Abstract
With the advent of superresolution imaging methods, fast dynamic imaging of biological processes in live cells remains a challenge. A subset of these methods requires the cellular targets to be labeled with spontaneously blinking probes. The delivery and specific targeting of cytosolic targets and the control of the probes’ blinking properties are reviewed for three types of blinking probes: quantum dots, synthetic dyes, and fluorescent proteins.
Keywords: Single molecule localization microscopy, Superresolution imaging, Optical nanoscopy, Quantum dots, Synthetic dyes, Fluorescent proteins
Optical microscopy and especially fluorescence microscopy have played key roles in studying cellular structures and functions, down to subcellular resolution. The high contrast, high sensitivity and minimal invasiveness attributes of fluorescence have afforded continuous imaging of live cell processes. However, due to the diffraction of light1, these methods were limited to spatial resolution of around 250–300 nm. In a quest to increase resolving power while maintaining the advantages of fluorescence microscopy, several far field super resolution (SR) techniques have been introduced during the past decade2–7.
A sub-set of these methods relies on single molecule localization, often referred to as “localization microscopy” methods (including PALM8, fPALM9, STORM10, dSTORM11, and GSD12). Localization microscopy methods are based on the precise localization of individual molecules via temporal separation, achieved either by photoactivation (PALM, fPALM, STORM, dSTORM and GSD), photobleaching (gSHRImP13), binding and dissociation (PAINT14, BALM15), or stochastic blinking (GSD, STORM and dSTORM).
Recently, we introduced a superresolution method dubbed ‘superresolution optical fluctuation imaging’, or SOFI16, that is based on stochastic blinking of probes, but does not rely on single molecule localization. Instead, a high-order statistical analysis of the probes’ temporal fluctuation is performed on the data set16–22. The method exhibits an attractive trade-off between achievable resolution and speed: Its resolution enhancement is not as high as in localization microscopies, but it can acquire and analyze the data much faster, and therefore it is suitable for SR imaging in live cells17.
Quite a few reports of SR imaging in live cells 23–43 and even in live organisms44–47 have been published in recent years. Specific labeling of molecular structures of interest with SR-compatible fluorophores in live cells is still quite challenging. Since probes that are conjugated to antibodies are not suitable for work in live cells, specific targeting has therefore been mostly limited to genetic markers (requiring genetic manipulation). However, the photophysical properties of such probes are not necessarily optimized for SR. Much work is still needed to improve SR probes and to develop better methods for targeting them to structures of interest in live cells.
This perspective focuses on targeting cytosolic structures in live cells with stochastically blinking probes that are suitable for SOFI and some of the other SR methods (STORM/dSTORM, GSD, PALM/fPALM). We will discuss several approaches, including delivery and targeting of blinking quantum dots (QDs), delivery and targeting of permeable blinking dyes to genetically encoded tags, delivery and targeting of permeable fluorogens to genetically encoded activating peptides (FAPs), and genetically encoded blinking fluorescence proteins (FPs). We conclude with a discussion and future outlook.
Quantum dots (QDs)48,49 have gained prominence in fluorescence imaging applications due to their unique photophysical properties. Some of their drawbacks (such as size, toxicity, non-specific binding) have, however, hindered their wider dissemination. Another QDs’ attribute that has been considered to be a drawback is their blinking50, a property that affects their brightness and undermines their performance in single molecule tracking experiments.
Blinking, though, could also be advantageous in some uses. Lidke et al. were first to take advantage of QDs' blinking to achieve SR imaging by single molecule localization51. However, since QDs' blinking is stochastic and is governed by a power-law statistics50 it is not ideal for localization microscopy because it is difficult to control the number of QDs in the ‘on’ and the ‘off’ states in each movie frame as is done with photoswitchable probes. In contrast, QDs blinking is ideal for the SOFI method (Fig. 1)16. Due to QDs’ high brightness and extraordinary photostability, long term acquisition can be achieved with low dose excitation light, possibly reducing phototoxicity in live cells. QDs could therefore serve as ideal probes for fast live cell SR imaging.
Fig. 1.
(A) A wide-field image of the microtubule network of a fixed 3T3 cell immunostained with QD800; (B) A second-order SOFI image of the same cell as in (A); (C) A Fourier reweighted second-order SOFI image of the same cell as in (A); Scale bar: 10 µm. Lower panel: Cross-sections as highlighted in (A) and compared for (A), (B) and (C): (A) in black, (B) in blue and (C) in red. (From ref 21, Reprinted with permission of the Optics Society for Optics Express.)
The big challenge is, of course, to label cytosolic structures in live cells with QDs. QDs conjugated to antibodies, streptavidin, and other small biomolecules or haptens have been successfully applied to fixed cells (immunocytochemistry) and to membrane proteins in live cells52–63. Although many works report on successful targeting of QDs to cytosolic structures64, this task is still very challenging. It requires an efficient delivery that escapes the endocytotic pathway, supplying freely diffusing QDs inside the cytosol. In addition, QDs should have minimal steric hindrance (i.e. small size) and minimal nonspecific binding so that they can find and specifically bind to their target(s). Extensive efforts have been vested in surface coating57,61,64–69, labeling strategy52–63,70 and developing cytosolic targeting methods64,66,71–77. As for the cytosolic delivery methods, they can be classified into two main categories: (i) Facilitated delivery strategies, using cell penetrating peptides76,77, proton sponge polymer carriers74,75, pinocytosis64,73,78, and transfection reagents64. Methods belonging to this category provide high throughput delivery, but suffer from low efficiency release of endosome-free QDs. As a result, most QDs still end up trapped in endosomes and provide background. (ii) Active delivery methods include electroporation71,72 and microinjection66. Electroporation offers an efficient way of delivery by temporally destabilizing the plasma membrane to create transient pores using high voltage electrical pulses. However, this method causes low cell viability, aggregation of the payload and low uptake of large objects64. The other active method, microinjection, is the most efficient and direct way to deliver QDs into the cytoplasm. For example, Lisse et al. delivered fluorescent nanoparticles to target intracellular structures through this technique79. The delivery is through a sharp glass microcapillary tip (with a diameter < 0.5µm) that mechanically penetrates the cell membrane80,81. However, the injection of QDs and QDs-protein conjugates is difficult and inconsistent due to QDs aggregation and repeated tip clogging. Recently we successfully delivered tubulin-QD conjugates into the cytoplasm of HeLa cell by the photothermal nanoblade82–84. As shown in figure 2a, a green laser pulse was applied on a titanium coated glass capillary pipette tip that was positioned in a close proximity to the cell membrane. Surface plasmon absorption of the metal coating conducted heat into the local liquid medium, which in turn generated explosive vapor bubbles right next to the cell membrane. Those bubbles produced temporal destabilization of the membrane. Simultaneously, a pressure pulse was applied to the capillary, generating a transient liquid flow through the transient membrane pores into the cytosol. The injected QDs-tubulin conjugates (Figure 2b) were incorporated into growing microtubules in the cell and showed clear filamentous structure as shown in Figure 2c. The photothermal nanoblade delivery technique greatly reduces the mechanical damage to cells (compared to conventional microinjection) since the tip does not puncture the cell membrane. Most importantly, the relatively large size of the tip’s orifice (2µm) enables it to deliver aggregation prone QDs-protein conjugate efficiently and consistently. The method suffers, however, from a low throughput (only one cell is injected at a time). Nonetheless, one can envision higher throughput delivery inspired by the photothermal nanoblade method.
Fig. 2.
(A) Photothermal nanoblade delivery of tubulin-QD conjugates into the cytosol; (B) Scheme for tubulin-QDs conjugation; (C) Wide-field image of a live HeLa cell after photothermal nanoblade delivery of tubulin-QD conjugates; (D) Zoom-in of boxed area in (C). Scale bar: 8 µm. (From ref 82, Reprinted with permission of the American Chemistry Society for Nano Letters.)
The example shown in Fig. 2 gives a hint to future possibilities that could be opened-up by dynamic live cell SR imaging with blinking QDs. For this vision to materialize, smaller, brighter, more inert QDs, and higher throughput delivery and targeting methods, need to be developed.
QDs are not the only fluorescent probes that blink. In fact, all conventional organic dyes blink as well, due to intersystem crossing to a shelved triplet state or other dark states85–90. STORM, dSTORM and GSD are based on these phenomena.
The Sauer group has extensively studied the mechanisms of dyes’ blinking using a combination of reducing and oxidizing agents in the buffer (Reducing Oxidizing System or ROXS buffer)85,86,88. These agents can greatly affect the radical anion and radical cation states of the fluorophore and hence modulate its blinking behavior.
Variants of ROXS buffers have been shown to work for Rhodamine, Cyanine and Oxazine dyes, covering the visible spectral range. However, for the localization microscopy methods, a stable off-state is required (e.g. the τoff/τon should be high enough for precise localization). In addition, independent tuning of ROXS buffers to achieve stochastic sparse blinking of multiple dyes simultaneously is not possible, making multicolor imaging very difficult. Of particular interest to this perspective, fine-tuning of the ROXS buffer and illumination intensity could generate blinking rates that are too fast or have an unfavorable τoff/τon ratio for localization microscopy, but are ideal for SOFI20,22, perhaps even allowing multicolor analysis in spite of the differences in blinking dynamics.
Although ROXS buffers have been successfully applied to in vitro sample imaging, the effect of exogenous ROXS to living cells is yet unknown. Fortunately, the presence of the endogenous molecules glutathione and oxidized glutathione (GSH and GSSG) in the cells serves as an effective, natural, ROXS. Indeed, several recent STORM and dSTORM works report on dyes blinking in live cells30,33. Fast blinking kinetics are essential for fast SR imaging. Blinking rates (kon = 1/τon and koff = 1/τoff) could be easily increased by increasing the lasers’ intensities for excitation and photoactivation. This, in turn, however, could increase phototoxicity and cell death, and therefore is suitable only for short observation times. Alternatively, one could take advantage of the fact that concentrations of GSH and GSSG vary in different cell types, and even within organelles in the same cell, in order to tailor the required blinking behavior.
As in the case of QDs, synthetic dyes also need to first be delivered into the cytosol in order to target structures in live cells. However, the size and chemical form of dyes can make them cell permeable, simplifying the cytosolic delivery substantially. In addition, typical dye-adducts are constitutively monovalent (achieving a 1:1 labeling stoichiometry), simplifying analysis of labeling density and reducing the chances of valency-based biological perturbation.
One approach for dye targeting can rely on the large body of work invested in developing various cellular indicators. These are permeable dyes that spontaneously recognize and non-covalently bind cytosolic structures (and / or specific proteins in them). In particular, some commercially available plasma membrane dyes, mitochondrial-tracker, ER tracker, and lysotracker have been shown to blink and to perform well for STORM imaging41, and DNA-based dyes have been used to visualize nanostructures and chromosome organization in fixed cells15.
Dyes can also be specifically targeted using genetically encoded tags that are fused to proteins of interest, in conjunction with the expression of post-translation modifying enzymes that catalyze covalent bonding between the dye and the target91. Some of these approaches, like Halo-tag92, SNAP-tag93,94 and CLIP-tag95 have even been commercialized. As an example, the SNAP-tag is engineered from the human repair protein O6-alkylguanine-DNA alkyltransferase (hAGT) that covalently reacts with benzylguanine derivatives. This tag protein can be genetically fused to any protein of interest. A dye labeled with benzylguanine recognizes the tagged protein and reacts with it to form a covalent bond (Figure 3a). This and similar technologies were recently used for live cell STORM33 and dSTORM30 imaging. Similarly, we used the dye Dy505 to label the histone protein H2B that was fused to the CLIP tag to image chromatin in live cells by SOFI (Fig. 3b–3c, and to be published).
Figure 3.
(A) Scheme for snap-tag conjugation with a Benzylguanine dye; (B) A wide-field image of dye505 labeled H2B clip tag in a live HeLa cell; (C) Second-order SOFI image of the cell in (B). Scale bar: 5 µm.
One disadvantage of tag-based technologies is the background due to non-specific binding. Sun et al.96 elegantly designed a molecule in which both a fluorescent dye and a quencher are conjugated to the benzylguanine moiety. Upon labeling to the SNAP-tag, the quencher with the benzylguanine group is cleaved away, leaving behind the dye which is now covalently conjugated to the tag while liberating its fluorescence. Another disadvantage of tag-based technologies is the irreversible nature of the covalent bond formation. Once the dye is bleached, the tag site is no longer available and therefore the overall occupancy of dyes on the targeted structure is diminished over time due to photobleaching. The FAPs technology that is described next solves this problem.
It is important to note that synthetic dyes are smaller than QDs and FPs, an attribute that can be important in some applications (but the tags themselves have a molecular weight ‘penalty’ similar to that of a fluorescent protein). Dyes have moderate brightnesses and photostabilities in comparison to QDs. Finding bright dyes that blink well under natural physiology conditions in live cell and methods for their specific targeting will greatly assist live cell SR imaging.
Stochastic blinking of probes could be achieved not only by controlling their photophysical properties, but also through binding and dissociation of probes freely diffusing in solution onto/from stationary targets. During the brief immobilization period, the diffused and dim signal of a mobile probe converts into a stable, high signal coming from a spot that can be localized with good accuracy. Furthermore, the quantum yield of the probe could increase during immobilization due to its rigidification when bound to the target. Blinking in this case is achieved by controlling the ‘on’ and ‘off’ rates of the probes to their targets. The localization microscopy method dubbed ‘point accumulation for imaging in nanoscale topography’, or PAINT, is based on this concept14.
In PAINT, normally quenched, freely diffusing probes brighten-up when they bind to their targets. This brightening enhances the contrast and signal-to-noise ratio. Fluorogen activating peptides (FAPs)97–99 are a realization of the PAINT concept that affords specific molecular targeting (in contrast to traditional approaches that are selective for chemical environments such as membranes or DNA)14,15.
FAPs are genetically encoded tags that noncovalently interact with weak (quenched) fluorophores (fluorogens) to activate bright fluorescence upon binding. The equilibrium process of binding and unbinding can be exploited to achieve stochastic blinking at the receptor (FAP) sites, where relative rates of binding and dissociation result in cycles of activation and de-activation at the single molecule level. The duty cycle of such protein-dye complexes can be controlled by the concentration of free dye and the rates of bleaching and/or dissociation of the protein-dye complex. Feasibility of this concept for SOFI imaging is shown in Fig. 4, where blinking frames were generated by labeling a fixed cell that expressed a FAP-actin fusion construct with a low concentration of fluorogen in vitro.
Fig. 4.
(A) A wide-field image of a malachite green ester analog dye bound to MG-FAPs fused to human β-actin in formaldehyde fixed and permeabilized HeLa cells; (B) Corresponding second-order SOFI image of the network in (A).
Current FAPs are based on single chain fragment (scFv) antibody molecules. Several cell permeable, normally dim dyes, such as malachite green, have been shown to reversibly bind, brighten-up, and dissociate (therefore ‘blink’) from the corresponding scFv99,100. scFv molecules have shown to be effective labels for the cell surface, and with suitable modifications can also function in the cytosol of living and fixed cells101.
Alternative affinity molecules and paired permeable dyes that function in the cytosol need to be developed and optimized in order for this concept to work well in live cells. The ability to control the equilibrium properties by adjustments of dye concentration makes this approach attractive for multicolor labels. Because kinetic properties control blinking rates, it may be more practical to generate stochastically blinking families of FAP-fluorogen pairs, than it would be to generate these under photoswitching or ROXS mediated blinking control typical of other SR imaging protocols.
Difficulties in delivery and targeting of QDs and synthetic dyes are all eliminated for fluorescent proteins (FPs), which are easily and specifically targeted to cytosolic structures (or proteins) via genetic manipulations. Mutations in the original green fluorescence protein (GFP) have led not only to a large color pallet of FPs, but also to the development of photoactivatable (or photoswitchable) FPs (PA-FPs)102–107 which are at the foundation of the localization microscopy techniques8,9.
PA-FPs can be switched from a dark state or a given wavelength fluorescence state to a different wavelength fluorescence state upon irradiation with a specific wavelength ‘switching’ laser which is usually different from the one used for fluorescence imaging. Adjusting the intensities of the two lasers could result in stochastic blinking of the FPs. For some of the PA-FPs, however, stochastic blinking could be achieved with a single wavelength source. Many options in colors and switching behaviors have been developed. Among them, the reversibly switchable FPs (rsFPs) have shown to be of great utility. For example, structured illumination microscopy (SIM)108 of nuclear pores and actin filaments in live cells labeled with the rsFPs Dronpa exhibited nonlinear response at relatively low intensity excitation, resulting in enhanced resolution at favorable live cell imaging conditions109.
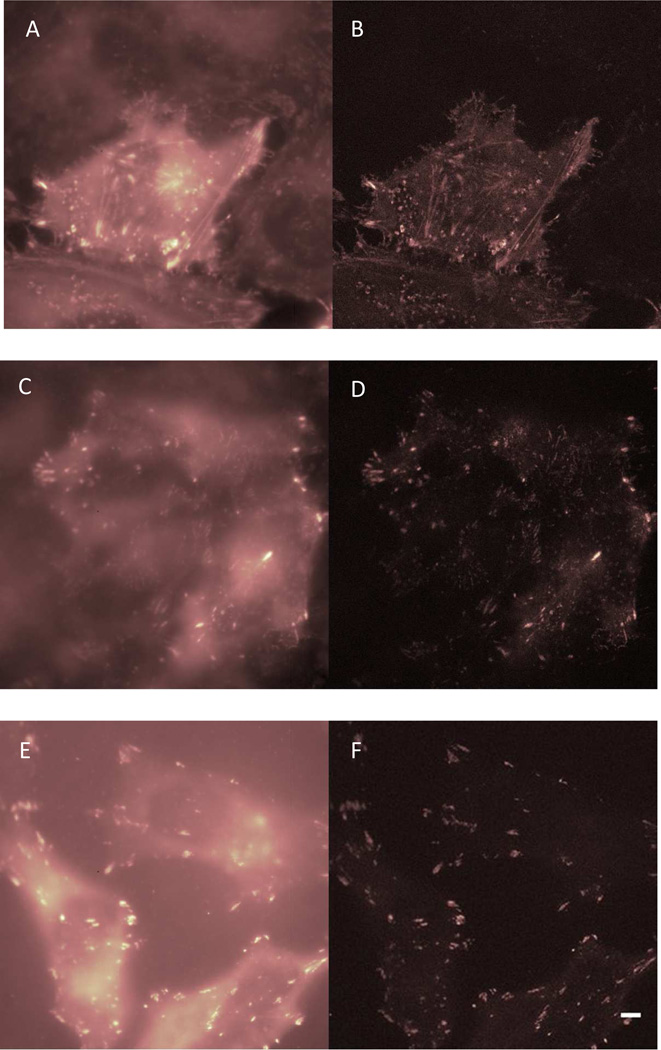
Dedecker et al. have recently demonstrated dual-color SOFI live cell imaging using the rsFPs Dronpa104 and rsTagRFP110. We have also used Dronpa labeling and a low dose LED excitation lamp in a simple wide-field fluorescence microscope to image the focal adhesion proteins FAK, zyxin and Hic6 in live HeLa cells (Fig. 5). The combination of a genetic marker (Dronpa in this case), a simple lamp-based microscope, and a simple computational algorithm (SOFI) greatly simplifies SR imaging and makes it more affordable.
Fig. 5.
Wide-field images of Dronpa fused to focal adhesion proteins (A) FAK, (C) Hic5 and (E) Zyxin in live HeLa cells; Corresponding second-order SOFI images are shown in (B), (D) and (F). Scale bar: 6 µm.
rsFPs suffer, however, from poor photostability and low blinking rate, limiting the speed and duration of the data acquisition. With better insight into their protein structure and blinking mechanism, more stable rsFPs have recently been developed111,112. Grotjohann et al. engineered novel rsFPs dubbed ‘reversibly switchable enhanced green fluorescent proteins’ (rsEGFP and rsEGFP2). When compared with Dronpa, rsEGFP displayed an exceptional photostablity (> 1000 on-off cycles) and fast blinking112. Using RESOLFT microscopy, they were able to image keratin and dendritic spines labeled with rsEGFP in live cells with a resolution of 40nm and minimal phototoxicity. Since rsEGFP and rsEGFPs can work either in a photoactivation mode or in a stochastic blinking mode, it is likely that they will preform well with other SR methods.
Traditionally, FPs have been optimized for high brightness and good photostability. With the advent of SR methods, several FPs have been evolved to display favorable photophysical properties for SR. Since they offer such an easy way to specifically target and label cytosolic structures of interest, more work is needed to allow specific tailoring of attributes to specific SR methods.
Various SR techniques have their own advantages and limitations. None of them can exhibit at the same time the highest spatial and the highest temporal resolutions and therefore a trade-off has to be tailored for a specific application. Dynamic SR imaging in live cells could be further improved by: (i) improving the photophysical properties of the probes. Brighter probes will allow longer observations with reduced phototoxicity and will result in better signals. Faster blinking will allow faster SR imaging; (ii) improving the delivery and specific targeting of the probes. Specific labeling reduces the background and enhances the image contrast; (iii) improving the speed and sensitivity of the cameras used for acquiring SR data, allowing faster SR imaging and better signals.
This perspective mainly focused on points (i) and (ii) by discussing several delivery and targeting approaches for dyes, QDs and FPs. Promising live cell dynamic SR imaging results have already been demonstrated with the various probes and delivery methods discussed above. However, long-term live cell SR imaging with both high spatial and temporal resolutions is still quite challenging. Luckily, there is ample of room for improvements. It is likely that better understanding of the probes’ structures and photophysical properties will lead to significant enhancements in their performance.
As for QDs, a major challenge is to develop efficient and high throughput endosome-free delivery methods64. The thermal nanoblade described here may provide inspiration for developing a transfection reagent that is based on plasmonic nanoparticles together with wide-field pulsed NIR illumination. Better control over QDs surfaces will enhance their photophysical properties and reduce non-specific binding. Thin coating will reduce their steric hindrance and increase targeting efficiency into crowded environments (such as organelles).
As for synthetic organic dyes, development of a larger pallet of bright and stable permeable dyes and the development of smaller molecular weight tags that act together with enzymes to catalyze conversion to covalent bonding (such as Sortase113, Formylglycine generating enzyme114, Transglutaminase115 and others) are needed. For long-term SR time-lapse microscopy, affinity binding such as PAINT and FAPs is an attractive approach for minimizing ill effects of photobleaching. This approach requires the development of a pallet of permeable fluorogens that are normally quenched, but brighten-up upon binding to their targets. These targets should consist of small molecular weight scaffolds that specifically bind and brighten-up the fluorogens. Molecular evolution techniques coupled to a photophysic-based screening assay could be used to evolve additional and/or more efficient such fluorogen-tag pairs with the desired specificities and association and dissociation rates.
As for FPs, intense effort is currently being conducted to evolve brighter, longer-lived photoswitchable FPs. Molecular evolution with photophysic-based screen will be very useful here too, and in particular for the evolution of spontaneously blinking FPs. Development of hybrid approaches could also provide powerful solutions. For example, Izeddin et al. have recently fused PA-FP to Lifeact, a weak actin binder116. The FP-Lifeact fusion reversibly binds and unbinds actin, allowing for the long-term PALM imaging of dendritic spines’ cytoskeleton.
The field of live cell SR imaging is at its infancy. Many more exciting technological advances are anticipated. Some of these advances will be along the lines presented in this perspective. Unexpected advances are also very likely for this fast-moving field. It is going to be a fun ride.
ACKNOWLEDGEMENTS
We thank Atsushi Miyawaki for the free gift of Dronpa constructs, Pei-Yu Chiou and Michael Teitell for assistance with thermal nanoblade delivery. This work was supported by NIH grant #5R01EB000312 and NIH grant #1R01GM086197 and NIH grant # 1R01GM086237.
Biographies
Jianmin Xu received his Ph.D in Chemistry from University of Miami in 2008. He is currently a postdoc scholar in the Department of Chemistry and Biochemistry at UCLA. His current interests include nanoparticle surface modification, bioconjugation, cellular fluorescent labeling and optical super resolution microscopy.
Jason Lin Chang is an undergraduate Biochemistry major at UCLA, performing research in the Weiss Lab. He studies photophysical properties of fluorescent dyes and fluorescent proteins and optimal conditions for super-resolution imaging. He is a recipient of the Bruce Merrifield Undergraduate Research Award at UCLA.
Qi Yan received her Ph.D in department of Biological Sciences at Carnegie Mellon University under mentoring of Marcel Bruchez in 2012. She is currently a Scientist at Sharp Edge Labs Inc. During her PhD study, she focused on characterizing the Fluorogen Activating Peptide (FAP) and fluorogen system and developed FAP-based fluorescent probes for performing super-resolution imaging and studying GPCR trafficking.
Thomas Dertinger received his PhD in Physics and Physical Chemistry at University of Cologne, Germany. He was a post-doctoral scholar in the Department of Chemistry and Biochemistry at UCLA from 2007–2011. During this time he developed SOFI. He is the founder of SOFast GmbH in Germany and currently puts his focus on intellectual property management.
Marcel Bruchez is an associate professor in Department of Chemistry and the Department of Biological Sciences at Carnegie Mellon University. He received his PhD at the University of California, Berkeley in Physical Chemistry in 1998. His research group focuses on developing new tools for specifically labeling biological targets in living cells and organisms with bright, stable fluorescent molecules, and delivery of active-sensing molecules to specific cellular sites. Prior to joining the faculty at Carnegie Mellon University in 2006, Marcel was the co-founder of Quantum Dot Corporation. His development of quantum dots as biological detection was recognized as one of the Top Ten Scientific Innovations of 2003. He was a TR100 awardee in 2004 and received the Lord Rank Prize for Optoelectronics in 2006 for the realization of quantum dots for biological labeling. He is also the founder and Chief Scientific Officer of Sharp Edge Laboratories.
Shimon Weiss is professor in the Department of Chemistry and Biochemistry and the Department of Physiology at UCLA. His lab has been working on ultrasensitive single molecule spectroscopy methods and applications. They were the first to introduce the single molecule FRET method and together with the Alivisatos group they were the first to introduce quantum dots to biological imaging. Shimon received his PhD from the Technion in Electrical Engineering in 1989. His postdoctoral training was in AT&T Bell Laboratories. He was a staff scientist at Lawrence Berkeley National Laboratory between 1991–2001. In 2001 he joined UCLA. Shimon is the Dean M. Willard Chair and a full Professor in the Department of Chemistry & Biochemistry. He is a fellow of the Optical Society of America, and received the 2001 Michael and Kate Barany Biophysical Society Award, the 2006 Rank Prize in opto-electronics, and the Humboldt Research Award in 2012.
REFERENCE
- 1.Abbe E. Beiträge zur Theorie des Mikroskops und der mikroskopischen Wahrnehmung. Archiv für mikroskopische Anatomie. 1873;9:413–418. [Google Scholar]
- 2.Ha T, Tinnefeld P. Photophysics of fluorescent probes for single-molecule biophysics and super-resolution imaging. Annu. Rev. Phys. Chem. 2012;63:595–617. doi: 10.1146/annurev-physchem-032210-103340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang B, Bates M, Zhuang X. Super-resolution fluorescence microscopy. Annu. Rev. Biochem. 2009;78:993–1016. doi: 10.1146/annurev.biochem.77.061906.092014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji N, Shroff H, Zhong H, Betzig E. Advances in the speed and resolution of light microscopy. Curr. Opin. Neurobiol. 2008;18:605–616. doi: 10.1016/j.conb.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Schermelleh L, Heintzmann R, Leonhardt H. A guide to super-resolution fluorescence microscopy. J. Cell Biol. 2010;190:165–175. doi: 10.1083/jcb.201002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van de Linde S, Heilemann M, Sauer M. Live-cell super-resolution imaging with synthetic fluorophores. Annu. Rev. Phys. Chem. 2012;63:519–540. doi: 10.1146/annurev-physchem-032811-112012. [DOI] [PubMed] [Google Scholar]
- 7.Vogelsang J, Steinhauer C, Forthmann C, Stein IH, Person-Skegro B, Cordes T, Tinnefeld P. Make them blink: probes for super-resolution microscopy. Chemphyschem. 2010;11:2475–2490. doi: 10.1002/cphc.201000189. [DOI] [PubMed] [Google Scholar]
- 8.Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–1645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 9.Hess ST, Girirajan TPK, Mason MD. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys. J. 2006;91:4258–4272. doi: 10.1529/biophysj.106.091116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rust MJ, Bates M, Zhuang XW. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM) Nature Methods. 2006;3:793–795. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heilemann M, van de Linde S, Schuttpelz M, Kasper R, Seefeldt B, Mukherjee A, Tinnefeld P, Sauer M. Subdiffraction-resolution fluorescence imaging with conventional fluorescent probes. Angew. Chem. Int. Ed. 2008;47:6172–6176. doi: 10.1002/anie.200802376. [DOI] [PubMed] [Google Scholar]
- 12.Folling J, Bossi M, Bock H, Medda R, Wurm CA, Hein B, Jakobs S, Eggeling C, Hell SW. Fluorescence nanoscopy by ground-state depletion and single-molecule return. Nat. Methods. 2008;5:943–945. doi: 10.1038/nmeth.1257. [DOI] [PubMed] [Google Scholar]
- 13.Simonson PD, Rothenberg E, Selvin PR. Single-molecule-based superresolution images in the presence of multiple fluorophores. Nano Lett. 2011;11:5090–5096. doi: 10.1021/nl203560r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharonov A, Hochstrasser RM. Wide-field subdiffraction imaging by accumulated binding of diffusing probes. Proc. Natl. Acad. Sci. U. S. A. 2006;103:18911–18916. doi: 10.1073/pnas.0609643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoen I, Ries J, Klotzsch E, Ewers H, Vogel V. Binding-Activated Localization Microscopy of DNA Structures. Nano Lett. 2011;11:4008–4011. doi: 10.1021/nl2025954. [DOI] [PubMed] [Google Scholar]
- 16.Dertinger T, Colyer R, Iyer G, Weiss S, Enderlein J. Fast, background-free,3D super-resolution optical fluctuation imaging (SOFI) Proc. Natl. Acad. Sci. U. S. A. 2009;106:22287–22292. doi: 10.1073/pnas.0907866106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dedecker P, Mo GCH, Dertinger T, Zhang J. Widely accessible method for superresolution fluorescence imaging of living systems. Proc. Natl. Acad. Sci. U. S. A. 2012;109:10909–10914. doi: 10.1073/pnas.1204917109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dertinger T, Xu J, Naini O, Vogel R, Weiss S. SOFI-based 3D superresolution sectioning with a widefield microscope. Opt. Nanosc. 2012;1:2. doi: 10.1186/2192-2853-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geissbuehler S, Bocchio N, Dellagiacoma C, Berclaz C, Leutenegger M, Lasser T. Mapping molecular statistics with balanced super-resolution optical fluctuation imaging (bSOFI) Opt. Nanosc. 2012;1:4. [Google Scholar]
- 20.Geissbuehler S, Dellagiacoma C, Lasser T. Comparison between SOFI and STORM. Biomed. Opt. Express. 2011;2:408–420. doi: 10.1364/BOE.2.000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dertinger T, Colyer R, Vogel R, Enderlein J, Weiss S. Achieving increased resolution and more pixels with Superresolution Optical Fluctuation Imaging (SOFI) Opt. Express. 2010;18:18875–18885. doi: 10.1364/OE.18.018875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dertinger T, Heilemann M, Vogel R, Sauer M, Weiss S. Superresolution Optical Fluctuation Imaging with Organic Dyes. Angew. Chem. Int. Ed. 2010;49:9441–9443. doi: 10.1002/anie.201004138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shroff H, Galbraith CG, Galbraith JA, Betzig E. Live-cell photoactivated localization microscopy of nanoscale adhesion dynamics. Nat. Methods. 2008;5:417–423. doi: 10.1038/nmeth.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biteen JS, Thompson MA, Tselentis NK, Bowman GR, Shapiro L, Moerner WE. Super-resolution imaging in live Caulobacter crescentus cells using photoswitchable EYFP. Nat. Methods. 2008;5:947–949. doi: 10.1038/NMETH.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hein B, Willig KI, Hell SW. Stimulated emission depletion (STED) nanoscopy of a fluorescent protein-labeled organelle inside a living cell. Proc. Natl. Acad. Sci. U. S. A. 2008;105:14271–14276. doi: 10.1073/pnas.0807705105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westphal V, Rizzoli SO, Lauterbach MA, Kamin D, Jahn R, Hell SW. Video-rate far-field optical nanoscopy dissects synaptic vesicle movement. Science. 2008;320:246–249. doi: 10.1126/science.1154228. [DOI] [PubMed] [Google Scholar]
- 27.Kner P, Chhun BB, Griffis ER, Winoto L, Gustafsson MG. Superresolution video microscopy of live cells by structured illumination. Nat. Methods. 2009;6:339–342. doi: 10.1038/nmeth.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hein B, Willig KI, Wurm CA, Westphal V, Jakobs S, Hell SW. Stimulated emission depletion nanoscopy of living cells using SNAP-tag fusion proteins. Biophys. J. 2010;98:158–163. doi: 10.1016/j.bpj.2009.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subach FV, Patterson GH, Renz M, Lippincott-Schwartz J, Verkhusha VV. Bright monomeric photoactivatable red fluorescent protein for two-color super-resolution sptPALM of live cells. J. Am. Chem. Soc. 2010;132:6481–6491. doi: 10.1021/ja100906g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wombacher R, Heidbreder M, van de Linde S, Sheetz MP, Heilemann M, Cornish VW, Sauer M. Live-cell super-resolution imaging with trimethoprim conjugates. Nat. Methods. 2010;7:717–719. doi: 10.1038/nmeth.1489. [DOI] [PubMed] [Google Scholar]
- 31.Cella Zanacchi F, Lavagnino Z, Perrone Donnorso M, Del Bue A, Furia L, Faretta M, Diaspro A. Live-cell 3D super-resolution imaging in thick biological samples. Nat. Methods. 2011;8:1047–1049. doi: 10.1038/nmeth.1744. [DOI] [PubMed] [Google Scholar]
- 32.Gunewardene MS, Subach FV, Gould TJ, Penoncello GP, Gudheti MV, Verkhusha VV, Hess ST. Superresolution imaging of multiple fluorescent proteins with highly overlapping emission spectra in living cells. Biophys. J. 2011;101:1522–1528. doi: 10.1016/j.bpj.2011.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones SA, Shim SH, He J, Zhuang X. Fast, three-dimensional superresolution imaging of live cells. Nat. Methods. 2011;8:499–508. doi: 10.1038/nmeth.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein T, Loschberger A, Proppert S, Wolter S, van de Linde S, Sauer M. Live-cell dSTORM with SNAP-tag fusion proteins. Nat. Methods. 2011;8:7–9. doi: 10.1038/nmeth0111-7b. [DOI] [PubMed] [Google Scholar]
- 35.Lew MD, Lee SF, Ptacin JL, Lee MK, Twieg RJ, Shapiro L, Moerner WE. Three-dimensional superresolution colocalization of intracellular protein superstructures and the cell surface in live Caulobacter crescentus. Proc. Natl. Acad. Sci. U. S. A. 2011;108:E1102–E1110. doi: 10.1073/pnas.1114444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shao L, Kner P, Rego EH, Gustafsson MG. Super-resolution 3D microscopy of live whole cells using structured illumination. Nat. Methods. 2011;8:1044–1046. doi: 10.1038/nmeth.1734. [DOI] [PubMed] [Google Scholar]
- 37.Wang W, Li GW, Chen C, Xie XS, Zhuang X. Chromosome organization by a nucleoid-associated protein in live bacteria. Science. 2011;333:1445–1449. doi: 10.1126/science.1204697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bakshi S, Siryaporn A, Goulian M, Weisshaar JC. Superresolution imaging of ribosomes and RNA polymerase in live Escherichia coli cells. Mol. Microbiol. 2012;85:21–38. doi: 10.1111/j.1365-2958.2012.08081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fiolka R, Shao L, Rego EH, Davidson MW, Gustafsson MG. Time-lapse two-color 3D imaging of live cells with doubled resolution using structured illumination. Proc. Natl. Acad. Sci. U. S. A. 2012;109:5311–5315. doi: 10.1073/pnas.1119262109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ries J, Kaplan C, Platonova E, Eghlidi H, Ewers H. A simple, versatile method for GFP-based super-resolution microscopy via nanobodies. Nat. Methods. 2012;9:582–584. doi: 10.1038/nmeth.1991. [DOI] [PubMed] [Google Scholar]
- 41.Shim SH, Xia C, Zhong G, Babcock HP, Vaughan JC, Huang B, Wang X, Xu C, Bi GQ, Zhuang X. Super-resolution fluorescence imaging of organelles in live cells with photoswitchable membrane probes. Proc. Natl. Acad. Sci. U. S. A. 2012;109:13978–13983. doi: 10.1073/pnas.1201882109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner E, Lauterbach MA, Kohl T, Westphal V, Williams GS, Steinbrecher JH, Streich JH, Korff B, Tuan HT, Hagen B, et al. Stimulated emission depletion live-cell super-resolution imaging shows proliferative remodeling of T-tubule membrane structures after myocardial infarction. Circ. Res. 2012;111:402–414. doi: 10.1161/CIRCRESAHA.112.274530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilmes S, Staufenbiel M, Lisse D, Richter CP, Beutel O, Busch KB, Hess ST, Piehler J. Triple-color super-resolution imaging of live cells: resolving submicroscopic receptor organization in the plasma membrane. Angew. Chem. Int. Ed. Engl. 2012;51:4868–4871. doi: 10.1002/anie.201200853. [DOI] [PubMed] [Google Scholar]
- 44.Testa I, Urban NT, Jakobs S, Eggeling C, Willig KI, Hell SW. Nanoscopy of living brain slices with low light levels. Neuron. 2012;75:992–1000. doi: 10.1016/j.neuron.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 45.York AG, Parekh SH, Dalle Nogare D, Fischer RS, Temprine K, Mione M, Chitnis AB, Combs CA, Shroff H. Resolution doubling in live, multicellular organisms via multifocal structured illumination microscopy. Nat. Methods. 2012;9:749–754. doi: 10.1038/nmeth.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rankin BR, Moneron G, Wurm CA, Nelson JC, Walter A, Schwarzer D, Schroeder J, Colon-Ramos DA, Hell SW. Nanoscopy in a living multicellular organism expressing GFP. Biophys. J. 2011;100:L63–L65. doi: 10.1016/j.bpj.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berning S, Willig KI, Steffens H, Dibaj P, Hell SW. Nanoscopy in a living mouse brain. Science. 2012;335:551. doi: 10.1126/science.1215369. [DOI] [PubMed] [Google Scholar]
- 48.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruchez M, Moronne M, Gin P, Weiss S, Alivisatos AP. Semiconductor nanocrystals as fluorescent biological labels. Science. 1998;281:2013–2016. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- 50.Nirmal M, Dabbousi BO, Bawendi MG, Macklin JJ, Trautman JK, Harris TD, Brus LE. Fluorescence intermittency in single cadmium selenide nanocrystals. Nature. 1996;383:802–804. [Google Scholar]
- 51.Lidke KA, Rieger B, Jovin TM, Heintzmann R. Superresolution by localization of quantum dots using blinking statistics. Opt. Express. 2005;13:7052–7062. doi: 10.1364/opex.13.007052. [DOI] [PubMed] [Google Scholar]
- 52.Bannai H, Levi S, Schweizer C, Dahan M, Triller A. Imaging the lateral diffusion of membrane molecules with quantum dots. Nat. Protoc. 2006;1:2628–2634. doi: 10.1038/nprot.2006.429. [DOI] [PubMed] [Google Scholar]
- 53.Dahan M, Levi S, Luccardini C, Rostaing P, Riveau B, Triller A. Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking. Science. 2003;302:442–445. doi: 10.1126/science.1088525. [DOI] [PubMed] [Google Scholar]
- 54.Pinaud F, Michalet X, Iyer G, Margeat E, Moore H-P, Weiss S. Dynamic Partitioning of a Glycosyl-Phosphatidylinositol-Anchored Protein in Glycosphingolipid-Rich Microdomains Imaged by Single-Quantum Dot Tracking. Traffic. 2009;10:691–712. doi: 10.1111/j.1600-0854.2009.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iyer G, Michalet X, Chang Y-P, Pinaud FF, Matyas SE, Payne G, Weiss S. High Affinity scFv-Hapten Pair as a Tool for Quantum Dot Labeling and Tracking of Single Proteins in Live Cells. Nano Lett. 2008;8:4618–4623. doi: 10.1021/nl8032284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pinaud F, Michalet X, Margeat E, Moore HP, Weiss S. Single molecule Imaging of raft associated GPI-anchored receptors using quantum dots. Biophys. J. 2005;88:587A–587A. [Google Scholar]
- 57.Pinaud F, King D, Moore HP, Weiss S. Bioactivation and cell targeting of semiconductor CdSe/ZnS nanocrystals with phytochelatin-related peptides. J. Am. Chem. Soc. 2004;126:6115–6123. doi: 10.1021/ja031691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu XY, Bruchez MP. Labeling cellular targets with semiconductor quantum dot conjugates. Cytometry, 4th Edition: New Developments. 2004;75:171–183. doi: 10.1016/s0091-679x(04)75007-4. [DOI] [PubMed] [Google Scholar]
- 59.Wu XY, Liu HJ, Liu JQ, Haley KN, Treadway JA, Larson JP, Ge NF, Peale F, Bruchez MP. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat.ure Biotechnol. 2003;21:41–46. doi: 10.1038/nbt764. [DOI] [PubMed] [Google Scholar]
- 60.Mason JN, Tomlinson ID, Rosenthal SJ, Blakely RD. Labeling cell-surface proteins via antibody quantum dot streptavidin conjugates In. In: Rosenthal SJ, Wright DW, editors. Methods in Molecular Biology. Vol. 303. 999 Riverview Dr, Ste 208, Totowa, Nj 07512-1165 USA: Humana Press Inc; 2005. p. 35.p. 50. [DOI] [PubMed] [Google Scholar]
- 61.Howarth M, Liu W, Puthenveetil S, Zheng Y, Marshall LF, Schmidt MM, Wittrup KD, Bawendi MG, Ting AY. Monovalent, reduced-size quantum dots for imaging receptors on living cells. Nat. Methods. 2008;5:397–399. doi: 10.1038/nmeth.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldman ER, Mattoussi H, Anderson GP, Medintz IL, Mauro JM. Fluoroimmunoassays using antibody-conjugated quantum dots. Methods Mol. Biol. 2005;303:19–34. doi: 10.1385/1-59259-901-X:019. [DOI] [PubMed] [Google Scholar]
- 63.Xing Y, Chaudry Q, Shen C, Kong KY, Zhau HE, Wchung L, Petros JA, O'Regan RM, Yezhelyev MV, Simons JW, et al. Bioconjugated quantum dots for multiplexed and quantitative immunohistochemistry. Nat. Protoc. 2007;2:1152–1165. doi: 10.1038/nprot.2007.107. [DOI] [PubMed] [Google Scholar]
- 64.Delehanty JB, Bradburne CE, Boeneman K, Susumu K, Farrell D, Mei BC, Blanco-Canosa JB, Dawson G, Dawson PE, Mattoussi H, et al. Delivering quantum dot-peptide bioconjugates to the cellular cytosol: escaping from the endolysosomal system. Integr. Biol. 2010;2:265–277. doi: 10.1039/c0ib00002g. [DOI] [PubMed] [Google Scholar]
- 65.Gao XH, Cui YY, Levenson RM, Chung LWK, Nie SM. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 66.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 67.Susumu K, Uyeda HT, Medintz IL, Pons T, Delehanty JB, Mattoussi H. Enhancing the stability and biological functionalities of quantum dots via compact multifunctional ligands. J. Am. Chem. Soc. 2007;129:13987–13996. doi: 10.1021/ja0749744. [DOI] [PubMed] [Google Scholar]
- 68.Clapp AR, Goldman ER, Mattoussi H. Capping of CdSe-ZnS quantum dots with DHLA and subsequent conjugation with proteins. Nat. Protoc. 2006;1:1258–1266. doi: 10.1038/nprot.2006.184. [DOI] [PubMed] [Google Scholar]
- 69.Xu J, Ruchala P, Ebenstain Y, Li JJ, Weiss S. Stable, Compact, Bright Biofunctional Quantum Dots with Improved Peptide Coating. J. Phys. Chem. B. 2012;116:11370–11378. doi: 10.1021/jp306453y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iyer G, Pinaud F, Xu J, Ebenstein Y, Li J, Chang J, Dahan M, Weiss S. Aromatic Aldehyde and Hydrazine Activated Peptide Coated Quantum Dots for Easy Bioconjugation and Live Cell Imaging. Bioconjugate Chem. 2011;22:1006–1011. doi: 10.1021/bc100593m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen FQ, Gerion D. Fluorescent CdSe/ZnS nanocrystal-peptide conjugates for long-term, nontoxic imaging and nuclear targeting in living cells. Nano Lett. 2004;4:1827–1832. [Google Scholar]
- 72.Derfus AM, Chan WCW, Bhatia SN. Intracellular delivery of quantum dots for live cell labeling and organelle tracking. Adv. Mater. 2004;16:961–966. [Google Scholar]
- 73.Buono C, Anzinger JJ, Amar M, Kruth HS. Fluorescent pegylated nanoparticles demonstrate fluid-phase pinocytosis by macrophages in mouse atherosclerotic lesions. J. Clin. Invest. 2009;119:1373–1381. doi: 10.1172/JCI35548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yezhelyev MV, Qi L, O'Regan RM, Nie S, Gao X. Proton-sponge coated quantum dots for siRNA delivery and intracellular imaging. J. Am. Chem. Soc. 2008;130:9006–9012. doi: 10.1021/ja800086u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bayles AR, Chahal HS, Chahal DS, Goldbeck CP, Cohen BE, Helms BA. Rapid Cytosolic Delivery of Luminescent Nanocrystals in Live Cells with Endosome-Disrupting Polymer Colloids. Nano Lett. 2010;10:4086–4092. doi: 10.1021/nl102172j. [DOI] [PubMed] [Google Scholar]
- 76.Medintz IL, Pons T, Delehanty JB, Susumu K, Brunel FM, Dawson PE, Mattoussi H. Intracellular delivery of quantum dot-protein cargos mediated by cell penetrating peptides. Bioconjugate Chem. 2008;19:1785–1795. doi: 10.1021/bc800089r. [DOI] [PubMed] [Google Scholar]
- 77.Ruan G, Agrawal A, Marcus AI, Nie S. Imaging and tracking of tat peptide-conjugated quantum dots in living cells: new insights into nanoparticle uptake, intracellular transport, and vesicle shedding. J. Am. Chem. Soc. 2007;129:14759–14766. doi: 10.1021/ja074936k. [DOI] [PubMed] [Google Scholar]
- 78.Courty S, Luccardini C, Bellaiche Y, Cappello G, Dahan M. Tracking Individual Kinesin Motors in Living Cells Using Single Quantum-Dot Imaging. Nano Lett. 2006;6:1491–1495. doi: 10.1021/nl060921t. [DOI] [PubMed] [Google Scholar]
- 79.Lisse D, Wilkens V, You C, Busch K, Piehler J. Selective targeting of fluorescent nanoparticles to proteins inside live cells. Angew. Chem. Int. Ed. Engl. 2011;50:9352–9355. doi: 10.1002/anie.201101499. [DOI] [PubMed] [Google Scholar]
- 80.Han S-W, Nakamura C, Kotobuki N, Obataya I, Ohgushi H, Nagamune T, Miyake J. High-efficiency DNA injection into a single human mesenchymal stem cell using a nanoneedle and atomic force microscopy. Nanomedicine. 2008;4:215–225. doi: 10.1016/j.nano.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Y. Microinjection technique and protocol to single cells. Protoc. Exchange. 2007 online. [Google Scholar]
- 82.Wu T-H, Teslaa T, Kalim S, French CT, Moghadam S, Wall R, Miller JF, Witte ON, Teitell MA, Chiou P-Y. Photothermal Nanoblade for Large Cargo Delivery into Mammalian Cells. Anal. Chem. 2011;83:1321–1327. doi: 10.1021/ac102532w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu T-H, Teslaa T, Teitell MA, Chiou P-Y. Photothermal nanoblade for patterned cell membrane cutting. Opt. Express. 2010;18:23153–23160. doi: 10.1364/OE.18.023153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu J, Teslaa T, Wu T-H, Chiou P-Y, Teitell MA, Weiss S. Nanoblade Delivery and Incorporation of Quantum Dot Conjugates into Tubulin Networks in Live Cells. Nano Lett. 2012;12:5669–5672. doi: 10.1021/nl302821g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van de Linde S, Loschberger A, Klein T, Heidbreder M, Wolter S, Heilemann M, Sauer M. Direct stochastic optical reconstruction microscopy with standard fluorescent probes. Nat. Protoc. 2011;6:991–1009. doi: 10.1038/nprot.2011.336. [DOI] [PubMed] [Google Scholar]
- 86.van de Linde S, Krstic I, Prisner T, Doose S, Heilemann M, Sauer M. Photoinduced formation of reversible dye radicals and their impact on super-resolution imaging. Photochem. Photobiol. Sci. 2011;10:499–506. doi: 10.1039/c0pp00317d. [DOI] [PubMed] [Google Scholar]
- 87.Heilemann M, van de Linde S, Mukherjee A, Sauer M. Super-Resolution Imaging with Small Organic Fluorophores. Angew. Chem. Int. Ed. 2009;48:6903–6908. doi: 10.1002/anie.200902073. [DOI] [PubMed] [Google Scholar]
- 88.van de Linde S, Kasper R, Heilemann M, Sauer M. Photoswitching microscopy with standard fluorophores. Appl. Phys. B. 2008;93:725–731. [Google Scholar]
- 89.Heilemann M, Margeat E, Kasper R, Sauer M, Tinnefeld P. Carbocyanine dyes as efficient reversible single-molecule optical switch. J. Am. Chem. Soc. 2005;127:3801–3806. doi: 10.1021/ja044686x. [DOI] [PubMed] [Google Scholar]
- 90.Bates M, Blosser TR, Zhuang XW. Short-range spectroscopic ruler based on a single-molecule optical switch. Phys. Rev. Lett. 2005;94:1–4. doi: 10.1103/PhysRevLett.94.108101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sunbul M, Yin J. Site specific protein labeling by enzymatic posttranslational modification. Org. Biomol. Chem. 2009;7:3361–3371. doi: 10.1039/b908687k. [DOI] [PubMed] [Google Scholar]
- 92.Los GV, Encell LP, McDougall MG, Hartzell DD, Karassina N, Zimprich C, Wood MG, Learish R, Ohana RF, Urh M, et al. HaloTag: A Novel Protein Labeling Technology for Cell Imaging and Protein Analysis. ACS Chem. Biol. 2008;3:373–382. doi: 10.1021/cb800025k. [DOI] [PubMed] [Google Scholar]
- 93.Keppler A, Pick H, Arrivoli C, Vogel H, Johnsson K. Labeling of fusion proteins with synthetic fluorophores in live cells. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9955–9959. doi: 10.1073/pnas.0401923101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Keppler A, Gendreizig S, Gronemeyer T, Pick H, Vogel H, Johnsson K. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat. Biotechnol. 2003;21:86–89. doi: 10.1038/nbt765. [DOI] [PubMed] [Google Scholar]
- 95.Gautier A, Juillerat A, Heinis C, Correa IR, Kindermann M, Beaufils F, Johnsson K. An engineered protein tag for multiprotein labeling in living cells. Chem. Biol. 2008;15:128–136. doi: 10.1016/j.chembiol.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 96.Sun X, Zhang A, Baker B, Sun L, Howard A, Buswell J, Maurel D, Masharina A, Johnsson K, Noren CJ, et al. Development of SNAP-Tag Fluorogenic Probes for Wash-Free Fluorescence Imaging. Chembiochem. 2011;12:2217–2226. doi: 10.1002/cbic.201100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schwartz SL, Yan Q, Huang F, Bruchez MP, Lidke DS, Lidke KA. Fluorogen Activating Peptides for Single Particle Tracking and Single Molecule Localization-Based Superresolution of FcRI Subunits. Biophys. J. 2011;100:257–257. [Google Scholar]
- 98.Yan Q, Schwartz SL, Maji S, Lidke DS, Lidke KA, Bruchez MP. Superresolution Localization Microscopy Using Fluorogen Activating Proteins. Biophys. J. 2011;100:476–476. [Google Scholar]
- 99.Szent-Gyorgyi C, Schmidt BA, Creeger Y, Fisher GW, Zakel KL, Adler S, Fitzpatrick JAJ, Woolford CA, Yan Q, Vasilev KV, et al. Fluorogen-activating single-chain antibodies for imaging cell surface proteins. Nat. Biotechnol. 2008;26:235–240. doi: 10.1038/nbt1368. [DOI] [PubMed] [Google Scholar]
- 100.Szent-Gyorgyi C, Schmidt BF, Fitzpatrick JAJ, Bruchez MP. Fluorogenic Dendrons with Multiple Donor Chromophores as Bright Genetically Targeted and Activated Probes. J. Am. Chem. Soc. 2010;132:11103–11109. doi: 10.1021/ja9099328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fitzpatrick JAJ, Yan Q, Sieber JJ, Dyba M, Schwarz U, Szent-Gyorgyi C, Woolford CA, Berget PB, Waggoner AS, Bruchez MP. STED Nanoscopy in Living Cells Using Fluorogen Activating Proteins. Bioconjugate Chem. 2009;20:1843–1847. doi: 10.1021/bc900249e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chudakov DM, Verkhusha VV, Staroverov DB, Souslova EA, Lukyanov S, Lukyanov KA. Photoswitchable cyan fluorescent protein for protein tracking. Nat. Biotechnol. 2004;22:1435–1439. doi: 10.1038/nbt1025. [DOI] [PubMed] [Google Scholar]
- 103.Tsutsui H, Karasawa S, Shimizu H, Nukina N, Miyawaki A. Semi-rational engineering of a coral fluorescent protein into an efficient highlighter. Embo. Reports. 2005;6:233–238. doi: 10.1038/sj.embor.7400361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Habuchi S, Ando R, Dedecker P, Verheijen W, Mizuno H, Miyawaki A, Hofkens J. Reversible single-molecule photoswitching in the GFP-like fluorescent protein Dronpa. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9511–9516. doi: 10.1073/pnas.0500489102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Adam V, Lelimousin M, Boehme S, Desfonds G, Nienhaus K, Field MJ, Wiedenmann J, McSweeney S, Nienhaus GU, Bourgeois D. Structural characterization of IrisFP, an optical highlighter undergoing multiple photo-induced transformations. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18343–18348. doi: 10.1073/pnas.0805949105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wiedenmann J, Ivanchenko S, Oswald F, Schmitt F, Rocker C, Salih A, Spindler KD, Nienhaus GU. EosFP, a fluorescent marker protein with UV-inducible green-to-red fluorescence conversion. Proc. Natl. Acad. Sci. U. S. A. 2004;101:15905–15910. doi: 10.1073/pnas.0403668101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ando R, Hama H, Yamamoto-Hino M, Mizuno H, Miyawaki A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc. Natl. Acad. Sci. U. S. A. 2002;99:12651–12656. doi: 10.1073/pnas.202320599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gustafsson MGL. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. Oxford. 2000;198:82–87. doi: 10.1046/j.1365-2818.2000.00710.x. [DOI] [PubMed] [Google Scholar]
- 109.Rego EH, Shao L, Macklin JJ, Winoto L, Johansson GA, Kamps-Hughes N, Davidson MW, Gustafsson MGL. Nonlinear structured-illumination microscopy with a photoswitchable protein reveals cellular structures at 50-nm resolution. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E135–E143. doi: 10.1073/pnas.1107547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Subach FV, Zhang L, Gadella TWJ, Gurskaya NG, Lukyanov KA, Verkhusha VV. Red Fluorescent Protein with Reversibly Photoswitchable Absorbance for Photochromic FRET. Chem. Biol. 2010;17:745–755. doi: 10.1016/j.chembiol.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tim G, Ilaria T, Matthias R, Tanja B, Christian E, Stefan WH, Stefan J. rsEGFP2 enables fast RESOLFT nanoscopy of living cells. eLife Sciences. 2012;1 doi: 10.7554/eLife.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grotjohann T, Testa I, Leutenegger M, Bock H, Urban NT, Lavoie-Cardinal F, Willig KI, Eggeling C, Jakobs S, Hell SW. Diffraction-unlimited all-optical imaging and writing with a photochromic GFP. Nature. 2011;478:204–208. doi: 10.1038/nature10497. [DOI] [PubMed] [Google Scholar]
- 113.Perry AM, Ton-That H, Mazmanian SK, Schneewind O. Anchoring of surface proteins to the cell wall of Staphylococcus aureus. III. Lipid II is an in vivo peptidoglycan substrate for sortase-catalyzed surface protein anchoring. J. Biol. Chem. 2002;277:16241–16248. doi: 10.1074/jbc.M109194200. [DOI] [PubMed] [Google Scholar]
- 114.Carrico IS, Carlson BL, Bertozzi CR. Introducing genetically encoded aldehydes into proteins. Nat. Chem. Biol. 2007;3:321–322. doi: 10.1038/nchembio878. [DOI] [PubMed] [Google Scholar]
- 115.Griffin M, Casadio R, Bergamini CM. Transglutaminases: nature's biological glues. Biochem. J. 2002;368:377–396. doi: 10.1042/BJ20021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Izeddin I, Specht CG, Lelek M, Darzacq X, Triller A, Zimmer C, Dahan M. Super-resolution dynamic imaging of dendritic spines using a low-affinity photoconvertible actin probe. PLoS One. 2011;6:e15611. doi: 10.1371/journal.pone.0015611. [DOI] [PMC free article] [PubMed] [Google Scholar]