Abstract
We have developed a method for direct, copper-catalyzed, auxiliary-assisted fluorination of β-sp2 C-H bonds of benzoic acid derivatives and γ-sp2 C-H bonds of α,α-disubstituted benzylamine derivatives. The reaction employs CuI catalyst, AgF fluoride source, and DMF, pyridine, or DMPU solvent at moderately elevated temperatures. Selective mono- or difluorination can be achieved by simply changing reaction conditions. The method shows excellent functional group tolerance and provides a straightforward way for the preparation of ortho-fluorinated benzoic acids.
Fluoroaromatic compounds possess inertness, high chemical, thermal, and metabolic stability, as well as unique electronic properties. They are widely used as pharmaceuticals, agrochemicals, and imaging materials.1 Classical methods for synthesis of fluoroaromatics such as Balz-Schiemann reaction employ prefunctionalized starting materials and typically require harsh reaction conditions thus limiting the scope of transformations.2,3 More recently, aryl-fluorine bonds have been created by using transition-metal catalysis. Specifically, Buchwald has shown that aryl triflates can be converted to aryl fluorides under palladium catalysis.4 Ritter has developed methods for stannane, boronic acid, and phenol conversion to fluoroaromatics.5 Hartwig has shown that aryl iodides can be converted to aryl fluorides by a combination of stoichiometric copper(I) complex and AgF.6 Aryl stannanes and aryl borates have been converted to aryl fluorides by employing Cu(I) in combination with electrophilic fluoride source.7 Many of these processes have been developed based on mechanistic studies.8 However, these methods require use of prefunctionalized starting materials. Non-directed fluorination of sp3 C-H bonds by radical methods has also been investigated.9 Only a few groups have reported directed catalytic sp2 C-H bond fluorination. In a pioneering work, Sanford has shown that pyridine directing group can be employed for a palladium-catalyzed arene fluorination by electrophilic fluorine sources. Mechanistic studies support involvement of high-valent palladium complexes in these reactions.10 Yu has demonstrated palladium-catalyzed benzylamine triflamide and benzoic acid perfluoroaniline amide ortho-fluorination by N-fluoropyridine derivatives.11 A recent paper by Sanford has shown that Pd(OAc)2/AgF/PhI(OPiv)2 system12 can be employed for 8-methylquinoline benzylic C-H bond fluorination.13 A general method for directed arene C-H bond fluorination by employing a first-row transition metal catalyst has not yet been reported.15 We disclose here a method for aminoquinoline and picolinamide-directed benzoic acid and benzylamine derivative ortho-fluorination under copper catalysis.
In 2005, we introduced 8-aminoquinoline and picolinic acid auxiliaries for palladium-catalyzed sp2 and sp3 C-H bond arylation.14a Recently copper-catalyzed sulfenylation and amination of C-H bonds in 8-aminoquinoline benzamides and benzylamine picolinamides was demonstrated.14b,c We hypothesized that 8-aminoquinoline and picolinic acid auxiliaries would promote copper-catalyzed ortho-fluorination of sp2 C-H bonds based on the following considerations: (1) copper-catalyzed aryl halide fluorination has been reported in a macrocyclic polyamine system,16a (2) copper-promoted C-H activation has been reported in the same system,16b and (3) it appears that both macrocyclic amine and 8-aminoquinoline benzamide ligands stabilize high-valent copper intermediates.
The reaction of 8-aminoquinoline p-trifluoromethyl-benzamide was investigated with respect to copper catalyst, fluorine source, oxidant, and solvent (Table 1). Best results for monofluorination were obtained by employing CuI catalyst, NMO oxidant, and DMF solvent. Use of PhI(OPiv)2 oxidant resulted in lower yields (entry 2). Dimethyl sulfoxide solvent is inferior to DMF due to starting material decomposition (entry 1 vs. 4). Reaction can be run in pyridine (entry 7) which slows decomposition of starting material, albeit at the expense of reaction rate. Selective difluorination can be achieved by employing higher loading of CuI (entries 9–11). Longer reaction times require the use of pyridine additive (2 equiv) to prevent decomposition of amide substrate (entry 11).
Table 1.
Optimization of Reaction Conditionsa

| |||||
|---|---|---|---|---|---|
| Entry | Catalyst (mol %) | T, °C | 1, % | 2, % | 3, % |
| 1b | Cu(OAc)2 (25%) | 100 | 4 | 17 | 5 |
| 2e,f | Cu(OAc)2 (25%) | 100 | <2 | 13 | 4 |
| 3 | Cu(OAc)2 (25%) | 100 | 20 | 42 | 8 |
| 4 | CuI (25%) | 100 | 11 | 54 | 6 |
| 5 | CuI (25%) | 80 | 20 | 60 | 5 |
| 6 | CuI (15%) | 80 | 18 | 70 | 3 |
| 7d | CuI (15%) | 80 | 4 | 79 | 7 |
| 8d,e | CuI (15%) | 80 | 16 | 80 | 3 |
| 9f | CuI (20%) | 80 | <2 | 17 | 38 |
| 10d,e | CuI (20%) | 80 | 10 | 52 | 33 |
| 11f,g | CuI (20%) | 80 | <2 | 5 | 78 |
Amide 0.25 mmol, AgF 3 equiv, NMO 4 equiv, DMF 1 mL. Yields were determined by GC analysis.
DMSO solvent.
PhI(OPiv)2 oxidant instead of NMO.
AgF 4 equiv, NMO 5 equiv.
Pyridine solvent.
AgF 6 equiv, 8 equiv NMO, 1.5 h.
Pyridine additive 2 equiv.
Reaction scope with respect to monofluorination of 8-aminoquinoline benzamides is presented in Table 2. Both electron-rich (entries 2 and 6) as well as electron-poor (entries 1, 3–5, 7, 8) benzamides are reactive. Heterocyclic carboxamides containing indole (entry 9) and pyridine (entry 10) moieties are fluorinated in good yields. The reaction is functional group tolerant, with carboxylate (entry 3), nitrile (entry 4), and nitro groups (entry 7) compatible with the fluorination conditions. For strongly electron-deficient substrates such as 4-nitrobezoyl and pyridyl derivatives (entries 7 and 10) reaction has to be run in pyridine solvent to prevent decomposition of product. However, somewhat longer reaction times are required if pyridine solvent is employed.
Table 2.
Monofluorination of Carboxylic Acid Derivativesa

| |||
|---|---|---|---|
| entry | Ar | product | yield, % |
| 1 | 4-CF3C6H4 |

|
71 |
| 2 | 4-MeC6H4 |
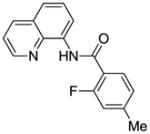
|
75 |
| 3 | 4-MeO2CC6H4 |
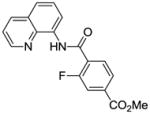
|
56 |
| 4 | 4-NCC6H4 |

|
62 60b |
| 5 | 2-CF3C6H4 |
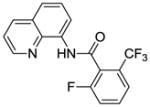
|
80 |
| 6 | 2-MeC6H4 |
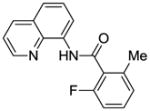
|
63 |
| 7c | 4-NO2C6H4 |
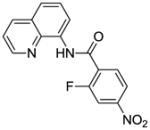
|
60 |
| 8 | 3-CF3C6H4 |

|
71 |
| 9 | 3-(N-Me-indolyl) |

|
54 |
| 10c | 4-Pyridyl |

|
62 |
Amide 0.25 mmol, DMF 1 mL. Yields are isolated yields. Please see Supporting information for details.
Reaction scale: 5 mmol.
Pyridine solvent.
Optimization results in Table 1 show that by increasing CuI and AgF loading and reaction time, clean difluorination can be obtained. Longer reaction times require the use of pyridine to prevent decomposition of aminoquinoline amides. Difluorination examples are presented in Table 3. Similar to monofluorination, electron-rich (entries 4, 5, 7), electron-poor (entries 1–3, 6) and heterocyclic (entry 8) amides can be efficiently difluorinated in good yields. Interestingly, difluorination of m-substituted amides is possible (entries 4, 7). This contrasts with palladium-catalyzed C-H bond functionalization where substitution at more hindered positions is typically not observed,17 and is consistent with results obtained in copper-promoted sulfenylation of sp2 C-H bonds where functionalization of hindered positions is possible.14b
Table 3.
Difluorination of Carboxylic Acid Derivativesa

| |||
|---|---|---|---|
| entry | Ar | product | yield, % |
| 1 | 4-CF3C6H4 |
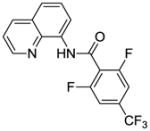
|
67 |
| 2 | 4-MeO2CC6H4 |

|
62 |
| 3 | NCC6H4 |
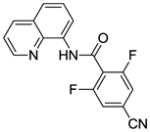
|
70 |
| 4 | 2-Naphthyl |

|
70 |
| 5 | 4-MeOC6H4 |

|
75 |
| 6 | 4-FC6H4 |

|
61 |
| 7 | 3-MeC6H4 |

|
77 |
| 8b | 4-Pyridyl |
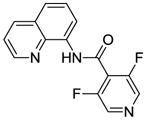
|
61 |
Amide 0.25 mmol, DMF 1 mL. Yields are isolated yields. Please see Supporting information for details.
Pyridine solvent.
Fluorination of benzylamine derivatives is also possible by employing a picolinamide directing group (Scheme 1). However, the reactions are substantially less efficient, requiring 50 mol% CuI catalyst, higher temperature, and DMPU solvent. Additionally, reasonable conversions could be obtained only with α,α-disubstituted benzylamines. This behavior is consistent with copper-catalyzed amination and sulfenylation of C-H bonds.14b,c
Scheme 1.
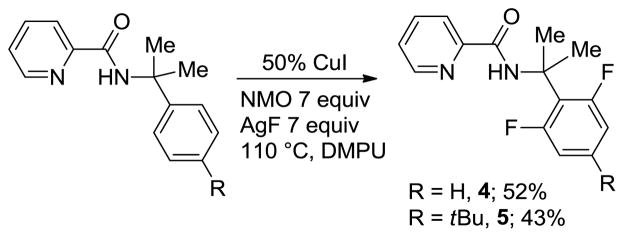
Fluorination of Picolinamides
Auxiliary can be cleaved by base hydrolysis. Thus, heating amide 6 with NaOH in ethanol for 24 h afforded high yield of trifluorobenzoic acid (Scheme 2).
Scheme 2.
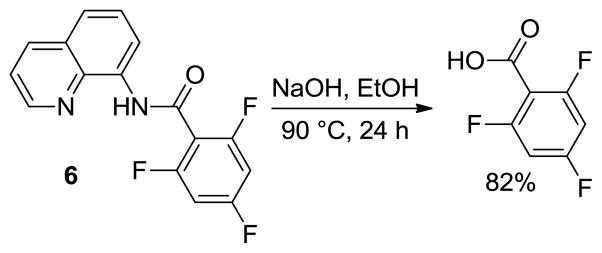
Auxiliary Cleavage
While speculations about the reaction mechanism are premature at this point, Ribas has shown that copper-catalyzed nucleophilic aryl fluorination and aryl halide exchange is possible in a highly geometrically constrained system.16a The reactions proceed via Cu(III) intermediates, thus showing that C-F reductive elimination from Cu(III) is possible under very mild conditions.7b Given that aminoquinoline amides stabilize high oxidation states in transition metals,14d it is likely that copper-catalyzed aminoquinoline amide fluorination also proceeds via Cu(III) intermediates.
In conclusion, we have developed a method for direct, copper-catalyzed, auxiliary-assisted fluorination of β-sp2 C-H bonds of benzoic acid derivatives and γ-sp2 C-H bonds of benzylamine derivatives. The reaction employs catalytic CuI, AgF as nucleophilic fluoride source, and DMF, pyridine, or DMPU solvent at moderately elevated temperatures. The method allows for selective mono- or difluorination of benzamide substrates. The reaction shows excellent functional group tolerance and provides a straightforward way for the preparation of ortho-fluorinated benzoic acids. Future directions of the work involve mechanistic studies of the transformation and attempts to isolate reaction intermediates.
Supplementary Material
Acknowledgments
We thank the Welch Foundation (Grant No. E-1571), National Institute of General Medical Science (Grant No. R01GM077635), Camille and Henry Dreyfus Foundation, and Norman Hackerman Advanced Research Program for supporting this research.
Footnotes
Detailed experimental procedures and characterization data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.(a) Hollingworth C, Gouverneur V. Chem Commun. 2012;48:2929. doi: 10.1039/c2cc16158c. [DOI] [PubMed] [Google Scholar]; (b) Coenen HH, Ermert J. Curr Radiopharm. 2010;3:163. [Google Scholar]; (c) Zhang XJ, Lai TB, Kong RYC. Top Curr Chem. 2012;308:365. doi: 10.1007/128_2011_270. [DOI] [PubMed] [Google Scholar]; (d) Purser S, Moore PR, Swallow S, Gouverneur V. Chem Soc Rev. 2008;37:320. doi: 10.1039/b610213c. [DOI] [PubMed] [Google Scholar]; (e) Jeschke P. Chem Bio Chem. 2004;5:570. [Google Scholar]
- 2.Balz G, Schiemann G. Chem Ber. 1927;60:1186. [Google Scholar]
- 3.(a) Dawood KM. Tetrahedron. 2004;60:1435. [Google Scholar]; (b) Adams DJ, Clark JH. Chem Soc Rev. 1999;28:225. [Google Scholar]; (c) Rozen S. Adv Synth Cat. 2010;352:2691. [Google Scholar]; (d) Yamada S, Knochel P. Synthesis. 2010:2490. [Google Scholar]; (e) Umemoto T, Tomizawa G. J Org Chem. 1995;60:6563. [Google Scholar]
- 4.(a) Watson DA, Su M, Teverovskiy G, Zhang Y, Garcia-Fortanet J, Kinzel T, Buchwald SL. Science. 2009;325:1661. doi: 10.1126/science.1178239. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Maimone TJ, Milner PJ, Kinzel T, Zhang Y, Takase MK, Buchwald SL. J Am Chem Soc. 2011;133:18106. doi: 10.1021/ja208461k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furuya T, Kaiser HM, Ritter T. Angew Chem, Int Ed. 2008;47:5993. doi: 10.1002/anie.200802164.Tang P, Wang W, Ritter T. J Am Chem Soc. 2011;133:11482. doi: 10.1021/ja2048072.Furuya T, Kaiser HM, Ritter T. J Am Chem Soc. 2010;132:12150. doi: 10.1021/ja105834t.Furuya T, Ritter T. J Am Chem Soc. 2008;130:10060. doi: 10.1021/ja803187x.Review: Furuya T, Kamlet AS, Ritter T. Nature. 2011;473:470. doi: 10.1038/nature10108.
- 6.Fier PS, Hartwig JF. J Am Chem Soc. 2012;134:10795. doi: 10.1021/ja304410x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Ye Y, Sanford MS. J Am Chem Soc. 2013;135:4648. doi: 10.1021/ja400300g. [DOI] [PubMed] [Google Scholar]; (b) Fier PS, Luo J, Hartwig JF. J Am Chem Soc. 2013;135:2552. doi: 10.1021/ja310909q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Grushin VV. Acc Chem Res. 2010;43:160. doi: 10.1021/ar9001763. [DOI] [PubMed] [Google Scholar]; (b) Vigalok A. Organometallics. 2011;30:4802. [Google Scholar]; (c) Dubinsky-Davidchik IS, Potash S, Goldberg I, Vigalok A, Vedernikov AN. J Am Chem Soc. 2012;134:14027. doi: 10.1021/ja3039272. [DOI] [PubMed] [Google Scholar]; (d) Zhao SB, Wang RY, Nguyen H, Becker JJ, Gagné MR. Chem Commun. 2012;48:443. doi: 10.1039/c1cc15006e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Mankad NP, Toste FD. Chem Sci. 2012;3:72. doi: 10.1039/C1SC00515D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloom S, Pitts CR, Miller DC, Haselton N, Holl MG, Urheim E, Lectka T. Angew Chem Int Ed. 2012;51:10580. doi: 10.1002/anie.201203642.Liu W, Huang X, Cheng M-J, Nielsen RJ, Goddard WA, III, Groves JT. Science. 2012;337:1322. doi: 10.1126/science.1222327.Yin F, Wang Z, Li Z, Li C. J Am Chem Soc. 2012;134:10401. doi: 10.1021/ja3048255.Amaoka Y, Nagatomo M, Inoue M. Org Lett. 2013;15:2160. doi: 10.1021/ol4006757.Katcher MK, Doyle AG. J Am Chem Soc. 2010;132:17402. doi: 10.1021/ja109120n.Review: Sibi MP, Landais Y. Angew Chem, Int Ed. 2013;52:3570. doi: 10.1002/anie.201209583.
- 10.(a) Hull KL, Anani WQ, Sanford MS. J Am Chem Soc. 2006;128:7134. doi: 10.1021/ja061943k. [DOI] [PubMed] [Google Scholar]; (b) Ball ND, Sanford MS. J Am Chem Soc. 2009;131:3796. doi: 10.1021/ja8054595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Mei TS, Yu YQ. J Am Chem Soc. 2009;131:7520. doi: 10.1021/ja901352k.Chan KSL, Wasa M, Wang X, Yu JQ. Angew Chem Int Ed. 2011;50:9081. doi: 10.1002/anie.201102985.Review: Engle KM, Mei TS, Wang X, Yu JQ. Angew Chem, Int Ed. 2011;50:1478. doi: 10.1002/anie.201005142.
- 12.Wu T, Yin G, Liu G. J Am Chem Soc. 2009;131:16354. doi: 10.1021/ja9076588. [DOI] [PubMed] [Google Scholar]
- 13.McMurtrey KB, Racowski JM, Sanford MS. Org Lett. 2012;14:4094. doi: 10.1021/ol301739f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Zaitsev VG, Shabashov D, Daugulis O. J Am Chem Soc. 2005;127:13154. doi: 10.1021/ja054549f. [DOI] [PubMed] [Google Scholar]; (b) Tran LD, Popov I, Daugulis O. J Am Chem Soc. 2012;134:18237. doi: 10.1021/ja3092278. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Tran LD, Roane J, Daugulis O. Angew Chem, Int Ed. 2013;52:6043. doi: 10.1002/anie.201300135. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Shabashov D, Daugulis O. J Am Chem Soc. 2010;132:3965. doi: 10.1021/ja910900p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CuF2-mediated fluorination of benzene: Subramanian MA, Manzer LE. Science. 2002;297:1665. doi: 10.1126/science.1076397.
- 16.(a) Casitas A, Canta M, Solá M, Costas M, Ribas X. J Am Chem Soc. 2011;133:19386. doi: 10.1021/ja2058567. [DOI] [PubMed] [Google Scholar]; (b) Ribas X, Jackson DA, Donnadieu B, Mahía J, Parella T, Xifra R, Hedman B, Hodgson KO, Llobet A, Stack TDP. Angew Chem Int Ed. 2002;41:2991. doi: 10.1002/1521-3773(20020816)41:16<2991::AID-ANIE2991>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]; (c) Huffman LM, Stahl SS. J Am Chem Soc. 2008;130:9196. doi: 10.1021/ja802123p. [DOI] [PubMed] [Google Scholar]
- 17.Daugulis O, Do HQ, Shabashov D. Acc Chem Res. 2009;42:1074. doi: 10.1021/ar9000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


