Abstract
Objective
Angiotensin II has a critical role in the regulation of blood pressure and cell growth and excess activity of the peptide is implicated in the pathogenesis of salt-induced cardiovascular injury. On the other hand, the role of counteracting angiotensin-(1–7) in cardiac structural and functional responses to high salt diet has not been elucidated. Therefore, the present study examined the changes in cardiac angiotensin-(1–7), its forming enzyme angiotensin converting enzyme 2 (ACE2) and receptor mas in response to a high salt diet in spontaneously hypertensive rats (SHR).
Methods
Eight-week-old male spontaneously hypertensive rats (SHR) were given an 8% salt diet for 5weeks (n = 8). Age- and gender-matched controls received standard chow (n = 6).
Results
Salt excess increased arterial pressure (p< 0.05) and plasma renin and angiotensin II concentrations (p<0.05). Salt-induced left ventricular remodeling and diastolic dysfunction were associated with diminished levels of angiotensin-(1–7) in the heart (p<0.05) and no changes in cardiac angiotensin II levels. Exposure to high salt intake decreased cardiac ACE2 mRNA and protein level (p< 0.05). There was no difference in the protein levels of angiotensin II type 1 and mas receptors between the two experimental groups.
Conclusion
The adverse cardiac effects of excessive salt intake may result not only from the undesirable action of angiotensin II but may also be a consequence of diminished protective effects of the angiotensin-(1–7).
Keywords: ACE2, angiotensin II, angiotensin-(1–7), hypertension, salt
Introduction
Angiotensin II (Ang II) has a critical role in the regulation of blood pressure and cellular growth and excess activity of the peptide is implicated in the pathogenesis of salt-induced cardiovascular and renal injury [Ahn et al. 2004; Inada et al. 2001; Takeda et al. 2001; Kreutz et al. 1995; Stier, Jr. et al. 1991]. However, the role of counteracting angiotensin-(1–7) (Ang-[1–7]) in cardiac structural and functional responses to high salt diet has not been elucidated. Ang-(1–7) exerts vasodilatory, antiproliferative, and antifibrotic action through activation of the mas receptor (mas) [Ferrario et al. 2005b; Tallant et al. 2005, 2003] and the reduced counterbalancing effects of the heptapeptide may be involved in salt-related cardiovascular injury. Various Ang-(1–7)-forming enzymes are part of the biochemical cascade of the renin angiotensin system, most of them using angiotensin I (Ang I) as a substrate [Ferrario et al. 2005b; Chappell et al. 1994; Yamamoto et al. 1992]. However, a recent study from our laboratory demonstrated a predominant role of the angiotensin converting enzyme (ACE) homologue, ACE2, in the cardiac production of Ang-(1–7) in hypertensive animals [Trask et al. 2007]. Therefore, the present study examined the changes in cardiac ACE2/Ang-(1–7)/mas axis in response to high salt diet in spontaneously hypertensive rats (SHR).
Method
Experimental protocol
Male SHR (Harlan Laboratories, Indianapolis, IN), were maintained in a temperature and humidity-controlled room with a 12 h light/dark cycle. All rats were handled in accordance with National Institute of Health guidelines; our Institutional Animal Care and Use Committee approved the study in advance. The rats were randomly divided into two groups to receive either a control (1% NaCl; n = 6) or a high salt (8% NaCl; n = 8) diet for the ensuing 5 weeks.
Blood pressure and urinary measurements
Systolic blood pressure was assessed by tail-cuff plethysmography (Hatteras Instruments, Cary, NC) during a baseline period followed by weekly measurements in rats trained beforehand. Twenty-four-hour urine output and protein (Bio-Rad protein assay) and sodium excretion (Nova Biomedical automated electrolyte analyzer, Waltham, MA) were assessed at the end of the study period.
Echocardiography
Transthoracic echocardiography was performed in rats anesthetized with a combination of ketamine/xylazine (50 mg/kg/5 mg/kg; i.m.) using a commercially available sector scanner equipped with a 12 MHz phased-array transducer (Envisor, Philips Medical Systems, Andover, MA) [Groban et al. 2008]. Left ventricular (LV) M-mode images were obtained in a two-dimensional short axis view close to the papillary muscles. Posterior wall thicknesses at end diastole (PWTd) and LV end-diastolic and end-systolic dimensions (ESD, EDD) were measured according to the American Society for Echocardiography leading-edge method [Sahn et al. 1978]. The percentage of LV fractional shortening (FS), an index of global systolic function, was calculated as ([LVDD –LVSD]/LVDD) × 100. Mitral inflow measurements of early and late filling velocities (E and A, respectively), deceleration slope (Edec slope), and early deceleration time (Edec time) were obtained using pulsed Doppler, with the sample volume placed at the tips of mitral leaflets from an apical four-chamber orientation. Doppler tissue imaging to assess early mitral valve septal annular velocities (e′) was also obtained from the four-chamber view. All systolic and diastolic indices are the average of at least five consecutive cardiac cycles to minimize beat-to-beat variability.
Histopathology and plasma and tissue measurements
After echocardiographic analysis was done the rats were allowed to recover for 5 days and trunk blood was collected from decapitated animals. After blood collection, the hearts were removed and weighed. The midportion of the heart was then fixed in 4% paraformaldehyde, dehydrated by routine methods, and embedded in paraffin for subsequent histological study. The investigator, blinded to the experimental groups, determined the myocardial volume fraction occupied by fibrillar collagen by quantitative morphometry using an automated image-analysis system (Spot Advanced software 4.0.9) in sections stained with collagen-specific picrosirius red [Varagic et al. 2006]. Paraffin-embedded kidney sections were also stained with trichrome for subsequent analysis of kidney structure.
Plasma was stored at −80°C for measurements of plasma renin concentration, Ang II, and Ang-(1–7) by radioimmunoassay (RIA) as described previously [Ferrario et al. 2005a; Ishiyama et al. 2004; Iyer et al. 1998]. For the measurement of angiotensin peptides, a cocktail of protease inhibitors was used for collecting blood to prevent peptide degradation and generation [Kohara et al. 1991]. Plasma renin concentration was done in blood collected in EDTA containing tubes. Plasma renin concentration was defined as the rate of Ang I generation from renin in the sample incubated at pH 6.5 for 90 min with excess exogenous substrate provided from nephrectomized rat plasma. Ang I generated in the sample was quantified by radioimmunoassay (Diosarin Corp, Stillwater, MN).
Tissue samples from the LV base were quickly frozen on dry ice. Frozen heart samples were then weighed, homogenized, and extracted using Sep-Pak columns as previously described [Senanayake et al. 1998]. The eluate was divided for two RIAs (Ang II and Ang-[1–7]), and the solvent was evaporated. Recoveries of radiolabeled angiotensin peptides added to the samples were determined following the extraction. Recovery of radiolabeled peptide averaged greater than 65% and results were corrected for recovery. Ang II was measured using the Alpco Diagnostics kit (Windham, NH). Ang-(1–7) was measured using an antibody produced by our laboratory and extensively documented elsewhere [Senanayake et al. 1998]. The minimum detectable levels (MDL) of these assays were 0.9 fmol/tube for Ang II and 1.39 fmol/tube for Ang-(1–7). Values at or below the MDL of the assay were assigned the value of the MDL for the respective peptide for statistical analysis. The results were adjusted for the protein content of the sample measured by the Bio-Rad protein assay.
For immunoblotting, samples of cardiac plasma membranes were subjected to SDS-PAGE in 10% polyacrylamide gels as previously described [Shaltout et al. 2007]. Proteins were then transferred onto polyvinylidene difluoride membrane, and subsequently blocked for 1 h with either 5% Bio-Rad Dry Milk and tris-buffered saline (TBS) with Tween for mas and ACE2 or Starting Block Blocking Buffer (Thermo Scientific, Rockford, IL) for angiotensin type 1 receptor (AT1) before incubation with primary antibodies against β-actin (1:2000; Sigma, St. Louis, MO); AT1 (1:250; Alomone Labs, Jerusalem, Israel), mas (1:200; Alomone labs, Jerusalem, Israel), and ACE2 (1:1000; Hypertension and Vascular Disease Center No. A2405). Immunoblots were then resolved with Pierce Super Signal West Pico Chemiluminescent substrates as described by the manufacturer and exposed to Amersham Hyperfilm enhanced chemiluminescence (Piscataway, NJ).
Additional LV tissue samples from the apical portion were snap-frozen in liquid nitrogen and stored at −80°C for reverse transcriptase realtime polymerase chain reaction analysis of mRNA for AT1, mas and ACE2 [Ferrario et al. 2005b; Tallant et al. 2005]. The following primer/probe sets were used: for AT1, the set was purchased from Applied Biosystems (Foster City, CA); for mas, forward primer 5_-TTCATAGCCATCCTCAGCTTCTTG-3; reverse primer 5_-GTTCTTCCGTATCTTCACCACCAA-3; and probe 5_-FAM-TTCACTCCGCTCATGT TAG-NFQ-3; for ACE2, forward primer 5_-CCCAGAGAACAGTGGACCAAAA-3; reverse primer 5_-GCTCCACCACACCAACGAT-3_; and probe -FAM-CTCCCGCTTCATCTCC-NFQ-3. All reactions were performed in triplicate and 18S ribosomal RNA served as an internal control. The results were quantified as cycle threshold (Ct) values, where Ct is defined as the threshold cycle of PCR at which the amplified product is first detected, and expressed as the ratio of target/18S rRNA control.
Statistics
All values are expressed as the mean ± 1SEM. Data were analyzed by use of Student's t-test except for the systolic blood pressure analysis for which ANOVA, followed by Newman-Keuls' post-test, was used for multiple comparisons among groups. A value of p<0.05 was considered to be of statistical significance.
Results
Effect of salt excess on blood pressure and heart weight and structure
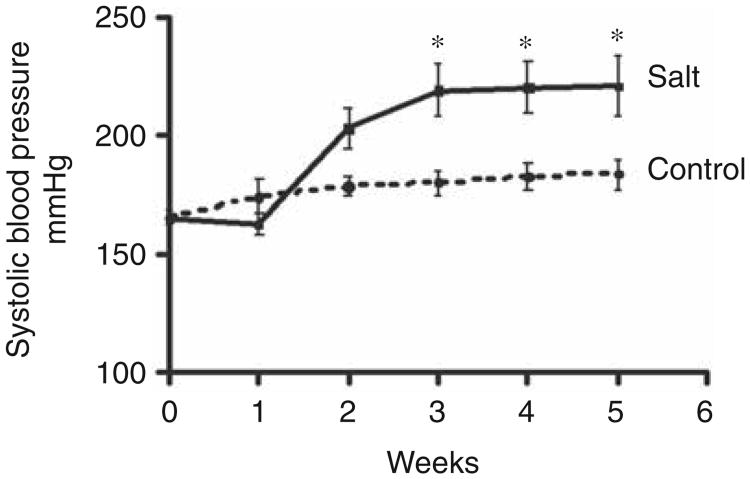
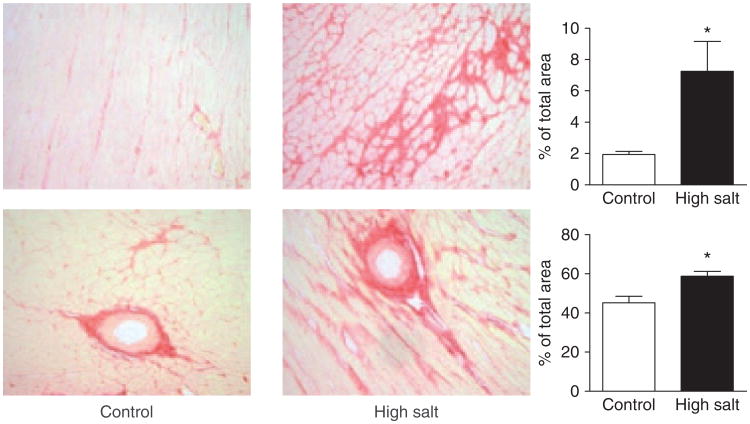
At baseline, systolic blood pressure averaged 166±3 mm Hg and 161±3 mmHg in the control and salt-loaded SHRs, respectively (p > 0.05). Exposure to a high salt diet was associated with a further increase in systolic blood pressure during the course of the experiment (Figure 1). Cardiac weight index (heart weight normalized to body weight) was increased in salt-loaded SHR, reflective of the increased LV posterior wall thickness (PWTd) but also, in part, of a lower body weight (Table 1). Unlike SHR fed a normal salt diet, prominent interstitial and perivascular fibro-sis was observed in the myocardium of SHR exposed to a high salt intake (Figure 2).
Figure 1.
Time course of systolic tail-cuff blood pressure in SHR given either a control or high salt diet over the 5 week observation period. *p<0.05 versus control.
Table 1.
Body weight, heart weight, and echocardiographic measurements.
| Control n=6 | Salt-loaded n=8 | p value | |
|---|---|---|---|
| Body weight (g) | 302 ± 3 | 212 ± 14 | <0.001 |
| Heart weight index (mg/g) | 3.45 ±0.09 | 5.25 ±0.14 | <0.001 |
| PWTd (cm) | 0.178 ± 0.013 | 0.221 ±0.011 | < 0.05 |
| ESD (cm) | 0.310 ±0.013 | 0.262 ±0.020 | n.s. |
| EDD (cm) | 0.624 ± 0.016 | 0.606 ±0.003 | n.s. |
| FS (%) | 50±1 | 58±3 | 0.06 |
| E (cm/s) | 77±2 | 65±3 | <0.001 |
| A (cm/s) | 41 ±2 | 43±3 | n.s. |
| E/A | 1.93 ±0.11 | 1.57 ±0.12 | < 0.05 |
| Edec slope (cm/s2) | 16±0.4 | 13±0.7 | < 0.05 |
| Edec time (s) | 0.051 ±0.002 | 0.054±0.002 | n.s. |
| e′ (cm/s) | 3.3 ± 0.1 | 2.9 ± 0.06 | < 0.01 |
| E/e′ | 24±1 | 22±1 | n.s. |
Values are expressed as mean±SEM; PWTd, posterior wall thickness at end diastole; ESD, end-systolic diameter; EDD, end-diastolic diameter; FS, fractional shortening; E = peak early filling velocity; A=peak velocity of late filling; Edec slope = early deceleration slope; Edec time=early deceleration time; e′ = early mitral annular velocity.
Figure 2.
Interstitial (top) and perivascular fibrosis (bottom) in the heart of control and salt-loaded SHR (picrosirius red staining; × 200 original magnification).
Effects of high salt intake on LV function
Echocardiographic analysis of the left ventricular structure and function is presented in Table 1. There were no differences between control and salt-loaded SHR with respect to ESD and EDD. Fractional shortening was higher in SHR fed a high sodium diet demonstrating preserved, if not enhanced, systolic function. Doppler transmitral flow and tissue imaging revealed signs of early diastolic dysfunction in salt-loaded rats manifested by decreases in E, E/A ratio, Edecslope, and e′. Left ventricular filling pressure or E/e′ and Edec time were not different between groups.
Effect of salt excess on urinary protein, sodium excretion and renal histology
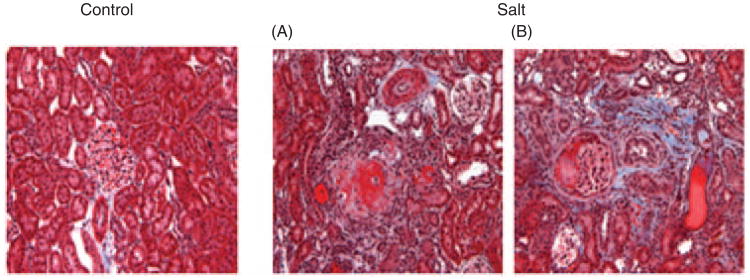
Twenty-four-hour urinary output (50±10 versus 6±1 ml/day; p < 0.001) and urinary protein (131±33 mg/day versus 14±2 mg/day; p < 0.001) and sodium (11.1±3.1 versus 1.0±0.1 mEq/day; p < 0.001) excretion were significantly higher in salt-loaded SHR compared to SHR controls. Histological examination of the kidney revealed that salt loading induced extensive nephrosclerosis as well as tubulo-interstitial changes (Figure 3).
Figure 3.
Renal light micrographic images showing changes in kidneys in salt-loaded rats. Left: SHR control rat; right: fibrinoid necrosis of afferent arterioles, with an ‘onion skin’ appearance (A), tubular cast, and interstitial fibrosis (B) can be seen in salt-loaded rats (Masson trichrome staining; × 200 original magnification).
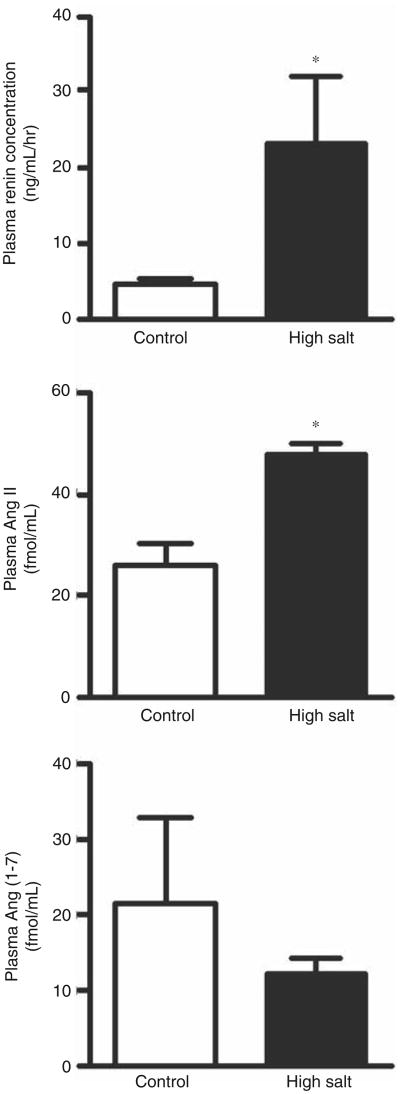
Effects of high salt intake on plasma and cardiac angiotensins
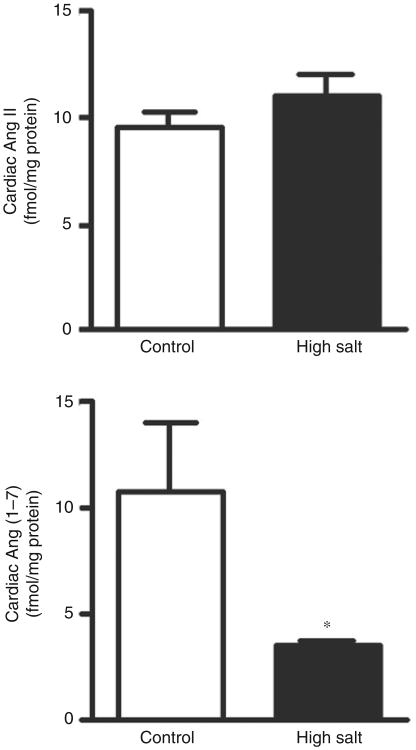
High dietary salt intake increased plasma renin and Ang II concentrations but not plasma Ang-(1–7) (Figure 4). While LV tissue Ang II content was not changed, high dietary salt intake dramatically reduced the cardiac content of Ang-(1–7) (Figure 5). Consequently, the Ang-(1–7)/Ang II ratio in the LV of salt-loaded SHR was reduced from 1.20 ±0.36 to 0.32 ±0.02 (p < 0.05) when compared with the rats fed a normal salt diet.
Figure 4.
Effect of a high salt diet on plasma renin concentration and plasma levels of angiotensin II (Ang II) and angiotensin-(1–7) (Ang-[1–7]). *p<0.05 versus control.
Figure 5.
Cardiac angiotensin II (Ang II) and angiotensin-(1–7) (Ang-[1–7]) concentrations in control and salt-loaded SHR. *p<0.05 versus control.
Effects of salt excess on cardiac AT1 and mas mRNA and protein levels
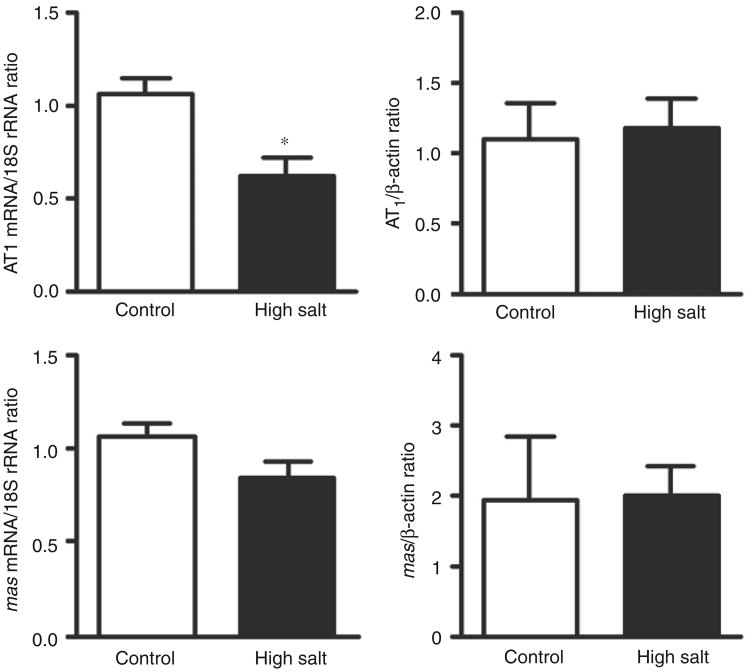
Cardiac expression of AT1 but not mas mRNA was reduced in SHR exposed to a high-salt diet (Figure 6). Cardiac receptor protein levels for both AT1 and mas receptors did not change.
Figure 6.
Cardiac angiotensin type 1 (AT1) and mas receptor mRNA and protein level in the left ventricle of spontaneously hypertensive rats (SHR) fed either a control or high salt diet. *p<0.05 versus control.
Effects of salt excess on cardiac ACE2
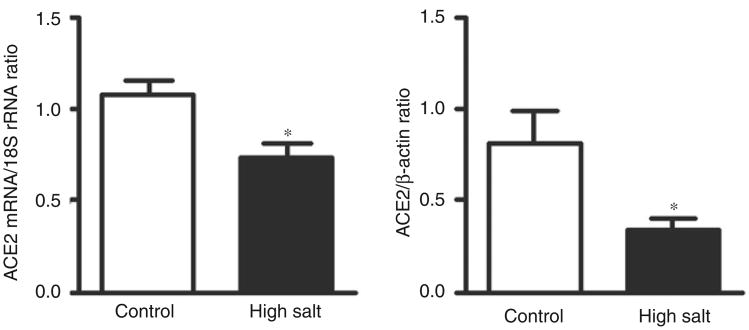
Salt excess reduced cardiac expression of ACE2 mRNA and protein (Figure 7).
Figure 7.
Effects of high salt diet on cardiac angiotensin converting enzyme 2 (ACE2) mRNA and protein level. *p < 0.05 versus control.
Discussion
Increased salt intake in SHR worsens hypertension as well as cardiac and renal remodeling as reflected in echocardiographic and structural evidence of cardiac hypertrophy, proteinuria and collagen deposition. It was previously demonstrated that the hypertension in SHR is in part salt sensitive and associated with a loss of the appropriate downregulatory effect of dietary sodium on circulating Ang II and angiotensino-gen [Hodge et al. 2002; Widimsky et al. 1991]. We now extend these studies showing that while salt excess did not significantly affect Ang-(1–7) in plasma it diminished cardiac levels of Ang-(1–7). Thus, high salt diet altered the balance between the two peptides facilitating the vasoconstrictor, proliferative and hypertrophic actions of Ang II in the heart. These data agree with our previous demonstration that an 8% salt diet induced cardiac remodeling and dysfunction, which may be, at least in part, independent of the effect of salt intake on arterial pressure [Varagic et al. 2006; du Cailar et al. 2002, 1989; Frohlich et al. 1993]. In agreement with this interpretation, concomitant treatment with an AT1 receptor antagonist ameliorated salt-induced cardiac injury in SHR without affecting the elevated blood pressure [Varagic et al. 2008].
Evidence suggests that Ang II exerts mitogenic and growth-promoting effects in cardiac myocytes and nonmyocytic elements and both of these actions significantly contribute to the development and progression of hypertensive heart disease [Weber and Brilla, 1991]. Indeed, in the present study salt excess was associated with further hemodynamic and structural evidence of cardiac hypertrophy and collagen deposition when compared with SHR maintained on a normal sodium intake. Although plasma renin activity may be suppressed in response to high dietary salt intake [Hodge et al. 2002; Yu et al. 1998], these changes were not associated with lower plasma angiotensinogen and Ang II levels [Hodge et al. 2002], suggesting dysregulation of the Ang II response to long-term high dietary salt intake. Increased salt intake in our study was accompanied by hyper-reninemia and higher levels of circulating Ang II that may be the result of the effects of salt on renal function and structure as well, as proteinuria and acceleration of glomerulosclerosis accompanied the exposure to a high salt diet. In agreement with this interpretation, we showed that intermediate levels of high salt intake (4% and 6% NaCl in food) promoted dramatic deterioration in renal and glomerular dynamics in SHR [Matavelli et al. 2007]. In those studies, high salt intake induced increased afferent and efferent arteriolar resistances associated with increased glomerular hydrostatic pressure that might favor the development of nephrosclerosis and, consequently, activation of the circulating renin–angiotensin system.
The increased cardiac work induced by the combination of the higher salt intake and the further augmentation in afterload was associated with increased systolic indices of ventricular contractility accompanied by nonsuppressed tissue levels of Ang II and AT1 receptor protein.
Evidence of downregulation of AT1 receptor mRNA in the heart of salt-loaded SHR may reflect a tissue response to the activated circulatory and nonsuppressed cardiac Ang II, providing further supporting evidence of inappropriately high Ang II activity in different models of salt-sensitive hypertension [Liang and Leenen, 2007; Zhu et al. 2004; Sumida et al. 1998; Kreutz et al. 1995].
Finally, salt loading diminished Ang-(1–7) level in the heart in association with reduced ACE2 mRNA and protein level. We previously showed that Ang II reduced ACE2 mRNA in cultured myocytes and fibroblasts [Gallagher et al. 2008]. In addition, although Ang-(1–7) did not affect ACE2 mRNA itself, it prevented the Ang II-mediated reduction in ACE2 mRNA in cultured cardiac cells, an effect blocked by a selective Ang-(1–7) receptor antagonist [Gallagher et al. 2008]. In agreement, in Dahl salt-sensitive rats fed a high salt diet, cardiac enlargement and fibrosis were associated with an increased cardiac angiotensinogen but reduced cardiac ACE2 mRNA. Tissue peptides were not determined in that study [Takeda et al. 2007]. To the best of our knowledge, we are the first to report a diminished level of cardiac Ang-(1–7) in response to salt loading. Thus, when tissue levels of Ang-(1–7) are decreased, the transcriptional downregulation of ACE2 by AngII may be left unopposed. In addition, besides its vasodilatory effects, Ang-(1–7) mediates antihypertrophic and antiproliferative effects by binding to its specific cell membrane receptor, mas [Tallant et al. 2005]. Thus, Ang-(1–7) inhibited protein incorporation into myocytes in culture and the antitrophic effects of the peptide were prevented following administration of antisense oligonucleotides directed against the mas receptor [Tallant et al. 2005]. In addition, Ang-(1–7) reduced proline incorporation into cardiac fibroblasts, indicating that the heptapep-tide also inhibits collagen synthesis as well [Tallant et al. 2003]. Furthermore, significant changes in the ACE2/Ang-(1–7)/mas receptor axis of the renin–angiotensin system may occur in some pathological states. For example, in a congenic rat model of hypertension (mRen2.Lewis), diminished cardiac expression and activity of ACE2 were demonstrated [Jessup et al. 2006]. Importantly, a recent study from our laboratory revealed a predominant role of ACE2 in Ang-(1–7) formation in the heart of hypertensive rats [Trask et al. 2007]. Along with studies showing that Ang-(1–7) supplementation reduces an increase in blood pressure in Dahl salt-sensitive rats fed a high salt diet [Eatman et al. 2001] and prevents Ang II-related cardiac hypertrophy and fibrosis [Grobe et al. 2007], our results suggest a critical role of the Ang-(1–7) in the development of salt-related hypertension and hypertensive heart disease.
In conclusion, salt excess increased circulating Ang II while reducing cardiac Ang-(1–7) shifting the balance in angiotensin peptides towards the vasoconstrictor, proliferative and prohypertrophic actions of Ang II. Our results, therefore, suggest that the adverse cardiac effects of excessive salt intake may result not only from the undesirable action of Ang II but also as a consequence of diminished protective effects of Ang-(1–7).
Acknowledgments
This study was supported by American Heart Association Mid-Atlantic Affiliate Grant 0765308U, the Wake Forest University School of Medicine Venture Fund, National Heart, Lung, and Blood Institute Grant HL-51952 and NIH KO8-AG026764-04 Paul Beeson award. Additionally, the authors gratefully acknowledge grant support in part provided by Unifi Inc., Greensboro, NC, and Farley-Hudson Foundation, Jacksonville, NC.
The authors acknowledge the technical assistance of Ms Jessica VonCannon and Mr Brian Bernish.
Footnotes
Reprints and permissions: http://www.sagepub.co.uk/journalsPermissions.nav
Conflict of interest statement: None declared.
References
- Ahn J, Varagic J, Slama M, Susic D, Frohlich ED. Cardiac structural and functional responses to salt loading in SHR. Am J Physiol Heart Circ Physiol. 2004;287:H767–H772. doi: 10.1152/ajpheart.00047.2004. [DOI] [PubMed] [Google Scholar]
- Chappell MC, Tallant EA, Brosnihan KB, Ferrario CM. Conversion of angiotensin I to angiotensin-(1–7) by thimet oligopeptidase (EC 3.4.24.15) in vascular smooth muscle cells. J Vasc Med Biol. 1994;5:129–137. [Google Scholar]
- du Cailar G, Ribstein J, Grolleau R, Mimran A. Influence of sodium intake on left ventricular structure in untreated essential hypertensives. J Hypertens. 1989;7(Suppl):S258–S259. doi: 10.1097/00004872-198900076-00125. [DOI] [PubMed] [Google Scholar]
- du Cailar G, Ribstein J, Mimran A. Dietary sodium and target organ damage in essential hypertension. Am J Hypertens. 2002;15:222–229. doi: 10.1016/s0895-7061(01)02287-7. [DOI] [PubMed] [Google Scholar]
- Eatman D, Wang M, Socci RR, Thierry-Palmer M, Emmett N, Bayorh MA. Gender differences in the attenuation of salt-induced hypertension by angiotensin (1–7) Peptides. 2001;22:927–933. doi: 10.1016/s0196-9781(01)00404-1. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Jessup JA, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, et al. Effect of angiotensin converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin converting enzyme 2. Circulation. 2005a;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1–7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005b;289:H2281–H2290. doi: 10.1152/ajpheart.00618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich ED, Chien Y, Sesoko S, Pegram BL. Relationship between dietary sodium intake, hemodynamics, and cardiac mass in SHR and WKY rats. Am J Physiol. 1993;264:R30–R34. doi: 10.1152/ajpregu.1993.264.1.R30. [DOI] [PubMed] [Google Scholar]
- Gallagher PE, Ferrario CM, Tallant EA. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am J Physiol Heart Circ Physiol. 2008;295:H2373–H2379. doi: 10.1152/ajpheart.00426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groban L, Yamaleyeva LM, Westwood BM, Houle TT, Lin M, Kitzman DW, et al. Progressive diastolic dysfunction in the female mRen(2). Lewis rat: influence of salt and ovarian hormones. J Gerontol A Biol Sci Med Sci. 2008;63:3–11. doi: 10.1093/gerona/63.1.3. [DOI] [PubMed] [Google Scholar]
- Grobe JL, Mecca AP, Lingis M, Shenoy V, Bolton TA, Machado JM, et al. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1–7) Am J Physiol Heart Circ Physiol. 2007;292:H736–H742. doi: 10.1152/ajpheart.00937.2006. [DOI] [PubMed] [Google Scholar]
- Hodge G, Ye VZ, Duggan KA. Dysregulation of angiotensin II synthesis is associated with salt sensitivity in the spontaneous hypertensive rat. Acta Physiol Scand. 2002;174:209–215. doi: 10.1046/j.1365-201x.2002.00937.x. [DOI] [PubMed] [Google Scholar]
- Inada Y, Tazawa S, Murakami M, Akahane M. KRH-594, a new angiotensin AT1 receptor antagonist, prevents end-organ damage in stroke-prone spontaneously hypertensive/Izm rats. Clin Exp Pharmacol Physiol. 2001;28:206–211. doi: 10.1046/j.1440-1681.2001.03430.x. [DOI] [PubMed] [Google Scholar]
- Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enyzme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- Iyer SN, Chappell MC, Averill DB, Diz DI, Ferrario CM. Vasodepressor actions of angiotensin-(1–7) unmasked during combined treatment with lisinopril and losartan. Hypertension. 1998;31:699–705. doi: 10.1161/01.hyp.31.2.699. [DOI] [PubMed] [Google Scholar]
- Jessup JA, Gallagher PE, Averill DB, Brosnihan KB, Tallant EA, Chappell MC, et al. Effect of angiotensin II blockade on a new congenic model of hypertension derived from transgenic Ren-2 rats. Am J Physiol Heart Circ Physiol. 2006;291:H2166–H2172. doi: 10.1152/ajpheart.00061.2006. [DOI] [PubMed] [Google Scholar]
- Kohara K, Tabuchi Y, Senanayake P, Brosnihan KB, Ferrario CM. Reassessment of plasma angiotensins measurement: effects of protease inhibitors and sample handling procedures. Peptides. 1991;12:1135–1141. doi: 10.1016/0196-9781(91)90070-6. [DOI] [PubMed] [Google Scholar]
- Kreutz R, Fernandez-Alfonso MS, Liu Y, Ganten D, Paul M. Induction of cardiac angiotensin I-converting enzyme with dietary NaCl-loading in genetically hypertensive and normotensive rats. J Mol Med. 1995;73:243–248. doi: 10.1007/BF00189924. [DOI] [PubMed] [Google Scholar]
- Liang B, Leenen FH. Prevention of salt induced hypertension and fibrosis by angiotensin converting enzyme inhibitors in Dahl S rats. Br J Pharmacol. 2007;152:903–914. doi: 10.1038/sj.bjp.0707472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matavelli LC, Zhou X, Varagic J, Susic D, Frohlich ED. Salt loading produces severe renal hemodynamic dysfunction independent of arterial pressure in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;292:H814–H819. doi: 10.1152/ajpheart.00671.2006. [DOI] [PubMed] [Google Scholar]
- Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- Senanayake PS, Smeby RR, Martins AS, Moriguchi A, Kumagai H, Ganten D, et al. Adrenal, kidney, and heart angiotensins in female murine Ren-2 transfected hypertensive rats. Peptides. 1998;19:1685–1694. doi: 10.1016/s0196-9781(98)00123-5. [DOI] [PubMed] [Google Scholar]
- Shaltout HA, Westwood BM, Averill DB, Ferrario CM, Figueroa JP, Diz DI, et al. Angiotensin metabolism in renal proximal tubules, urine, and serum of sheep: evidence for ACE2-dependent processing of angiotensin II. Am J Physiol Renal Physiol. 2007;292:F82–F91. doi: 10.1152/ajprenal.00139.2006. [DOI] [PubMed] [Google Scholar]
- Stier CT, Jr, Chander P, Gutstein WH, Levine S, Itskovitz HD. Therapeutic benefit of captopril in salt-loaded stroke-prone spontaneously hypertensive rats is independent of hypotensive effect. Am J Hypertens. 1991;4:680–687. doi: 10.1093/ajh/4.8.680. [DOI] [PubMed] [Google Scholar]
- Sumida Y, Umemura S, Kobayashi S, Kihara M, Tamura K, Ishigami T, et al. Angiotensin II receptors in cardiac left ventricles of Dahl rats. Clin Exp Pharmacol Physiol. 1998;25:252–256. doi: 10.1111/j.1440-1681.1998.t01-16-.x. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Yoneda T, Demura M, Furukawa K, Miyamori I, Mabuchi H. Effects of high sodium intake on cardiovascular aldosterone synthesis in stroke-prone spontaneously hypertensive rats. J Hypertens. 2001;19:635–639. doi: 10.1097/00004872-200103001-00017. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Zhu A, Yoneda T, Usukura M, Takata H, Yamagishi H. Effects of aldosterone and angiotensin II receptor blockade on cardiac angiotensinogen and angiotensin-converting enzyme 2 expression in Dahl salt-sensitive hypertensive rats. Am J Hypertens. 2007;20:1119–1124. doi: 10.1016/j.amjhyper.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Tallant EA, Ferrario CM, Gallagher PE. Angiotensin-(1–7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am J Physiol Heart Circ Physiol. 2005;289:H1560–H1566. doi: 10.1152/ajpheart.00941.2004. [DOI] [PubMed] [Google Scholar]
- Tallant EA, Howard TL, Dancho B, Gallagher PE. Angiotensin-(1–7) reduces hypertrophy in cardiomyocytes and hyperplasia in cardiac fibroblasts. Hypertension. 2003;42:389A. [Google Scholar]
- Trask AJ, Averill DB, Ganten D, Chappell MC, Ferrario CM. Primary role of angiotensin-converting enzyme-2 in cardiac production of angiotensin-(1–7) in transgenic Ren-2 hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;292:H3019–H3024. doi: 10.1152/ajpheart.01198.2006. [DOI] [PubMed] [Google Scholar]
- Varagic J, Frohlich ED, Diez J, Susic D, Ahn J, Gonzalez A, et al. Myocardial fibrosis, impaired coronary hemodynamics, and biventricular dysfunction in salt-loaded SHR. Am J Physiol Heart Circ Physiol. 2006;290:H1503–H1509. doi: 10.1152/ajpheart.00970.2005. [DOI] [PubMed] [Google Scholar]
- Varagic J, Frohlich ED, Susic D, Ahn J, Matavelli L, Lopez B, et al. AT1 receptor antagonism attenuates target organ effects of salt excess in SHRs without affecting pressure. Am J Physiol Heart Circ Physiol. 2008;294:H853–H858. doi: 10.1152/ajpheart.00737.2007. [DOI] [PubMed] [Google Scholar]
- Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83:1849–1865. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
- Widimsky J, Kuchel O, Tremblay J, Hamet P. Distinct plasma atrial natriuretic factor, renin and aldosterone responses to prolonged high-salt intake in hypertensive and normotensive rats. J Hypertens. 1991;9:241–247. doi: 10.1097/00004872-199103000-00008. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Chappell MC, Brosnihan KB, Ferrario CM. In vivo metabolism of angiotensin I by neutral endopeptidase (EC 3.4.24.11) in spontaneously hypertensive rats. Hypertension. 1992;19:692–696. doi: 10.1161/01.hyp.19.6.692. [DOI] [PubMed] [Google Scholar]
- Yu HC, Burrell LM, Black MJ, Wu LL, Dilley RJ, Cooper ME, et al. Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation. 1998;98:2621–2628. doi: 10.1161/01.cir.98.23.2621. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Zhu S, Wu Z, Liu D, Yang Y, Wang X, et al. Effect of sodium on blood pressure, cardiac hypertrophy, and angiotensin receptor expression in rats. Am J Hypertens. 2004;17:21–24. doi: 10.1016/j.amjhyper.2003.08.004. [DOI] [PubMed] [Google Scholar]