Abstract
Rationale: Pseudomonas aeruginosa is the most commonly isolated gram-negative bacterium after lung transplantation and has been shown to up-regulate glutamic acid–leucine–arginine–positive (ELR+) CXC chemokines associated with bronchiolitis obliterans syndrome (BOS), but the effect of pseudomonas on BOS and death has not been well defined.
Objectives: To determine if the influence of pseudomonas isolation and ELR+ CXC chemokines on the subsequent development of BOS and the occurrence of death is time dependent.
Methods: A three-state model was developed to assess the likelihood of transitioning from lung transplant (state 1) to BOS (state 2), from transplant (state 1) to death (state 3), and from BOS (state 2) to death (state 3). This Cox semi-Markovian approach determines state survival rates and cause-specific hazards for movement from one state to another.
Measurements and Main Results: The likelihood of transition from transplant to BOS was increased by acute rejection, CXCL5, and the interaction between pseudomonas and CXCL1. The pseudomonas effect in this transition was due to infection rather than colonization. Movement from transplant to death was facilitated by pseudomonas infection and single lung transplant. Transition from BOS to death was affected by the length of time in state 1 and by the interactions between any pseudomonas isolation and CXCL5 and aspergillus, either independently or in combination.
Conclusions: Our model demonstrates that common post-transplantation events drive movement from one post-transplantation state to another and influence outcomes differently depending upon when after transplantation they occur. Pseudomonas and the ELR+ CXC chemokines may interact to negatively influence lung transplant outcomes.
Keywords: transplantation, lung, BOS, pseudomonas, chemokine
At a Glance Commentary
Scientific Knowledge on the Subject
Pseudomonas is the most commonly isolated bacterium after lung transplantation and has been shown to up-regulate glutamic acid–leucine–arginine–positive (ELR+) CXC chemokines associated with bronchiolitis obliterans syndrome, but the effect of pseudomonas on long-term outcomes is controversial.
What This Study Adds to the Field
In this study, we have shown that the effect of pseudomonas isolation after lung transplantation is dependent upon when after transplantation it is isolated and that pseudomonas infection, but not colonization, increases the risk of chronic lung allograft rejection and death. Higher levels of bronchoalveolar fluid ELR+ CXC chemokines further augment the risk of developing chronic rejection or death. Our data suggest that pseudomonas infection and the ELR+ CXC chemokines may interact to negatively influence lung transplant outcomes in a time-sensitive manner.
Lung transplantation is a life-saving procedure for end-stage lung disease. Long-term success is limited by the high incidence of chronic lung allograft dysfunction, predominantly due to bronchiolitis obliterans syndrome (BOS). Acute rejection is perhaps the most widely recognized event to increase the risk of chronic lung allograft dysfunction. There is expanding evidence that a variety of infections increase the chances of developing BOS. Fungal pneumonia or colonization (1, 2) and viral pneumonitis (1, 3, 4) are among infectious conditions shown to increase the risk of BOS. However, the most commonly isolated pathogens after lung transplantation are bacteria, broadly separated into gram-negative and gram-positive organisms (5–8). The gram-negative Pseudomonas aeruginosa is the most frequently isolated bacterium after lung transplantation (5–7, 9–12). Moreover, bacterial pulmonary infections have been associated with increased bronchoalveolar lavage fluid (BALF) levels of inflammatory markers, including the glutamic acid–leucine–arginine–positive (ELR+) CXC chemokine IL-8 (CXCL8) (13–16). Prior studies have suggested that pseudomonas may influence the development of BOS by de novo colonization after lung transplantation (17) or by persistent colonization (18).
Based on the above studies, we hypothesized that pseudomonas colonization and infection interact with the allograft inflammatory milieu and effect the recipient’s post-transplant state. As patients move between the states of post-transplantation, BOS, and death, different post-transplant events, such as pseudomonas infection or acute rejection, have varying impacts. Such impacts from post-transplant events are state-specific or state dependent; that is, their influence on the outcome is dependent upon when after transplantation they occur. These impacts can be investigated via a Markovian analysis that measures the likelihood of moving from one state to another. The objectives of this study were to use a multistate Cox semi-Markov approach to assess cause-specific hazards for state transitions after lung transplantation to determine the impact of acute events, including pseudomonas isolation and BALF ELR+ CXC chemokine levels on BOS, death, and death after BOS.
Methods
Patient Population
A total of 355 recipients of lung transplants at the University of California, Los Angeles who had BALF specimens collected between January 1, 2000 and December 31, 2008 were evaluated. Of these, 323 had available BALF chemokine data and 281 had adequate pulmonary function test results thereby providing the final 260 lung transplant recipients that are included for analysis. Follow-up data through December 31, 2009 are included. Each participant provided written, informed consent under a UCLA Institutional Review Board-approved protocol.
Diagnostic Definitions and Specimen Processing
Post-transplant immunosuppression, prophylaxis, pulmonary function testing, and bronchoscopy were performed according to protocols as previously reported (19). Acute rejection ≥ 1 and BOS ≥ Stage 1 were determined according to standard criteria (20–22). All chemokine data were obtained from BALF specimens.
“Pseudomonas infection” required isolation of P. aeruginosa and documentation of new shortness of breath, hypoxia, altered sputum production, or radiographic infiltrate. Isolations of P. aeruginosa lacking any of these findings are considered “colonization.” BALF preparation, determination of chemokine concentration, and immunohistochemistry staining was performed as previously reported (2, 19, 23).
Statistical Methods
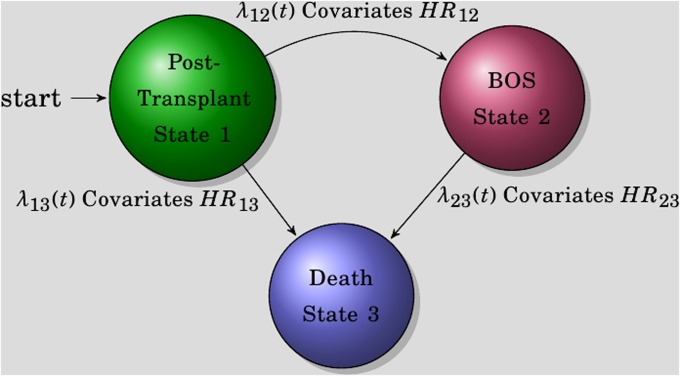
The movement of lung transplant recipients between states of transplantation, BOS, and death can be characterized by a multistate process (Figure 1). In this process, individuals start as recipients of a lung transplant (denoted by state 1). After transplantation, patients may develop BOS (from state 1 to state 2), die (from state 1 to state 3), or die after developing BOS (from state 2 to state 3). Cox-type models are used to investigate the effects of transplant type, acute rejection, aspergillus, pseudomonas, and BALF ELR+ CXC chemokines (growth-related protein-α [CXCL1], epithelial-derived neutrophil attractant-78 [CXCL5], neutrophil activating peptide-2 [CXCL7], and IL-8 [CXCL8]) on each transition (24, 25). Episodes of acute rejection, aspergillus, and pseudomonas are treated as time-dependent covariates to assess the effects of zero to multiple episodes. For transition hazards λ12 and λ13, we have and . For the transition from BOS to death, transition hazard λ23 is modeled as a function of the time spent in state 1 through a Cox semi-Markov model: . In the above formulae, λij,0(t) are the baseline hazards from state i to j, Zij,k(t) are vectors of covariates of interest at time t for the kth patient, βij are the corresponding vectors of regression coefficients, and T2 is the time of BOS .
Figure 1.
Markovian model diagram. The three states of the models are shown as individual balls. Transition is denoted by λ (e.g., transition from state 1 to state 2 is represented by λ12).
We further investigated the effects of pseudomonas infection and colonization separately with the above models and covariates to determine whether the pseudomonas effect is attributable to infections only or to colonizations only or whether any type of pseudomonas isolation is important.
Tests for significance are two-tailed with a statistically significant P value threshold of 0.05. The median is used, and the interquartile range (IQR) is given. The lower and upper limits of the hazard ratio (HR) are given in parentheses after the HR. Analyses are performed in SAS version 9.2 (SAS Institute Inc., Cary, NC) (26) and R version 2.12 (27–29).
Results
Lung Transplant Patient Population and Clinical Outcomes
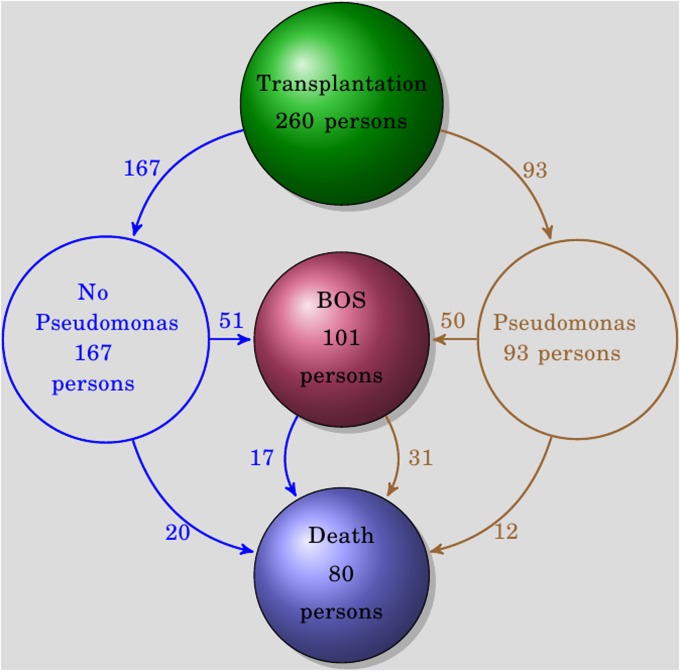
We analyzed data from 260 lung transplant recipients for whom microbiologic, acute rejection, BALF for chemokine analysis, and BOS outcomes were available (Table 1). Of these individuals, 101 (38.8%) developed BOS of grade ≥1 at a median post-transplantation time of 651 days (IQR, 397–943) (Figure 2). There were 290 episodes of acute rejection at a median of 147 days (IQR, 40–409) after transplant in 152 individuals (58%). The median number of bronchoscopies was not greater in those with BOS or any episode of acute rejection compared with those without either, and there was no significant difference in number of bronchoscopies between those with and without pseudomonas or aspergillus isolation (nine bronchoscopies [IQR, 7–11] vs. eight bronchoscopies [IQR, 6–8]; P = 1, Wilcoxon rank-sum test). Bronchoscopy specimens were obtained for a mixture of surveillance (61%), clinical (29%), follow-up for abnormal findings (8%), and undocumented (2%) indications. Respiratory specimens for microbiologic evaluation included BALF (69%), sputum (16%), donor bronchus (11%), and tracheal aspirate (4%). Eighty lung transplant recipients died during the follow-up time, with 48 of those having developed BOS before death (980 d; IQR, 532–1,491) (Figure 2). For recipients with BOS, the most frequent causes of death were graft failure (48%), infection (19%), and malignancy (13%). For recipients without BOS, the most frequent causes of death were infection (22%), malignancy (19%), and multiorgan failure (13%).
TABLE 1.
DEMOGRAPHIC/DESCRIPTIVES BY BRONCHIOLITIS OBLITERANS SYNDROME OUTCOME
| No BOS (n = 159) | BOS (n = 101) | Combined (n = 260) | Test Statistic | |
| Single transplant | 40% | 40% | 40% | χ12 = 0; P = 1* |
| Diagnosis: CF/bronchiectasis | 7% | 4% | 6% | χ32 = 4.2; P = 0.24* |
| Obstructive | 33% | 44% | 37% | |
| Restrictive | 57% | 48% | 53% | |
| Vascular | 3% | 5% | 4% | |
| Aspergillus episodes | ||||
| 0 | 64% | 40% | 55% | χ62 = 20; P = 0.003* |
| 1 | 22% | 36% | 27% | |
| 2 | 10% | 13% | 11% | |
| 3 | 1% | 4% | 2% | |
| 4 | 2% | 2% | 2% | |
| 6 | 1% | 4% | 2% | |
| 8 | 0% | 2% | 1% | |
| Pseudomonas episodes | ||||
| 0 | 73% | 50% | 64% | χ82 = 19; P = 0.017* |
| 1 | 14% | 22% | 17% | |
| 2 | 6% | 12% | 8% | |
| 3 | 2% | 2% | 2% | |
| 4 | 1% | 7% | 3% | |
| 5 | 3% | 4% | 3% | |
| 6 | 0% | 1% | 0% | |
| 7 | 1% | 1% | 1% | |
| 8 | 0% | 1% | 0% | |
| Pseudomonas colonization | ||||
| 0 | 76% | 56% | 68% | χ62 = 14; P = 0.031* |
| 1 | 13% | 21% | 16% | |
| 2 | 7% | 11% | 8% | |
| 3 | 1% | 5% | 2% | |
| 4 | 2% | 4% | 3% | |
| 5 | 1% | 2% | 1% | |
| 7 | 1% | 1% | 1% | |
| Pseudomonas infection | ||||
| 0 | 93% | 80% | 88% | χ52 = 14; P = 0.017* |
| 1 | 4% | 14% | 8% | |
| 2 | 1% | 2% | 1% | |
| 3 | 1% | 4% | 2% | |
| 4 | 1% | 0% | 0% | |
| 5 | 1% | 0% | 0% | |
| Acute rejection episodes | ||||
| 0 | 44% | 33% | 40% | χ72 = 17; P = 0.016* |
| 1 | 33% | 31% | 32% | |
| 2 | 15% | 17% | 16% | |
| 3 | 7% | 7% | 7% | |
| 4 | 1% | 8% | 4% | |
| 5 | 0% | 3% | 1% | |
| 7 | 0% | 1% | 0% | |
| 9 | 0% | 1% | 0% | |
| Time to BOS or censure | F1,258 = 2.5; P = 0.12† | |||
| Lower quartile‡ | 403 | 397 | 399 | |
| Median | 743 | 651 | 704 | |
| Upper quartile | 1,386 | 943 | 1,202 | |
| Median (SD) | 934 (690) | 749 (474) | 862 (621) | |
| Time from state 1 to state 3 | ||||
| Lower quartile | 480 | |||
| Median | 884 | |||
| Upper quartile | 1,410 | |||
| Median (SD) | 1,029 (717) | |||
| Time from state 2 to state 3 | ||||
| Lower quartile | 811 | |||
| Median | 1,087 | |||
| Upper quartile | 1,773 |
Definition of abbreviations: BOS = bronchiolitis obliterans syndrome; CF = cystic fibrosis.
Pearson test (χ2).
Wilcoxon test.
Lower quartile, median, and upper quartile are for continuous variables.
Figure 2.
The overall outcomes for study subjects. The number of individuals reaching each state is shown within each state ball. Ninety-three of the subjects had an isolation of pseudomonas; 167 had no isolation of pseudomonas.
Episodes of Pseudomonas and Aspergillus
Among the 260 patients included for analysis, there were 238 episodes of bacterial infection (279 d after transplant; IQR, 42–620) and 629 respiratory samples that were considered colonization without infection (65 d; IQR, 2–243). From 93 of the 260 patients (36%), there were 212 respiratory cultures positive for pseudomonas, of which 51 were classified as infection and 161 as colonization (Figure 2). The median time of all pseudomonas-positive cultures was 203 days (IQR, 42–581) after transplantation, with no significant difference between infection and colonization samples (P = 0.20, Wilcoxon rank-sum test). There were 213 cultures positive for aspergillus (259 d; IQR, 95–734) from 118 lung transplant recipients (42%) with Aspergillus fumigatus (n = 134 [63%]) and Aspergillus niger (n = 35 [16%]) being the most frequently isolated species.
Movement from Post-Transplantation to BOS (State 1 to State 2)
The likelihood of transition from transplant to BOS is significantly increased by acute cellular rejection, aspergillus, CXCL5, and the interaction between pseudomonas infection and CXCL1 (Table 2). For acute cellular rejection, there is a moderate dose-dependent effect, with those having two or more episodes (HR, 2.79 [1.67–15.28]; P < 0.0001) at greater risk of BOS than those with only one (HR, 1.86 [1.10–5.28]; P = 0.02) (Table 2). Likewise, as BALF concentrations of CXCL5 increase, the likelihood of BOS does too (HR, 1.10 per ng/ml increases [1.02–1.18]; P = 0.01). Further analysis demonstrates that pseudomonas infection, but not colonization, increases the risk of BOS via an interaction with BALF CXCL1 (Table 2). In this transition, the pseudomonas effect is due to infection (P = 0.04) rather than colonization (P > 0.10).
TABLE 2.
STATE 1 TO STATE 2, ONLY PSEUDOMONAS INFECTIONS CONSIDERED*
| Event | Lower Limit | Hazard Ratio† | Upper Limit | P Value |
| Pseudomonas infection × CXCL1 | — | — | — | 0.04 |
| Infection 1 vs. 0 | CXCL1 = 2 ng | 1.46 | 3.33 | 7.61 | 0.004 |
| CXCL5 per ng increase | 1.02 | 1.10 | 1.18 | 0.01 |
| Acute rejection overall | — | — | — | 0.0005 |
| Acute rejection ≥ 1 vs. 0 | 1.10 | 1.86 | 5.28 | 0.02 |
| Acute rejection ≥ 1 vs. 0 | 1.67 | 2.79 | 15.28 | <0.0001 |
| Aspergillus overall | — | — | — | 0.04 |
| Aspergillus 1 vs. 0 | 1.13 | 1.80 | 2.87 | 0.01 |
The interaction between pseudomonas infections and bronchoalveolar lavage fluid (BALF) CXCL1 increases the risk of bronchiolitis obliterans syndrome (BOS). Greater BALF concentrations of CXCL5 also increase the risk of BOS, as do episodes of acute rejection independently of pseudomonas.
Hazard ratios for transition from transplantation to bronchiolitis obliterans syndrome for pseudomonas infections only.
Movement to Death before BOS (State 1 to State 3)
Movement from transplant to death is facilitated by pseudomonas infection (HR, 3.84 [1.41–10.47]; P = 0.008), not colonization, and by transplant type, with single-lung having a deleterious effect (HR, 2.97 [1.40–6.31]; P = 0.005) (Table 3). None of the BALF ELR+ CXC chemokines is influential in this transition because there were many recipients who died from nonlung allograft–related causes. For example, one would not expect lung transplant allograft chemokine concentrations to predict death from leukemia. When considering just pseudomonas colonizations, only transplant type remains significant.
TABLE 3.
STATE 1 TO STATE 3, PSEUDOMONAS INFECTIONS*
| Event | Lower Limit | Hazard Ratio† | Upper Limit | P Value |
| Pseudomonas infection ≥ 1 episode vs. 0 | 1.41 | 3.84 | 10.47 | 0.008 |
| Single vs. double transplant | 1.40 | 2.97 | 6.31 | 0.005 |
Only pseudomonas infections and type of transplant are significant. None of the bronchoalveolar lavage fluid glutamic acid–leucine–arginine–positive CXC chemokines were significant in this transition.
Hazard ratios for transition from transplantation to death; only pseudomonas infections were considered.
Movement to Death after BOS (State 2 to State 3)
Significant covariates in the transition from BOS to death include interactions between pseudomonas, CXCL5, and aspergillus as well as the time spent in state 1 (Table 4). Longer duration of time spent in the post-transplantation state (state 1) leads to decreased risk of entering the death state (state 3). The interaction of all pseudomonas isolations with CXCL5 is driven by pseudomonas colonization, not infection (Table 5). The interaction between pseudomonas colonization and CXCL5 in this state suggests that as BALF levels of CXCL5 increase, the importance of the pseudomonas colonization decreases (Table 5). For example, when there is at least one pseudomonas colonization and the CXCL5 concentration is 10 ng/ml, the effect of the pseudomonas isolation is greater (HR, 7.61 [3.38–17.17]; P < 0.0001) than when CXCL5 concentration is 100 ng/ml (HR, 6.28 [2.90–13.61]; P < 0.0001) (Table 5). Higher concentrations of CXCL5 in the BALF increase the risk of death after BOS even in the absence of pseudomonas colonization (HR, 1.23 per 0.1 ng/ml [1.04–6.03]; P = 0.01) (Table 5).
TABLE 4.
STATE 2 TO STATE 3, PSEUDOMONAS INFECTIONS*
| Event | Lower Limit | Hazard Ratio† | Upper Limit | P Value |
| Pseudomonas infection × aspergillus overall | — | — | — | 0.007 |
| Infection ≥ 1 | vs. 0 | Aspergillus = 0 | 2.07 | 6.93 | 23.18 | 0.002 |
| Infection ≥ 1 | vs. 0 | Aspergillus = 1 | 0.03 | 0.24 | 1.99 | 0.19 |
| Aspergillus ≥ 1 | vs. 0 | Infection = 0 | 1.21 | 2.39 | 4.75 | 0.01 |
| Aspergillus ≥ 1 | vs. 0 | Infection = 1 | 0.16 | 0.52 | 1.73 | 0.29 |
Model includes pseudomonas infection and aspergillus × acute rejection interaction. Here there is no interaction between pseudomonas and CXCL5, but there is a reaction between aspergillus and pseudomonas.
Hazard ratios for transition from bronchiolitis obliterans syndrome to death.
TABLE 5.
STATE 2 TO STATE 3, PSEUDOMONAS COLONIZATIONS*
| Event | Lower Limit | Hazard Ratio† | Upper Limit | P Value |
| Pseudomonas colonization × aspergillus overall | — | — | — | 0.013 |
| Colonization ≥ 1 | aspergillus = 0; CXCL5 = 10 | 3.38 | 7.61 | 17.17 | <0.0001 |
| Colonization ≥ 1 | aspergillus = 0; CXCL5 = 25 | 3.30 | 7.37 | 16.49 | <0.0001 |
| Colonization ≥ 1 | aspergillus = 0; CXCL5 = 50 | 3.17 | 7.00 | 15.44 | <0.0001 |
| Colonization ≥ 1 | aspergillus = 0; CXCL5 = 100 | 2.90 | 6.28 | 13.61 | <0.0001 |
| CXCL5 per 0.1 ng pseudomonas = 0 | 1.04 | 1.23 | 6.03 | 0.01 |
Model includes pseudomonas colonization and pseudomonas × aspergillus interaction.
Hazard ratios for transition from bronchiolitis obliterans syndrome to death.
Pseudomonas infection and aspergillus affect the transition from BOS to death via their interaction, which can be characterized as an inclusive disjunction (Table 4). This inclusive disjunction means there is increased risk after the first isolation of either organism, but further isolations of either organism are not of additive importance. More specifically, any pseudomonas infection before the isolation of aspergillus is an important risk for death (HR, 6.93 [2.07–23.18]; P = 0.002), and any aspergillus isolation before that of pseudomonas is also significant (HR, 2.39 [1.21–4.75]; P = 0.01). However, after the isolation of either pseudomonas or aspergillus, further pseudomonas or aspergillus isolations are not important, such as pseudomonas infection after aspergillus (HR, 0.24 [0.03–1.99]; P = 0.19). This remains true when considering only pseudomonas colonizations (P = 0.01) or only pseudomonas infections (P = 0.007).
Expression of the ELR+ CXC Chemokines and Their Receptors in the Lung Allograft
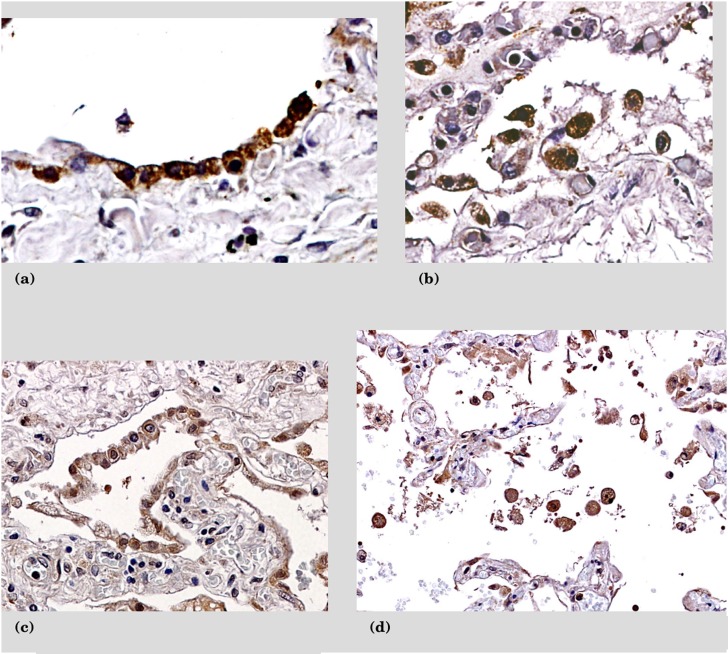
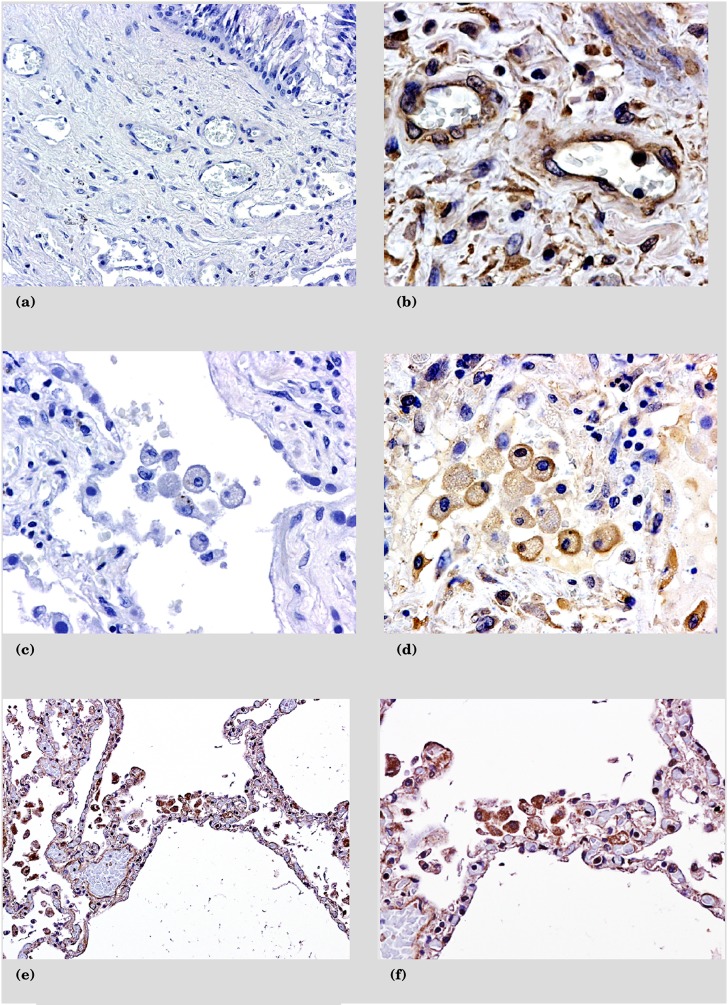
Based on the above findings, we investigated the cellular sources of these chemokines and their receptors. The protein expression of CXCL1 and CXCL5 is on hyperplastic type II pneumocytes as well as infiltrating mononuclear cells (Figures 3a–3d). The receptors for these chemokines are CXCR1 and CXCR2. CXCR1 is localized to alveolar macrophages, whereas CXCR2 is found on alveolar macrophages, infiltrating mononuclear cells, and endothelial cells (Figures 4a–4f).
Figure 3.
Immunohistochemistry sections from patients with recent pseudomonas infections stained for CXCL1 and CXCL5. (a) CXCL1 expression on type 2 pneumocytes. (b) Alveolar macrophages positive for CXCL1. (c) Type II pneumocytes positive for CXCL5. (d) Alveolar macrophages positive for CXCL5.
Figure 4.
Immunohistochemistry sections from patients with recent pseudomonas infections stained for CXC receptors. (a) Negative control for CXC receptors. (b) Mononuclear and endothelial cells positive for CXCR2. (c) Negative control for CXC receptors with macrophages. (d) Macrophages positive for CXCR2. (e) Predominantly alveolar macrophages are positive for CXCR1. (f) Detail of macrophages positive for CXCR1.
Discussion
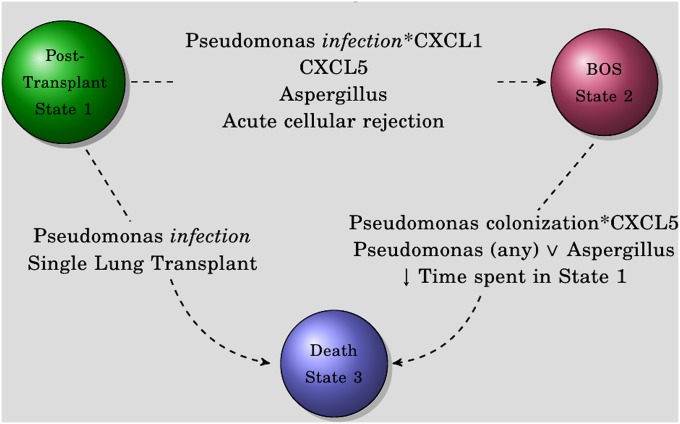
In this study we have shown via multistate Cox semi-Markov models that the effect of pseudomonas isolation after lung transplantation is state specific and that pseudomonas infection, but not colonization, increases the risk of BOS (state 1 to state 2) and death before BOS (state 1 to state 3) (Figure 5). Elevated levels of BALF ELR+ CXC chemokines further increase the risk of developing BOS (state 1 to state 2) and death after BOS (state 2 to state 3). This is seen with BALF CXCL1 via an interaction with pseudomonas infection (state 1 to state 2) and with CXCL5 alone (state 1 to state 2 and state 2 to state 3) (Figure 5). Our data suggest that pseudomonas and the ELR+ CXC chemokines interact to negatively influence lung transplant outcomes in a time-sensitive manner.
Figure 5.
Markovian model results as a diagram. The three states of the models are shown. Covariates that in our models increase the hazard of moving from one state to the other are shown. ∨ refers to an inclusive disjunction.
The Effect of Pseudomonas on Clinical Outcomes Is State Dependent
Clinical care of post-transplant patients proceeds through various states, and the impact of clinical events in each of these states is important to understand. Our intent was to model events in such a way as to replicate these clinical states. The multistate modeling approach provides transition hazards for movement from one state to another, thereby allowing the possibility of differentiating between the effects of covariates on the transition hazard to BOS, and to death in those with BOS and those without. As examples, we have shown that colonization with pseudomonas shortly after transplantation is not nearly as concerning as pseudomonas infections in increasing the patient’s risk of developing BOS. Conversely, after development of BOS, both pseudomonas colonization and infection increase the risk of death. We also investigated whether repeated exposure to risk factors was an important consideration. For some recurrent exposures, a dose-dependent effect does exist, such as for acute rejection in transition from transplant to BOS (state 1 to state 2). Therefore, prevention of recurrence is of clinical significance.
Only Pseudomonas Infection and Type of Transplant Effect Transition to Death before BOS
These two covariates have plausible biologic explanations for their effect. First, pseudomonas infection may be fatal, whereas colonization should not directly cause death. Second, double-lung transplantation in a small, single-center study like ours improved survival over single-lung transplantation for those with restrictive lung disease (30). Approximately 50% of our cohort had restrictive lung disease as an indication for lung transplant. In contrast, a large registry study found no survival benefit of double-lung transplantation for idiopathic pulmonary fibrosis (31). Many recipients moving from transplant to death died from nonallograft insults, such as malignancy and nonpulmonary infections, that are not expected to elevate graft levels of ELR+ CXC chemokines. Indeed, BALF chemokines in this transition are not expected to predict a nonlung allograft–related outcome. These findings suggest internal and external validation for our model.
Transition from Transplantation to BOS Is Affected by Pseudomonas Infection, Acute Rejection, and ELR+ CXC Chemokines
In our analysis, acute cellular rejection is a significant risk factor for BOS, and, in accordance with previous studies, recurrent acute rejection has a dose-response effect (32). There is controversy regarding the effect of pseudomonas on long-term outcomes after lung transplantation (17, 18, 33). Botha and associates evaluated 155 lung transplant recipients, of which 64 were culture positive for pseudomonas at some time after lung transplantation (17). Forty-four patients had “persistent” culture positivity, meaning that they had pseudomonas isolations before and after transplantation, and 20 lung transplant recipients had cultures positive for pseudomonas only after transplantation, or “de novo” pseudomonas. Botha found that only de novo development of pseudomonas was associated with the development of BOS. Conversely, Vos and associates found no effect from de novo pseudomonas but showed that persistent, post–lung transplant pseudomonas colonization was associated with BOS (18). Many of the prior studies did not discriminate between pseudomonas colonization and infection, which our data reveal is an important distinction because only infections increase the risk of BOS. Botha’s and Vos’ studies raise concerns of concentrating on the microbe itself as compared with the interaction between the microbe, allograft, and host. In our model, it is the interaction between pseudomonas infections and BALF CXCL1 that increases the likelihood that a patient will develop BOS. This finding indicates that there is an important interaction between the host response, the allograft, and pseudomonas that ultimately generates ELR+ CXC chemokines that are associated with clinical outcomes. The use of our state model to assess these interactions is a deciding factor in determining the effects of post-transplant events.
Colonization or Infection with Pseudomonas after BOS Is an Important Risk for Death
The cystic fibrosis literature documents an association of persistent pseudomonas colonization with decreased lung function and time to death or first lung transplantation (34, 35). Other studies in lung transplantation found a trend for pseudomonas colonization as a risk factor for death (P = 0.07), but it is not clear if this was death before or after BOS (18). Similar to the association between pseudomonas infection and BOS, we find that pseudomonas infection after BOS leads to a marked increase risk of death. However, contrary to our finding that only pseudomonas infection was associated with moving from transplant to BOS (state 1 to state 2), we found an association between pseudomonas colonization after BOS and death (state 2 to state 3). Persons with BOS may have a lower threshold for continued immune-mediated graft damage due to the chemokine-rich inflammatory milieu; thus, mild insults may result in progression of disease. Hence, pseudomonas colonization in combination with elevated graft CXCL5 is a risk for death after BOS. We also found that there is no additive effect if a patient with BOS develops pseudomonas after aspergillus or aspergillus after pseudomonas. Furthermore, our analysis demonstrates that the longer the lung transplant recipient stays in state 1, the lower the risk of transition from state 2 to death, a finding previously noted by Finlen Copeland and colleagues (36). Retarding the onset of BOS may offer survival advantages beyond what is seen by delaying the onset of a fatal syndrome alone.
BALF ELR+ CXC Chemokines Interact with Pseudomonas and Increase the Risk of BOS and Death after BOS
Most studies have concentrated on the ELR+ CXC chemokine CXCL8 and the numbers of neutrophils within the BALF. Multiple authors have found that CXCL8 levels and BALF neutrophilia is increased in lung transplant recipients with pseudomonas colonization of the airways (13–16, 18). In this present study, elevated BALF CXCL8 levels were associated with pseudomonas infection but not with colonization (data not shown). Pseudomonas stimulates lung epithelial cells to excrete multiple ELR+ CXC chemokines (37–40), which act via their receptors CXCR1 (IL-8 receptor α) and CXCR2 (IL-8 receptor β). Affinities for the receptors vary by CXC chemokine, but CXCL1 and CXCL5 have about 10 times greater affinity for CXCR2 than for CXCR1 (41). We found an interaction between pseudomonas and BALF concentrations of CXCL1 and CXCL5. This finding suggests that the inflammatory response and pseudomonas together influence clinical outcomes. Similarly, the higher the CXCL5 BALF levels, the more likely that the lung transplant recipient will develop BOS independent of pseudomonas isolation. We determined the cellular sources of these chemokines during pseudomonas infection and found that they are expressed by hyperplastic type 2 pneumocytes, infiltrating mononuclear cells, and alveolar macrophages. This cellular pattern is distinctly different from the cellular sources previously found for CXCL8, which were airway smooth muscles cells from patients with BOS (42). CXCR1 is found on infiltrating mononuclear cells and alveolar macrophages, whereas CXCR2 localizes to infiltrating mononuclear cells, alveolar macrophages, and endothelial cells. We have previously shown that multiple ELR+ CXC chemokines interacting with their receptors is important during lung allograft dysfunction in rodent model systems (23). For instance, some ELR+ CXC chemokines recruit leukocytes that lead to allograft injury, whereas others are linked with vascular remodeling that supports fibroobliterative lesions found in obliterative bronchiolitis (23, 43–46). These data suggest that strategies to inhibit multiple ELR+ CXC chemokines or their receptors could prolong the survival of the lung allograft.
Studies have found protection against BOS or death after BOS with azithromycin therapy (47, 48), and azithromycin has been shown to reduce the production of ELR+ CXC chemokines by lung epithelial cells (49). It is possible that persistent or recurrent elevation of the same ELR+ CXC chemokines, initially from primary graft dysfunction (43) and then via colonization or infection with pseudomonas, results in continuation of a profibrotic/angiogenic milieu within the lung, promoting development or progression of BOS. Moreover, targeted therapy to reduce the burden of pseudomonas and aspergillus within the allograft may reduce the inflammatory milieu that leads to allograft dysfunction and death due to BOS. There may be additional benefit in appropriate reduction of immunosuppression in selected individuals to decrease the risk of infection and the associated increases in BALF ELR+ CXC chemokines, thereby increasing longevity of the allograft and decreasing the risk of death after the development of BOS.
Limitations of the present study include the sample size and the single-center design, which may limit the generalizability of our findings. Furthermore, we were not able to determine if pseudomonas isolations were de novo due to a lack of universal pretransplant screening.
In conclusion, we have shown that pseudomonas isolation after lung transplantation has state-specific effects and that infection, not colonization, increases the risk of BOS and death before BOS. Furthermore, pseudomonas infection interacts with allograft-derived ELR+ CXC chemokines, resulting in an profibrotic/angiogenic allograft milieu that increases the risk of BOS and death.
Acknowledgments
The authors thank the patients for their participation in this study and the physicians for providing clinical care.
Footnotes
This work was supported by National Institutes of Health grants K23HL102220 and HL112990 (J.A.B.), by a Cystic Fibrosis Foundation Pilot and Feasibility Grant (A.L.G.), and by National Center for Advancing Translational Sciences through UCLA CTSI grant UL1TR000124.
Author Contributions: A.L.G.: Conception, hypothesis, design, data collection, analysis, interpretation, manuscript writing/revision. X.W.: Design, analysis, interpretation, manuscript writing/revision. S.S.W. and V.P.: Data collection, interpretation, manuscript revision. J.P.L., D.J.R., and R.S.: Data collection. B.M.K.: Data collection. M.C.F.: Data collection and interpretation, manuscript revision. A.A.: Data collection. G.L.: Design, interpretation, manuscript revision. R.E.: Design, interpretation, manuscript revision. J.A.B.: Hypothesis, design, data collection, interpretation, manuscript writing/revision.
Originally Published in Press as DOI: 10.1164/rccm.201207-1228OC on January 17, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Valentine VG, Gupta MR, Walker JE Jr, Seoane L, Bonvillain RW, Lombard GA, Weill D, Dhillon GS. Effect of etiology and timing of respiratory tract infections on development of bronchiolitis obliterans syndrome. J Heart Lung Transplant 2009;28:163–169 [DOI] [PubMed] [Google Scholar]
- 2.Weigt SS, Elashoœ RM, Huang C, Ardehali A, Gregson AL, Kubak B, Fishbein MC, Saggar R, Keane MP, Saggar R, et al. Aspergillus colonization of the lung allograft is a risk factor for bronchiolitis obliterans syndrome. Am J Transplant 2009;9:1903–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weigt SS, Elashoœ RM, Keane MP, Strieter RM, Gomperts BN, Xue YY, Ardehali A, Gregson AL, Kubak B, Fishbein MC, et al. Altered levels of CC chemokines during pulmonary CMV predict BOS and mortality post-lung transplantation. Am J Transplant 2008;8:1512–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weigt SS, Derhovanessian A, Liao E, Hu S, Gregson AL, Kubak BM, Saggar R, Saggar R, Plachevskiy V, Fishbein MC, et al. CXCR3 chemokine ligands during respiratory viral infections predict lung allograft dysfunction. Am J Transplant 2012;12:477–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valentine VG, Bonvillain RW, Gupta MR, Lombard GA, LaPlace SG, Dhillon GS, Wang G. Infections in lung allograft recipients: ganciclovir era. J Heart Lung Transplant 2008;27:528–535 [DOI] [PubMed] [Google Scholar]
- 6.Parada MT, Alba A, Sepulveda C. Early and late infections in lung transplantation patients. Transplant Proc 2010;42:333–335 [DOI] [PubMed] [Google Scholar]
- 7.Aguilar-Guisado M, Givalda J, Ussetti P, Ramos A, Morales P, Blanes M, Bou G, Torre-Cisneros de la J, Roman A, Borro JM, Lama R, Cisneros JM; RESITRA Cohort Pneumonia after lung transplantation in the RESITRA Cohort: a multicenter prospective study. Am J Transplant 2007;7:1989–1996 [DOI] [PubMed] [Google Scholar]
- 8.Horvath J, Dummer S, Loyd J, Walker B, Merrill WH, Frist WH. Infection in the transplanted and native lung after single lung transplantation. Chest 1993;104:681–685 [DOI] [PubMed] [Google Scholar]
- 9.Zeglen S, Wojarski J, Wozniak-Grygiel E, Siola M, Jastrzebski D, Kucewicz-Czech E, Zembala M. Frequency of Pseudomonas aeruginosa colonizations/infections in lung transplant recipients. Transplant Proc 2009;41:3222–3224 [DOI] [PubMed] [Google Scholar]
- 10.Campos S, Caramori M, Teixeira R, Jr JA, Carraro R, Strabelli T, Samano M, Pego-Fernandes P, Jatene F. Bacterial and fungal pneumonias after lung transplantation. Transplant Proc 2008;40:822–824 [DOI] [PubMed] [Google Scholar]
- 11.Deusch E, End A, Grimm M, Graninger W, Klepetko W, Wolner E. Early bacterial infections in lung transplant recipients. Chest 1993;104:1412–1416 [DOI] [PubMed] [Google Scholar]
- 12.Dummer JS, Montero CG, Griffith BP, Hardesty RL, Paradis IL, Ho M. Infections in heart-lung transplant recipients. Transplantation 1986;41:725–729 [DOI] [PubMed] [Google Scholar]
- 13.Nunley DR, Grgurich W, Iacono AT, Yousem S, Ohori NP, Keenan RJ, Dauber JH. Allograft colonization and infections with pseudomonas in cystic fibrosis lung transplant recipients. Chest 1998;113:1235–1243 [DOI] [PubMed] [Google Scholar]
- 14.Dosanjh AK, Elashoœ D, Robbins RC. The bronchoalveolar lavage fluid of cystic fibrosis lung transplant recipients demonstrates increased interleukin-8 and elastase and decreased IL-10. J Interferon Cytokine Res 1998;18:851–854 [DOI] [PubMed] [Google Scholar]
- 15.Vos R, Vanaudenaerde BM, Dupont LJ, Raemdonck DEV, Verleden GM. Transient airway colonization is associated with airway inflammation after lung transplantation. Am J Transplant 2007;7:1278–1287 [DOI] [PubMed] [Google Scholar]
- 16.Vos R, Blondeau K, Vanaudenaerde BM, Mertens V, Raemdonck DEV, Sifrim D, Dupont LJ, Verleden GM. Airway colonization and gastric aspiration after lung transplantation: do birds of a feather flock together? J Heart Lung Transplant 2008;27:843–849 [DOI] [PubMed] [Google Scholar]
- 17.Botha P, Archer L, Anderson RL, Lordan J, Dark JH, Corris PA, Gould K, Fisher AJ. Pseudomonas aeruginosa colonization of the allograft after lung transplantation and the risk of bronchiolitis obliterans syndrome. Transplantation 2008;85:771–774 [DOI] [PubMed] [Google Scholar]
- 18.Vos R, Vanaudenaerde BM, Geudens N, Dupont LJ, Raemdonck DEV, Verleden GM. Pseudomonal airway colonisation: risk factor for bronchiolitis obliterans syndrome after lung transplantation? Eur Respir J 2008;31:1037–1045 [DOI] [PubMed] [Google Scholar]
- 19.Gregson AL, Hoji A, Palchevskiy V, Hu S, Weigt SS, Liao E, Derhovanessian A, Saggar R, Song S, Elashoff R, et al. Protection against bronchiolitis obliterans syndrome is associated with allograft CCR7+ CD45RA- T regulatory cells. PLoS ONE 2010;5:e11354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, Glanville A, Gould FK, Magro C, Marboe CC, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant 2007;26:1229–1242 [DOI] [PubMed] [Google Scholar]
- 21.Cooper JD, Billingham M, Egan T, Hertz MI, Higenbottam T, Lynch J, Mauer J, Paradis I, Patterson GA, Smith C. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. International Society for Heart and Lung Transplantation. J Heart Lung Transplant 1993;12:713–716 [PubMed] [Google Scholar]
- 22.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, Mallory GB, Snell GI, Yousem S. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant 2002;21:297–310 [DOI] [PubMed] [Google Scholar]
- 23.Belperio JA, Keane MP, Burdick MD, Gomperts B, Xue YY, Hong K, Mestas J, Ardehali A, Mehrad B, Saggar R, et al. Role of CXCR2/CXCR2 ligands in vascular remodeling during bronchiolitis obliterans syndrome. J Clin Invest 2005;115:1150–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersen PK, Gill RD. Cox’s regression model for counting processes: a large sample study. Ann Stat 1982;10:1100–1120 [Google Scholar]
- 25.Cox DR. Regression models and life tables. J Roy Stat Soc B Met 1972;34:187–220 [Google Scholar]
- 26.SAS Institute Inc SAS/STAT9.2 user’s guide. Cary, NC: SAS Institute Inc. [Google Scholar]
- 27.R Development Core Team. R: a language and environment for statistical computing. 2009 [accessed 2013 Feb 6]. Available from: http://www.R-project.org
- 28.Harrell FE., Jr. RMS: regression modeling strategies. 2011 [accessed 2013 Feb 6]. Available from: http://biostat.mc.vanderbilt.edu/rms.
- 29.Sarkar D. Lattice: lattice graphics. 2009 [accessed 2013 Feb 6]. Available from: http://lattice.r-forge.r-project.org/
- 30.Mason DP, Brizzio ME, Alster JM, McNeill AM, Murthy SC, Budev MM, Mehta AC, Minai OA, Pettersson GB, Blackstone EH. Lung transplantation for idiopathic pulmonary fibrosis. Ann Thorac Surg 2007;84:1121–1128 [DOI] [PubMed] [Google Scholar]
- 31.Thabut G, Christie JD, Ravaud P, Castier Y, Dauriat G, Jebrak G, Fournier M, Lesèche G, Porcher R, Mal H. Survival after bilateral versus single-lung transplantation for idiopathic pulmonary fibrosis. Ann Intern Med 2009;151:767–774 [DOI] [PubMed] [Google Scholar]
- 32.Hopkins PM, Aboyoun CL, Chhajed PN, Malouf MA, Plit ML, Rainer SP, Glanville AR. Association of minimal rejection in lung transplant recipients with obliterative bronchiolitis. Am J Respir Crit Care Med 2004;170:1022–1026 [DOI] [PubMed] [Google Scholar]
- 33.Gottlieb J, Mattner F, Weissbrodt H, Dierich M, Fuehner T, Strueber M, Simon A, Welte T. Impact of graft colonization with gram-negative bacteria after lung transplantation on the development of bronchiolitis obliterans syndrome in recipients with cystic fibrosis. Respir Med 2009;103:743–749 [DOI] [PubMed] [Google Scholar]
- 34.Aaron SD, Vandemheen KL, Ramotar K, Giesbrecht-Lewis T, Tullis E, Freitag A, Paterson N, Jackson M, Lougheed MD, Dowson C, et al. Infection with transmissible strains of Pseudomonas aeruginosa and clinical outcomes in adults with cystic fibrosis. JAMA 2010;304:2145–2153 [DOI] [PubMed] [Google Scholar]
- 35.Rosenbluth DB, Wilson K, Ferkol T, Schuster DP. Lung function decline in cystic fibrosis patients and timing for lung transplantation referral. Chest 2004;126:412–419 [DOI] [PubMed] [Google Scholar]
- 36.Finlen Copeland CA, Snyder LD, Zaas DW, Turbyøll WJ, Davis WA, Palmer SM. Survival after bronchiolitis obliterans syndrome among bilateral lung transplant recipients. Am J Respir Crit Care Med 2010;182:784–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cobb LM, Mychaleckyj JC, Wozniak DJ, Lopez-Boado YS. Pseudomonas aeruginosa flagellin and alginate elicit very distinct gene expression patterns in airway epithelial cells: implications for cystic fibrosis disease. J Immunol 2004;173:5659–5670 [DOI] [PubMed] [Google Scholar]
- 38.Perez A, Davis PB. Gene profile changes after Pseudomonas aeruginosa exposure in immortalized airway epithelial cells. J Struct Funct Genomics 2004;5:179–194 [DOI] [PubMed] [Google Scholar]
- 39.Reiniger N, Ichikawa JK, Pier GB. Influence of cystic fibrosis transmembrane conductance regulator on gene expression in response to Pseudomonas aeruginosa infection of human bronchial epithelial cells. Infect Immun 2005;73:6822–6830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alam DA, Deslee G, Tournois C, Lamkhioued B, Lebargy F, Merten M, Belaaouaj A, Guenounou M, Gangloff SC. Impaired interleukin-8 chemokine secretion by staphylococcus aureus-activated epithelium and T-cell chemotaxis in cystic fibrosis. Am J Respir Cell Mol Biol 2010;42:644–650 [DOI] [PubMed] [Google Scholar]
- 41.Ahuja SK, Murphy PM. The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J Biol Chem 1996;271:20545–20550 [DOI] [PubMed] [Google Scholar]
- 42.DiGiovine B, Lynch JP, Martinez FJ, Flint A, Whyte RI, Iannettoni MD, Arenberg DA, Burdick MD, Glass MC, Wilke CA, et al. Bronchoalveolar lavage neutrophilia is associated with obliterative bronchiolitis after lung transplantation: role of IL-8. J Immunol 1996;157:4194–4202 [PubMed] [Google Scholar]
- 43.Belperio JA, Keane MP, Burdick MD, Gomperts BN, Xue YY, Hong K, Mestas J, Zisman D, Ardehali A, Saggar R, et al. CXCR2/CXCR2 ligand biology during lung transplant ischemia-reperfusion injury. J Immunol 2005;175:6931–6939 [DOI] [PubMed] [Google Scholar]
- 44.Keane MP, Donnelly SC, Belperio JA, Goodman RB, Dy M, Burdick MD, Fishbein MC, Strieter RM. Imbalance in the expression of CXC chemokines correlates with bronchoalveolar lavage fluid angiogenic activity and procollagen levels in acute respiratory distress syndrome. J Immunol 2002;169:6515–6521 [DOI] [PubMed] [Google Scholar]
- 45.Sue RD, Belperio JA, Burdick MD, Murray LA, Xue YY, Dy MC, Kwon JJ, Keane MP, Strieter RM. CXCR2 is critical to hyperoxia-induced lung injury. J Immunol 2004;172:3860–3868 [DOI] [PubMed] [Google Scholar]
- 46.Keane MP, Belperio JA, Xue YY, Burdick MD, Strieter RM. Depletion of CXCR2 inhibits tumor growth and angiogenesis in a murine model of lung cancer. J Immunol 2004;172:2853–2860 [DOI] [PubMed] [Google Scholar]
- 47.Jain R, Hachem RR, Morrell MR, Trulock EP, Chakinala MM, Yusen RD, Huang HJ, Mohanakumar T, Patterson GA, Walter MJ. Azithromycin is associated with increased survival in lung transplant recipients with bronchiolitis obliterans syndrome. J Heart Lung Transplant 2010;29:531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vos R, Vanaudenaerde BM, Verleden SE, Vleeschauwer SID, Willems-Widyastuti A, Raemdonck DEV, Schoonis A, Nawrot TS, Dupont LJ, Verleden GM. A randomised controlled trial of azithromycin to prevent chronic rejection after lung transplantation. Eur Respir J 2011;37:164–172 [DOI] [PubMed] [Google Scholar]
- 49.Murphy DM, Forrest IA, Corris PA, Johnson GE, Small T, Jones D, Fisher AJ, Egan JJ, Cawston TE, Lordan JL, et al. Azithromycin attenuates effects of lipopolysaccharide on lung allograft bronchial epithelial cells. J Heart Lung Transplant 2008;27:1210–1216 [DOI] [PubMed] [Google Scholar]