularly within the NAc, came in the 1980s from a series of microdialysis studies. In this work, intravenous cocaine (1.0 – 2.0 mg/kg) produced a transient increase in extracellular dopamine in the NAc which peaked within 10 min and returned to baseline levels within 30 min (90,91). Likewise, intratissue infusions of cocaine directly into the NAc also increased dopamine levels (90). The effects of systemic cocaine administration (10 – 40 mg/kg) are similar to those observed with intravenous injection with the exception that the time-course is protracted for both onset and offset of drug effects (92,93) and often the magnitude of peak effects are somewhat lower. Interestingly, the effects of long-term cocaine experience appear to result in physiological changes that lead to tolerance, given that repeated daily treatment with systemic cocaine or continuous intravenous cocaine reduces the magnitude of cocaine-induced increases in dopamine levels following acute cocaine challenge (see 1.6). As shown in Figure 4A, microdialysis studies in mice conducted in this laboratory largely confirm the above results. Using a 20 min sampling time, we showed that 10 mg/kg intraperitoneal cocaine elicits a robust increase in extracellular levels of dopamine that are observed within the first 20 min following cocaine injection and peak at 40 min post cocaine injection. A return to baseline levels of dopamine was reached after approximately 100 minutes. Together these results indicate that cocaine administration via several routes produces an enhancing effect of cocaine on dopamine levels within the NAc.
Figure 4. Cocaine and amphetamine increase extracellular levels of dopamine in the NAc core.
Shown are the mean ± SEMs for microdialysis measurements of extracellular dopamine prior to and following (A) cocaine or (B) amphetamine administration. Intraperitoneal cocaine (10 mg/kg) and intravenous amphetamine (0.56 mg/kg) significantly increased extracellular concentrations of dopamine in rats relative to pre-drug levels. This effect was observed within the first post-drug dialysis sample and was maintained for extended period of time before returning to baseline levels. The arrows indicate the time of drug injection.
As mentioned above, amphetamine is distinct from cocaine because it acts as a substrate for the DAT and results in release of dopamine via reverse transport. Using microdialysis measures of dopamine in the NAc, both intraperitoneal (0.5 mg/kg) and intravenous (1.0 mg/kg) amphetamine delivery elicited a dramatic increase in dopamine levels within 20 min of injection (85,91,92). Peak effects were observed at 30 min post injection and levels returned to baseline after 2 hrs. Figure 4B shows the effects of a single intravenous amphetamine injection on extracellular levels of dopamine in the NAc. In general the effects of amphetamine on dopamine levels are more potent than high doses of cocaine, likely due to the dual actions of amphetamine at the DAT.
4.4. Differences in dopamine signaling within the nucleus accumbens
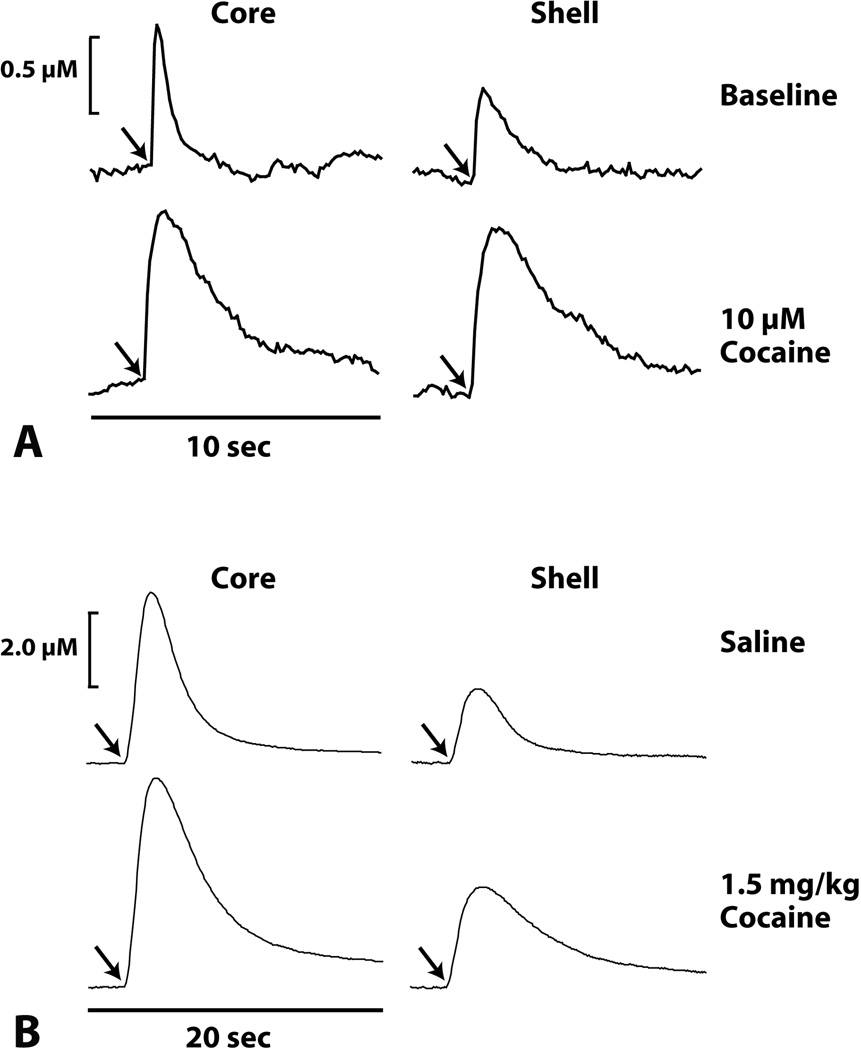
As mentioned above, the NAc core and shell participate in related, yet distinctive, aspects of reward processing. Therefore it is not surprising that several neurochemical observations have shown differences in dopamine release and uptake dynamics in NAc core vs shell. For example, experiments from this laboratory have generally shown higher levels of stimulated dopamine release and higher uptake rates in the NAc core relative to the shell (83). In those studies, the NAc core or shell in rat brain slices were stimulated with a single electrical pulses and resulting dopamine responses were compared under baseline conditions and following cocaine application. Prior to cocaine, electrical stimulation elicited a rapid increase in dopamine responses in both the core and shell of the NAc, however, release was greater and uptake was faster in the core (Figure 5A). When cocaine (10 microM) was applied, both the core and shell showed increased dopamine levels and inhibited uptake with no major differences between regions in the relative magnitude of uptake inhibition. A similar pattern of effects was also observed when comparing dopamine dynamics between the core and shell in anesthetized rats (Figure 5B). In those studies rats were anesthetized with urethane (1.5 g/kg, intraperitoneal) and implanted with a carbon fiber working electrode in the NAc core or shell, and a stimulating electrode in the VTA. Similar to the slice, electrical stimulation produced greater dopamine release and faster uptake in the core relative to the shell. However, unlike the slice studies, the trend of greater release and faster uptake was maintained following intravenous cocaine.
Figure 5. The NAc core displays greater levels of dopamine release and faster uptake than the NAc shell.
(A) Shown are representative concentration-time plots of dopamine responses in the presence of 10 microM cocaine applied to a brain slice containing the NAc core or shell. Prior to cocaine application, the NAc core displayed nearly two-fold greater dopamine release and faster uptake than the NAc shell. Interestingly however, this difference in dopamine response was not maintained following application of cocaine, which produced a similar decrease in uptake rate in both of these regions. However, the relative change in dopamine uptake following cocaine was greater in the NAc core. Arrows in (A) indicate time of electrical stimulation (single pulse, 300 microA). (B) Shown are representative concentration-time plots of dopamine responses in NAc core or shell following intravenous saline or 1.5 mg/kg cocaine. Following saline injection the NAc core showed a two-fold greater release of dopamine and moderately faster dopamine uptake than the NAc shell. This difference in dopamine release and uptake was maintained following cocaine injection, such that the core continued to show greater release and faster uptake relative to the shell. Arrows indicate time of electrical stimulation (60Hz, 1 sec, 300 microA).
Differences in baseline and cocaine-induced dopamine signaling between the core and shell have also been observed using microdialysis, however, the direction of the effects often varies across experiments. For example, although many papers suggest that the core displays greater extracellular levels of dopamine than the shell (15,94–97), other observations indicate no differences between regions (98–101), and others report the opposite effect (102). This discrepancy is also observed following treatment with stimulant drugs. Thus, in one experiment amphetamine induced a 2–3 fold higher increase in the core than the shell (97), yet the opposite is true in several other studies which show that the shell is more responsive to cocaine and amphetamine than the core (99,103).
The reasons for inconsistencies across experiments are unclear although it is likely that methodological differences such as dose, probe placement and route of administration may be to blame. Nevertheless, the majority of observations reveal that differences in dopamine signaling exist the between the core and shell and tend to support the hypothesis that the core displays greater levels of dopamine release and faster uptake rates than the shell, which is in direct agreement with observations showing that the core has a denser dopaminergic innervation, and displays higher DAT density than the shell (83,104–106).
4.5. Time of onset of cocaine and other dopamine transporter inhibitors
Despite general agreement that cocaine and other uptake inhibitors increase extracellular dopamine via inhibitory actions on the DAT, it remains unclear whether a direct relationship exists between transporter blockade and the reinforcing affects of cocaine, particularly in terms of the temporal relationship between these two phenomena (74,107–109). For example, until recently, it was unclear how quickly dopamine uptake inhibition occurs following intravenous cocaine administration. Behavioral estimates of cocaine effects demonstrate enhanced locomotor activation within 6–10 s of intravenous cocaine administration (85,110). Initial neurochemical estimates, however, suggested that the latency to a measurable neurochemical cocaine response vary widely. The reason for this hypothesis originated from the use of microdialysis as a measure of dopamine responsivity to cocaine. In those studies it was estimated that that intravenous cocaine elevates dopamine levels beginning 2–5 min after injection, yet as described above, a reason for the slow effects of cocaine on dopamine levels could simply be attributed to the slow responsivity of microdialysis techniques rather than the dopamine system itself. Electrophysiological studies suggested a faster time-frame of cocaine effects. These studies showed that cocaine inhibits VTA neurons within a minute of intravenous administration, due to increased extracellular dopamine levels and consequent activation of dopamine autoreceptors (111–115). Although these electrophysiological studies provide a more accurate sampling of dopamine activity due to enhanced temporal resolution, they do not directly measure dopamine levels at the synapse but rather the consequences of accumulated dopamine, and therefore incorporate in the measurement the time it takes for dopamine to accumulate, interact with presynaptic autoreceptors, and inhibit dopamine cell firing. Thus the incongruity amongst behavioral, early neurochemical, and electrophysiological studies concerning the time-course of cocaine effects reflects technique-based limitations that foment disagreements on how quickly cocaine exerts its initial effects.
As discussed above, the high temporal resolution of voltammetry permits rapid assessment of dopamine release and uptake processes, and therefore this technique is ideal to evaluate the onset of cocaine effects. This benefit allowed us to examine the effects of intravenous cocaine (1.5 mg/kg) on dopamine dynamics in the NAc of anesthetized rats (85). In these studies we found that intravenous cocaine begins inhibiting dopamine uptake (i.e., increased apparent Km) in the rat NAc within 4–6 s after infusion. The peak uptake inhibition was reached in approximately 1 min, and uptake levels returned to baseline in approximately 1 hr. The changes observed in dopamine uptake were on a similar time scale to alterations in the peak height of dopamine overflow suggesting that cocaine effects on dopamine peak height are related to decreases in the rates of dopamine uptake.
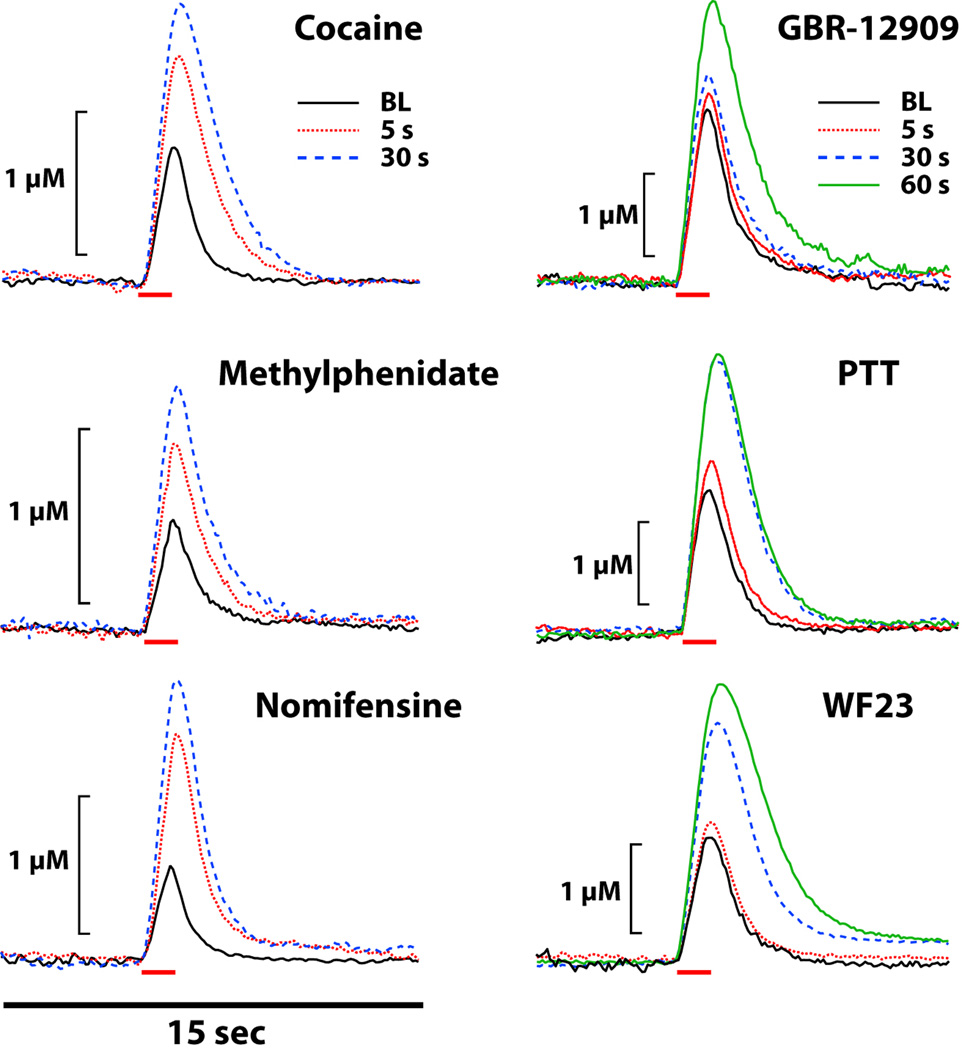
Another set of studies from our lab extended these observations by examining the onset of dopamine uptake inhibition across several doses of cocaine (116). In those studies, a dose as low as 0.75 mg/kg of cocaine also produced rapid uptake inhibition within 5 sec of the injection, although the effects were less robust than those observed following 1.5 or 3.0 mg/kg of cocaine. Finally, to explore the onset kinetics of both low and high affinity DAT inhibitors, a third set of studies was conducted using uptake inhibitors with varying affinities and specificity for the DAT. Specifically, we compared the onset effects of cocaine, methylphenidate, nomifensine, GBR-12909, 2β-propanoyl-3β-(4-tolyl)-tropane (PTT), and 2β-propanoyl-3β-(2-naphthyl)-tropane (WF-23). As shown in Figure 6, all examined DAT inhibitors elicited measurable dopamine uptake inhibition within 5 sec of intravenous injection. Uptake inhibition following cocaine, methylphenidate and nomifensine peaked between 30 sec and 1 min following injection, whereas peak effects for GBR-12909, PTT and WF23 occurred between 20 and 60 mins after the injection.
Figure 6.
Shown are representative concentration-time traces of dopamine responses from rats following intravenous injections of cocaine (1.5 mg/kg), methylphenidate (1.5 mg/kg), nomifensine (1.5 mg/kg), GBR-12909 (1.5 mg/kg), PTT (0.5 mg/kg), and WF23 (0.5 mg/kg). Electrical stimulation of the VTA (60 Hz, 1 sec, 300 microA, red bars) rapidly induced dopamine release in the NAc for all drugs. These DAT blockers increased dopamine peak height and uptake inhibition within 5 sec, despite differences in the magnitude of effects.
In contrast to our observations, several other studies using voltammetry have suggested that intravenous cocaine exerts its effects on dopamine uptake in tens of seconds, and that the time to reach peak inhibition occurs in minutes (117,118). An even bigger discrepancy with our findings is observed in one study using voltammetry in conjunction with exogenously applied dopamine. In that study intravenous cocaine was reported to inhibit dopamine uptake in approximately 2 min and peak effects took nearly 6–7 min to occur (110). The differences between those findings and our previous observations are likely related to the methods employed. In our studies, we used electrical stimulation of the VTA to elicit action potential-mediated endogenous dopamine release. By comparison, the Kiyatkin study used high concentrations of exogenous dopamine which may disrupt DAT function in a manner similar to what is observed with amphetamine (see below). Therefore we posit that with high concentrations of dopamine, the DAT takes up dopamine into the presynaptic terminal resulting in saturation of vesicular uptake and leading to excessive concentrations of dopamine inside the terminal. Under these conditions the DAT is thought to transport dopamine in both the forward and reverse direction, which complicates kinetic analysis of dopamine uptake and may contribute to a longer latency to observe measurable changes in dopamine uptake (119).
Although the described studies do not show a direct relationship between alterations in DAT function and the rewarding effects of cocaine, these studies do demonstrate conclusively that cocaine elicits rapid uptake inhibition within a few seconds of intravenous injection. Importantly, this time course of initial cocaine effects is in line with the behavioral and subjective effects of this drug (120–122).
5. NEUROCHEMICAL CONSEQUENCES OF COCAINE AND AMPHETAMINE SELF-ADMINISTRATION
Chronic exposure to stimulants is known to produce neurobiological adaptations in the dopamine system, but the changes vary according to the paradigm used. Some important variables include whether the administration is contingent or non-contingent, continuous or intermittent exposure, and the dose of the drug. In order to approximate human “binge” stimulant usage as closely as possible, we have focused on contingent, intermittent self-administration paradigms with high doses of drug. Using neurochemical techniques, it is possible to examine changes in the functional status of the dopamine system after binge self-administration of stimulants, including cocaine and amphetamine.
5.1. Reduced responsivity to cocaine following cocaine self-administration
Many paradigms have been developed to investigate drug-seeking and drug-taking in rodents, however, intravenous self-administration of drugs is generally accepted as the gold standard for modeling drug abuse. Consequently, many self-administration procedures have been developed to model distinct aspects of the addiction process and to assess the consequences of cocaine on neural function. To examine the relationship between drug reinforcement and dopamine signaling within the NAc, we have tested the effects of cocaine and amphetamine self-administration on dopamine neurotransmission in the NAc (123,124). In the first set of these studies, we used the discrete trials (DT) paradigm to examine whether contingent exposure to cocaine was associated with alterations in dopamine signaling within the NAc core. In general, the DT schedule is an extension of a fixed ratio (FR) paradigm that allows for 24hr access to cocaine, while limiting cocaine availability to a designated number of trials per hour. In this manner, animals are restricted to a limited number of injections which engenders varying patterns of cocaine intake across a 24 hr light/dark cycle (125). For example, when using the DT procedure with one of its least restricted access conditions (e.g., 1.5 mg/kg cocaine, 5 trials/per hour – DT5) rats are presented with a lever every 12 minutes during 24 hr sessions. Under these conditions rats can readily attain preferred blood levels of cocaine, and for the majority of cases the DT5 schedule results in behavior similar to that observed with an FR1 schedule at the same dose of cocaine. Additionally, with this level of cocaine access rats typically exhibit dysregulated binge-like patterns of intake that span both sleep and waking portions of the light/dark cycle (125). In contrast, when access to cocaine is restricted to every 20 or 30 minutes (3 trials/per hour - DT3 and 2 trials/per hour - DT2) rats are unable to maximize their blood levels of cocaine and are disinclined to self-administer cocaine during the light phase, which results in reduced cocaine consumption and a characteristic pattern of intake that is limited largely to the dark phase. The complex interaction between dose and availability occurring during the different DT paradigms renders this schedule more vulnerable to pharmacological and physiological influences than schedules with less restricted access to cocaine (126–129).
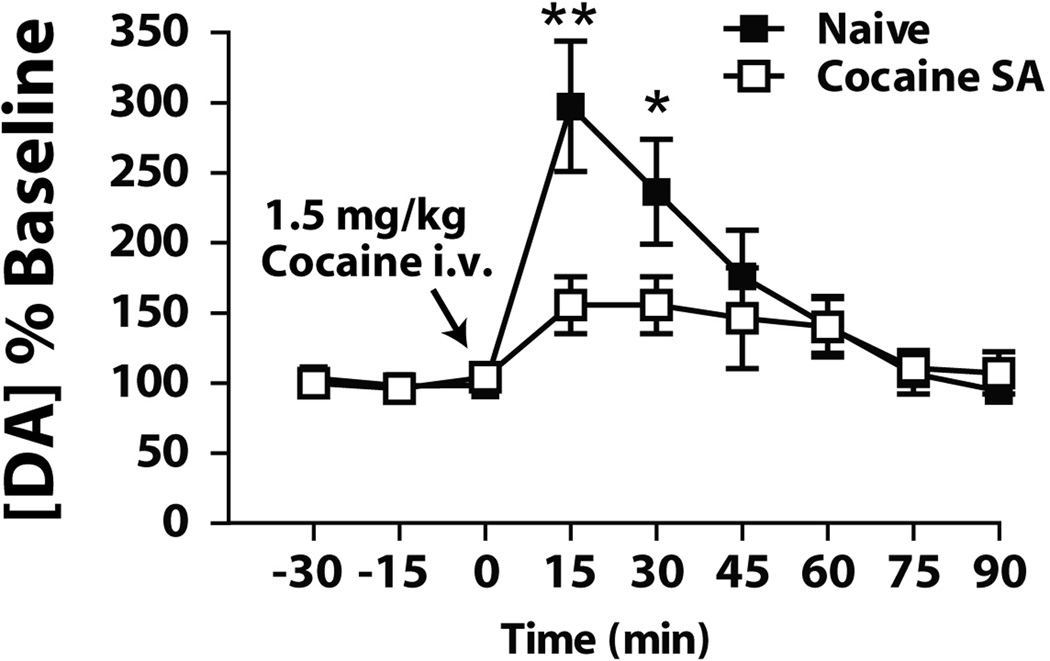
Using a moderate DT schedule that allows rats the opportunity to self-administer cocaine once every 15 minutes (DT4), we exposed rats to 10 days of 24-hr access to 1.5 mg/kg cocaine. As shown in Figure 7, microdialysis indicated that intravenous injections of 1.5 mg/kg cocaine increased extracellular concentrations of dopamine in the NAc core of naïve rats, but failed to do so in rats exposed to 10 days of DT4 cocaine self-administration. Furthermore, cocaine experienced rats displayed significantly lower baseline levels of dopamine, suggesting a down-regulation of tonic dopamine signaling. These findings are similar to previous studies showing decreased dopamine release following a self-administration regimen of unlimited access cocaine (93). A similar insensitivity to cocaine challenge was also observed in studies using chronic daily cocaine administration (130).
Figure 7. Cocaine has reduced effects on extracellular levels of dopamine in cocaine self-administering rats.
Microdialysis measurements of changes in dopamine levels after injection of 1.5 mg/kg intravenous cocaine in naïve and 5×40 cocaine self-administering rats. There is a significant difference in the dopamine response to cocaine, with a markedly reduced increase after cocaine in the rats with a history of cocaine self-administration, indicating tolerance to the effects of cocaine in these animals.
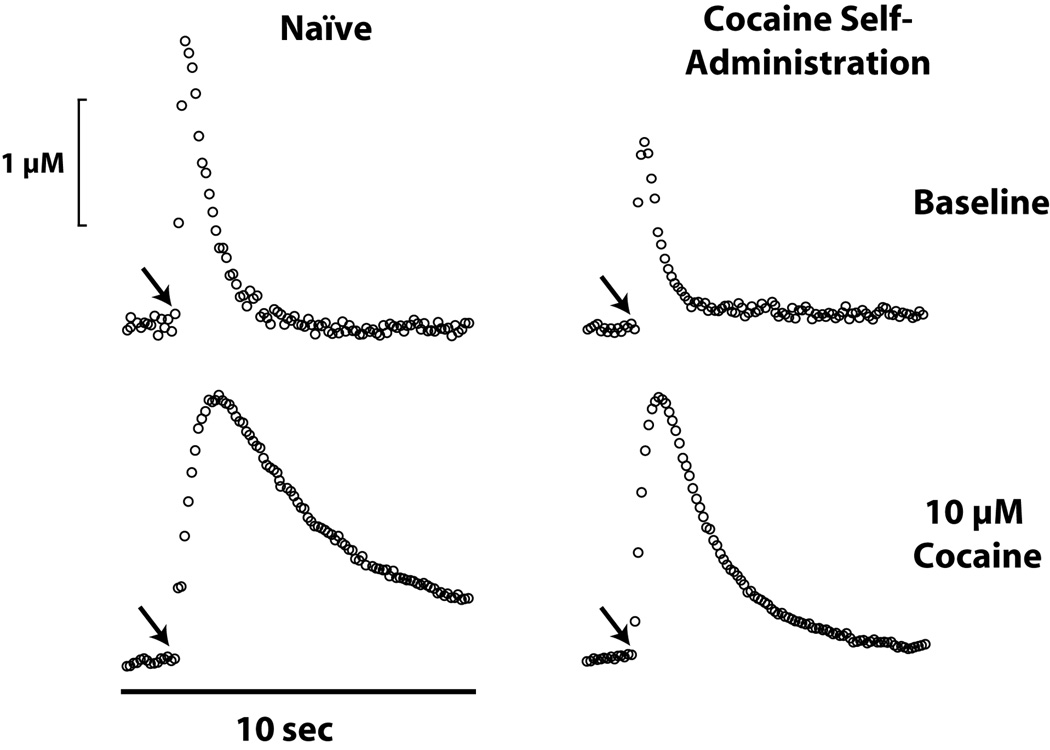
In addition to alterations in extracellular dopamine responses to cocaine, rats with a history of cocaine under the DT4 schedule also displayed reduced dopamine uptake inhibition following cocaine challenge (Figure 8). Surprisingly, the dose-response curves for cocaine inhibition of dopamine uptake were shifted to the right and showed reductions in the maximal cocaine-induced uptake inhibition. A similar insensitivity to cocaine’s uptake inhibiting effects was also observed in animals that had self-administered cocaine under an FR1 schedule restricted to 40 cocaine (1.5 mg/kg) injections per session (5×40). In those studies, animals with a history of cocaine showed reduced responsivity to cocaine when compared to naïve controls (124). Although baseline uptake rates were different between cocaine-experienced and cocaine-naïve rats in both the DT4 and 5×40 studies, the effects were in the opposite directions. Thus, the insensitivity of cocaine in rats with a history of cocaine is likely to be independent of any change in basal dopamine uptake rate (123,124). Rather, we hypothesize that decreased uptake inhibition in animals with a history of cocaine may be associated with allosteric and/or conformational or covalent alterations in DATs that allow dopamine to be transported normally but result in a reduction of cocaine-induced uptake inhibition.
Figure 8. Decreased dopamine release and uptake following cocaine self-administration.
Shown are representative concentration-time plots of dopamine responses in the presence of 10 microM cocaine applied to a brain slice containing the NAc core. Dopamine release is greater and uptake is faster in naïve rats (left) relative to rats which have self-administered cocaine (right). Further, the effects of cocaine are greatly reduced in animals with a history of cocaine self-administration.
In addition to DAT insensitivity, cocaine-induced locomotor activation was drastically reduced following cocaine self-administration, indicating that reduced DAT sensitivity, is accompanied by behavioral tolerance in activity assays (123). These findings are consistent with the literature on human stimulant and alcohol abuse and addiction, which shows that after chronic usage, drug abusers are tolerant to the behaviorally stimulating, euphoria-producing and dopamine-elevating effects of stimulants. Unfortunately, the motivation to take the drugs is not similarly reduced but rather is enhanced, highlighting the need for additional studies to elucidate the contributions of dopamine and other neurotransmitters in the NAc and elsewhere to the complex addiction process.
5.2. No Changes following amphetamine self-administration
In order to determine whether the marked changes caused by cocaine self-administration were specific to cocaine itself, or would be common to other psychostimulants, we undertook a study investigating potential changes to presynaptic dopamine parameters after amphetamine self-administration. To match the behavioral protocol as closely as possible to the 5 day, 40 injections per day (1.5 mg/kg/injection) of cocaine intake, we matched the number of days and amount of time per day spent self-administering instead of matching the number of injections taken per session. This decision was based on the fact that amphetamine has a longer half-life than cocaine, and thus, if we had matched the number of injections with the cocaine study it would have resulted in sessions that were twice as long as what we encounter with cocaine.
In contrast to cocaine, amphetamine exposure did not produce any changes in the baseline or drug-induced responses of the dopamine system. As measured by in vitro voltammetry, the magnitude of electrically stimulated release, the maximal rate of uptake and the amount of uptake inhibition following cocaine (100 nM – 30 microM) or amphetamine (100 nM – 10 microM) application to NAc slices were all unchanged. In addition, the baseline levels of extracellular dopamine measured by microdialysis were not different from naïve rats. Cocaine and amphetamine both produced identical, significant increases in dopamine levels in naïve and amphetamine-exposed rats. Thus, 5 days of binge-like amphetamine self-administration did not produce any of the changes observed with the 5 day cocaine protocol (124). The cocaine effects appear to be specific to cocaine, and not due to elevations in dopamine. We speculate that amphetamine did not produce any changes in dopamine system responsivity or pharmacological sensitivity to cocaine or amphetamine because it is recognized by the DAT as dopamine. Amphetamine is structurally very similar to dopamine, and is a substrate for the DAT, being actively transported across the plasma membrane. It is also recognized as a substrate and transported into vesicles by the vesicular monoamine transporter. Due to amphetamine’s similarity to dopamine, we postulate that the DAT does not recognize it as foreign and therefore no changes in pharmacology are induced.
When taken together, the microdialysis and voltammetry experiments described above show convincingly that cocaine, but not amphetamine, self-administration produces an intrinsic insensitivity of the DAT to cocaine. Previous studies using rodents show that repeated administration of cocaine alters DAT measures, although the nature of these changes are influenced by route and pattern of administration, dose, treatment duration, and length of deprivation period (105,131,132).
6. POTENTIAL MECHANISMS FOR ALTERED DOPAMINE SIGNALING FOLLOWING COCAINE SELF-ADMINISTRATION
The neural mechanisms underlying the alterations in dopamine responsivity to cocaine are not well understood. Regulation of the function of the DAT occurs under many circumstances, including acute and chronic drug administration (105,131,132), stress, exercise, learning and reward related events (133–135). To date, the vast majority of changes in DAT function have been attributed to redistribution of DAT molecules to and from the plasma membrane and cytoplasmic vesicles. This process of DAT trafficking, as the movement into and out of the plasma membrane is called, is highly regulated by protein kinase activity, with phosphorylation by PKC, CamKII and ERK1/2 receiving a great deal of attention (for review see (136). Additional regulation is provided by glycosylation of the DAT which also can alter its sensitivity (137).
Another mechanism of DAT regulation is oligomerization. DAT molecules have been shown to form complexes of dimers, tetramers or higher order complexes of DAT molecules (138–140). In DAT-transfected cells, oligomers of DAT have been shown to exhibit different kinetics for dopamine uptake and different sensitivity to cocaine-induced dopamine uptake inhibition, making this process an attractive one for explaining the reduced sensitivity to cocaine found after cocaine self-administration (140). The reports from our lab, however, are the only demonstrations of altered cocaine potency of the DAT shown in tissue taken from animals, thus the mechanism is relatively unexplored and will require additional studies.
7. CONCLUSIONS
The mesolimbic dopamine system, projecting from the VTA to the NAc, plays a central role in reward learning and hedonic/emotional processing. Extensive evidence indicates that dopamine signaling is an important regulator of the activity of accumbens neurons and consequently, alterations in dopamine release, uptake, and extracellular levels are of critical importance to understanding the neural correlates underlying drug addiction. Neurochemical tools such as microdialysis and voltammetry, particularly when combined with self-administration techniques, are highly valuable for uncovering the alterations in dopamine neurophysiology that occur in animal models of addiction. The studies reviewed here show that binge-like self-administration of cocaine and amphetamine have very different effects on the DAT, and that the effects appear to be driven by different molecular interactions with the DAT, such that cocaine binding at one site leads to dramatic tolerance, while amphetamine binding to another site does not induce any changes in potency. Finding of such disparate chronic effects of stimulants that have similar dopamine-elevating acute effects urges caution in making generalizations across classes of abused stimulants such as cocaine and amphetamine.
ACKNOWLEDGEMENTS
Much of the published research on the effects of drug self-administration upon which this review is based was the work of Drs. Yolanda Mateo and Mark Ferris. This work was supported by R01 DA021325, R01 DA030161 and P50 DA06634 (SRJ), K01 DA025279 and NARSAD GTS 35482 (RAE).
REFERENCES
- 1.Woolverton WL, Johnson KM. Neurobiology of cocaine abuse. Trends Pharmacol Sci. 1992;13:193–200. doi: 10.1016/0165-6147(92)90063-c. [DOI] [PubMed] [Google Scholar]
- 2.Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- 3.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 4.Ljungberg T, Apicella P, Schultz W. Responses of monkey midbrain dopamine neurons during delayed alternation performance. Brain Res. 1991;567:337–341. doi: 10.1016/0006-8993(91)90816-e. [DOI] [PubMed] [Google Scholar]
- 5.Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol. 1992;67:145–163. doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- 6.Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci. 1993;13:900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirenowicz J, Schultz W. Importance of unpredictability for reward responses in primate dopamine neurons. J Neurophysiol. 1994;72:1024–1027. doi: 10.1152/jn.1994.72.2.1024. [DOI] [PubMed] [Google Scholar]
- 8.Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1:304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- 9.Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- 10.Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- 11.Carboni E, Imperato A, Perezzani L, Di Chiara G. Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience. 1989;28:653–661. doi: 10.1016/0306-4522(89)90012-2. [DOI] [PubMed] [Google Scholar]
- 12.Adams BW, Bradberry CW, Moghaddam B. NMDA antagonist effects on striatal dopamine release: microdialysis studies in awake monkeys. Synapse. 2002;43:12–18. doi: 10.1002/syn.1114. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Paredes W, Lowinson JH, Gardner EL. Delta 9-tetrahydrocannabinol enhances presynaptic dopamine efflux in medial prefrontal cortex. Eur J Pharmacol. 1990;190:259–262. doi: 10.1016/0014-2999(90)94136-l. [DOI] [PubMed] [Google Scholar]
- 14.Riegel AC, Zapata A, Shippenberg TS, French ED. The abused inhalant toluene increases dopamine release in the nucleus accumbens by directly stimulating ventral tegmental area neurons. Neuropsychopharmacology. 2007;32:1558–1569. doi: 10.1038/sj.npp.1301273. [DOI] [PubMed] [Google Scholar]
- 15.Zocchi A, Girlanda E, Varnier G, Sartori I, Zanetti L, Wildish GA, Lennon M, Mugnaini M, Heidbreder CA. Dopamine responsiveness to drugs of abuse: A shell-core investigation in the nucleus accumbens of the mouse. Synapse. 2003;50:293–302. doi: 10.1002/syn.10271. [DOI] [PubMed] [Google Scholar]
- 16.Self DW, Stein L. The D1 agonists SKF 82958 and SKF 77434 are self-administered by rats. Brain Res. 1992;582:349–352. doi: 10.1016/0006-8993(92)90155-3. [DOI] [PubMed] [Google Scholar]
- 17.Weed MR, Woolverton WL. The reinforcing effects of dopamine D1 receptor agonists in rhesus monkeys. J Pharmacol Exp Ther. 1995;275:1367–1374. [PubMed] [Google Scholar]
- 18.Self DW, Belluzzi JD, Kossuth S, Stein L. Self-administration of the D1 agonist SKF 82958 is mediated by D1, not D2, receptors. Psychopharmacology (Berl) 1996;123:303–306. doi: 10.1007/BF02246638. [DOI] [PubMed] [Google Scholar]
- 19.Caine SB, Negus SS, Mello NK, Bergman J. Effects of dopamine D(1-like) and D(2-like) agonists in rats that self-administer cocaine. J Pharmacol Exp Ther. 1999;291:353–360. [PubMed] [Google Scholar]
- 20.Spealman RD, Bergman J, Madras BK, Melia KF. Discriminative stimulus effects of cocaine in squirrel monkeys: involvement of dopamine receptor subtypes. J Pharmacol Exp Ther. 1991;258:945–953. [PubMed] [Google Scholar]
- 21.Sinnott RS, Mach RH, Nader MA. Dopamine D2/D3 receptors modulate cocaine's reinforcing and discriminative stimulus effects in rhesus monkeys. Drug Alcohol Depend. 1999;54:97–110. doi: 10.1016/s0376-8716(98)00162-8. [DOI] [PubMed] [Google Scholar]
- 22.Sinnott RS, Nader MA. Modulation of cocaine's discriminative stimulus effects by dopamine D(1) agonists in rhesus monkeys. Pharmacol Biochem Behav. 2001;68:301–309. doi: 10.1016/s0091-3057(00)00462-7. [DOI] [PubMed] [Google Scholar]
- 23.Caine SB, Koob GF. Effects of dopamine D-1 and D-2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J Pharmacol Exp Ther. 1994;270:209–218. [PubMed] [Google Scholar]
- 24.White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- 25.Kalivas PW, Sorg BA, Hooks MS. The pharmacology and neural circuitry of sensitization to psychostimulants. Behav Pharmacol. 1993;4:315–334. [PubMed] [Google Scholar]
- 26.Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 27.Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 28.Zahm DS, Brog JS. On the significance of subterritories in the "accumbens" part of the rat ventral striatum. Neuroscience. 1992;50:751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
- 29.McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 30.Groenewegen HJ, Vermeulen-Van der Zee E, te KA, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- 31.Groenewegen HJ, Berendse HW, Wolters JG, Lohman AH. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization. Prog Brain Res. 1990;85:95–116. doi: 10.1016/s0079-6123(08)62677-1. [DOI] [PubMed] [Google Scholar]
- 32.Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the "accumbens" part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- 33.Wright CI, Beijer AV, Groenewegen HJ. Basal amygdaloid complex afferents to the rat nucleus accumbens are compartmentally organized. J Neurosci. 1996;16:1877–1893. doi: 10.1523/JNEUROSCI.16-05-01877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- 35.Zahm DS, Heimer L. Specificity in the efferent projections of the nucleus accumbens in the rat: comparison of the rostral pole projection patterns with those of the core and shell. J Comp Neurol. 1993;327:220–232. doi: 10.1002/cne.903270205. [DOI] [PubMed] [Google Scholar]
- 36.Usuda I, Tanaka K, Chiba T. Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res. 1998;797:73–93. doi: 10.1016/s0006-8993(98)00359-x. [DOI] [PubMed] [Google Scholar]
- 37.Carelli RM, King VC, Hampson RE, Deadwyler SA. Firing patterns of nucleus accumbens neurons during cocaine self-administration in rats. Brain Res. 1993;626:14–22. doi: 10.1016/0006-8993(93)90557-4. [DOI] [PubMed] [Google Scholar]
- 38.Uzwiak AJ, Guyette FX, West MO, Peoples LL. Neurons in accumbens subterritories of the rat: phasic firing time-locked within seconds of intravenous cocaine self-infusion. Brain Res. 1997;767:363–369. doi: 10.1016/s0006-8993(97)00752-x. [DOI] [PubMed] [Google Scholar]
- 39.Carlezon WA, Jr, Devine DP, Wise RA. Habit-forming actions of nomifensine in nucleus accumbens. Psychopharmacology (Berl) 1995;122:194–197. doi: 10.1007/BF02246095. [DOI] [PubMed] [Google Scholar]
- 40.Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci. 1997;17:8580–8587. doi: 10.1523/JNEUROSCI.17-21-08580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hitchcott PK, Phillips GD. Double dissociation of the behavioural effects of R(+) 7-OH-DPAT infusions in the central and basolateral amygdala nuclei upon Pavlovian and instrumental conditioned appetitive behaviours. Psychopharmacology (Berl) 1998;140:458–469. doi: 10.1007/s002130050790. [DOI] [PubMed] [Google Scholar]
- 42.McKinzie DL, Rodd-Henricks ZA, Dagon CT, Murphy JM, McBride WJ. Cocaine is self-administered into the shell region of the nucleus accumbens in Wistar rats. Ann N Y Acad Sci. 1999;877:788–791. doi: 10.1111/j.1749-6632.1999.tb09323.x. [DOI] [PubMed] [Google Scholar]
- 43.Roberts DCS, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav. 1977;6:615–620. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- 44.McGregor A, Roberts DCS. Effect of medial prefrontal cortex injections of SCH 23390 on intravenous cocaine self-administration under both a fixed and progressive ratio schedule of reinforcement. Behav Brain Res. 1995;67:75–80. doi: 10.1016/0166-4328(94)00106-p. [DOI] [PubMed] [Google Scholar]
- 45.Carr GD, White NM. Conditioned place preference from intra-accumbens but not intra-caudate amphetamine injections. Life Sci. 1983;33:2551–2557. doi: 10.1016/0024-3205(83)90165-0. [DOI] [PubMed] [Google Scholar]
- 46.Hemby SE, Jones GH, Justice JB, Jr, Neill DB. Conditioned locomotor activity but not conditioned place preference following intra-accumbens infusions of cocaine. Psychopharmacology (Berl) 1992;106:330–336. doi: 10.1007/BF02245413. [DOI] [PubMed] [Google Scholar]
- 47.Schildein S, Agmo A, Huston JP, Schwarting RK. Intraaccumbens injections of substance P, morphine and amphetamine: effects on conditioned place preference and behavioral activity. Brain Res. 1998;790:185–194. doi: 10.1016/s0006-8993(98)00062-6. [DOI] [PubMed] [Google Scholar]
- 48.Lyness WH, Friedle NM, Moore KE. Destruction of dopaminergic nerve terminals in nucleus accumbens: effect on d-amphetamine self-administration. Pharmacol Biochem Behav. 1979;11:553–556. doi: 10.1016/0091-3057(79)90040-6. [DOI] [PubMed] [Google Scholar]
- 49.Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berl) 1984;84:167–173. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- 50.Groenewegen HJ, Russchen FT. Organization of the efferent projections of the nucleus accumbens to pallidal, hypothalamic, and mesencephalic structures: a tracing and immunohistochemical study in the cat. J Comp Neurol. 1984;223:347–367. doi: 10.1002/cne.902230303. [DOI] [PubMed] [Google Scholar]
- 51.Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boye SM, Grant RJ, Clarke PB. Disruption of dopaminergic neurotransmission in nucleus accumbens core inhibits the locomotor stimulant effects of nicotine and D-amphetamine in rats. Neuropharmacology. 2001;40:792–805. doi: 10.1016/s0028-3908(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 53.Sellings LH, Clarke PB. Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J Neurosci. 2003;23:6295–6303. doi: 10.1523/JNEUROSCI.23-15-06295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- 55.Alderson HL, Parkinson JA, Robbins TW, Everitt BJ. The effects of excitotoxic lesions of the nucleus accumbens core or shell regions on intravenous heroin self-administration in rats. Psychopharmacology (Berl) 2001;153:455–463. doi: 10.1007/s002130000634. [DOI] [PubMed] [Google Scholar]
- 56.Hutcheson DM, Parkinson JA, Robbins TW, Everitt BJ. The effects of nucleus accumbens core and shell lesions on intravenous heroin self-administration and the acquisition of drug-seeking behaviour under a second-order schedule of heroin reinforcement. Psychopharmacology (Berl) 2001;153:464–472. doi: 10.1007/s002130000635. [DOI] [PubMed] [Google Scholar]
- 57.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodd-Henricks ZA, McKinzie DL, Li TK, Murphy JM, McBride WJ. Cocaine is self-administered into the shell but not the core of the nucleus accumbens of Wistar rats. J Pharmacol Exp Ther. 2002;303:1216–1226. doi: 10.1124/jpet.102.038950. [DOI] [PubMed] [Google Scholar]
- 59.Ikemoto S, Qin M, Liu ZH. The functional divide for primary reinforcement of D-amphetamine lies between the medial and lateral ventral striatum: is the division of the accumbens core, shell, and olfactory tubercle valid? J Neurosci. 2005;25:5061–5065. doi: 10.1523/JNEUROSCI.0892-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nicola SM. The flexible approach hypothesis: unification of effort and cue-responding hypotheses for the role of nucleus accumbens dopamine in the activation of reward-seeking behavior. J Neurosci. 2010;30:16585–16600. doi: 10.1523/JNEUROSCI.3958-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25:341–360. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- 63.Floresco SB, McLaughlin RJ, Haluk DM. Opposing roles for the nucleus accumbens core and shell in cue-induced reinstatement of food-seeking behavior. Neuroscience. 2008;154:877–884. doi: 10.1016/j.neuroscience.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 64.Chaudhri N, Sahuque LL, Schairer WW, Janak PH. Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology. 2010;35:783–791. doi: 10.1038/npp.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salamone JD. The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav Brain Res. 1994;61:117–133. doi: 10.1016/0166-4328(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 66.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 67.Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- 68.Di Chiara G, Valentini V, Fenu S. Behavioral and neurochemical pharmacology of 5-HT6 receptors related to reward and reinforcement. Int Rev Neurobiol. 2011;96:111–139. doi: 10.1016/B978-0-12-385902-0.00005-X. [DOI] [PubMed] [Google Scholar]
- 69.Richard JM, Berridge KC. Nucleus accumbens dopamine/glutamate interaction switches modes to generate desire versus dread: D(1) alone for appetitive eating but D(1) and D(2) together for fear. J Neurosci. 2011;31:12866–12879. doi: 10.1523/JNEUROSCI.1339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hilario MR, Costa RM. High on habits. Front Neurosci. 2008;2:208–217. doi: 10.3389/neuro.01.030.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roberts DC, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1980;12:781–787. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- 73.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 74.Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- 75.Heikkila RE, Orlansky H, Mytilineou C, Cohen G. Amphetamine: evaluation of d- and l-isomers as releasing agents and uptake inhibitors for 3H-dopamine and 3H-norepinephrine in slices of rat neostriatum and cerebral cortex. J Pharmacol Exp Ther. 1975;194:47–56. [PubMed] [Google Scholar]
- 76.Sulzer D, Rayport S. Amphetamine and other psychostimulants reduce pH gradients in midbrain dopaminergic neurons and chromaffin granules: a mechanism of action. Neuron. 1990;5:797–808. doi: 10.1016/0896-6273(90)90339-h. [DOI] [PubMed] [Google Scholar]
- 77.Sulzer D, Pothos E, Sung HM, Maidment NT, Hoebel BG, Rayport S. Weak base model of amphetamine action. Ann N Y Acad Sci. 1992;654:525–528. doi: 10.1111/j.1749-6632.1992.tb26020.x. [DOI] [PubMed] [Google Scholar]
- 78.Raiteri M, Cerrito F, Cervoni AM, Levi G. Dopamine can be released by two mechanisms differentially affected by the dopamine transport inhibitor nomifensine. J Pharmacol Exp Ther. 1979;208:195–202. [PubMed] [Google Scholar]
- 79.Schmitz Y, Lee CJ, Schmauss C, Gonon F, Sulzer D. Amphetamine distorts stimulation-dependent dopamine overflow: effects on D2 autoreceptors, transporters, and synaptic vesicle stores. J Neurosci. 2001;21:5916–5924. doi: 10.1523/JNEUROSCI.21-16-05916.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramsson ES, Howard CD, Covey DP, Garris PA. High doses of amphetamine augment, rather than disrupt, exocytotic dopamine release in the dorsal and ventral striatum of the anesthetized rat. J Neurochem. 2011;119:1162–1172. doi: 10.1111/j.1471-4159.2011.07407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramsson ES, Covey DP, Daberkow DP, Litherland MT, Juliano SA, Garris PA. Amphetamine augments action potential-dependent dopaminergic signaling in the striatum in vivo. J Neurochem. 2011;117:937–948. doi: 10.1111/j.1471-4159.2011.07258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Michael DJ, Wightman RM. Electrochemical monitoring of biogenic amine neurotransmission in real time. J Pharm Biomed Anal. 1999;19:33–46. doi: 10.1016/s0731-7085(98)00145-9. [DOI] [PubMed] [Google Scholar]
- 83.Jones SR, Odell SJ, Marshall JF, Wightman RM. Functional and anatomical evidence for different dopamine dynamics in the core and shell of the nucleus accumbens in slices of rat brain. Synapse. 1996;23:224–231. doi: 10.1002/(SICI)1098-2396(199607)23:3<224::AID-SYN12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 84.Budygin EA, John CE, Mateo Y, Jones SR. Lack of cocaine effect on dopamine clearance in the core and shell of the nucleus accumbens of dopamine transporter knock-out mice. J Neurosci. 2002;22:RC222. doi: 10.1523/JNEUROSCI.22-10-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mateo Y, Budygin EA, Morgan D, Roberts DCS, Jones SR. Fast onset of dopamine uptake inhibition by intravenous cocaine. Eur J Neurosci. 2004;20:2838–2842. doi: 10.1111/j.1460-9568.2004.03736.x. [DOI] [PubMed] [Google Scholar]
- 86.John CE, Jones SR. Voltammetric characterization of the effect of monoamine uptake inhibitors and releasers on dopamine and serotonin uptake in mouse caudate-putamen and substantia nigra slices. Neuropharmacology. 2007;52:1596–1605. doi: 10.1016/j.neuropharm.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wieczorek WJ, Kruk ZL. Differential action of (+)-amphetamine on electrically evoked dopamine overflow in rat brain slices containing corpus striatum and nucleus accumbens. Br J Pharmacol. 1994;111:829–836. doi: 10.1111/j.1476-5381.1994.tb14813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18:1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iravani MM, Kruk ZL. Effects of amphetamine on carrier-mediated and electrically stimulated dopamine release in slices of rat caudate putamen and nucleus accumbens. J Neurochem. 1995;64:1161–1168. doi: 10.1046/j.1471-4159.1995.64031161.x. [DOI] [PubMed] [Google Scholar]
- 90.Hurd YL, Ungerstedt U. Cocaine: an in vivo microdialysis evaluation of its acute action on dopamine transmission in rat striatum. Synapse. 1989;3:48–54. doi: 10.1002/syn.890030107. [DOI] [PubMed] [Google Scholar]
- 91.Moghaddam B, Bunney BS. Differential effect of cocaine on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens: comparison to amphetamine. Synapse. 1989;4:156–161. doi: 10.1002/syn.890040209. [DOI] [PubMed] [Google Scholar]
- 92.Kuczenski R, Segal DS, Aizenstein ML. Amphetamine, Cocaine, and Fencamfamine-Relationship Between Locomotor and Stereotypy Response Profiles and Caudate and Accumbens-Dopamine Dynamics. J Neurosci. 1991;11:2703–2712. doi: 10.1523/JNEUROSCI.11-09-02703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weiss F, Paulus MP, Lorang MT, Koob GF. Increases in extracellular dopamine in the nucleus accumbens by cocaine are inversely related to basal levels: effects of acute and repeated administration. J Neurosci. 1992;12:4372–4380. doi: 10.1523/JNEUROSCI.12-11-04372.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pierce RC, Kalivas PW. Amphetamine produces sensitized increases in locomotion and extracellular dopamine preferentially in the nucleus accumbens shell of rats administered repeated cocaine. J Pharmacol Exp Ther. 1995;275:1019–1029. [PubMed] [Google Scholar]
- 95.King D, Zigmond MJ, Finlay JM. Effects of dopamine depletion in the medial prefrontal cortex on the stress-induced increase in extracellular dopamine in the nucleus accumbens core and shell. Neuroscience. 1997;77:141–153. doi: 10.1016/s0306-4522(96)00421-6. [DOI] [PubMed] [Google Scholar]
- 96.Heidbreder C, Feldon J. Amphetamine-induced neurochemical and locomotor responses are expressed differentially across the anteroposterior axis of the core and shell subterritories of the nucleus accumbens. Synapse. 1998;29:310–322. doi: 10.1002/(SICI)1098-2396(199808)29:4<310::AID-SYN3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 97.McKittrick CR, Abercrombie ED. Catecholamine mapping within nucleus accumbens: differences in basal and amphetamine-stimulated efflux of norepinephrine and dopamine in shell and core. J Neurochem. 2007;100:1247–1256. doi: 10.1111/j.1471-4159.2006.04300.x. [DOI] [PubMed] [Google Scholar]
- 98.Kalivas PW, Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res. 1995;675:325–328. doi: 10.1016/0006-8993(95)00013-g. [DOI] [PubMed] [Google Scholar]
- 99.Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the "shell" as compared with the "core" of the rat nucleus accumbens. Proc Natl Acad Sci U S A. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sokolowski JD, Conlan AN, Salamone JD. A microdialysis study of nucleus accumbens core and shell dopamine during operant responding in the rat. Neuroscience. 1998;86:1001–1009. doi: 10.1016/s0306-4522(98)00066-9. [DOI] [PubMed] [Google Scholar]
- 101.Cadoni C, Solinas M, Di Chiara G. Psychostimulant sensitization: differential changes in accumbal shell and core dopamine. Eur J Pharmacol. 2000;388:69–76. doi: 10.1016/s0014-2999(99)00824-9. [DOI] [PubMed] [Google Scholar]
- 102.Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le MM, Piazza PV. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci. 2000;12:973–979. doi: 10.1046/j.1460-9568.2000.00996.x. [DOI] [PubMed] [Google Scholar]
- 103.Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- 104.Marshall JF, O'Dell SJ, Navarrete R, Rosenstein AJ. Dopamine high-affinity transport site topography in rat brain: major differences between dorsal and ventral striatum. Neuroscience. 1990;37:11–21. doi: 10.1016/0306-4522(90)90187-9. [DOI] [PubMed] [Google Scholar]
- 105.Pilotte NS, Sharpe LG, Rountree SD, Kuhar MJ. Cocaine withdrawal reduces dopamine transporter binding in the shell of the nucleus accumbens. Synapse. 1996;22:87–92. doi: 10.1002/(SICI)1098-2396(199601)22:1<87::AID-SYN10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 106.Nirenberg MJ, Chan J, Pohorille A, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter: comparative ultrastructure of dopaminergic axons in limbic and motor compartments of the nucleus accumbens. J Neurosci. 1997;17:6899–6907. doi: 10.1523/JNEUROSCI.17-18-06899.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 108.Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1995;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- 109.Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, Hitzemann R, Shea CE. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997;386:827–830. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- 110.Kiyatkin EA, Kiyatkin DE, Rebec GV. Phasic inhibition of dopamine uptake in nucleus accumbens induced by intravenous cocaine in freely behaving rats. Neuroscience. 2000;98:729–741. doi: 10.1016/s0306-4522(00)00168-8. [DOI] [PubMed] [Google Scholar]
- 111.Pitts DK, Marwah J. Cocaine modulation of central monoaminergic neurotransmission. Pharmacol Biochem Behav. 1987;26:453–461. doi: 10.1016/0091-3057(87)90147-x. [DOI] [PubMed] [Google Scholar]
- 112.Einhorn LC, Johansen PA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: studies in the ventral tegmental area. J Neurosci. 1988;8:100–112. doi: 10.1523/JNEUROSCI.08-01-00100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Batsche K, Granoff MI, Wang RY. 5-HT3 receptor antagonists fail to block the suppressant effect of cocaine on the firing rate of A10 dopamine neurons in the rat. Brain Res. 1992;592:273–277. doi: 10.1016/0006-8993(92)91685-8. [DOI] [PubMed] [Google Scholar]
- 114.Hinerth MA, Collins HA, Baniecki M, Hanson RN, Waszczak BL. Novel in vivoelectrophysiological assay for the effects of cocaine and putative "cocaine antagonists" on dopamine transporter activity of substantia nigra and ventral tegmental area dopamine neurons. Synapse. 2000;38:305–312. doi: 10.1002/1098-2396(20001201)38:3<305::AID-SYN9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 115.Ahmed SH, Lin D, Koob GF, Parsons LH. Escalation of cocaine self-administration does not depend on altered cocaine-induced nucleus accumbens dopamine levels. J Neurochem. 2003;86:102–113. doi: 10.1046/j.1471-4159.2003.01833.x. [DOI] [PubMed] [Google Scholar]
- 116.España RA, Roberts DCS, Jones SR. Short-acting cocaine and long-acting GBR-12909 both elicit rapid dopamine uptake inhibition following intravenous delivery. Neuroscience. 2008;155:250–257. doi: 10.1016/j.neuroscience.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Samaha AN, Mallet N, Ferguson SM, Gonon F, Robinson TE. The rate of cocaine administration alters gene regulation and behavioral plasticity: implications for addiction. J Neurosci. 2004;24:6362–6370. doi: 10.1523/JNEUROSCI.1205-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Heien ML, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Wassum KM, Wightman RM. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc Natl Acad Sci U S A. 2005;102:10023–10028. doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jones SR, Joseph JD, Barak LS, Caron MG, Wightman RM. Dopamine neuronal transport kinetics and effects of amphetamine. J Neurochem. 1999;73:2406–2414. doi: 10.1046/j.1471-4159.1999.0732406.x. [DOI] [PubMed] [Google Scholar]
- 120.Seecof R, Tennant FS., Jr Subjective perceptions to the intravenous "rush" of heroin and cocaine in opioid addicts. Am J Drug Alcohol Abuse. 1986;12:79–87. doi: 10.3109/00952998609083744. [DOI] [PubMed] [Google Scholar]
- 121.Evans SM, Cone EJ, Henningfield JE. Arterial and venous cocaine plasma concentrations in humans: relationship to route of administration, cardiovascular effects and subjective effects. J Pharmacol Exp Ther. 1996;279:1345–1356. [PubMed] [Google Scholar]
- 122.Zernig G, Giacomuzzi S, Riemer Y, Wakonigg G, Sturm K, Saria A. Intravenous drug injection habits: drug users' self-reports versus researchers' perception. Pharmacology. 2003;68:49–56. doi: 10.1159/000068731. [DOI] [PubMed] [Google Scholar]
- 123.Mateo Y, Lack CM, Morgan D, Roberts DCS, Jones SR. Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacology. 2005;30:1455–1463. doi: 10.1038/sj.npp.1300687. [DOI] [PubMed] [Google Scholar]
- 124.Ferris MJ, Mateo Y, Roberts DC, Jones SR. Cocaine-insensitive dopamine transporters with intact substrate transport produced by self-administration. Biol Psychiatry. 2011;69:201–207. doi: 10.1016/j.biopsych.2010.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roberts DCS, Brebner K, Vincler M, Lynch WJ. Patterns of cocaine self-administration in rats produced by various access conditions under a discrete trials procedure. Drug Alcohol Depend. 2002;67:291–299. doi: 10.1016/s0376-8716(02)00083-2. [DOI] [PubMed] [Google Scholar]
- 126.Brebner K, Phelan R, Roberts DCS. Effect of baclofen on cocaine self-administration in rats reinforced under fixed-ratio 1 and progressive-ratio schedules. Psychopharmacology (Berl) 2000;148:314–321. doi: 10.1007/s002130050056. [DOI] [PubMed] [Google Scholar]
- 127.Smith MA, Yancey DL, Morgan D, Liu Y, Froestl W, Roberts DCS. Effects of positive allosteric modulators of the GABAB receptor on cocaine self-administration in rats. Psychopharmacology (Berl) 2004;173:105–111. doi: 10.1007/s00213-003-1706-5. [DOI] [PubMed] [Google Scholar]
- 128.España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DCS, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.España RA, Melchior JR, Roberts DCS, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology (Berl) 2011;214:415–426. doi: 10.1007/s00213-010-2048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Parsons LH, Smith AD, Justice JB., Jr Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence from chronic cocaine. Synapse. 1991;9:60–65. doi: 10.1002/syn.890090109. [DOI] [PubMed] [Google Scholar]
- 131.Gerrits MA, Petromilli P, Westenberg HG, Di Chiara G, van Ree JM. Decrease in basal dopamine levels in the nucleus accumbens shell during daily drug-seeking behaviour in rats. Brain Res. 2002;924:141–150. doi: 10.1016/s0006-8993(01)03105-5. [DOI] [PubMed] [Google Scholar]
- 132.Chefer VI, Shippenberg TS. Changes in basal and cocaine-evoked extracellular dopamine uptake and release in the rat nucleus accumbens during early abstinence from cocaine: quantitative determination under transient conditions. Neuroscience. 2002;112:907–919. doi: 10.1016/s0306-4522(02)00099-4. [DOI] [PubMed] [Google Scholar]
- 133.Teixeira A, Muller LG, Reckziegel P, Boufleur N, Pase CS, Villarinho JG, Fachinetto R, Ferreira J, Rocha JB, Burger ME. Beneficial effects of an innovative exercise model on motor and oxidative disorders induced by haloperidol in rats. Neuropharmacology. 2011;60:432–438. doi: 10.1016/j.neuropharm.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 134.Novick AM, Forster GL, Tejani-Butt SM, Watt MJ. Adolescent social defeat alters markers of adult dopaminergic function. Brain Res Bull. 2011;86:123–128. doi: 10.1016/j.brainresbull.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- 136.Ramamoorthy S, Shippenberg TS, Jayanthi LD. Regulation of monoamine transporters: Role of transporter phosphorylation. Pharmacol Ther. 2011;129:220–238. doi: 10.1016/j.pharmthera.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li LB, Chen N, Ramamoorthy S, Chi L, Cui XN, Wang LC, Reith ME. The role of N-glycosylation in function and surface trafficking of the human dopamine transporter. J Biol Chem. 2004;279:21012–21020. doi: 10.1074/jbc.M311972200. [DOI] [PubMed] [Google Scholar]
- 138.Torres GE, Carneiro A, Seamans K, Fiorentini C, Sweeney A, Yao WD, Caron MG. Oligomerization and trafficking of the human dopamine transporter. Mutational analysis identifies critical domains important for the functional expression of the transporter. J Biol Chem. 2003;278:2731–2739. doi: 10.1074/jbc.M201926200. [DOI] [PubMed] [Google Scholar]
- 139.Chen N, Reith ME. Substrates dissociate dopamine transporter oligomers. J Neurochem. 2008;105:910–920. doi: 10.1111/j.1471-4159.2007.05195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li Y, Cheng SY, Chen N, Reith ME. Interrelation of dopamine transporter oligomerization and surface presence as studied with mutant transporter proteins and amphetamine. J Neurochem. 2010;114:873–885. doi: 10.1111/j.1471-4159.2010.06818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]