Abstract
Rationale: Tuberculosis (TB) is characterized by a subclinical phase (symptoms absent or not considered abnormal); prediagnostic phase (symptoms noticed but diagnosis not pursued); and clinical phase (care actively sought). Diagnostic capacity during these phases is limited.
Objectives: To estimate the population-level impact of TB case-finding strategies in the presence of subclinical and prediagnostic disease.
Methods: We created a mathematical epidemic model of TB, calibrated to global incidence. We then introduced three prototypical diagnostic interventions: increased sensitivity of diagnosis in the clinical phase by 20% (“passive”); early diagnosis during the prediagnostic phase at a rate of 10% per year (“enhanced”); and population-based diagnosis of 5% of undiagnosed prevalent cases per year (“active”).
Measurements and Main Results: If the subclinical phase was ignored, as in most models, the passive strategy was projected to reduce TB incidence by 18% (90% uncertainty range [UR], 11–32%) by year 10, compared with 23% (90% UR, 14–35%) for the enhanced strategy and 18% (90% UR, 11–28%) for the active strategy. After incorporating a subclinical phase into the model, consistent with population-based prevalence surveys, the active strategy still reduced 10-year TB incidence by 16% (90% UR, 11–28%), but the passive and enhanced strategies’ impact was attenuated to 11% (90% UR, 8–25%) and 6% (90% UR, 4–13%), respectively. The degree of attenuation depended strongly on the transmission rate during the subclinical phase.
Conclusions: Subclinical disease may limit the impact of current diagnostic strategies for TB. Active detection of undiagnosed prevalent cases may achieve greater population-level TB control than increasing passive case detection.
Keywords: tuberculosis, diagnostic techniques and procedures, models, theoretical, epidemiology
At a Glance Commentary
Scientific Knowledge on the Subject
The duration of subclinical and prediagnostic tuberculosis (TB) can be estimated from representative prevalence surveys, but the influence of these phases on the impact of novel TB diagnostics, often used late in the disease course, remains poorly studied.
What This Study Adds to the Field
This study uses realistic estimates of the prevalence and duration of subclinical and prediagnostic TB to demonstrate that active TB case detection is likely to have substantially greater impact on population-level TB epidemiology than passive or “enhanced” diagnostic strategies.
Improved diagnosis is a cornerstone of current efforts to control tuberculosis (TB) (1). For the last 15 years, the World Health Organization (WHO) and Stop TB Partnership have focused on improving TB case detection and treatment success, in part because key mathematical models predicted that meeting these targets would result in improved disease control (2). Although the directly observed therapy, short-course strategy has been scaled up globally and has saved more than 1 million lives (3), 1.4 million people still die every year from TB (4). Most of these deaths reflect diagnosis that is either delayed, missed, or never attempted. Strategies for improving TB diagnosis generally take one of three forms: (1) improvement of the “passive” diagnostic system (e.g., deploying new diagnostic tools, such as Xpert MTB/RIF [5, 6] for diagnosis of people who present with TB symptoms); (2) “enhanced” diagnosis that aims to reduce delays in diagnosis for patients with recognizable symptoms, such as community awareness campaigns (7) and improved access to TB diagnostic services (8); and (3) “active” strategies that do not rely on patient presentation (e.g., household surveys [9], contact investigations [10]). The factors that determine the comparative effectiveness of these strategies in reducing population-level TB incidence remain largely unknown.
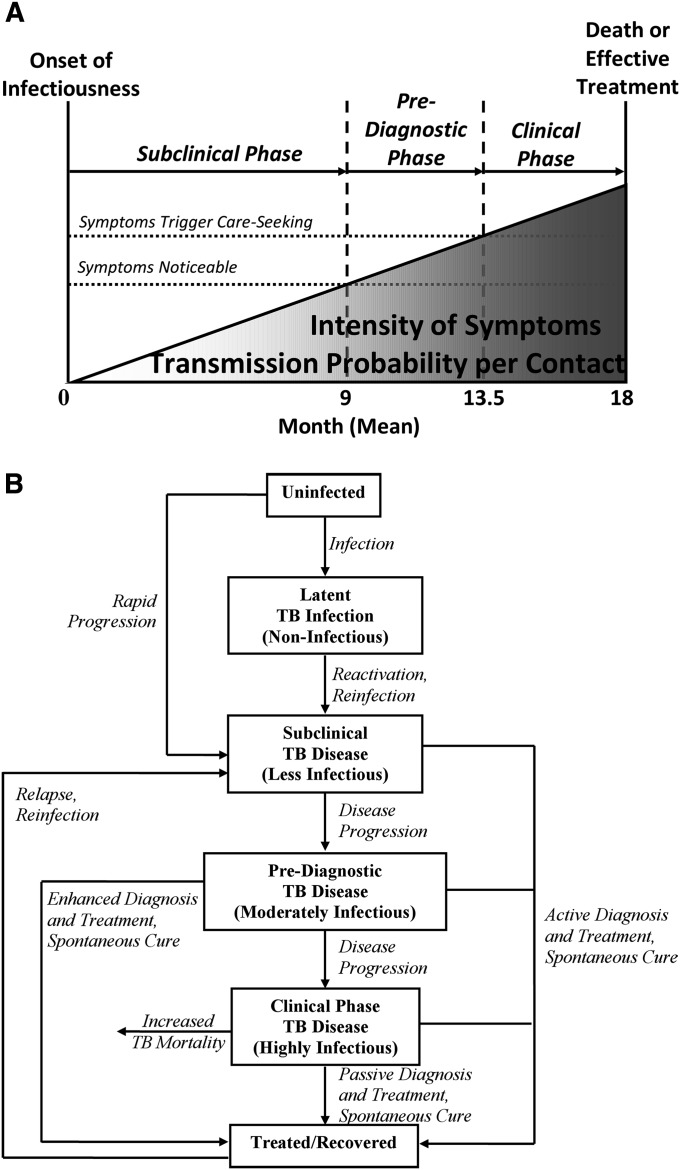
The clinical course of TB symptoms and infectiousness represents a spectrum that varies between individual patients. However, for purposes of evaluating the interplay between disease and diagnosis, this progression may be conceptualized in simplified terms as a series of discrete (although clinically artificial) phases. These phases include latent infection (asymptomatic and noninfectious); subclinical TB (negative TB symptom screen, but cultures are positive and TB is presumably infectious) (11, 12); “prediagnostic” disease (symptoms sufficiently noticeable to screen positive on symptom screening, but not sufficiently severe to seek medical care); and “clinical” disease (during which patients actively seek care for their symptoms). In this conceptual framework, “passive” diagnosis cannot occur before the clinical phase, whereas “enhanced” diagnosis seeks to shorten the prediagnostic phase. Population-based TB prevalence surveys have found that more than half of all prevalent culture-positive disease, even in people infected with HIV, is asymptomatic (13–15), and that individuals with asymptomatic TB generally progress to symptomatic disease (13, 16). These patients, described as “subclinical” in our conceptual framework, may experience symptoms (particularly cough, with a reported prevalence >30% in some high-burden settings [17]); however, these symptoms are not recognized as requiring medical attention. Other indicators of TB, most notably abnormalities on chest radiograph (15), may be detectable at this early stage. During this period, bacillary lung burden may be low, thereby reducing the risk of TB transmission per infectious contact (Figure 1). However, most TB transmission may still occur during this time if the relative duration of subclinical disease is long, or if individuals with subclinical disease “saturate” their close contacts with TB infection, take fewer precautions to prevent infection, or reduce their mobility (e.g., less time at work) after they develop noticeable symptoms.
Figure 1.
Subclinical, prediagnostic, and clinical periods in tuberculosis (TB). Mycobacterium tuberculosis can be cultured from the sputum of individuals before the onset of noticeable symptoms, which in turn occurs before those people seek diagnosis. During this time, the lung bacillary burden, an important determinant of symptom intensity and per-contact transmission probability, increases. (The linear plane is not intended to imply that this increase is necessarily linear.) The subclinical phase lasts from the onset of infectiousness (for which we take the ability to culture M. tuberculosis as a proxy) to the onset of noticeable symptoms (i.e., the presence of which patients would affirm if asked). The prediagnostic phase lasts from the onset of noticeable symptoms to the point at which those symptoms begin to trigger diagnostic attempts. (A) Clinical progression. (B) Compartmental model.
Mathematical models that simplify disease progression into conceptual stages are frequently used to inform decision-making related to TB and other infectious diseases. To date, most models have ignored the existence of subclinical TB, thereby potentially overlooking transmission events during this period and overestimating new diagnostic strategies’ effectiveness. To quantify the relationships between duration of the subclinical and prediagnostic periods, relative transmission rate during those periods, and modeled population-level impact of strategies for improved TB diagnosis, we constructed a mathematical model that accounts for the possibility of subclinical and prediagnostic disease.
Methods
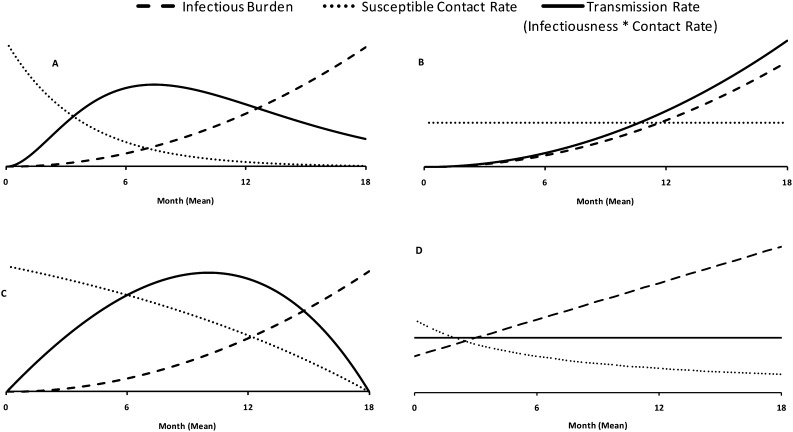
Using widely cited TB models as a guide (2, 18), we constructed a compartmental model described by a series of ordinary differential equations and parameter values from the literature (Table 1) (2, 4, 19–27). We incorporated two additional compartments to represent the progression of TB disease (Figure 1; see online supplement). Although infectiousness per contact increases throughout the subclinical, prediagnostic, and clinical phases as lung bacillary load increases, the relative transmission rate depends on the contact rate, such that transmission may occur more frequently (Figure 2A), near-equally (Figure 2B), or less frequently (Figure 2C) in the preclinical versus clinical phases. In our reference scenario, we assumed equal mean rates of transmission per person-day spent in each compartment, thereby allowing the time trajectory of transmission to take any shape that results in equal mean transmission rates across phases.
TABLE 1.
PARAMETERS FOR TRANSMISSION MODEL OF TB
| Parameter | Baseline Value (Uncertainty Range) | References |
| TB incidence, per 100,000 per year* | 112 (101–128) | 4 |
| Endogenous reactivation rate, per year | 0.0005 (0.0001–0.001) | 2, 21, 22 |
| Proportion of recent infections resulting in rapid progression | 0.14 (0.08–0.25) | 2, 23 |
| Reduction in TB rapid progression probability in people with latent TB infection† | 0.5 (0.1–0.8) | 2, 23–25 |
| TB mortality rate, per year | 0.2 (0.15–0.3) | 2, 4 |
| Spontaneous cure rate, per year | 0.2 (0.15–0.25) | 2, 19, 20 |
| Life expectancy, yr | 65 (55–75) | 26 |
| Relapse rate, per year | 0.001 (0–0.002) | 2, 27 |
| Total duration of TB infectiousness, yr | 1.5 (1.0–2.0) | 4 |
Definition of abbreviation: TB = tuberculosis.
The model fits a transmission rate to TB incidence, first by determining the transmission rate that would produce an equilibrium incidence of 128 per 100,000 per year, then reducing that rate by a value sufficient to generate a 1.3% annual decline in TB incidence (i.e., 112 per 100,000 after 10 years), the global estimate (4). The baseline value of this transmission parameter is five infections per infectious person-year.
This parameter reflects the partial immunologic protection provided by initial TB infection (i.e., latent infection) against primary progression to active TB on reexposure to Mycobacterium tuberculosis.
Figure 2.
Tuberculosis transmission rate as a function of time. Although the infectious burden (probability of successful infection per contact) likely increases with time over the course of disease, the rate of transmission (infectious burden × susceptible contact rate) may peak during the subclinical, prediagnostic, or clinical phases. (A) Cases of tuberculosis infect all susceptible close contacts (e.g., household members) early in their disease course, such that the transmission rate peaks during the subclinical phase. (B) The effective contact rate remains constant, such that the transmission rate increases throughout the disease course. (C) The effective contact rate decreases slowly over time as individuals infect close contacts, reduce mobility, and take simple measures (e.g., covering cough with hands) to prevent transmission. The primary model described in the text assumes equal mean rates of transmission in the subclinical, prediagnostic, and diagnostic periods (without specifying the shape of the transmission curves). (D) One such formulation is shown.
We used data from published population-based TB prevalence surveys to inform the duration of each diagnostic stage in the reference scenario, with the goal of meeting four important criteria. First, the overall duration of active TB disease should be similar to the ratio of prevalence/incidence of culture-confirmed TB in prevalence surveys and as estimated globally by the WHO. Second, the proportion of individuals with subclinical TB should reflect the proportion of individuals screening negative on standard symptom screens in published population-based surveys. Third, the duration of prediagnostic TB should be similar to that of clinical TB, such that “patient delay” (corresponding to the prediagnostic stage) is similar in duration to “health system delay” (clinical stage) (28). Finally, the durations of each stage should be clinically meaningful. The model that best fit all four of these criteria was one in which the subclinical stage lasted 9 months, followed by a prediagnostic stage of 4.5 months and a clinical stage of 4.5 months. This model gives a point prevalence of subclinical TB of 55% (13–15), overall duration of active TB of 1.35 years (4, 13, 14, 29), equal prediagnostic and clinical phases among people who are diagnosed, and clinically meaningful cut-points. Notably, each phase is modeled as an exponential distribution and thus implicitly includes some individuals who remain in that phase for much longer (and others much shorter) than this mean duration. We fit each simulation by solving analytically to provide an equilibrium TB incidence of 128 per 100,000 per year in 2009 (4), adjusting the transmission parameter downward to produce a geometric mean 1.3% annual decline in incidence (4). We studied three different archetypal diagnostic interventions:
Improved passive case finding: a 20% increase in the sensitivity of diagnosis during the clinical phase only (e.g., increase in sensitivity from 0.70 to 0.84, as might be achievable with implementation of the Xpert MTB/RIF test [6]).
Enhanced case finding: successful diagnosis and treatment of individuals in the prediagnostic phase (but not clinical phase) at a rate of 10% per year (e.g., community awareness campaign covering 10% of the population every year, obtaining sputum from all individuals with noticeable symptoms but not yet seeking care).
Active case finding: annual diagnosis of 5% of all TB cases in subclinical, prediagnostic, and clinical phases (e.g., screening 5% of the population per year using a highly sensitive diagnostic algorithm, such as radiograph and interview followed by culture, similar to a population-based survey of one-sixth of the population [30] every 3 years).
We varied each parameter in one-way sensitivity analysis across the ranges of values used by the WHO in its models of TB control (2). We also conducted multivariate sensitivity analysis by calculating partial rank correlation coefficients (31, 32) using 1,000 simulations of Latin Hypercube samples from uniform distributions defined by the sensitivity ranges in Table 1. We also report 90% uncertainty ranges as the range between the 5th and 95th percentile of results from all simulations. We calculated simple (Pearson) correlation coefficients between the impact of each intervention and the proportion of transmission occurring from each infectious compartment (subclinical, prediagnostic, and clinical) under variation of the duration and relative transmissibility of TB in each compartment. Finally, to extend model findings to areas of higher or lower TB burden, we considered alternative scenarios fit to TB incidence of 40 per 100,000 per year and 300 per 100,000 per year.
Results
Standard versus Subclinical Model
In the model that assumed no subclinical period (prediagnostic and clinical periods each 9 mo), TB incidence and mortality at the end of Year 10 were projected at 112 cases and 20 deaths per 100,000 per year, respectively, corresponding to a 1.3% annual decline from current incidence estimates. In this model, improved TB case finding and treatment through passive (20% improved sensitivity for clinical TB), “enhanced” (detecting and treating 10% of prediagnostic TB per year), and active (5% of all TB cases per year) strategies each resulted in similar epidemiologic impact by the end of Year 10 (Table 2).
TABLE 2.
PROJECTED EPIDEMIOLOGIC IMPACT OF DIAGNOSTIC INTERVENTIONS AFTER TEN YEARS
| Intervention | Reduction in Incidence (90% Uncertainty Range)* | Reduction in Mortality (90% Uncertainty Range)* |
| Model 1: No Subclinical Phase, Prediagnostic Phase 9 Months, Clinical Phase 9 Months | ||
| Increase sensitivity of passive diagnosis by 20% | 18% (11–32) | 26% (19–39) |
| Diagnose individuals with prediagnostic TB at a rate of 10% per year | 23% (14–35) | 27% (19–37) |
| Diagnose all people with TB at a rate of 5% per year | 18% (11–28) | 22% (16–31) |
| Model 2: Subclinical Phase 9 Months, Prediagnostic Phase 4.5 Months, Clinical Phase 4.5 Months† | ||
| Increase sensitivity of passive diagnosis by 20% | 11% (8–25) | 21% (18–32) |
| Diagnose individuals with prediagnostic TB at a rate of 10% per year | 6% (4–13) | 9% (7–15) |
| Diagnose all people with TB at a rate of 5% per year | 16% (11–28) | 21% (15–31) |
Definition of abbreviation: TB = tuberculosis.
Relative to the baseline scenario in which the current trajectory of incidence (i.e., 1.3% annual decline in incidence) continues.
Estimated based on the duration ratio (prevalence/incidence) and subclinical disease proportion from population-based TB prevalence surveys.
We next incorporated a subclinical phase of 9 months, prediagnostic phase of 4.5 months, and clinical phase of 4.5 months, consistent with population-based prevalence surveys as described in the Methods section and an equivalent transmission rate in each phase. In this model, when incidence was maintained at equivalent levels, projected mortality was reduced to 12 per 100,000 per year at 10 years, reflecting lower mortality risk during subclinical and prediagnostic disease. Under these circumstances, the projected impact of passive and enhanced diagnosis was substantially attenuated (Table 2). Failure to account for the subclinical phase caused a large overestimation of the 10-year incidence impact of passive (65% overestimation, [18–11]/11 in Table 2) and enhanced (270% overestimation, [23–6]/6) diagnosis. By contrast, active case detection had nearly equivalent impact on TB incidence regardless of the duration of the subclinical phase (7% overestimation). The degree of overestimation was substantially less (6% for passive, 89% for enhanced, and 0% for active case-finding) if the relative transmission rate during the subclinical phase was reduced to 0.25.
In summary, we took three TB diagnostic interventions (passive, enhanced, and active) that were projected to have similar impact on TB incidence in a model that ignored subclinical TB. After incorporating a realistic subclinical phase of 9 months (relative transmission rate = 1.0), the reduction in TB incidence achieved through active case-finding strategy was 1.5 times greater than the passive diagnostic intervention, and 2.7 times greater than the enhanced case-finding intervention.
Effect of Duration of Subclinical, Prediagnostic, and Clinical TB
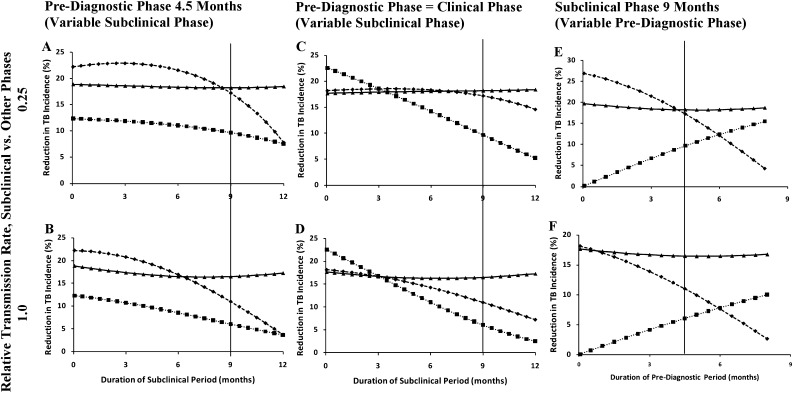
Figure 3 shows the projected reduction in TB incidence as a function of the relative duration of the subclinical, prediagnostic, and clinical phases. As the subclinical phase increased while decreasing the length of the clinical phase (Figures 3A and 3B), the impact declined for the enhanced and passive strategies, with steeper declines occurring after the subclinical period was lengthened beyond 6 months. If the subclinical phase increased while decreasing the length of prediagnostic and clinical phases (Figures 3C and 3D), the impact of enhanced diagnosis declined almost linearly, whereas that of passive diagnosis declined more slowly. Under the assumption of 0.25 relative transmission during the subclinical period (Figure 3C), the impact of passive diagnosis remained nearly constant regardless of the subclinical period, unless the subclinical period was lengthened beyond 9 months. When the prediagnostic phase was increased while shortening the clinical phase, enhanced diagnosis became more effective while the effectiveness of passive diagnosis decreased accordingly. In none of these scenarios was the impact of active diagnosis substantially affected by the length of the three diagnostic phases.
Figure 3.
Epidemiologic impact of diagnostic strategies, assuming different subclinical and prediagnostic phases. Graphs show the reduction in tuberculosis (TB) incidence at the end of 10 years, relative to continuation of current trajectories, after increasing the rate of symptom-driven diagnosis by 20% (passive, diamonds), diagnosing 10% of all individuals with prediagnostic TB per year (enhanced, squares), and diagnosing 5% of all prevalent cases in the population per year (active, triangles). Vertical lines denote the baseline scenario (subclinical phase of 9 mo, prediagnostic phase of 4.5 mo, and clinical phase of 4.5 mo). For every 1-month increase in the subclinical phase, A and B assume a 1-month decrease in the clinical phase, whereas the prediagnostic phase remains constant. C and D assume that both the prediagnostic and clinical phases decrease by 0.5 month. In E and F, a 1-month increase in the prediagnostic phase results in a 1-month decrease in the clinical phase. A, C, and E assume that the TB transmission rate in the subclinical phase is 25% of that in the prediagnostic and clinical phases. B, D, and F assume that transmission is constant throughout (i.e., the primary analysis).
Sensitivity Analysis
On inclusion of a “smear-negative clinical” compartment, the model projected somewhat greater impact from active diagnosis relative to passive diagnosis, but our primary conclusions remained the same. Results from a “high HIV prevalence model” that incorporated shorter disease duration and higher TB mortality showed similar trends as those seen in Figure 3, but active and enhanced diagnosis saw their impact reduced by approximately 50%, reflecting the shorter disease duration (6 vs. 18 mo). In models fit to TB incidence of 40 per 100,000 per year and 300 per 100,000 per year, no intervention’s relative impact varied by more than 10% (although absolute number of cases and deaths averted varied proportionally), and the “overestimation factor” from ignoring the subclinical phase in evaluating a passive diagnostic remained at 65% in both scenarios. The impact of improved passive diagnosis was almost perfectly correlated with the proportion of transmission that occurred during the clinical phase (R2 = 0.98); similarly, the impact of enhanced case finding was strongly correlated with the proportion of transmission that occurred during the clinical plus prediagnostic phases (R2 = 0.91). By contrast, active case finding was relatively independent of the proportion of transmission occurring in each phase (all R2 <0.90). In 98.6% of model simulations, ignoring the subclinical phase caused greater error in estimating passive diagnostics’ impact on TB incidence than that of active case finding.
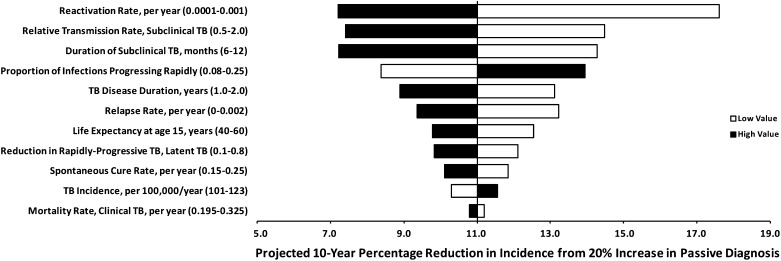
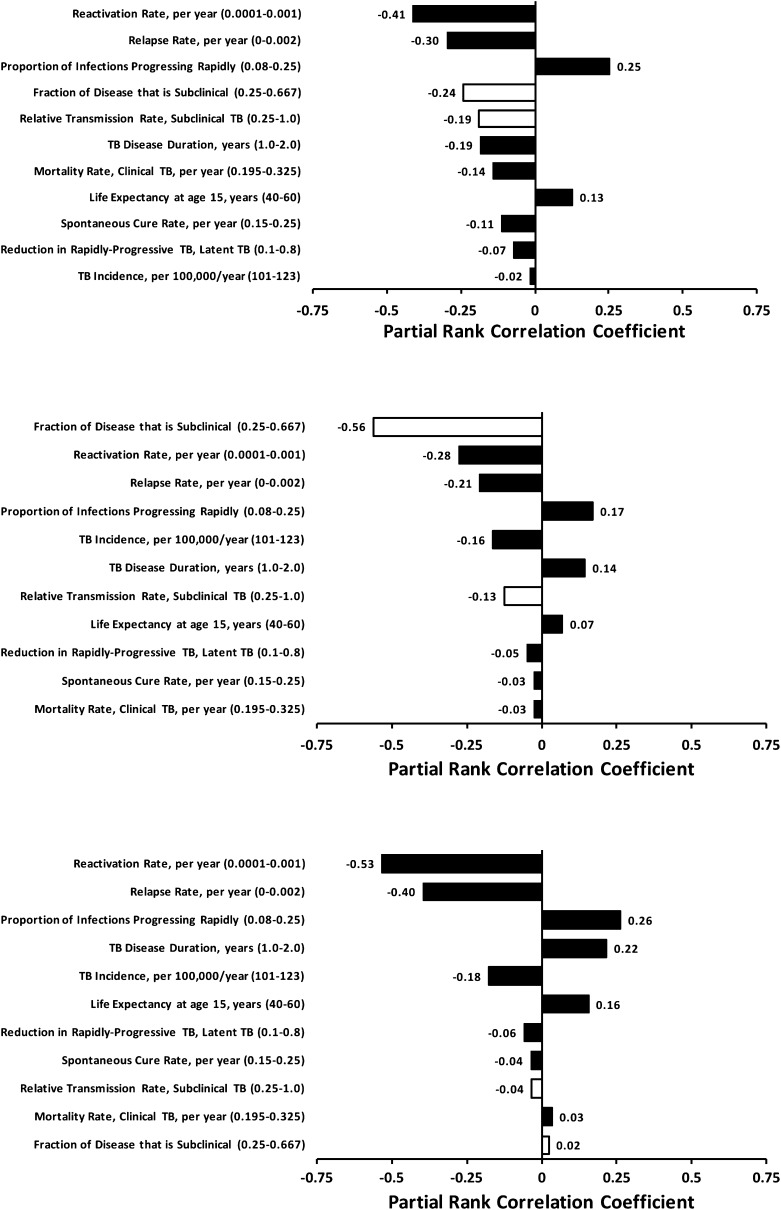
Relative to other model parameters, the duration and relative transmission rate of the subclinical period were important determinants of passive and enhanced TB diagnostics’ impact (Figure 4). Passive diagnostics were least effective when the subclinical period was long (as might occur in a setting with high prevalence of chronic cough) and the proportion of transmission from subclinical cases was high. In multivariable sensitivity analysis, the fraction of TB disease spent in the subclinical state was the single-most important determinant of enhanced diagnostics’ impact, but had virtually no effect on the impact of active diagnostics (Figure 5). Increasing the proportion of TB incidence because of recent infection (relative to reactivation or relapse) substantially reduced all diagnostic interventions’ impact (seen in Figure 4 for passive diagnosis).
Figure 4.
One-way sensitivity analysis: epidemiologic impact of improved passive diagnosis. Bars represent the 10-year percentage reduction in incidence after improving passive case detection and treatment by a factor of 20% under the baseline scenario (subclinical phase 9 mo; prediagnostic phase 4.5 mo; clinical phase 4.5 mo), with the vertical line at 11% reduction representing the reference scenario in Table 2. Solid bars denote variation of the corresponding parameter to its high value in Table 1, open bars to the low value. Parameters to which the model is most sensitive appear at the top of the diagram. The corresponding analysis for enhanced diagnosis is similar in the rank-ordering of parameters’ importance; active diagnosis is less sensitive to the duration or relative transmission rate of subclinical tuberculosis (TB).
Figure 5.
Multivariable sensitivity analysis. Partial rank correlation coefficients (shown on the x axis) describe the degree of correlation between the corresponding parameter and 10-year reduction in tuberculosis (TB) incidence for passive (top), enhanced (middle), and active (bottom) diagnostic strategies after adjustment for the effects of other parameters in the model. Larger bars (i.e., parameters appearing at the top of each diagram) suggest that the outcome is more sensitive to the corresponding parameter value. Open bars correspond to those parameters that describe the subclinical infectious phase.
Discussion
This conceptual model demonstrates that the population-level impact of passive and enhanced TB diagnostic strategies may depend strongly on the duration and transmission rate of subclinical and prediagnostic TB. For example, in a scenario that ignores subclinical TB, a strategy that actively diagnoses and treats 5% of all TB cases each year has similar impact on incidence as strategies that increase the rate of passive diagnosis and treatment by 20% or diagnose and treat 10% of prediagnostic TB cases per year. However, under more realistic assumptions about the duration of subclinical disease, the active diagnostic strategy is at least 1.5 times more effective in reducing incidence than the passive strategy and 2.7 times more effective than the enhanced strategy. Ultimately, the impact of improved passive diagnosis is strongly correlated with the proportion of TB transmission that occurs after individuals begin to seek care for their symptoms. In contrast, active case finding is likely to have important impact on TB epidemiology regardless of whether most transmission occurs early or late in the disease process.
This simplistic model is not meant to provide accurate projections of diagnostic interventions’ impact under specific epidemiologic scenarios, but rather to illustrate the potential flaws of making such projections (particularly for passive and enhanced diagnostic strategies) without accounting for the reality of subclinical TB. Most existing models of the epidemiologic impact of TB diagnostics (33–36) do not account for transmission during a subclinical phase, and very few (37) explicitly account for a prediagnostic period. Our results demonstrate that these models may overestimate the epidemiologic impact of passively implemented TB diagnostics by a factor of 50% or more. A number of policy decisions related to scale-up of TB diagnostics (e.g., WHO recommendation for global roll-out of Xpert MTB/RIF [38]) have been made without explicit consideration of the relative impact of diagnostics deployed in active, passive, or enhanced fashion. Our analysis provides preliminary guidance in this regard: active detection of 5% of all prevalent TB cases per year should have substantially greater impact than a 20% increase in passive case detection rates in most epidemiologic settings. To improve on these estimates, it is important to estimate the proportion of TB transmission occurring from subclinical cases. Prevalence surveys (based on more sensitive screening techniques, such as chest radiograph) coupled with contact investigations (e.g., screening with tuberculin skin test) of all TB cases detected could provide an early estimate of this proportion. Without such data, the population-level impact of TB diagnostics is difficult to accurately project, and likely overestimated by most existing modeling techniques.
Although potentially essential for TB elimination, active diagnostic strategies capable of detecting subclinical cases are resource-intensive, and several questions remain for further research if such a strategy were to be considered. For example, our model is limited in that it does not explicitly consider costs. Prevalence surveys using radiography rather than simple interview are two to six times more expensive to conduct on a per-person basis (39). Because subclinical cases are unlikely to be smear-positive, more expensive diagnostic tests (e.g., culture or molecular testing, which are nearly 20 times more expensive than sputum smear [40]) are also required. In a population with TB prevalence of 250 per 100,000, chest radiograph screening of the population ($25 per person [39]) with follow-up culture ($20 per person [40]) of the 4% of individuals screening positive (15) could cost in excess of $100,000 per case detected, clearly beyond the resources available in most high-burden settings. Recurrent efforts at active case finding may prove cheaper on a per-person basis; future studies should estimate the costs and cost-effectiveness of such efforts more precisely. Importantly, although we calibrated our model to a global-average incidence, concentrating efforts on those at highest risk (including people previously treated for TB [15] and residents of geographic “hotspots” [41]) could improve efficiency substantially. In areas characterized by prolonged patient delay (i.e., long prediagnostic period), “enhanced” strategies aiming to reduce that delay are also important considerations (Figures 3E and 3F), but these strategies should not be universally assumed to have substantial population-level impact.
Our analysis offers other important new insights. First, other than the relative duration of the subclinical and prediagnostic periods, the balance between recent infection and reactivation or relapse was a primary determinant of all diagnostic strategies’ effectiveness (Figures 4 and 5). This finding, which reflects the fact that more timely diagnosis can prevent transmission but not reactivation, suggests that diagnostics have greater effect on incidence in settings with higher proportions of incident TB caused by recent transmission (42). Second, whereas the impact of enhanced diagnostic strategies depends almost linearly on the duration of the preclinical period (Figures 3E and 3F), the importance of the subclinical and preclinical periods on passive diagnostics’ effectiveness is much greater at longer durations of subclinical disease (Figure 3A). Thus, the impact of passive diagnosis may be especially overestimated in settings where many individuals do not think that mild symptoms, being common, merit medical attention (e.g., high levels of air pollution or wasting).
Although we did not seek to generate quantitative projections from any single intervention, our findings may nonetheless provide some direction to decision-makers. Specifically, diagnostic strategies should be prioritized in areas of high ongoing transmission, whereas other interventions (e.g., preventive therapy) are likely to have greater impact in lower-burden settings. Within high-transmission areas, active case finding is likely to have greatest impact if the frequency and coverage of screening can be raised relative to the duration of disease. For example, if 7.5% of cases can be detected within the mean duration of disease (i.e., our reference case), a 15–20% reduction in incidence may be feasible, regardless of whether subclinical cases account for most transmission. Improved passive diagnosis is generally more feasible from a logistical and resource perspective, but the impact of passive diagnostic strategies depends heavily on the amount of transmission that occurs during the clinical period; better data on subclinical disease are therefore required before accurate projections of passive diagnostics’ impact can be made.
As with any model-based analysis, our study has limitations that reflect the assumptions inherent to modeling. First, because we sought to describe generalizable behavior likely to be common to many epidemiologic settings and diagnostic testing strategies, we used a simple model with a minimum number of parameters. Our model therefore simplifies the complexities of TB diagnosis and does not account for such operational realities as linkage of patients from diagnosis to treatment initiation. Second, given the absence of data on the relative transmission rate in subclinical versus prediagnostic and clinical phases, we are limited to providing sensitivity analyses around this parameter and are unable to say which set of projections in Figure 3 is most accurate. It is possible that subclinical TB is not infectious and may therefore be safely ignored; in this regard, our analysis argues for better data to inform this assumption. Third, for demonstrative purposes, we used a model fit to global TB incidence. This formulation simplifies important (e.g., geospatial or HIV-related) heterogeneities in TB transmission and our degree of uncertainty may be greater when applied to real populations of diverse TB incidence. Finally, our model assumes homogeneous mixing and does not formally incorporate other biologic modulators of TB (e.g., HIV infection, smoking) that may have important effects on the impact of diagnostic strategies for TB.
In conclusion, the population-level impact of diagnostic strategies for TB may depend strongly on the duration of subclinical and prediagnostic disease and the relative rate of transmission during those intervals. When accounting for these phases, active diagnostic strategies that detect 5% of prevalent undiagnosed cases every year may have greater epidemiologic impact than passive strategies that improve existing diagnostic rates by 20% or more. To better project the impact of improved TB diagnosis, investigators conducting population-based TB prevalence surveys should consider performing contact investigations of subclinical cases to estimate the relative rate of TB transmission from those individuals. As decisions to implement improved diagnostic strategies are increasingly made on the basis of projected epidemiologic impact, we must also prioritize better understanding of TB transmission from individuals other than those who have symptoms and are seeking care.
Footnotes
Supported in part by grants T32 AI007433 (J.R.A.) and 1R21AI101152 (D.W.D.) from the National Institutes of Health.
Author Contributions: D.W.D., S.B., and J.R.A. designed the study. D.W.D. conducted the experiments and wrote the first draft of the manuscript. D.W.D., S.B., and J.R.A. revised the manuscript for important intellectual content and approved the final version.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201207-1217OC on December 21, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Stop TB. Partnership. The global plan to stop TB, 2011–2015. Transforming the fight: towards elimination of tuberculosis. Geneva: World Health Organization; 2011.
- 2.Dye C, Garnett GP, Sleeman K, Williams BG. Prospects for worldwide tuberculosis control under the WHO DOTS strategy. Lancet 1998;352:1886–1891 [DOI] [PubMed] [Google Scholar]
- 3.Komatsu R, Korenromp EL, Low-Beer D, Watt C, Dye C, Steketee RW, Nahlen BL, Lyerla R, Garcia-Calleja JM, Cutler J, et al. Lives saved by Global Fund-supported HIV/AIDS, tuberculosis and malaria programs: estimation approach and results between 2003 and end-2007. BMC Infect Dis 2010;10:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. Global tuberculosis control: WHO report 2011. Geneva: WHO Press; 2011.
- 5.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010;363:1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, Gler MT, Blakemore R, Worodria W, Gray C. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 2011;377:1495–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayles HM, Sismanidis C, Beyers N, Hayes RJ, Godfrey-Faussett P. ZAMSTAR, The Zambia South Africa TB and HIV Reduction study: design of a 2 × 2 factorial community randomized trial. Trials 2008;9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rifat M, Rusen ID, Islam MA, Enarson DA, Ahmed F, Ahmed SM, Karim F. Why are tuberculosis patients not treated earlier? A study of informal health practitioners in Bangladesh. Int J Tuberc Lung Dis 2011;15:647–651 [DOI] [PubMed] [Google Scholar]
- 9.Corbett EL, Bandason T, Duong T, Dauya E, Makamure B, Churchyard GJ, Williams BG, Munyati SS, Butterworth AE, Mason PR. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. Lancet 2010;376:1244–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro AE, Variava E, Rakgokong MH, Moodley N, Luke B, Salimi S, Chaisson RE, Golub JE, Martinson NA. Community-based targeted case finding for tuberculosis and HIV in household contacts of patients with tuberculosis in South Africa. Am J Respir Crit Care Med 2012;185:1110–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barry CE, III, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, Schnappinger D, Wilkinson RJ, Young D. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol 2009;7:845–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mack U, Migliori GB, Sester M, Rieder HL, Ehlers S, Goletti D, Bossink A, Magdorf K, Holscher C, Kampmann B, et al. TBNET. LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J 2009;33:956–973 [DOI] [PubMed] [Google Scholar]
- 13.Corbett EL, Bandason T, Cheung YB, Munyati S, Godfrey-Faussett P, Hayes R, Churchyard G, Butterworth A, Mason P. Epidemiology of tuberculosis in a high HIV prevalence population provided with enhanced diagnosis of symptomatic disease. PLoS Med 2007;4:e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood R, Middelkoop K, Myer L, Grant AD, Whitelaw A, Lawn SD, Kaplan G, Huebner R, McIntyre J, Bekker LG. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med 2007;175:87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoa NB, Cobelens FG, Sy DN, Nhung NV, Borgdorff MW, Tiemersma EW. Yield of interview screening and chest X-ray abnormalities in a tuberculosis prevalence survey. Int J Tuberc Lung Dis 2012;16:762–767 [DOI] [PubMed] [Google Scholar]
- 16.Lawn SD, Kerkhoff AD, Wood R. Progression of subclinical culture-positive tuberculosis to symptomatic disease in HIV-infected individuals. AIDS 2011;25:2190–2191 [DOI] [PubMed] [Google Scholar]
- 17.Nriagu J, Robins T, Gary L, Liggans G, Davila R, Supuwood K, Harvey C, Jinabhai CC, Naidoo R. Prevalence of asthma and respiratory symptoms in south-central Durban, South Africa. Eur J Epidemiol 1999;15:747–755 [DOI] [PubMed] [Google Scholar]
- 18.Blower SM, Small PM, Hopewell PC. Control strategies for tuberculosis epidemics: new models for old problems. Science 1996;273:497–500 [DOI] [PubMed] [Google Scholar]
- 19.Tiemersma EW, van der Werf MJ, Borgdorff MW, Williams BG, Nagelkerke NJ. Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: a systematic review. PLoS ONE 2011;6:e17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med 2003;163:1009–1021 [DOI] [PubMed] [Google Scholar]
- 21.Horsburgh CR, Jr, O'Donnell M, Chamblee S, Moreland JL, Johnson J, Marsh BJ, Narita M, Johnson LS, von Reyn CF. Revisiting rates of reactivation tuberculosis: a population-based approach. Am J Respir Crit Care Med 2010;182:420–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis: a general review. Bibl Tuberc 1970;26:28–106 [PubMed] [Google Scholar]
- 23.Vynnycky E, Fine PEM. The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol Infect 1997;119:183–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutherland I, Svandová E, Radhakrishna S. The development of clinical tuberculosis following infection with tubercle bacilli: 1. A theoretical model for the development of clinical tuberculosis following infection, linking from data on the risk of tuberculous infection and the incidence of clinical tuberculosis in the Netherlands. Tubercle 1982;63:255–268 [DOI] [PubMed] [Google Scholar]
- 25.Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, Horsburgh CR. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin Infect Dis 2012;54:784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Bank. Life expectancy [accessed 2012 July 11]. Available at: http://www.google.com/publicdata/explore?ds=d5bncppjof8f9_&ctype=l&strail=false&nselm=h&hl=en&dl=en#ctype=l&strail=false&bcs=d&nselm=h&met_y=sp_dyn_le00_in&scale_y=lin&ind_y=false&rdim=region&ifdim=region&tdim=true&hl=en_US&dl=en&ind=false
- 27.Lambert ML, Hasker E, Van Deun A, Roberfroid D, Boelaert M, Van der Stuyft P. Recurrence in tuberculosis: relapse or reinfection? Lancet Infect Dis 2003;3:282–287 [DOI] [PubMed] [Google Scholar]
- 28.Sreeramareddy CT, Panduru KV, Menten J, Van den Ende J. Time delays in diagnosis of pulmonary tuberculosis: a systematic review of literature. BMC Infect Dis 2009;9:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corbett EL, Charalambous S, Moloi VM, Fielding K, Grant AD, Dye C, De Cock KM, Hayes RJ, Williams BG, Churchyard GJ. Human immunodeficiency virus and the prevalence of undiagnosed tuberculosis in African gold miners. Am J Respir Crit Care Med 2004;170:673–679 [DOI] [PubMed] [Google Scholar]
- 30.Yimer S, Holm-Hansen C, Yimaldu T, Bjune G. Evaluating an active case-finding strategy to identify smear-positive tuberculosis in rural Ethiopia. Int J Tuberc Lung Dis 2009;13:1399–1404 [PubMed] [Google Scholar]
- 31.Kendall MG. Partial rank correlation. Biometrika 1942;32:277–283 [Google Scholar]
- 32.Sanchez MA, Blower SM. Uncertainty and sensitivity analysis of the basic reproductive rate: tuberculosis as an example. Am J Epidemiol 1997;145:1127–1137 [DOI] [PubMed] [Google Scholar]
- 33.Uys PW, Warren R, van Helden PD, Murray M, Victor TC. Potential of rapid diagnosis for controlling drug-susceptible and drug-resistant tuberculosis in communities where Mycobacterium tuberculosis infections are highly prevalent. J Clin Microbiol 2009;47:1484–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dowdy DW, Chaisson RE, Moulton LH, Dorman SE. The potential impact of enhanced diagnostic techniques for tuberculosis driven by HIV: a mathematical model. AIDS 2006;20:751–762 [DOI] [PubMed] [Google Scholar]
- 35.Keeler E, Perkins MD, Small P, Hanson C, Reed S, Cunningham J, Aledort JE, Hillborne L, Rafael ME, Girosi F. Reducing the global burden of tuberculosis: the contribution of improved diagnostics. Nature 2006;444:49–57 [DOI] [PubMed] [Google Scholar]
- 36.Murray CJL, Salomon JA. Modeling the impact of global tuberculosis control strategies. Proc Natl Acad Sci USA 1998;95:13881–13886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abu-Raddad LJ, Sabatelli L, Achterberg JT, Sugimoto JD, Longini IM, Dye C, Halloran ME. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc Natl Acad Sci USA 2009;106:13980–13985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. World Health Organization. Roadmap for rolling out Xpert MTB/RIF for rapid diagnosis of TB and MDR-TB. Geneva: WHO; 2010.
- 39.Glaziou P, van der Werf MJ, Onozaki I, Dye C, Borgdorff MW, Chiang CY, Cobelens F, Enarson DA, Gopi PG, Holtz TH, et al. Tuberculosis prevalence surveys: rationale and cost. Int J Tuberc Lung Dis 2008;12:1003–1008 [PubMed] [Google Scholar]
- 40.Vassall A, van Kampen S, Sohn H, Michael JS, John KR, den Boon S, Davis JL, Whitelaw A, Nicol MP, Gler MT, et al. Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high burden countries: a cost-effectiveness analysis. PLoS Med 2011;8:e1001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dowdy DW, Golub JE, Chaisson RE, Saraceni V. Heterogeneity in tuberculosis transmission and the role of geographic hotspots in propagating epidemics. Proc Natl Acad Sci USA 2012;109:9557–9562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin HH, Dowdy DW, Dye C, Murray MB, Cohen T. The impact of new tuberculosis diagnostics on transmission: why context matters. Bull World Health Organ 2012;90:739–747A [DOI] [PMC free article] [PubMed] [Google Scholar]