Abstract
Rationale: Alveolar epithelial cells (AECs) play central roles in the response to lung injury and the pathogenesis of pulmonary fibrosis.
Objectives: We aimed to determine the role of β-catenin in alveolar epithelium during bleomycin-induced lung fibrosis.
Methods: Genetically modified mice were developed to selectively delete β-catenin in AECs and were crossed to cell fate reporter mice that express β-galactosidase (βgal) in cells of AEC lineage. Mice were given intratracheal bleomycin (0.04 units) and assessed for AEC death, inflammation, lung injury, and fibrotic remodeling. Mouse lung epithelial cells (MLE12) with small interfering RNA knockdown of β-catenin underwent evaluation for wound closure, proliferation, and bleomycin-induced cytotoxicity.
Measurements and Main Results: Increased β-catenin expression was noted in lung parenchyma after bleomycin. Mice with selective deletion of β-catenin in AECs had greater AEC death at 1 week after bleomycin, followed by increased numbers of fibroblasts and enhanced lung fibrosis as determined by semiquantitative histological scoring and total collagen content. However, no differences in lung inflammation or protein levels in bronchoalveolar lavage were noted. In vitro, β-catenin–deficient AECs showed increased bleomycin-induced cytotoxicity as well as reduced proliferation and impaired wound closure. Consistent with these findings, mice with AEC β-catenin deficiency showed delayed recovery after bleomycin.
Conclusions: β-Catenin in the alveolar epithelium protects against bleomycin-induced fibrosis. Our studies suggest that AEC survival and wound healing are enhanced through β-catenin–dependent mechanisms. Activation of the developmentally important β-catenin pathway in AECs appears to contribute to epithelial repair after epithelial injury.
Keywords: apoptosis, idiopathic pulmonary fibrosis, wound healing
At a Glance Commentary
Scientific Knowledge on the Subject
The alveolar epithelial cell population plays critical roles in the pathogenesis of pulmonary fibrosis. Lung developmental pathways are reactivated after lung injury and fibrosis.
What This Study Adds to the Field
This study provides evidence on the role of β-catenin in the alveolar epithelium in the response to lung injury and fibrosis.
Alveolar epithelial cell (AEC) dysfunction is believed to contribute to idiopathic pulmonary fibrosis (IPF) pathogenesis by enhancing susceptibility to injurious stimuli and impairing reepithelialization (1). It has long been known that epithelial abnormalities are common in IPF lung biopsy specimens, including hyperplastic AECs lining areas of fibrosis, but observations that mutations in genes encoding surfactant protein C and A2 are associated with familial IPF provide strong evidence that AECs are critical to disease pathogenesis (2). AECs likely contribute to lung fibrosis in multiple ways. Numerous studies have shown that AEC apoptosis is common in humans with IPF and can initiate fibrotic remodeling in animal models (3, 4). In addition, AECs are known to produce key profibrotic cytokines (5). However, the mechanisms by which AECs guide successful or aberrant alveolar repair after injury remains largely undefined.
In evaluating potential mechanisms important for responding to AEC injury, it is attractive to consider that pathways involved in lung development could be reactivated to mediate repair. β-Catenin is a key component to patterning of the alveolar epithelium in development with mouse models revealing prominent roles for β-catenin in type II AEC differentiation (6), branching morphogenesis, and alveolarization (7). The role for β-catenin in the alveolar epithelium in the adult lung is less clear, but recent evaluations demonstrated that β-catenin and other pathway components are found in hyperplastic alveolar epithelium lining areas of fibrosis in IPF (8, 9), raising the question of whether β-catenin may also be important in the adult lung for recovery after injury (10). To address this issue, we developed a transgenic mouse model in which β-catenin can be selectively deleted in the alveolar epithelium in the adult mouse. Our studies indicate an important role for β-catenin in AECs in lung repair and protection from fibrosis. Some of these results have been presented previously in abstract form (11, 12).
Methods
Detailed methods are in the online supplement.
Models
Transgenic mice (C57BL/6J background) used include (1) mice expressing reverse tetracycline transactivator (rtTA) under human surfactant protein C promoter (SFTPC.rtTA) (13), (2) mice expressing Cre recombinase under tetracycline operator (tet-O)7 and minimal CMV promoter (tetO.Cre) (13), (3) mice with β-catenin exons 2 to 6 flanked by loxP sites (βcatfl/fl) (14), (4) R26Rosa.Stop.LacZ mice in which R26Rosa promoter drives a construct with a loxP flanked STOP cassette upstream of lacZ (gene product βgal) (15), (5) R26Rosa.Tomato.GFP mice in which R26Rosa promoter drives a loxP flanked construct expressing fluorescent Tomato protein upstream of green fluorescent protein (GFP) (16), and (6) TOPGAL mice expressing lacZ under a LEF-1/TCF (lymphoid enhancer factor-1/T cell factor) regulatory binding sequence (17).
Combining SFTPC.rtTA, tetO.Cre, and R26Rosa.Stop.LacZ mice yielded lung epithelium cell fate reporter mice, abbreviated STR. Mating STR with βcatfl/fl mice generated mice in which β-catenin could be selectively deleted in alveolar epithelium, abbreviated STBR. SFTPC.rtTA and tetO.Cre mice were also crossed to βcatfl/fl and R26Rosa.Tomato.GFP mice.
Mice were housed in animal care facilities at Vanderbilt University (Nashville, TN) with food/water ad libitum. Protocol was approved by Vanderbilt’s institutional animal care and use committee. Doxycycline (Sigma-Aldrich, St. Louis, MO) was administered in drinking water (2 g/dl with 2% sucrose). Bleomycin (Bedford Laboratories, Bedford, Ohio) 0.04 units in 100 μl saline was administered intratracheally after intubation as previously described (4, 18).
Histology, Immunostaining, Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling, Western Blot, Lung Lavage, and Antibodies
Lungs were harvested (18–21); sections prepared (4, 19, 20, 22); immunohistochemistry, immunocytochemistry, immunofluorescence, Xgal staining, and terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining performed (19–21, 23); Western blots performed (19, 23); and bronchoalveolar lavage (BAL) performed (18, 22) as previously described. See online methods for antibodies used in these studies.
Type II AEC Isolation and Polymerase Chain Reaction
Type II AECs were isolated as previously described (19, 21, 22). Type II AECs from fluorescent reporter mice underwent flow sorting (BD-FACsAria; Becton Dickinson, Franklin Lakes, NJ). GFP+ AECs were exposed to Wnt3a (100 ng/ml) (R&D Systems, Minneapolis, MN) with analysis by Wnt/β-catenin polymerase chain reaction (PCR) array (SABiosciences, Frederick, MD) and quantitative real-time PCR per online methods.
Semiquantitative Scoring, Collagen Content, Morphometry, and Lung Mechanics
Semiquantitative lung fibrosis scoring (4, 18, 20, 22); quantitation of TUNEL, S100A4-positive, and α-smooth muscle actin (αSMA)-positive cells (21, 22); hydroxyproline microplate assay (4); measurement of alveolar diameter and perimeter (22); and measurement of static compliance and airway resistance (4, 24) were performed as previously described.
Small Interfering RNA
Mouse lung epithelial (MLE12) cells and rat lung epithelial (RLE6TN) cells underwent small interfering RNA (siRNA) transfection against β-catenin per online methods and as previously described (23). Lactate dehydrogenase cytotoxicity, wound closure, proliferation, and immunofluorescence studies were performed as outlined in online methods.
Statistics
Statistical analyses were performed using GraphPad InStat (GraphPad, San Diego, CA). Differences among groups were assessed using one-way analysis of variance or Kruskal-Wallis rank analysis of variance. Differences between pairs were assessed using Student t test or Mann-Whitney test. Survival differences were evaluated using Fisher exact test. Results are presented as mean ± SEM. P less than 0.05 was considered significant.
Results
β-Catenin Expression Increases after Bleomycin
To evaluate β-catenin expression in bleomycin-induced lung fibrosis, adult wild-type C57BL/6J mice were given intratracheal bleomycin and lungs harvested for evaluation of β-catenin by immunohistochemistry. In untreated lung, β-catenin was detected at low levels in many cells but was most easily observed in corners of some alveoli, suggestive of type II AECs (see Figure E1A in the online supplement). After bleomycin, cytoplasmic and nuclear staining for β-catenin increased in multiple cells types, including epithelial cells in areas of injury, inflammation, and fibrosis (Figures E1B–E1D). Dual immunofluorescence studies indicated that β-catenin was detected in type II AECs, fibroblasts, endothelial cells, and macrophages (Figure E2). In addition, Xgal staining was detected in both epithelial and mesenchymal cells after bleomycin in TOPGAL mice, revealing LEF1/TCF pathway activation (Figure E3). Although β-catenin was detected in multiple cell populations, we elected to analyze its role specifically in the alveolar epithelium.
Mice with Selective Deletion of β-Catenin in the Alveolar Epithelium in Adulthood Have Normal Lung Architecture
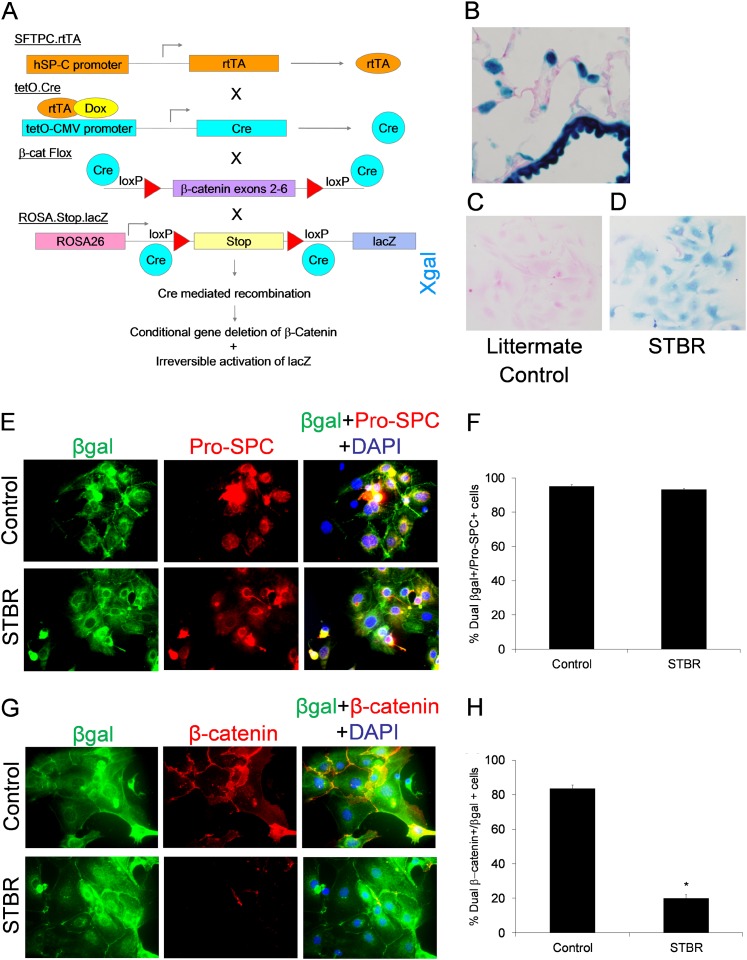
We developed a transgenic mouse model in which β-catenin can be selectively deleted in the alveolar epithelium. To eliminate potential for lung development effects, the tet-on system was used, and β-catenin was deleted by treatment with doxycycline beginning at 8 weeks of age. Transgenic mice (SFTPC.rtTA, tetO.Cre, βcatfl/fl, Rosa.Stop.lacZ) were crossed to generate the desired model, abbreviated STBR (Figure 1A). SFTPC.rtTA+/tetO.Cre−, SFTPC.rtTA−/tetO.Cre+, and SFTPC.rtTA−/tetO.Cre− littermates served as controls.
Figure 1.
Development of transgenic model for deficiency of β-catenin in the alveolar epithelium. (A) Schematic illustrating how the combination of the four individual transgenic mice results in the desired model. SFTPC.rtTA and tetO.Cre were maintained in the heterozygous state, but βcatfl/fl and Rosa.Stop.lacZ were maintained in the homozygous state; thus one-fourth of the resulting mice were the desired transgenic combination. (B) Xgal staining in a lung section from an adult SFTPC.rtTA.tetO.Cre.β-cateninfl/fl.R26Rosa.Stop.LacZ (STBR) mouse treated with doxycycline in the drinking water for 1 week. Magnification, ×600. (C, D) Xgal staining on type II alveolar epithelial cells (AECs) isolated from transgenic mice with deficiency of β-catenin the alveolar epithelium (STBR) demonstrated positive blue staining (D) as opposed to type II AECs from littermate control mice, which were not Xgal-positive (C). (E, F) Lung epithelium cell fate mapping mice with intact β-catenin in the alveolar epithelium (Control) and deficient in β-catenin in the alveolar epithelium (STBR) had a high percentage of recombination events in the type II AEC population as detected by βgal expression in isolated type II AECs. (E) Confocal immunofluorescence images for βgal (green), Pro-SPC (red), and 4′,6-diamidino-2-phenylindole (DAPI) (blue) in isolated type II AECs from STBR and control reporter mice at baseline. (F) Graph representing the percentage of pro-SPC+ AECs that were also βgal+ in control and STBR mice at baseline. n = 3 per group. (G, H) Control and STBR mice had a marked difference in β-catenin expression in isolated type II AECs. (G) Confocal immunofluorescence images for βgal (green), β-catenin (red), and DAPI (blue) in isolated type II AECs from STBR and control reporter mice at baseline. (H) Graph representing the percentage of βgal+ AECs that were also β-catenin+ in control and STBR mice at baseline. As illustrated, transgene efficiency for recombination events in STBR mice was not 100%, with approximately 20% βgal+ AECs still having evidence of β-catenin expression. n = 3 per group. *P < 0.0001 between columns.
Because of the reporter construct, type II AECs (and distal bronchial epithelial cells) express β-galactosidase (βgal) after doxycycline exposure, allowing for lung epithelial cell fate mapping. To verify recombination, we evaluated βgal expression in lung sections and in isolated type II AECs from STBR mice after 1 week of doxycycline (Figures 1B–1D). Xgal staining was identified in the alveolar epithelium in a distribution consistent with type II AECs as well as in distal bronchiolar cells. Isolated type II AECs from AEC β-catenin deficient (STBR) and control cell fate mapping mice were more than 90% βgal+ (Figures 1E and 1F). Dual immunofluorescence studies revealed that approximately 80% of βgal+ AECs from control cell fate mapping mice expressed β-catenin, whereas approximately 20% of βgal+ AECs from STBR mice expressed β-catenin (Figures 1G and 1H), indicating incomplete efficiency of β-catenin gene deletion. Dual immunofluorescence for βgal and the cell markers pro-SPC, S100A4, CD34, and F4/80 revealed that βgal expression was specific to the epithelium (Figures E4 and E5).
To further characterize model integrity, we evaluated downstream effects of AEC deletion of β-catenin on the Wnt/β-catenin pathway. We generated cell fate mapping mice with fluorescent expression to optimize purity of the type II AEC isolation. SFTPC.rtTA.tetO.Cre.βcatfl/fl.R26Rosa.Tomato.GFP (STBR-TGFP) mice had targeted deletion of β-catenin (Figures E6A and E6B), whereas SFTPC.rtTA.tetO.Cre.R26Rosa.Tomato.GFP mice served as fluorescent reporter controls. Mice were given doxycycline for 1 week and a type II AEC isolation was performed with flow sorting to isolate GFP+ (and Tomato+) cells. PCR for genomic β-catenin (Ctnnb1) revealed that GFP+ AECs from STBR-TGFP mice were deficient in Ctnnb1 (Figure E6C). GFP+ AECs were placed in culture with Wnt3a (100 ng/ml) for 24 hours, followed by harvest. Next, we performed a targeted Wnt/β-catenin–specific array (SA Biosciences) (Table E1), with several Wnt/β-catenin–regulated genes showing down-regulation in AECs isolated from AEC β-catenin–deficient mice compared with control mice, including confirmation of β-catenin deletion (Figure E7). In addition, quantitative real-time PCR was performed for β-catenin (Ctnnb1) and the classic downstream targets cyclin D1 (ccnd1) and axin 1, revealing that each was down-regulated in AECs from STBR mice (Figure E8).
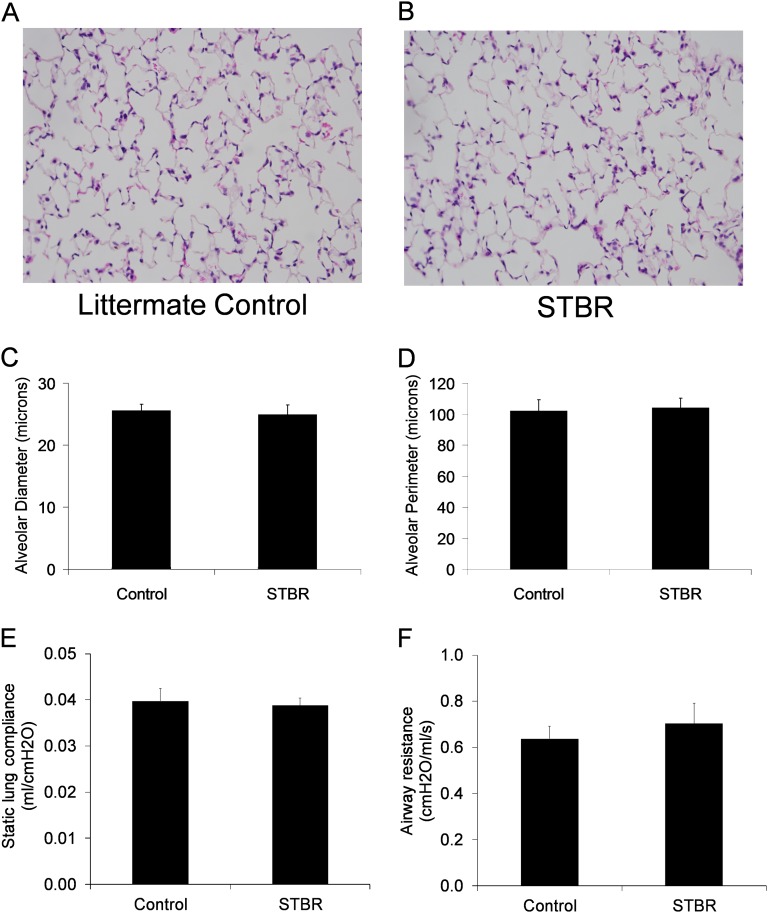
In absence of doxycycline, STBR mice and littermate control mice appeared normal at birth and into adulthood. After reaching adulthood (> 8 wk age), mice were exposed to doxycycline for up to 6 weeks with no evidence of respiratory distress or effects on general appearance or weight. After 6 weeks of doxycycline, lungs from STBR mice and control mice had normal lung architecture by light microscopy (Figures 2A and 2B) and no difference in measurements of alveolar size (Figures 2C and 2D). In separate studies, measurements of lung mechanics, including static lung compliance and airway resistance, were similar between STBR mice and control mice (Figures 2E and 2F). Thus, at least over a short time frame, β-catenin deletion did not appear to impact lung structure or function.
Figure 2.
Mice with deficiency of β-catenin in the alveolar epithelium (STBR) had normal-appearing lung architecture by light microscopy after 6 weeks of doxycycline. Hematoxylin and eosin sections of lung from (A) a mouse with intact β-catenin in the alveolar epithelium, and (B) a mouse with alveolar epithelial β-catenin deficiency (STBR). Magnification, ×200. (C) Mean alveolar diameter and (D) mean alveolar perimeter were similar between STBR mice and control mice. n = 4 for each column. (E) Static lung compliance and (F) airway resistance were similar between control and STBR mice. n = 4 per column.
Mice with Deficiency of β-Catenin in the Alveolar Epithelium Have Greater Mortality and Enhanced Lung Fibrosis after Intratracheal Bleomycin
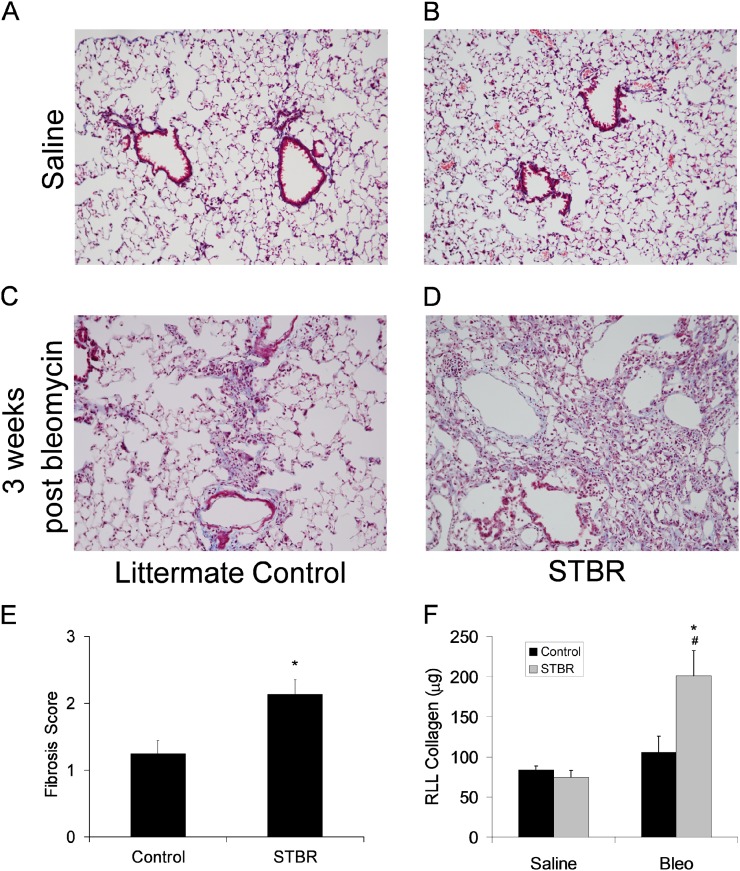
To investigate the impact that AEC-specific deletion of β-catenin may have on lung injury and remodeling, we treated STBR mice and littermate control mice with intratracheal bleomycin. Mice received doxycycline for 1 week before bleomycin. After intratracheal bleomycin 0.04 units, STBR mice had increased mortality compared with littermate control mice (Figure E9). At 3 weeks after bleomycin, lungs from surviving STBR mice and littermate control mice were harvested to evaluate lung fibrosis. On trichrome blue–stained sections, mice with AEC deletion of β-catenin had greater lung fibrosis compared with littermate control mice. STBR mice had increased lung parenchymal distortion and enlarged fibrotic areas (Figures 3A–3D) with increased lung fibrosis by semiquantitative scoring and hydroxyproline quantitation (Figures 3E and 3F).
Figure 3.
Lung fibrosis was increased in mice with deficiency of β-catenin in the alveolar epithelium at 3 weeks after bleomycin. Representative images of trichrome-stained lung sections from (A) a mouse with intact β-catenin expression in the alveolar epithelium (control) with saline injection, (B) a mouse with deficiency of β-catenin in the alveolar epithelium (STBR) with saline injection, (C) control with bleomycin injection, and (D) STBR with bleomycin injection. Magnification, ×100. (E) Lung fibrosis was scored on trichrome blue–stained lung sections, revealing a greater score with deficiency of β-catenin in the alveolar epithelium. n = 9–10 per column. *P < 0.01 between columns. (F) By microplate hydroxyproline assay, right lower lobe collagen content was greater in lungs from mice with deficiency of β-catenin in the alveolar epithelium at 3 weeks after bleomycin. n = 6–7 per column. *P < 0.05 between bleomycin-treated control and STBR mice. #P < 0.05 between bleomycin-treated and saline-treated STBR mice. RLL = right lower lobe.
Mice with Deficiency of β-Catenin in the Alveolar Epithelium Have Greater AEC Death after Intratracheal Bleomycin
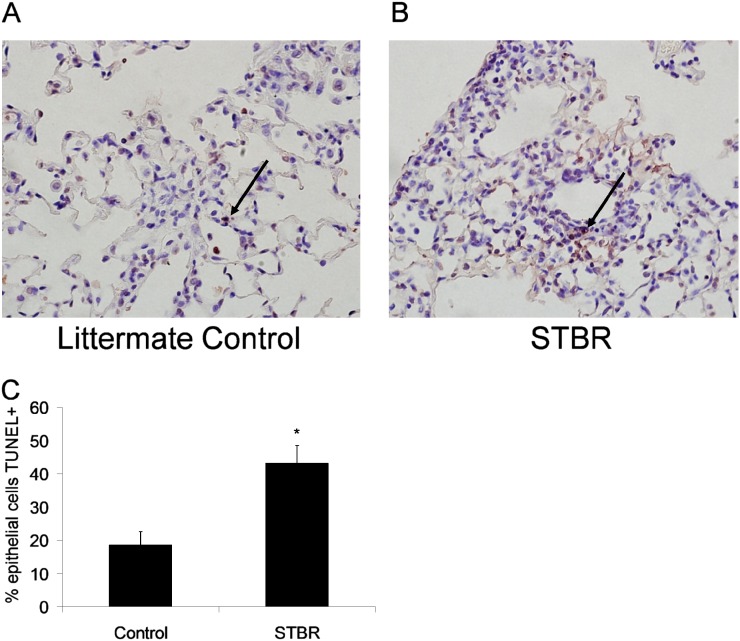
To determine if β-catenin deficiency alters AEC survival after bleomycin, lungs from STBR mice and littermate control mice were harvested at 1 week after bleomycin or vehicle (saline) injection, a time point characterized by prominent AEC death (18). In saline-treated animals, TUNEL+ cells were rare, and no difference was observed between STBR and control mice. At 1 week after bleomycin, STBR mice had markedly greater TUNEL+ epithelial cells compared with littermate control mice (Figure 4). Dual immunofluorescence for TUNEL and pro-SPC confirmed that AECs were the predominant TUNEL+ cell population (Figure E10). Thus, deletion of β-catenin predisposes to AEC death after bleomycin.
Figure 4.
Mice with deficiency of β-catenin in the alveolar epithelium had greater alveolar epithelial cell (AEC) death after bleomycin. Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)+ epithelial cells were more frequent in mice with deficiency of β-catenin in the alveolar epithelium at 1 week after bleomycin. Representative lung sections from (A) a mouse with intact β-catenin expression in the alveolar epithelium, and (B) a mouse with deficiency of β-catenin in the alveolar epithelium (STBR). Arrows point to representative TUNEL+ cells. Magnification, ×400. (C) When quantitated per high-power field, a greater percentage of epithelial cells were TUNEL+ with AEC β-catenin deficiency. n = 5 per column. *P < 0.05 between columns.
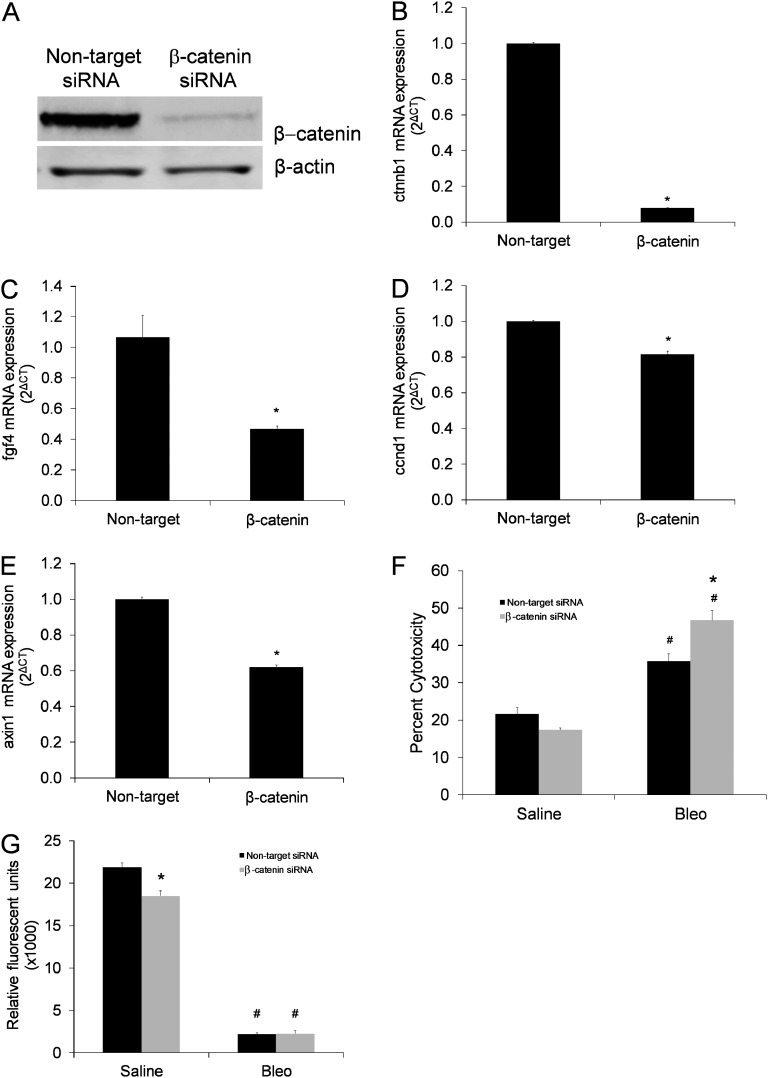
To further investigate this relationship, we performed siRNA studies targeting β-catenin in MLE12 cells, resulting in appropriate knockdown of β-catenin and attenuation of expression of the classic downstream targets fibroblast growth factor 4 (fgf4), cyclin D1 (ccnd1), and axin 1 (Figures 5A–5E). MLE12 cells with targeted knockdown of β-catenin had greater cytotoxicity after bleomycin exposure compared with MLE12 cells with nontarget siRNA (Figure 5F), results that were consistent with in vivo TUNEL staining and indicative of a direct susceptibility to bleomycin cytotoxicity in β-catenin–deficient AECs. Furthermore, MLE12 cells with targeted knockdown of β-catenin showed reduced 5-bromo-2′-deoxyuridine (BrdU) incorporation compared with MLE12 cells with nontarget siRNA (Figure 5G). In isolated type II AECs from STBR and control mice, lactate dehydrogenase cytotoxicity was similar between groups, whereas BrdU incorporation was less in AECs from STBR mice compared with control mice (Figure E11).
Figure 5.
siRNA knockdown of β-catenin in mouse lung epithelial (MLE12) cells increased cell death after bleomycin and attenuated proliferation. (A) Western blot for β-catenin from whole-cell lysates of MLE12 cells transfected with nontarget control small interfering RNA (siRNA) or siRNA targeting β-catenin. β-actin band shown as loading control. (B–D) MLE12 cells with siRNA knockdown of β-catenin had decreased expression of β-catenin (ctnnb1) and downstream targets fibroblast growth factor 4 (fgf4), cyclin D1 (ccnd1), and axin1 compared with nontarget siRNA. Results of quantitative real-time polymerase chain reaction for expression of (B) ctnnb1, (C) fgf4, (D) ccnd1, and (E) axin1, normalized to the housekeeping gene GAPDH. n = 6 for each column. *P < 0.005 between columns. (F) Cytotoxicity based on lactate dehydrogenase assay was increased after bleomycin in MLE 12 cells with siRNA knockdown of β-catenin compared with cells with nontarget siRNA. *P < 0.05 for β-catenin siRNA versus nontarget siRNA after bleomycin. #P < 0.05 compared with respective saline column. n = 8 per column. (G) Cell proliferation was decreased as determined by 5-bromo-2′-deoxyuridine (BrdU) incorporation in MLE cells with siRNA knockdown of β-catenin compared with cells with nontarget siRNA. After bleomycin, BrdU incorporation was markedly attenuated in both groups. *P < 0.001 for β-catenin siRNA versus nontarget siRNA in cells in standard culture conditions (saline vehicle exposure). #P < 0.001 compared with respective saline column. n = 4 per column.
To determine if AEC deletion of β-catenin impacted lung inflammation, BAL was performed at 1 week after saline or bleomycin injection and cell counts and differentials performed. With saline, total BAL leukocytes, macrophages, neutrophils, and lymphocytes were similar between the two groups (Figure E12A–E12D). After bleomycin, inflammatory cells increased in both groups but with no statistical difference between STBR mice and control mice (Figure E12A–E12D). BAL protein levels increased with bleomycin exposure in both groups, but no differences were found between STBR and control mice (Figure E12E).
Deficiency of β-Catenin in Lung Epithelial Cells Impairs Wound Healing
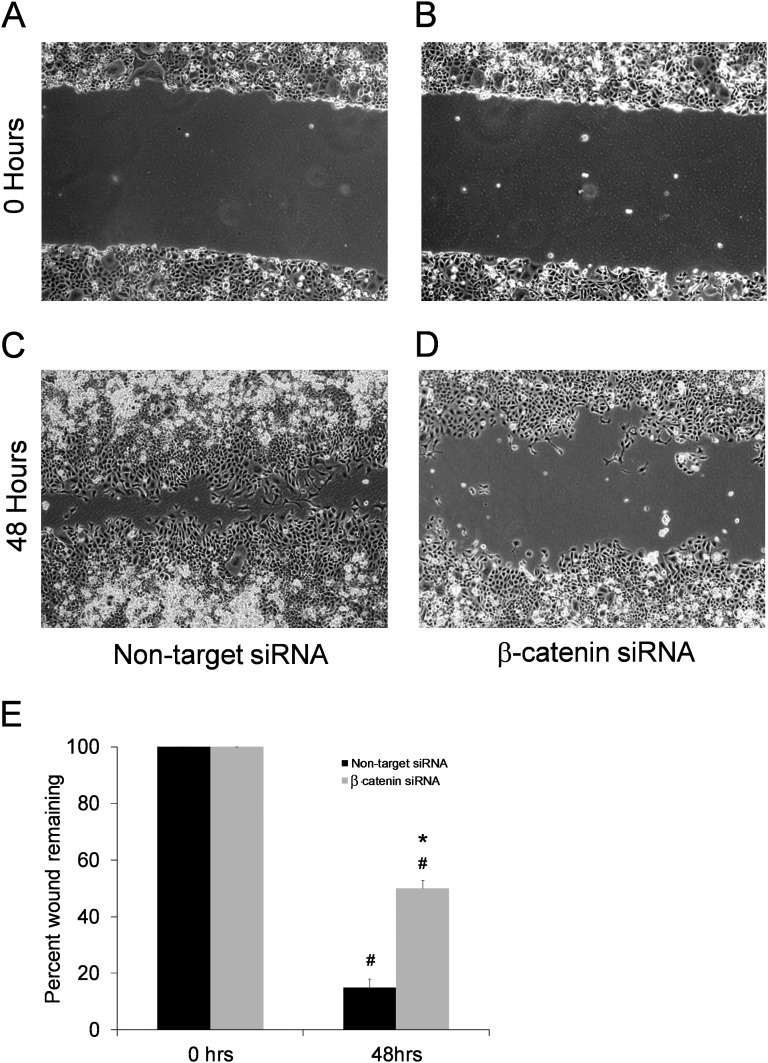
We speculated that β-catenin is important in wound healing functions of the alveolar epithelium. To investigate this possibility, MLE12 cells transfected with siRNA against β-catenin or nontarget siRNA were analyzed in an in vitro two-dimensional monolayer wound closure assay. MLE12 cells with siRNA knockdown of β-catenin had diminished wound closure compared with nontarget siRNA controls (Figure 6). Recent investigations have suggested synergistic interactions between the transforming growth factor β (TGFβ) and Wnt/β-catenin pathways during lung fibrosis (25, 26). Thus, wound healing studies were performed evaluating the effects of TGFβ1 administration in the presence of siRNA knockdown of β-catenin. Although TGFβ augmented wound closure in nontarget siRNA MLE12 cells, this effect was abrogated by siRNA against β-catenin (Figure E13). Taken together, these studies suggest that AEC regulation of β-catenin plays a role in wound healing, which could in part explain the enhanced lung fibrosis noted with AEC β-catenin deficiency after bleomycin.
Figure 6.
Targeted knockdown of β-catenin in mouse lung epithelial cells (MLE12) impairs wound healing. Wound closure in the scratch assay was impaired in MLE12 cells with siRNA knockdown of β-catenin. Representative images at scratch initiation for (A) nontarget siRNA and (B) β-catenin siRNA and the same plates 48 hours later for (C) nontarget siRNA and (D) β-catenin siRNA. (E) Graph showing the percentage of wound closure. *P < 0.001 for β-catenin siRNA versus nontarget siRNA at 48 hours. #P < 0.001 compared with respective 0 h column. n = 9 per column.
Mice with Deficiency of β-Catenin in the Alveolar Epithelium Have Increased Numbers of Lung Fibroblasts
Given the differences in lung fibrosis, we next evaluated numbers of fibroblasts recruited to the lung. Given the prominent role that β-catenin has been shown to have in epithelial–mesenchymal transition (EMT) (26), we further evaluated interactions between TGFβ and β-catenin on EMT in RLE6TN cells. Even with siRNA knockdown of β-catenin, TGFβ1 exposure still reduced E-cadherin and zonula occludens 1 (ZO-1) expression and increased S100A4 expression. In contrast, TGFβ1-induced expression of αSMA was attenuated with siRNA knockdown of β-catenin (Figure E14), complementing recent studies by other investigators (26, 27). Confocal immunofluorescence on lung sections from cell fate mapping mice revealed that total S100A4+ and EMT-derived βgal+/S100A4+ lung fibroblasts increased at 2 weeks after bleomycin (Figure E15A). STBR mice had greater numbers of S100A4+ fibroblasts (Figure E15B), with a lower percentage of EMT-derived fibroblasts compared with control mice (Figure E15C). Thus, two important points emerged: (1) EMT still occurred with β-catenin deficiency, and (2) despite greater lung fibrosis, the relative percentage of EMT-derived lung fibroblasts was lower with AEC β-catenin deficiency. Immunofluorescence for βgal and αSMA was also performed, with dual βgal+/αSMA+ cells rare in both groups, a pattern we have noted previously (21). However, greater numbers of αSMA+ cells were present in alveolar parenchyma of STBR mice, consistent with differences in lung fibrosis (Figure E16).
Resolution of Lung Fibrosis Is Delayed in Mice with Deficiency of β-Catenin
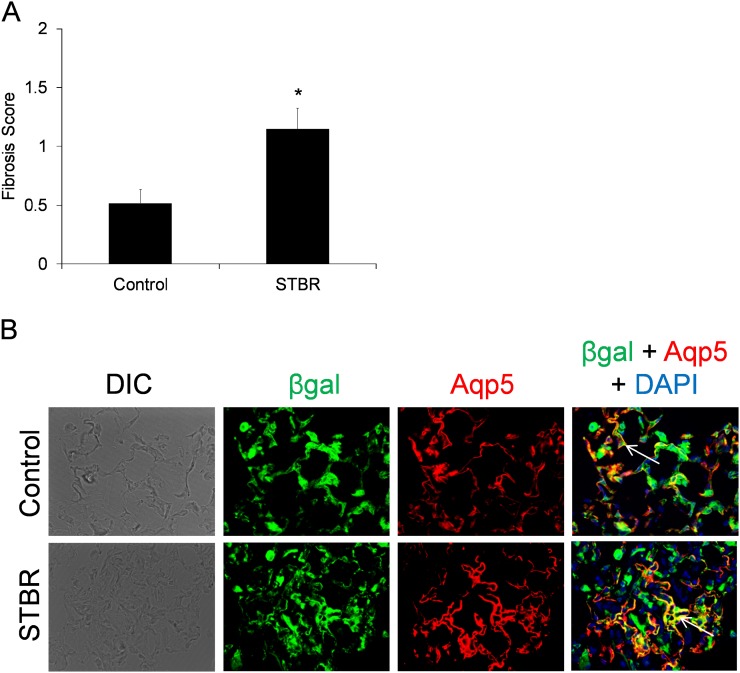
Given that immunostaining for β-catenin was strongly positive at 5 weeks after bleomycin, a time frame in which fibrosis is resolving, experiments were performed with mice harvested at 5 weeks after bleomycin. Trichrome blue–stained lung sections were evaluated and scored, revealing that fibrosis was still greater in STBR mice compared with littermate control mice (Figure 7A). However, scores for both STBR mice and control mice were lower than their respective 3-week scores (Figure 3E). These results indicated that although delayed to some degree with AEC β-catenin deficiency, resolution still proceeds. Because of β-catenin’s prominent role in alveolarization in lung development, we next evaluated whether AEC deficiency of β-catenin impacted regeneration of the alveolar epithelium, specifically whether type I AEC regeneration was impaired, during the resolution phase of the bleomycin model, with aquaporin-5 as a type I AEC marker. Despite AEC deficiency of β-catenin, STBR mice still had evidence of type II to I AEC transition demonstrated by dual βgal+/aquaporin-5+ cells in areas of resolving fibrosis (Figure 7B), a pattern also seen with wild-type cell fate mapping control mice. In contrast, saline-treated control mice had only rare dual βgal+/aquaporin-5+ cells.
Figure 7.
With deficiency of β-catenin in the alveolar epithelium, resolution of fibrosis is delayed, but repair of the alveolar epithelium occurs. (A) Lung fibrosis remained greater in mice with deficiency of β-catenin in the alveolar epithelium (STBR) compared with control mice at 5 weeks after bleomycin. Graph of lung fibrosis scores shown. n = 12–16 per column. *P < 0.01 between columns. (B) Characterization of type I alveolar epithelial cell (AEC) regeneration from type II AECs or more proximal progenitors at 5 weeks after bleomycin. Despite their deficiency of β-catenin in AECs, STBR mice still had evidence of type II to type I AEC transition at 5 weeks after bleomycin. Confocal immunofluorescence images for βgal (green), aquaporin-5 (Aqp5) (red), and 4′,6-diamidino-2-phenylindole (DAPI) (blue) in lungs from STBR and control reporter mice 5 weeks after bleomycin. Dual Aqp5+/βgal+ cells are yellow (arrows to representative cells). Magnification, ×600. DIC = differential interference contrast.
In the studies above, AEC β-catenin gene deletion was induced before bleomycin administration. To determine whether AEC β-catenin gene deletion after initiation of fibrosis is detrimental to repair, we performed repetitive bleomycin injections as previously reported (18). STBR mice and littermate control mice received intratracheal bleomycin 0.04 units every other week for six doses with target for harvest at 2 weeks after the final dose. Doxycycline was started concurrent with the third dose and continued for the remainder of the experiment. Trichrome-stained lung sections revealed prominent lung fibrosis in both groups, with fibrosis scores greater and a trend toward greater lung collagen content in the STBR group (Figure E17). These results suggest that AEC deficiency of β-catenin augments aberrant lung remodeling even when gene deletion occurs after the injury/fibrosis cycle is already initiated.
Discussion
Increasing evidence implicates AEC dysfunction in IPF pathogenesis, including studies suggesting aberrant wound repair of injured epithelium. In these studies, deficiency of β-catenin in AECs caused greater AEC death, increased numbers of lung fibroblasts, and exaggerated fibrosis after bleomycin treatment. In vitro, MLE12 cells with targeted knockdown of β-catenin had impaired wound healing, suggesting an important role for β-catenin in AEC repair processes. Taken together, these studies reveal that AEC regulation of β-catenin plays a critical role in the alveolar epithelium’s reparative response to injury.
β-Catenin exists as a component of the adherens junction, where it is bound to E-cadherin, and in the cytoplasm where it exerts signaling functions (28, 29). In absence of Wnt signaling, cytosolic β-catenin is phosphorylated and undergoes proteasome-mediated degradation (28, 30, 31). With Wnt binding to the Frizzled receptor, phosphorylation is prevented, increasing intracytoplasmic β-catenin levels (28, 30). β-Catenin can then be transported to the nucleus, where it interacts with other proteins including cAMP-response element binding protein (CBP) or p300 with subsequent binding to LEF-1/TCF, leading to gene transcription (32, 33). Thus, β-catenin has two prominent roles, one as a component of the adherens junction and the other as a transcriptional regulator. In our model, β-catenin is genetically deleted in AECs, which could impact both functions, but it is difficult to determine what components of the observed phenotype are due to effects on the adherens junctions or transcription.
β-Catenin is a key component to patterning of the alveolar epithelium in lung development (34, 35). Overexpression of β-catenin in epithelial cells in the developing lung results in epithelial cell dysplasia and aberrant type II AEC differentiation (6). In contrast, lung epithelial cell deletion of β-catenin results in disruption of normal branching morphogenesis and failure of alveolarization (7). Furthermore, Wnt inhibition via overexpression of DKK1 in the developing lung epithelium leads to aberrancies in proximal–distal lung patterning (7), whereas deficiencies in specific Wnt proteins also lead to impairments in lung development (36–38).
In adult lung, β-catenin influences airway repair mechanisms (39). Using a transgenic model in which exon 3 of β-catenin was selectively deleted in CC10+ cells, leading to stabilization and persistent activation of β-catenin, an increased number of undifferentiated airway epithelial cells was observed with enhanced bronchiolar epithelial cell reparative capacity after naphthalene airway injury (40). In contrast, selective deletion of β-catenin in CC10+ cells did not impact airway epithelial recovery and repair after naphthalene (41).
The role for β-catenin in the adult alveolar epithelium is less clear, but recent studies demonstrated expression of β-catenin and other pathway components in hyperplastic AECs in IPF (8, 9). In 2009, Konigshoff and colleagues reported that administration of neutralizing antibodies against Wnt1-inducible signaling protein 1 (WISP1) attenuated bleomycin-induced lung fibrosis (42). Subsequently, Henderson and colleagues demonstrated that administration of a small molecule (ICG-001) that inhibits CBP/β-catenin–dependent transcription attenuated bleomycin-induced lung fibrosis (43). In 2011, Kim and colleagues reported that intratracheal administration of siRNA targeting β-catenin also decreased lung fibrosis after bleomycin (44). In contrast, our studies demonstrated that targeted AEC deficiency of β-catenin predisposes to lung fibrosis. We suspect the principal reason for this discrepancy is that we specifically targeted deletion of β-catenin to the alveolar epithelium. Inhibition modalities such as those in the studies above likely affect other cell populations in addition to epithelium. Wnt/β-catenin has been implicated in fibroblast function in lung fibrosis (9, 42, 45). Thus, global inhibition models could impact fibroblasts in a favorable manner to attenuate fibrosis, whereas epithelial cell–specific targeting could be detrimental. In vitro studies from Flozak and colleagues support our findings that β-catenin is required for AEC survival and wound healing (46). Using adenoviral transfection in rat type II AECs, these investigators demonstrated that β-catenin was required for cell survival and monolayer wound closure (46), consistent with our siRNA studies. Another reason for differences between our studies and previous studies is that our model also has potential to affect the adherens junctions, whereas the studies by Konigshoff and colleagues (42) and Henderson and colleagues (43) specifically would not.
However, other in vivo studies support our observations. A recent study by Zemans and colleagues revealed that inhibition of p300/β-catenin–dependent transcription with the small molecule IQ-1 was detrimental to lung epithelial repair in experimental inflammation (47). Furthermore, the ICG-001 study above (43) emphasized the inhibitory effect on CBP/β-catenin. However, ICG-001 does not inhibit the p300/β-catenin interaction, and subsequent transcription can still occur (33). Thus, ICG-001 may have potential beneficial effects, not only because of the CBP/β-catenin inhibition, but because of promotion of p300/β-catenin interactions. If so, that would be complementary to our findings.
AEC apoptosis has been prominently implicated in pulmonary fibrosis (3, 48), including a study by Sisson and colleagues in which targeted type II AEC death resulted in lung fibrosis (49). In our study, mice with AEC deficiency of β-catenin had enhanced AEC death at 1 week after bleomycin, which could explain, at least in part, increased lung fibrosis. In addition, our in vivo evaluations and in vitro scratch assay studies suggest that β-catenin may affect epithelial wound healing. However, it should be noted that these studies do not suggest that alveolar repair processes are blocked completely. Rather, resolution, though delayed, still occurs with β-catenin deficiency. Furthermore, regeneration of the alveolar epithelium still proceeds, as evidenced by type I AECs with the βgal lineage tag, indicating they arose from type II AECs or other proximal epithelial cell progenitors. Therefore, it is likely that other pathways are also involved and can mediate resolution and regeneration in β-catenin’s absence. However, given our incomplete transgene efficiency for gene deletion, it is possible that AECs still expressing β-catenin were responsible for alveolar repair, potentially a limitation to this study.
Although these evaluations reveal a prominent role for AEC regulation of β-catenin in pulmonary fibrosis, questions arise that need to be addressed with future investigations. Considering the discrepancy between more global approaches to down-regulating β-catenin versus those that are AEC targeted, future studies are needed to investigate the functions of β-catenin in lung fibroblasts and determine whether fibroblast-targeted deletion of β-catenin attenuates lung fibrosis. Furthermore, to better understand mechanisms by which β-catenin in AECs protects from fibrosis, it will be important to differentiate structural effects of β-catenin on adherens junctions from phenotypic effects resulting from transcriptional activity. Finally, considering its role in airway stem cell maintenance (39), investigations are needed to determine whether β-catenin has similar stem cell functions in alveolar repair.
In summary, our studies demonstrate that β-catenin regulates alveolar repair and remodeling in experimental lung fibrosis. Although some have proposed therapeutically inhibiting the β-catenin pathway in IPF and other fibrotic diseases (50), our findings suggest that this approach could be counterproductive, particularly if the alveolar epithelium is a prominent target of these interventions. In addition, our studies support the hypothesis that AEC dysfunction is central to the pathogenesis of fibrotic lung diseases such as IPF, providing additional rationale for specifically targeting the repair and regenerative abilities of this cell population as a goal for development of new therapies.
Footnotes
Supported by National Institutes of Health National Heart, Blood, and Lung Institute grants HL85406 (W.E.L.), HL105479 (W.E.L.), HL85317 (T.S.B.), HL92870 (T.S.B.), HL87738 (A.L.D., P.F.C.); NIH National Center for Research Resources grant UL1 RR024975; American Thoracic Society/Coalition for Pulmonary Fibrosis Research Grant (H.T.), Francis Families Foundation (H.T.), IPFNet Cowlin Career Development Award (A.L.D.), and the Department of Veterans Affairs (W.E.L., T.S.B.). H.T. is a Parker B. Francis Fellow in Pulmonary Research.
Author Contributions: H.T. designed and performed research, analyzed data, and wrote the paper. A.L.D. designed and performed research and analyzed data. P.F.C. designed and performed research and analyzed data. X.C.X. performed research and analyzed data. M.E.M. performed research and analyzed data. B.R.J. performed research and analyzed data. V.V.P. performed research and analyzed data. A.J.B. performed research and analyzed data. D.-S.C. performed research and analyzed data. D.C.N. performed research and analyzed data. F.B.M. performed research and analyzed data. L.A.G. performed research and analyzed data. T.S.B designed and performed research, analyzed data, and wrote the paper. W.E.L. designed and performed research, analyzed data, and wrote the paper.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201205-0972OC on January 10, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Zoz DF, Lawson WE, Blackwell TS. Idiopathic pulmonary fibrosis: a disorder of epithelial cell dysfunction. Am J Med Sci 2011;341:435–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawson WE, Loyd JE, Degryse AL. Genetics in pulmonary fibrosis–familial cases provide clues to the pathogenesis of idiopathic pulmonary fibrosis. Am J Med Sci 2011;341:439–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uhal BD. Epithelial apoptosis in the initiation of lung fibrosis. Eur Respir J Suppl 2003;44:7s–9s [DOI] [PubMed] [Google Scholar]
- 4.Lawson WE, Cheng DS, Degryse AL, Tanjore H, Polosukhin VV, Xu XC, Newcomb DC, Jones BR, Roldan J, Lane KB, et al. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci USA 2011;108:10562–10567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khalil N, O’Connor RN, Unruh HW, Warren PW, Flanders KC, Kemp A, Bereznay OH, Greenberg AH. Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 1991;5:155–162 [DOI] [PubMed] [Google Scholar]
- 6.Mucenski ML, Nation JM, Thitoff AR, Besnard V, Xu Y, Wert SE, Harada N, Taketo MM, Stahlman MT, Whitsett JA. Beta-catenin regulates differentiation of respiratory epithelial cells in vivo. Am J Physiol Lung Cell Mol Physiol 2005;289:L971–L979 [DOI] [PubMed] [Google Scholar]
- 7.Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA. Beta-catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem 2003;278:40231–40238 [DOI] [PubMed] [Google Scholar]
- 8.Konigshoff M, Balsara N, Pfaff EM, Kramer M, Chrobak I, Seeger W, Eickelberg O. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS ONE 2008;3:e2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, et al. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol 2003;162:1495–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beers MF, Morrisey EE. The three R’s of lung health and disease: repair, remodeling, and regeneration. J Clin Invest 2011;121:2065–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Degryse AL, Crossno PF, Tanjore H, Xu C, Jones BR, McMahon FB, Gleaves LA, Blackwell TS, Lawson WE. Deletion of beta-catenin in type II alveolar epithelial cells leads to enhanced lung injury and fibrosis following intratracheal bleomycin. Am J Respir Crit Care Med 2010;181:A1966 [Google Scholar]
- 12.Tanjore H, Degryse AL, Crossno PF, Xu XC, Polosukhin VV, Jones BR, Bryant AJ, Gleaves LA, Blackwell TS, Lawson WE. Selective loss of beta-catenin in alveolar epithelial cells enhances bleomycin induced lung injury and fibrosis. Am J Respir Crit Care Med 2012;185:A5543 [Google Scholar]
- 13.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA 2002;99:10482–10487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 2001;128:1253–1264 [DOI] [PubMed] [Google Scholar]
- 15.Soriano P. Generalized LacZ expression with the ROSA26 Cre reporter strain. Nat Genet 1999;21:70–71 [DOI] [PubMed] [Google Scholar]
- 16.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis 2007;45:593–605 [DOI] [PubMed] [Google Scholar]
- 17.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 1999;126:4557–4568 [DOI] [PubMed] [Google Scholar]
- 18.Degryse AL, Tanjore H, Xu XC, Polosukhin VV, Jones BR, McMahon FB, Gleaves LA, Blackwell TS, Lawson WE. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2010;299:L442–L452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawson WE, Polosukhin VV, Zoia O, Stathopoulos GT, Han W, Plieth D, Loyd JE, Neilson EG, Blackwell TS. Characterization of fibroblast-specific protein 1 in pulmonary fibrosis. Am J Respir Crit Care Med 2005;171:899–907 [DOI] [PubMed] [Google Scholar]
- 20.Lawson WE, Polosukhin VV, Stathopoulos GT, Zoia O, Han W, Lane KB, Li B, Donnelly EF, Holburn GE, Lewis KG, et al. Increased and prolonged pulmonary fibrosis in surfactant protein C-deficient mice following intratracheal bleomycin. Am J Pathol 2005;167:1267–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanjore H, Xu XC, Polosukhin VV, Degryse AL, Li B, Han W, Sherrill TP, Plieth D, Neilson EG, Blackwell TS, et al. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med 2009;180:657–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Degryse AL, Tanjore H, Xu XC, Polosukhin VV, Jones BR, Boomershine CS, Ortiz C, Sherrill TP, McMahon FB, Gleaves LA, et al. TGFbeta signaling in lung epithelium regulates bleomycin-induced alveolar injury and fibroblast recruitment. Am J Physiol Lung Cell Mol Physiol 2011;300:L887–L897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanjore H, Cheng DS, Degryse AL, Zoz DF, Abdolrasulnia R, Lawson WE, Blackwell TS. Alveolar epithelial cells undergo epithelial-to-mesenchymal transition in response to endoplasmic reticulum stress. J Biol Chem 2011;286:30972–30980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polosukhin VV, Degryse AL, Newcomb DC, Jones BR, Ware LB, Lee JW, Loyd JE, Blackwell TS, Lawson WE. Intratracheal bleomycin causes airway remodeling and airflow obstruction in mice. Exp Lung Res 2012;38:135–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulsamer A, Wei Y, Kim KK, Tan K, Wheeler S, Xi Y, Thies RS, Chapman HA. Axin pathway activity regulates in vivo PY654-beta-catenin accumulation and pulmonary fibrosis. J Biol Chem 2012;287:5164–5172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou B, Liu Y, Kahn M, Ann DK, Han A, Wang H, Nguyen C, Flodby P, Zhong Q, Krishnaveni MS, et al. Interactions between beta-catenin and transforming growth factor-beta signaling pathways mediate epithelial-mesenchymal transition and are dependent on the transcriptional co-activator cAMP-response element-binding protein (CREB)-binding protein (CBP). J Biol Chem 2012;287:7026–7038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Velden JL, Guala AS, Leggett SE, Sluimer J, Badura EC, Janssen-Heininger YM. Induction of a mesenchymal expression program in lung epithelial cells by wingless protein (Wnt)/beta-catenin requires the presence of c-Jun N-terminal kinase-1 (JNK1). Am J Respir Cell Mol Biol 2012;47:306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bienz M. Beta-catenin: a pivot between cell adhesion and Wnt signalling. Curr Biol 2005;15:R64–R67 [DOI] [PubMed] [Google Scholar]
- 29.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science 2004;303:1483–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet 2004;5:691–701 [DOI] [PubMed] [Google Scholar]
- 31.He X. A Wnt-Wnt situation. Dev Cell 2003;4:791–797 [DOI] [PubMed] [Google Scholar]
- 32.Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9–2 in the switch between beta-catenin’s adhesive and transcriptional functions. Genes Dev 2004;18:2225–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma H, Nguyen C, Lee KS, Kahn M. Differential roles for the coactivators CBP and P300 on TCF/beta-catenin-mediated survivin gene expression. Oncogene 2005;24:3619–3631 [DOI] [PubMed] [Google Scholar]
- 34.Weng T, Liu L. The role of pleiotrophin and beta-catenin in fetal lung development. Respir Res 2010;11:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Langhe SP, Reynolds SD. Wnt signaling in lung organogenesis. Organogenesis 2008;4:100–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li C, Xiao J, Hormi K, Borok Z, Minoo P. Wnt5a participates in distal lung morphogenesis. Dev Biol 2002;248:68–81 [DOI] [PubMed] [Google Scholar]
- 37.Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, Yamaguchi TP, Morrisey EE. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell 2009;17:290–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development 2002;129:4831–4842 [DOI] [PubMed] [Google Scholar]
- 39.Reynolds SD, Malkinson AM. Clara cell: progenitor for the bronchiolar epithelium. Int J Biochem Cell Biol 2010;42:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reynolds SD, Zemke AC, Giangreco A, Brockway BL, Teisanu RM, Drake JA, Mariani T, Di PY, Taketo MM, Stripp BR. Conditional stabilization of beta-catenin expands the pool of lung stem cells. Stem Cells 2008;26:1337–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zemke AC, Teisanu RM, Giangreco A, Drake JA, Brockway BL, Reynolds SD, Stripp BR. Beta-catenin is not necessary for maintenance or repair of the bronchiolar epithelium. Am J Respir Cell Mol Biol 2009;41:535–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konigshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, Rose F, Fink L, Seeger W, Schaefer L, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest 2009;119:772–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henderson WR, Jr, Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, Borok Z, Knight DA, Kahn M. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci USA 2010;107:14309–14314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim TH, Kim SH, Seo JY, Chung H, Kwak HJ, Lee SK, Yoon HJ, Shin DH, Park SS, Sohn JW. Blockade of the Wnt/beta-catenin pathway attenuates bleomycin-induced pulmonary fibrosis. Tohoku J Exp Med 2011;223:45–54 [DOI] [PubMed] [Google Scholar]
- 45.Lam AP, Flozak AS, Russell S, Wei J, Jain M, Mutlu GM, Budinger GR, Feghali-Bostwick CA, Varga J, Gottardi CJ. Nuclear beta-catenin is increased in systemic sclerosis pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am J Respir Cell Mol Biol 2011;45:915–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flozak AS, Lam AP, Russell S, Jain M, Peled ON, Sheppard KA, Beri R, Mutlu GM, Budinger GR, Gottardi CJ. Beta-catenin/T-cell factor signaling is activated during lung injury and promotes the survival and migration of alveolar epithelial cells. J Biol Chem 2010;285:3157–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zemans RL, Briones N, Campbell M, McClendon J, Young SK, Suzuki T, Yang IV, De LS, Reynolds SD, Mason RJ, et al. Neutrophil transmigration triggers repair of the lung epithelium via beta-catenin signaling. Proc Natl Acad Sci USA 2011;108:15990–15995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X, Shu R, Filippatos G, Uhal BD. Apoptosis in lung injury and remodeling. J Appl Physiol 2004;97:1535–1542 [DOI] [PubMed] [Google Scholar]
- 49.Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A, Dave A, Engelhardt JF, Liu X, White ES, et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med 2010;181:254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lam AP, Gottardi CJ. Beta-catenin signaling: a novel mediator of fibrosis and potential therapeutic target. Curr Opin Rheumatol 2011;23:562–567 [DOI] [PMC free article] [PubMed] [Google Scholar]