Abstract
Since kallikrein was discovered as a vasodilatory substance in human urine, the kallikrein–kinin system (KKS) has been considered to play a physiological role in controlling blood pressure. Gene targeting experiments in mice in which the KKS has been inactivated to varying degrees have, however, questioned this role, because basal blood pressures are not altered. Rather, these experiments have shown that the KKS has a different and important role in preventing changes associated with normal senescence in mice, and in reducing the nephropathy and accelerated senescence-associated phenotypes induced in mice by diabetes. Other experiments have shown that the KKS suppresses mitochondrial respiration, partly by nitric oxide and prostaglandins, and that this suppression may be a key to understanding how the KKS influences senescence-related diseases. Here we review the logical progression and experimental data leading to these conclusions, and discuss their relevance to human conditions.
Keywords: ACE inhibitors, aging, bradykinin, DNA damage, electron transport chain, oxidative stress
BACKGROUND
Angiotensin-I-converting enzyme and bradykinin
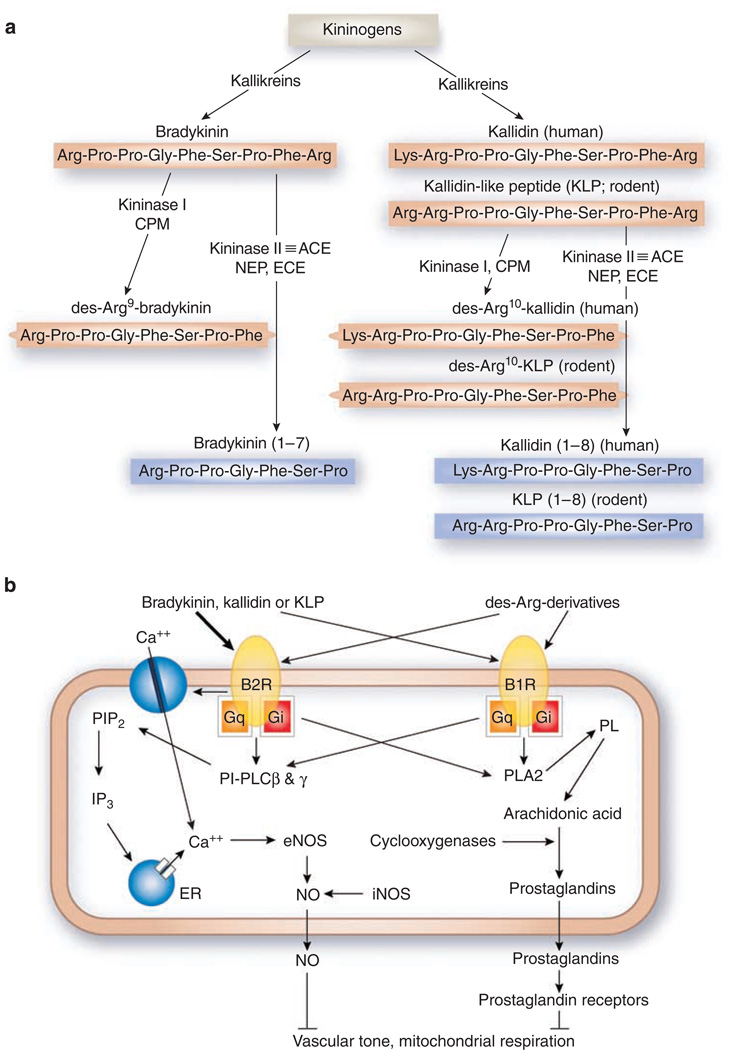
The angiotensin-I-converting enzyme (ACE, also known as kininase II) is a carboxydipeptidase that removes two amino acids from the carboxyl terminus of the inactive peptide angiotensin I and converts it into the active blood pressure-raising peptide, angiotensin II. ACE also converts the active blood pressure-lowering kinins, bradykinin (1–9) and kallidin (1–10), into inactive bradykinin (1–7) and kallidin (1–8) (Figure 1a). ACE has a 30 times lower Km and 10 times higher kcat for the kinins than for angiotensin I.
Figure 1. Components and signaling of the KKS.
(a) Biosynthesis and metabolism of kinins. CPM, carboxypeptidase-M; ACE, angiotensin I-converting enzyme; NEP, neprilysin (endopeptidase 24.11); ECE, endothelin-converting enzyme; red, active peptides; blue, inactive peptides. (b) Binding of kinins to bradykinin receptors and two intracellular mechanisms for suppression of oxidative metabolism. The thickness of arrows arising from the kinins indicates the relative potency of each peptide to elevate intracellular calcium concentrations. PIP2, phosphatidylinositol-4,5-bisphosphate; PI-PLC, phosphatidylinositol-specific phospholipase C; IP3, 1,4,5-inositol triphosphate; ER, endoplasmic reticulum; PL, phospholipids; PLA2, phospholipase A2.
A very common insertion/deletion (I/D) polymorphism of the ACE gene is associated with different relative plasma levels of the enzyme, ranging from about 0.75–1.0 to 1.25 in I/I, I/D, and D/D individuals. The ACE I/D polymorphism in humans does not significantly affect blood pressure,3 nor does a modest genetically induced decrease in expression of the Ace gene (to 0.5 × normal) or a modest increase (to 1.5 × normal) affect blood pressure in mice.4 Nevertheless, the two human alleles are associated with different risks for developing a wide constellation of diseases, including diabetic nephropathy,5 breast cancer,6–8 prostate cancer,9,10 gastric cancer,11,12 Alzheimer’s disease,13 Parkinson’s disease, 14 congestive heart failure, 15 myocardial infarction,16 stroke, 17 and retinal macular degeneration18. In all these many senescence-associated human disorders, it is the D allele with its higher levels of ACE that confers the increased risk.
A clear example of this association is provided by the demonstration that the D allele is an independent risk factor for both the onset and progression of nephropathy in type I diabetic patients.5 This association led to experiments in which diabetes was induced with streptozotocin (STZ) in mice having different genetically determined levels of ACE.19 These experiments showed that, when diabetic, mice having the higher levels of the enzyme (about 1.5 × normal) developed significantly more urinary albumin excretion than their siblings with normal or reduced (0.5 × normal) Levels of ACE. These experiments consequently established a causative link between genetically increased levels of ACE and the nephropathy induced by type I diabetes. Yet earlier experiments with varying degrees of ACE inhibition,20 and computer simulations of the effects of genetically altering the levels of ACE,21 had shown that such modest changes in ACE levels have little effect on the levels of its products (including the active peptide angiotensin II), although they change the levels of its substrates (including the active peptides bradykinin (1–9) and kallidin (1–10)). This consideration led to the inference that decreases in the level of the active ACE substrate bradykinin probably mediate the harmful effects of the ACE D allele.
The kinins
The kinins, bradykinin and kallidin in humans or bradykinin and the kallidin-like peptide in rodents, are generated from kininogens by kallikreins (Figure 1a). Humans have one kininogen gene; rodents have two closely linked kininogen genes.22 Kallidin can be converted into bradykinin by a plasma aminopeptidase. All the kinins are strong agonists of the bradykinin 2 receptor (B2R, Bdkrb2), although less so of the B1 receptor (B1R, Bdkrb1). Kininase I (carboxypeptidase-N) and carboxypeptidase-M remove arginine from the carboxyl terminus of the kinins and generate their des-Arg derivatives, which are agonists mainly of B1R. Kininase II (a synonym for ACE),23 neprilysin (endopeptidase 24.11),23 and endothelin-converting enzyme24 all remove two amino acids (Phe and Arg) from the carboxyl terminus of the kinins, and inactivate them.
Bradykinin B1 and B2 receptors
In mammals, as indicated above, two bradykinin receptors have been identified: B1R and B2R, both of which are G protein-coupled receptors with seven transmembrane domains. Mice deficient in both B1R and B2R have no contractile response to bradykinin in isolated smooth muscle tissues, suggesting that there are no other major receptors for bradykinin, at least in smooth muscle cells.25 The B2R protein is constitutively expressed in most tissues. Vascular endothelial cells express B2R abundantly, where it is functionally linked to activation of endothelial nitric oxide (NO) synthase (eNOS, Nos3). Expression of B1R is minimal under normal circumstances, but is induced by inflammation,26 diabetes,27 ischemia/reperfusion injury,28 and by the absence of B2R.29 B2R mRNA is expressed in all segments of the kidney under physiological conditions, and lipopolysac-charide (LPS) increases this expression. In contrast, no B1R mRNA levels can be detected in any segments of the kidney under physiological conditions, although treatment with LPS induces the expression of B1R mRNA in all renal segments except the outer medullary collecting ducts.30
The transcriptional regulation of the two receptor genes differ, although the intracellular signals that follow stimulation of B1R and B2R are quite similar (Figure 1b).31,32 Stimulation of bradykinin receptors by kinins elevates [Ca+ +]i by activation of phosphatidylinositol (PI)-specific phospholipase C (PI-PLC) in Gq-protein-dependent and Gq-protein-independent ways. Allosteric activation of PI-PLCβ isoforms by Gq/11 proteins and direct tyrosine phosphorylation of PI-PLCγ isoforms by bradykinin receptors33,34 both play an important role in the bradykinin-induced changes in [Ca+ +]i (Figure 1b).
Stimulation of either B1R or B2R increases eNOS activity and prostacyclin synthesis in endothelial cells, at least partly by elevating intracellular calcium levels ([Ca+ + ]i).35,36 And it has recently been shown that bradykinin, kallidin, and kallidin-like peptide are equipotent at eliciting Ca+ + -transients by B2R in humans and rodents.37 As the concentration of kallidin or kallidin-like peptide is much higher than that of bradykinin in humans38 and rats,39 kallidin and kallidin-like peptide rather than bradykinin may be the major endogenous agonists of the KKS.
The NO release in response to agonist binding by B1R is slow in onset, but desensitization of B1R does not occur.40 In contrast, the NO response of the B2R is rapid40 and agonist-occupied phosphorylated B2R41 is internalized into endo-somes in a β-arrestin 2- and clathrin-dependent manner42,43. This uncouples B2R from the G proteins and desensitizes the second messenger-mediated signal.42 Interestingly, eNOS also reversibly translocates from the cell membrane into the cell cytosol following B2R stimulation or administration of a calcium ionophore.44 Although the kinins promote B2R endocytosis, they delay B1R endocytosis, which is clathrin-dependent but β-arrestin 2-independent.43
In transgenic mice, over expression of B2R results in hypotension,45 but overexpression of B1R does not change blood pressure.46 In contrast, the absence of either B2R orB1R does not change blood pressure,47,48 nor does lack of both bradykinin B1R and B2R, which abolishes most ofbradykinin signaling25,49. Furthermore, mice lacking tissue kallikrein and kininogen-deficient Brown Norway Katholiek rats have normal blood pressure.50,51 These observations suggest that the KKS plays only a minor physiological role in regulating chronic blood pressure in mammals, even though transient decreases in blood pressure are caused by administration of kallikrein or the kinins.
Bradykinin and nitric oxide (NO)
The absence of B2R decreases the urinary excretion of stable metabolites of NO ( and NO3−),52 and lack of both B1Rand B2R reduces fasting plasma concentration.49
Stimulation of the bradykinin receptors by the kinins elevates [Ca+ +]i and activates the Ca+ +-dependent iso-forms of the NOS (eNOS and neuronal NOS).53,54 Bradykinin through its receptors also leads sequentially to activation of PI3-kinase, phosphorylation of Akt, and phosphorylation of eNOS, which sensitizes it to [Ca+ +]i.55 Furthermore, B2R forms a complex with eNOS from which the active enzyme is released following receptor activation.56 Bradykinin also increases the association of heat-shock protein 90 with eNOS, which is required for NO formation by eNOS.57
The expression of inducible NOS (the Ca+ +-independent isoform of the NOS) is also increased by bradykinin through both B1R58, and B2R.59 The bradykinin-induced expression of inducible NOS is dependent on intranuclear calcium and Akt signaling in rat hepatocytes.59
Lipopolysaccharide causes hypotension in normal rats, which is diminished by a B2R antagonist60. Furthermore, kininogen-deficient rats and mice lacking both B1R and B2R are resistant to LPS-induced septic shock.25,60 Inducible NOS mRNA levels are increased by LPS in wildtype, but in mice lacking both B1R and B2R this increase is diminished. 25 Thus, bradykinin plays a role in the development of septic shock, in part by inducible NOS.
Together these various studies show that the KKS is important in controlling NO production through all the isoforms of NOS. The NO so formed has the potential of acting on the cells that produced it or on neighboring cells.
Bradykinin and the prostaglandins
Most of the nonsteroidal anti-inflammatory drugs, including aspirin, indomethacin, ibuprofen, and the more isoform-specific cyclooxygenase inhibitors, exert their anti-inflammatory effects by inhibiting the formation of arachidonic acid metabolites, including the prostaglandins (PGs). Bradykinin acts through its receptors in at least three ways to increase production of PGs. First, it leads to the Ca+ +-dependent phosphorylation and translocation into the cell membrane of cytosolic phospholipase A2.61,62 Second, bradykinin stimulates membrane-associated Ca+ +-independent phospholipase A2.63 Both the Ca+ + -dependent and -independent A2 phospholipases liberate arachidonic acid from membrane phospholipids. Third, bradykinin leads to the induction of cyclooxygenase-2,64–66 which converts arachidonic acid into PGs. The bradykinin-stimulated formation of PGE2 is inhibited by pertussis toxin,67 suggesting that this response is mediated by the Gi protein that is associated with the bradykinin receptors.68 The PGs formed following stimulation of the bradykinin receptors, acting through PG receptors, mediate some of the effects of the kinins on vascular tone and on mitochondrial respiration. When kinins are injected in supra-physiological amounts, they cause inflammation, pain, and increased vascular permeability,69 at least partly through the PGs. KKS antagonists are therefore effective for suppressing excessive inflammatory responses.70
Bradykinin and other second messengers
The endothelium-dependent vasodilatory effect of bradykinin is not completely abolished by the simultaneous administration of NOS inhibitors and cyclooxygenase inhibitors. However, this unexplained vasodilation is inhibited by blockers of Ca+ +-activated K+-channels or high external [K+], suggesting the presence of an endothelium-derived hyperpolarizing factor.71 Epoxyeicosatrienoic acids, P450 epoxygenase metabolites of arachidonic acid, are most likely candidates for the endothelium-derived hyperpolarizing factor.72
The C-terminal part of B2R interacts with the protein-tyrosine phosphatase SH2 domain-containing phosphatase-2 (SHP-2), and activates it in rat mesangial cells.73 As the antimitogenic effect of bradykinin is abolished by transfection of dominant-negative SHP-2 in mesangial cells,73 it is possible that bradykinin, acting through SHP-2, plays a role in controlling the mesangial expansion that occurs in many glomerular diseases.
Bradykinin, oxidative stress, and senescence
Mitochondrial oxidative respiration is a much more efficient source of energy than anaerobic glycolysis. However, oxidative metabolism generates reactive oxygen species, which can have deleterious effects. Several studies have shown that NO reversibly suppresses mitochondrial oxidative metabolism,74,75 in part by inhibiting cytochrome c oxidase, a key enzyme in electron transport chain.76,77 Recent studies have also shown that cAMP decreases mitochondrial respiration by activating NADH-ubiquinone oxidoreductase activity of complex I and by inhibiting cytochrome c oxidase.78,79 As bradykinin, acting through B1R and B2R, stimulates eNOS activity in vascular endothelial cells and increases cAMP levels in kidney epithelial cells, it is not surprising that the KKS can influence the level of oxidative stress. For example, when bradykinin is administered to rats that were made hyperglycemic with STZ, it reduces their oxidative stress phenotype, as judged by hydrogen peroxide and malondialdehyde levels.80 Furthermore, because the binding of B2R to eNOS, referred to above, is through the oxygenase domain of the enzyme, the ability of eNOS to catalyze uncoupled NADPH oxygenation is blocked by B2R,81 suggesting that B2R can affect the generation of reactive oxygen species by eNOS even without agonist stimulation.
In the kidney, proximal tubular epithelial cells are densely packed with mitochondria, which supply the energy for the active transport of sodium ions by Na+/K+ ATPase. These cells are among the most oxygen-using cells of the body, and are therefore at risk for oxidative damage. It is consequently again not surprising that several indicators of oxidative damage are increased in these and other high oxygen-using cells when the KKS is impaired by the absence of B2R. Figure 2a illustrates this by showing that lack of B2R greatly enhanced the accumulation in diabetic mice of lipofuscin-like intracellular inclusions (an indicator of mitochondrial damage) in renal proximal tubules.82
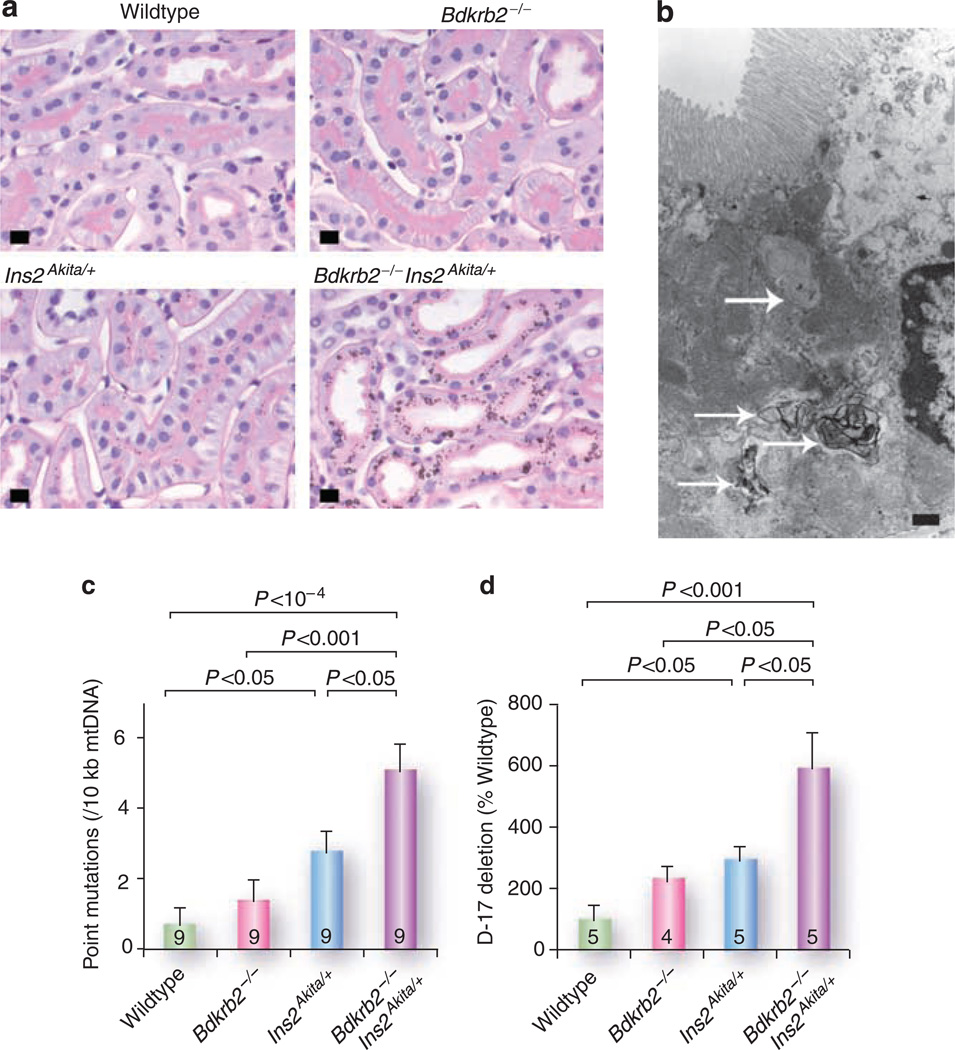
Figure 2. Senescence-associated indices in the kidney of B2R-null and/or Akita diabetic mice.
(a) Periodic acid-Schiff-stained kidneys of 12-month-old male mice (bar = 10 µm). Note the intracellular pigmented vacuoles in the cytoplasm of the proximal tubular epithelial cells of the Akita diabetic mice (Ins2 Akita/+), and their greater prominence when the diabetic mice also lack B2R (bdkrb2−/−). (b) Transmission electron micrograph of a renal proximal tubule cell from a 12-month-old doubly mutant mouse (bar= 1 µm). There are numerous phagolysosomes containing lipid debris with focal lamination (arrows). (c) Frequencies of point mutations in mitochondrial DNA. (d) Relative proportion of D-17 deletions in mitochondrial DNA.
Lipofuscins are electron dense substances contained in autophagolysosomes derived from damaged organelles (Figure 2b). They are a manifestation of senescence as well as of oxidative stress.83 Mice lacking the senescence marker protein-30 have systemic premature senescence, and lipofuscin accumulation is observed in their proximal tubular cells preceded by senescence-associated β-galactosidase activity,84 an established hallmark of aging.85 We have also observed that the deposition of lipofuscin in the proximal tubule cells of B2R-null Akita mice aged 12 months is associated with the presence of senescence-associated β-galactosidase activity (unpublished observation).
Point mutations and deletions in mitochondrial DNA, which are also known reflections of aging,86,87 were increased in the kidney by the absence of B2R even in non-diabetic mice, although additively more so with diabetes82 (Figure 2b and c).
Transforming growth factor β1 enhances autophagy,88 and its expression is increased in aged cells89 and in a number of fibrogenic kidney diseases.90 We have found that the absence of B2R and/or presence of diabetes enhances the renal expression of transforming growth factor β1.82 Thus, the increase in transforming growth factor β1 expression may play a causative role in lipofuscin accumulation in the kidney of B2R-null Akita mice.
EXPERIMENTAL STUDIES ON KKS IN RENAL DISEASES
Diabetic nephropathy
We have described above how genetically increased ACE levels, which do not change blood pressure or angiotensin II levels,4 nevertheless enhance urinary excretion of albumin in mice with diabetes induced by STZ.19 In the reverse direction, ACE inhibition is protective in many models of diabetic nephropathy.91–94 That this protection is mediated in part by the KKS is strongly suggested by experiments showing that the beneficial effects of ACE inhibitors were attenuated by a B2R antagonist in rats that were made diabetic with STZ,91,92 in obese Zucker diabetic fatty rats,93 and in C57BLKS db/db mice.94
We have found that albuminuria, glomerular sclerosis, interstitial fibrosis, lipofuscin accumulation in proximal tubules, and lifespan shortening in Akita diabetic mice are enhanced by the absence of B2R.82,95 In agreement with these observations, adeno-associated virus-mediated expression of the human tissue kallikrein has been shown to mitigate nephropathy induced by STZ and high-fat diet as assessed by urinary albumin excretion, histological changes, creatinine clearance, and urinary osmolarity.96 However, Tan et al.97 have reported that deletion of B2R protects against the albuminuria and histological changes which develop in diabetic nephropathy induced by STZ. These opposite results may be due to the differences in the strains of mice used and/ or in the method of induction of diabetes.
The importance of NO in relation to experimental diabetic nephropathy is well documented. Thus, L-arginine, the substrate of the NO synthases, reduces the proteinuria that develops in STZ-induced diabetic rats.98 Furthermore, L-NAME, an NOS inhibitor, aggravates the proteinuria and histological changes that occur in the diabetic nephropathy of Otsuka Long-Evans Tokushima Fatty rats.99 eNOS deficiency also accelerates the severity of diabetic nephropathy in C57BLKS/J db/db mice,100 lepr(db/db) mice,101 and STZ-treated C57BL/6 mice.102 As bradykinin induces eNOS activity,40 these observations bear directly on how the KKS exerts its protective effects.
Cicaprost, a PGI2 analog, also attenuates the progression of diabetic renal injury in STZ-treated rats,103 suggesting that the beneficial effects of the KKS also involve the PG arm of the system.
Hypertensive glomerulosclerosis and other chronic fibrogenic kidney diseases
In Dahl salt-sensitive rats, an animal model of salt-sensitive hypertension, an ACE inhibitor reduced the proteinuria, urinary excretion of N-acetyl-β-d-glucosaminidase (an indicator of damages in proximal tubules), and renal fibrotic changes caused by high salt. The protective effects of an angiotensin II type 1 receptor blocker (ARB) were, however, much smaller than those of the ACE inhibitor, despite the same reduction of blood pressure.104 In Ren-2 transgenic rats, an ACE inhibitor or a vasopeptidase inhibitor significantly reduced tubulointerstitial fibrosis, and this reduction was annulled by a B2R antagonist.105 Long-term infusion of rat urinary kallikrein106 or bradykinin107 into Dahl salt-sensitive rats attenuated their urinary protein excretion and glomerulosclerosis, despite no changes in blood pressure. The beneficial effect of infused kallikrein was attenuated by a B2R antagonist.108 Adenovirus-mediated human tissue kallikrein gene delivery caused a similar favorable effect in both Dahl salt-sensitive rats and Goldblatt hypertensive rats, again without affecting blood pressure.109,110
In rats with passive Heymann nephritis, an experimental model of human membranous nephropathy, an ACE inhibitor exerted antiproteinuric action, which was prevented by a B2R antagonist.111
Alport syndrome is a hereditary cause of endstage renal disease (ESRD) due to defects in type IV collagen genes. In mice lacking the COL4A3 gene, an animal model of Alport syndrome, an ACE inhibitor reduced the proteinuria and renal interstitial fibrosis.112 In subsequent experiments, it was shown that the ACE inhibitor extended the lifespan of the COL4A3-null mice by 111%, whereas an ARB resulted in only a 38% prolongation of the lifespan,113 emphasizing the importance of non-angiotensin II mechanisms in helping survival of these animals.
Unilateral ureteral obstruction in rodents is a well-established non-immune inflammatory experimental model that results in tubulointerstitial fibrosis in the obstructed kidney. ACE inhibitors,114 and to a lesser extent ARBs,115 prevent the progression of tubulointerstitial fibrosis in this unilateral ureteral obstruction models. Co-treatment of the animals with an ACE inhibitor and a NOS inhibitor reversed the beneficial effect of the ACE inhibitor in the obstructed kidney.116 Unilateral ureteral obstruction-induced fibrosis is more severe in B2R-null mice than wild-type mice, and less severe in the human tissue kallikrein-transgenic rats than in control Sprague Dawley rats.117 These several experiments together provide a strong case for the importance of the KKS in limiting the tubulointerstitial fibrosis caused by unilateral ureteral obstruction.
Aminoglycoside-induced renal injury
Aminoglycoside antibiotics have been used primarily to repress infection by aerobic Gram-negative bacteria. They are, however, notorious for their ototoxicity and nephrotoxi-city, although how they cause the proximal tubular cell necrosis that constitutes their renal effects is largely unknown. However, in this context it is intriguing that the administration of tissue kallikrein protein118 or tissue kallikrein-expressing adenovirus119 are both protective against gentamicin-induced renal injury in rats. NAD(P)H oxidase activity and superoxide production in the kidney are elevated by gentamicin treatment.120 Administration of tissue kallikrein partially reverses these increases,118 indicating that enhancing KKS activity can reduce gentamicin-induced renal injury.
Other studies have shown that the NOS substrate L-arginine prevents gentamicin-induced tubular damage in rats,121 whereas the NOS inhibitor NG-nitro-l-arginine-methyl ester increases the damage.121,122 PGI2 overexpression in renal tubular cells also prevented apoptosis induced by gentamicin, and decreased the generation of reactive oxygen species induced by the drug.123 Thus, the protective effect of tissue kallikrein against gentamicin-induced renal injury is probably mediated by its ability to increase both NO and PGI2.
Ischemia-reperfusion injury
The angiotensin-I-converting enzyme inhibitors markedly and consistently reduce the tissue injury that occurs in ischemic acute renal failure (iARF), including the tubular necrosis, loss of endothelium-dependent vasorelaxation, and excretory dysfunction.124,125 In contrast, it is still debatable whether ARBs have any beneficial effects on the tissue damage caused by iARF.126,127 There is agreement, however, that ACE inhibitors are more effective than ARBs in protecting against ischemia-reperfusion injury.127–130 Furthermore, a B2R antagonist and NOS blocker markedly attenuate the protective effects of the ACE inhibitors.128,129 These observations support the concept that, in many contexts, ACE inhibitors are beneficial more by inhibiting the inactivation of the kinins than by suppressing angiotensin II formation.
Recent studies are beginning to provide a more detailed explanation for this protection. Thus, it is now known that ischemia-reperfusion injuries are associated with mitochondrial Ca2+ overload consequent to a burst of reactive oxygen species, which together trigger the opening of mitochondrial permeability transition pores leading to cell apoptosis.131 This opening of mitochondrial pores is suppressed by bradykinin.132 As NO, a second messenger of both bradykinin receptors, reversibly suppresses mitochondrial oxidative metabolism,74,75 it is possible that the KKS can reduce or prevent the burst of reactive oxygen species.
In contrast to the consensus that ACE inhibitors have beneficial effects on ischemia-reperfusion injury, in part mediated by the KKS, there are conflicting reports on the effects of administered bradykinin on iARF. Thus, exogenous supplementation of bradykinin has been reported to aggravate iARF,133 yet suppression of the endogenous KKS49 is detrimental to functional recovery after iARF. These findings suggest that physiological levels of bradykinin produced endogenously, but not the presumably higher levels achieved with exogenously administered bradykinin, play a beneficial role in iARF. In agreement with this interpretation, adenovirus-mediated gene transfer of tissue kallikrein has been shown to protect against ischemic stroke in rats.134 Similarly, transgenic expression of tissue kallikrein in mice attenuates ischemic cardiac damage,135 whereas knocking out tissue kallikrein aggravates the damage.28
We have found that lack of B2R alone or lack of both receptors, B1R and B2R, aggravates the renal morphological and functional damages and mortality following iARF.49 Furthermore, the absence of both B1R and B2R had more detrimental effects than deficiency in B2R only, showing that both B1R and B2R are protective in iARF.49 As NO donors attenuate136 and NOS inhibitors aggravate tissue damage in iARF,137,138 endothelium-derived NO is most likely to be one of the mediators of the beneficial effect of bradykinin. In addition, earlier studies have shown that prostaglandins E1, E2, and I2139–143 are protective in mitigating renal injury caused by iARF, suggesting that phospholipase A2-derived products are involved in bradykinin-induced protection following iARF.
Autosomal dominant polycystic kidney disease
Autosomal dominant polycystic kidney disease (ADPKD) accounts for 10% of cases of ESRD. In the heterozygous cystic Han:Sprague-Dawley-cy rat, which is an animal model of ADPKD, urinary kallikrein and bradykinin levels are increased compared with age-matched controls.144 ACE inhibition significantly reduces the albuminuria and prevents the enlargement of kidney size and decline in glomerular filtration rate, independently of its effects on blood pressure,145–148 suggesting that the KKS has a protective role in this ADPKD model. However, it has been reported that a B2R antagonist significantly reduced proteinuria and albu-minuria in this model,148 and in human ADPKD patients, the KKS system is not activated.144
Chronic renal failure
Irrespective of their etiology, most renal diseases eventually lead to a reduction in the number of nephrons and to insufficiency of renal excretory function and chronic renal failure (CRF). The rate of decline in creatinine clearance accelerates as the absolute value of creatinine clearance is reduced, suggesting overload on the remaining nephrons (‘hyperfiltration theory’).149 In the 5/6 nephrectomy animal model of CRF, ACE inhibitors,118,150 adenovirus virus-mediated tissue kallikrein gene delivery,151 or adeno-associated virus delivery,152 all decelerate the decline in renal function. However, whether a B2R antagonist abrogates the beneficial efficacy of ACE inhibitors in this 5/6 renal mass reduction model is debatable.118,150 Nevertheless, as renal pathology and mortality in this model are both exaggerated by eNOS deficiency,153 it is most likely that changes in eNOS activity are partly responsible for the beneficial effect of KKS in CRF.
RELEVANCE OF THE KKS TO RENAL DISEASES IN HUMANS
Studies of mice in which genes have been knocked out have proved invaluable in determining the role of many genes in mammals. However, the common human diseases, such as diabetes and hypertension, do not appear to be prevalent because of the complete absence of any gene function. Rather they appear to be due to a variety of combinations of genetic differences that individually have relatively small quantitative effects.154 Furthermore, humans are genetically more hetero-genous than inbred experimental animals. For these reasons, it has been proved difficult to identify genetic differences in humans that influence these common multifactorial conditions. Nevertheless, polymorphisms in several KKS-related genes have been associated with the risks of developing a number of renal problems. Thus, the insertion/deletion ACE polymorphism has been clearly shown to influence diabetic nephropathy.5 And a polymorphism in the human B2R gene has been correlated with altered urinary albumin/creatinine values in diabetic patients.155 Similarly, a polymorphism in intron 4 of the Nos3 gene has been associated with an increased risk for nephropathy in patients with either type 1156 or type 2 diabetes.157 Other reports have shown that the ACE D/D genotype is a risk factor for progression to CRF in IgA nephropathy.158,159 ADPKD patients with the ACE D/D genotype have a 5–10 years earlier onset of ESRD than those with the I/I genotype.160–162 The progression of IgA nephropathy and ADPKD into ESRD are both influenced by the Nos3 polymorphism.163 In humans, polymorphisms in most of the genes in the KKS have also been associated with the progression of CRF into ESRD, including ACE,164,165 Bdkrb1,166,167 Bdkrb2,167,168 and Nos3.169,170
THERAPEUTIC IMPLICATIONS
Of the presently available drugs, the ACE inhibitors are most effective in enhancing the KKS, and many of their benefits are independent of blood pressure lowering. Besides retarding the decline in renal function, the KKS may be of particular relevance in the processes of angiogenesis and cardiac regeneration, following myocardial infarction.171 The recently developed vasopeptidase inhibitors that inhibit ACE, neprilysin and/or endothelin-converting enzyme, all of which degrade the kinins, may also prove to be beneficial, when they become available for clinical use.172 Yet the ACE inhibitors are not suitable for use in pregnancy because of teratogeni-city, nor are they recommended in patients with CRF who are not under dialysis therapy, because they are susceptible to cardiotoxic hyperkalemia.173 Additionally, some individuals have to discontinue their usage because of coughing. At present, no drugs are available that enhance KKS specifically without affecting the renin–angiotensin–aldoster-one system.
In principle, KKS-specific antagonists and agonists could both be useful, although for different purposes. KKS antagonists could be used for acute life-threatening inflammatory conditions including septic shock,174 asthma,175 acute pancreatitis,176 and attacks in patients with hereditary angioedema.177 However, because the KKS is important for retaining renal blood flow and suppressing oxidative stress by NO and the PGs, long-term usage of KKS antagonists is most likely to be undesirable. KKS-specific agonists (represented currently almost exclusively by ACE inhibitors) have, in contrast, proved effective in long-term usage for the treatment of senescence-associated renal diseases. Two of the adverse effects of ACE inhibitors, teratogenicity and cardiotoxic hyperkalemia, might be avoidable by developing bradykinin receptor-specific agonists, which would probably have minimal effects on the renin–angiotensin–aldosterone system. If the receptor-specific agonists proved not to have the adverse effects of the ACE inhibitors, they could be useful for the treatment of diabetes and/or fibrogenic renal diseases in pregnant women, and in non-dialysed patients with advanced CRF.
CONCLUSIONS
The KKS affects a variety of physiological and pathophysiological functions in mammals including pain, inflammation, vascular permeability, oxidative stress, and calcium home-ostasis.178,179 Of the currently available agents that affect the KKS, the ACE inhibitors are most important. Thus, although ACE was initially discovered as a component of renin– angiotensin–aldosterone system, it has a greater affinity for bradykinin than for angiotensin I. This accounts for the fact that ACE inhibitors and the ACE insertion polymorphism have beneficial effects on a number of fibrogenic kidney diseases independently of changes in blood pressure and angiotensin II levels. The importance of the KKS in renoprotection is now well established. Thus, many studies have shown that the KKS inhibits the development and progression of a variety of kidney diseases and senescence partly by NO and PGs, both of which shift metabolism away from mitochondrial respiration towards glycolysis. An interesting possibility is that KKS-specific drugs could be used to alter the balance between oxidative and non-oxidative metabolism, thereby providing a new way of decreasing oxidative stress.
ACKNOWLEDGMENTS
This study was supported by Career Development Award (no. 2-2006-108) from Juvenile Diabetes Research Foundation International to M.K. and National Institutes of Health grants HL49277, HL70523, and HL71266 to O.S. We are grateful to Dr J. Charles Jennette for his critical reading of our manuscript.
Footnotes
DISCLOSURE
The authors declared no competing interests.
REFERENCES
- 1.Jaspard E, Wei L, Alhenc-Gelas F. Differences in the properties and enzymatic specificities of the two active sites of angiotensin I-converting enzyme (kininase II). Studies with bradykinin and other natural peptides. J Biol Chem. 1993;268:9496–9503. [PubMed] [Google Scholar]
- 2.Rigat B, Hubert C, Alhenc-Gelas F, et al. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lachurie ML, Azizi M, Guyene TT, et al. Angiotensin-converting enzyme gene polymorphism has no influence on the circulating renin-angiotensin-aldosterone system or blood pressure in normotensive subjects. Circulation. 1995;91:2933–2942. doi: 10.1161/01.cir.91.12.2933. [DOI] [PubMed] [Google Scholar]
- 4.Krege JH, Kim HS, Moyer JS, et al. Angiotensin-converting enzyme gene mutations, blood pressures, and cardiovascular homeostasis. Hypertension. 1997;29(1 Part 2):150–157. doi: 10.1161/01.hyp.29.1.150. [DOI] [PubMed] [Google Scholar]
- 5.Marre M, Bernadet P, Gallois Y, et al. Relationships between angiotensin I converting enzyme gene polymorphism, plasma levels, and diabetic retinal and renal complications. Diabetes. 1994;43:384–388. doi: 10.2337/diab.43.3.384. [DOI] [PubMed] [Google Scholar]
- 6.Koh WP, Yuan JM, Sun CL, et al. Angiotensin I-converting enzyme (ACE) gene polymorphism and breast cancer risk among Chinese women in Singapore. Cancer Res. 2003;63:573–578. [PubMed] [Google Scholar]
- 7.Gonzalez-Zuloeta Ladd AM, Vasquez AA, Sayed-Tabatabaei FA, et al. Angiotensin-converting enzyme gene insertion/deletion polymorphism and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:2143–2146. doi: 10.1158/1055-9965.EPI-05-0045. [DOI] [PubMed] [Google Scholar]
- 8.Yaren A, Turgut S, Kursunluoglu R, et al. Insertion/deletion polymorphism of the angiotensin I-converting enzyme gene in patients with breast cancer and effects on prognostic factors. J Investig Med. 2007;55:255–261. doi: 10.2310/6650.2007.00006. [DOI] [PubMed] [Google Scholar]
- 9.Medeiros R, Vasconcelos A, Costa S, et al. Linkage of angiotensin I-converting enzyme gene insertion/deletion polymorphism to the progression of human prostate cancer. J Pathol. 2004;202:330–335. doi: 10.1002/path.1529. [DOI] [PubMed] [Google Scholar]
- 10.Yigit B, Bozkurt N, Narter F, et al. Effects of ACE I/D polymorphism on prostate cancer risk, tumor grade and metastatis. Anticancer Res. 2007;27:933–936. [PubMed] [Google Scholar]
- 11.Ebert MP, Lendeckel U, Westphal S, et al. The angiotensin I-converting enzyme gene insertion/deletion polymorphism is linked to early gastric cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2987–2989. doi: 10.1158/1055-9965.EPI-05-0411. [DOI] [PubMed] [Google Scholar]
- 12.Rocken C, Lendeckel U, Dierkes J, et al. The number of lymph node metastases in gastric cancer correlates with the angiotensin I-converting enzyme gene insertion/deletion polymorphism. Clin Cancer Res. 2005;11:2526–2530. doi: 10.1158/1078-0432.CCR-04-1922. [DOI] [PubMed] [Google Scholar]
- 13.Farrer LA, Sherbatich T, Keryanov SA, et al. Association between angiotensin-converting enzyme and Alzheimer disease. Arch Neurol. 2000;57:210–214. doi: 10.1001/archneur.57.2.210. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Zagato L, Kuznetsova T, et al. Angiotensin-converting enzyme I/D and alpha-adducin Gly460Trp polymorphisms: from angiotensin-converting enzyme activity to cardiovascular outcome. Hypertension. 2007;49:1291–1297. doi: 10.1161/HYPERTENSIONAHA.106.085498. [DOI] [PubMed] [Google Scholar]
- 15.Raynolds MV, Bristow MR, Bush EW, et al. Angiotensin-converting enzyme DD genotype in patients with ischaemic or idiopathic dilated cardiomyopathy. Lancet. 1993;342:1073–1075. doi: 10.1016/0140-6736(93)92061-w. [DOI] [PubMed] [Google Scholar]
- 16.Cambien F, Poirier O, Lecerf L, et al. Deletion polymorphism in the gene for angiotensin-converting enzyme is a potent risk factor for myocardial infarction. Nature. 1992;359:641–644. doi: 10.1038/359641a0. [DOI] [PubMed] [Google Scholar]
- 17.Doi Y, Yoshinari M, Yoshizumi H, et al. Polymorphism of the angiotensin-converting enzyme (ACE) gene in patients with thrombotic brain infarction. Atherosclerosis. 1997;132:145–150. doi: 10.1016/s0021-9150(97)00080-4. [DOI] [PubMed] [Google Scholar]
- 18.Hamdi HK, Reznik J, Castellon R, et al. Alu DNA polymorphism in ACE gene is protective for age-related macular degeneration. Biochem Biophys Res Commun. 2002;295:668–672. doi: 10.1016/s0006-291x(02)00728-3. [DOI] [PubMed] [Google Scholar]
- 19.Huang W, Gallois Y, Bouby N, et al. Genetically increased angiotensin I-converting enzyme level and renal complications in the diabetic mouse. Proc Natl Acad Sci USA. 2001;98:13330–13334. doi: 10.1073/pnas.231476798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell DJ, Kladis A, Duncan AM. Effects of converting enzyme inhibitors on angiotensin and bradykinin peptides. Hypertension. 1994;23:439–449. doi: 10.1161/01.hyp.23.4.439. [DOI] [PubMed] [Google Scholar]
- 21.Smithies O, Kim HS, Takahashi N, et al. Importance of quantitative genetic variations in the etiology of hypertension. Kidney Int. 2000;58:2265–2280. doi: 10.1046/j.1523-1755.2000.00411.x. [DOI] [PubMed] [Google Scholar]
- 22.Shesely EG, Hu CB, Alhenc-Gelas F, et al. A second expressed kininogen gene in mice. Physiol Genomics. 2006;26:152–157. doi: 10.1152/physiolgenomics.00244.2005. [DOI] [PubMed] [Google Scholar]
- 23.Skidgel RA, Engelbrecht S, Johnson AR, et al. Hydrolysis of substance p and neurotensin by converting enzyme and neutral endopeptidase. Peptides. 1984;5:769–776. doi: 10.1016/0196-9781(84)90020-2. [DOI] [PubMed] [Google Scholar]
- 24.Hoang MV, Turner AJ. Novel activity of endothelin-converting enzyme: hydrolysis of bradykinin. Biochem J. 1997;327(Part 1):23–26. doi: 10.1042/bj3270023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cayla C, Todiras M, Iliescu R, et al. Mice deficient for both kinin receptors are normotensive and protected from endotoxin-induced hypotension. FASEB J. 2007;21:1689–1698. doi: 10.1096/fj.06-7175com. [DOI] [PubMed] [Google Scholar]
- 26.Schremmer-Danninger E, Offner A, Siebeck M, et al. B1 bradykinin receptors and carboxypeptidase M are both upregulated in the aorta of pigs after LPS infusion. Biochem Biophys Res Commun. 1998;243:246–252. doi: 10.1006/bbrc.1997.7999. [DOI] [PubMed] [Google Scholar]
- 27.Spillmann F, Altmann C, Scheeler M, et al. Regulation of cardiac bradykinin B1- and B2-receptor mRNA in experimental ischemic, diabetic, and pressure-overload-induced cardiomyopathy. Int Immunopharmacol. 2002;2:1823–1832. doi: 10.1016/s1567-5769(02)00174-1. [DOI] [PubMed] [Google Scholar]
- 28.Griol-Charhbili V, Messadi-Laribi E, Bascands JL, et al. Role of tissue kallikrein in the cardioprotective effects of ischemic and pharmacological preconditioning in myocardial ischemia. FASEB J. 2005;19:1172–1174. doi: 10.1096/fj.04-3508fje. [DOI] [PubMed] [Google Scholar]
- 29.Duka I, Kintsurashvili E, Gavras I, et al. Vasoactive potential of the b(1) bradykinin receptor in normotension and hypertension. Circ Res. 2001;88:275–281. doi: 10.1161/01.res.88.3.275. [DOI] [PubMed] [Google Scholar]
- 30.Marin-Castano ME, Schanstra JP, Praddaude F, et al. Differential induction of functional B1-bradykinin receptors along the rat nephron in endotoxin induced inflammation. Kidney Int. 1998;54:1888–1898. doi: 10.1046/j.1523-1755.1998.00201.x. [DOI] [PubMed] [Google Scholar]
- 31.Couture R, Girolami JP. Putative roles of kinin receptors in the therapeutic effects of angiotensin 1-converting enzyme inhibitors in diabetes mellitus. Eur J Pharmacol. 2004;500:467–485. doi: 10.1016/j.ejphar.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 32.Riad A, Zhuo JL, Schultheiss HP, et al. The role of the renal kallikrein-kinin system in diabetic nephropathy. Curr Opin Nephrol Hypertens. 2007;16:22–26. doi: 10.1097/MNH.0b013e328011a20c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venema VJ, Ju H, Sun J, et al. Bradykinin stimulates the tyrosine phosphorylation and bradykinin B2 receptor association of phospholipase C gamma 1 in vascular endothelial cells. Biochem Biophys Res Commun. 1998;246:70–75. doi: 10.1006/bbrc.1998.8574. [DOI] [PubMed] [Google Scholar]
- 34.Duchene J, Chauhan SD, Lopez F, et al. Direct protein-protein interaction between PLCgamma1 and the bradykinin B2 receptor–importance of growth conditions. Biochem Biophys Res Commun. 2005;326:894–900. doi: 10.1016/j.bbrc.2004.11.126. [DOI] [PubMed] [Google Scholar]
- 35.D’Orleans-Juste P, de Nucci G, Vane JR. Kinins act on B1 or B2 receptors to release conjointly endothelium-derived relaxing factor and prostacyclin from bovine aortic endothelial cells. Br J Pharmacol. 1989;96:920–926. doi: 10.1111/j.1476-5381.1989.tb11903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drummond GR, Cocks TM. Endothelium-dependent relaxations mediated by inducible B1 and constitutive B2 kinin receptors in the bovine isolated coronary artery. Br J Pharmacol. 1995;116:2473–2481. doi: 10.1111/j.1476-5381.1995.tb15098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zubakova R, Gille A, Faussner A, et al. Ca2+ signalling of kinins in cells expressing rat, mouse and human B1/B2-receptor. Int Immunopharmacol. 2008;8:276–281. doi: 10.1016/j.intimp.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Hilgenfeldt U, Linke R, Riester U, et al. Strategy of measuring bradykinin and kallidin and their concentration in plasma and urine. Anal Biochem. 1995;228:35–41. doi: 10.1006/abio.1995.1311. [DOI] [PubMed] [Google Scholar]
- 39.Liu X, Lukasova M, Zubakova R, et al. Kallidin-like peptide mediates the cardioprotective effect of the ACE inhibitor captopril against ischaemic reperfusion injury of rat heart. Br J Pharmacol. 2006;148:825–832. doi: 10.1038/sj.bjp.0706799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sangsree S, Brovkovych V, Minshall RD, et al. Kininase I-type carboxypeptidases enhance nitric oxide production in endothelial cells by generating bradykinin B1 receptor agonists. Am J Physiol Heart Circ Physiol. 2003;284:H1959–H1968. doi: 10.1152/ajpheart.00036.2003. [DOI] [PubMed] [Google Scholar]
- 41.Pizard A, Blaukat A, Muller-Esterl W, et al. Bradykinin-induced internalization of the human B2 receptor requires phosphorylation of three serine and two threonine residues at its carboxyl tail. J Biol Chem. 1999;274:12738–12747. doi: 10.1074/jbc.274.18.12738. [DOI] [PubMed] [Google Scholar]
- 42.Simaan M, Bedard-Goulet S, Fessart D, et al. Dissociation of beta-arrestin from internalized bradykinin B2 receptor is necessary for receptor recycling and resensitization. Cell Signal. 2005;17:1074–1083. doi: 10.1016/j.cellsig.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Enquist J, Skroder C, Whistler JL, et al. Kinins promote B2 receptor endocytosis and delay constitutive B1 receptor endocytosis. Mol Pharmacol. 2007;71:494–507. doi: 10.1124/mol.106.030858. [DOI] [PubMed] [Google Scholar]
- 44.Prabhakar P, Thatte HS, Goetz RM, et al. Receptor-regulated translocation of endothelial nitric-oxide synthase. J Biol Chem. 1998;273:27383–27388. doi: 10.1074/jbc.273.42.27383. [DOI] [PubMed] [Google Scholar]
- 45.Wang DZ, Chao L, Chao J. Hypotension in transgenic mice overexpressing human bradykinin B2 receptor. Hypertension. 1997;29(1 Part 2):488–493. doi: 10.1161/01.hyp.29.1.488. [DOI] [PubMed] [Google Scholar]
- 46.Ni A, Yin H, Agata J, et al. Overexpression of kinin B1 receptors induces hypertensive response to des-Arg9-bradykinin and susceptibility to inflammation. J Biol Chem. 2003;278:219–225. doi: 10.1074/jbc.M209490200. [DOI] [PubMed] [Google Scholar]
- 47.Milia AF, Gross V, Plehm R, et al. Normal blood pressure and renal function in mice lacking the bradykinin B(2) receptor. Hypertension. 2001;37:1473–1479. doi: 10.1161/01.hyp.37.6.1473. [DOI] [PubMed] [Google Scholar]
- 48.Pesquero JB, Araujo RC, Heppenstall PA, et al. Hypoalgesia and altered inflammatory responses in mice lacking kinin B1 receptors. Proc Natl Acad Sci USA. 2000;97:8140–8145. doi: 10.1073/pnas.120035997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kakoki M, McGarrah RW, Kim HS, et al. Bradykinin B1 and B2 receptors both have protective roles in renal ischemia/reperfusion injury. Proc Natl Acad Sci USA. 2007;104:7576–7581. doi: 10.1073/pnas.0701617104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meneton P, Bloch-Faure M, Hagege AA, et al. Cardiovascular abnormalities with normal blood pressure in tissue kallikrein-deficient mice. Proc Natl Acad Sci USA. 2001;98:2634–2639. doi: 10.1073/pnas.051619598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rhaleb NE, Yang XP, Nanba M, et al. Effect of chronic blockade of the kallikrein-kinin system on the development of hypertension in rats. Hypertension. 2001;37:121–128. doi: 10.1161/01.hyp.37.1.121. [DOI] [PubMed] [Google Scholar]
- 52.Schanstra JP, Duchene J, Praddaude F, et al. Decreased renal NO excretion and reduced glomerular tuft area in mice lacking the bradykinin B2 receptor. Am J Physiol Heart Circ Physiol. 2003;284:H1904–H1908. doi: 10.1152/ajpheart.01150.2002. [DOI] [PubMed] [Google Scholar]
- 53.Mayer B, Hemmens B. Biosynthesis and action of nitric oxide in mammalian cells. Trends Biochem Sci. 1997;22:477–481. doi: 10.1016/s0968-0004(97)01147-x. [DOI] [PubMed] [Google Scholar]
- 54.Talukder MA, Fujiki T, Morikawa K, et al. Up-regulated neuronal nitric oxide synthase compensates coronary flow response to bradykinin in endothelial nitric oxide synthase-deficient mice. J Cardiovasc Pharmacol. 2004;44:437–445. doi: 10.1097/01.fjc.0000139450.64337.cd. [DOI] [PubMed] [Google Scholar]
- 55.Harris MB, Ju H, Venema VJ, et al. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J Biol Chem. 2001;276:16587–16591. doi: 10.1074/jbc.M100229200. [DOI] [PubMed] [Google Scholar]
- 56.Ju H, Venema VJ, Marrero MB, et al. Inhibitory interactions of the bradykinin B2 receptor with endothelial nitric-oxide synthase. J Biol Chem. 1998;273:24025–24029. doi: 10.1074/jbc.273.37.24025. [DOI] [PubMed] [Google Scholar]
- 57.Harris MB, Ju H, Venema VJ, et al. Role of heat shock protein 90 in bradykinin-stimulated endothelial nitric oxide release. Gen Pharmacol. 2000;35:165–170. doi: 10.1016/s0306-3623(01)00104-5. [DOI] [PubMed] [Google Scholar]
- 58.Ignjatovic T, Stanisavljevic S, Brovkovych V, et al. Kinin B1 receptors stimulate nitric oxide production in endothelial cells: signaling pathways activated by angiotensin I-converting enzyme inhibitors and peptide ligands. Mol Pharmacol. 2004;66:1310–1316. doi: 10.1124/mol.104.001990. [DOI] [PubMed] [Google Scholar]
- 59.Savard M, Barbaz D, Belanger S, et al. Expression of endogenous nuclear bradykinin B2 receptors mediating signaling in immediate early gene activation. J Cell Physiol. 2008;216:234–244. doi: 10.1002/jcp.21398. [DOI] [PubMed] [Google Scholar]
- 60.Ueno A, Ishida H, Oh-ishi S. Comparative study of endotoxin-induced hypotension in kininogen-deficient rats with that in normal rats. Br J Pharmacol. 1995;114:1250–1256. doi: 10.1111/j.1476-5381.1995.tb13340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lal MA, Kennedy CR, Proulx PR, et al. Bradykinin-stimulated cPLA2 phosphorylation is protein kinase C dependent in rabbit CCD cells. Am J Physiol. 1997;273(6 Part 2):F907–F915. doi: 10.1152/ajprenal.1997.273.6.F907. [DOI] [PubMed] [Google Scholar]
- 62.Kennedy C, Proulx PR, Hebert RL. Bradykinin-induced translocation of cytoplasmic phospholipase A2 in MDCK cells. Can J Physiol Pharmacol. 1997;75:563–567. [PubMed] [Google Scholar]
- 63.Libano-Soares JD, Gomes-Quintana E, Melo HK, et al. B2 receptor-mediated dual effect of bradykinin on proximal tubule Na+ -ATPase: sequential activation of the phosphoinositide-specific phospholipase Cbeta/protein kinase C and Ca2+ -independent phospholipase A2 pathways. Biochim Biophys Acta. 2008;1778:1316–1323. doi: 10.1016/j.bbamem.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez JA, De la Cerda P, Collyer E, et al. Cyclooxygenase-2 induction by bradykinin in aortic vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;290:H30–H36. doi: 10.1152/ajpheart.00349.2005. [DOI] [PubMed] [Google Scholar]
- 65.Rodriguez JA, Vio CP, Pedraza PL, et al. Bradykinin regulates cyclooxygenase-2 in rat renal thick ascending limb cells. Hypertension. 2004;44:230–235. doi: 10.1161/01.HYP.0000136751.04336.e9. [DOI] [PubMed] [Google Scholar]
- 66.Imig JD, Zhao X, Orengo SR, et al. The bradykinin B2 receptor is required for full expression of renal COX-2 and renin. Peptides. 2003;24:1141–1147. doi: 10.1016/j.peptides.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 67.Welsh C, Dubyak G, Douglas JG. Relationship between phospholipase C activation and prostaglandin E2 and cyclic adenosine monophosphate production in rabbit tubular epithelial cells. Effects of angiotensin, bradykinin, and arginine vasopressin. J Clin Invest. 1988;81:710–719. doi: 10.1172/JCI113376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liao JK, Homcy CJ. The G proteins of the G alpha i and G alpha q family couple the bradykinin receptor to the release of endothelium-derived relaxing factor. J Clin Invest. 1993;92:2168–2172. doi: 10.1172/JCI116818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dray A, Perkins M. Bradykinin and inflammatory pain. Trends Neurosci. 1993;16:99–104. doi: 10.1016/0166-2236(93)90133-7. [DOI] [PubMed] [Google Scholar]
- 70.Uknis AB, DeLa Cadena RA, Janardham R, et al. Bradykinin receptor antagonists type 2 attenuate the inflammatory changes in peptidoglycan-induced acute arthritis in the Lewis rat. Inflamm Res. 2001;50:149–155. doi: 10.1007/s000110050739. [DOI] [PubMed] [Google Scholar]
- 71.Hecker M, Bara AT, Bauersachs J, et al. Characterization of endothelium-derived hyperpolarizing factor as a cytochrome P450-derived arachidonic acid metabolite in mammals. J Physiol. 1994;481(Part 2):407–414. doi: 10.1113/jphysiol.1994.sp020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Campbell WB, Gebremedhin D, Pratt PF, et al. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 73.Duchene J, Schanstra JP, Pecher C, et al. A novel protein-protein interaction between a G protein-coupled receptor and the phosphatase SHP-2 is involved in bradykinin-induced inhibition of cell proliferation. J Biol Chem. 2002;277:40375–40383. doi: 10.1074/jbc.M202744200. [DOI] [PubMed] [Google Scholar]
- 74.Adler S, Huang H, Loke KE, et al. Endothelial nitric oxide synthase plays an essential role in regulation of renal oxygen consumption by NO. Am J Physiol Renal Physiol. 2001;280:F838–F843. doi: 10.1152/ajprenal.2001.280.5.F838. [DOI] [PubMed] [Google Scholar]
- 75.Brown GC, Bolanos JP, Heales SJ, et al. Nitric oxide produced by activated astrocytes rapidly and reversibly inhibits cellular respiration. Neurosci Lett. 1995;193:201–204. doi: 10.1016/0304-3940(95)11703-y. [DOI] [PubMed] [Google Scholar]
- 76.Sarti P, Lendaro E, Ippoliti R, et al. Modulation of mitochondrial respiration by nitric oxide: investigation by single cell fluorescence microscopy. FASEB J. 1999;13:191–197. doi: 10.1096/fasebj.13.1.191. [DOI] [PubMed] [Google Scholar]
- 77.Brunori M, Giuffre A, Forte E, et al. Control of cytochrome c oxidase activity by nitric oxide. Biochim Biophys Acta. 2004;1655:365–371. doi: 10.1016/j.bbabio.2003.06.008. [DOI] [PubMed] [Google Scholar]
- 78.Lee I, Salomon AR, Ficarro S, et al. cAMP-dependent tyrosine phosphorylation of subunit I inhibits cytochrome c oxidase activity. J Biol Chem. 2005;280:6094–6100. doi: 10.1074/jbc.M411335200. [DOI] [PubMed] [Google Scholar]
- 79.Piccoli C, Scacco S, Bellomo F, et al. cAMP controls oxygen metabolism in mammalian cells. FEBS Lett. 2006;580:4539–4543. doi: 10.1016/j.febslet.2006.06.085. [DOI] [PubMed] [Google Scholar]
- 80.Mikrut K, Paluszak J, Kozlik J, et al. The effect of bradykinin on the oxidative state of rats with acute hyperglycaemia. Diabetes Res Clin Pract. 2001;51:79–85. doi: 10.1016/s0168-8227(00)00222-9. [DOI] [PubMed] [Google Scholar]
- 81.Golser R, Gorren AC, Leber A, et al. Interaction of endothelial and neuronal nitric-oxide synthases with the bradykinin B2 receptor. Binding of an inhibitory peptide to the oxygenase domain blocks uncoupled NADPH oxidation. J Biol Chem. 2000;275:5291–5296. doi: 10.1074/jbc.275.8.5291. [DOI] [PubMed] [Google Scholar]
- 82.Kakoki M, Kizer CM, Yi X, et al. Senescence-associated phenotypes in Akita diabetic mice are enhanced by absence of bradykinin B2 receptors. J Clin Invest. 2006;116:1302–1309. doi: 10.1172/JCI26958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brunk UT, Terman A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic Biol Med. 2002;33:611–619. doi: 10.1016/s0891-5849(02)00959-0. [DOI] [PubMed] [Google Scholar]
- 84.Yumura W, Imasawa T, Suganuma S, et al. Accelerated tubular cell senescence in SMP30 knockout mice. Histol Histopathol. 2006;21:1151–1156. doi: 10.14670/HH-21.1151. [DOI] [PubMed] [Google Scholar]
- 85.Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Michikawa Y, Mazzucchelli F, Bresolin N, et al. Aging-dependent large accumulation of point mutations in the human mtDNA control region for replication. Science. 1999;286:774–779. doi: 10.1126/science.286.5440.774. [DOI] [PubMed] [Google Scholar]
- 87.Tanhauser SM, Laipis PJ. Multiple deletions are detectable in mitochondrial DNA of aging mice. J Biol Chem. 1995;270:24769–24775. doi: 10.1074/jbc.270.42.24769. [DOI] [PubMed] [Google Scholar]
- 88.Gajewska M, Gajkowska B, Motyl T. Apoptosis and autophagy induced by TGF-B1 in bovine mammary epithelial BME-UV1 cells. J Physiol Pharmacol. 2005;56(Suppl 3):143–157. [PubMed] [Google Scholar]
- 89.Kim KH, Park GT, Lim YB, et al. Expression of connective tissue growth factor, a biomarker in senescence of human diploid fibroblasts, is up-regulated by a transforming growth factor-beta-mediated signaling pathway. Biochem Biophys Res Commun. 2004;318:819–825. doi: 10.1016/j.bbrc.2004.04.108. [DOI] [PubMed] [Google Scholar]
- 90.Sharma K, Ziyadeh FN. The emerging role of transforming growth factor-beta in kidney diseases. Am J Physiol. 1994;266(6 Part 2):F829–F842. doi: 10.1152/ajprenal.1994.266.6.F829. [DOI] [PubMed] [Google Scholar]
- 91.Tschope C, Seidl U, Reinecke A, et al. Kinins are involved in the antiproteinuric effect of angiotensin-converting enzyme inhibition in experimental diabetic nephropathy. Int Immunopharmacol. 2003;3:335–344. doi: 10.1016/S1567-5769(02)00273-4. [DOI] [PubMed] [Google Scholar]
- 92.Allard J, Buleon M, Cellier E, et al. ACE inhibitor reduces growth factor receptor expression and signaling but also albuminuria through B2-kinin glomerular receptor activation in diabetic rats. Am J Physiol Renal Physiol. 2007;293:F1083–F1092. doi: 10.1152/ajprenal.00401.2006. [DOI] [PubMed] [Google Scholar]
- 93.Schafer S, Schmidts HL, Bleich M, et al. Nephroprotection in Zucker diabetic fatty rats by vasopeptidase inhibition is partly bradykinin B2 receptor dependent. Br J Pharmacol. 2004;143:27–32. doi: 10.1038/sj.bjp.0705884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Buleon M, Allard J, Jaafar A, et al. Pharmacological blockade of B2-kinin receptor reduces renal protective effect of angiotensin-converting enzyme inhibition in db/db mice model. Am J Physiol Renal Physiol. 2008;294:F1249–F1256. doi: 10.1152/ajprenal.00501.2007. [DOI] [PubMed] [Google Scholar]
- 95.Kakoki M, Takahashi N, Jennette JC, et al. Diabetic nephropathy is markedly enhanced in mice lacking the bradykinin B2 receptor. Proc Natl Acad Sci USA. 2004;101:13302–13305. doi: 10.1073/pnas.0405449101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yuan G, Deng J, Wang T, et al. Tissue kallikrein reverses insulin resistance and attenuates nephropathy in diabetic rats by activation of phosphatidylinositol 3-kinase/protein kinase B and adenosine 5′-monophosphate-activated protein kinase signaling pathways. Endocrinology. 2007;148:2016–2026. doi: 10.1210/en.2006-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tan Y, Keum JS, Wang B, et al. Targeted deletion of B2-kinin receptors protects against the development of diabetic nephropathy. Am J Physiol Renal Physiol. 2007;293:F1026–F1035. doi: 10.1152/ajprenal.00203.2007. [DOI] [PubMed] [Google Scholar]
- 98.Reyes AA, Karl IE, Kissane J, et al. L-arginine administration prevents glomerular hyperfiltration and decreases proteinuria in diabetic rats. J Am Soc Nephrol. 1993;4:1039–1045. doi: 10.1681/ASN.V441039. [DOI] [PubMed] [Google Scholar]
- 99.Kamijo H, Higuchi M, Hora K. Chronic inhibition of nitric oxide production aggravates diabetic nephropathy in Otsuka Long-Evans Tokushima Fatty rats. Nephron Physiol. 2006;104:p12–p22. doi: 10.1159/000093276. [DOI] [PubMed] [Google Scholar]
- 100.Zhao HJ, Wang S, Cheng H, et al. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol. 2006;17:2664–2669. doi: 10.1681/ASN.2006070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mohan S, Reddick RL, Musi N, et al. Diabetic eNOS knockout mice develop distinct macro- and microvascular complications. Lab Invest. 2008;88:515–528. doi: 10.1038/labinvest.2008.23. [DOI] [PubMed] [Google Scholar]
- 102.Nakagawa T, Sato W, Glushakova O, et al. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol. 2007;18:539–550. doi: 10.1681/ASN.2006050459. [DOI] [PubMed] [Google Scholar]
- 103.Villa E, Rabano A, Ruilope LM, et al. Effects of cicaprost and fosinopril on the progression of rat diabetic nephropathy. Am J Hypertens. 1997;10:202–208. doi: 10.1016/s0895-7061(96)00319-6. [DOI] [PubMed] [Google Scholar]
- 104.Hirawa N, Uehara Y, Kawabata Y, et al. Mechanistic analysis of renal protection by angiotensin converting enzyme inhibitor in Dahl salt-sensitive rats. J Hypertens. 1994;12:909–918. [PubMed] [Google Scholar]
- 105.Seccia TM, Belloni AS, Guidolin D, et al. The renal antifibrotic effects of angiotensin-converting enzyme inhibition involve bradykinin B2 receptor activation in angiotensin II-dependent hypertension. J Hypertens. 2006;24:1419–1427. doi: 10.1097/01.hjh.0000234124.94013.ac. [DOI] [PubMed] [Google Scholar]
- 106.Uehara Y, Hirawa N, Kawabata Y, et al. Long-term infusion of kallikrein attenuates renal injury in Dahl salt-sensitive rats. Hypertension. 1994;24:770–778. doi: 10.1161/01.hyp.24.6.770. [DOI] [PubMed] [Google Scholar]
- 107.Chao J, Li HJ, Yao YY, et al. Kinin infusion prevents renal inflammation, apoptosis, and fibrosis via inhibition of oxidative stress and mitogen-activated protein kinase activity. Hypertension. 2007;49:490–497. doi: 10.1161/01.HYP.0000255925.01707.eb. [DOI] [PubMed] [Google Scholar]
- 108.Hirawa N, Uehara Y, Suzuki T, et al. Regression of glomerular injury by kallikrein infusion in Dahl salt-sensitive rats is a bradykinin B2-receptor-mediated event. Nephron. 1999;81:183–193. doi: 10.1159/000045275. [DOI] [PubMed] [Google Scholar]
- 109.Chao J, Zhang JJ, Lin KF, et al. Adenovirus-mediated kallikrein gene delivery reverses salt-induced renal injury in Dahl salt-sensitive rats. Kidney Int. 1998;54:1250–1260. doi: 10.1046/j.1523-1755.1998.00104.x. [DOI] [PubMed] [Google Scholar]
- 110.Yayama K, Wang C, Chao L, et al. Kallikrein gene delivery attenuates hypertension and cardiac hypertrophy and enhances renal function in Goldblatt hypertensive rats. Hypertension. 1998;31:1104–1110. doi: 10.1161/01.hyp.31.5.1104. [DOI] [PubMed] [Google Scholar]
- 111.Hutchison FN, Cui X, Webster SK. The antiproteinuric action of angiotensin-converting enzyme is dependent on kinin. J Am Soc Nephrol. 1995;6:1216–1222. doi: 10.1681/ASN.V641216. [DOI] [PubMed] [Google Scholar]
- 112.Gross O, Beirowski B, Koepke ML, et al. Preemptive ramipril therapy delays renal failure and reduces renal fibrosis in COL4A3-knockout mice with Alport syndrome. Kidney Int. 2003;63:438–446. doi: 10.1046/j.1523-1755.2003.00779.x. [DOI] [PubMed] [Google Scholar]
- 113.Gross O, Schulze-Lohoff E, Koepke ML, et al. Antifibrotic, nephroprotective potential of ACE inhibitor vs AT1 antagonist in a murine model of renal fibrosis. Nephrol Dial Transplant. 2004;19:1716–1723. doi: 10.1093/ndt/gfh219. [DOI] [PubMed] [Google Scholar]
- 114.Kaneto H, Morrissey J, McCracken R, et al. Enalapril reduces collagen type IV synthesis and expansion of the interstitium in the obstructed rat kidney. Kidney Int. 1994;45:1637–1647. doi: 10.1038/ki.1994.215. [DOI] [PubMed] [Google Scholar]
- 115.Ishidoya S, Morrissey J, McCracken R, et al. Angiotensin II receptor antagonist ameliorates renal tubulointerstitial fibrosis caused by unilateral ureteral obstruction. Kidney Int. 1995;47:1285–1294. doi: 10.1038/ki.1995.183. [DOI] [PubMed] [Google Scholar]
- 116.Morrissey JJ, Ishidoya S, McCracken R, et al. Nitric oxide generation ameliorates the tubulointerstitial fibrosis of obstructive nephropathy. J Am Soc Nephrol. 1996;7:2202–2212. doi: 10.1681/ASN.V7102202. [DOI] [PubMed] [Google Scholar]
- 117.Schanstra JP, Neau E, Drogoz P, et al. In vivo bradykinin B2 receptor activation reduces renal fibrosis. J Clin Invest. 2002;110:371–379. doi: 10.1172/JCI15493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bledsoe G, Crickman S, Mao J, et al. Kallikrein/kinin protects against gentamicin-induced nephrotoxicity by inhibition of inflammation and apoptosis. Nephrol Dial Transplant. 2006;21:624–633. doi: 10.1093/ndt/gfi225. [DOI] [PubMed] [Google Scholar]
- 119.Murakami H, Yayama K, Chao L, et al. Human kallikrein gene delivery protects against gentamycin-induced nephrotoxicity in rats. Kidney Int. 1998;53:1305–1313. doi: 10.1046/j.1523-1755.1998.00867.x. [DOI] [PubMed] [Google Scholar]
- 120.Martinez-Salgado C, Eleno N, Tavares P, et al. Involvement of reactive oxygen species on gentamicin-induced mesangial cell activation. Kidney Int. 2002;62:1682–1692. doi: 10.1046/j.1523-1755.2002.00635.x. [DOI] [PubMed] [Google Scholar]
- 121.Ghaznavi R, Faghihi M, Kadkhodaee M, et al. Effects of nitric oxide on gentamicin toxicity in isolated perfused rat kidneys. J Nephrol. 2005;18:548–552. [PubMed] [Google Scholar]
- 122.Rivas-Cabanero L, Rodriguez-Barbero A, Arevalo M, et al. Effect of NG-nitro-L-arginine methyl ester on nephrotoxicity induced by gentamicin in rats. Nephron. 1995;71:203–207. doi: 10.1159/000188713. [DOI] [PubMed] [Google Scholar]
- 123.Hsu YH, Chen CH, Hou CC, et al. Prostacyclin protects renal tubular cells from gentamicin-induced apoptosis via a PPARalpha-dependent pathway. Kidney Int. 2008;73:578–587. doi: 10.1038/sj.ki.5002704. [DOI] [PubMed] [Google Scholar]
- 124.Oosterlinck W, Roelandt R, De Sy WA, et al. Captopril: a protective agent in renal warm ischemia in rats. Eur Urol. 1985;11:36–39. doi: 10.1159/000472446. [DOI] [PubMed] [Google Scholar]
- 125.Kakoki M, Hirata Y, Hayakawa H, et al. Effects of vasodilatory antihypertensive agents on endothelial dysfunction in rats with ischemic acute renal failure. Hypertens Res. 2000;23:527–533. doi: 10.1291/hypres.23.527. [DOI] [PubMed] [Google Scholar]
- 126.Lopau K, Hefner L, Bender G, et al. Haemodynamic effects of valsartan in acute renal ischaemia/reperfusion injury. Nephrol Dial Transplant. 2001;16:1592–1597. doi: 10.1093/ndt/16.8.1592. [DOI] [PubMed] [Google Scholar]
- 127.Pazoki-Toroudi HR, Hesami A, Vahidi S, et al. The preventive effect of captopril or enalapril on reperfusion injury of the kidney of rats is independent of angiotensin II AT1 receptors. Fundam Clin Pharmacol. 2003;17:595–598. doi: 10.1046/j.1472-8206.2003.00188.x. [DOI] [PubMed] [Google Scholar]
- 128.Liu YH, Yang XP, Sharov VG, et al. Paracrine systems in the cardioprotective effect of angiotensin-converting enzyme inhibitors on myocardial ischemia/reperfusion injury in rats. Hypertension. 1996;27:7–13. doi: 10.1161/01.hyp.27.1.7. [DOI] [PubMed] [Google Scholar]
- 129.Kitakaze M, Minamino T, Node K, et al. Beneficial effects of inhibition of angiotensin-converting enzyme on ischemic myocardium during coronary hypoperfusion in dogs. Circulation. 1995;92:950–961. doi: 10.1161/01.cir.92.4.950. [DOI] [PubMed] [Google Scholar]
- 130.Guba M, Steinbauer M, Buchner M, et al. Differential effects of short-term ace- and AT1-receptor inhibition on postischemic injury and leukocyte adherence in vivo and in vitro . Shock. 2000;13:190–196. doi: 10.1097/00024382-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 131.Brookes PS, Yoon Y, Robotham JL, et al. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 132.Park SS, Zhao H, Mueller RA, et al. Bradykinin prevents reperfusion injury by targeting mitochondrial permeability transition pore through glycogen synthase kinase 3beta. J Mol Cell Cardiol. 2006;40:708–716. doi: 10.1016/j.yjmcc.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 133.Chiang WC, Chien CT, Lin WW, et al. Early activation of bradykinin B2 receptor aggravates reactive oxygen species generation and renal damage in ischemia/reperfusion injury. Free Radic Biol Med. 2006;41:1304–1314. doi: 10.1016/j.freeradbiomed.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 134.Xia CF, Yin H, Borlongan CV, et al. Kallikrein gene transfer protects against ischemic stroke by promoting glial cell migration and inhibiting apoptosis. Hypertension. 2004;43:452–459. doi: 10.1161/01.HYP.0000110905.29389.e5. [DOI] [PubMed] [Google Scholar]
- 135.Koch M, Spillmann F, Dendorfer A, et al. Cardiac function and remodeling is attenuated in transgenic rats expressing the human kallikrein-1 gene after myocardial infarction. Eur J Pharmacol. 2006;550:143–148. doi: 10.1016/j.ejphar.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 136.Matsumura Y, Nishiura M, Deguchi S, et al. Protective effect of FK409, a spontaneous nitric oxide releaser, on ischemic acute renal failure in rats. J Pharmacol Exp Ther. 1998;287:1084–1091. [PubMed] [Google Scholar]
- 137.Chintala MS, Chiu PJ, Vemulapalli S, et al. Inhibition of endothelial derived relaxing factor (EDRF) aggravates ischemic acute renal failure in anesthetized rats. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:305–310. doi: 10.1007/BF00169160. [DOI] [PubMed] [Google Scholar]
- 138.Kakoki M, Hirata Y, Hayakawa H, et al. Effects of tetrahydrobiopterin on endothelial dysfunction in rats with ischemic acute renal failure. J Am Soc Nephrol. 2000;11:301–309. doi: 10.1681/ASN.V112301. [DOI] [PubMed] [Google Scholar]
- 139.Lifschitz MD, Barnes JL. Prostaglandin I2 attenuates ischemic acute renal failure in the rat. Am J Physiol. 1984;247(5 Part 2):F714–F717. doi: 10.1152/ajprenal.1984.247.5.F714. [DOI] [PubMed] [Google Scholar]
- 140.Tobimatsu M, Konomi K, Saito S, et al. Protective effect of prostaglandin E1 on ischemia-induced acute renal failure in dogs. Surgery. 1985;98:45–53. [PubMed] [Google Scholar]
- 141.Finn WF, Hak LJ, Grossman SH. Protective effect of prostacyclin on postischemic acute renal failure in the rat. Kidney Int. 1987;32:479–487. doi: 10.1038/ki.1987.235. [DOI] [PubMed] [Google Scholar]
- 142.Tobimatsu M, Ueda Y, Saito S, et al. Effects of a stable prostacyclin analog on experimental ischemic acute renal failure. Ann Surg. 1988;208:65–70. doi: 10.1097/00000658-198807000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Paller MS, Manivel JC. Prostaglandins protect kidneys against ischemic and toxic injury by a cellular effect. Kidney Int. 1992;42:1345–1354. doi: 10.1038/ki.1992.426. [DOI] [PubMed] [Google Scholar]
- 144.Braun C, Kleemann T, Birck R, et al. Increased activity of the renal kallikrein–kinin system in autosomal dominant polycystic kidney disease in rats, but not in humans. Int Immunopharmacol. 2002;2:1949–1956. doi: 10.1016/s1567-5769(02)00171-6. [DOI] [PubMed] [Google Scholar]
- 145.Kennefick TM, Al-Nimri MA, Oyama TT, et al. Hypertension and renal injury in experimental polycystic kidney disease. Kidney Int. 1999;56:2181–2190. doi: 10.1046/j.1523-1755.1999.00783.x. [DOI] [PubMed] [Google Scholar]
- 146.Ecder T, Chapman AB, Brosnahan GM, et al. Effect of antihypertensive therapy on renal function and urinary albumin excretion in hypertensive patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000;35:427–432. doi: 10.1016/s0272-6386(00)70195-8. [DOI] [PubMed] [Google Scholar]
- 147.Ecder T, Edelstein CL, Fick-Brosnahan GM, et al. Diuretics versus angiotensin-converting enzyme inhibitors in autosomal dominant polycystic kidney disease. Am J Nephrol. 2001;21:98–103. doi: 10.1159/000046231. [DOI] [PubMed] [Google Scholar]
- 148.Braun C, Kleemann T, Hilgenfeldt U, et al. Activity and functional significance of the renal kallikrein–kinin-system in polycystic kidney disease of the rat. Kidney Int. 2002;61:2149–2156. doi: 10.1046/j.1523-1755.2002.00385.x. [DOI] [PubMed] [Google Scholar]
- 149.Hostetter TH, Olson JL, Rennke HG, et al. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981;241:F85–F93. doi: 10.1152/ajprenal.1981.241.1.F85. [DOI] [PubMed] [Google Scholar]
- 150.MacLaughlin M, Monserrat AJ, Muller A, et al. Role of kinins in the renoprotective effect of angiotensin-converting enzyme inhibitors in experimental chronic renal failure. Kidney Blood Press Res. 1998;21:329–334. doi: 10.1159/000025890. [DOI] [PubMed] [Google Scholar]
- 151.Wolf WC, Yoshida H, Agata J, et al. Human tissue kallikrein gene delivery attenuates hypertension, renal injury, and cardiac remodeling in chronic renal failure. Kidney Int. 2000;58:730–739. doi: 10.1046/j.1523-1755.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- 152.Tu L, Xu X, Wan H, et al. Delivery of recombinant adeno-associated virus-mediated human tissue kallikrein for therapy of chronic renal failure in rats. Hum Gene Ther. 2008;19:318–330. doi: 10.1089/hum.2007.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yamashita C, Tazawa N, Ohkita M, et al. Exaggerated renal pathology of partial ablation-induced chronic renal failure in eNOS deficient mice. Biol Pharm Bull. 2008;31:1029–1031. doi: 10.1248/bpb.31.1029. [DOI] [PubMed] [Google Scholar]
- 154.Smithies O. Many little things: one geneticist’s view of complex diseases. Nat Rev Genet. 2005;6:419–425. doi: 10.1038/nrg1605. [DOI] [PubMed] [Google Scholar]
- 155.Maltais I, Bachvarova M, Maheux P, et al. Bradykinin B2 receptor gene polymorphism is associated with altered urinary albumin/creatinine values in diabetic patients. Can J Physiol Pharmacol. 2002;80:323–327. doi: 10.1139/y02-036. [DOI] [PubMed] [Google Scholar]
- 156.Zanchi A, Moczulski DK, Hanna LS, et al. Risk of advanced diabetic nephropathy in type 1 diabetes is associated with endothelial nitric oxide synthase gene polymorphism. Kidney Int. 2000;57:405–413. doi: 10.1046/j.1523-1755.2000.00860.x. [DOI] [PubMed] [Google Scholar]
- 157.Neugebauer S, Baba T, Watanabe T. Association of the nitric oxide synthase gene polymorphism with an increased risk for progression to diabetic nephropathy in type 2 diabetes. Diabetes. 2000;49:500–503. doi: 10.2337/diabetes.49.3.500. [DOI] [PubMed] [Google Scholar]
- 158.Harden PN, Geddes C, Rowe PA, et al. Polymorphisms in angiotensin-converting-enzyme gene and progression of IgA nephropathy. Lancet. 1995;345:1540–1542. doi: 10.1016/s0140-6736(95)91088-3. [DOI] [PubMed] [Google Scholar]
- 159.Yoshida H, Mitarai T, Kawamura T, et al. Role of the deletion of polymorphism of the angiotensin converting enzyme gene in the progression and therapeutic responsiveness of IgA nephropathy. J Clin Invest. 1995;96:2162–2169. doi: 10.1172/JCI118270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Baboolal K, Ravine D, Daniels J, et al. Association of the angiotensin I converting enzyme gene deletion polymorphism with early onset of ESRF in PKD1 adult polycystic kidney disease. Kidney Int. 1997;52:607–613. doi: 10.1038/ki.1997.373. [DOI] [PubMed] [Google Scholar]
- 161.Perez-Oller L, Torra R, Badenas C, et al. Influence of the ACE gene polymorphism in the progression of renal failure in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1999;34:273–278. doi: 10.1016/s0272-6386(99)70355-0. [DOI] [PubMed] [Google Scholar]
- 162.Konoshita T, Miyagi K, Onoe T, et al. Effect of ACE gene polymorphism on age at renal death in polycystic kidney disease in Japan. Am J Kidney Dis. 2001;37:113–118. doi: 10.1053/ajkd.2001.20595. [DOI] [PubMed] [Google Scholar]
- 163.Merta M, Reiterova J, Tesar V, et al. Influence of the endothelial nitric oxide synthase polymorphism on the progression of autosomal dominant polycystic kidney disease and IgA nephropathy. Ren Fail. 2002;24:585–593. doi: 10.1081/jdi-120013961. [DOI] [PubMed] [Google Scholar]
- 164.Lovati E, Richard A, Frey BM, et al. Genetic polymorphisms of the renin-angiotensin-aldosterone system in end-stage renal disease. Kidney Int. 2001;60:46–54. doi: 10.1046/j.1523-1755.2001.00769.x. [DOI] [PubMed] [Google Scholar]
- 165.Gumprecht J, Zychma MJ, Grzeszczak W, et al. Angiotensin I-converting enzyme gene insertion/deletion and angiotensinogen M235T polymorphisms: risk of chronic renal failure. End-Stage Renal Disease Study Group. Kidney Int. 2000;58:513–519. doi: 10.1046/j.1523-1755.2000.00197.x. [DOI] [PubMed] [Google Scholar]
- 166.Bachvarov DR, Landry M, Pelletier I, et al. Characterization of two polymorphic sites in the human kinin B1 receptor gene: altered frequency of an allele in patients with a history of end-stage renal failure. J Am Soc Nephrol. 1998;9:598–604. doi: 10.1681/ASN.V94598. [DOI] [PubMed] [Google Scholar]
- 167.Zychma MJ, Gumprecht J, Zukowska-Szczechowska E, et al. Polymorphisms in the genes encoding for human kinin receptors and the risk of end-stage renal failure: results of transmission/disequilibrium test. The End-Stage Renal Disease Study Group. J Am Soc Nephrol. 1999;10:2120–2124. doi: 10.1681/ASN.V10102120. [DOI] [PubMed] [Google Scholar]
- 168.Jozwiak L, Drop A, Buraczynska K, et al. Association of the human bradykinin B2 receptor gene with chronic renal failure. Mol Diagn. 2004;8:157–161. doi: 10.1007/BF03260059. [DOI] [PubMed] [Google Scholar]
- 169.Asakimori Y, Yorioka N, Yamamoto I, et al. Endothelial nitric oxide synthase intron 4 polymorphism influences the progression of renal disease. Nephron. 2001;89:219–223. doi: 10.1159/000046071. [DOI] [PubMed] [Google Scholar]
- 170.Wang Y, Kikuchi S, Suzuki H, et al. Endothelial nitric oxide synthase gene polymorphism in intron 4 affects the progression of renal failure in non-diabetic renal diseases. Nephrol Dial Transplant. 1999;14:2898–2902. doi: 10.1093/ndt/14.12.2898. [DOI] [PubMed] [Google Scholar]
- 171.Westermann D, Schultheiss HP, Tschope C. New perspective on the tissue kallikrein–kinin system in myocardial infarction: role of angiogenesis and cardiac regeneration. Int Immunopharmacol. 2008;8:148–154. doi: 10.1016/j.intimp.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 172.Daull P, Jeng AY, Battistini B. Towards triple vasopeptidase inhibitors for the treatment of cardiovascular diseases. J Cardiovasc Pharmacol. 2007;50:247–256. doi: 10.1097/FJC.0b013e31813c6ca5. [DOI] [PubMed] [Google Scholar]
- 173.Desai AS, Swedberg K, McMurray JJ, et al. Incidence and predictors of hyperkalemia in patients with heart failure: an analysis of the CHARM Program. J Am Coll Cardiol. 2007;50:1959–1966. doi: 10.1016/j.jacc.2007.07.067. [DOI] [PubMed] [Google Scholar]
- 174.Ridings PC, Sugerman HJ, Blocher CR, et al. Hemodynamic effects of bradykinin antagonism in porcine gram-negative sepsis. J Invest Surg. 1995;8:115–122. doi: 10.3109/08941939509016514. [DOI] [PubMed] [Google Scholar]
- 175.Abraham WM, Scuri M, Farmer SG. Peptide and non-peptide bradykinin receptor antagonists: role in allergic airway disease. Eur J Pharmacol. 2006;533:215–221. doi: 10.1016/j.ejphar.2005.12.071. [DOI] [PubMed] [Google Scholar]
- 176.Griesbacher T, Rainer I, Tiran B, et al. Kallikrein inhibitors limit kinin B(2) antagonist-induced progression of oedematous to haemorrhagic pancreatitis in rats. Br J Pharmacol. 2008;155:865–874. doi: 10.1038/bjp.2008.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Levy JH, O’Donnell PS. The therapeutic potential of a kallikrein inhibitor for treating hereditary angioedema. Expert Opin Investig Drugs. 2006;15:1077–1090. doi: 10.1517/13543784.15.9.1077. [DOI] [PubMed] [Google Scholar]
- 178.Blanchard A, Azizi M, Peyrard S, et al. Partial human genetic deficiency in tissue kallikrein activity and renal calcium handling. Clin J Am Soc Nephrol. 2007;2:320–325. doi: 10.2215/CJN.02630706. [DOI] [PubMed] [Google Scholar]
- 179.Picard N, Van Abel M, Campone C, et al. Tissue kallikrein-deficient mice display a defect in renal tubular calcium absorption. J Am Soc Nephrol. 2005;16:3602–3610. doi: 10.1681/ASN.2004110923. [DOI] [PubMed] [Google Scholar]