Abstract
Rationale: Studies have demonstrated that angiotensin-converting enzyme 2 (ACE2) plays a protective role against lung diseases, including pulmonary hypertension (PH). Recently, an antitrypanosomal drug, diminazene aceturate (DIZE), was shown to exert an “off-target” effect of enhancing the enzymatic activity of ACE2 in vitro.
Objectives: To evaluate the pharmacological actions of DIZE in experimental models of PH.
Methods: PH was induced in male Sprague Dawley rats by monocrotaline, hypoxia, or bleomycin challenge. Subsets of animals were simultaneously treated with DIZE. In a separate set of experiments, DIZE was administered after 3 weeks of PH induction to determine whether the drug could reverse PH.
Measurements and Main Results: DIZE treatment significantly prevented the development of PH in all of the animal models studied. The protective effects were associated with an increase in the vasoprotective axis of the lung renin-angiotensin system, decreased inflammatory cytokines, improved pulmonary vasoreactivity, and enhanced cardiac function. These beneficial effects were abolished by C-16, an ACE2 inhibitor. Initiation of DIZE treatment after the induction of PH arrested disease progression. Endothelial dysfunction represents a hallmark of PH pathophysiology, and growing evidence suggests that bone marrow–derived angiogenic progenitor cells contribute to endothelial homeostasis. We observed that angiogenic progenitor cells derived from the bone marrow of monocrotaline-challenged rats were dysfunctional and were repaired by DIZE treatment. Likewise, angiogenic progenitor cells isolated from patients with PH exhibited diminished migratory capacity toward the key chemoattractant stromal-derived factor 1α, which was corrected by in vitro DIZE treatment.
Conclusions: Our results identify a therapeutic potential of DIZE in PH therapy.
Keywords: pulmonary hypertension, ACE2, angiogenic progenitor cells, diminazene
At a Glance Commentary
Scientific Knowledge on the Subject
The angiotensin-converting enzyme 2 (ACE2)/angiotensin-(1–7)/Mas axis has been shown to exert protection against pulmonary hypertension (PH). Recently, bone marrow (BM)-derived angiogenic progenitor cells (APCs) have been reported to be dysfunctional in PH, contributing to impaired vascular homeostasis. Strategies that can favorably modulate the activity of this axis and correct APC dysfunction could bear therapeutic potential for PH treatment.
What This Study Adds to the Field
We show that diminazene, an antitrypanosomal drug, attenuates hemodynamic changes, prevents maladaptive right ventricular remodeling, and enhances pulmonary vasorelaxation in experimental models of PH through activation of ACE2. Furthermore, diminazene improves the functions of APCs obtained from experimental animals and patients with PH. This study identifies a new application for an existing drug, which could be successfully developed for PH therapeutics.
Pulmonary hypertension (PH) is a life-threatening disease characterized by elevated pressure in the pulmonary arteries and abnormal remodeling of the lung vasculature, which leads to right-heart failure (1). PH can be of idiopathic origin or can arise in association with an underlying interstitial lung disease, such as pulmonary fibrosis (PF) (2). Currently available therapeutic strategies rely on calcium channel blockers, prostanoids, endothelin receptor antagonists, and phosphodiesterase-5 blockers, all of which induce pulmonary vasodilation (3). However, despite these therapeutic advances, the morbidity and mortality rates remain high in patients with PH. Hence, innovative approaches and novel drugs must be discovered in an attempt to improve PH treatment. It is in this regard that the recent discovery of angiotensin-converting enzyme 2 (ACE2) and its role in pulmonary diseases is of immense relevance. ACE2 is a mono-carboxypeptidase that metabolizes angiotensin-II (Ang-II) to angiotensin-(1–7) [Ang-(1–7)], thereby maintaining a balance between the deleterious axis (ACE–AngII-AT1R) and the vasoprotective axis [ACE2–Ang-(1–7)-Mas] of the renin-angiotensin system (RAS) (4). ACE2 is distributed throughout the lungs and offers a therapeutic potential in the management of PH. The rationale for this concept stems from the following evidence: (1) we have previously demonstrated that lung overexpression of ACE2 renders protection against PH (5, 6), (2) ACE2 has been shown to exert beneficial effects against acute lung injury (7), and (3) levels of ACE2 are altered in failing human heart ventricles from subjects with PH (8). Collectively, these findings have led several groups to propose that administration of ACE2 would be useful in treating lung diseases like PH and heart failure (9–11). Recently, an antitrypanosomal drug, diminazene aceturate (DIZE), was shown to exert “off-target” effects of activating ACE2 in vitro (12), which prompted us to evaluate the pharmacological actions of this drug against PH. DIZE is used in tropical countries for the treatment of early-stage human African trypanosomiasis, also called sleeping sickness (13). Our objectives in this study were to assess the pharmacological effects of DIZE in rat models of PH. The drug was injected subcutaneously because higher plasma concentrations were reported to be achieved with this route as compared with oral administration (14). Recent evidence suggests that the bone marrow (BM)-derived angiogenic progenitor cells (APCs) are dysfunctional in PH, contributing to impaired vascular homeostasis (15–18). Therefore, we also evaluated the outcome of DIZE treatment on the function of APCs obtained from experimental animals and patients with PH. Some of the results of these studies have been previously reported in the form of an abstract (19).
Methods
Reagents and Antibodies
Monocrotaline (MCT) and α-smooth muscle actin (clone 1A4) were purchased from Sigma Aldrich (St. Louis, MO). Bleomycin sulfate was purchased from Calbiocem Labs (San Diego, CA). Compound 16 (C-16), a selective ACE2 inhibitor, was obtained from Millennium Pharmaceuticals Inc. (Cambridge, MA). AT1-receptor rabbit polyclonal antibody and ACE rabbit polyclonal antibody were procured from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). DIZE was purchased from LKT Laboratories Inc. (St. Paul, MN). Terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) assay was performed using the TMR red in situ cell death detection kit from Roche Diagnostics (Indianapolis, IN). Cell culture media was purchased from STEMCELL Technologies Inc. (Vancouver, BC, Canada). Recombinant stromal-derived factor 1 α (SDF1-α) and vascular endothelial growth factor (VEGF) were procured from R&D Systems (Minneapolis, MN). Vialight Plus cell proliferation bioassay kit was procured from Lonza Inc. (Rockland, ME).
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Florida and complied with National Institutes of Health guidelines. PH was induced by administration of MCT or bleomycin or hypoxia exposure in 8-week-old male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) as detailed in the online supplement.
Hemodynamic Measurements and Histological Analysis
Right ventricular systolic pressure (RVSP) was measured in anesthetized, closed-chest rats using a fluid-filled silastic catheter as detailed in the online supplement. Right ventricular hypertrophy (RVH) was expressed as the ratio of right ventricle (RV) to left ventricle plus ventricular septum (LV+S) weights (RV/LV+S). Myocardial fibrosis was evaluated using picrosirius red staining as previously described (6). Formalin-fixed lung sections were stained for α-smooth muscle actin (1:600), and the medial wall thickness was measured as reported elsewhere (5).
Apoptosis Assay
Apoptosis was determined using the TUNEL assay according to the manufacturer’s protocol. Nuclei were labeled with 4′,6-diamidino-2-phenylindole. For each slide, five different fields were evaluated at high magnification (×400). The apoptotic index was calculated as the number of TUNEL-positive apoptotic nuclei divided by the total number of 4′,6-diamidino-2-phenylindole–stained nuclei multiplied by 100.
Isolation of Human CD34+ Cells
Peripheral blood was obtained from healthy subjects (n = 15) or individuals with PH (n = 31) visiting clinics at the Shands Teaching Hospital at the University of Florida in accordance with the approved protocol by the Institutional Review Board. The research followed the tenets of the Declaration of Helsinki, and informed consent was obtained from the subjects. Peripheral blood mononuclear cells were enriched for CD34+ cells by immunomagnetic selection.
Proliferation and Migration Assays
Freshly sorted human CD34+ cells or animal BM-derived CD90+ cells were used to carry out proliferation and migration assays in the presence of 100 nM SDF1-α or 50 ng/ml VEGF as detailed in the online supplement. Refer the online supplement for additional details on ACE2 activity assay, real-time RT-PCR analysis, Western blot analysis, pulmonary vasoreactivity, and systemic blood pressure measurement.
Statistical Analysis
All data are reported as means ± SEM. Statistical analysis was performed with the Prism software package (GraphPad v4). Data were analyzed using one-way ANOVA followed by the Newman-Keuls test for multiple comparisons. P values less than 0.05 were considered statistically significant.
Results
DIZE Treatment Prevents the Development of PH
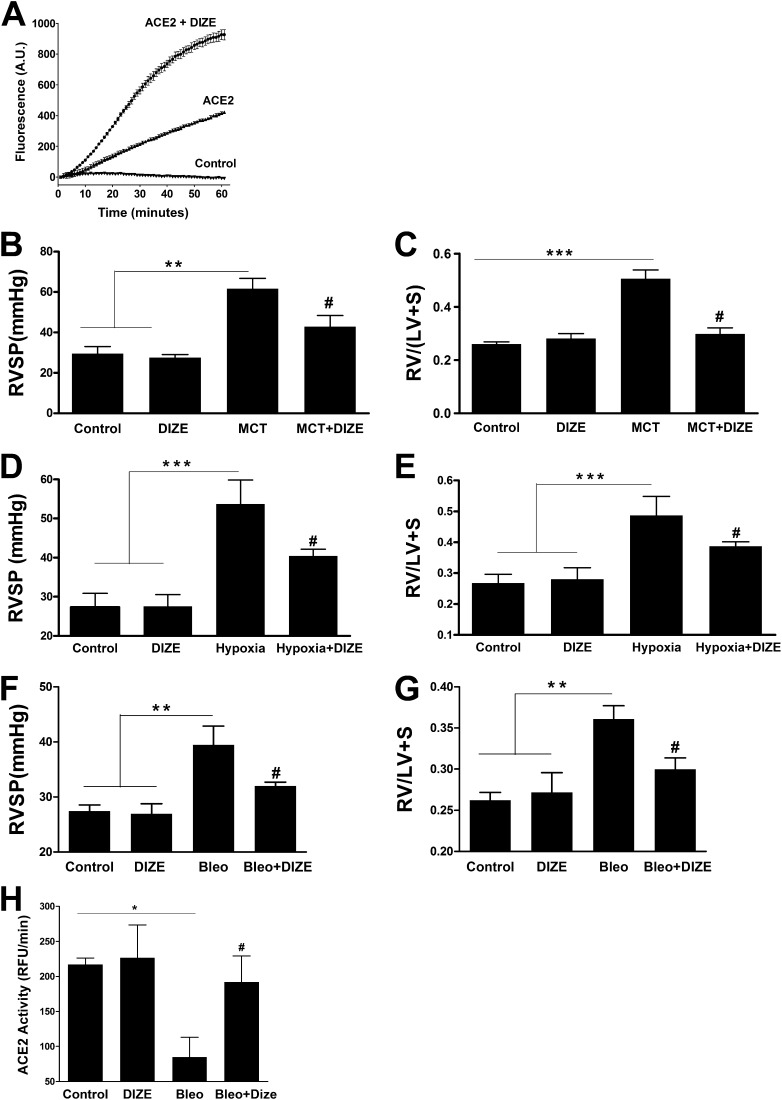
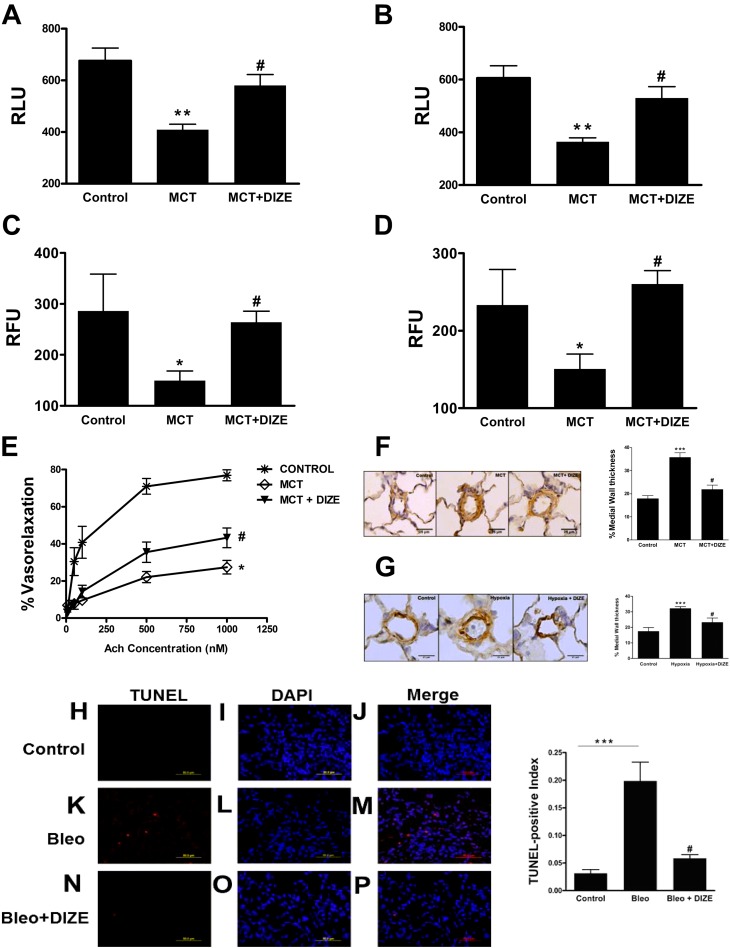
Incubation of recombinant human ACE2 with 100 μM DIZE resulted in a twofold increase in its enzymatic activity in vitro (Figure 1A), confirming previous reports (12). Subsequently, we evaluated the chronic effects of DIZE treatment in the MCT-induced PH model. Four weeks of MCT challenge induced marked increases in RVSP, signifying the development of PH (Figure 1B). In addition, these animals exhibited a 92% increase in RV to left ventricle plus septum (LV+S) weight ratio, an index of RVH (Figure 1C). However, DIZE-treated MCT animals displayed 31% and 40% lower RVSP and RVH, respectively (Figures 1B and 1C). Next, we validated the beneficial effects of DIZE in the hypoxia-induced PH model. Exposure of rats to hypoxia significantly elevated RVSP (Figure 1D), along with an 81% increase in RVH (Figure 1E). In contrast, DIZE treatment significantly reduced RVSP and RVH (Figures 1D and 1E). We further explored DIZE’s protective role on the development of secondary PH. Two weeks of bleomycin administration induced severe lung fibrosis that was associated with an 11% decrease in body weight (Figures E1A–E1C ) and a 44% increase in RVSP, followed by the development of RVH (Figures 1F and 1G). Coadministration of DIZE significantly attenuated all these parameters (Figures 1F and 1G). Furthermore, bleomycin-treated animals exhibited a 61% decrease in lung ACE2 activity, which was significantly prevented by DIZE treatment (Figure 1H).
Figure 1.
Diminazene aceturate (DIZE) treatment prevents pulmonary hypertension (PH) and associated cardiac hypertrophy. (A) Coincubation with DIZE (100 μM) increases the enzymatic activity of recombinant human angiotensin-converting enzyme 2 (ACE2) in vitro. (B) Measurement of right ventricular systolic pressure (RVSP) in monocrotaline (MCT)-challenged rats. (C) Right ventricle (RV) hypertrophy reflected by the ratio of RV to left ventricle (LV) plus interventricular septum (S) weight ratio [RV/(LV + S)] in the MCT-induced PH study. (D) RVSP measurement in the hypoxia model of PH. (E) RV/(LV + S) values in hypoxia-exposed rats. (F) Measurement of RVSP in the model of PH secondary to bleomycin-induced lung fibrosis. (G) RV hypertrophy in the bleomycin study. (H) Effect of chronic DIZE treatment on lung ACE2 activity in the bleomycin model of lung injury. Data represent mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control rats or rats treated with DIZE group. #P < 0.05 compared with MCT/hypoxia/bleomycin-challenged rats (n = 10 per group for MCT study; n = 6 per group for hypoxia experiments; n = 5 per group for bleomycin study). Bleo = bleomycin.
DIZE Inhibits PH-associated Right Ventricular Dysfunction and Fibrosis
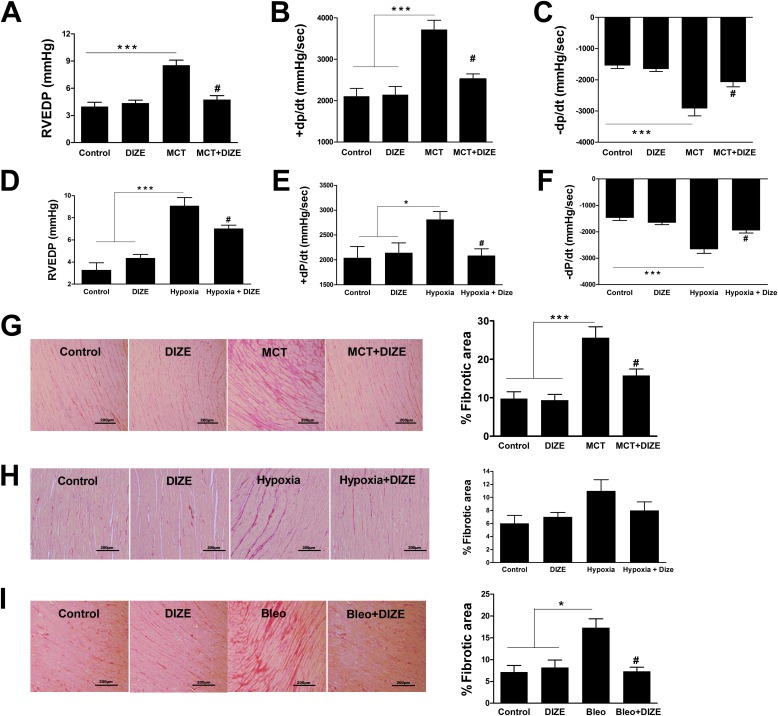
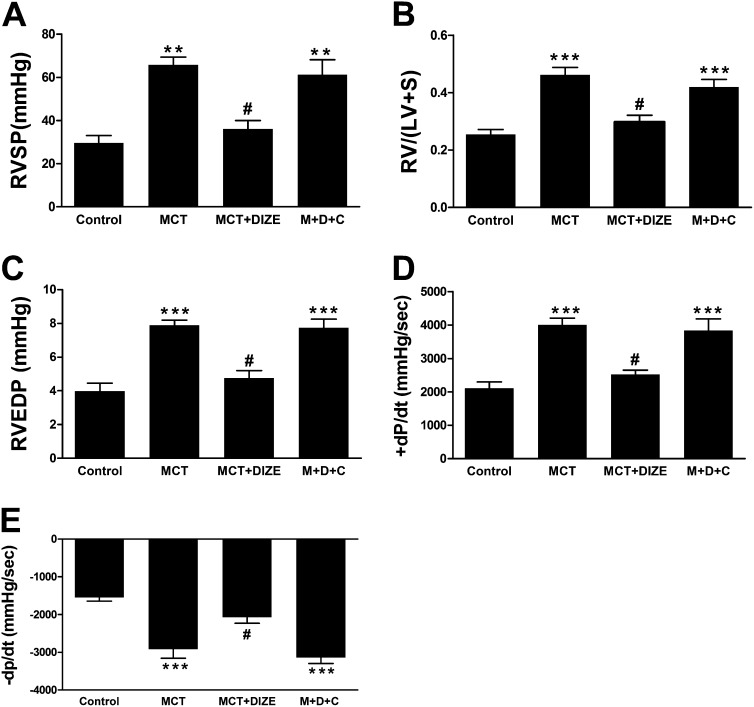
Measurement of cardiac functions in MCT animals revealed higher right ventricular end diastolic pressure (114%), elevated +dP/dt (76%), and –dP/dt (89%) as compared with normal control animals. However, these parameters were recovered to near control levels by DIZE treatment, suggesting normalization of ventricular performance (Figures 2A–2C). Furthermore, DIZE treatment resulted in similar beneficial effects on cardiac function in the hypoxia model of PH (Figures 2D–2F). In addition to improved cardiac function, DIZE treatment decreased myocardial collagen accumulation. MCT administration caused an approximately threefold increase in myocardial interstitial fibrosis as assessed by picrosirius red staining, and this increase was significantly attenuated by DIZE (Figure 2G). Similar results were obtained with DIZE treatment in the hypoxia and bleomycin models of PH (Figures 2H and 2I). However, development of ventricular fibrosis was not associated with compromised cardiac function in the bleomycin model (Figures E2A–E2C).
Figure 2.
Attenuation of pulmonary hypertension (PH)-induced right ventricular dysfunction and cardiac fibrosis. Measurement of (A) right ventricular end diastolic pressure (RVEDP), (B) +dP/dt, and (C) −dP/dt in the monocrotaline (MCT) model of PH. Similar measurements of (D) RVEDP, (E) +dP/dt, and (F) −dP/dt in the hypoxia model of PH. (G) Collagen staining and quantitative analysis of right ventricular fibrosis from the MCT study. (H) Quantification of right heart fibrosis from the hypoxia study. (I) Representative picrosirius red–stained sections and quantitative analysis of myocardial fibrosis from the bleomycin study. Data represent mean ± SEM. *P < 0.05 and ***P < 0.001 compared with control rats or the DIZE-alone group. #P < 0.05 compared with MCT/hypoxia/bleomycin-challenged rats. +dP/dt and −dP/dt refer to rate of change in ventricular pressure with respect to time and characterize the systolic and diastolic function of the right ventricle, respectively. Scale bars: 200 μm.
DIZE Favorably Modulates Pulmonary RAS and Proinflammatory Cytokines
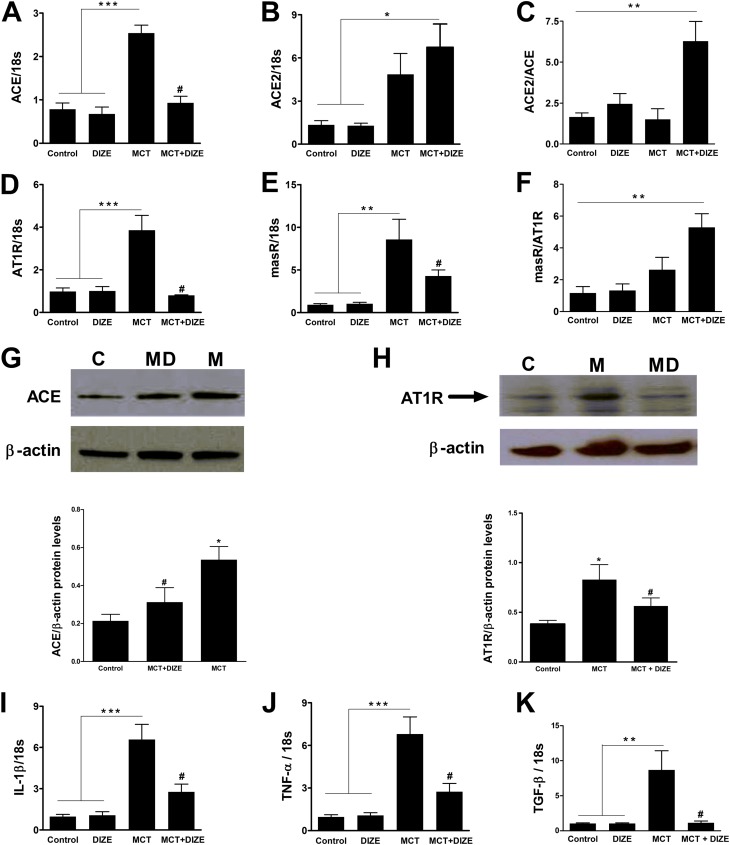
We have previously shown that the beneficial effects of ACE2 overexpression are associated with shifting the balance of the RAS from the proinflammatory, fibrotic, and hypertrophic axis toward the antiinflammatory, antifibrotic, and vasoprotective axis (5, 6). Thus, we sought to determine if DIZE treatment could cause a similar shift. Pulmonary ACE and AT1R mRNA levels were increased by 170% and 400%, respectively, by MCT administration (Figures 3A and 3D). Similarly, elevated levels of ACE2 mRNA and Mas receptor (masR) were observed in MCT-challenged animals (Figures 3B and 3E). DIZE treatment of MCT rats demonstrated a 60% decrease in ACE, a 75% decrease in AT1R, a 30% increase in ACE2, and a 38% decrease in masR mRNA levels as compared with MCT-challenged animals (Figures 3A, 3B, 3D, and 3E). As a result, DIZE-treated MCT rats displayed an approximately threefold increase in the ACE2/ACE ratio and a fivefold increase in the masR/AT1R ratio (Figures 3C and 3F). Furthermore, Western blot analysis confirmed the mRNA findings with a significant increase in ACE and AT1R protein expression in the lung lysates of MCT animals, which was prevented by DIZE (Figures 3G and 3H). Consistent with these changes were our observations for the key proinflammatory cytokines (IL-1β, TNF-α, and TGF-β) that are known to increase during PH (20, 21). The MCT-induced increases in mRNA levels of IL-1β (approximately sevenfold), TNF-α (approximately sevenfold), and TGF-β (approximately ninefold) were significantly attenuated in DIZE-treated MCT rats (Figures 3I–3K). Likewise, Western blot analysis confirmed these mRNA findings with significant increases in IL-1β and TNF-α protein expression in MCT animals, which was decreased by DIZE treatment (see Figure E3 in the online supplement). We observed similar effects of DIZE on pulmonary RAS and proinflammatory cytokines in the bleomycin model (Figure E4).
Figure 3.
Diminazene aceturate (DIZE) modulates pulmonary renin-angiotensin system (RAS) and proinflammatory cytokines. Relative change in lung mRNA levels of the RAS genes. (A) Angiotensin-converting enzyme (ACE), (B) angiotensin-converting enzyme 2 (ACE2), (C) ACE2/ACE ratio, (D) AT1R, (E) Mas receptor (masR), and (F) masR/AT1R ratio in the monocrotaline (MCT) model of pulmonary hypertension. Representative Western blot and quantification of protein expression for (G) ACE and (H) AT1R from total lung extracts of MCT-challenged rats. β-actin was used as a loading control. Immunoblots are representative of four individual lungs for each group. Arbitrary densitometric values are given as ratio of ACE or AT1R to β-actin. Relative mRNA levels of lung proinflammatory cytokines, (I) IL-1β, (J), tumor necrosis factor (TNF)-α, and (K) tumor growth factor (TGF)-β from the MCT study. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 versus control rats or the DIZE-alone group. #P < 0.05 versus MCT group. For Western blots, C = control, M = MCT, and MD = MCT + DIZE.
DIZE Arrests the Progression of Established PH and Ameliorates Cardiac Dysfunction
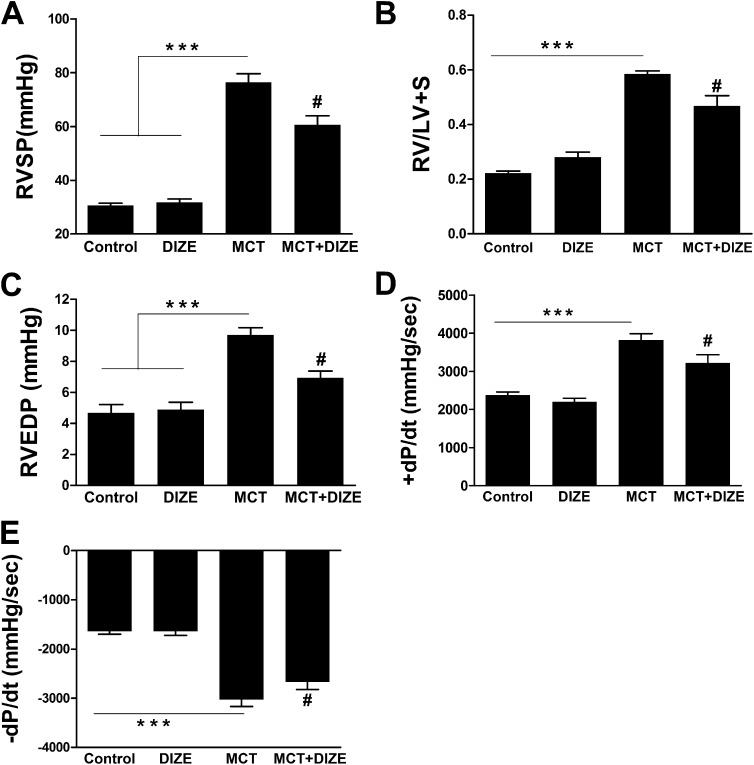
We next determined whether it is possible to reverse MCT-induced PH by DIZE treatment. We observed that 3 weeks of MCT challenge induced significant elevation in RVSP (Figure E5). Hence, in these experiments, DIZE was injected after 3 weeks of MCT challenge, and the drug treatment continued for an additional 2 weeks. DIZE showed a significant attenuation of RVSP (Figure 4A) and a 20% reduction in RVH (Figure 4B). Improved cardiac function was observed with DIZE administration (Figures 4C–4E).
Figure 4.
Diminazene aceturate (DIZE) arrests the progression of established pulmonary hypertension and improves cardiac function. Initiation of DIZE treatment after 3 weeks of monocrotaline (MCT) challenge arrests progression of (A) RVSP and attenuates worsening of (B) right ventricular hypertrophy. Measurement of (C) right ventricular end diastolic pressure (RVEDP), (D) +dP/dt, and (E) −dP/dt for the same study groups. Data represent mean ± SEM. ***P < 0.001 versus control subjects or the DIZE-alone group. #P < 0.05 compared with MCT-challenged rats. +dP/dt and −dP/dt refer to rate of change in ventricular pressure with respect to time and characterize the systolic and diastolic function of the right ventricle, respectively.
C-16, an ACE2 Inhibitor, Abolishes the Protective Effects of DIZE
We had hypothesized that the beneficial effects of DIZE are mediated via its actions on ACE2. Therefore, we coadministered C-16, a selective ACE2 inhibitor (22), along with DIZE in the MCT-induced PH model. Four weeks of C-16 injection abolished the improvements in RVSP, RV/(LV+S) ratio, and cardiac function produced by DIZE (Figures 5A–5E).
Figure 5.
Coadministration of C-16, an angiotensin-converting enzyme 2 (ACE2) inhibitor, abolishes the protective effects of diminazene aceturate (DIZE). C-16 reversed the beneficial effects of DIZE on (A) right ventricular systolic pressure (RVSP), (B) right ventricular hypertrophy, (C) right ventricular end diastolic pressure (RVEDP), (D) +dP/dt, and (E) −dP/dt in the monocrotaline (MCT)-induced pulmonary hypertension model. Data represent mean ± SEM. ***P < 0.001 versus control subjects. #P < 0.05 compared with MCT and MDC groups (MDC represents the MCT+DIZE+C-16 group) (n = 6–8 per experimental group). +dP/dt and −dP/dt refer to rate of change in ventricular pressure with respect to time and characterize the systolic and diastolic function of the right ventricle, respectively.
DIZE Rectifies Dysfunctional Angiogenic Progenitor Cells, Improves Pulmonary Endothelial Function, and Inhibits Apoptosis
Previous studies have established that rat BM-derived cells positive for the CD90 marker exhibit angiogenic properties and participate in tube formation (23, 24). Hence, we decided to isolate these cells and study their functional features. CD90+ cells obtained from the BM of MCT rats exhibited approximately twofold decreases in proliferation and migration in response to the key chemoattractants, SDF-1α and VEGF, reflecting significant dysfunction of these progenitors (Figures 6A–6D). However, the reduced proliferation and migration was normalized in CD90+ cells isolated from DIZE-treated MCT rats. No significant difference in the number of BM-derived CD90+ cells was observed between the experimental groups (control, 2.7%; MCT, 2.8%; and MCT+DIZE, 2.8% of mononuclear cells) as analyzed by flow cytometry. We then examined the role of DIZE on pulmonary vasorelaxation. Acetylcholine-induced vasorelaxation was decreased by 64% in the pulmonary arteries of MCT-challenged rats. Conversely, DIZE-treated animals showed 32% improvement in vasoreactivity (Figure 6E). DIZE therapy was associated with considerable reduction in pulmonary vessel wall thickening in MCT and hypoxia models of PH (Figures 6F and 6G). Next, we examined whether DIZE could exert antiapoptotic effects in response to tissue damage. Lung sections of bleomycin-treated animals showed increased TUNEL staining, which was prevented by DIZE treatment (Figures 6H–6P).
Figure 6.
Improvement in angiogenic progenitor cell (APC) function, better pulmonary vasoreactivity, and decreased apoptosis by diminazene aceturate (DIZE) treatment. Proliferative capacity of bone marrow–derived CD90+ cells from different experimental groups in response to (A) 100 nM stromal-derived factor 1α (SDF-1α) treatment and (B) 50 ng/ml vascular endothelial growth factor (VEGF) treatment. (C) Migratory capacity of CD90+ cells in response to SDF-1α and (D) VEGF treatment. (E) Percentage vasorelaxation of preconstricted pulmonary vessels from control, monocrotaline (MCT), and MCT + diminazene aceturate (DIZE) rats in response to acetyl-choline (1–1,000 nM). The vessels were preconstricted with phenylephrine (10 μM). Representative microphotographs of pulmonary vessels stained for α-smooth muscle actin and quantification of medial wall thickness with an external diameter of 25 to 50 μm from the MCT study (F) and the hypoxia study (G). (H–P) Representative lung sections and quantification of apoptosis from the bleomycin study. Apoptotic cell death was expressed as terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling (TUNEL)-positive index, calculated by dividing the TUNEL-positive cells (red) by nuclear-stained 4′,6-diamidino-2-phenylindole–positive cells (blue). Data represent mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control subjects. #P < 0.05, compared with MCT/bleomycin-challenged rats. Scale bars: 25 μm.
CD34+ Cells from Patients with PH Are Dysfunctional, and In Vitro Treatment with DIZE Improves Their Migratory Capacity
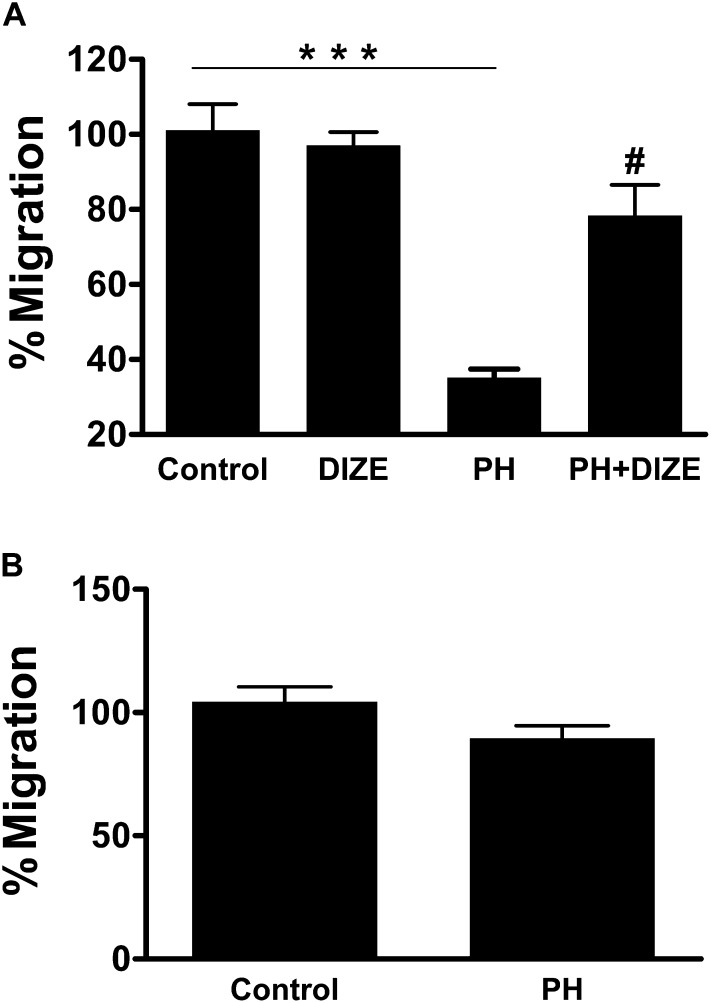
Our objective in this study was to determine whether hematopoietic progenitor cells with the CD34+ marker are dysfunctional in patients with PH and, if so, whether in vitro treatment with DIZE would improve their function. Numerous studies have shown that circulating human CD34+ cells hold great promise for tissue repair and regeneration (25–28). In fact, a recent clinical trial by Losordo and colleagues demonstrated that administration of autologous CD34+ cells rendered protection against refractory angina (29). Hence, for our experiments we used this cell type. Table E2 provides characteristics of patients with PH from whom the cells were obtained. CD34+ cells collected from these patients showed a 63% decrease in the migratory capacity toward SDF-1α (Figure 7A). On the other hand, in vitro treatment of these cells with 100 nM DIZE resulted in a significant increase in SDF-1α–induced migration that was comparable to control subjects. Next, we examined the migratory potential of these cells toward Ang-(1–7), a known stimulator of progenitor cell function (30, 31). Ang-(1–7) induced a similar migratory response in cells harvested from patients with PH and normal control subjects (Figure 7B). These observations support our data from animal studies and suggest that modification of the vasoprotective axis of the RAS may be important in PH therapy.
Figure 7.
Functional impairment of CD34+ cells isolated from patients with pulmonary hypertension (PH) is corrected by diminazene aceturate (DIZE) treatment in vitro. (A) Migratory capacity of CD34+ cells isolated from patients with PH (n = 14) and age- and sex-matched control subjects (n = 6) in response to stromal-derived factor 1α (SDF-1α). Coincubation of CD34+ cells from patients with PH with 100 nM DIZE for up to 10 hours improved migration toward SDF-1α (n = 5) that was similar to the response observed with control cells. (B) Migration toward 100 nM angiotensin-(1–7) [Ang-(1–7)] was similar in cells from control subjects (n = 6) and patients with PH (n = 5). Data represent mean ± SEM. ***P < 0.001 compared with control cells or DIZE-treated normal control cells. #P < 0.05 compared with patients with PH.
Discussion
The most significant finding of our study is that chronic administration of DIZE prevents and arrests the progression of PH in established experimental models of lung injury. DIZE targets the cardiopulmonary system to cause a decrease in RVSP, reduction in right ventricular remodeling accompanied by improved cardiac functions, a decrease in medial wall thickness along with enhanced pulmonary vasodilation, and restoration of normal APC function in experimental animals and in patients with PH in vitro.
DIZE is an antitrypanosomal drug that was recently reported to increase the enzymatic activity of ACE2 (12). Here, we confirm these in vitro findings and further demonstrate the in vivo protective effects of this drug in MCT-induced, hypoxic-mediated, and bleomycin models of PH. We found that DIZE treatment reduces elevated RVSP in all the three experimental models studied. More importantly, DIZE attenuates established PH in the MCT model. It is well known that sustained PH exerts pressure overload on the right heart to cause ventricular dysfunction, which culminates in cardiac failure and death (32, 33). In this study, we show an improvement in ventricular function along with attenuation of maladaptive cardiac remodeling (hypertrophy and fibrosis) in response to DIZE treatment. Conversely, inhibition of ACE2 abolished the cardiopulmonary protective effects of DIZE in the MCT-induced PH model, suggesting that this drug mediates its effects via ACE2 activation. Another prominent effect of DIZE treatment was against lung fibrogenesis. Earlier reports have shown that genetic ablation or down-regulation of ACE2 is associated with the development of PF (34, 35). However, in the bleomycin model of PF, DIZE treatment preserved lung ACE2 levels and its enzymatic activity. More importantly, DIZE prevented the development of secondary PH. This has clinical significance because secondary PH correlates with poor prognosis and reduced survival in patients with PF (36, 37). An important aspect of DIZE therapy is that the drug treatment did not adversely affect basal systemic blood pressure (Figure E5). This is important because drug-induced systemic hypotension may prove detrimental for patients with PH who already suffer from compromised cardiopulmonary functions (38). Collectively, our results suggest that DIZE treatment may represent a promising therapeutic strategy for treating PH.
A prominent feature of PH pathology is inflammation followed by abnormal changes within the lung vasculature that together cause endothelial dysfunction (39). Elevated levels of proinflammatory cytokines and growth factors such as IL-1β, TNF-α, and TGF-β1 contribute significantly to the development and progression of PH (40–42). These cytokines are involved in pulmonary vascular remodeling, which leads to narrowing of the lumen area with a subsequent decrease in vasodilator responsiveness. We found that DIZE treatment causes notable reduction in inflammatory response accompanied by decreased medial wall thickness and improved pulmonary vasorelaxation. A key event involved in the pathogenesis of PF is apoptotic cell death of the lung parenchyma (43). It is believed that an altered RAS is a potent inducer of pulmonary apoptosis (44, 45). Recent data suggest that ACE2 inhibits apoptosis of alveolar epithelial cells to exert antifibrotic effects (46). In our study, bleomycin-challenged animals that were treated with DIZE exhibited decreased cell apoptosis of the lungs. Also, DIZE favorably modulated the pulmonary RAS to shift the balance from the deleterious, ACE–AngII-AT1R axis toward the protective, ACE2–Ang-(1–7)-Mas axis, which may in part be responsible for its beneficial actions. An increased expression of ACE2 mRNA levels was observed in DIZE-treated bleomycin and MCT rats. Although the precise mechanism behind this increase was not investigated, we speculate that a positive feed-forward mechanism initiated by ACE2 activation may be involved. Similar increases in ACE2 levels were noted with XNT treatment, another ACE2 activator (47), and by the overexpression of Ang-(1–7) (6) in the MCT model. However, a direct effect of DIZE on ACE2 gene expression cannot be ruled out. Surprisingly, the masR was significantly increased in the lungs of MCT-challenged animals. masR is known to exert vasoprotective actions against cardiovascular diseases (48). Thus, it is likely that this increase in the MCT group could be a compensatory response to protect the lungs against elevated pulmonary pressure. On the contrary, masR levels were lowered in DIZE-treated MCT rats, possibly due to decreased lung injury. Together, a combination of decreases in lung proinflammatory cytokines and a shift of the RAS toward the vasoprotective axis may account for the beneficial effects of DIZE.
There is mounting interest in testing the concept that APC therapy could be important in the treatment of PH (49–51). This view is supported by observations that circulating progenitors are dysfunctional in patients with PH (15, 16). Thus, correcting this dysfunction may hold promise in restoring endothelial integrity and establishing pulmonary vascular homeostasis. We observed that APCs (CD90+) from the BM of MCT-challenged rats are dysfunctional and that in vivo DIZE treatment corrects this dysfunction. Our data on patients with PH also support this contention. Circulating CD34+ cells isolated from patients with PH show reduced migratory capacity toward SDF-1α. However, coincubation of these cells with DIZE in vitro restores their migratory capacity, similar to that observed in normal control subjects. Likewise, Ang-(1–7) induces a similar migratory response in CD34+ cells harvested from patients with PH and normal control subjects, suggesting that the masR signaling may be intact in these cells. Overall, DIZE might have a direct effect on the mobilization of APCs, apart from its actions on the peripheral tissues.
Apart from its actions on ACE2, DIZE also exerts other pharmacological effects, such as DNA binding and inhibition of topoisomerase enzyme (mechanisms that are related to its trypanocidal activity) (52, 53). We do not know whether these effects of DIZE play a role in attenuating PH. Our current data support the view that DIZE renders protection against PH through its actions on ACE2. Further investigation is warranted to better understand the modes of action of this drug for PH therapy.
In summary, our study illustrates that DIZE exerts cardiopulmonary protective effects and corrects APC dysfunction in PH. This has several therapeutic implications: (1) Clinically, the use of recombinant ACE2 protein for pulmonary disorders may be limited due to its peptide nature and possible metabolic degradation, if administered orally. However, these limitations could be overcome by the use of synthetic small molecules like DIZE. (2) Autologous cell therapy for PH has met with limited success since clinical studies have shown that APCs obtained from these patients are already dysfunctional. Thus, our strategy to correct APC dysfunction by coincubation with DIZE in vitro could be an important advancement in cell therapy for PH treatment. (3) DIZE therapy offers the advantage of being cost effective because it is inexpensive and easily obtainable. Moreover, it has been successfully used to treat patients with trypanosomiasis infections in tropical and subtropical countries. However, in the United States, this drug is approved only for animal use because the parent manufacturer (Hoechst Pharmaceuticals) did not pursue the registration procedure due to poor profit prospects. Nonetheless, given that DIZE has been used elsewhere on human subjects, our findings provide an opportunity to develop this drug for PH therapeutics in the near future.
Acknowledgments
The authors thank Ms. Marda Jorgensen for technical assistance and help with immunohistochemistry, Ms. Robin Carrie for collection of blood samples from human subjects, and Ms. Lin Ai for helping with the hypoxia studies.
Footnotes
This work was supported by National Institutes of Health grants HL99980 and HL102033 (M.K.R.) and by AHA grant SDG12080302 (V.S.).
Author Contributions: V.S. and A.G. designed animal experiments, performed hemodynamic measurements, analyzed data, and wrote the manuscript. Y.P.J. performed experiments related to human CD34+ cells. A.A., Y.Q., K.R., and J.Y.J. isolated and performed functional assays with rat CD90 cells. C.B. and W.R.K. performed and analyzed Western blot data. D.A. and J.F. were responsible for the gene expression data (quantitative real-time PCR) and Western blot data. D.G., A.R., and E.B. performed animal experiments and analyzed data involving the bleomycin study. A.J.F. and R.A.F.-S. performed enzymatic activity assays and provided intellectual input. P.K. and A.J.C. were responsible for the pulmonary vasoreactivity experiments. J.M.P. supervised the hypoxia experiments. K.K.M. is a pulmonary physician responsible for patient selection and collection of blood samples from human subjects. J.M., M.B.G., M.J.K., and M.K.R. supervised experiments, analyzed data, and helped with preparation of the manuscript. All coauthors read and edited the manuscript; J.Y.D. revised and rewrote the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201205-0880OC on January 31, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol 2011;8:443–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathan SD, Noble PW, Tuder RM. Idiopathic pulmonary fibrosis and pulmonary hypertension: connecting the dots. Am J Respir Crit Care Med 2007;175:875–880 [DOI] [PubMed] [Google Scholar]
- 3.O'Callaghan DS, Savale L, Montani D, Jaïs X, Sitbon O, Simonneau G, Humbert M. Treatment of pulmonary arterial hypertension with targeted therapies. Nat Rev Cardiol 2011;8:526–538 [DOI] [PubMed] [Google Scholar]
- 4.Raizada MK, Ferreira AJ. ACE2: a new target for cardiovascular disease therapeutics. J Cardiovasc Pharmacol 2007;50:112–119 [DOI] [PubMed] [Google Scholar]
- 5.Yamazato Y, Ferreira AJ, Hong KH, Sriramula S, Francis J, Yamazato M, Yuan L, Bradford CN, Shenoy V, Oh SP, et al. Prevention of pulmonary hypertension by angiotensin-converting enzyme-2 gene transfer. Hypertension 2009;54:365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shenoy V, Ferreira AJ, Qi Y, Fraga-Silva RA, Díez-Freire C, Dooies A, Jun JY, Sriramula S, Mariappan N, Pourang D, et al. The angiotensin-converting enzyme 2/angiotensin-(1–7)/Mas axis confers cardiopulmonary protection against lung fibrosis and pulmonary hypertension. Am J Respir Crit Care Med 2010;182:1065–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005;436:112–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zisman LS, Keller RS, Weaver B, Lin Q, Speth R, Bristow MR, Canver CC. Increased angiotensin-(1–7)-forming activity in failing human heart ventricles: evidence for upregulation of the angiotensin-converting enzyme Homologue ACE2. Circulation 2003;108:1707–1712 [DOI] [PubMed] [Google Scholar]
- 9.Shenoy V, Qi Y, Katovich MJ, Raizada MK. ACE2, a promising therapeutic target for pulmonary hypertension. Curr Opin Pharmacol 2011;11:150–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oudit GY, Penninger JM. Recombinant human angiotensin-converting enzyme 2 as a new renin-angiotensin system peptidase for heart failure therapy. Curr Heart Fail Rep 2011;8:176–183 [DOI] [PubMed] [Google Scholar]
- 11.Iwata M, Cowling RT, Yeo SJ, Greenberg B. Targeting the ACE2-Ang-(1–7) pathway in cardiac fibroblasts to treat cardiac remodeling and heart failure. J Mol Cell Cardiol 2011;51:542–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulemina LV, Ostrov DA. Prediction of off-target effects on angiotensin-converting enzyme 2. J Biomol Screen 2011;16:878–885 [DOI] [PubMed] [Google Scholar]
- 13.Abaru DE, Liwo DA, Isakina D, Okori EE. Retrospective long-term study of effects of Berenil by follow-up of patients treated since 1965. Tropenmed Parasitol 1984;35:148–150 [PubMed] [Google Scholar]
- 14.Raether W, Hajdu P, Seidenath H, Damm D. Pharmacokinetic and chemoprophylactic studies on Berenil in Wistar rats. Z Tropenmed Parasitol 1974;23:418–427 [PubMed] [Google Scholar]
- 15.Toshner M, Voswinckel R, Southwood M, Al-Lamki R, Howard LS, Marchesan D, Yang J, Suntharalingam J, Soon E, Exley A, et al. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am J Respir Crit Care Med 2009;180:780–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Junhui Z, Xingxiang W, Guosheng F, Yunpeng S, Furong Z, Junzhu C. Reduced number and activity of circulating endothelial progenitor cells in patients with idiopathic pulmonary arterial hypertension. Respir Med 2008;102:1073–1079 [DOI] [PubMed] [Google Scholar]
- 17.Marsboom G, Pokreisz P, Gheysens O, Vermeersch P, Gillijns H, Pellens M, Liu X, Collen D, Janssens S. Sustained endothelial progenitor cell dysfunction after chronic hypoxia-induced pulmonary hypertension. Stem Cells 2008;26:1017–1026 [DOI] [PubMed] [Google Scholar]
- 18.Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res 2005;96:442–450 [DOI] [PubMed] [Google Scholar]
- 19.Shenoy V, Yagna J, Gjymishka A, Afzal A, Rigatto K, Qi Y, Bradford C, Ely D, Kearns P, Carrie R, et al. An ACE2 activator (DIZE) improves endothelial progenitor cell function in patients with pulmonary hypertension. Circulation 2011;124:A13757 [Google Scholar]
- 20.Dorfmüller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J 2003;22:358–363 [DOI] [PubMed] [Google Scholar]
- 21.Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, Trembath RC, Jennings S, Barker L, Nicklin P, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 2010;122:920–927 [DOI] [PubMed] [Google Scholar]
- 22.Dales NA, Gould AE, Brown JA, Calderwood EF, Guan B, Minor CA, Gavin JM, Hales P, Kaushik VK, Stewart M, et al. Substrate-based design of the first class of angiotensin-converting enzyme-related carboxypeptidase (ACE2) inhibitors. J Am Chem Soc 2002;124:11852–11853 [DOI] [PubMed] [Google Scholar]
- 23.Valarmathi MT, Davis JM, Yost MJ, Goodwin RL, Potts JD. A three-dimensional model of vasculogenesis. Biomaterials 2009;30:1098–1112 [DOI] [PubMed] [Google Scholar]
- 24.Busik JV, Tikhonenko M, Bhatwadekar A, Opreanu M, Yakubova N, Caballero S, Player D, Nakagawa T, Afzal A, Kielczewski J, et al. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J Exp Med 2009;206:2897–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–967 [DOI] [PubMed] [Google Scholar]
- 26.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, et al. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation 2006;114:2163–2169 [DOI] [PubMed] [Google Scholar]
- 27.Tei K, Matsumoto T, Mifune Y, Ishida K, Sasaki K, Shoji T, Kubo S, Kawamoto A, Asahara T, Kurosaka M, et al. Administrations of peripheral blood CD34-positive cells contribute to medial collateral ligament healing via vasculogenesis. Stem Cells 2008;26:819–830 [DOI] [PubMed] [Google Scholar]
- 28.Blocklet D, Toungouz M, Berkenboom G, Lambermont M, Unger P, Preumont N, Stoupel E, Egrise D, Degaute JP, Goldman M, et al. Myocardial homing of nonmobilized peripheral-blood CD34+ cells after intracoronary injection. Stem Cells 2006;24:333–336 [DOI] [PubMed] [Google Scholar]
- 29.Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Traverse JH, et al. ACT34-CMI Investigators. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res 2011;109:428–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heringer-Walther S, Eckert K, Schumacher SM, Uharek L, Wulf-Goldenberg A, Gembardt F, Fichtner I, Schultheiss HP, Rodgers K, Walther T. Angiotensin-(1–7) stimulates hematopoietic progenitor cells in vitro and in vivo. Haematologica 2009;94:857–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellefson DD, diZerega GS, Espinoza T, Roda N, Maldonado S, Rodgers KE. Synergistic effects of co-administration of angiotensin-(1–7) and Neupogen on hematopoietic recovery in mice. Cancer Chemother Pharmacol 2004;53:15–24 [DOI] [PubMed] [Google Scholar]
- 32.Bradley SP, Auger WR, Moser KM, Fedullo PF, Channick RN, Bloor CM. Right ventricular pathology in chronic pulmonary hypertension. Am J Cardiol 1996;78:584–587 [DOI] [PubMed] [Google Scholar]
- 33.Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest 2009;135:794–804 [DOI] [PubMed] [Google Scholar]
- 34.Rey-Parra GJ, Vadivel A, Coltan L, Hall A, Eaton F, Schuster M, Loibner H, Penninger JM, Kassiri Z, Oudit GY, et al. Angiotensin converting enzyme 2 abrogates bleomycin-induced lung injury. J Mol Med 2012;90:637–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Molina-Molina M, Abdul-Hafez A, Uhal V, Xaubet A, Uhal BD. Angiotensin converting enzyme-2 is protective but downregulated in human and experimental lung fibrosis. Am J Physiol Lung Cell Mol Physiol 2008;295:L178–L185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel NM, Lederer DJ, Borczuk AC, Kawut SM. Pulmonary hypertension in idiopathic pulmonary fibrosis. Chest 2007;132:998–1006 [DOI] [PubMed] [Google Scholar]
- 37.Nadrous HF, Pellikka PA, Krowka MJ, Swanson KL, Chaowalit N, Decker PA, Ryu JH. The impact of pulmonary hypertension on survival in patients with idiopathic pulmonary fibrosis. Chest 2005;128:616S–617S [DOI] [PubMed] [Google Scholar]
- 38.Rubin LJ, Badesch DB. Evaluation and management of the patient with pulmonary arterial hypertension. Ann Intern Med 2005;143:282–292 [DOI] [PubMed] [Google Scholar]
- 39.Hassoun PM, Mouthon L, Barberà JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 2009;54(Suppl)S10–S19 [DOI] [PubMed] [Google Scholar]
- 40.Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, Duroux P, Galanaud P, Simonneau G, Emilie D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 1995;151:1628–1631 [DOI] [PubMed] [Google Scholar]
- 41.Fujita M, Mason RJ, Cool C, Shannon JM, Hara N, Fagan KA. Pulmonary hypertension in TNF-alpha-overexpressing mice is associated with decreased VEGF gene expression. J Appl Physiol 2002;93:2162–2170 [DOI] [PubMed] [Google Scholar]
- 42.Zaiman AL, Podowski M, Medicherla S, Gordy K, Xu F, Zhen L, Shimoda LA, Neptune E, Higgins L, Murphy A, et al. Role of TGF-β/ALK5 kinase in monocrotaline-induced pulmonary hypertension. Am J Respir Crit Care Med 2008;177:896–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drakopanagiotakis F, Xifteri A, Polychronopoulos V, Bouros D. Apoptosis in lung injury and fibrosis. Eur Respir J 2008;32:1631–1638 [DOI] [PubMed] [Google Scholar]
- 44.Uhal BD, Abdul-Hafez A. Angiotensin II in apoptotic lung injury: potential role in meconium aspiration syndrome. J Perinatol 2008;S108–S112 [DOI] [PubMed] [Google Scholar]
- 45.Uhal BD, Li X, Piasecki CC, Molina-Molina M. Angiotensin signaling in pulmonary fibrosis. Int J Biochem Cell Biol 2012;44:465–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uhal BD, Li X, Xue A, Gao X, Abdul-Hafez A. Regulation of alveolar epithelial cell survival by the ACE-2/angiotensin 1–7/Mas axis. Am J Physiol Lung Cell Mol Physiol 2011;301:L269–L274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferreira AJ, Shenoy V, Yamazato Y, Sriramula S, Francis J, Yuan L, Castellano RK, Ostrov DA, Oh SP, Katovich MJ, et al. Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am J Respir Crit Care Med 2009;179:1048–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santos RA, Ferreira AJ, Pinheiro SV, Sampaio WO, Touyz R, Campagnole-Santos MJ. Angiotensin-(1–7) and its receptor as a potential targets for new cardiovascular drugs. Expert Opin Investig Drugs 2005;14:1019–1031 [DOI] [PubMed] [Google Scholar]
- 49.Mirsky R, Jahn S, Koskenvuo JW, Sievers RE, Yim SM, Ritner C, Bernstein HS, Angeli FS, Boyle AJ, De Marco T, et al. Treatment of pulmonary arterial hypertension with circulating angiogenic cells. Am J Physiol Lung Cell Mol Physiol 2011;301:L12–L19 [DOI] [PubMed] [Google Scholar]
- 50.Toshner M, Morrell NW. Endothelial progenitor cells in pulmonary hypertension: dawn of cell-based therapy? Int J Clin Pract Suppl 2010;165:7–12 [DOI] [PubMed] [Google Scholar]
- 51.Fadini GP, Avogaro A, Ferraccioli G, Agostini C. Endothelial progenitors in pulmonary hypertension: new pathophysiology and therapeutic implications. Eur Respir J 2010;35:418–425 [DOI] [PubMed] [Google Scholar]
- 52.Pilch DS, Kirolos MA, Liu X, Plum GE, Breslauer KJ. Berenil [1,3-bis(4'-amidinophenyl)triazene] binding to DNA duplexes and to a RNA duplex: evidence for both intercalative and minor groove binding properties. Biochemistry 1995;34:9962–9976 [DOI] [PubMed] [Google Scholar]
- 53.Portugal J. Berenil acts as a poison of eukaryotic topoisomerase II. FEBS Lett 1994;344:136–138 [DOI] [PubMed] [Google Scholar]