Abstract
Background
Various studies support the inclusion of cannabis withdrawal to the diagnosis of cannabis use disorders in the upcoming DSM-5. The aims of the current study were to (1) estimate the prevalence of DSM-5 cannabis withdrawal (Criterion B), (2) estimate the role of genetic and environmental influences on individual differences in cannabis withdrawal, and (3) determine the extent to which genetic and environmental influences on cannabis withdrawal overlap with those on DSM-IV defined abuse/dependence.
Methods
The sample included 2276 lifetime cannabis-using adult Australian twins. Cannabis withdrawal was defined in accordance with Criterion B of the proposed DSM-5 revisions. Cannabis abuse/dependence was defined as endorsing one or more DSM-IV criteria of abuse or three or more dependence criteria. The classical twin model was used to estimate the genetic and environmental influences on variation in cannabis withdrawal, as well as its covariation with abuse/dependence.
Results
Of all cannabis users 11.9% met criteria for cannabis withdrawal. Around 50% of between-individual variation in withdrawal could be attributed to additive genetic variation, and the rest of the variation was mostly due to non-shared environmental influences. Importantly, the genetic influences on cannabis withdrawal almost completely (99%) overlapped with those on abuse/dependence.
Conclusions
We showed that cannabis withdrawal symptoms exist among cannabis users, and that cannabis withdrawal is moderately heritable. Genetic influences on cannabis withdrawal are the same as those influencing abuse/dependence. These results add to the wealth of literature that recommends the addition of cannabis withdrawal to the diagnosis of DSM-5 cannabis use disorders.
Keywords: Cannabis, dependence, abuse, DSM-5, genetics, twins, withdrawal
Introduction
Cannabis is the most widely consumed illicit drug in the world (United Nations Office on Drugs and Crime, 2008) and its prolonged use is associated with various adverse effects including anxiety, paranoia, depression, tiredness, lack of motivation and low energy (Reilly et al. 1998). Furthermore, recurrent cannabis use appears to have negative psychosocial consequences, including poor work- and school performance (Lynskey & Hall, 2000; Hall, 2009), and physical impairments like decreased infection resistance, respiratory system problems and adverse reproductive effects (Hall & Solowij, 1998; Tashkin et al. 2002; Hall, 2009). Cannabis use is addictive and cessation attempts often result in withdrawal symptoms, such as anger, aggression, anxiety, decreased appetite, irritability, restlessness and sleep difficulty (Budney et al. 2008; Pruess et al. 2010). In turn, these withdrawal symptoms make successful long-term cessation difficult (Coffey et al. 2002; Budney & Hughes, 2006; Budney et al. 2008). Although the cannabis withdrawal syndrome is not recognized in the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV; APA, 2000), recent research has found consistent evidence for the existence of a reliable and valid withdrawal syndrome (Budney et al. 2004; Crowley, 2006; Vandrey et al. 2008; Agrawal et al. 2008; Hasin et al. 2008; Preuss et al. 2010). For instance, findings from Hasin et al. (2008) indicate that 57.7% of frequent cannabis users in a U.S. population cohort report one or more symptoms of cannabis withdrawal. The most prevalent symptoms were feeling weak/tired, hypersomnia, yawning, psychomotor retardation, anxiety, and depressed mood.
Numerous factor- and item-response analyses also indicate a high factor-loading for cannabis withdrawal on the underlying liability to cannabis use disorders (Lachenbucher et al. 2004; Lynskey & Agrawal, 2007; Compton et al. 2008; Gillespie et al. 2011) – as such, it is now well recognized that cannabis withdrawal is, phenotypically, an integral aspect of cannabis use disorders (abuse/dependence). These factor analytic findings, together with clinical observations, resulted in the recommended inclusion of the cannabis withdrawal syndrome in the fifth iteration of the Diagnostic and Statistical Manual (DSM-5, see www.dsm5.org). Accordingly, the proposed DSM-5 definition of cannabis withdrawal includes (A) cessation of prolonged (or less frequent but chronic) pattern of use; (B) emergence of 3 or more of 7 withdrawal symptoms within a week of cessation; (C) impairment or distress attributable to (B); and (D) the symptoms are not attributable to other medical or psychiatric conditions.
Despite growing agreement that cannabis withdrawal contributes to the liability to cannabis use disorders, little is known of its etiology, particularly from a genetic perspective. With regard to cannabis abuse and dependence, several lines of research suggest a genetic influence. A meta-analysis of twin studies by Verweij et al. (2010) estimated a heritability (h2) of 51% in males and 59% in females for problematic cannabis use. Recently, Gillespie et al. (2011) found that a general latent factor representing cannabis use disorders, including withdrawal criteria, was also substantially heritable (h2=54% for males and 53% for females). However, only a few studies have specifically examined the heritability of cannabis withdrawal symptomatology. Agrawal et al. (2008) demonstrated a strong association between cannabis withdrawal symptoms and parental drug and alcohol problems, after controlling for the intensity of cannabis use in the past 12 months. This finding suggests that cannabis withdrawal symptoms are partly influenced by familial (possibly including genetic) factors not overlapping with heritability of use and heavy use of cannabis. Furthermore, using a family-based sample, Ehlers et al. (2010) found a heritability of 26% for experiencing cannabis withdrawal symptoms. However, their sample was ascertained for alcoholism making the results less generalizable, they took a genomic variance components approach to compute heritability which can be less robust than the classical twin design, and their measure of cannabis withdrawal did not include all DSM-5 symptoms.
In addition to examining genetic variation in cannabis withdrawal, it is also of interest to investigate the extent to which this genetic variation is shared with that of cannabis abuse/dependence. A high overlap in the genetic variation in both variables would indicate common underlying biological mechanisms and would provide further support for the inclusion of withdrawal as a criterion for cannabis abuse/dependence in the DSM-5.
For cigarette smoking, Pergadia et al. (2006) found that ~45% of the variance in nicotine withdrawal was due to genetic influences, with both genetic effects specific to smoking withdrawal and genetic effects shared with smoking progression and quantity smoked. No twin study to date has specifically examined whether genetic factors underlying cannabis withdrawal are specific or shared with the genetic factors influencing cannabis abuse and dependence.
Using data from a large, community-based Australian twin sample, the present study aimed to (1) estimate the prevalence of the upcoming DSM-5 cannabis withdrawal (Criterion B) in lifetime cannabis users; (2) estimate the magnitude of genetic and environmental sources of variance in cannabis withdrawal; and (3) determine the extent to which the genetic and environmental variation in cannabis withdrawal overlaps with that of DSM-IV defined abuse/dependence.
Methods
Participants
The Australian Twin Registry (ATR) includes a total of 4131 twin pairs born between 1972 and 1979. The ATR approached 7850 twin individuals of which 3876 (49%) consented to participate, 1971 declined and the remainder gave passive refusals. Attempts to recruit these individuals to participate in the present study were made using a two tiered process, as required by the ATR’s ethics committee: first, the ATR contacted twins (by mail and subsequently by telephone) and asked if they were willing to have their name and contact details forwarded to the Queensland Institute of Medical Research (QIMR) for potential participation in an interview based study of substance use and mental health. Contact details of those consenting were then forwarded to QIMR who recontacted potential subjects to explain the purposes of the study and enrol them in the study. Further details regarding the recruitment procedure and study characteristics can be found in Lynskey et al. (2012).
Twins were interviewed between 2006 and 2009; a total of 3326 twin individuals completed the full interview. Of these, 2276 (69% of 3302; 24 missing) reported lifetime (ever) cannabis use. The final breakdown of the sample, including gender and zygosity is presented in Table 1. The mean age of the final sample was 31.9 (SD=2.5), ranging from 27–37 years.
Table 1.
Prevalence, twin pair probandwise concordance rates and tetrachoric twin pair correlations for cannabis withdrawal and abuse/dependence in 2276 cannabis users.
| Zygosity | Complete pairs |
Single twins |
Withdrawal | Abuse/dependence | ||||
|---|---|---|---|---|---|---|---|---|
| Prevalence % |
Probandwise concordance rates % |
Tetrachoric twin pair correlations ρ 95% CI |
Prevalence % |
Probandwise concordance rates % |
Tetrachoric twin pair correlations ρ 95% CI |
|||
| MZF | 208 | 191 | 9.72 | 54.17 | 0.76 | 15.82 | 61.33 | 0.76 |
| 0.55 – 0.89 | 0.59 – 0.88 | |||||||
| MZM | 98 | 161 | 14.29 | 40.00 | 0.52 | 31.09 | 64.62 | 0.68 |
| 0.13 – 0.79 | 0.43 – 0.85 | |||||||
| DZF | 149 | 174 | 8.90 | 35.71 | 0.56 | 19.70 | 43.33 | 0.49 |
| 0.19 – 0.81 | 0.21 – 0.71 | |||||||
| DZM | 77 | 134 | 15.97 | 36.36 | 0.48 | 31.60 | 48.98 | 0.40 |
| 0.01 – 0.80 | 0.04 – 0.69 | |||||||
| DOS | 132 | M:108 | 20.00 | 21.28 | 0.09 | 38.75 | 26.83 | 0.03 |
| F: 180 | 7.69 | −0.27 – 0.42 | 17.31 | −0.27 – 0.34 | ||||
MZF Monozygotic females. MZM Monozygotic males. DZF Dizygotic females. DZM Dizygotic males. DOS Dizygotic opposite-sex.
Measures
The computer-assisted telephone interview was based on a modified version of the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA-OZ; Bucholz et al. 1994; Heath et al. 1997). The revised version of the SSAGA obtained more diagnostic information on cannabis and other illicit drug abuse and dependence, and questions assessing cannabis use disorders were supplemented with detailed questions, based on those described by Budney et al. (2004), to assess potential symptoms of withdrawal.
Cannabis withdrawal
The cannabis withdrawal items used in this study are in accordance with the proposed symptoms included in Criterion B of the upcoming DSM-5 cannabis withdrawal syndrome (see www.dsm5.org/ProposedRevision/Pages/proposed-revision.aspx?rid=430). All symptoms are shown in Table 2. Items assessing withdrawal were asked of those who reported a lifetime history of using cannabis 11 or more times and monthly use (when using the most) and had attempted cessation. For each item, participants were asked to indicate on a 4-point scale, ranging from ‘not at all’, ‘mildly’, ‘moderately’ to ‘severely’, how much they experienced that specific symptom after cutting down or going without using marijuana. An item was scored positive if it was experienced at least mildly. In accordance to the DSM-5 guideline, to meet criterion B of cannabis withdrawal, participants were required to endorse 3 symptoms or more. All other lifetime users, including those who had used cannabis less than 11 times, did not use monthly, did not attempt to quit or endorsed 1–2 symptoms were coded negatively.
Table 2.
Prevalence of proposed DSM-5 withdrawal symptoms, and abuse/dependence in 2276 lifetime cannabis users.
| After you cut down or went without marijuana, how much did you experience: | |||
|---|---|---|---|
| Symptoms | Prevalence (%) | ||
| - Sub-symptoms | Males (N=885) |
Females (N=1391) |
|
| 1. Irritability, anger or aggression | 16.8 | 8.3 | |
| 1.1 Irritability | 15.9 | 7.9 | |
| 1.2 Increased anger | 10.7 | 5.1 | |
| 1.3 Increased aggression | 9.6 | 4.5 | |
| 2. Nervousness or anxiety | 10.5 | 6.7 | |
| 3. Sleep difficulty | 15.9 | 7.5 | |
| 4. Decreased appetite | 9.5 | 5.8 | |
| 5. Restlessness | 15.6 | 7.7 | |
| 6. Depressed mood | 14.0 | 7.8 | |
| 7. Physical symptoms or discomfort | 11.2 | 5.6 | |
| 7.1 Headaches | 5.1 | 3.9 | |
| 7.2 Shakiness | 4.1 | 2.4 | |
| 7.3 Stomach pains | 2.0 | 0.9 | |
| 7.4 Sweating | 8.5 | 3.1 | |
| Proposed DSM-5 Withdrawal Criterion B (≥3 symptoms) | 16.4 | 9.0 | |
| Abuse (≥1 DSM-IV abuse symptoms) | 31.9 | 16.3 | |
| Dependence (≥3 DSM-IV dependence symptoms) | 19.4 | 9.5 | |
| Abuse/dependence (≥1 DSM-IV abuse symptoms and/or ≥3 | 33.3 | 17.5 | |
| DSM_IV dependence symptoms) | |||
Note: Symptom 1 and 7 are subdivided in multiple sub-symptoms, in which one of these sub symptoms was sufficient to be diagnosed as having that particular criterion.
Cannabis abuse/dependence
We used the DSM-IV abuse and dependence symptoms to obtain a measure of cannabis abuse/dependence. Questions to assess abuse/dependence were also only asked on those using 11 or more times and at least monthly during the heaviest period of use. For cannabis abuse the criteria were: (1) recurrent use resulting in a failure to fulfill major obligations at work, school, or home, (2) recurrent use in situations which are physically hazardous, (3) continued use despite significant social or interpersonal problems caused by the substance use, and (4) continued use despite legal problems. The criteria for cannabis dependence were: (1) an increase in use to achieve an effect (tolerance), (2) using more frequently or for longer periods than intended, (3) persistent desire or unsuccessful efforts to cut down or control substance use, (4) a great deal of time is spent in activities necessary to obtain the substance, use the substance, or recover from its effects, (5) important social, occupational, or recreational activities are given up or reduced because of substance use, and (6) the substance use is continued despite knowledge of having a persistent physical or psychological problem that is likely to have been caused or exacerbated by the substance. Each item could be answered with yes or no, and participants were considered to have cannabis abuse/dependence if they endorsed at least one or more of four DSM-IV abuse or three or more of six DSM-IV dependence criteria (not including withdrawal, and regardless of whether the criteria clustered in a 12 month period or not [American Psychiatric Association, 2000]).
Statistical analyses
Prevalences, sex differences, and probandwise concordance rates of cannabis withdrawal and DSM-IV abuse/dependence were calculated in SAS (version 9.1). Tetrachoric twin pair correlations and corresponding confidence intervals were estimated in the statistical software package Mx by maximum likelihood (Neale et al. 1999).
Next, we used the classical twin model to partition the total variance in cannabis withdrawal into additive genetic (A), common (or shared) environmental (C), and residual/non-shared environmental (E) variance. A denotes the variance resulting from the sum of allelic effects across all segregating genes. C refers to environmental influences shared by family members and may include shared home environment, parental style and uterine environment. E includes environmental factors not shared by twin pairs (e.g. idiosyncratic experiences), stochastic biological effects, and also measurement error. These variance components can be estimated using twin data because identical (monozygotic, MZ) twins share all their genes, while nonidentical (dizygotic, DZ) twins share on average half their segregating genes. A, C, and E influences predict different patterns of MZ and DZ twin pair correlations, and structural equation modeling is used to determine the combination of influences that best matches the observed data.
Note that nonadditive genetic variance (D; including dominant genetic effects and epistasis) can be modeled in place of C, but based on the pattern of twin pair correlations (the DZ twin pair correlations for cannabis withdrawal were greater than half the MZ twin pair correlations), we modeled A, C, and E.
Using the cross-twin cross-trait correlations, we also partitioned the covariance between cannabis withdrawal and abuse/dependence into its additive genetic, shared environmental and nonshared environmental sources in the same way as we did for the variance in cannabis withdrawal. We calculated the genetic correlation, a measure of the overlap in the genetic variation underlying cannabis withdrawal and abuse/dependence. Further, we partitioned genetic and environmental influences on cannabis withdrawal into those that were shared with abuse/dependence and those that were unique for cannabis withdrawal.
Structural equation modelling of twin data was performed in the flexible matrix algebra program Mx (Neale et al., 2006), which employs maximum likelihood modelling procedures to determine the combination of A, C, and E that best explains the observed data. We fitted univariate and bivariate models (for more information about this model see Rijswijk et al., 2002) to the raw dichotomous data, where it is assumed that a normally distributed continuum of liability underlies the dichotomous observed categories. Age and sex effects were accounted for in the model by including them as covariates to adjust the thresholds.
The goodness-of-fit of a model to the observed data is summarized by a statistic distributed as chi-square (χ2). By testing the change in model fit (Δχ2) against the change in degrees of freedom (Δdf), we can test whether constraining parameters to zero or constraining them to be equal, significantly worsens the model fit. In this way we can test hypotheses regarding those parameters. Further details of the classical twin design can be found elsewhere (Neale & Cardon, 1992; Posthuma et al. 2003).
For ease of interpretation, the models were transformed from Cholesky forms into ‘correlated factors’ models as suggested by Loehlin (1996).
Results
Prevalence of lifetime cannabis use, withdrawal, and abuse/dependence
Of the entire sample, 68.9% (N=2276) reported lifetime cannabis use with a significantly higher prevalence for males (76.5%) than females (64.9%) (OR = 1.76, 95% confidence interval [CI] 1.50–2.07). This prevalence is comparable to the prevalence obtained from a large household survey in Australia, which showed that 55% of Australians aged between 20 and 39 years have used cannabis during their lives (McLaren & Mattick, 2007). The slightly higher estimate obtained in our sample may be the result of the somewhat younger age group.
Of all lifetime users, 21.9% reported a lifetime history of using cannabis 100 or more times, 14.2% reported using cannabis every day when using it the most, and 20% reported cannabis use in the past 12 months prior to the interview. Male and female prevalences of cannabis withdrawal, the individual withdrawal symptoms, and DSM-IV abuse/dependence are presented in Table 2. Of all lifetime cannabis users, 23.6% (N=538) reported cannabis abuse or dependence, and 11.9% (N=270) met the criteria for cannabis withdrawal. Evidently, cannabis withdrawal was more common in those who endorsed more abuse/dependence symptoms. Of all individuals reporting cannabis abuse/dependence, 48.0% also experienced cannabis withdrawal, while 95.6% of participants with cannabis withdrawal also reported abuse/dependence. Of those reporting cannabis withdrawal (criterion B), 42.6% reported social impairment attributable to it and 38.2% reported using cannabis or a related medication or drug for withdrawal relief.
Of all lifetime users, significantly more males (16.4%) than females (9.0%) reported cannabis withdrawal (OR = 1.99, 95% CI 1.54–2.56), and met DSM-IV criteria for cannabis abuse/dependence (males=33.3%, females=17.5%; OR = 2.36, 95%; CI 1.94–2.88).
Genetic analyses
Preliminary analyses
For each zygosity group, Table 1 summarizes the prevalence, probandwise concordance rates and tetrachoric twin pair correlations for withdrawal and abuse/dependence in lifetime cannabis users. Before modeling the variance components, we tested the effects of age, sex, and zygosity on the prevalences (thresholds) of cannabis withdrawal and abuse/dependence (α=0.05). We found a significant age effect on the prevalence of withdrawal (Δχ21=11.26, P<0001), indicating that younger participants were more likely to report cannabis withdrawal than older participants. No such age effect was found for abuse/dependence (Δχ21=2.36, P=0.12).
Further, as mentioned above, we found a significant sex effect on the prevalence of cannabis withdrawal (Δχ21=29.39, P<0.001), as well as abuse/dependence (Δχ21=67.62, P<0.001), such that males exhibited more withdrawal and abuse/dependence symptoms than females. Effects of sex and age were accounted for in subsequent modeling.
For both sexes, MZ and DZ twins did not differ significantly with respect to the prevalence of withdrawal or cannabis abuse/dependence. Also, we found no sex differences between male and female MZ nor male and female same sex DZ twin pair correlations for both variables, suggesting genetic and environmental effects of similar magnitude in males and females. However, the twin pair correlation for opposite-sex DZ twin pairs for abuse/dependence was significantly lower than that for the same-sex DZ pairs (Δχ21=4.94, P=0.03), suggesting that there may be qualitative sex differences in sources of familial aggregation in abuse/dependence. In a univariate twin model, though, we were unable to detect significant sex differences in the source of genetic or shared environmental influences, so we do not deal with this further.
Table 1 shows that, for both sexes, the probandwise concordance rate and tetrachoric twin-pair correlations for withdrawal and cannabis abuse/dependence were higher in MZ versus DZ twins, suggesting a role for genetic influences on both phenotypes. However, the twin pair correlations were only significantly higher for MZ females than DZ same sex females for abuse/dependence (Δχ21=4.13, P=0.04), while for withdrawal for females and, for both variables, for males the differences in twin pair correlations did not reach significance (all P>0.10).
The genetic and environmental influences on cannabis withdrawal
Results of the univariate ACE models for cannabis withdrawal are presented in Table 3. Given that the DOS twin pair correlation is lower (although not significantly) than the DZ same sex correlations we first fitted a general sex-limitation model, which allows for qualitative and quantitative differences in the sources of variation in cannabis withdrawal between sexes (see Neale and Cardon, 1992). To model qualitative differences in genetic influences between males and females, the genetic correlation for DOS twins was freely estimated in the model instead of fixed at 0.5 as it is for same sex DZ twin pairs. In the same way, to model qualitative differences in shared environmental influences between sexes, the C correlation for DOS twins was freely estimated in the model instead of fixed at 1.0. Under this latter model (which fitted slightly better than the genetic sex-limited model; difference in AIC=0.30), the A, C, and E estimates for males were 9%, 44%, and 47%, respectively, and for females 38%, 36%, and 26%, respectively. However, fixing the genetic or shared environmental correlation at 0.5 or 1 respectively (common effects sex-limitation model) did not lead to a significant deterioration of model fit, indicating no evidence for qualitative sex differences. Subsequently, we equated the A,C, and E estimates between the sexes (general ACE model), and results show no significant deterioration of model fit, indicating there were no significant magnitude differences in effects of A, C, and E on variance in cannabis withdrawal between males and females. Based on the general ACE model, estimates of the influence of A, C, and E (95% CIs) on cannabis withdrawal are 55% (0.1–81%), 12% (0–57%) and 33% (19–51%) respectively, where the A influences were just significant (p<0.05), but the C influences were not significantly different from zero (p=0.61).
Table 3.
Goodness-of-fit statistics for univariate models of cannabis withdrawal.
| Model | Comparison model |
Δχ2 | Δdf | p-value | |
|---|---|---|---|---|---|
| 1 | General sex limitation model (C correlation for DOS twins estimated in the model) | ||||
| 2 | Common effects sex-limitation model | 1 | 0.79 | 1 | 0.37 |
| 3 | General ACE model | 2 | 2.46 | 2 | 0.29 |
| 4 | AE model | 3 | 0.26 | 1 | 0.61 |
| 5 | CE model | 3 | 3.85 | 1 | 0. 0497 |
A= additive genetic influences, C = shared environmental influences, E = non-shared environmental influences
Phenotypic and genetic correlation between cannabis withdrawal and abuse/dependence
A series of bivariate models were tested in order to partition the covariation between cannabis abuse/dependence and cannabis withdrawal into that due to A, C, and E. Table 4 summarizes the model fitting steps. Based on the results from the univariate models where we did not find evidence for sex-limitation, we fitted a general ACE model (model 1) with the A, C, and E parameters equated between sexes. In this model, the phenotypic correlation between withdrawal and abuse/dependence was estimated to be very high (r=0.92, p<0.001). Accordingly, the genetic correlation between the traits was also very high and significant (r=0.99, p<0.001), and so was the unshared environmental correlation (r=0.84, p<0.001). The shared environmental correlation was not significant (r=1.0, p=0.55), due to the weak influence of the shared environment on both traits. Figure 1 shows the parameter estimates in the bivariate model (transformed into a correlated factors model (Loehlin 1996)), including the proportions of variance in withdrawal and abuse/dependence accounted for by genetic effects (heritability; h2) and shared and unshared environmental influences, along with the genetic, shared environmental, and unshared environmental correlations between the two traits.
Table 4.
Goodness-of-fit statistics for bivariate models of cannabis withdrawal and abuse/dependence.
| Model | Comparison model |
Δχ2 | Δdf | p-value | |
|---|---|---|---|---|---|
| 1 | General ACE model; A, C, and E parameters equated between sexes | ||||
| 2 | Drop the phenotypic correlation (drop all cross-paths) | 1 | 696.23 | 3 | <0.001 |
| 3 | Drop genetic cross | 1 | 11.64 | 1 | <0.001 |
| 4 | Drop shared environmental cross | 1 | 0.36 | 1 | 0.55 |
| 5 | Drop unique | 1 | 57.04 | 1 | <0.001 |
A= additive genetic influences, C = shared environmental influences, E = non-shared environmental influences
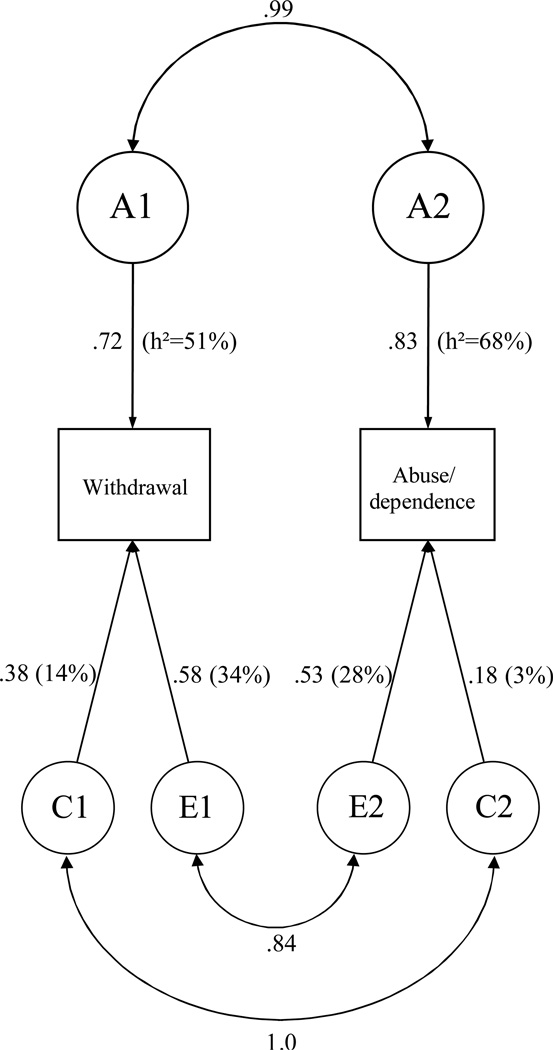
Figure 1.
Correlated factor model. Graphical presentation of the parameter estimates and proportions of variance in cannabis withdrawal and abuse/dependence accounted for by genes (heritability; h2), shared and unshared environmental influences. The double-headed arrows represent the genetic, shared and unshared environmental correlations.
Results of the model show that individual differences in both cannabis withdrawal and abuse /dependence are substantially attributable to additive genetic factors (51% for withdrawal, and 68% for abuse/dependence), and the remaining variance was mainly due to unshared environmental factors. Variance in both variables overlaps greatly, due to very high genetic and environmental correlations between both variables. Of the total phenotypic variance between withdrawal and abuse/dependence, 64% is due to overlapping genetic, 8% to shared environmental, and 28% to unshared environmental influences.
Discussion
This study is amongst the first to use a genetically informative sample to look at the proposed inclusion of cannabis withdrawal in the upcoming DSM-5. We demonstrated that the proposed DSM-5 withdrawal criterion B is commonly experienced by lifetime cannabis users, and that withdrawal is moderately heritable. Furthermore, we found a very high correlation between cannabis withdrawal and abuse/dependence due to near-complete sharing of genetic influences and, to a lesser extent, unshared environmental influences on the two traits. This finding underscores the general cohesiveness of cannabis withdrawal with existing DSM-IV and upcoming DSM-5 definitions of cannabis use disorders and shows that a general genetic vulnerability to cannabis use disorder criteria, including withdrawal, may exist. By providing evidence for the shared genetic background with cannabis use disorders according to DSM-IV our findings add to the wealth of psychometric literature that recommends the addition of cannabis withdrawal to the definition of DSM-5 cannabis use disorder.
There has been an ongoing debate as to whether the cannabis withdrawal syndrome exists, which is one of the reasons that cannabis withdrawal was not part of the diagnostic criteria for cannabis dependence in the DSM-IV. We show that of all lifetime cannabis users, 11.9% experienced three or more symptoms of cannabis withdrawal, and therefore met criterion B of DSM-5 cannabis withdrawal. Irritability, sleep difficulty, restlessness, depressed mood and nervousness were the most common withdrawal symptoms (see Table 2), consistent with findings from previous studies investigating cannabis withdrawal (Budney & Hughes, 2006; Vandrey et al. 2008; Pruess et al. 2010; Ehlers et al. 2010; Gillespie et al. 2011). Males were more likely to have exhibited cannabis withdrawal and abuse/dependence than females. Our study and previous research findings (e.g. Hasin et al. 2008; Budney et al. 2004; Agrawal et al 2008) support the existence of cannabis withdrawal (symptoms) in cannabis users, providing support for its addition to the DSM-5.
Our findings demonstrated that additive genetic factors explained approximately half the variance in DSM-5 cannabis withdrawal and the remaining variance was mainly due to unshared environmental factors. The shared environmental influences on cannabis withdrawal were not significant, partly due to a lack of power (see below). The estimate for genetic variance was slightly higher for abuse/dependence, while the C estimate was somewhat lower. Our estimates are in accordance with the results from a meta-analysis of twin studies by Verweij et al. (2010), which showed that A, C, and E estimates of problematic cannabis use are, respectively, 51%, 20% and 29% for males and 59%, 15% and 26% for females. Large variation across studies in A, C, and E estimates of cannabis abuse and dependence exists (Verweij et al. 2010), due to the different (aged) populations from which research samples are gathered and different measures used. A low or absent shared environmental influence, as estimated in the present study, has also been found in several previous studies (Kendler & Prescott, 1998; van der Bree et al. 1998; Kendler et al. 2006; Agrawal et al. 2007). Furthermore, multi-stage modeling has revealed that the influence of shared environmental influences on the symptoms of cannabis abuse was indirect and mediated entirely by cannabis initiation (Gillespie et al. 2009).
We found a very high correlation (r=0.92) between cannabis withdrawal and DSM-IV abuse/dependence; approximately 96% of participants that met criteria for cannabis withdrawal also exhibited DSM-IV abuse/dependence. This is in line with extensive research findings supporting a single cannabis use disorder (CUD) latent factor which includes symptoms of withdrawal, indicating withdrawal to be part of the CUD construct (Lachenbucher et al. 2004; Lynskey & Agrawal, 2007; Compton et al. 2008; Gillespie et al. 2011). Our finding that a substantial majority of those endorsing cannabis withdrawal already met criteria for cannabis abuse/dependence supports the observation that withdrawal is common among individuals with a cannabis use disorder. Therefore it is unlikely that its addition to the repertoire of diagnostic criteria will substantially elevate rates of CUD diagnoses.
The near-complete overlap between the genetic variation in withdrawal and that in abuse/dependence supports a common biological basis for the various cannabis use disorder criteria. This finding should also be reassuring for those genetically informed studies that do not assess cannabis withdrawal. Our analyses indicate that an overwhelming majority of the genetic influences on cannabis withdrawal are shared with those influencing current DSM-IV assessments of cannabis abuse/dependence.
There are a few important methodological limitations to be considered for this study. Firstly, there are some shortcomings in relation to the data used for this study. We were unable to estimate the heritability of the proposed DSM-5 definition of cannabis use disorders. This was because the twin study had completed data collection before the DSM-5 proposal was announced and, consequently, we did not collect data on the craving criterion. Furthermore, withdrawal was measured using the proposed symptoms for cannabis withdrawal Criterion B of the DSM-5 but, in the interest of sample size, heavy or prolonged use (criterion A) and impairment (criterion C) were not used. For cannabis abuse/dependence, we did not require that the criteria cluster within the same 12 month period – however, only 19 individuals did not satisfy the clustering requirement. This potentially makes our findings less applicable for clinicians – for example, prevalence rates of the full diagnosis of withdrawal may be lower in other populations. However, this would not have biased the variance component estimates in any particular direction. Additionally, our measurements of cannabis withdrawal, abuse, and dependence symptoms were limited by the potential for bias and inaccuracy in retrospective self-reports. Finally, the sample was predominantly Caucasian, aged 27–37 which may limit, to some extent, the generalizability of the results.
Most importantly, our study has a lack of statistical power. Although the sample size is fairly large, both variables had to be analyzed as ordinal data thus limiting the range of potential genetic analyses. Neale et al. (1994) showed that a threshold trait requires at least three times the sample size needed for the same power using an equivalent continuous trait. In addition, as this is a general population sample, rates of cannabis withdrawal and abuse/dependence were relatively low, further limiting our statistical power.
This lack of power may have precluded the detection of qualitative or quantitative sex differences in the variance components for withdrawal and abuse/dependence. Hence, while the very low DOS twin pair correlations for both variables point to possible differences in the sources of variance between sexes, the sample was not adequately powered to detect sex-limitation in the source of genetic or shared environmental influences. By equating the same sex DZ twins with the OS twins we may have inflated the heritability estimates and underestimated the role of shared environment. Furthermore, for males but not for females, the same-sex twin pair correlations point to a substantial influence of C factors on individual differences in withdrawal, but our sample size did not enable us to differentiate between male and females ACE parameters, resulting in a lower C and larger A estimate for withdrawal than expected based on the same sex twin pair correlations.
Despite these limitations, this study yielded several important findings supporting the proposed changes in the DSM-5 to include withdrawal criteria to cannabis use disorders criteria. We found that cannabis withdrawal symptoms exist and are frequently reported by cannabis users. Also, there is a high correlation between cannabis withdrawal and abuse/dependence. Individual differences in withdrawal symptoms are partly due to genetic influences, which are the same as those influencing abuse/dependence.
Acknowledgments
Declaration of interest: AA receives funding from ABMRF/The Foundation for Alcohol Research
Funding: This research was funded by National Institute on Drug Abuse (NIDA) grant: DA18267 (ML) and facilitated through access to the Australian Twin Registry, a national resource supported by an Enabling Grant (ID 628911) from the National Health & Medical Research Council. We thank Anjali Henders, Dixie Statham, Richard Parker, Soad Hancock, Judith Moir, Sally Rodda, Pieta-Maree Shertock, Heather Park, Jill Wood, Pam Barton, Fran Husband and Adele Somerville for data collection and data management. Also supported by DA18660 (MTL), DA23668 and K02DA32573 (AA). Work on this paper was further supported by the Netherlands Organization for Health Research and Development, ZonMW 31160212 (NON, HEC).
References
- Agrawal A, Pergadia ML, Lynskey MT. Is there evidence for symptoms of cannabis withdrawal in the national epidemiologic survey of alcohol and related conditions? American Journal on Addictions. 2008;17:199–208. doi: 10.1080/10550490802019519. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT, Bucholz KK, Martin NG, Madden PAF, Heath AC. Contrasting models of genetic co-morbidity for cannabis and other illicit drugs in adult Australian twins. Psychological Medicine. 2007;37:49–60. doi: 10.1017/S0033291706009287. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4th edn. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report of the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR. The cannabis withdrawal syndrome. Current Opinion in Psychiatry. 2006;19:233–238. doi: 10.1097/01.yco.0000218592.00689.e5. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. American Journal of Psychiatry. 2004;161:1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: Severity and contribution to relapse. Journal of Substance Abuse Treatment. 2008;35:362–368. doi: 10.1016/j.jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey C, Carlin BB, Degenhardt L, Lynskey MT, Danci L, Patton GC. Cannabis dependence in young adults: an Australian population study. Addiction. 2002;97:187–194. doi: 10.1046/j.1360-0443.2002.00029.x. [DOI] [PubMed] [Google Scholar]
- Compton WM, Saha TD, Conway KP, Grant BF. The role of cannabis use within a dimensional approach to cannabis use disorders. Drug and Alcohol Dependence. 2009;100(3):221–227. doi: 10.1016/j.drugalcdep.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley TJ. Adolescents and substance-related disorders: research agenda to guide decisions on Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) Addiction. 2006;101(suppl. 1):115–124. doi: 10.1111/j.1360-0443.2006.01594.x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Gizer IR, Vieten C, Gilder DA, Stouffer GM, Lau P, Wilhelmsen KC. Cannabis dependence in the San Francisco Family Study: Age of onset of use, DSM-IV symptoms, withdrawal, and heritability. Addictive Behaviors. 2010;35:102–110. doi: 10.1016/j.addbeh.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie NA, Kendler KS, Neale MC. Psychometric modeling of cannabis initiation and use and the symptoms of cannabis abuse, dependence and withdrawal in a sample of male and female twins. Drug and Alcohol Dependence. 2011;118:166–172. doi: 10.1016/j.drugalcdep.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie NA, Neale MC, Kendler KS. Pathways to cannabis abuse: a multi-stage model from cannabis availability, cannabis initiation and progression to abuse. Addiction. 2009;104:430–438. doi: 10.1111/j.1360-0443.2008.02456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. The adverse health effects of cannabis use: What are they, and what are their implications for policy? International Journal of drug policy. 2009;20:458–466. doi: 10.1016/j.drugpo.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Hall W, Solowij N. Adverse effects of cannabis. Lancet. 1998;352:1611–1616. doi: 10.1016/S0140-6736(98)05021-1. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Keyes KM, Alderson D, Wang S, Aharonovich E, Grant BE. Cannabis withdrawal in the United States: Results from NESARC. Journal of Clinical Psychiatry. 2008;69:1354–1363. doi: 10.4088/jcp.v69n0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PAF, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychological Medicine. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Tambs K, Reichborn-Kjennerud T. Illicit psychoactive substance use, abuse and dependence in a population-based sample of Norwegian twins. Psychological Medicine. 2006;36:955–962. doi: 10.1017/S0033291706007720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Cannabis use, abuse, and dependence in a population-based sample of female twins. American Journal of Psychiatry. 1998;155:1016–1022. doi: 10.1176/ajp.155.8.1016. [DOI] [PubMed] [Google Scholar]
- Lachenbucher JW, Labouvie E, Martin CS, Sanjuan PM, Bavly L, Kirisci L, Chung T. An application of item response theory analysis to alcohol, cannabis, and cocaine criteria in DSM-IV. Journal of Abnormal Psychology. 2004;113:72–80. doi: 10.1037/0021-843X.113.1.72. [DOI] [PubMed] [Google Scholar]
- Loehlin JC. The Cholesky approach: A cautionary note. Behaviour Genetics. 1996;26:65–69. [Google Scholar]
- Lynskey MT, Agrawal A, Henders A, Nelson EC, Madden PAF, Martin NG. An Australian twin study of cannabis and other illicit drug use and misuse, and other psychopathology. Twin Research and Human Genetics. 2012;15:631–641. doi: 10.1017/thg.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey MT, Agrawal A. Psychometric properties of DSM assessments of illicit drug abuse and dependence: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Psychological Medicine. 2007;37:1345–1355. doi: 10.1017/S0033291707000396. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Hall W. The effects of adolescent cannabis use on educational attainment: A review. Addiction. 2000;95:1621–1630. doi: 10.1046/j.1360-0443.2000.951116213.x. [DOI] [PubMed] [Google Scholar]
- Pergadia ML, Heath AC, Martin NG, Madden PAF. Genetic analyses of DSM-IV nicotine withdrawal in adult twins. Psychological Medicine. 2006;36:963–972. doi: 10.1017/S0033291706007495. [DOI] [PubMed] [Google Scholar]
- Preuss UW, Watzke AB, Zimmermann J, Wong JWM, Schmidt CO. Cannabis withdrawal severity and short-term course among cannabis-dependent adolescent and young adult inpatients. Drug and Alcohol Dependence. 2010;106:133–141. doi: 10.1016/j.drugalcdep.2009.08.008. [DOI] [PubMed] [Google Scholar]
- McLaren J, Mattick RP. National Drug Strategy Australia. Canberra: Australian Government Department of Health and Ageing; 2007. Cannabis in Australia; Use, supply, harms, and responses. Monograph series no. 57. doi: http://www.health.gov.au/internet/drugstrategy/publishing.nsf/Content/4FDE76ABD582C84ECA257314000BB6EB/$File/mono-57.pdf. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 7th ed. Richmond, VA: VCU Department of Psychiatry, Medical College of Virginia; 2006. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Boston: Kluwer; 1992. [Google Scholar]
- Neale MC, Eaves LJ, Kendler KS. The power of the classical twin study to resolve variation in threshold traits. Behaviour Genetics. 1994;24:239–258. doi: 10.1007/BF01067191. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Beem AL, de Geus EJC, van Baal GCM, von Hjelmborg JB, Lachine I, Boomsma DI. Theory and practice in quantitative genetics. Twin Research. 2003;6:361–376. doi: 10.1375/136905203770326367. [DOI] [PubMed] [Google Scholar]
- Reilly D, Didcott P, Swift W, Hall W. Long term cannabis use: characteristics of users in an Australian rural area. Addiction. 1998;93:837–846. doi: 10.1046/j.1360-0443.1998.9368375.x. [DOI] [PubMed] [Google Scholar]
- Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Briefings in Bioinformatics. 2002;3:119–133. doi: 10.1093/bib/3.2.119. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Baldwin GC, Sarafian T, Dubinett S, Roth MD. Respiratory and immunologic consequences of marijuana smoking. Journal of clinical pharmacology. 2002;42:71s–81s. doi: 10.1002/j.1552-4604.2002.tb06006.x. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC) [Accessed 28 November 2010];World Drug Report. 2008 2008. ( http://www.unodc.org/unodc/en/data-and-analysis/WDR-2008.html). [Google Scholar]
- Van den Bree MBM, Johnson EO, Neale MC, Pickens RW. Genetic and environmental influences on drug use and abuse/dependence in male and female twins. Drug and Alcohol Dependence. 1998;52:231–241. doi: 10.1016/s0376-8716(98)00101-x. [DOI] [PubMed] [Google Scholar]
- Vandrey RG, Budney AJ, Hughes JR, Liguori A. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug and Alcohol Dependence. 2008;92:48–54. doi: 10.1016/j.drugalcdep.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij KJH, Zietsch BP, Lynskey MT, Medland SE, Neale MC, Martin NG, Boomsma DI, Vink JM. Genetic and environmental influences on cannabis use initiation and problematic use: a meta-analysis of twin studies. Addiction. 2010;105:417–430. doi: 10.1111/j.1360-0443.2009.02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]