Abstract
Objectives
We report two simultaneous cases of Staphylococcus aureus sepsis initially consistent with and diagnosed as transfusion related acute lung injury (TRALI). The sepsis in both cases resulted from transfusion of two split products from a single contaminated plateletpheresis unit. In each case the platelets were given along with numerous other blood products during posterior spine surgery. The discussion includes presentation, clinical course, diagnosis and similarities between sepsis and TRALI. The cases and discussion highlight the importance of considering sepsis as part of the differential for any patient believed to have TRALI with clinical features of sepsis.
Data Sources
Data were collected from the patients’ electronic medical records and the hospital laboratory medicine database.
Conclusions
Our cases highlight the importance of vigilant investigation in patients suspected of TRALI, as septic transfusions are easily missed and may mimic or coexist with TRALI. Sepsis should be strongly considered whenever clinical features such as hypotension, leucopenia and fever are noted in patients with suspected TRALI. In comparison to patients receiving red blood cells or plasma, platelet transfusion recipients are at a greater risk for sepsis from a contaminated unit. Patients developing sepsis from a contaminated blood product may meet the clinical definition of TRALI. In such cases, if the clinical syndrome is attributed solely to TRALI and bacterial sepsis is not suspected, the correct diagnosis may be missed or delayed. Consequently, appropriate treatment for sepsis would also be delayed or not provided and likely result in increased morbidity and mortality.
Keywords: TRALI, sepsis, transfusion, platelets, bacterial contamination
Introduction
The risk of most transfusion-transmitted infectious vectors has decreased substantially during past decades(1–4). Transfusion related acute lung injury (TRALI)(5) and bacterial sepsis(6) are among the leading causes of transfusion-associated mortality(7). The former has been attributed to plasma containing leukocyte antibody or other bioreactive mediators, while the latter is frequently associated with platelet transfusion, owing to a storage temperature (22°C) that while maintaining platelet function, permits bacterial growth.
We report two cases of Staphylococcus aureus sepsis caused by transfusion of two split products from one contaminated plateletpheresis collection unit. In both cases, the initial clinical presentation was consistent with, and diagnosed as TRALI. These cases illustrate the possibility of misdiagnosing a septic transfusion reaction as only due to TRALI, especially those associated with platelet transfusion. Additionally, they demonstrate a need for vigilant investigation including culture of the patient and the platelet product whenever TRALI is suspected but the clinical picture has strong features of sepsis. The IRB was contacted regarding use of the two cases presented below as part of this Clinical Case Report and were excused from needing formal approval.
Case 1
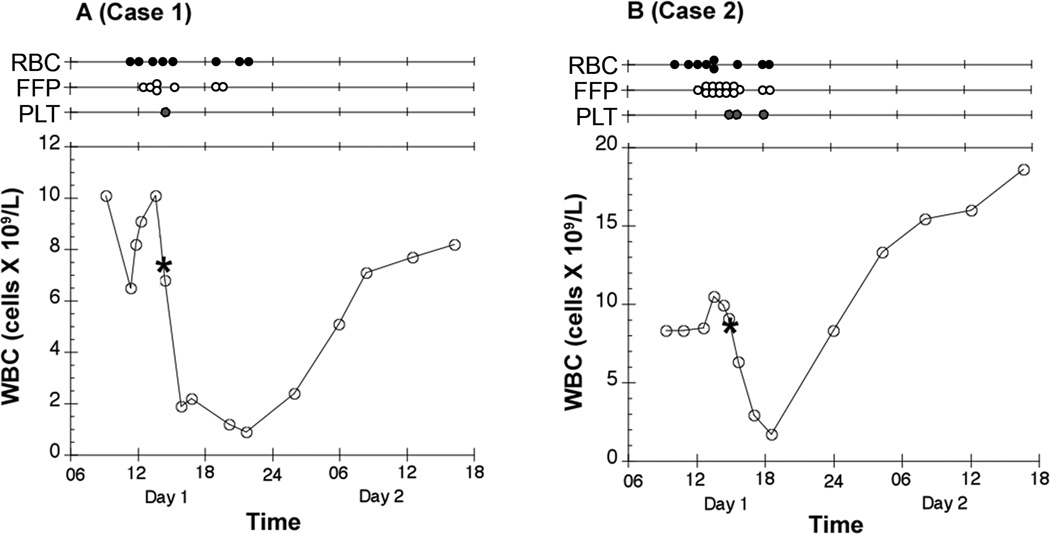
A 47 year-old woman with scoliosis underwent a revision posterior spinal fusion (PSF) with an estimated blood loss (EBL) of 6.0 liters. Her history included hypertension, chronic back pain, and two prior PSFs. Prophylactic vancomycin was given pre-operatively secondary to a penicillin allergy. Intraoperatively, she received one liter salvaged blood, 5 units red blood cells (RBC), 5 units fresh frozen plasma (FFP), and one plateletpheresis unit transfused 45 minutes before the end of surgery. In the post-anesthesia care unit (PACU), she received an additional RBC unit and 2 units FFP secondary to anemia and increasing coagulopathy. Central venous pressures (CVP) remained 14 to 16 mmHg during this time with less than 200 mL wound drain output. After 4-hours in the PACU she became acutely tachycardic (heart rate 130 bpm), tachypneic, hypoxic (oxygen saturation 84% on 4L oxygen by nasal cannula) and hypotensive (systolic blood pressure near 70 mmHg). Her trachea was intubated, vasopressor infusion started and 2 additional units RBCs transfused. A rash was noted on her chest, and during the next 8-hours, she became febrile to 39.2°C (PACU arrival temperature was 37.2°C). Her white blood cell count (WBC) decreased from 10.0 ×109/L (preoperatively) to 0.9 × 109/L 4.5 hrs after surgery (Figure 1A). Chest x-ray (CXR) showed new-onset diffuse, bilateral pulmonary edema in contrast to a normal CXR just two hours prior. CVPs remained 12 to 17 mmHg. Given the acute hypoxemia, bilateral infiltrates on CXR, and no evidence of increased left atrial pressure or cardiac dysfunction, acute lung injury (ALI) was suspected(8). The diagnosis of TRALI was thought likely(5), given the ALI within 6-hours of transfusion. Overnight the patient remained febrile, hypotensive, with evidence of increasing multi-organ dysfunction including respiratory and renal failure.
Figure 1.
Time course of the white blood count (WBC) in peripheral blood of the two patients is presented. Above each graph, the markers displayed on each timeline note the transfusion of each red blood cell (RBC), fresh frozen plasma (FFP), and platelet (PLT) unit. The asterisk (*) corresponds to the time at which the contaminated unit of platelets was transfused. It should be noted that the transfusion of colloid and salvaged red blood cells are not displayed.
Initial blood bank evaluation excluded an acute hemolytic transfusion reaction and supported a clinical diagnosis consistent with TRALI. All transfused blood products were identified and the empty transfusion bags from the operating room and PACU were recovered. Other products from these same donors were identified and those not yet transfused were quarantined. However, the platelet unit transfused to this patient was one of two parts from a split plateletpheresis collection; the second unit from this same split collection had already been transfused to the patient in Case 2 (below).
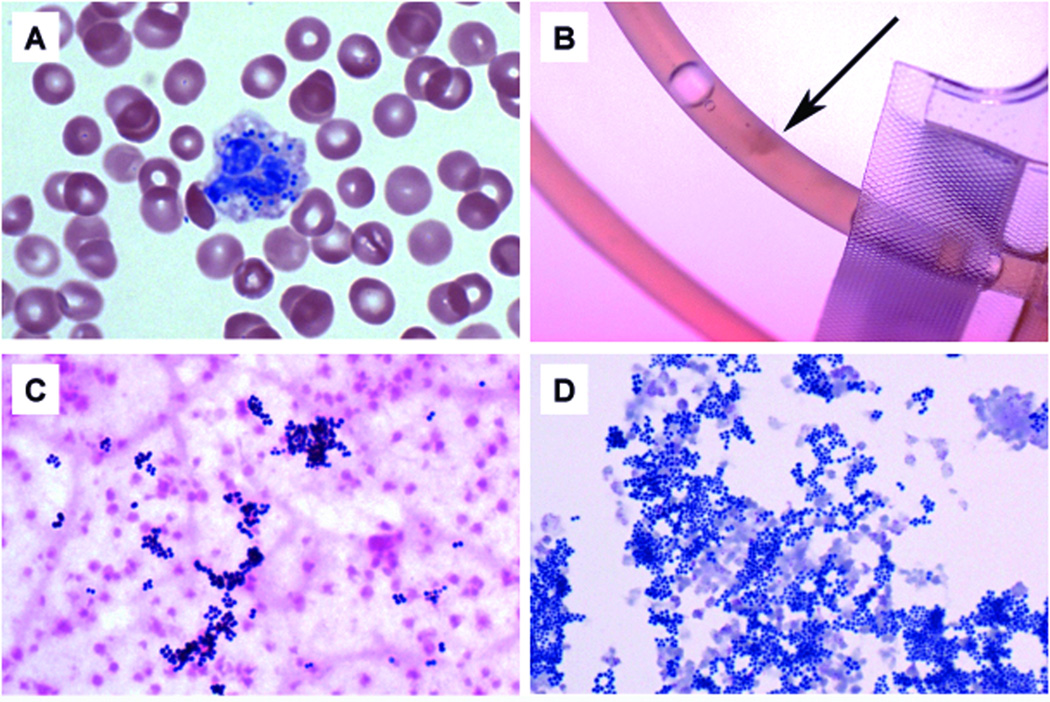
Although the initial presentation was consistent with TRALI, the patient remained febrile and severely hypotensive with vasopressor support, an unusually severe clinical picture for TRALI. Sepsis was suspected when a peripheral blood smear of a sample drawn 30 minutes after surgery (1-hour after platelet transfusion) showed rare leukocytes with intracellular bacteria (Figure 2A). As bacteria in the platelet unit was suspected, a gram-stain of the spent platelet unit was performed and showed numerous gram-positive cocci in clusters amidst numerous platelets. A culture from this platelet unit grew methicillin-sensitive Staphylococcus aureus (MSSA), as did a blood culture of the patient’s blood taken 12-hours after platelet administration. Isolates from the patient's blood and platelet bag showed identical antibiotic susceptibilities.
Figure 2.
(A) Giemsa-Wright stained blood smear at 100× magnification from a CBC sample (Case 1) one hour after receiving the contaminated platelet unit. Note the bacterial cocci present within the leukocyte. This smear was performed to obtain a more accurate manual platelet count as the CBC analyzer noted platelet clumps. (B) The recovered platelet bag approximately 24 hours post-infusion (Case 2), revealing a small clump within the unit tubing (arrow), suggestive of bacterial contamination. (C) Gram stain at 100× from the recovered platelet bag (Case 2) showing clumps of gram-postive cocci in clusters, consistent with a Staphylococcus species. (D) Concentrated Giemsa-Wright stain at 100× from the same recovered platelet bag (~24 hours post-infusion), again showing clumps of cocci consistent with Staphylococcus.
Upon investigation, the implicated plateletpheresis unit had been collected with proper aseptic technique at the local blood donation center, by apheresis from a healthy, afebrile donor. Standard unit collection culture included sampling and innoculation 24 hours after collection. The product was split into two parts and both released for patient transfusion following no bacterial growth after 24 hours of incubation. Further review noted that the culture remained negative at the end of 7 days of incubation. Internal and external quality assurance reviews of the donor center procedures showed no deficiencies. Although a follow-up examination of the donor noted no illness reported before or after donation, a nasal swab revealed carrier status of MSSA. Pulse field gel electropheresis of the MSSA isolates obtained from the platelet donor, both spent platelet bags, and the blood culture from the patient (Case 1) all showed identical banding patterns for all isolates. This result made it highly likely that the donor (skin colonization or transient bacteremia) was the source of platelet unit contamination. Testing of this split platelet unit from the male donor showed antibody to human neutrophil antigen (no identifiable specificity), and a cognate antibody against a class II human leukocyte antigen (anti-DR4).
Case 2
A 76-year old man with a history of coronary artery disease, aortic valve replacement, atrial fibrillation, and hypertension underwent uncomplicated anterior spinal fusion, followed five days later by a PSF with EBL of 8.5 liters. Intraoperatively, he received 1.8 liters salvaged blood, 7 units RBCs, 12 units FFP, and 2 units of plateletpheresis. Two hours prior to surgical completion, he had received the second part of the split platelet collection unit previously transfused to the patient in “Case 1." Both patients had undergone surgery the same day. His condition was initially stable in the PACU, but two hours later he became acutely hypotensive, tachycardic, tachypneic, and hypoxic with increasing surgical wound drain output to over a liter. He received an additional 2 units FFP, 2 units RBCs, one platelet unit, and recombinant activated coagulation factor VII (Novo Nordisk, Bagsvaerd, Denmark) secondary to worsening coagulopathy and anemia. Although the initial CVP was 6 mmHg, it remained 11 to 16 mmHg following fluid resuscitation and initiation of vasopressor support. He remained afebrile throughout. WBC count fell from 9.3 × 109/L preoperatively to a nadir of 1.7 ×109/L (Figure 1B).
The initial course was consistent with a diagnosis of post-surgical bleeding. However, his failure to respond to blood products and his acute respiratory compromise suggested the possibility of TRALI. A CXR showed mild, bilateral pulmonary edema. A transthoracic echocardiogram performed in the PACU was not significantly changed from evaluation before surgery (mild left ventricular dysfunction and aortic dilation).
Overnight, renal function deteriorated and the patient became febrile. Blood cultures drawn 19-hours postoperatively were negative. However, the patient was receiving cefazolin every 4-hours during the case and postoperatively per surgical protocol. When Gram stain results from the platelet bag of "Case 1" noted staphalococcus contamination, recipients of co-components were identified as part of the septic transfusion reaction work up. It was determined that this patient "Case 2" had received the other half of the same contaminated platelet collection unit. Following retrieval of the spent unit, a gram stain and culture of this empty bag also revealed MSSA (Figure 2B–D). Testing of this split platelet unit from the male donor showed similar results to the other unit with antibody to human neutrophil antigen (no identifiable specificity), and no cognate antibody against a class I or class II human leukocyte antigen. As would be expected, the HLA cognate antibodies determined for each patient were different. Cognate antibodies represent the subset of HLA donor antibodies that match the recipient’s HLA antigens. Although samples from each of the two platelet products contained the same antibodies (same donor), since each patient had different HLA antigens, the matching set of cognate antibodies reported were different for each case.
Both patients remained critically ill in the ICU for several weeks and were hospitalized for more than a month. The first patient had continual fevers and persistently positive blood cultures, requiring multiple antibiotic regimens to treat her bacteremia. The second patient had renal failure requiring dialysis and recovered slowly. Both patients required extensive rehabilitation but eventually recovered for discharge.
Discussion
These cases demonstrate that recipients of bacteria-containing platelet units can develop sepsis and present with clinical features of ALI. Until the diagnosis of sepsis was determined, both these transfusion reactions met the clinical definition of TRALI(5), defined(8) as new ALI occurring within 6-hours of transfusion in the absence of other risk factors for ALI. ALI is defined as acutely impaired oxygenation (PaO2/FiO2≤300 mmHg); bilateral infiltrates on CXR; and Paw ≤18 mmHg or no clinical evidence of elevated left atrial pressure(8). Thus the clinical diagnosis of TRALI is generally one of exclusion. One of the clinical features that caused these cases to be considered as TRALI was the acute decrease in WBC (noted in both cases), which has been reported previously in TRALI(9) and is also consistent with sepsis. Although septic transfusion reactions are rare and most diagnosis of TRALI are likely correct, these cases demonstrate that a septic transfusion reaction should be considered in the differential diagnosis of patients with TRALI, especially if the patient has clinical features of sepsis such as hypotension, leucopenia and fever. Early consideration of a septic transfusion allows initiation of an appropriate clinical and laboratory investigation(10) and timely treatment for sepsis including the use of pre-emptive broad-spectrum antibiotic therapy. Although specific policies and protocols differ between blood banks, notification of suspected TRALI might only ensure co-components of the implicated products are not transfused to the same patient, whereas notification of a possible septic transfusion reaction would result in quarantine of co-components from release to any patient.
If the clinical syndrome were attributed solely to TRALI and bacterial sepsis from the contaminated blood products were not suspected, the diagnosis of sepsis would have been missed or delayed, and would likely have resulted in increased morbidity and mortality. As both patients received platelet products from a donor positive for antibodies to human leukocytes, it is possible that these antibodies contributed in some part to the ALI in addition to sepsis.
It is standard blood bank practice to collect blood products aseptically and apheresis platelet products are routinely tested for bacterial contamination. However, sampling error may prevent the detection of small numbers of bacteria found in the product in the first 24 hours post collection. These bacteria can multiply during the remaining 3 or 4 days of shelf life. The residual rate of platelet bacterial contamination is approximately one in 40,000 apheresis units with most due to Staphylococcus species(11).
Septic transfusion reactions are easily missed and underreported (12). The diagnosis is often not considered due to underlying and superimposed medical illness. and the transfusion bag discarded and not available for gram stain and bacterial culture. Many institutions discard transfused bags routinely without returning them to the blood bank unless reactions are noted during infusion. Our institution was performing a prospective study of TRALI and we were fortunate that all transfused bags were routinely returned to the blood bank. This practice facilitated definitive diagnosis of these two cases.
Critical care physicians, surgeons, and anesthesiologists should be aware that:
ALI due to sepsis from transfusion may mimic or coexist with TRALI. Concordance of bacterial species from patient blood culture and the spent blood product is ideally required for diagnosis.
Septic fever in operative patients may be delayed or absent due to perioperative antibiotics or while under general anesthesia.
Blood cultures may also be negative due to perioperative antibiotic administration.
As both TRALI and bacterial sepsis can be associated with hypotension, leucopenia, fever and ALI after transfusion, both diagnoses should be considered.
Acknowledgments
Disclosure of Funding:
This work was supported in part by Award Number P50 HL081027 from the National Heart, Lung and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have not disclosed any potential conflicts of interest
Contributor Information
Mark D. Rollins, Department of Anesthesia & Perioperative Care, University of California San Francisco.
Ari B. Molofsky, Department of Laboratory Medicine, University of California San Francisco.
Ashok Nambiar, Department of Laboratory Medicine, University of California San Francisco.
Suchitra Pandey, Department of Laboratory Medicine, University of California San Francisco.
Richard B. Weiskopf, Department of Anesthesia & Perioperative Care, University of California San Francisco.
Pearl Toy, Department of Laboratory Medicine, University of California San Francisco.
References
- 1.Busch MP, Glynn SA, Stramer SL, et al. A new strategy for estimating risks of transfusion-transmitted viral infections based on rates of detection of recently infected donors. Transfusion. 2005;45(2):254–264. doi: 10.1111/j.1537-2995.2004.04215.x. [DOI] [PubMed] [Google Scholar]
- 2.Busch MR. Evolving approaches to estimate risks of transfusion-transmitted viral infections: incidence-window period model after ten years. Dev Biol (Basel) 2007;127:87–112. [PubMed] [Google Scholar]
- 3.Zou S, Dorsey KA, Notari EP, et al. Prevalence, incidence, and residual risk of human immunodeficiency virus and hepatitis C virus infections among United States blood donors since the introduction of nucleic acid testing. Transfusion. 2010;50(7):1495–1504. doi: 10.1111/j.1537-2995.2010.02622.x. [DOI] [PubMed] [Google Scholar]
- 4.Zou S, Stramer SL, Notari EP, et al. Current incidence and residual risk of hepatitis B infection among blood donors in the United States. Transfusion. 2009 doi: 10.1111/j.1537-2995.2009.02195.x. [DOI] [PubMed] [Google Scholar]
- 5.Toy P, Popovsky MA, Abraham E, et al. Transfusion-related acute lung injury: definition and review. Crit Care Med. 2005;33(4):721–726. doi: 10.1097/01.ccm.0000159849.94750.51. [DOI] [PubMed] [Google Scholar]
- 6.Kuehnert MJ, Roth VR, Haley NR, et al. Transfusion-transmitted bacterial infection in the United States, 1998 through 2000. Transfusion. 2001;41(12):1493–1499. doi: 10.1046/j.1537-2995.2001.41121493.x. [DOI] [PubMed] [Google Scholar]
- 7. [Accessed on August 2011];Fatalities Reported to FDA Following Blood Collection and Transfusion: Annual Summary for Fiscal Year 2010: U.S. department of Health and Human Services and the U.S. Food and Drug Administration. www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ReportaProblem/TransfusionDonationFatalities/ucm254802.htm. Web Site.
- 8.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa M, Toy P. Acute and transient decrease in neutrophil count in transfusion-related acute lung injury: cases at one hospital. Transfusion. 2004;44(12):1689–1694. doi: 10.1111/j.0041-1132.2004.04194.x. [DOI] [PubMed] [Google Scholar]
- 10.Eder AF, Goldman M. How do I investigate septic transfusion reactions and blood donors with culture-positive platelet donations? Transfusion. 2011;51(8):1662–1668. doi: 10.1111/j.1537-2995.2011.03083.x. [DOI] [PubMed] [Google Scholar]
- 11.Eder AF, Kennedy JM, Dy BA, et al. Bacterial screening of apheresis platelets and the residual risk of septic transfusion reactions: the American Red Cross experience (2004–2006) Transfusion. 2007;47(7):1134–1142. doi: 10.1111/j.1537-2995.2007.01248.x. [DOI] [PubMed] [Google Scholar]
- 12.Palavecino EL, Yomtovian RA, Jacobs MR. Bacterial contamination of platelets. Transfus Apher Sci. 2010;42(1):71–82. doi: 10.1016/j.transci.2009.10.009. [DOI] [PubMed] [Google Scholar]