Abstract
BACKGROUND
A new miniature high-resolution pocket-mobile echocardiographic (PME) device has become available to clinicians, but there are no data available comparing this technology with standard transthoracic echo (TTE) examination.
OBJECTIVE
To assess the potential validity of PME imaging as a quick assessment of cardiovascular disease by direct comparison to standard TTE.
DESIGN
Ultrasonographers attempted to acquire seven standard echocardiography views with the PME prior to performing comprehensive standard TTEs. In blinded fashion, images from the two modalities were compared by two experienced echocardiographers and two cardiology fellows.
PRIMARY FUNDING SOURCE
This work was funded in part by Scripps Health and the NIH UL1 RR025774 (Scripps Translational Science Institute, Clinical and Translational Science Award).
SETTING
Scripps Clinic/Green Hospital
PATIENTS
97 consecutive unselected patients
MEASUREMENTS
Comparisons were made in regards to ejection fraction (EF), segmental wall motion abnormalities (WMA), left ventricular end-diastolic dimension (LVEDD), inferior vena cava (IVC) size, aortic and mitral valve pathology, and pericardial effusion.
RESULTS
PME images were adequate for interpretation of EF in 95% of the studies, LVEDD 95%, mitral valve 90%, WMA 83%, aortic valve 83%, and IVC 75%. Compared to standard TTE, PME interpretation by attendings and fellows had an accuracy of 97% and 93% for EF, respectively. Likewise, accuracy for WMA was 90% and 87% ; LVEDD 94% and 91%; aortic stenosis 97% and 95%; mitral abnormality 88% and 82%; and IVC size 81% and 74%.
LIMITATIONS
As this was a validation study of imaging alone, further evaluation with clinician image acquisition is needed.
CONCLUSIONS
PME images obtained rapidly by skilled ultrasonographers provide excellent visualization in the vast majority of patients and correlate well with standard, comprehensive TTE. Such validation needs to be extended to untrained clinicians in larger and diverse patient populations before broad dissemination of this technology can be recommended.
Keywords: cardiovascular disease, echocardiography, imaging
Introduction
In the past decade, advancements in ultrasound technology have led to the development of smaller ultrasound devices and increased use of those devices at the point-of-care. Several studies in a variety of clinical settings have shown incremental benefit when hand-carried ultrasound is “added-on” to the general physical exam, and many investigators have suggested that these devices will someday become an integral part of the physical exam (1-5).
Recently, the same advances in technology implemented for ultrasonography have been implemented for echocardiography. A pocket-mobile echocardiographic (PME) device roughly the size of a mobile phone and easily fitting within a physician’s pocket (weight 13.8 oz, dimensions 5.3” × 2.9” × 1.1” with a 3.8 MHz phased array transducer) was released directly to the medical community in February 2010 without documentation of its accuracy compared to standard transthoracic echocardiography (TTE) machines. In this study we compared the accuracy of PME as a quick assessment for clinical and subclinical cardiovascular disease to standard TTE using blinded assessments by multiple cardiologists, and calculated inter-observer variability for PME image interpretation for experienced echocardiographers and cardiology fellows with less than two months of training in echocardiographic interpretation.
Methods
Population
This study, which was approved by the Scripps Institutional Review Board, included a convenience sample of 97 inpatients and outpatients referred for TTE at the Scripps Clinic Torrey Pines and Scripps Green Hospital between February 22 and March 16, 2010. The patients were selected according to a “next-available” model with even-numbered days dedicated primarily to inpatients, and odd-numbered days primarily outpatients, regardless of the indication for the study. We sought a roughly equal number of inpatients and outpatients to ensure variability of clinical presentation and indications for imaging (assuming more inpatients would undergo imaging for assessment of ischemia and more outpatients for murmurs). Clinicians ordering echocardiography were not aware that patients referred for TTE would also undergo PME.
Study Acquisition
Study ultrasonographers (n=14) attempted to acquire standard echocardiography parasternal long-axis, parasternal short axis, subcostal, and apical two-, three-, and four-chamber views with a PME (Vscan, GE Healthcare, Milwaukee, Wisconsin, Figure 1) in five minutes or less immediately prior to performing a comprehensive standard TTE with the Philips iE33 Echocardiograph System (Philips Medical Systems, Andover, Massachusetts). Ultrasonographers were encouraged to complete the PME exam in 5 minutes or less in an attempt to simulate the length of time a physician might use the PME device as part of the physical examination in a clinical encounter. The Doppler flow function of the device was turned off to facilitate rapid acquisition of images in keeping with a first-pass screening exam.
Figure 1.
Panel (a) shows the GE Vscan device, panel (b) is a perspective of the pocket-size Vscan device compared to a traditional stethoscope.
Blinding
Every PME study was assigned a number, and patients were identifiable only by their medical record number. The ultrasonographers obtaining the images were not blind to the clinical indication for the imaging study and were not blind to the PME images when obtaining the standard TTE images. The physicians interpreting the PME images were blinded to the indication for the study and the results of the corresponding standard TTE but knew that the images they were interpreting came from a PME rather than standard TTE device. Standard TTE images were interpreted by physician readers in keeping with standard clinical care, who were blind to the results of the corresponding PME exam. Physicians interpreting the PME images had no involvement in the clinical care of patients.
Study Interpretation
PME images were individually interpreted by two cardiology fellows with two months of basic echocardiography training and two faculty cardiologists with advanced echocardiography training (Level 3) in the seven pre-specified image elements: ejection fraction (EF) (“normal” or “low”), segmental wall motion abnormality (WMA) (“yes” or “no”), left ventricular end-diastolic dimension (LVEDD) (“normal” or “enlarged”), pericardial effusion (“clinically significant” or “not clinically significant”), mitral valve (“normal” or “abnormal”), aortic valve (“normal/sclerotic” or “stenotic”), and inferior vena cava (IVC) size (“normal” or “enlarged”). EF was considered low if less than 45% by visual estimation. Segmental wall abnormality of hypokinesis was defined as a lack of translational motion toward the centerline or lack of normal systolic thickening. The wall motion abnormality was categorized as hypokinetic, akinetic, or dyskinetic. LVEDD was measured in the parasternal long axis view with electronic calipers built into the software of the PME device and was considered enlarged if greater than 5.3cm for women or 5.9cm for men. Pericardial effusion was considered clinically significant if it was at least moderate or associated with evidence of hemodynamic changes. The mitral valve was considered structurally abnormal if it appeared to have severe mitral annular calcification, prolapse, flail, or at least moderately thickened leaflets and/or subvalvular apparatus using accepted criteria (6). Aortic valve was considered stenotic if leaflet opening appeared restricted, if the valve appeared thickened or abnormally echo-bright in the representative views. Color flow and mitral regurgitation were not assessed for this study. IVC was considered dilated if its diameter was greater than 1.5cm and there was less than 50% collapse during inspiration (corresponding to a right atrial pressure > 10 mmHg). Finally, each clinical element could also be classified as “not well visualized” if the images were not adequate for interpretation. If a clinical element was not visualized with the standard TTE machine, the interpretation of that element on the corresponding PME was excluded from further analysis.
Statistical Methods
The ability to visualize images (yes/no) is summarized as a proportion for each parameter. To address the influence of body mass index (BMI) on the ability of PME to obtain images adequate for interpretation, the proportions of abnormal BMI (BMI < 18.5 or BMI > 30) in visualizable scans to not visualizable scans was compared by chisquare test. Estimates of accuracy of PME methods were calculated based on the proportion of accurate interpretations (sum of true positives and true negatives) over the total number of scans visualized by both TTE and PME, with TTE serving as the gold standard. A more conservative estimate of accuracy was also calculated using total scans as the denominator. Cohen’s kappa was used to estimate agreement between 2 raters and Fleiss’s kappa was used to estimate agreement across all 4 raters (7). Kappa values were estimated using interpretations on scans visualized by both or all of the raters being compared. Analyses were performed with SAS version 9.2 (SAS Institute Inc, Cary, NC) software.
Results
Baseline Characteristics
We recruited 97 patients, whose characteristics are outlined in Table 1. All standard TTE exams were adequate for interpretation of EF, LVEDD, aortic and mitral valves, and pericardial effusion. Only one standard TTE was not adequate for interpretation of EF, and two of the exams did not assess the IVC. Echo contrast (Definity™, Lantheus Medical Imaging, North Billerica, Massachusetts) was required to assist interpretation of 8.2% of the standard TTE studies, after the PME images had already been obtained.
Table 1.
Summary of patient and echocardiography characteristics.
| Patient characteristics (n=97) | |
| Age (mean [SD]) | 68 +/− 17 |
| Sex (male) | 45 % |
| Body Mass Index (mean [SD])* | 27 +/− 5 |
| <18.5 | 4 % |
| 18.5 – 30 | 72 % |
| >30 | 24 % |
| Inpatient | 19 % |
| Echocardiography characteristics | |
| Indications | |
| Coronary artery disease | 19 % |
| Congestive heart failure | 19 % |
| Arrhythmia | 21 % |
| Valve evaluation | 22 % |
| Other | 18% |
| Time to complete pocket mobile exam (mean [SD]) |
4.7 +/− 1.5 minutes |
| % completed < 5 minutes | 59 % |
| Ejection Fraction < 45% | 14 % |
| Segmental Wall Motion Abnormalities |
13 % |
| Enlarged LVEDD | 16 % |
| Dilated Inferior Vena Cava | 12 % |
| Abnormal Mitral Valve | 7 % |
| Aortic Stenosis | 6 % |
| Significant Pericardial Effusions |
0 % |
BMI Data not available for one patient.
Abbreviations: SD, Standard Deviation; LV-EDD, left-ventricular end diastolic dimension.
Visualization
The proportion of PME images adequate for estimation of EF, LVEDD, mitral valve, WMA, aortic valve and IVC are summarized in Table 2. There were no clinically significant pericardial effusions but images were adequate for assessing effusions 94% of the time. Visualizability in individuals with normal BMI was generally greater than in those with abnormal BMI (odds ratio (95% CI): IVC =1.16 (0.48-2.82), WMA=1.78 (0.73-4.36)). None of these comparisons reached statistical significance due to small group sizes. IVC and WMA results are summarized because these parameters had the two largest numbers of scans that could not be visualized.
Table 2. Pocket Mobile Echocardiography (PME) and Transthoracic Echocardiography (TTE) interpretation.
Includes visualization, concordance, and inter-observer agreement by kappa values.
| Transthoracic echo parameter (% abnormal) |
Visualized (%) | True Positive + True Negative (% of visualized/% of total scanned) |
Variability (κ) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Attendings | Fellows | Overall | Attendings | Fellows | Overall (4 raters) |
Attendings (2 raters) |
Fellows (2 raters) |
|
|
EF Low – 14 % |
95 | 93 | 97 | 95/91 | 97/91 | 93/91 | 0.71 | 0.95 | 0.68 |
|
WMA* Abnormal – 13 % |
83 | 85 | 81 | 89/74 | 90/77 | 87/71 | 0.72 | 0.90 | 0.47 |
|
LVEDD Enlarged – 15 % |
95 | 95 | 94 | 92/87 | 94/90 | 91/85 | 0.67 | 0.82 | 0.55 |
|
Pericardial effusion Significant – 0 % |
94 | 94 | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
|
Mitral valve Abnormal – 7 % |
90 | 90 | 90 | 85/77 | 88/79 | 82/74 | 0.35 | 0.59 | 0.29 |
|
Aortic valve Abnormal – 6 % |
82 | 86 | 80 | 96/79 | 97/83 | 95/76 | 0.76 | 0.84 | 0.75 |
|
IVC size Dilated – 12 % |
75 | 73 | 77 | 78/58 | 81/59 | 74/57 | 0.42 | 0.84 | 0.39 |
TTE comparison image missing for WMA assessment in 1 patient
TTE comparison image missing for IVC assessment in 2 patients
Abbreviations: EF, ejection fraction; LV- EDD, left-ventricular end diastolic dimension; MV, mitral valve; WMA, wall motion abnormality; AV, aortic valve; IVC, inferior vena cava; k, kappa value.
PME Accuracy and Inter-observer Agreement
Accuracy of attending and fellow interpretation of EF, aortic valve, LVEDD, WMA, mitral valve and IVC are described in Table 2. The kappa statistics for fellow agreement ranged from (0.29-0.75) while the kappas for attending agreement were consistently higher for all six parameters and ranged from (0.59-0.95). The largest difference between fellow and attending agreement was for IVC size (fellows: k=0.39, attendings: k=0.84). Of the six cases of aortic stenosis read by the two attendings, the abnormality was identified on PME on eleven of twelve interpretations. Kappa values were calculated between all reviewers, results also included in Table 2. These numbers suggested fair to moderate agreement for mitral valve abnormality and IVC size, and substantial agreement for LVEDD, EF, WMA, and the aortic valve (8). The false positive rate ranged from 1-14% for attendings and 2-21% for fellows. The false negative rate ranged from 1-13% for attendings and 2-8% for fellows. The highest false positive rates were for IVC (7-21%), mitral valve (6-21%) and WMA (5-13%) and highest false negative rates were for IVC (6-13%), LVEDD (4-8%) and WMA (3-5%).
Representative Images
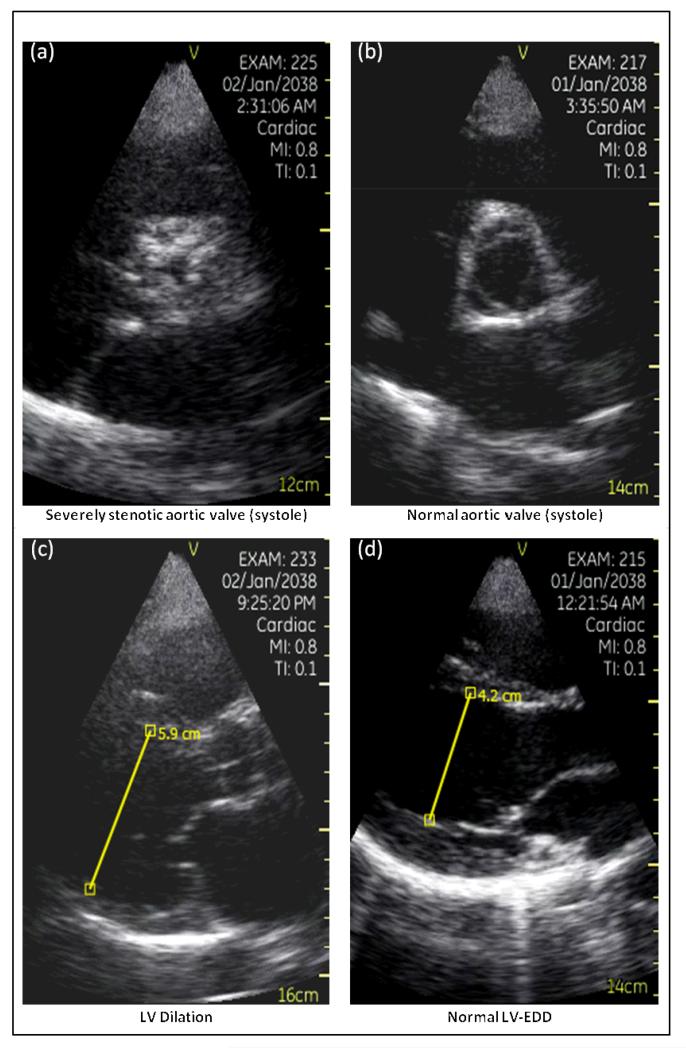
Figure 2 presents aortic valve stop frames from a patient with (a) critical aortic stenosis and (b) one with a normal aortic valve, as well as stop frame images of a (c) dilated and a (d) normal sized left ventricle.
Figure 2.
Pocket-mobile echo (PME) images of (a) severely stenotic aortic valve and (b) a normal aortic valve, both images recorded from a parasternal short-axis view during ventricular systole. PME images of (c) enlarged LVEDD and (d) a normal LVEDD in the parasternal long axis view as measured by electronic calipers built into the ultrasound device’s software.
Discussion
We compared images acquired from a pocket mobile and standard transthoracic echocardiogram and found that those obtained with the PME device, when obtained by the same ultrasound technician performing the TTE exam, were of comparable visualizability and accuracy when physicians blinded to TTE images interpreted the PME images. This was true for experienced echocardiographers and fellows with less than two months of training in echocardiographic interpretation. Of note, while the images of cardiac structures and left ventricular global and segmental function had good accuracy, the imaging of the inferior vena cava was suboptimal by PME when compared with TTE interrogation. Furthermore, the concordance between PME and TTE was highest among experienced cardiologists who read large number of TTE studies on a daily basis.
We encouraged study ultrasonographers to complete the PME exam in 5 minutes or less to simulate a rapid screening exam during a clinical encounter. While 41% of the PME exams in this study took longer than the intended five minutes to complete, the ultrasonographers were also charged with assessing our seven pre-specified parameters. In a real-world clinical setting, the PME will likely be used to perform a more focused study directed by a specific clinical question, and five minutes appears to be a reasonable approximation of the time such an exam might take. A recent group using the same device demonstrated the ability to perform a PME exam in an average of 3 minutes (9).
The good visualizability and accuracy of PME images is notable given that we built in an advantage for TTE by encouraging rapid PME image acquisition, by using echo contrast to better delineate endocardial borders in 8% of the TTE studies, and by not utilizing the PME color flow capabilities. We did not formally calculate sensitivity and specificity because of the relatively small number of patients and of abnormal echocardiographic parameters.
We deemed it essential for this study to have the same ultrasound technicians acquire all echocardiographic images (both PME and TTE) to maximize blinding and minimize confounding for the image comparison analysis. However ultrasound technicians are unlikely to be the primary users of PME in clinical practice, and the results of the present study using skilled ultrasonographers cannot be generalized to untrained clinicians. While our own limited experience suggests a clinician with even minor experience in echocardiographic image acquisition (i.e. first year cardiology fellow) can use the PME device to acquire quality images, a direct comparison of the accuracy of clinician and ultrasonographer-acquired images would be desirable before the technology is disseminated for routine use by practicing clinicians.
Of interest, the traditional stethoscope was invented by Laennec in 1816, but for more than 20 years there were strong protests from the medical community about incorporating use of the instrument into the routine physical examination (10) because of physicians’ unwillingness to learn heart sounds. Now, nearly 200 years later, PME provides an opportunity to quickly acquire non-invasive imaging of the major cardiac structures. The word stethoscope is derived from “steth” the Greek word for chest, and scope, “to look in.” Clearly the current “stethoscope” is actually a stethophone, as it does not afford any looking into the chest. While CT scans and magnetic resonance imaging looks into the chest, the lack of portability, exposure to radiation, and length of time required for such studies make them impractical substitutes for a pocket device that could be incorporated to the physical examination. Thus, it does appear that PME may ultimately fulfill, with more extensive proof of accuracy, the actual concept of a pocket stethoscope.
The role for the PME device in contemporary US health systems is unclear and will require consideration of economics. The device presently costs $7900 and there is no reimbursement category for performing or interpreting PME exams. Rapid PME studies are free, in contrast to TTE, which is associated with a technical and professional fee exceeding $1500 and an average of 40 minutes of ultrasonographer’s time per study. The potential of the PME device to reduce the need for unnecessary TTEs requires assessment in a large health system in which incentives are aligned between the hospital and professional staff. The financial costs of and savings from using the device might be balanced against the convenience for patients of obtaining echo images quickly during the physical exam, rather than having to make a subsequent appointment and return to the clinical setting represents a desirable feature.
In summary, PME images obtained rapidly by skilled ultrasonographers in this relatively small study provided excellent visualization of cardiac structural features and ejection fraction in the majority of patients. Further testing of the accuracy of the device, in untrained clinicians, and much larger patient cohorts with diverse cardiac abnormalities, will be required before wide dissemination can be recommended.
Supplementary Material
Acknowledgements
The corresponding author had full access to all data in the study and takes responsibility for the integrity and accuracy of the data. EJT conceived and designed the study and edited the manuscript; DSR and MRS analyzed the echoes blinded fashion and edited the manuscript; EL performed statistical analysis of the data; MJL and RLI contributed equally to this manuscript, drafted the manuscript, collected the data and analyzed the echoes.
Funding Sources
This work was funded by NIH UL1 RR025774 (Scripps Translational Science Institute, Clinical and Translational Science Award).
Footnotes
Publisher's Disclaimer: This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
Disclosures
There are no conflicts of interest to disclose.
Protocol: Not available.
Statistical Code: Available to interested readers by contacting, Dr. Eric J. Topol, 3344 North Torrey Pines Ct, La Jolla, CA 92037
Data: Not available.
References
- (1).Roelandt JR. Ultrasound stethoscopy: a renaissance of the physical examination? Heart. 2003;89:971–973. doi: 10.1136/heart.89.9.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Kimura BJ, Demaria AN. Empowering physical examination: the “laying on” of ultrasound. JACC Cardiovasc Imaging. 2008;1(5):602–604. doi: 10.1016/j.jcmg.2008.06.004. [DOI] [PubMed] [Google Scholar]
- (3).Kobal SL, Atar S, Siegel RJ. Hand-carried ultrasound improves bedside cardiovascular examination. Chest. 2004;126(3):693–701. doi: 10.1378/chest.126.3.693. [DOI] [PubMed] [Google Scholar]
- (4).Brennan JM, Blair JE, Goonewardena S, Ronan A, Shah D, Vasaiwala S, Kirkpatrick JN, Spencer KT. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J Am Soc Echocardiogr. 2007;20(7):857–861. doi: 10.1016/j.echo.2007.01.005. [DOI] [PubMed] [Google Scholar]
- (5).Popp RL. The physical exam of the future: echocardiography as part of the assessment. ACC Curr. J. Rev. 1998;7:79–81. [Google Scholar]
- (6).Armstrong WF. Feigenbaum’s Echocardiography. 7th ed. Lippincott Williams & Wilkins; Philadelphia: 2010. [Google Scholar]
- (7).Fleiss JL. Wiley Series in probability and mathematical statistics. second edition. Statistical methods for rates and proportions; pp. 212–236. Chapter 13. [Google Scholar]
- (8).Landis JR, Koch GG. The Measurement of Observer Agreement for Categorical Data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- (9).Cardim N, Golfin CF, Ferreira D, Aubele A, Toste J, Cobos MA, Carmelo V, Nunes I, Oliveira AG, Zamorano J. Usefulness of a New Miniature Echocardiographic System in Outpatient Cardiology Consultations as an Extension of Physical Examination. Journal of the American Society of Echocardiography. 2011;24(2):117–124. doi: 10.1016/j.echo.2010.09.017. [DOI] [PubMed] [Google Scholar]
- (10).Reiser SL. Technological Medicine. Cambridge University Press; New York: 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.