Abstract
Homotypic fusion of immature secretory granules (ISGs) gives rise to mature secretory granules (MSGs), the storage compartment in endocrine and neuroendocrine cells for hormones and neuropeptides. With the use of a cell-free fusion assay, we investigated which soluble N-ethylmaleimide-sensitive fusion protein attachment receptor (SNARE) molecules are involved in the homotypic fusion of ISGs. Interestingly, the SNARE molecules mediating the exocytosis of MSGs in neuroendocrine cells, syntaxin 1, SNAP-25, and VAMP2, were not involved in homotypic ISG fusion. Instead, we have identified syntaxin 6 as a component of the core machinery responsible for homotypic ISG fusion. Subcellular fractionation studies and indirect immunofluorescence microscopy show that syntaxin 6 is sorted away during the maturation of ISGs to MSGs. Although, syntaxin 6 on ISG membranes is associated with SNAP-25 and SNAP-29/GS32, we could not find evidence that these target (t)-SNARE molecules are involved in homotypic ISG fusion. Nor could we find any involvement for the vesicle (v)-SNARE VAMP4, which is known to be associated with syntaxin 6. Importantly, we have shown that homotypic fusion requires the function of syntaxin 6 on both donor as well as acceptor membranes, which suggests that t–t-SNARE interactions, either direct or indirect, may be required during fusion of ISG membranes.
INTRODUCTION
Cellular organization requires accurate protein transport throughout the entire secretory pathway. Key requirements for protein transport are vesicular carriers with a full complement of machinery to enable them to find and fuse with the correct downstream compartment. This machinery includes the soluble N-ethylmaleimide-sensitive fusion protein (NSF) attachment protein (SNAP) receptors (SNAREs). The SNAREs and SNAPs together with NSF are the core components of the highly conserved machinery involved in all docking and fusion steps in membrane traffic pathways so far described (Robinson and Martin, 1998; Jahn and Sudhof, 1999; Mayer, 1999). There are two classes of SNAREs, vesicle (v)-SNAREs and target (t)-SNAREs, which are defined according to their localization on vesicles or target membranes, respectively, although t-SNAREs have also been detected on vesicles (Tagaya et al., 1995; Walch-Solinema et al., 1995; Gaisano et al., 1996). Typically two t-SNAREs and one v-SNARE build a 7S complex composed of a bundle of 4 α-helices (Sutton et al., 1998). SNAREs have been shown to be the minimal machinery needed to drive the fusion of lipid bilayers (Weber et al., 1998) and to provide an inherent level of specificity (McNew et al., 2000, summarized in Clague and Herrmann, 2000).
Although the majority of membrane fusion events are heterotypic, i.e., between membranes from, or derived from, different intracellular compartments, there are several membrane fusion events, which are homotypic. Two well-described homotypic fusion events occur after cell division when cells exit mitosis, whereupon both the Golgi complex and the endoplasmic reticulum reassemble in the daughter cells (Rabouille et al., 1998; Roy et al., 2000). Homotypic fusion events also have been described in cells in interphase and are used to alter the composition and size of compartments, such as the yeast vacuole (Conradt et al., 1992), the early endosome (Gruenberg and Howell, 1986), and the immature secretory granule (ISG) (Tooze et al., 1991).
Yeast vacuolar fusion is perhaps the best characterized homotypic fusion event (for recent review see Wickner and Haas, 2000). Briefly, yeast vacuolar fusion occurs through a series of priming, docking, and fusion reactions. Priming, triggered by Sec18p (the ortholog of NSF)-catalyzed ATP hydrolysis, results in the dissociation of the cis SNARE complex (Ungermann et al., 1998a) and production of a “primed” t-SNARE, Vam7p, in association with a chaperone-like molecule LMA1 (Xu et al., 1998). Docking involves tethering the vacuoles together, followed by an irreversible trans-SNARE pairing. Tethering requires a complimentary set of proteins on both vacuoles, including a member of the rab family of proteins ypt7 (Mayer and Wickner, 1997). Trans-SNARE pairing occurs between the primed t-SNARE Vam7p and a complex of at least 3 v-SNAREs (Ungermann et al., 1998b). Two of these v-SNAREs, Vam3p and Vti1p, actually function as the light chains together with the heavy chain of the t-SNARE Vam3p to make the complete t-SNARE complex (Fukuda et al., 2000). The remaining v-SNARE, Nyv1p, which is present on the other vacuolar membrane, provides the fourth α-helix for the SNARE complex.
ISGs derive from the trans-Golgi network (TGN), the major sorting and recycling system for secretory proteins. ISGs can fuse homotypically to build mature secretory granules (MSGs), the storage compartment for secretory proteins such as hormones or neuropeptides. During the maturation of ISGs, a variety of events specific to the regulated secretory pathway take place within the granules, including prohormone processing by endopeptidases (for review see Arvan and Castle, 1998; Tooze, 1998). ISG maturation also allows the remodeling and removal of excess membrane through the formation of ISG-derived clathrin-coated vesicles (CCVs). The formation of these CCVs leads to a further “proofreading” and/or sorting step for membrane proteins with destinations other than MSGs. The CCVs derived from the ISGs contain the adaptor protein AP-1 (Dittiéet al., 1996; Klumperman et al., 1998). Recruitment of the AP-1 complex requires the small molecular GTP-binding protein ARF1 (Austin et al., 2000) as well as the mannose-6-phosphate receptor (M6PR) and furin. Both furin and M6PR belong to a group of membrane proteins that are removed during maturation from the ISG (Dittiéet al., 1996; Dittiéet al., 1999; Klumperman et al., 1998). In addition to the M6PR and furin, the AP-1 containing CCVs budding from ISGs also contain syntaxin 6 (Klumperman et al., 1998). Recent experiments in AtT20 cells (Eaton et al., 2000) suggest that VAMP4 is present on ISGs and is removed in a BFA-sensitive manner during maturation, strongly suggesting that VAMP4 also may be incorporated in the ISG-derived CCVs.
To study the molecules involved in homotypic ISG fusion, we developed an in vitro fusion assay that reconstitutes ISG–ISG fusion (Urbéet al., 1998). This assay is based on an enzyme/substrate processing reaction that reports on the fusion between a donor ISG vesicle population providing the prohormone convertase 2 (PC2) enzyme and a [35SO4]-labeled acceptor membrane population providing the substrate secretogranin II (SgII). Fusion is measured by the quantification of a cleavage product of [35SO4]-SgII produced by the PC2 enzyme. With the use of this assay we have shown that the process of ISG–ISG fusion requires NSF (Urbéet al., 1998). Here we demonstrate that syntaxin 6 is required for homotypic fusion of ISGs. Furthermore, we have identified two syntaxin 6 complexes on the ISG, containing either SNAP-25 or SNAP-29, which suggests that syntaxin 6 may form multiple SNARE complexes. We have used the fusion assay to examine whether these and previously described SNARE molecules that have been shown to form complexes with syntaxin 6 also have a role in ISG–ISG fusion. We find that none of the partners of syntaxin 6 are involved in ISG–ISG fusion. Finally, we provide evidence that syntaxin 6 is necessary on the membranes of both fusion partners for efficient homotypic ISG fusion.
MATERIALS AND METHODS
Proteins
Recombinant syntaxin 4 (Bennett et al., 1993), syntaxin 6 (Bock et al., 1996), and VAMP4 (Advani et al., 1998) proteins lacking their transmembrane domains fused to GST in pGEX vectors (Amersham Pharmacia Biotech, Buckinghamshire, UK) are from R. Scheller (Stanford University, Palo Alto, CA). Syntaxin 1b (Bennett et al., 1992) fused to GST in pGEX, as well as α-SNAP (Whiteheart et al., 1993), myc-NSF (Söllner et al., 1993), and SNAP25 (Oyler et al., 1989) cloned in the pQE vectors (Qiagen, UK) providing a his6-tag are from J. Rothman (Memorial Sloan Kettering Cancer Center, New York, NY). Purification and thrombin cleavage of GST-fusion protein was performed according to the manufacturer's protocols (Amersham Pharmacia Biotech). His6-tagged proteins were purified as described elsewhere (Whiteheart et al., 1993). Botulinum neurotoxins (BotNTs) A and E (Binz et al., 1994), C (Land et al., 1997), and D (Weber et al., 1998) were prepared as His-tagged constructs, expressed in XL1Blue, induced by 100 μM IPTG, and purified by Ni-NTA to homogeneity by affinity chromatography.
Antibodies
Rabbit antiserum against syntaxin 6 (amino acid residues 2–231) or VAMP4 (amino acid residues 2–115) was increased by the subcutaneous injection of bacterially expressed cytoplasmic domains of syntaxin 6 or VAMP4 after cleavage from GST. For affinity purification, the antiserum was incubated with the soluble fusion protein covalently coupled to cyanogen bromide (CNBr)-activated sepharose and was washed extensively, and bound antibodies were eluted with the use of 0.1 M glycine, pH 2.8. Eluates containing the affinity-purified antibodies were neutralized and stored at –70°C. The specificity of the affinity-purified antibodies was tested by Western blot analysis and competition experiments as well as by immunofluorescence microscopy. Monoclonal anti-syntaxin 6 was purchased from Transduction Laboratories (Lexington, KY), monoclonal anti-syntaxin 1 antibody (HPC-1) from Sigma (Dorset, UK), monoclonal anti-SNAP-25 antibody SM181 from Sternberger Monoclonals (Lutherville, MD), polyclonal anti-VAMP and anti-VAMP2 antibodies from Synaptic Systems GmbH (Gottingen, Germany), anti-SNAP29 antiserum was a gift from WS Hong (Singapore), and anti-SNAP-23 antiserum a gift from P. Roche (National Cancer Institute, Bethesda, MD). Monoclonal antip18 antibody and polyclonal antibody 175 (anti-SgII) are described elsewhere (Dittié and Tooze, 1995; Tooze et al., 1994). Cy3-conjugated antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA) and Alexa Fluor 488-conjugated antibodies from Molecular Probes (Eugene, OR) for immunofluorescence microscopy. HRP-conjugated antibodies were from (Amersham Pharmacia-Biotech).
Preparation of ISGs and MSGs
ISG and MSG fractions were prepared from PC12/PC2 cells by velocity and equilibrium sucrose gradient centrifugation, as described previously (Dittiéet al., 1996).
Homotypic ISG–ISG Fusion Assay
ISG–ISG fusion was assayed by the formation of p18, a cleavage product of SgII, as described previously (Urbéet al., 1998). Complete fusion reactions were preincubated with or without antibodies for 30 min on ice before starting the fusion reaction. In the case of preincubation of one ISG population or membrane pellet alone, the antibody was added to only one or the other or both membrane populations and was incubated for 30 min on ice. To remove unbound antibodies, membranes were harvested by ultracentrifugation for 1 h 05 min at 100,000 × g through a sucrose cushion (0.5 M sucrose in 10 mM HEPES, pH 7.2) and were resuspended in specific fusion conditions as described (Urbéet al., 1998). To confirm that the antibodies added to the fusion assay reaction bound their antigen under the conditions of the fusion assay, we assayed for the presence of the antibody in the membrane pellet. Membrane pellets were resuspended and solubilized in SDS-PAGE sample buffer then were subjected to immunoblotting. The heavy and light chains were detected with the use of HRP-conjugated antibody specific for rabbit IgG (VAMP4, SNAP-29) or mouse IgG (SNAP-25).
Immunoisolation
Immunoisolation was performed with the use of affinity-purified anti-syntaxin 6 antibody covalently coupled with the use of dimethyl pimelimidate-2HCl (Pierce, Rockford, IL) to protein A magnetic microspheres (ProZyme, San Leandro, CA) at a final density of 0.1 μg of IgG per microliter of beads. One hundred fifty microliters of ISGs, or MSGs containing an equivalent amount of SgII, were used with 100 μl of antibody beads, or 100 μl of beads treated identically but without antibody. Membranes were diluted in IB buffer (i.e., 150 mM NaCl, 50 mM Tris, pH 8.0, 5 mM MgCl2, and 1% bovine serum albumin), the beads were added, and the mixture was incubated with gentle agitation at 4°C for 2 h. After incubation, the samples were washed five times by binding to a magnetic support. After washing, the bound material was eluted from the beads in Laemmli sample buffer and was subjected to SDS-PAGE and immunoblotting.
Immunoprecipitation
Membranes, typically from 1 ml of ISG fractions, were diluted with 2 volumes of 10 mM HEPES, pH 7.2, were sedimented at 100,000 × g for 1 h and 5 min, and were solubilized in 750 μl of immunoprecipitation (IP) buffer (i.e., 10 mM HEPES, pH 7.2, 100 mM KCl, 2 mM EDTA, 0.5% Triton X-100, 0.25 mM PMSF, and 10 μl/ml leupeptin). Unsolubilized membranes were pelleted by centrifugation at 100, 000 × g for 15 min, and 150 μl of the supernatant was used for each reaction condition. Monoclonal (SNAP-25, syntaxin 6) or polyclonal (SNAP-29) antibodies were prebound to protein A or G Sepharose beads (Amersham Pharmacia Biotech), and 30 μl of antibody beads were added to the samples and incubated for at least 2 h at 4°C, rotating end over end. For coimmunoprecipitation of VAMP4, polyclonal syntaxin 6 antibodies were covalently coupled to magnetic beads as described above, and 500 μl of ISGs were used per condition. After binding, the immunoprecipitates were washed four times in IP buffer. The bound material was eluted with Laemmli sample buffer, analyzed by SDS-PAGE and subjected to immunoblotting.
For samples in which assembly or disassembly conditions for SNARE complexes were applied, recombinant His-tagged-myc-NSF (1 μg) and His-tagged α-SNAP (2 μg) were added to the samples and were incubated for 30 min at 4°C in the presence of 0.5 mM ATP for assembly conditions or 0.5 mM ATP/8 mM MgCl2 for disassembly conditions, respectively, before the addition of antibody beads. Immunoprecipitates were washed and analyzed as above.
Indirect Immunofluorescence Microscopy
PC12/PC2 cells were fixed with 3% paraformaldehyde in phosphate-buffered saline, permeabilized with 0.2% Triton X-100, and then blocked with 0.2% gelatin and incubated with the appropriate antibodies. Antibodies were used at the following dilutions: syntaxin 6 mAb at a 1:1000 dilution; syntaxin 6 affinity-purified antibody (described herein) at a 1:400 dilution; p18 mAb at 1 μg/ml; and STO 175 at a 1:400 dilution. Images were collected with a confocal laser scanning microscope (model LSM510, Zeiss, Hertsfordshire, UK) and represent the projection of z sections (1.6 μm thick) through each cell. In all cases, images were exported to Photoshop (Adobe, San Jose, CA) for figure preparation.
Gel Filtration
Recombinant his6-tagged SNAP-25 or the cytoplasmic domains of syntaxin 4 (amino acid residues 5–274) or syntaxin 6 (amino acid residues 2–231) were prepared by purification on glutathione–sepharose (Amersham Pharmacia Biotech) and cleavage from GST by 0.25U/μl thrombin (Sigma) in 50 mM Tris, pH 8.0, 150 mM NaCl, 2.5 mM CaCl2, and 0.1% β-mercaptoethanol. One milliliter of the cleaved fusion protein preparation containing either 250 μg of SNAP-25, 860 μg of syntaxin 4, or 500 μg of syntaxin 6 then was fractionated by gel filtration in buffer A (i.e., 10 mM HEPES, pH 7.2, and 100 mM KCl) with the use of a Superdex 200 HR10/30 column (Amersham Pharmacia Biotech). Fractions of 1 ml were collected. To calibrate the column, a gel filtration calibration kit (Amersham Pharmacia Biotech) was used.
RESULTS
Neurotoxin Cleavage of Syntaxin 1 and VAMP2 Does Not Inhibit ISG–ISG Fusion
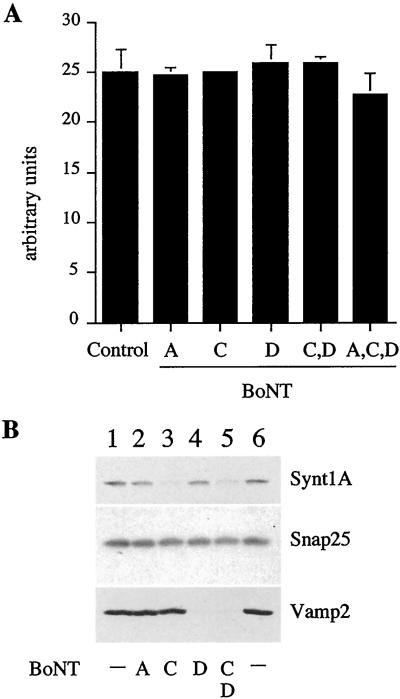
VAMP1/2, syntaxin 1, and SNAP-25 are the SNAREs involved in the fusion of synaptic vesicles with the presynaptic membrane in neuronal cells. The same SNAREs also have been found to be involved in exocytosis of chromaffin granules (Glenn and Burgoyne, 1996) and secretory granules in PC12 cells (Banerjee et al., 1996). Because secretory granules derive from ISGs, we asked whether any of the SNAREs involved in exocytosis are also involved in ISG–ISG fusion. To address this question, we studied the effect of BotNTs on ISG–ISG fusion with the use of an in vitro fusion assay that reconstitutes ISG–ISG fusion (Urbéet al., 1998). It has been established that syntaxin 1 is cleaved by BotNT serotype C and that SNAP-25 is cleaved by BotNT serotype A as well as by serotype C, whereas VAMP1/2 is cleaved by BotNT serotype D (Schiavo et al., 2000). PC2-ISGs were preincubated with the recombinant light chains of BotNT A, C, D, or C and D together in the presence of an ATP-regenerating system to ensure that all the SNAREs on the ISG membrane were accessible to the toxins. As shown in Figure 1A, pretreatment of PC2-ISGs with the BotNT A, C, and D in the presence of an ATP-regenerating system did not have any effect on ISG–ISG fusion, although all of the VAMP2 and the majority of the syntaxin 1 on the ISG membranes is cleaved (Figure 1B). Surprisingly, cleavage of SNAP-25 by either BotNT A or C is undetectable (Figure 1B). Lack of SNAP-25 cleavage was not due to neurotoxin inactivity as BotNT C was able to cleave syntaxin 1 and the activity of both toxins had been confirmed in independent experiments (G. Schiavo, personal communication).
Figure 1.
BotNT treatment does not inhibit ISG–ISG fusion. PC2 ISGs were treated with 20 nM BotNT A, C, D, or C and D, or A, C, and D for 30 min at 37°C in the presence of 0.5 mM ATP. (A) Pretreated or untreated ISGs were used in a fusion assay containing [35S]-PC12 ISGs, as described in MATERIALS AND METHODS. Control ISGs were incubated in buffer alone. The extent of fusion is compared with controls and represents the signal obtained after subtraction of the background signal obtained in the absence of ISGs containing PC2. (B) Aliquots of untreated or treated ISG were solubilized and were subjected to SDS-PAGE and immunoblotting with the indicated antibodies. As expected, BotNT C cleaved syntaxin 1 (Synt 1) and BotNT D cleaved VAMP2. SNAP-25 was resistant to cleavage by BotNT A or C.
Syntaxin 6 Is Present on ISGs
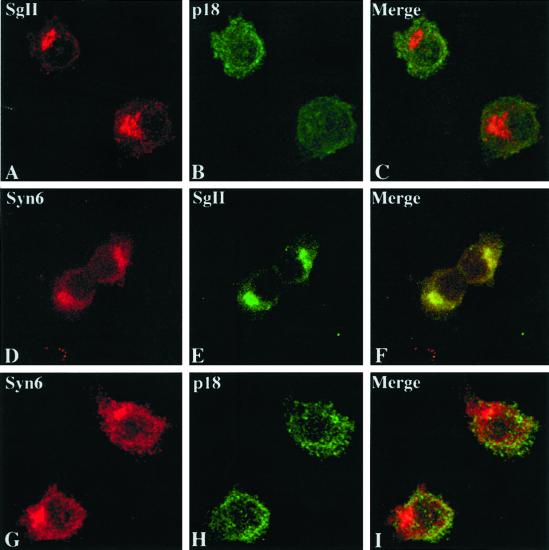
Because inactivation of the neuronal SNAREs known to be involved in exocytosis in PC12 cells did not inhibit ISG–ISG fusion, we have taken a “candidate” protein approach to define the SNAREs necessary for ISG–ISG fusion. Syntaxin 6 was shown earlier to be localized to the TGN and to be part of the regulated secretory pathway (Klumperman et al., 1998). To confirm and extend these findings, we investigated whether syntaxin 6 was present on ISGs in PC12 cells with the use of several approaches. First, we established that the subcellular localization of syntaxin 6 is consistent with it being present in vivo on early ISGs with the use of indirect immunofluorescence and confocal microscopy in PC12/PC2 cells, a PC12 cell line that is stably transfected with PC2 (Dittié and Tooze, 1995). We used two antibodies directed against SgII, one that only recognizes the full-length protein and one that only recognizes a shorter form of SgII, p18, that is produced from the full length by PC2 cleavage. PC2 processing of SgII, which requires an acidic pH, begins in the ISG but is optimal in the MSG. Processing in the ISG is slow compared with maturation, resulting in the majority of p18 being present in MSGs (Urbéet al., 1997). Thus, the antibody (called 175) specific for the full-length SgII should label the Golgi apparatus and newly formed ISGs, while the antibody specific for p18 is expected to label predominantly MSGs. With the use of the former antibody a perinuclear labeling was detected in PC12/PC2 cells corresponding to the Golgi apparatus and early ISG populations (Figure 2A). The latter antibody directed against p18, labels maturing ISG populations and MSGs (Figure 2B). Syntaxin 6 (Figure 2D) was found to colocalize with full-length SgII (Figure 2E) in the Golgi apparatus and early ISG populations (Figure 2F), Additional punctate syntaxin 6 staining was found over the entire cell, which represents the labeling of endosomes (Bock et al., 1997). Importantly, syntaxin 6 immunoreactivity (Figure 2G) did not significantly overlap with that of p18 (Figure 2, H and I), suggesting that syntaxin 6 is not present on MSGs.
Figure 2.
Codistribution of syntaxin 6 with a marker of ISGs but not MSGs. The distribution of endogenous SgII (A and E), p18 (B and H), and syntaxin 6 (D and G) in PC12/PC2 cells is shown by double labeling in confocal images, with the use of the anti-SgII antibody 175, the anti-p18 antibody 6B1/3, and anti-syntaxin 6 antibodies (D, mAb; G, polyclonal antibody), respectively. Superimposed images (C, F, and I) demonstrate the overlapping distribution of the syntaxin 6 only with SgII in TGN and ISGs. Full-length SgII, recognized by 175, appears mainly in the perinuclear region containing Golgi membranes and early ISGs (A), whereas p18 staining is found mainly distributed in maturing ISGs and MSGs distributed throughout the cytoplasm (B). Syntaxin 6 immunostaining can be found in the Golgi apparatus (D), where it colocalizes with SgII (E and F). In addition to the perinuclear Golgi staining, a punctate syntaxin 6 staining appears over the entire cell (D and G), which does not colocalize with p18 (H and I). Selected images from three independent experiments are shown.
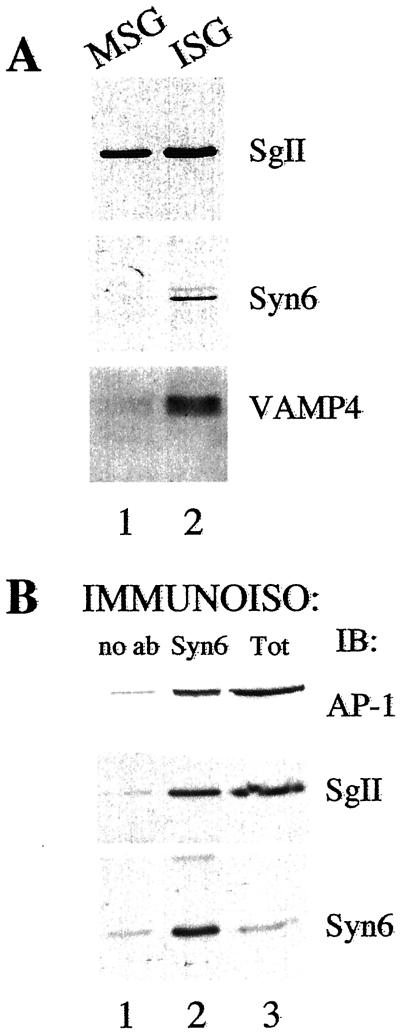
To confirm this result, we determined by immunoblotting with antibodies specific for syntaxin 6 the distribution of syntaxin 6 in the two secretory granule populations obtained by subcellular fractionation. ISGs and MSGs were isolated from PC12 cells with the use of sequential sucrose velocity and equilibrium gradient centrifugation (Dittiéet al., 1997). Syntaxin 6 immunoreactivity is distributed across the sequential gradients in a profile that is very similar to that previously observed for furin and M6PR (Dittiéet al., 1997, 1999), two proteins that are present in the TGN ISGs but not in MSGs. As seen in Figure 3A, when ISGs and MSGs containing equivalent amounts of SgII are compared with the use of antibodies specific for syntaxin 6, syntaxin 6 is only detectable in the ISG fraction. In addition, we find that the v-SNARE VAMP4, which has been reported to interact with syntaxin 6 (Steegmaier et al., 1999) and is found on ISGs in AtT20 cells (Eaton et al., 2000), also is present on ISGs but not MSGs in PC12 cells.
Figure 3.
Syntaxin 6 is present on ISGs from PC12 cells. PC12 ISGs and MSGs were isolated by subcellular fractionation involving two sequential sucrose gradients, as detailed in MATERIALS AND METHODS. (A) MSGs and ISGs were solubilized, subjected to SDS-PAGE, and immunoblotted with antibodies to SgII, syntaxin 6, and VAMP4. The number of MSGs and ISGs used were normalized to contain the same amount of the content protein SgII. (B) ISGs were subjected to immunoisolation with the use of empty beads (lane 1) or anti-syntaxin 6 beads (lane 2). The ISGs bound to the beads, and 1/10 the starting material (lane 3) was solubilized, subjected to SDS-PAGE, and subjected to immunoblotting with the antibodies indicated.
To demonstrate directly that the syntaxin 6 immunoreactivity detected in the ISG fractions was present on ISGs, and not on contaminating membranes present in the fraction, we performed immunoisolation experiments. Immunoisolation was performed with the use of the ISG fraction and the anti-syntaxin 6 antibodies, and the bound membranes were solubilized and analyzed. Anti-syntaxin 6 antibodies could efficiently immunoisolate ISGs containing the secretory granule marker, SgII, from the fraction while the control beads could not (Figure 3B). Importantly, the ISGs immunoisolated with anti-syntaxin 6 antibodies are positive for the clathrin adaptor protein AP-1 as expected from previous results in PC12 cells and β-cells (Dittiéet al., 1996; Kuliawat et al., 1997).
Syntaxin 6 Is Required for ISG–ISG Fusion
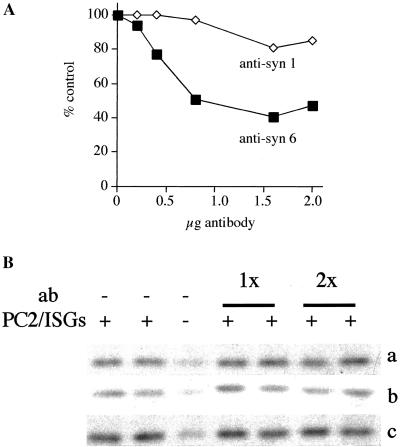
The evidence presented above indicates that syntaxin 6 must be removed from maturing ISGs because it is not present on MSGs. To test whether or not syntaxin 6 is involved in ISG–ISG fusion, we used the in vitro fusion assay, which reconstitutes ISG–ISG fusion (Urbéet al., 1998). Preincubation of the complete fusion reaction with monoclonal or affinity-purified polyclonal anti-syntaxin 6 antibody to the fusion assay resulted in a concentration-dependent inhibition of fusion up to a maximum of 60% (Figure 4A). This inhibition is successfully eliminated through competition by preincubation of the antibody with recombinant syntaxin 6.
Figure 4.
Antibodies to syntaxin 6, but not SNAP-25, SNAP-29, or VAMP4, inhibit ISG–ISG fusion. A standard fusion assay was performed with PC2 ISGs and [35S]-sulfate-labeled ISGs. Both ISG populations were preincubated with (A) monoclonal anti-syntaxin 6 or syntaxin 1, or (B) SNAP-25 (line a), SNAP-29 (line b), or VAMP4 (line c) then were supplemented with cytosol and nucleotides. In (B) 5 μl and 10 μl (1× and 2×, respectively) of monoclonal SNAP-25, 10 μl and 20 μl of polyclonal SNAP-29 (1× and 2×, respectively), and 10 μl and 20 μl (1× and 2×, respectively) of affinity-purified polyclonal VAMP4 (0.2 μg/μl) antibodies were used. In (line b) the controls shown are done in the presence 20 μl of nonspecific preimmune sera. Fusion was assayed by determining the amount of p18 produced, as detailed in MATERIALS AND METHODS, and was quantitated as shown in (A) or as shown in (B). For quantitation, the signals used are those obtained after subtraction of the background obtained in the absence of PC2 ISGs. In (A), a representative experiment from a total of three independent experiments, performed in duplicate, is shown. The experiments in (B) were repeated two times (lines a and b) and three times (line c).
With the use of recombinant fusion proteins as standards, we have estimated that ISGs have roughly equivalent amounts (∼ 200 pg/μg ISG protein) of syntaxin 6 and syntaxin 1. To rule out the possibility that the antibody inhibited fusion nonspecifically, an mAb against syntaxin 1 or affinity-purified rabbit polyclonal antibody against phogrin, a secretory granule membrane protein (Wasmeier and Hutton, 1996), were used as controls (Figure 4A and our unpublished results) and had no effect on fusion. Finally, the anti-syntaxin 6 antibodies did not block binding of α-SNAP to syntaxin 6, eliminating the possibility that the inhibition of fusion was due to the inhibition of α-SNAP and NSF binding.
Because antibodies against syntaxin 6 could inhibit ISG–ISG fusion, it should also be possible to inhibit fusion by the addition of soluble recombinant syntaxin 6 protein, which would be expected to assemble with the appropriate SNARE partners that are present on ISG membranes. We did not see any inhibition of fusion by the addition of the purified, recombinant soluble syntaxin 6 protein at a final concentration in the fusion reaction of up to 0.25 μg/μl (∼ 7.5 μM), under conditions that would allow for disassembly and/or reassembly of SNARE complexes. However, the soluble syntaxin 6 protein, either as a recombinant GST-fusion protein that is cleaved from GST by thrombin, or exogenously expressed in cells as a myc-tagged protein, did not form complexes with any SNAREs under any condition tested and, so, would be unable to act as a dominant negative reagent to inhibit SNARE assembly and fusion.
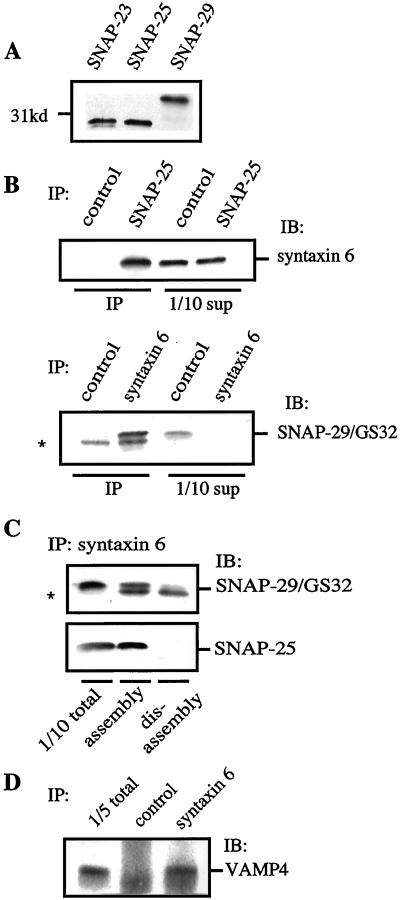
SNAP-25, SNAP-29/GS32, and VAMP4 Exist in a Protein Complex with Syntaxin 6 on ISG Membranes
SNAREs can assemble into complexes with defined SNARE partners depending on, and restricted by, where they are localized in the cell (Scales et al., 2000). Syntaxin 6 has been shown to assemble in detergent extracts with several SNAREs, including SNAP-29/GS32 (Wong et al., 1999), VAMP2 (Bock et al., 1997), and VAMP4 (Steegmaier et al., 1999). Because we identified syntaxin 6 as one component of the SNARE complex involved in ISG fusion, we asked which other SNAREs might take part in this process. We found SNAP-23, SNAP-25, and SNAP-29/GS32 by immunoblotting in the ISG fraction (Figure 5A), and therefore we investigated their possible interaction with syntaxin 6. When immunoprecipitation with syntaxin 6 antibodies was done with the use of solubilized ISG membrane fractions, both SNAP-25, and SNAP-29, but not SNAP-23, could be found in the immunoprecipitates (Figure 5, B and C, and our unpublished results). The reciprocal coimmunoprecipitation of syntaxin 6 with antibodies to SNAP-25 (Figure 5B) and SNAP-29 (our unpublished results) further confirmed these interactions and clearly established that both SNAP-25 and SNAP-29 can exist in a protein complex with syntaxin 6. Both SNAP-25:syntaxin 6 and SNAP-29:syntaxin 6 SNARE complexes can be dissociated by the concerted actions of α-SNAP and NSF, as shown in Figure 5C. Likewise, we have found that VAMP4 could be coimmunoprecipitated with syntaxin 6 from ISG membrane fractions (Figure 5D)
Figure 5.
Syntaxin 6 is in a SNARE complex with SNAP-25 as well as SNAP-29/GS32, and both are regulated by NSF and α-SNAP. (A) ISG fractions (100 μg of protein) were subjected to immunoblotting with the use of SNAP-23, SNAP-25, or SNAP-29/GS32 antibodies. (B) Detergent-solubilized ISG membrane fractions were subjected to immunoprecipitation with the use of monoclonal antibodies against SNAP-25, syntaxin 6, or a nonspecific mAb as a control. Immunoprecipitates were analyzed by SDS-PAGE and immunoblotting with polyclonal antibodies to syntaxin 6 or SNAP-29/GS32, respectively. Immunoblotting with SNAP-29/GS32 antibody revealed that SNAP-29/GS32 was detected in the syntaxin 6 immunoprecipitates. An unspecific band was detected in both the control and syntaxin 6 immunoprecipitations (*). SUP corresponds to the supernatant left after the immunoprecipiatation reaction. (C) Detergent-solubilized ISG membrane fractions (100 μg) were incubated for 30 min at 4°C with recombinant NSF (2 μg) and α-SNAP (2 μg) and EDTA/ATP or Mg/ATP to provide assembly or disassembly conditions, respectively, followed by immunoprecipitation with syntaxin 6 antibodies. Immunoprecipitates were analyzed by SDS-PAGE and immunoblotting with the use of antibodies against SNAP-25 or SNAP-29/GS32, respectively. (D) Detergent solubilized ISGs (750 μg) were incubated with anti-syntaxin 6 magnetic beads, or magnetic beads alone. Immunoprecipitates were analyzed by SDS-PAGE and immunoblotting with polyclonal antibodies to VAMP4.
Although we have found both SNAP-25 and SNAP-29 in a complex with syntaxin 6, we could not find an involvement of these molecules in homotypic ISG–ISG fusion. Antibodies specific for both SNAP-25 and SNAP-29 (Figure 4B, lines a and b) or the recombinant fusion proteins (our unpublished results) did not result in a detectable inhibition of the fusion. In similar experiments, we could not identify a requirement for VAMP4 in ISGs fusion (Figure 4B, line c, and our unpublished results). The anti-SNAP-25 antibody used here previously has been shown to inhibit Ca2+-dependent secretion of glutamate from synaptosomes (Mehta et al., 1996). Regarding the remaining antibodies, the failure of these antibodies to inhibit fusion was not due to their inability to bind ISGs, because we could demonstrate that the antibodies bound to the intact ISGs under the conditions used for the fusion assay.
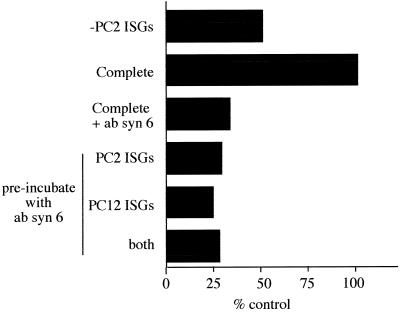
Homotypic ISG–ISG Fusion Requires Syntaxin 6 on Both Donor as well as Acceptor ISG Membranes
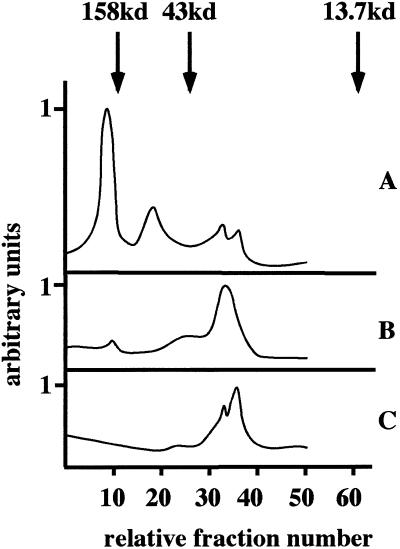
The inability of SNAP-25, SNAP-29 reagents, and the soluble syntaxin 6 protein to inhibit fusion raises a possibility either that syntaxin 6 is the only t-SNARE required for fusion or that syntaxin 6 forms a SNARE complex with SNAREs yet to be localized to ISGs. The former implies that syntaxin 6 could have an intrinsic ability to form homotypic t-t-SNARE pairs, and thus would be required on both donor and acceptor membranes. t-t-SNARE pairing was shown previously for Ufe1, a yeast SNARE involved in ER membrane fusion (Patel et al., 1998). To address this question, monoclonal syntaxin 6 antibodies were added to either the donor or acceptor ISGs. After a short incubation at 4°C, the ISGs were reisolated to remove the excess antibody, then were incubated under conditions that allow fusion to proceed. As shown in Figure 6, the incubation of either donor or acceptor membranes alone caused an efficient inhibition of fusion. These data suggest that syntaxin 6 is required on both donor and acceptor membranes for fusion to occur. One explanation would be a t-t-SNARE pairing that would also prevent an efficient inhibition of ISG fusion with recombinant syntaxin 6 protein due to the lack of syntaxin 6 monomers in the recombinant protein preparation. Indeed, gel filtration experiments with recombinant syntaxin 6 confirmed that this recombinant protein exists mainly as an oligomer. Recombinant syntaxin 6 was subjected to gel filtration with the use of a Superdex 200 column and was compared with SNAP-25 and syntaxin 4. Both SNAP-25 and the bulk of syntaxin 4 protein eluted at a position consistent with monomers, while syntaxin 6 under the same conditions eluted with a molecular weight consistent with an oligomeric state, corresponding mainly to a hexameric form (Figure 7).
Figure 6.
Anti-syntaxin 6 antibodies inhibit ISG–ISG fusion if added to only one population of ISGs. Anti-syntaxin 6 antibodies (concentration, 0.25 μg/μl) were added to the complete incubation on ice (complete + syn 6 ab). Alternatively, the PC2 ISGs, the PC12 ISGs, or both were preincubated with anti-syntaxin 6 antibodies, subjected to centrifugation, resuspended, and supplemented with the required components for fusion. A standard fusion assay was performed, and the amount of p18 produced was quantitated, as detailed in MATERIALS AND METHODS. A representative experiment performed in duplicate from three independent experiments is shown.
Figure 7.
The schematic representation of gel filtration analysis of syntaxin 6, syntaxin 4, and SNAP-25. The soluble recombinant (A) syntaxin 6, (B) syntaxin 4, or (C) full-length SNAP-25 proteins were subjected to gel filtration with the use of a Superdex 200 column. The fractions shown correspond to fraction nos. 60 through 120, with dextran blue eluting at fraction no. 32. For calibration of the gel filtration column, ribonuclease A (13.7 kDa), ovalbumin (43 kDa), and aldolase (158 kDa) were used as size standards.
DISCUSSION
We have investigated which SNAREs are involved in the homotypic fusion of ISGs in the neuroendocrine cell PC12. Initially, we focused on the neuronal SNARE complex, which consists of two t-SNAREs, syntaxin 1 and SNAP-25, that are associated with the neuronal plasma membrane, and the v-SNARE synaptobrevin/VAMP localized to synaptic vesicles. The same SNAREs are involved in the exocytosis of chromaffin granules (Glenn and Burgoyne, 1996) and secretory granules in PC12 cells (Banerjee et al., 1996). Furthermore, it is known that ∼ 20% of both syntaxin 1 and SNAP-25 are present on vesicles, although the majority are localized to the plasma membrane (Tagaya et al., 1995; Walch-Solinema et al., 1995; Gaisano et al., 1996). Pretreatment of ISG membrane fractions with BotNTs or specific antibodies directed against syntaxin 1, SNAP-25, or VAMP2 before fusion did not have any obvious effects on fusion. It is unlikely, therefore, that these SNAREs play a role in ISG maturation.
Surprisingly, SNAP-25 on the ISG membrane could not be cleaved by BotNT/A and C, although disassembly conditions for SNARE complexes were applied. This is in contrast to the situation at the plasma membrane, where BotNT/A efficiently cleaves SNAP-25 (Gerona et al., 2000). A pool of SNAP-25 that is resistant to toxin cleavage also has been found on chromaffin granules (Höhne-Zell and Gratzl, 1996). This result raises the possibility that on ISG membranes SNAP-25 could be protected from cleavage by BotNT/A by association with another protein. However, because syntaxin 1 is cleaved by BONT/C it is unlikely that the toxin-resistant SNAP-25 is found in a complex with syntaxin 1.
We have taken a candidate protein approach, which led to the finding that the t-SNARE syntaxin 6 is involved in homotypic ISG fusion. Syntaxin 6 previously was localized to the TGN, to vesicles in the vicinity of endosome-like structures, and to endosomes (Bock et al., 1997). Moreover, it has been reported that syntaxin 6 is part of the regulated secretory pathway and colocalizes with AP-1 containing clathrin-coated buds on the TGN and ISG in endocrine and exocrine cells (Klumperman et al., 1998). The indirect immunofluorescence, subcellular fractionation, and immunoisolation shown here confirm and extend these findings in PC12 cells. The absence of syntaxin 6 on MSGs strongly suggests that syntaxin 6 is removed during transit from ISGs by AP1 containing CCVs. This notion is supported by the presence of several potential sorting signals in the cytoplasmic domain of syntaxin 6, including two di-leucine motifs (at position 31–32 and 123–124) as well as one tyrosine-based sorting signal motif (YGRL at position 140–143). Recently, it was reported (Watson and Pessin, 2000) that the tyrosine-based motif in syntaxin 6 plays a role in the retrieval of syntaxin 6 from the plasma membrane back to the TGN in 3T3L1 adipocytes. Furthermore, it has been shown recently in human neutrophil cells, which are terminally differentiated cells and are largely depleted of Golgi membranes, that syntaxin 6 is mainly localized to the plasma membrane and plays a role in granule exocytosis (Martin-Martin et al., 2000). Taken together, these data support the following model in PC12 cells: Syntaxin 6 is sorted in the TGN into ISGs, then removed from the maturing secretory granule by CCVs, which are targeted to the early endosomes (Turner and Arvan, 2000). Syntaxin 6 then could shuttle to and from the plasma membrane or possibly could return back to the TGN via the endosomal system.
Syntaxin 6 in detergent-solubilized membrane extracts can be copurified with the SNARE molecules SNAP-29/GS32 (Wong et al., 1999), and VAMP4 (Steegmaier et al., 1999). In addition, VAMP2, cellubrevin, or both were found to coimmunoprecipitate with syntaxin 6 with the use of rat brain membranes (Bock et al., 1997). Our results confirmed these findings and showed for the first time that these syntaxin 6-containing SNARE complexes are also present in solubilized ISG membrane fractions. In addition, SNAP-25 was found in two separate complexes, either with syntaxin 1 or with syntaxin 6 in the ISG membrane fractions (our unpublished results). Although syntaxin 6 shows a promiscuous behavior and can build different SNARE complexes, we could not find syntaxin 6 in a SNARE complex with SNAP-23 on ISG membranes. The promiscuity of syntaxin 6 SNARE interactions is supported by data from the sedimentation analysis of SNAREs in detergent-solubilized rat brain membranes on glycerol gradients (Steegmaier et al., 1999). In contrast to VAMP2, VAMP4 and syntaxin 1, which all showed large stable complexes with defined sedimentation values, syntaxin 6 had a broad distribution across the entire gradient. None of the syntaxin 6–SNARE complexes were involved in ISG–ISG fusion: With the use of the available antibodies, and those we describe here, we could not find evidence for an involvement of VAMP2, VAMP4, SNAP-25, or SNAP-29.
Apart from the ability of syntaxin 6 to form several SNARE complexes with different members of the SNAP and VAMP families, we present evidence that ISG–ISG fusion requires syntaxin 6 on both ISG membranes. Similar results were observed during ER homotypic fusion obtained with the use of antibodies specific for Ufe1p (Patel et al., 1998). One possible explanation for the requirement for syntaxin 6 on both membranes is that ISG homotypic fusion requires a t-t-SNARE pair. If syntaxin 6 forms t-t-SNARE pairs during ISG membrane fusion, what would be the nature of this t-t-SNARE complex on ISG membranes? Syntaxin 6 is an unusual member of the syntaxin family because it appears to be more closely related to SNAP-25 than to other syntaxins (Bock et al., 1996). In addition, α-SNAP binds to the N-terminal coil-coil domain of syntaxin 6 (Bock et al., 1996), as has been found for SNAP-25, whereas α-SNAP has been shown to bind the C-terminal H3 helix of syntaxin 1a. This suggests that perhaps syntaxin 6 could assemble into an unusual SNARE complex and could supply either the light chain or the heavy chain of the t-SNARE. Gel-filtration experiments with recombinant syntaxin 6 protein lacking the trans-membrane domain revealed that syntaxin 6 can form dimers, however, the bulk of the recombinant protein is found predominantly in a molecular range corresponding to the predicted size of hexamers. An issue to address in future experiments is whether the oligomeric complexes identified here represent the relevant physiological functional complexes. In support of our data, Tishgarten et al. (1999) recently have shown a difference in the oligomerization state of different recombinant SNARE proteins under a variety of solution conditions. Although light-scattering results indicated that syntaxin 1 and the yeast ortholog Sso1p are monomeric, they suggested that the closest ortholog of syntaxin 6, Pep12p, predominantly forms dimers and trimers (Tishgarten et al., 1999).
The yeast SNARE Ufe1p was shown previously to undergo homomeric as well as heteromeric SNARE interactions (Patel et al., 1998). Ufe1p is implicated in the retrograde transport of vesicles to the ER (Lewis and Pelham, 1996) as well as in the homotypic fusion of ER membranes (Patel et al., 1998). Although homotypic ER fusion requires a homomeric t-t-SNARE pairing of Ufe1p, which is regulated by Cdc48/p97, Ufe1p functions in a v-t-SNARE pair during retrograde transport of vesicles to the ER and is sensitive to Sec18p/NSF and Sec17/α-SNAP. We have observed that the homotypic fusion of ISGs only requires NSF and does not need the action of the p97/p47 complex (Urbéet al., 1998). So, whereas syntaxin 6, like Ufe1p, may form different SNARE complexes during its transport through the ISG, the endosomal compartment, and the plasma membrane, the regulation of the different syntaxin 6 SNARE complexes cannot be due to a difference in the requirement for p97 vs. that for NSF.
There are two further alternative explanations for the inhibition that we have observed. First, the inhibition by antibodies on either ISG membrane could be due to a disruption of the symmetry of SNAREs that is required for efficient membrane fusion. The efficient homotypic fusion of yeast vacuoles requires the presence of the Nyv1p (v-SNARE) and Vam3p (t-SNARE) pair on each vacuole (Nichols et al., 1997). By isolating vacuoles from yeast strains missing one or both SNAREs, Nichols et al. (1997) demonstrated that the fusion efficiency decreased dramatically (down to 25% of the control) when a v-SNARE was only present on one and a t-SNARE was only present on the other. This result suggests that efficient fusion requires a pair of SNAREs on each membrane. This hypothesis is supported by the observation that vacuoles that have only a v-SNARE or only a t-SNARE undergo fusion inefficiently (between 25% and 40% of the control) with wild-type vacuoles. Thus, it is possible that the inhibition of fusion that we observe results from a loss of symmetry between vt-SNARE pairs on opposing membranes. Anti-syntaxin 6 antibodies added to one population may effectively be removing the t-SNARE from the treated ISGs, which when added to normal ISGs under fusion conditions results in an inefficient fusion between ISGs with one v-SNARE and one vt-SNARE pair.
Alternatively, the anti-syntaxin 6 antibody may be disrupting protein–protein interactions mediated by syntaxin 6. Homotypic membrane fusion, like heterotypic membrane fusion, requires a series of coordinated protein–protein interactions to complete priming, docking, and finally fusion. Docking requires the tethering of membranes containing primed t-SNAREs (Wickner and Haas, 2000) and is facilitated by molecules such as EEA1 and Rabenosyn 5, which bind to both phosphatidylinositol 3-phosphate and rab5-GTP on early endosomes (Christoforidis et al., 1999; Nielsen et al., 2000). Both EEA1 and Rabenosyn 5 are required for endosome–endosome fusion (Mills et al., 1998; Nielsen et al., 2000). EEA1 has been shown to be assembled into oligomeric complexes containing NSF and SNAREs (McBride et al., 1999), and, intriguingly, Rabenosyn 5 also has been shown to bind the SNAREs required for endosome–endosome fusion, as well as vps45, an sec−1-like molecule that binds syntaxin 6 (Nielsen et al., 2000). Thus, it is possible that anti-syntaxin 6 antibodies inhibit the recruitment of tethering molecules, which are required to bind to both membranes, as has been previously observed with EEA1 (Mills et al., 1998) and Rabenosyn 5 (Nielsen et al., 2000).
Understanding why syntaxin 6 is required on both membranes requires more information about the syntaxin 6 complex on the ISGs. In future experiments, we will investigate if the homo-oligomerization of syntaxin 6 represents the situation on intact ISG membranes. Our experiments also will be focused on the composition of the syntaxin 6 SNARE complex and accessory molecules interacting with syntaxin 6 during homotypic ISG fusion.
ACKNOWLEDGMENTS
We thank W. Hong for the GS32/SNAP-29 antibodies, P. Roche for anti-SNAP23 antibodies, Claire Thomas and Giovanna Lalli for help with confocal microscopy, and Giampietro Schiavo and Graham Warren for advice, stimulating discussions, and reading of the manuscript. We thank John Tooze, Dave Shima, Jim Shorter, and Joyce Müller for reading the manuscript and members of the Tooze laboratory for help and advice. F.W. was supported by a Schrödinger fellowship from the Austrian FWF.
Abbreviations used:
- AP-1
adaptor protein-1
- CCV
clathrin-coated vesicle
- ISG
immature secretory granule
- M6PR
mannose-6-phosphate receptor
- MSG
mature secretory granule
- NSF
N-ethylmaleimide-sensitive fusion protein
- PC2
prohormone convertase 2
- SgII
secretogranin II
- SNAP
soluble N-ethylmaleimide-sensitive fusion protein attachment protein
- SNARE
soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor
REFERENCES
- Advani RJ, Bae H-R, Bock JB, Chao DS, Doung Y-C, Prekeris R, Yoo J-S, Scheller RH. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J Biol Chem. 1998;273:10317–10324. doi: 10.1074/jbc.273.17.10317. [DOI] [PubMed] [Google Scholar]
- Arvan P, Castle D. Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem J. 1998;332:593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin C, Hinners I, Tooze SA. Direct and GTP-dependent Interaction of ADP-ribosylation factor 1 with clathrin adaptor protein AP-1 on immature secretory granules. J Biol Chem. 2000;275:21862–21869. doi: 10.1074/jbc.M908875199. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Barry VA, Bibhuti DR, Martin TFJ. N-Ethylmaleimide-sensitive FACTOR acts at a prefusion ATP-dependent step in Ca2+-activated exocytosis. J Biol Chem. 1996;271:20223–20226. doi: 10.1074/jbc.271.34.20223. [DOI] [PubMed] [Google Scholar]
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesices at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Bennett MK, Garcia-Arraras JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, Scheller RH. The syntaxin family of vesicular transport receptors. Cell. 1993;74:863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- Binz T, Blasi J, Yamasaki S, Baumeister A, Link E, Sudhof TC, Jahn R, Niemann H. Proteolysis of SNAP-25 by types E and A botulinal neurotoxins. J Biol Chem. 1994;269:1617–20. [PubMed] [Google Scholar]
- Bock JB, Klumperman J, Davanger S, Scheller RH. Syntaxin 6 functions in trans Golgi network vesicle trafficking. Mol Biol Cell. 1997;8:1261–1271. doi: 10.1091/mbc.8.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock JB, Lin RC, Scheller RH. A new syntaxin family member implicated in targeting of intracellular transport vesicles. J Biol Chem. 1996;271:17961–17965. doi: 10.1074/jbc.271.30.17961. [DOI] [PubMed] [Google Scholar]
- Christoforidis S, McBride HM, Burgoyne RD, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999;397:621–625. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- Clague MJ, Herrmann A. Membrane transport: deciphering fusion. Curr Biol. 2000;10:R750–R752. doi: 10.1016/s0960-9822(00)00741-7. [DOI] [PubMed] [Google Scholar]
- Conradt B, Shaw J, Vida T, Emr S, Wickner W. In vitro reactions of vacuole inheritance in Saccharomyces cerevisiae. J Cell Biol. 1992;119:1469–1479. doi: 10.1083/jcb.119.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittié A, Tooze S. Characterization of the endopeptidase PC2 activity towards SgII in stably transfected PC12 cells. Biochem J. 1995;310:777–787. doi: 10.1042/bj3100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittié AS, Hajibagheri N, Tooze SA. The AP-1 adaptor complex binds to immature secretory granules from PC12 cells, and is regulated by ADP-ribosylation factor. J Cell Biol. 1996;132:523–536. doi: 10.1083/jcb.132.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittié AS, Klumperman J, Tooze SA. Differential distribution of mannose-6-phosphate receptors and furin in immature secretory granules, J. Cell Sci. 1999;112:3955–3966. doi: 10.1242/jcs.112.22.3955. [DOI] [PubMed] [Google Scholar]
- Dittié AS, Thomas L, Thomas G, Tooze SA. Interaction of furin in immature secretory granules from neuroendocrine cells with the AP-1 adaptor complex is modulated by casein kinase II phosphorylation. EMBO J. 1997;16:4859–4870. doi: 10.1093/emboj/16.16.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton BA, Haugwitz M, Lau D, Moore HP. Biogenesis of regulated exocytotic carriers in neuroendocrine cells. J Neurosci. 2000;20:7334–7344. doi: 10.1523/JNEUROSCI.20-19-07334.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda R, McNew JA, Weber T, Parlati F, Engel T, Nickel W, Rothman JE, Sollner TH. Functional architecture of an intracellular membrane t-SNARE. Nature. 2000;407:198–202. doi: 10.1038/35025084. [DOI] [PubMed] [Google Scholar]
- Gaisano HY, Ghai M, Malkus PN, Sheu L, Bouquillion A, Bennett MK, Trimble WS. Distinct cellular locations of the syntaxin family of proteins in rat pancreatic acinar cells. Mol Biol Cell. 1996;7:2019–2027. doi: 10.1091/mbc.7.12.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerona RRL, Larsen EC, Kowalchyk JA, Martin TFJ. The C terminus of SNAP25 is essential for Ca2+-dependent binding of synaptotagmin to SNARE complexes. J Biol Chem. 2000;275:6328–6336. doi: 10.1074/jbc.275.9.6328. [DOI] [PubMed] [Google Scholar]
- Glenn DE, Burgoyne RD. Botulinum neurotoxin light chains inhibit both Ca2+-induced and GTP analogue induced catecholamine release from permeabilized adrenal chromaffin cells. FEBS Lett. 1996;386:137–140. doi: 10.1016/0014-5793(96)00432-2. [DOI] [PubMed] [Google Scholar]
- Gruenberg JE, Howell KE. Reconstitution of vesicle fusions occurring in endocytosis with a cell-free system. EMBO J. 1986;5:3091–101. doi: 10.1002/j.1460-2075.1986.tb04615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhne-Zell B, Gratzl M. Adrenal chromaffin cells contain functionally different SNAP-25 monomers and SNAP-25/syntaxin heterodimers. FEBS Lett. 1996;394:109–116. doi: 10.1016/0014-5793(96)00931-3. [DOI] [PubMed] [Google Scholar]
- Jahn R, Sudhof TC. Membrane fusion and exocytosis. Annu Rev Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- Klumperman J, Kuliawat R, Griffith JM, Geuze HJ, Arvan P. Mannose 6-phosphate receptors are sorted from immature secretory granules via adaptor protein AP-1, clathrin, and syntaxin 6-positive vesicles. J Cell Biol. 1998;141:359–371. doi: 10.1083/jcb.141.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuliawat R, Klumperman J, Ludwig T, Arvan P. Differential sorting of lysosomal enzymes out of the regulated secretory pathway in pancreatic β-cells. J Cell Biol. 1997;137:595–608. doi: 10.1083/jcb.137.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land J, Zhang H, Vaidyanathan VV, Sadoul K, Niemann H, Wollheim CB. Transient expression of botulinum neurotoxin C1 light chain differentially inhibits calcium and glucose induced insulin secretion in clonal beta-cells. FEBS Lett. 1997;419:13–17. doi: 10.1016/s0014-5793(97)01411-7. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Pelham HR. SNARE-mediated retrograde traffic from the Golgi complex to the endoplasmic reticulum. Cell. 1996;19:205–215. doi: 10.1016/s0092-8674(00)81097-1. [DOI] [PubMed] [Google Scholar]
- Martin-Martin B, Nabokina SM, Blasi J, Lazo PA, Mollinedo F. Involvement of SNAP-23 and syntaxin 6 in human neutrophil exocytosis. Blood. 2000;96:2574–2583. [PubMed] [Google Scholar]
- Mayer A. Intracellular membrane fusion: SNAREs only? Curr Opin Cell Biol. 1999;11:447–52. doi: 10.1016/S0955-0674(99)80064-7. [DOI] [PubMed] [Google Scholar]
- Mayer A, Wickner W. Docking of yeast vacuoles is catalyzed by the ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF), J. Cell Biol. 1997;136:307–317. doi: 10.1083/jcb.136.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride HM, Rybin V, Murphy C, Giner A, Teasdale R, Zerial M. Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell. 1999;98:377–386. doi: 10.1016/s0092-8674(00)81966-2. [DOI] [PubMed] [Google Scholar]
- McNew JA, Parlati F, Fukuda R, Lohnston RJ, Paz K, Paumet F, Solliner TH, Rothman JE. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature. 2000;407:153–159. doi: 10.1038/35025000. [DOI] [PubMed] [Google Scholar]
- Mehta PP, Battenberg E, Wilson MC. SNAP-25 and synaptotagmin involvement in the final Ca(2+)-dependent triggering of neurotransmitter exocytosis. Proc Natl Acad Sci USA. 1996;93:10471–10476. doi: 10.1073/pnas.93.19.10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills I, Jones A, Clague MJ. Involvement of the endosomal autoantigen EEA1 in homotypic fusion of early endosomes. Curr Biol. 1998;16:881–884. doi: 10.1016/s0960-9822(07)00351-x. [DOI] [PubMed] [Google Scholar]
- Nichols JB, Ungermann C, Pelham HRB, Wickner WT, Haas A. Homotypic vacuolar fusion mediated by t-and v-SNAREs. Nature. 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]
- Nielsen E, Christoforidis S, Uttenweiler-Joseph S, Miaczynska M, Dewitte F, Wilm M, Hoflack B, Zerial M. Rabenosyn-5, a NOVEL Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain. J Cell Biol. 2000;151:601–612. doi: 10.1083/jcb.151.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyler GA, Higgins GA, Hart RA, Battenberg E, Billingley M, Bloom FE, Wilson MC. The identification of a novel synaptosomal-associated protein SNAP-25, differentially expressed by neuronal subpopulations, J. Cell Biol. 1989;109:3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SK, Indig FE, Olivieri N, Levine ND, Latterich M. Organelle membrane fusion: a novel function for the syntaxin homolog Ufe1p in ER membrane fusion. Cell. 1998;92:611–620. doi: 10.1016/s0092-8674(00)81129-0. [DOI] [PubMed] [Google Scholar]
- Rabouille C, Kondo H, Newman R, Hui N, Freemont P, Warren G. Syntaxin 5 is a common component of the NSF- and p97-mediated reassembly pathways of Golgi cisternae from mitotic Golgi fragments in vitro. Cell. 1998;92:603–610. doi: 10.1016/s0092-8674(00)81128-9. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Martin TF. Docking and fusion in neurosecretion. Curr Opin Cell Biol. 1998;10:483–492. doi: 10.1016/s0955-0674(98)80063-x. [DOI] [PubMed] [Google Scholar]
- Roy L, Bergeron JJ, Lavoie C, Hendriks R, Gushue J, Fazel A, Pelletier A, Morre DJ, Subramaniam VN, Hong W, Paiement J. Role of p97 and syntaxin 5 in the assembly of transitional endoplasmic reticulum. Mol Biol Cell. 2000;11:2529–2542. doi: 10.1091/mbc.11.8.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales SJ, Bock JB, Scheller RH. The specifics of membrane fusion. Nature. 2000;407:144–146. doi: 10.1038/35025176. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol Rev. 2000;80:717–766. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- Söllner T, Whiteheart S, Brunner M, H. E-B, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Steegmaier M, Klumperman J, Foletti DL, Yoo J-S, Scheller RH. Vesicle-associated membrane protein 4 is implicated in trans-Golgi network vesicle trafficking. Mol Biol Cell. 1999;10:1957–1972. doi: 10.1091/mbc.10.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Tagaya M, Toyonaga S, Takahashi M, Yamamoto A, Fujiwara T, Akagawa K, Moriyama Y, Mizushima S. Syntaxin 1 (HPC-1) is associated with chromaffin granules. J Biol Chem. 1995;270:15930–15933. doi: 10.1074/jbc.270.27.15930. [DOI] [PubMed] [Google Scholar]
- Tishgarten T, Yin FF, Faucher KM, Dluhy RA, Grant TR, Fischer von Mollard G, Stevens TH, Lipscomb LA. Structures of yeast vesicle trafficking proteins. Protein Sci. 1999;8:2465–2473. doi: 10.1110/ps.8.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze SA. Biogenesis of secretory granules in the trans-Golgi network of neuroendocrine and endocrine cells. Biochim Biophys Acta. 1998;1404:231–244. doi: 10.1016/S0167-4889(98)00059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze SA, Flatmark T, Tooze J, Huttner WB. Characterization of the immature secretory granule, an intermediate in granule biogenesis. J Cell Biol. 1991;115:1491–1503. doi: 10.1083/jcb.115.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze SA, Hollinshead M, Dittié AS. Antibodies to secretogranin II reveal potential processing sites. Biochimie. 1994;76:271–276. doi: 10.1016/0300-9084(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Turner MD, Arvan P. Protein traffic from the secretory pathway to the endosomal system in pancreatic beta-cells. J Biol Chem. 2000;275:14025–14030. doi: 10.1074/jbc.275.19.14025. [DOI] [PubMed] [Google Scholar]
- Ungermann C, Nicholls BJ, Pelham HRB, Wickner W. A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion. J Cell Biol. 1998a;140:61–69. doi: 10.1083/jcb.140.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C, Sato K, Wickner W. Defining the functions of trans-SNARE pairs. Nature. 1998b;396:543–548. doi: 10.1038/25069. [DOI] [PubMed] [Google Scholar]
- Urbé S, Dittié S, Tooze SA. pH-Dependent processing of secretogranin II by the endopeptidase PC2 in isolated immature secretory granules. Biochem J. 1997;321:65–74. doi: 10.1042/bj3210065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbé S, Page LJ, Tooze SA. Homotypic fusion of immature secretory granules during maturation in a cell-free assay. J Cell Biol. 1998;143:1831–1844. doi: 10.1083/jcb.143.7.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solinema C, Blasi J, Edelman L, Chapman ER, Fischer von Mollard G, Jahn R. The t-SNARE syntaxin 1 and SNAP25 are present on organelles that participate in synaptic vesicle recycling. J Cell Biol. 1995;128:637–645. doi: 10.1083/jcb.128.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmeier C, Hutton JC. Molecular cloning of phogrin, a protein-tyrosine phosphatase homologue localized to insulin secretory granule membranes. J Biol Chem. 1996;271:18161–18170. doi: 10.1074/jbc.271.30.18161. [DOI] [PubMed] [Google Scholar]
- Watson RT, Pessin JE. Functional cooperation of two independent targeting domains in syntaxin 6 is required for its efficient localization in the trans-Golgi network of 3T3L1 adipocytes. J Biol Chem. 2000;275:1261–1268. doi: 10.1074/jbc.275.2.1261. [DOI] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westerman B, Gmachi M, Parlati F, Söllner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Whiteheart SW, Griff IC, Brunner M, Clary DO, Mayer T, Buhrov SA, Rothman JE. SNAP family of NSF attachment proteins includes a brain-specific isoform. Nature. 1993;362:353–355. doi: 10.1038/362353a0. [DOI] [PubMed] [Google Scholar]
- Wickner W, Haas A. Yeast homotypic vacuole fusion: a window on organelle trafficking mechanisms. Annu Rev Biochem. 2000;69:247–275. doi: 10.1146/annurev.biochem.69.1.247. [DOI] [PubMed] [Google Scholar]
- Wong SH, Xu Y, Zhang T, Griffiths G, Lowe SL, Subramaniam VN, Seow KT, Hong W. GS32, a novel Golgi SNARE of 32 kDa, interacts preferentially with syntaxin 6. Mol Biol Cell. 1999;10:119–134. doi: 10.1091/mbc.10.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Sato K, Wickner W. LMA1 binds to vacuoles at Sec18p (NSF), transfers upon ATP hydrolysis to a t-SNARE (Vam3p) complex, and is released during fusion. Cell. 1998;93:1125–1134. doi: 10.1016/s0092-8674(00)81457-9. [DOI] [PubMed] [Google Scholar]