Abstract
There are three types of mouse Mutyh mRNAs (type a, b and c) generated by alternative splicing, and type b mRNA is a major form among the three in most of the tissues examined. The level of type c mRNA is relatively high in brain. Type a and b mRNAs were expected to encode 57.7 kDa protein (MUTYHα), while type c mRNA had a partly different open reading frame encoding a 50.2 kDa protein (MUTYHβ). An in vitro translation of type b and c mRNAs produced a 50 kDa MUTYHα and 47 kDa MUTYHβ, respectively. MUTYHα and MUTYHβ were detected in wild-type embryonic stem (ES) cells or thymocytes prepared from wild-type mice, but neither MUTYH-null ES cells nor thymocytes prepared from MUTYH-null mice. Both MUTYHα and MUTYHβ were mainly localized in the nuclei and some in mitochondria in wild-type ES cells. Recombinant MUTYHα and β were expressed as fusion proteins with thioredoxin in Escherichia coli, but only MUTYHα was partly soluble and thus could be purified. Recombinant MUTYHα possessed DNA glycosylase activities to excise adenine opposite 8-oxoguanine and guanine but not AP lyase activity.
INTRODUCTION
Cellular DNA is at high risk of being oxidized by reactive oxygen species, which are inevitably generated by normal metabolic functions such as mitochondrial respiration or by environmental exposure to ionizing radiation and chemicals. The oxidation of DNA has been shown to apparently result in either spontaneous mutagenesis or cell death (1,2), thus causing various age-related diseases such as cancer and neurodegeneration (3,4). Among the various types of oxidized damage in DNA, 8-oxoguanine (8-oxoG), an oxidized form of guanine, can pair with adenine as well as cytosine during DNA replication, and this action is considered to be one of the spontaneous causes of a G:C to T:A transversion mutation (5).
To minimize such spontaneous mutagenesis, organisms come equipped with elaborated repair enzymes, namely 8-oxoG DNA glycosylase which excises 8-oxoG opposite cytosine in DNA, and adenine DNA glycosylase which removes adenine incorporated opposite 8-oxoG in template DNA (6). In Escherichia coli, mutM (fpg) gene encoding the former DNA glycosylase, and mutY gene encoding the latter play important roles in the prevention of such spontaneous mutagenesis, especially in G:C to T:A transversion mutation (7). Most eukaryotic cells also possess either a MutM homolog or its functional homolog, OGG1 for the repair of 8-oxoG, and a MutY homolog (MUTYH or MYH) for the repair of adenine opposite 8-oxoG (3,8).
Recently, familial alterations in the human MUTYH gene have been reported to be possible causative mutations for certain types of autosomal recessive colorectal adenomatous polyposis (9–12), thus suggesting that the absence of the MUTYH function in human cells might also result in a mutator phenotype. Among the many genes involved in base excision repair (BER), MUTYH is the first candidate gene for a hereditary neoplasm in human beings. We recently generated MUTYH-null mouse embryonic stem (ES) cell lines (2), and reported that the spontaneous mutation rate in the MUTYH-null cells increased 2-fold in comparison to wild-type cells, thus indicating that the absence of the MUTYH function in mammalian cells results in a moderate mutator phenotype.
In human cells, we previously reported that there are three major MUTYH transcripts, namely type α, β and γ with a different 5′ sequence or first exon and each transcript is alternatively spliced, thus multi-forms of human MUTYH (hMUTYH) proteins are present in the nuclei and in the mitochondria (13). hMUTYH protein encoded by type α mRNA possesses a mitochondrial targeting sequence (MTS), consisting of the amino terminal 14 residues which are required for its localization in the mitochondria (14), while those encoded by type β and γ mRNAs lack the MTS, and are localized in the nuclei. As a result, the subcellular localization of hMUTYH in human cells indicates that mitochondrial DNA is an important target for BER initiated by MUTYH as well as OGG1, probably because of their increased oxidative stress (15,16). Interestingly, rodent MUTYH proteins deduced from mouse and rat MUTYH cDNA clones lack an amino-terminal sequence corresponding to the MTS in hMUTYH (17), thus raising the question as to whether or not mitochondrial forms of MUTYH exist in rodents.
In the present study, we identified three alternatively spliced Mutyh mRNAs from mouse ES cells and mouse tissues, and found these Mutyh mRNAs to encode two different forms of mouse MUTYH (mMUTYH) protein. An analysis of the subcellular distribution of the two mMUTYH proteins revealed that these proteins are mostly localized in the nuclei and to some extent in the mitochondria.
MATERIALS AND METHODS
Oligonucleotides
Oligonucleotides shown in Table 1, were obtained from Greiner Japan, and Hokkaido System Science.
Table 1. Oligonucleotides used in this study.
| Oligonucleotides | Sequencesa |
|---|---|
| mY5B1 |
TCGGAGACTGCGCAGGAG |
| mY5ATG |
ATTATCATGAAGAAACTCCAAGCAT |
| mY5A1 |
ATACTCGATGGATGCAGAAGT |
| mY5A2 |
CCTGGTGCAAAGGCCTGA |
| mY3TGA |
GTATCACTGGGTAGTACTGTTG |
| mY3A1 |
GGGAAGCGCTGGCCAGGT |
| mY3A2 |
CCTCGAGAATAGTAGCCCAG |
| mYe255-B1 |
CGGGGATCCGATTATTATCATGAAGAAACTCCAAGCAT |
| mY3B-N1 |
TAAGCGGCCGCCTGGGTAGTACTGTTGGGTTTGT |
| YN13F |
GTAAAACGACGGCCAGT |
| YNK |
CGCTCTAGAACTAGTGGATC |
| mGA5-1 |
CTGCCATTTGCAGTGGCAAAG |
| mGA3-1 |
TGGTATTCAAGAGAGTAGGGA |
| NSpE5-1 |
TAACCATGGGTCGACAAATGGCCTGGCCAAGCAGAAGA |
| BpE3-2 |
ATTGGATCCTTACAGCATAGGCCCTCCTGTCC |
| *A |
FAM-AGCGGCCATCGATACCGTCAACCTCGAGGAATTCC |
| GO |
GGAATTCCTCGAGGTGOGACGGTATCGATGGCCGCT |
| G |
GGAATTCCTCGAGGTGGACGGTATCGATGGCCGCT |
| 19-P |
FAM-AGCGGCCATCGATACCGTC-phosphate |
| 19-OH | FAM-AGCGGCCATCGATACCGTC-OH |
aFAM, 5′-end was labeled with FAM; GO, 8-oxoG; phosphate, 3′-end was attached to a phosphate group; OH, 3′-end was attached to a hydroxyl group; bold letter indicates a target base for nicking assay.
Isolation of genomic clones for Mutyh
Using the Mutyh type b cDNA as a probe, three independent phage clones were obtained from the λ phage genomic library for 129SvJ mouse (Stratagene).
RNA
Total RNA from the cultured cells and various tissues of C57BL6/J mice was prepared using ISOGEN (Nippon Gene), and total RNA from tissues of BALB/c mice were purchased from Clontech.
Quantitative RT–PCR
RT–PCR for Mutyh and Gapdh mRNA was performed as follows. First-strand cDNA, prepared using First-Strand cDNA Synthesis Kits (Amersham Biosciences) according to the manufacturer’s instructions, was subjected to PCR. PCR was performed in 20 µl of a reaction mixture containing 10 mM Tris–HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.5 µl of the first-strand cDNA, 0.4 U of recombinant Taq DNA polymerase (Takara), 0.2 µM each primer, and 200 µM each deoxynucleoside triphosphate. The initial denaturation was performed at 94°C for 1 min and the amplification was performed by 27, 32, 37 and 40 cycles of denaturation at 94°C for 20 s, annealing at 55°C (mY5A2 + mY3TGA, mGA5 + mGA3), or at 58°C (mY5B1 + mY3A1), for 20 s and extension at 72°C for 35 s (mY5B1 + mY3A1), or for 60 s (mY5A2 + mY3TGA, mGA5 + mGA3), followed by additional extension at 72°C for 5 min. PCR products were subjected to agarose gel electrophoresis, and the band intensity on the gel stained with ethidium bromide was measured using LAS1000-plus Luminescent Image Analysis System (FUJIFILM).
Plasmids
Mutyh cDNAs (type b, c) obtained by RT–PCR of total RNA prepared from CCE28 ES cells using a primer set of mY5ATG + mY3TGA, were cloned into pT7BlueR (Novagen), generating pT7:mMUTYHα and pT7:mMUTYHβ, respectively. For the expression of two forms of mouse MUTYH protein in E.coli, type b and type c Mutyh cDNA were subcloned into pET32a(+) (Novagen), and resultant plasmids were designated as pET32a:mMUTYHα and pET32a:mMUTYHβ, and these plasmids encoded fusion proteins with thioredoxin (Trx-mMUTYHα, Trx-mMUTYHβ). For the stable expression of two forms of MUTYH protein in mouse ES cells, type b and type c Mutyh cDNA were subcloned into pcDNA3.1/Hygro(+) (Invitrogen). pcDEBΔ:EGFP and pcDEBΔ:EGFPm were previously described (18). A cDNA fragment with BamHI site (5′ end) and NotI site (3′ end) which encodes the MUTYHα was prepared by PCR using a primer set (mYe255-B1 + mY3B-N1) and pT7:mMUTYHα or pT7:mMUTYHβ as a template, and inserted into the HindIII–NotI site of pcDEBΔ:EGFPm to construct pcDEBΔ:mMUTYHα-EGFPm or pcDEBΔ:mMUTYHβ-EGFPm, respectively. A plasmid pcDEBΔ:COX8-EGFPm was prepared by inserting cDNA encoding the N-terminal 34 amino acid residues of cytochrome c oxidase subunit VIII into the HindIII–NotI site of pcDEBΔ:EGFPm (18). For the production of MUTYHβ specific antigen, a DNA fragment with NcoI site (5′ end) and BamHI site (3′ end) which encodes the unique N-terminal residues for MUTYHβ (1–64 aa) was prepared by PCR using a primer set (NSpE5-1 + BpE3-2) and pT7:mMUTYHβ as a template, and subcloned into NcoI–BamHI sites of pET8c-TrpE to generate pET8c:TrpE-mMUTYHβN which encodes TrpE-mMUTYHβN fusion protein, or into NcoI–BamHI sites of pET32a(+) to generate pET32a:mMUTYHβN which encodes Trx-mMUTYHβN fusion protein.
DNA sequencing
The nucleotide sequence was determined using either Dye Terminator or Dye Primer Cycle Sequencing FS Ready Reaction Kits and a model 377 automated DNA sequencer (Perkin Elmer), according to the manufacturer’s instructions.
Antibodies against mMUTYHβN
Rabbit polyclonal antibodies against the fusion protein TrpE-mMUTYHβN were prepared as previously described (19). The antibodies were purified with the aid of antigen-affinity columns (Trx-mMUTYHβN-Sepharose column) (18,20). Briefly, antiserum against TrpE-mMUTYHβN was loaded onto the Trx-mMUTYHβN-Sepharose column, and the column was washed with Low E buffer [50 mM sodium phosphate buffer (pH 7.6), 0.1% Triton X-100] and 10 mM sodium phosphate buffer (pH 7.6). The bound antibodies were then eluted using buffer E [0.2 M glycine–HCl (pH 2.3), 0.1 M NaCl, 0.1% Triton X-100]. The eluted fraction was dialyzed against TBS [10 mM Tris–HCl (pH 7.5), 0.9% NaCl] and the purified antibodies were designated as anti-mMUTYHβN.
In vitro transcription and translation
Each type of Mutyh cDNA was placed under the control of the T7 promoter in pT7Blue vector (Novagen). The transcripts were synthesized from BamHI linearized plasmids by T7 RNA polymerase at 30°C for 15 min using Single Tube Protein System 2 (Novagen). These mRNAs were translated in rabbit reticulocyte lysates in a total volume of 50 µl at 30°C for 60 min.
Cell culture
Wild-type mouse ES cell line, CCE28 and MUTYH-null ES cell line, YDK15, and COS-1 cells were maintained as previously described (2,21). For the expression of MUTYH protein in mouse ES cells, plasmid was electroporated into YDK15 cells, and stable transfectants grown in the presence of hygromycin B (140 and 160 µg/ml, Wako Pure Chemicals) were established. YDK15 cells expressing wild-type mMUTYHα was designated YDKα cells. YDK15 cells expressing a mutant mMUTYHβ was designated YDKβ.
Laser scanning fluorescence microscopy
COS-1 cells were cultured on slides and transfected with pcDEBΔ:EGFPm derivatives with the aid of LipofectAMINE (Invitrogen) according to the manufacturer’s instructions. After 48 h of incubation at 37°C, cells were incubated with 2 µM MitoTracker Red CM-H2XROS (Molecular Probes) for 30 min, then the slides were observed under an Eclipse TE300 (Nikon) equipped with a Radiance 2100 laser scanning fluorescence microscope system (Bio-Rad).
Cell fractionation
The preparations of nuclear, cytosolic and mitochondrial fractions were all performed as previously described by Kang et al. (22).
Western blotting analysis
Protein samples were separated by SDS–PAGE, and then were transferred onto Immobilon-P membrane (Millipore), and western blotting analyses were performed as previously described (18), using anti-hMUTYH (13) and anti-mMUTYHβN.
Electron microscopic immunocytochemistry
Thin sections of fixed cells were prepared and processed for electron microscopic immunocytochemistry, as previously described (1,21).
Image processing
All digitized images were processed for publication using Adobe Photoshop 5.5J (Adobe Systems).
Statistical analysis
We performed the statistical analysis using StatView Version 5.0 (SAS Institute).
Purification of recombinant Trx-mMUTYH proteins
E.coli BL21(codonplus RIL) cells transformed with pET32a-mMUTYH were grown in LB medium (250 ml × 12 flasks), which was supplemented with ampicillin (50 µg/ml), 0.2 mM ammonium iron II and 0.2 mM ammonium sulfide, to an OD600 of 0.6–0.7 at 37°C with vigorous shaking. Protein expression was induced by addition of 0.4 mM isopropyl β-d-thiogalactoside (IPTG) to the cultures and incubation at 20°C for 10 h. The cells were washed, harvested and stored at –80°C. The cells (13 g wet weight from 3 liter culture) were suspended at room temperature for 30 min in 36 ml of buffer B containing 200 mM KCl, 5 mM β-mercaptoethanol and 20 mM HEPES–KOH (pH 7.8) with 0.1 mM PMSF, leupeptin (0.5 µg/ml), pepstatin (0.5 µg/ml), and chymostatin (0.5 µg/ml). The cells were disrupted by sonication and the cell lysates were clarified by centrifugation at 40 000 g for 30 min at 4°C. Two milliliters of 50% suspension of TALON Superflow metal affinity resin (Clontech) equilibrated with buffer B, was mixed with the cell lysate (FI, 20 ml) and gently rotated for 20 min at 4°C. Next, the resin was washed twice with 20 ml of buffer B containing 10 mM imidazole, and the resin was transferred into a column, washed with 10 ml of the same buffer. Elution was performed with buffer B containing 150 mM imidazole. Fractions (FII) containing Trx-MUTYH protein were pooled and proteins were precipitated with 70% saturated ammonium sulfate. Precipitated proteins were resuspended in 100 µl of buffer B containing 500 mM KCl and 10% glycerol. The resuspended proteins were further separated through superose 12/30 (Amersham Biosciences) equilibrated with the same buffer, and 300 µl of fractions were collected. Fractions (FIII) containing a single band of Trx-MUTYH were stored at –80°C.
Determination of the protein concentration
The protein concentration was determined using a Bio-Rad protein assay kit (Bio-Rad) and BSA as the standard.
Nicking assay
Double-stranded oligonucleotide substrates summarized in Table 1 were prepared and then the standard nicking assay was performed, as previously described (2,13,15). Briefly, duplex oligonucleotides (20 nM) were incubated in 12.5 µl of a reaction mixture containing 10 mM Tris–HCl (pH 7.6), 5 µM ZnCl2, 0.5 mM DTT, 0.5 mM EDTA, 1.5% glycerol, 100 µg/ml BSA and 40 nM Trx-mMUTYH at 37°C for 60 min. The reactions were heated in the presence of 0.67 N NaOH at 95°C for 5 min, and the products were extracted with phenol/chloroform, and precipitated with ethanol. Precipitated products were dissolved in 30 µl of 40% formamide containing 3 µg/ml BlueDextran (Sigma) and 10 mM EDTA, then 3 µl of the mixture was fractionated on 8% long Ranger denatured gel (24 cm length) containing 7 M urea at 30 W for 2 h.
RESULTS
Alternative splicing transcripts from Mutyh gene
To examine whether the mouse Mutyh gene encodes multiple transcripts as with the human MUTYH gene, we performed RT–PCR analysis of the total RNA prepared from mouse ES cells using two different sets of primers, mY5A1 + mY3TGA, and mY5ATG + mY3TGA, and an additional smaller cDNA was amplified with the latter set of primers, which cover the entire open reading frame of mMUTYH, in addition to the major 1.55 kb band which corresponds to the size expected from Mutyh cDNAs reported (Fig. 1A and B) (2). To obtain sequences for most 5′-regions of Mutyh mRNA, we next amplified Mutyh cDNA from mouse spleen cDNA library constructed in λZAPII vector, by nested PCR using two sets of primers (YN13F + mY3A2, YN7 + mY3A1), in which YN13F and YN7 hybridized to the multi-cloning site of the vector. Multiple bands were amplified and those were subcloned and sequenced (Fig. 1C). Sequence analysis revealed that there are three different Mutyh cDNAs (368, 440, 533 bp) corresponding to the bands shown with arrowheads in Figure 1C, with or without 93- and/or 74-base insertion, and that other smaller bands are incompletely elongated Mutyh cDNAs.
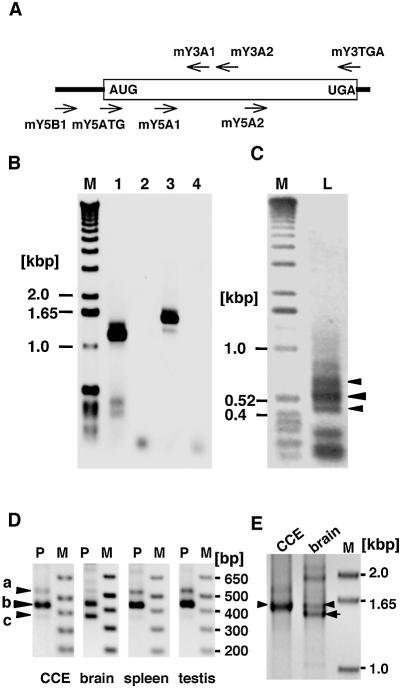
Figure 1.
The detection of multiple forms of Mutyh mRNA in mouse embryonic stem cells and tissues. (A) Schematic diagram of Mutyh mRNA structure and primers used for the amplification of its cDNA. The primer sequences are listed in Table 1. (B) RT–PCR analysis of total RNA prepared from a mouse ES cell line, CCE28 cells. cDNA prepared from total RNA were amplified using two different sets of primers, mY5A1 + mY3TGA (lanes 1, 2), and mY5ATG + mY3TGA (lanes 3, 4). Template cDNA was omitted in the reactions for lanes 2 and 4. Lane M, markers. (C) Amplification of 5′-region of Mutyh cDNA. The mouse spleen cDNA library in λZAPII was initially amplified using a set of primers, YN13F + mY3A2, then a nested PCR was performed using a set of primers, YNK + mY3A1. YN13F and YNK hybridize to the multi-cloning site in the vector. Lane M, markers; lane L, PCR product. Three major PCR products are shown with arrowheads. (D) Detection of three types of Mutyh mRNA in ES cells and mouse tissues. cDNA prepared from total RNA extracted from CCE28 cells (CCE), brain, spleen and testis of BALB/c mice were amplified using a primer set of mY5B1 and mY3A1. The bands corresponding to types a, b and c are shown with arrowheads. Lane M, markers; lane P, PCR product. (E) Detection of full-length Mutyh mRNAs from mouse ES cells and tissue. cDNA prepared from total RNA prepared from CCE28 cells (CCE) and the brain were amplified using a set of primers, mY5B1 + mY3TGA, for 40 cycles. Lane M, markers. Arrowheads indicate 1.6 kb bands, and an arrow indicates the smaller cDNA amplified from brain RNA.
Based on the most 5′-sequence of the Mutyh cDNA obtained, we further performed a RT–PCR analysis of total RNA prepared from mouse ES cells and tissues of BALB/c mice using a primer set, mY5B1 + mY3A1. As shown in Figure 1D, three bands corresponding to 529, 436 and 362 bp fragments were detected, and thus transcripts corresponding to these cDNAs were designated as type a, b and c Mutyh mRNA, respectively. In each sample, type b mRNA was a major form of Mutyh mRNA and very low levels of type a and c mRNAs were detected, but in brain, type c was as abundant as type b. These cDNA fragments were again subcloned, and their sequences were confirmed (data not shown). To examine whether there were full-length mRNAs carrying these alternative 5′ sequences, we again performed RT–PCR analysis of total RNA prepared from CCE28 cells and brain using a primer set, mY5B1 + mY3TGA. A major 1.6 kb band corresponding to the full-length type b cDNA was amplified from CCE28 RNA, while a slightly smaller band corresponding to the full-length type c cDNA, as well as the 1.6 kb band was amplified from brain RNA (Fig. 1E), thus confirming that there are at least two types of full-length Mutyh mRNAs in mouse brain.
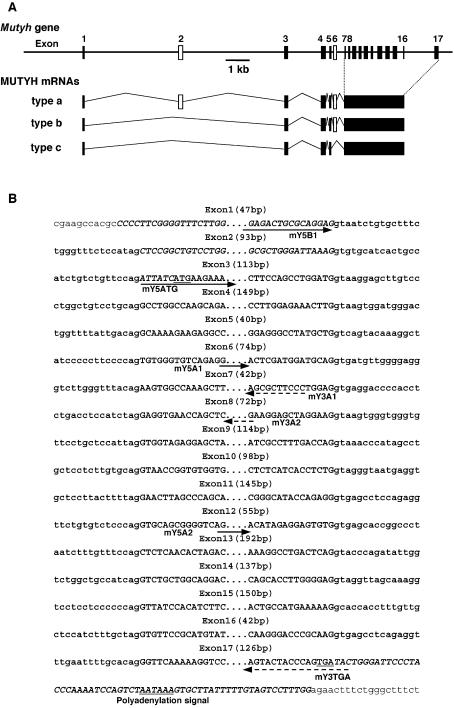
Then we isolated several independent phage clones from a λ phage genomic DNA library derived from 129SvJ mouse, and determined sequences flanking each exon. The Mutyh gene spans 12 kb and consists of 17 exons (Fig. 2B), and sequence comparison with the three types of Mutyh cDNA revealed that exon 2 and exon 6 are alternatively spliced to generate three types of Mutyh transcripts, namely type a, b and c (Fig. 2A). The sequence of each exon–intron junction was completely conserved in Mutyh gene of C57BL/6J strain [S.Blakey (2002) DDBJ/EMBL/GenBank accession no. AL683847].
Figure 2.
Genomic organization and alternative splicing of Mutyh gene. (A) Schematic diagram of the structure of Mutyh gene and its transcripts. The structure of the gene is shown in the upper part. Each exon is shown as a box, and alternative exons, 2 and 6 are shown in open boxes. Three types of alternatively spliced Mutyh mRNAs are shown. (B) The nucleotide sequences of intron/exon boundaries of Mutyh gene. The nucleotide sequences of exons and parts of introns determined by comparison of sequences of three types of Mutyh cDNAs [Y.Nakabeppu and A.Ichinoe (2003) DDBJ/EMBL/GenBank accession nos. AB117937, AB117938 and AB117939] with mouse genomic sequence [S.Blakey (2002) DDBJ/EMBL/GenBank accession no. AL683847], are shown in bold uppercase and lowercase, respectively, and the flanking sequences in plain lowercase. The genomic sequences shown were confirmed by the sequencing of phage clones from a λ phage genomic DNA library derived from 129SvJ mouse. The start of exon 1 was based on the longest EST clone for Mutyh mRNA [Y.Hayashizaki (2002) DDBJ/EMBL/GenBank accession no. BY181655]. The initiation codon ATG, the termination codon TGA and a putative polyadenylation signal are underlined. Positions of primers shown in Figure 1A are also shown with arrows: solid arrows, 5′-side primers; dotted arrows, 3′-side primers.
To compare the expression level of each Mutyh transcript in various mouse tissue, quantitative RT–PCR for Mutyh and Gapdh mRNAs was performed using RNA prepared from C57BL/6J mouse tissues. The expression level of Mutyh mRNA was determined by amplifying the common 3′-region for the three types of Mutyh mRNA with a primer set of mY5A2 and mY3TGA, and was normalized to that of Gapdh mRNA (Fig. 3, top and middle panels). Thymus, small intestine, lung and heart expressed high levels of Mutyh mRNAs, and cerebellum, large intestine, liver and cerebrum expressed slightly lower levels of Mutyh mRNA than did the former. The expression level of Mutyh mRNA in thymus was the highest among all examined tissues.
Figure 3.
The expression of Mutyh mRNA in various mouse tissues. cDNAs were prepared from total RNA extracted from various mouse tissues, and the common 3′-region for the three types of Mutyh mRNA including exons 13–17 were amplified with a set of primers of mY5A2 + mY3TGA (top panel). The relative amount of total Mutyh mRNA in each tissue to that in thymus, which was normalized by the level of Gapdh mRNA (middle panel), is shown under each lane. Data from amplification for 32 cycles are shown, which achieved a linear range of amplification. The 5′-parts of three types of Mutyh cDNA (type a, b, c) were competitively amplified with a primer set of mY5B1 + mY3A1 for each tissue, and data from amplification for 40 cycles are shown (bottom panel).
In order to compare expression levels of the three types of Mutyh mRNAs in each tissue, RT–PCR with a primer set of mY5B1 + mY3A1 was performed (Fig. 3, bottom panel). In most tissues examined, type b mRNA was the major form, while in the cerebellum almost equal amounts of type b and c Mutyh mRNAs were detected. Relatively high levels of type c mRNA were detected in the cerebrum, heart, lung and thymus in addition to cerebellum, while much lower levels of type a Mutyh mRNA were detected in some tissues such as the large intestine. As a result, we concluded that three types of Mutyh mRNA formed by alternative splicing were indeed expressed in vivo, and their expression levels vary among tissues.
Two types of mouse MUTYH protein encoded by alternatively spliced transcripts
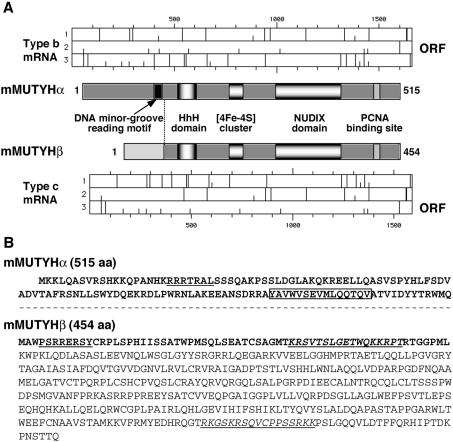
Among the three types of Mutyh mRNAs, type a and type b completely share their open reading frames, indicating that they encode the same mMUTYH protein, namely mMUTYHα (516 aa, MW: 57 686), while type c mRNA shares only the C-terminal part of its open reading frame with those of type a and b (Fig. 4A), and thus encoding mMUTYH protein with a unique N-terminus, designated as mMUTYHβ (429 aa, MW: 47 197) (Fig. 4B). As shown in Figure 5A, bands corresponding to 50–54 kDa, which reacted with anti-hMUTYH antibody, were produced by an in vitro translation of type b Mutyh mRNA, while 45–47 kDa bands were detected in an in vitro translation product of type c Mutyh mRNA by the anti-hMUTYH. mRNA for the nuclear type of hMUTYH produced a polypeptide which co-migrated with mMUTYHα.
Figure 4.
Alternatively spliced transcripts of the Mutyh gene encode two different forms of mMUTYH proteins. (A) Two forms of mMUTYH protein encoded by type b and c Mutyh mRNAs. The functional motifs in mMUTYHα encoded by type a and b mRNAs and mMUTYHβ encoded by type c mRNA are shown in the middle part. mMUTYHβ lacks the DNA minor groove reading motif (black box) present in mMUTYHα (25). An initiation codon (AUG, short bar) and stop codons (long bar) in each three reading frames (1, 2, 3) of type b and type c Mutyh mRNAs are shown in the upper (type b), and the lower part (type c). ORF, open reading frame for each mRNA. (B) The amino acid sequences of mMUTYHα and mMUTYHβ proteins. Differences in the amino acid sequences between the two MUTYH proteins are shown in bold. In MUTYHα, the common sequences with MUTYHβ are omitted (---). MTS-like sequences are underlined, while NLS-like sequences are shown in italic and are underlined. The boxed sequence in mMUTYHα indicates the DNA minor groove reading motif missing in mMUTYHβ.
Figure 5.
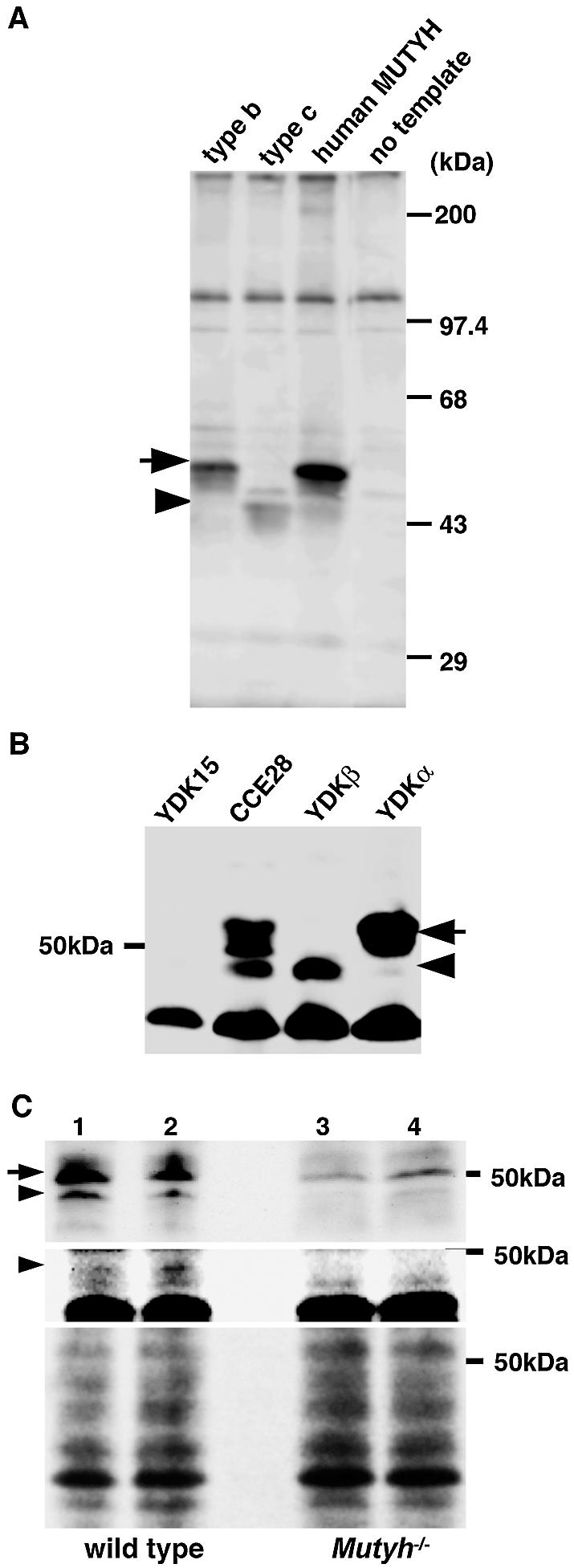
Two forms of mMUTYH protein encoded by the alternatively spliced transcripts. (A) In vitro translation of type b and type c Mutyh mRNAs. RNAs synthesized from pT7Blue plasmids carrying type b and type c Mutyh cDNA or human MUTYH cDNA for nuclear hMUTYH (13) by T7 RNA polymerase were translated using rabbit reticulocyte lysate, and translation products were subjected to a western blot analysis with anti-hMUTYH antibody. No template, during in vitro translation, template RNA was omitted. An arrow indicates 50 kDa mMUTYHα encoded by type b mRNA, and an arrowhead indicates the 47 kDa mMUTYHβ encoded by type c mRNA, respectively. (B) The detection of two forms of MUTYH in mouse ES cells. Whole cell extracts prepared from wild-type ES cell line, CCE28 cells, MUTYH-null YDK15 cells, and YDKα or YDKβ cells to which an expression construct for type b or type c cDNA was stably introduced, respectively, were subjected to western blot analysis with anti-hMUTYH antibody. An arrow indicates mMUTYHα and an arrowhead indicates mMUTYHβ, respectively. (C) The detection of two forms of mMUTYH protein in mouse thymocytes. Thymocyte extracts prepared from two independent wild-type (lanes 1, 2) and MUTYH-null mice (Mutyh–/–; lanes 3, 4) were subjected to western blot analysis with anti-hMUTYH antibody (top panel), or with anti-mMUTYHβN (middle panel). An arrow indicates mMUTYHα and an arrowhead indicates mMUTYHβ, respectively. Stained filter with Coomassie Brilliant Blue is shown (bottom) for loading control.
By western blotting analysis using anti-hMUTYH (Fig. 5B), two upper bands whose sizes were approximately 50–54 kDa and one lower band corresponding to a 47 kDa polypeptide were detected in the wild-type ES cell line, CCE28 cells but not a MUTYH-null ES cell line, YDK15 cells. YDKα cells which received an expression vector for type b Mutyh cDNA restored expression of the upper bands but not the lower band, while YDKβ cells which received an expression vector for type c Mutyh cDNA restored only expression of the lower band, thus confirming that the two forms of mMUTYH protein encoded by alternatively spliced Mutyh mRNAs were indeed expressed in ES cells. The expression of the two forms of mMUTYH protein was confirmed in thymocytes prepared from wild-type but not MUTYH-null mice (Fig. 5C), although in MUTYH-null thymocytes, there was a minor 50 kDa band reactive with anti-hMUTYH. We raised antibodies against the amino-terminal region of mMUTYHβ, namely anti-mMUTYHβN, and the anti-mMUTYHβN detected a 47 kDa band only in wild-type thymocytes, thus supporting the expression of mMUTYHβ in wild-type mice, as expected from the RT–PCR analysis findings shown in Figure 3.
Subcellular localization of two forms of mMUTYH protein in mouse ES cells
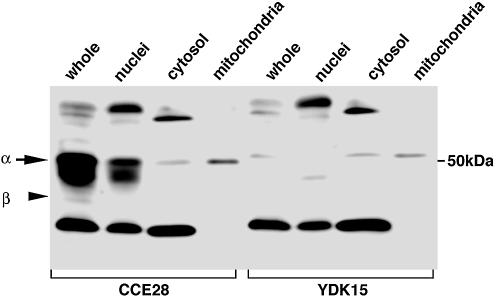
Whole cell extracts, nuclear, cytosolic and mitochondrial fractions were prepared from CCE28 and YDK15 cells, and subjected to western blot analysis using anti-hMUTYH. As shown in Figure 6, a few bands were detected in each fraction, and 50–54 kDa bands detected only in CCE28 cells were mostly recovered in the nuclear fraction, and some in the mitochondrial fraction. However, a faint 54 kDa band was also seen in YDK15 cells. It is thus likely that a mitochondrial mMUTYH, if any, comigrates with the nonspecific band detected in YDK15 cells. A 47 kDa mMUTYHβ, was hardly detected in any fraction, because its expression level was very low.
Figure 6.
Subcellular localization of mMUTYH in mouse ES cells. Whole cell lysate (whole, 100 µg protein), nuclear fraction (nuclei, 100 µg protein), cytosolic fraction (cytosol, 25 µg protein) and mitochondrial fraction (mitochondria, 5 µg protein) prepared from wild-type CCE28 cells and MUTYH-null YDK15 cells were subjected to western blotting with anti-hMUTYH.
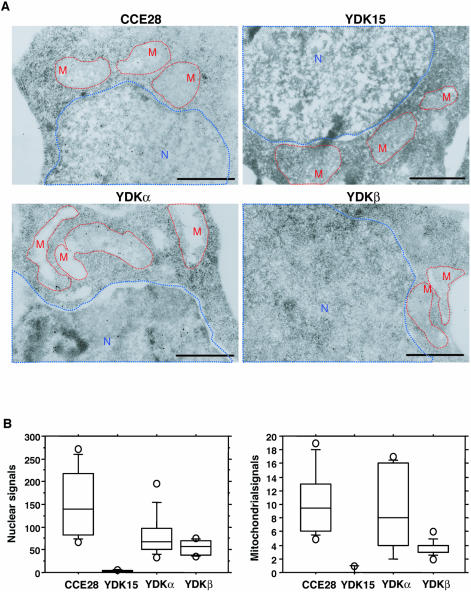
To further evaluate the subcellular localization of mMUTYH in mouse cells, we next prepared thin sections from fixed CCE28, YDK15, YDKα and YDKβ cells and the intracellular localization of the authentic mMUTYH was analyzed by electron microscopic immunocytochemistry, using anti-hMUTYH in combination with protein A-gold (Fig. 7A). In each of the nuclear and mitochondrial regions from CCE28 cells, specific signals were observed. However, no such signal was observed in YDK15 cells, thus confirming that mMUTYH is indeed localized in the mitochondria as well as in nuclei.
Figure 7.
Intracellular localization of mMUTYH proteins. (A) Electron microscopic immunocytochemistry. Thin sections were prepared from fixed wild-type CCE28 cells, MUTYH-null YDK15 cells, mMUTYHα-expressing YDKα cells, and mMUTYHβ-expressing YDKβ cells. MUTYH signals were examined by electron microscopic immunocytochemistry, using anti-hMUTYH. Nuclear and mitochondrial structures are shown with blue and red dotted lines, respectively. Bars: 1 μm. (B) The distribution of MUTYH signals in nuclear and mitochondrial region determined by electron microscopic immunocytochemistry. Gold signals present in nuclear and mitochondrial regions as seen in (A), from 10 sections of each sample were counted, and the number of signals in nuclear or mitochondrial region per section is shown in a box-and-whisker-plot. Each plot shows a box with ends at quartiles, and the statistical median as a horizontal line in the box. The whiskers extend to the farthest points that are not outliers (circles). Both in nuclei and mitochondria, the number of gold signals detected in CCE28, YDKα and YDKβ cells were significantly higher than those in YDK cells (Mann–Whitney U-test, P ≤ 0.0002).
In YDKα and YDKβ cells, slightly less signals were observed both in nuclei and mitochondria. We counted the number of signals in the nuclear and mitochondrial regions in each section (Fig. 7B), and the number of both nuclear and mitochondrial signals in CCE28, YDKα and YDKβ cells were significantly higher than those in YDK15 cells.
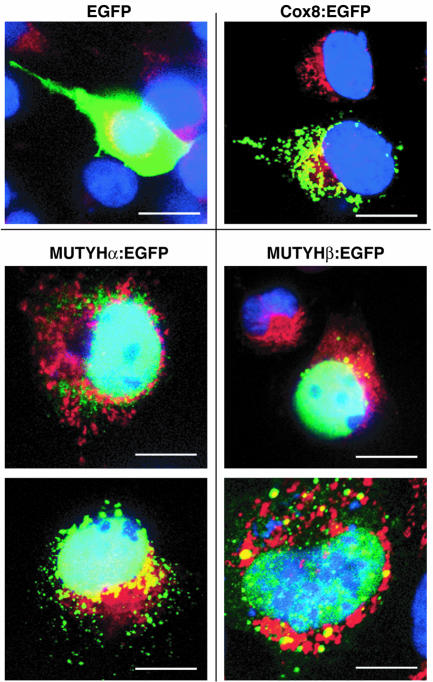
Recombinant mMUTYHα and mMUTYHβ fused to EGFP were expressed in COS-1 cells, and their fluorescence was mostly detected in the nuclei and to a lesser extent in the cytoplasm, and was partly merged with MitoTracker, a mitochondrial fluorescent marker (Fig. 8). EGFP itself was evenly distributed in COS-1 cells, while EGFP fused to COX8 mitochondrial targeting sequence was exclusively localized in mitochondria, thus indicating that mMUTYHα and mMUTYHβ are both mostly localized in the nuclei and to a much lesser extent in the mitochondria in mouse ES cells.
Figure 8.
Expression of Mutyh cDNA in COS-1 cells. COS-1 cells transfected with plasmids encoding EGFP, COX8:EGFP, mMUTYHα:EGFP and mMUTYHβ:EGFP were incubated with MitoTracker (red) and fixed, then nuclei were stained with DAPI (blue). Cells were subjected to laser- scanning fluorescence microscopy. EGFP signals are shown in green and merged signals with MitoTracker (red) are seen in yellow. Bars: 20 µm.
Mouse MUTYHα specifically excises adenine opposite 8-oxoguanine and guanine, but lacks AP lyase activity
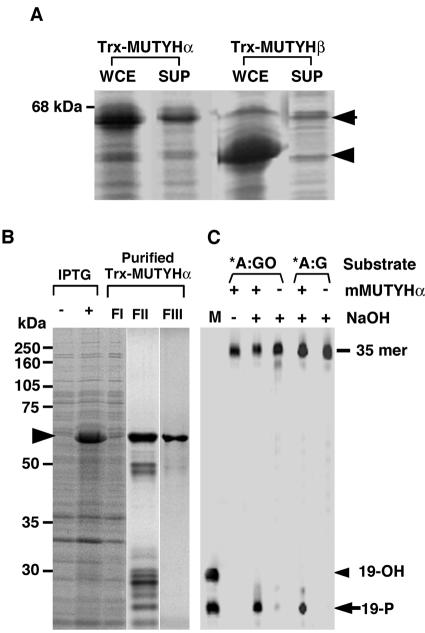
In order to characterize the biochemical properties of mMUTYHα and mMUTYHβ, we expressed these proteins as thioredoxin fusion proteins (Trx-mMUTYHα, Trx-mMUTYHβ) in E.coli. As shown in Figure 9A, both fusion proteins were abundantly expressed, however only a part of Trx-mMUTYHα, but not Trx-mMUTYHβ, was able to be recovered in a soluble fraction. We purified Trx-mMUTYHα through two-step column chromatography to near homogeneity (Fig. 9B).
Figure 9.
Expression and purification of recombinant mMUYTH proteins. (A) The expression of recombinant mMUTYH proteins. E.coli cells transformed with pET32a-mMUTYHα and pET32a-mMUTYHβ were grown in the presence of IPTG, and harvested cells were disrupted with sonication as described in Materials and Methods. Whole cell extracts (WCE) or supernatant after centrifugation (SUP) were subjected to western blotting with anti-hMUTYH. (B) Purification of recombinant Trx-mMUTYHα. E.coli cells transformed with pET32a-mMUTYHα were grown in the presence (+) or absence (–) of IPTG, and supernatant fraction (FI) was prepared from the harvested cells. Trx-mMUTYHα protein was purified using cobalt beads (FII) followed by gel filtration (FIII), as described in Materials and Methods. Each fraction was analyzed by SDS–PAGE and the gel was stained with Coomassie Brilliant Blue. (C) Biochemical characterization of purified Trx-mMUTYHα. Duplex oligonucleotide substrates containing A:8-oxoG (*A:GO) and adenine:guanine (*A:G) pairs, in which the 5′-end of one strand carrying target base adenine (*A) was labeled with FAM, were incubated with (+) or without (–) the purified Trx-mMUTYHα protein (FIII), as described in Materials and Methods, and the reaction products were fractionated by gel electrophoresis with (+) or without (–) NaOH treatment. M, marker oligonucleotides whose 5′-end was labeled with FAM (19-OH and 19-P).
The activities introducing a single strand nick into substrates containing adenine opposite 8-oxoG, or adenine opposite guanine, were detected in the purified Trx-mMUTYHα preparation, only after NaOH treatment (Fig. 9C). The cleaved product after NaOH treatment comigrated with a marker oligonucleotide with the 3′-phosphate group (19-P) but not with the 3′-hydroxy group (19-OH), indicating that Trx-mMUTYHα does not possess AP-lyase activity at all.
DISCUSSION
Among three genes, MTH1, OGG1 and MUTYH which play essential roles in preventing spontaneous mutagenesis due to oxidative damage in nucleic acids, MUTYH gene has been widely noticed because of its alteration in patients with autosomal recessive colorectal adenomatous polyposis and colorectal cancer (9–12). We recently demonstrated that a disruption of Mutyh gene in mouse embryonic stem cells resulted in a mutator phenotype (2), and we have obtained data supporting the view that MUTYH-null mice are indeed predisposed to colorectal adenomatous polyposis and colorectal cancer (K.Sakamoto, Y.Tominaga, K.Yamauchi, Y.Nakatsu, K.Sakumi, K.Yoshiyama, A.Asaeda, A.Egashira, S.Kura, T.Yao, M.Tsuneyoshi, H.Maki, Y.Nakabeppu and T.Tsuzuki, manuscript in preparation). In the present study, we intensively characterized the expression of mouse Mutyh gene and its products both in embryonic stem cells and various mouse tissues, to gain an understanding of the physiological role of MUTYH protein in vivo.
The mouse Mutyh gene is located on chromosome 4, and consists of 17 exons among which exons 2 and 6 are alternatively spliced to generate three types of transcripts, type a, b and c. Type a and b transcripts of Mutyh produce 50 kDa polypeptide, namely mMUTYHα, while type c produces 47 kDa polypeptide, mMUTYHβ. In human cells, the MUTYH gene encodes more than 10 transcripts generated by alternative transcription initiation as well as alternative splicing, and those mRNAs produce more than seven different forms of hMUTYH proteins (13). We and others have demonstrated that hMUTYH polypeptide (522 aa) encoded by type β3, β5 or γ3 mRNAs are localized in the nucleus and other isoforms of hMUTYH (546 aa, 536 aa, 535 aa) encoded by type α1, α2 and α3 mRNA are localized in the mitochondria, and that the latter possess MTS at their amino terminal ends, while the former, which are translated from an initiation codon corresponding to the second AUG in the latter, lack MTS (13,14). There is a further amino-terminally truncated form of hMUTYH (429 aa) encoded by type α4 and type γ4 mRNAs in human cells (13). mMUTYHα corresponds to the nuclear form of hMUTYH, while mMUTYHβ corresponds to the last form of hMUTYH whose expression is also high in brain.
We demonstrated the expression of two forms of mMUTYH polypeptide in mouse ES cells and thymocytes as well as expression of three forms of Mutyh mRNA in various mouse tissues. The expression levels of Mutyh mRNAs are highest in the thymus, small intestine and lung among the tissues examined, and in any tissue, type b mRNA encoding mMUTYHα is a major form, while type a also encoding mMUTYHα is a minor form. The expression levels of type c mRNA encoding mMUTYHβ are highest in the cerebellum, cerebrum, heart and lung, thus suggesting that the efficiency of alternative splicing to produce each transcript varies among those tissues.
In ES cells and thymocytes, mMUTYHα is more abundant than mMUTYHβ, and both forms are mostly localized in the nuclei, and to some extent in the mitochondria in ES cells. Initially, we could not deduce any MTS-like sequence in either mMUTYHα or mMUTYHβ, however, we now suggest that both forms of mMUTYH protein possess different MTS-like sequences as shown in Figure 4B, which were predicted using the iPSORT prediction system (http://hypothesiscreator.net/iPSORT). In addition, it was predicted that both forms share a typical bipartite nuclear localization signal (NLS) in the common C-terminal region while mMUTYHβ possesses an additional bipartite NLS in its unique N-terminal region, by PSORT II server (http://psort.nibb.ac.jp) (Fig. 4B). Probably because of these NLSs, the majority of both forms of mMUTYH seem to be localized in the nuclei. Since oxidative stress is likely to be very high in the mitochondria, and thus the level of 8-oxoG in mitochondrial DNA is known to be higher than that in nuclear DNA (23), mitochondria come equipped with defense enzymes such as OGG1, MUTYH and MTH1 which play essential roles in avoiding errors caused by 8-oxoG (16). Human cell is equipped with hOGG1-2a which is exclusively located in mitochondria (15), however, only one form of OGG1 in rodent cell is likely to be located both in mitochondria as well as nuclei (8). Our results indicate that MUTYH protein also behaves similarly in rodent cells, suggesting that rodent cells may have a different sorting mechanism(s) for such repair proteins between nuclei and mitochondria. Nevertheless, our data indicate that MUTYH plays an important role in maintaining the genetic information in the mitochondrial genome as well as in the nuclear genome in mammalian cells.
To investigate the biochemical function of the two forms of mMUTYH protein, we prepared their recombinant form; however, we could only obtain mMUTYHα as a soluble form. Using recombinant protein, we demonstrated that mMUTYHα efficiently excises adenine opposite 8-oxoG and guanine as expected, but it lacks AP lyase activity. In the present study, we could not show any biochemical activity of mMUTYHβ, because of its unavailability in vitro. We expected that mMUTYHβ expressed in mouse ES cells may be available for such an in vitro study: however, it was somehow hard to establish cell lines expressing a large amount of mMUTYHβ in comparison to mMUTYHα. As a result, other expression systems should be examined for this purpose. It is noteworthy that the expression level of type c mRNA in brain or especially cerebellum is higher than in other tissues, thus suggesting that mMUTYHβ has a tissue-specific function. It was recently reported that hypoxia induces expression of MUTYH in rat brain, and further that there are at least two isoforms of rat MUTYH (57, 50 kDa) and the 50 kDa isoform is abundant in neuronal mitochondria in the cerebrum and cerebellum (17,24).
It is very likely that the 50 kDa isoform of rat MUTYH corresponds to mMUTYHβ thus suggesting that mMUTYHβ or its equivalents in human and rat may have some function to protect the brain or neurons from DNA damage in mitochondria caused by hypoxia. Judging by their amino acid sequence (Fig. 4), such MUTYH isoforms may retain their binding activity to their substrate; however, it still remains to be clarified as to whether or not they retain DNA glycosylase activity, because they all lack the DNA minor groove reading motif (25).
It has now been established that patients with MUTYH mutations in both alleles are highly predisposed to tumorigenesis in the colon, and our long-term observation of MUTYH-null mice is likely to support this observation. In mice, the highest expression level of type b Mutyh mRNA was observed in the small intestine and to a lesser extent in the large intestine, where no type c mRNA was detected, thus suggesting that MUTYHα may be sufficient to prevent the occurrence of mutations in these tissues.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr Masato Furuichi for helpful discussions, Akemi Matsuyama and Masafumi Sasaki for technical assistance, and Dr B. Quinn for comments on the manuscript. This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (grant numbers: 15025257, 10480218, 1169420).
DDBJ/EMBL/GenBank accession nos+ AB117937–AB117939
REFERENCES
- 1.Yoshimura D., Sakumi,K., Ohno,M., Sakai,Y., Furuichi,M., Iwai,S. and Nakabeppu,Y. (2003) An oxidized purine nucleoside triphosphatase, MTH1 suppresses cell death caused by oxidative stress. J. Biol. Chem., 278, 37965–37973. [DOI] [PubMed] [Google Scholar]
- 2.Hirano S., Tominaga,Y., Ichinoe,A., Ushijima,Y., Tsuchimoto,D., Honda-Ohnishi,Y., Ohtsubo,T., Sakumi,K. and Nakabeppu,Y. (2003) Mutator phenotype of MUTYH-null mouse embryonic stem cells. J. Biol. Chem., 278, 38121–38124. [DOI] [PubMed] [Google Scholar]
- 3.Nakabeppu Y., Tominaga,Y., Tsuchimoto,D., Ide,Y., Hirano,S., Sakai,Y., Sakumi,K. and Furuichi,M. (2001) Mechanisms protecting genomic integrity from damage caused by reactive oxygen species: implications for carcinogenesis and neurodegeneration. Environ. Mutagen. Res., 23, 197–209. [Google Scholar]
- 4.Sakumi K., Tominaga,Y., Furuichi,M., Xu,P., Tsuzuki,T., Sekiguchi,M. and Nakabeppu,Y. (2003) Ogg1 knockout-associated lung tumorigenesis and its suppression by Mth1 gene disruption. Cancer Res., 63, 902–905. [PubMed] [Google Scholar]
- 5.Shibutani S., Takeshita,M. and Grollman,A.P. (1991) Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature, 349, 431–434. [DOI] [PubMed] [Google Scholar]
- 6.Michaels M.L., Cruz,C., Grollman,A.P. and Miller,J.H. (1992) Evidence that MutY and MutM combine to prevent mutations by an oxidatively damaged form of guanine in DNA. Proc. Natl Acad. Sci. USA, 89, 7022–7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tajiri T., Maki,H. and Sekiguchi,M. (1995) Functional cooperation of MutT, MutM and MutY proteins in preventing mutations caused by spontaneous oxidation of guanine nucleotide in Escherichia coli. Mutat. Res., 336, 257–267. [DOI] [PubMed] [Google Scholar]
- 8.Boiteux S. and Radicella,J.P. (1999) Base excision repair of 8-hydroxyguanine protects DNA from endogenous oxidative stress. Biochimie, 81, 59–67. [DOI] [PubMed] [Google Scholar]
- 9.Al-Tassan N., Chmiel,N.H., Maynard,J., Fleming,N., Livingston,A.L., Williams,G.T., Hodges,A.K., Davies,D.R., David,S.S., Sampson,J.R. et al. (2002) Inherited variants of MYH associated with somatic G:C→T:A mutations in colorectal tumors. Nature Genet., 30, 227–232. [DOI] [PubMed] [Google Scholar]
- 10.Jones S., Emmerson,P., Maynard,J., Best,J.M., Jordan,S., Williams,G.T., Sampson,J.R. and Cheadle,J.P. (2002) Biallelic germline mutations in MYH predispose to multiple colorectal adenoma and somatic G:C→T:A mutations. Hum. Mol. Genet., 11, 2961–2967. [DOI] [PubMed] [Google Scholar]
- 11.Sieber O.M., Lipton,L., Crabtree,M., Heinimann,K., Fidalgo,P., Phillips,R.K., Bisgaard,M.L., Orntoft,T.F., Aaltonen,L.A., Hodgson,S.V. et al. (2003) Multiple colorectal adenomas, classic adenomatous polyposis and germ-line mutations in MYH. N. Engl. J. Med., 348, 791–799. [DOI] [PubMed] [Google Scholar]
- 12.Sampson J.R., Dolwani,S., Jones,S., Eccles,D., Ellis,A., Evans,D.G., Frayling,I., Jordan,S., Maher,E.R., Mak,T. et al. (2003) Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet, 362, 39–41. [DOI] [PubMed] [Google Scholar]
- 13.Ohtsubo T., Nishioka,K., Imaiso,Y., Iwai,S., Shimokawa,H., Oda,H., Fujiwara,T. and Nakabeppu,Y. (2000) Identification of human MutY homolog (hMYH) as a repair enzyme for 2-hydroxyadenine in DNA and detection of multiple forms of hMYH located in nuclei and mitochondria. Nucleic Acids Res., 28, 1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takao M., Zhang,Q.M., Yonei,S. and Yasui,A. (1999) Differential subcellular localization of human MutY homolog (hMYH) and the functional activity of adenine:8-oxoguanine DNA glycosylase. Nucleic Acids Res., 27, 3638–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishioka K., Ohtsubo,T., Oda,H., Fujiwara,T., Kang,D., Sugimachi,K. and Nakabeppu,Y. (1999) Expression and differential intracellular localization of two major forms of human 8-oxoguanine DNA glycosylase encoded by alternatively spliced OGG1 mRNAs. Mol. Biol. Cell, 10, 1637–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakabeppu Y. (2001) Regulation of intracellular localization of human MTH1, OGG1 and MYH proteins for repair of oxidative DNA damage. Prog. Nucleic Acid Res. Mol. Biol., 68, 75–94. [DOI] [PubMed] [Google Scholar]
- 17.Englander E.W., Hu,Z., Sharma,A., Lee,H.M., Wu,Z.H. and Greeley,G.H. (2002) Rat MYH, a glycosylase for repair of oxidatively damaged DNA, has brain-specific isoforms that localize to neuronal mitochondria. J. Neurochem., 83, 1471–1480. [DOI] [PubMed] [Google Scholar]
- 18.Tsuchimoto D., Sakai,Y., Sakumi,K., Nishioka,K., Sasaki,M., Fujiwara,T. and Nakabeppu,Y. (2001) Human APE2 protein is mostly localized in the nuclei and to some extent in the mitochondria, while nuclear APE2 is partly associated with proliferating cell nuclear antigen. Nucleic Acids Res., 29, 2349–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakabeppu Y. and Nathans,D. (1991) A naturally occurring truncated form of FosB that inhibits Fos/Jun transcriptional activity. Cell, 64, 751–759. [DOI] [PubMed] [Google Scholar]
- 20.Nakabeppu Y., Oda,S. and Sekiguchi,M. (1993) Proliferative activation of quiescent Rat-1A cells by delta FosB. Mol. Cell. Biol., 13, 4157–4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ide Y., Tsuchimoto,D., Tominaga,Y., Iwamoto,Y. and Nakabeppu,Y. (2003) Characterization of the genomic structure and expression of the mouse Apex2 gene. Genomics, 81, 47–57. [DOI] [PubMed] [Google Scholar]
- 22.Kang D., Nishida,J., Iyama,A., Nakabeppu,Y., Furuichi,M., Fujiwara,T., Sekiguchi,M. and Takeshige,K. (1995) Intracellular localization of 8-oxo-dGTPase in human cells, with special reference to the role of the enzyme in mitochondria. J. Biol. Chem., 270, 14659–14665. [DOI] [PubMed] [Google Scholar]
- 23.Ames B., Shigenaga,M. and Hagen,T. (1993) Oxidants, antioxidants and the degenerative diseases of aging. Proc. Natl Acad. Sci. USA, 90, 7915–7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee H.M., Wang,C., Hu,Z., Greeley,G.H., Makalowski,W., Hellmich,H.L. and Englander,E.W. (2002) Hypoxia induces mitochondrial DNA damage and stimulates expression of a DNA repair enzyme, the Escherichia coli MutY DNA glycosylase homolog (MYH), in vivo, in the rat brain. J. Neurochem., 80, 928–937. [DOI] [PubMed] [Google Scholar]
- 25.Guan Y., Manuel,R.C., Arvai,A.S., Parikh,S.S., Mol,C.D., Miller,J.H., Lloyd,S. and Tainer,J.A. (1998) MutY catalytic core, mutant and bound adenine structures define specificity for DNA repair enzyme superfamily. Nat. Struct. Biol., 5, 1058–1064. [DOI] [PubMed] [Google Scholar]