Abstract
Introduction:
Smoking cessation is typically verified biochemically by expired-air carbon monoxide (CO) levels below 9 ppm (i.e., ≤8 ppm), but this CO criterion may lead many who have smoked within 24hr to be misclassified as abstinent.
Methods:
Adult dependent smokers (N = 261; 124 men, 137 women) prospectively recorded each cigarette smoked and provided CO on five consecutive days during each of two short-term attempts to quit smoking. Participants were those recruited for crossover tests of the effects of placebo versus medication (nicotine patch or varenicline) on the ability to initiate 24-hr abstinence. All had either a high or low interest in permanently quitting smoking soon (within 3 months) and were randomized to the presence or absence of daily ($12) monetary reinforcement of abstinence.
Results:
Total accuracy of sensitivity to detect smoking (83%) plus specificity to verify abstinence (87%) was optimal at a CO criterion for abstinence below 5 ppm (≤4 ppm), compared with below 9 ppm (sensitivity of 60%, specificity of 97%). Overall CO detection of sensitivity and specificity was higher in those with high versus low quit interest, but reinforcement of abstinence made no difference.
Conclusions:
Results indicate a CO criterion half that used in most clinical research may optimally validate 24-hr cessation and reduce misclassification of smokers as “abstinent.”
INTRODUCTION
Complete abstinence from smoking over at least 24hr is typically the minimum duration necessary to define an ex-smoker as “quit” in clinical research (e.g., Ockene et al., 2000). This duration of abstinence is supported by observations that a recent 24-hr quit predicts greater subsequent quit success (Balmford, Borland, & Burney, 2010), and any smoking at all within 24hr of trying to quit increases the risk of relapse to regular smoking (Juliano, Donny, Houtsmuller, & Stitzer, 2006; Westman, Behm, Simel, & Rose, 1997). Among those trying to quit, the majority who fail to abstain for at least 24hr will not even report the effort as a true quit attempt (Berg et al., 2010). Cotinine’s 16-hr half-life may allow verification of abstinence over the past several days (SRNT, 2002). However, a very common and immediate method of biochemically verifying 24-hr abstinence in clinical research is an expired-air carbon monoxide reading equal to or less than 8 ppm (CO < 8 ppm), or perhaps 10 ppm (Bailey, Bryson, & Killen, 2011; SRNT, 2002). CO’s typical 4-hr half-life makes it more sensitive for verifying 24-hr abstinence.
However, recent research suggests that smokers may be able to smoke one or more cigarettes within the prior 24hr and still meet such a CO criterion (“cutoff”) for abstinence. For example, Javors, Hatch, and Lamb (2005) found that, compared with the typical criterion of ≤8 ppm, a CO cutoff for abstinence of <3 ppm increased sensitivity for accurately identifying smoking (i.e., the percentage of smoking days with CO at or above 3 ppm) from about 40% to 70%. Thus, at least half of those with a CO ≤ 8 ppm would have been incorrectly identified as abstinent, despite smoking in the prior 24hr. Although specificity for correctly identifying abstinence (i.e., the percentage of nonsmoking days with CO below 3 ppm) was moderately reduced from 98% to 85%, the lower CO criterion of 3 ppm enhanced the total accuracy of sensitivity plus specificity. These results may be specific to the conditions of this study, which assessed smokers varying in quit interest, all of whom were monetarily reinforced for gradually reducing their smoking over a period of 3 months. Yet, they suggest that a CO criterion much lower than that of the typical criterion of 8 ppm may have better sensitivity for detecting recent (24-hr) smoking in studies examining successful quitting. If broadly applicable, use of a lower CO criterion to verify abstinence could have important implications. Misclassification of those who have smoked in the prior 24hr as “abstinent” may complicate the interpretation of results of controlled studies assessing the efficacy of medications or other treatments for cessation.
The current analyses examined sensitivity and specificity of different CO criterion levels for biochemically verifying 24-hr abstinence during two short-term (5-day) quit attempts on active medication or placebo. To examine the potential influence of quit interest and abstinence reinforcement on CO sensitivity and specificity, we assessed daily CO and number of cigarettes among smokers (a) with a high or a low interest in making a permanent quit attempt after the study (within 3 months), and (b) randomized to receive reinforcement ($12/day) or no reinforcement for each day of abstinence.
METHODS
Participants
Participants were 261 adult smokers (124 men, 137 women) recruited for two very similar studies of ability to quit for at least 24hr during two short-term periods while on active (varenicline; 21mg nicotine patch) or placebo medication in a crossover design. All met DSM-IV nicotine dependence criteria (adapted from Breslau, Kilbey, & Andreski, 1994), smoked >10 cigarettes per day for >1 year, and provided a screening CO of at least 10 ppm. They also completed the Fagerstrom Test of Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991), although inclusion did not depend on FTND score. Mean (SD) sample characteristics were 31.9 (11.6) years old, 16.7 (5.3) cigarettes/day, 13.5 (10.7) years of smoking, 23.5 (10.2) ppm CO at screening, and 4.7 (1.5) FTND score. Participants self-identified mostly as Caucasian (77.4%), with 13.8% as African-American, 2.7% as Asian, less than 1% as Hispanic, and 5.4% as more than one ethnicity. Participant characteristics did not differ between studies.
All were recruited partly based on their high or low interest in making a permanent quit attempt on completion of the study, defined as either wanting to quit within 3 months (high; n = 103) or having no interest in quitting for at least 6 months (low; n = 158). Procedures for assessing and validating high and low quit interest at screening are described elsewhere (Perkins et al., 2008, 2010).
Procedure
As described below, expired-air CO and the number of cigarettes smoked were assessed daily (Mon–Fri) during the two 5-day quit attempt periods (i.e., a total of 10 daily assessments), separated by at least 1 week of ad lib smoking without any medication. These medication conditions are not particularly relevant in these analyses since our focus was on the sensitivity and specificity of CO level for verifying 24-hr abstinence, regardless of the method by which abstinence was obtained. Sessions were scheduled between noon and 4:00 p.m. at the same time of the day for each participant. Daily expired-air CO was assessed at each session via Breathco CO monitor (Lenexa, KS) following a 20-s breath hold prior to slowly expiring air into the monitor mouthpiece (Javors et al., 2005; Raiff, Faix, Turturici, & Dallery, 2010). Subjects were not informed of their CO readings (in ppm), which were recorded away from the participants’ view.
All 261 participants were randomly assigned to monetary reinforcement of daily abstinence ($12/day; n = 127) or to no reinforcement (n = 134) during both quit periods. Reinforcement was provided for those with CO <5 (≤4 ppm) and who reported no smoking in the last 24hr, although none was informed of their CO values or this specific CO criterion for reinforcement. Rather, they were told they would be reinforced for “being quit.” For this analysis, this manipulation enabled us to assess whether the presence or absence of reinforcement for quitting influenced the association of CO with abstinence (see Javors et al., 2005). These studies were approved by the University of Pittsburgh Institutional Review Board, and all participants provided written informed consent for participation after the nature and the consequences of the relevant study were explained. Other details of the study procedures are described in Perkins et al. (2008, 2010).
Assessing Daily Abstinence or Smoking
Abstinence was defined as zero cigarettes smoked in the 24hr prior to each weekday session (Monday–Friday) during the quit attempt periods. To assess the amount of daily smoking, participants were given “tally” cards to monitor all cigarette use. Tallies were returned at the next session and the number of cigarettes smoked over precisely the 24hr prior to the session was recorded, based on tallies for that day and the previous day. The rows of each tally card listed the 24 individual hours of the day (e.g., “noon to 1:00 p.m.”), with a space to mark the tallies every time they smoked a cigarette at that time. (The size and the shape of the card were intentionally designed to fit inside the cellophane wrapper of a cigarette pack, to facilitate compliance.) To encourage compliance, all received $5 for turning in the tally cards at each session. Note that falsely reporting no smoking (i.e., no cigarettes) over the prior 24hr, when smoking had in fact occurred, would be expected to overestimate the optimum CO to verify abstinence since such subjects would be counted as quit despite high CO levels.
For these 261 subjects, CO and cigarette tallies were assessed on a total of 2,572 days, with abstinence (0 cigarettes) identified during 1,012 days (39.4%), and nonabstinence (>0 cigarettes) identified during the remaining 1,560 days. Total days comprised 1,378 from the patch study, involving 563 abstinent (40.9%) and 815 nonabstinent days, and 1,194 days from the varenicline study, involving 449 abstinent (37.6%) and 745 nonabstinent days. A tally and/or a CO reading was missing for a total of 38 days, or 1.6% of the total possible (2,610 days), from a total of 30 subjects. (Three other potential participants, or 1.1%, were excluded from all analyses due to repeated noncompliance with tally recording, as defined by CO > 10 ppm despite tallies indicating 0 cigarettes in the prior 24hr during three or more sessions.) This method has been shown to be valid and reliable in assessments of daily nicotine nasal spray medication use (Perkins et al., 1996).
Statistical Analysis
Consistent with prior research (e.g., Cropsey, Eldridge, Weaver, Villalobos, & Stitzer, 2006; Javors et al., 2005), sensitivity was determined by the percentage of CO values that were above the designated criterion for abstinence (e.g., above 4 ppm, above 8 ppm) on the days in which at least one cigarette (>0) was smoked during the prior 24hr. Similarly, specificity was determined by the percentage of CO values below the abstinence criterion on the days in which zero cigarettes were smoked in those prior 24hr. To further compare subgroups on how well CO discriminated between abstinent and nonabstinent days, we assessed the area under the curve (AUC) for the receive-operator characteristics (ROC), using the nonparametric method (Zweig & Campbell, 1993). To do so, we plotted sensitivity by specificity for all obtained CO levels, with AUC of 1.0 indicating perfect identification of abstinence and nonabstinence and 0.5 demonstrating no discrimination between the two (see Javors et al., 2005). Nonoverlapping 95% confidence intervals (CIs) for AUC values indicated subgroup differences.
RESULTS
Over the two 5-day quit attempt periods, the mean ± SD number of abstinent and nonabstinent days was 3.9±3.6 versus 6.0±3.6, respectively. Corresponding mean CO values were 3.2±2.2 versus 12.3±7.3 ppm, respectively. In the ROC analysis, AUC ± SEM was 0.910 ± .006, p < .001, with a 95% CI of 0.899–0.921, showing very strong ability of CO to discriminate between days of smoking (sensitivity) and abstinence (specificity), as expected.
More importantly, a CO criterion of 5 ppm for smoking (i.e., “cutoff”; ≤4 for abstinence) resulted in the highest overall combined sensitivity to detect smoking (83%) and specificity to verify abstinence (87%), as indicated in Table 1. An even more stringent cutoff of 3 ppm produced 95% sensitivity, but only 54% specificity. By contrast, the standard clinical CO cutoff of 9 ppm (i.e., ≤8 for abstinence) produced a sensitivity of only 60%, although specificity was 97% as detection of abstinence was improved. In other words, nearly all abstinent days resulted in a CO <9 ppm, but about 40% of those who smoked within 24hr also had CO <9 ppm, compared to 17% who had CO <5 ppm. Consistent with these findings, mean CO for the 27% of all nonabstinent days involving just one or just two cigarettes in the prior 24hr was 5.3±4.1 ppm and 7.3±5.2 ppm, respectively, essentially below the standard cutoff of 9 ppm but above the cutoff of 5 ppm.
Table 1.
Sensitivity to Detect Smoking and Specificity for Verifying Abstinence in All Subjects, by Carbon Monoxide (CO) Cutoff Level (i.e., Below Which Designates Abstinence)
| CO cutoff (ppm) | Sensitivity | Specificity | Sensitivity + specificity |
|---|---|---|---|
| 3 | 0.948 | 0.542 | 1.490 |
| 4 | 0.885 | 0.757 | 1.642 |
| 5 | 0.826 | 0.873 | 1.699 |
| 6 | 0.769 | 0.911 | 1.680 |
| 7 | 0.710 | 0.942 | 1.652 |
| 8 | 0.658 | 0.955 | 1.613 |
| 9 | 0.602 | 0.967 | 1.569 |
| 10 | 0.560 | 0.979 | 1.539 |
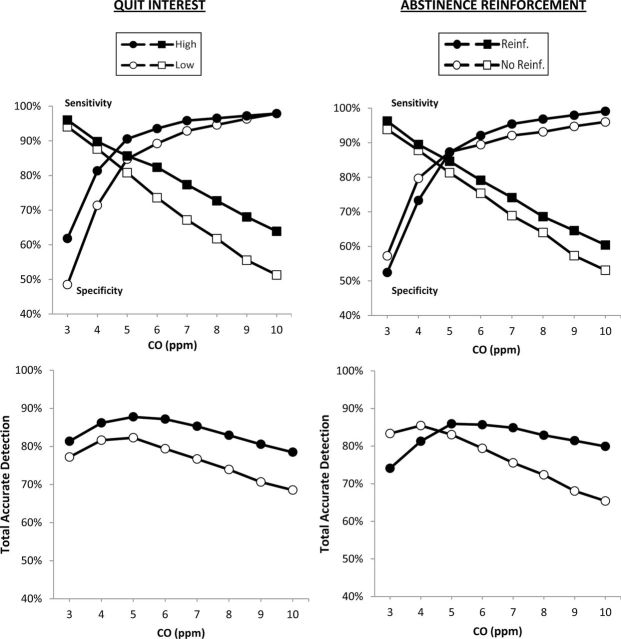
Separate and total results for sensitivity and specificity by CO cutoffs are shown in Figure 1, by high or low quit interest, as well as by the presence or absence of abstinence reinforcement. Notably, a CO cutoff of 5 ppm was optimal for total sensitivity and specificity in each subgroup, except for those not reinforced, for whom a cutoff of 4 ppm was optimal. Also as shown, a CO <5 ppm was also the point at which the sensitivity and specificity plots intersected for all subgroups. ROC analyses showed AUC values of 0.936 ± .008 (CI of 0.921–0.951) for those high in quit interest, compared to 0.890 ± .008 (CI of 0.874–0.906) for those low in quit interest, with nonoverlapping CIs indicating a significant difference. For example, at a CO cutoff of 5 ppm, respective sensitivity and specificity were 86% and 91% for high quit interest, compared to 81% and 85% for low quit interest. At a CO of 9 (≤8 ppm for abstinence), specificity was similar but sensitivity to detect smoking was 68% versus 56% for high versus low quit interest, respectively. However, for reinforcement of abstinence, AUC was 0.924 ± .008 (CI of 0.909–0.939) for those receiving reinforcement, and 0.900 ± .009 (CI of 0.882–0.918) for those not reinforced, as overlapping CIs indicated no difference.
Figure 1.
Sensitivity to detect smoking and specificity for verifying abstinence in the prior 24hr are shown by carbon monoxide (CO) cutoff values (i.e., minimum criterion to designate smoking), separately (top) and as total accurate detection (i.e., the percentage of true detections of smoking and abstinence; bottom). Plots are presented for smokers who are high (n = 103) versus low (n = 158) in quit interest and for those reinforced (n = 127) versus not reinforced (n = 134) for abstinence.
DISCUSSION
We found an optimal CO cutoff to detect smoking and verify 24-hr abstinence of 5 ppm, meaning a criterion of ≤4 ppm, half that of the standard clinical criterion (≤8 ppm), which may provide the most accurate biochemical verification of a successful quit attempt. These results are very consistent with those of Javors et al. (2005), who found an optimum CO cutoff of 3 ppm among smokers reinforced for gradually reducing CO across several months. Because we generally found no differences in CO sensitivity or specificity due to monetary reinforcement for abstinence, the results of Javors et al. (2005) likely were not limited to providing reinforcement to reduce CO. Thus, our CO cutoff for smoking of 5 ppm may be broadly applicable in comparable studies whether or not abstinence is reinforced. However, we did find better sensitivity and specificity of CO among those high in quit interest, and this more stringent CO criterion may be even more effective for verifying successful abstinence in clinical research on permanent quit attempts. Yet, which CO cutoff is most appropriate may depend on whether sensitivity in detecting recent smoking, as in most cessation research, or specificity in validating abstinence is of more concern. The optimum CO cutoff may also be influenced by the potential for infrequent nontobacco sources of CO exposure in the study sample, such as recent or chronic exposure to severe air pollution or impaired pulmonary function (Kotz, 2012).
Consistent with our findings, similar research suggests a lower criterion for another method of biochemical verification of abstinence, cotinine, may be warranted (Benowitz, Bernert, Caraballo, Holiday, & Wang, 2009). Other research has suggested that cotinine assessment, which has a longer half-life, may be more sensitive than CO in detecting smoking (Gariti, Alterman, Ehrman, Mulvaney, & O’Brien, 2002). However, in that study, cotinine was compared to a CO criterion for abstinence of <10 ppm, and the use of the lower CO criterion here (<5 ppm) may show less difference in sensitivity between these biochemical measures of verifying abstinence. Finally, although we assessed CO only among smokers making a short-term quit attempt, our findings may be relevant for determining optimum CO criteria to verify general smoking status in nonquitting smokers and nonsmokers, as suggested by other research (e.g., Cropsey et al., 2006; Middleton & Morice, 2000).
FUNDING
This research was supported by National Institute of Health grants CA143187 and DA031218.
DECLARATION OF INTERESTS
KAP has consulted with Embera Neurotherapeutics on the development of smoking cessation medications unrelated to this paper. The other authors have no disclosures.
ACKNOWLEDGMENTS
The authors thank Carolyn Fonte, Jessica Briski, and Melissa Mercincavage for their helpful assistance in assessing CO and cigarette tallies.
REFERENCES
- Bailey S. R., Bryson S. W., Killen J. D. (2011). Predicting successful 24-hr quit attempt in a smoking cessation intervention. Nicotine & Tobacco Research, 13, 1092–1097. 10.1093/ntr/ntr151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmford J., Borland R., Burney S. (2010). The role of prior quitting experience in the prediction of smoking cessation. Psychological Health, 25, 911–924ISSN: 1476-8321 [DOI] [PubMed] [Google Scholar]
- Benowitz N. L., Bernert J. T., Caraballo R. S., Holiday D. B., Wang J. T. (2009). Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. American Journal of Epidemiology, 169, 236–24810.1093/aje/kwn301 [DOI] [PubMed] [Google Scholar]
- Berg C. J., An L. C., Kirch M., Guo H., Thomas J. L., Patten C. A. … West R. (2010). Failure to report attempts to quit smoking. Addictive Behaviors, 35, 900–90410.1016/j.addbeh.2010.06.009 [DOI] [PubMed] [Google Scholar]
- Breslau N., Kilbey M. M., Andreski P. (1994). DSM-IIIR nicotine dependence in young adults: Prevalence, correlates and associated psychiatric disorders. Addiction, 89, 743–75410.1111/j.1360-0443.1994.tb00960.x [DOI] [PubMed] [Google Scholar]
- Cropsey K. L., Eldridge G. D., Weaver M. F., Villalobos G. C., Stitzer M. L. (2006). Expired carbon monoxide levels in self-reported smokers and nonsmokers in prison. Nicotine & Tobacco Research, 8, 653–65910.1080/14622200600789684 [DOI] [PubMed] [Google Scholar]
- Gariti P., Alterman A. I., Ehrman R., Mulvaney F. D., O’Brien C. P. (2002). Detecting smoking following smoking cessation treatment. Drug and Alcohol Dependence, 65, 191–19610.1016/S0376-8716(01)00162-4 [DOI] [PubMed] [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerstrom K. O. (1991). The fagerstrom test for nicotine dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127ISSN: 09520481 [DOI] [PubMed] [Google Scholar]
- Javors M. A., Hatch J. P., Lamb R. J. (2005). Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction, 100, 159–16710.1111/j.1360-0443 [DOI] [PubMed] [Google Scholar]
- Juliano L. M., Donny E. C., Houtsmuller E. J., Stitzer M. L. (2006). Experimental evidence for a causal relationship between smoking lapse and relapse. Journal of Abnormal Psychology, 115, 166–17310.1037/0021-843X.115.1.166 [DOI] [PubMed] [Google Scholar]
- Kotz D. (2012). Possible reasons for elevated carbon monoxide levels in self-reported ex-smokers. Nicotine & Tobacco Research, 14, 834–83810.1093/ntr/ntr305 [DOI] [PubMed] [Google Scholar]
- Middleton E. T., Morice A. H. (2000). Breath carbon monoxide as an indication of smoking habit. Chest, 117, 758–76310.1378/chest.117.3.758 [DOI] [PubMed] [Google Scholar]
- Ockene J. K., Emmons K. M., Mermelstein R. J., Perkins K. A., Bonollo D. S., Hollis J. F. (2000). Relapse and maintenance issues for smoking treatment. Health Psychology, 19(Suppl.)17–3110.1037/0278-6133.19.1(suppl.).17 [DOI] [PubMed] [Google Scholar]
- Perkins K. A., Grobe J. E., D’Amico D., Fonte C., Wilson A., Stiller R. L. (1996). Low-dose nicotine nasal spray use and effects during initial smoking cessation. Experimental and Clinical Psychopharmacology, 4, 157–16510.1037/ 1064-1297.4.2.157 [Google Scholar]
- Perkins K. A., Lerman C., Fonte C., Mercincavage M., Stitzer M. L., Chengappa K. R. N., Jain A. (2010). Cross-validation of a new procedure for early screening of smoking cessation medications in humans. Clinical Pharmacology and Therapeutics, 88, 109–11410.1038/clpt.2010.65 [DOI] [PubMed] [Google Scholar]
- Perkins K. A., Lerman C., Stitzer M. L., Fonte C. A., Briski J. L., Scott J. A., Chengappa K. N. R. (2008). Development of procedures for early screening of smoking cessation medications in humans. Clinical Pharmacology and Therapeutics, 84, 216–22110.1038/clpt2008.30 [DOI] [PubMed] [Google Scholar]
- Raiff B.R., Faix C., Turturici M., Dallery J. (2010). Breath carbon monoxide output is affected by speed of emptying the lungs: Implications for laboratory and smoking cessation research. Nicotine & Tobacco Research, 12, 834–83810.1093/ntr/ntq090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification (2002). Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research, 4, 149–159ISSN: 1469-2203. [DOI] [PubMed] [Google Scholar]
- Westman E. C., Behm F. M., Simel D. L., Rose J. E. (1997). Smoking behavior on the first day of a quit attempt predicts long-term abstinence. Archives of Internal Medicine, 157, 335–34010.1001/archinte.157.3.335. [PubMed] [Google Scholar]
- Zweig M. H., Campbell G. (1993). Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clinical Chemistry, 39, 561–577ISSN: 0009-9147. [PubMed] [Google Scholar]