Abstract
New linker phosphoramidite reagents containing a cleavable 3′-ester linkage are used for attaching the first nucleoside to the surface of a solid- phase support. Inexpensive, underivatized amino supports, such as long chain alkylamine controlled-pore glass, can serve as universal supports. No modifications to phosphoramidite coupling conditions are required and, after synthesis, treatment with NH4OH releases the products with 3′-OH ends. No 3′-dephosphorylation is required. Phosphoramidite reagents containing a succinate and sulfonyl diethanol linkage between the nucleoside and phosphoramidite group are particularly advantageous and can be used to create both 3′-OH and 5′-phosphate ends on oligonucleotides. Reproducibility and quality of oligonucleotide synthesis is demonstrated for either column and 96-well plate formats on low-, medium- or high-loading CPG supports.
INTRODUCTION
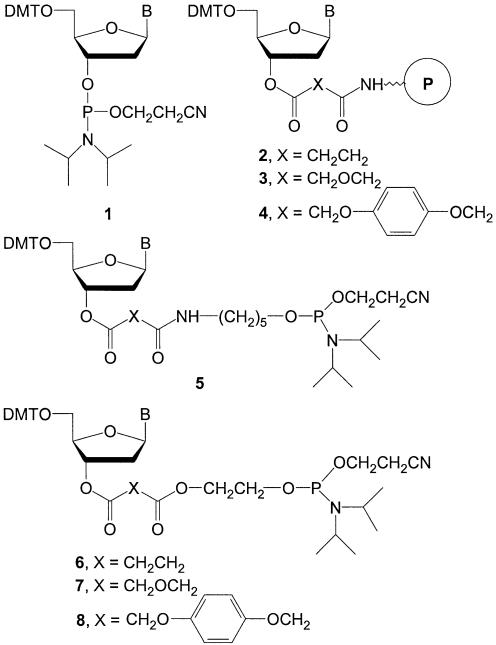
Synthetic oligonucleotides are widely produced by the stepwise addition of nucleoside-3′-phosphoramidite monomers (1) (see Figure 1) to solid-phase supports using automated DNA synthesizers. Synthetic oligonucleotides have found numerous applications as tools and diagnostics in molecular biology, and so, many millions of oligonucleotides are now produced annually. Additionally, growing use of modified oligonucleotides as therapeutic agents has lead to a separate large-scale synthesis industry (1). The phosphoramidite coupling chemistry with its high yields and ease of use has proven to be extremely satisfactory for both these industries. However, this approach to oligonucleotide synthesis requires a solid-phase support with the first nucleoside residue already attached to it (a ‘prederivatized’ support) before synthesis can begin (2).
Figure 1.
Structures of conventional phosphoramidites (1) and derivatized supports (2–4) compared with linker phosphoramidites (5–8).
The first nucleoside is usually attached to an amino support, such as long chain alkylamine controlled-pore glass (LCAA-CPG), through a succinic (3), diglycolic (4) or hydroquinone-O,O′-diacetic acid (Q-Linker) (5) (2–4) (see Fig. 1) linker arm. The ester linkage joining the 3′-OH position of the nucleoside to the dicarboxylic acid linker arm in 2–4 is of critical significance. This is because eventual hydrolysis at this position will yield oligonucleotides with terminal 3′-OH groups, a structural requirement for activity with DNA polymerases. Since most methods of adding the first nucleoside to the support are not fast enough to perform in situ on automated synthesizers, prederivatized supports containing the correct nucleoside must be manually selected and installed before each synthesis begins.
As part of a project to develop reusable supports (6), we found that fast, automated coupling reactions could be performed using O-benzotriazol-1-yl-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HBTU) and 4-dimethylaminopyridine (DMAP) as coupling reagents (7,8). This allowed selection of the first nucleoside to be performed automatically while only producing products with the desired 3′-terminal OH groups. However, the need for both HBTU and phosphoramidite coupling chemistries on the same instrument limited the utility of this approach.
It would be much better if only phosphoramidite chemistry could be used to produce oligonucleotides with terminal 3′-OH groups. Although, effective strategies for doing this have been sought for over 20 years, the recent development of high-throughput oligonucleotide synthesizers using 96- and 384-well synthesis plates (9–11) has made this even more urgent. This is because sorting prederivatized supports into the correct locations on multi-well synthesis plates is very tedious and error-prone. Previous methods for using phosphoramidites to add the first nucleoside have been referred to as ‘universal’ support strategies (12–17) because they allow a single support to be used in every synthesis position. However, all of these strategies have focused on modifying the linker arm on the support, usually with some type of cyclic or acyclic diol configuration, so nucleoside-3′-phosphoramidite reagents (1) can be used in every step. This produces a 3′-phosphate linkage to the support which must be removed through some type of post-synthesis cyclization and elimination reaction. Unfortunately, the 3′-dephosphorylation step has not been satisfactory because of the additional reagents and processing time needed. Also, because the dephosphorylation is often not quantitative, the product is either obtained in reduced yield or in the presence of unwanted 3′-phosphorylated impurities.
We decided to approach this problem from a different route. Instead of changing the support, we decided to synthesize a new set of linker phosphoramidite reagents which would be used solely for attachment of the first nucleoside to the solid-phase support [conventional phosphoramidites (1) are still used for the rest of the synthesis]. The linker phosphoramidites differ from conventional phosphoramidites by having an internal cleavage site (a 3′-ester) which can be easily hydrolyzed after synthesis to generate products with only the desired 3′-OH groups. In this way, all of the previous problems related to the post-synthesis 3′-dephosphorylation steps can be eliminated, while still retaining the desirable properties of phosphoramidite coupling chemistry.
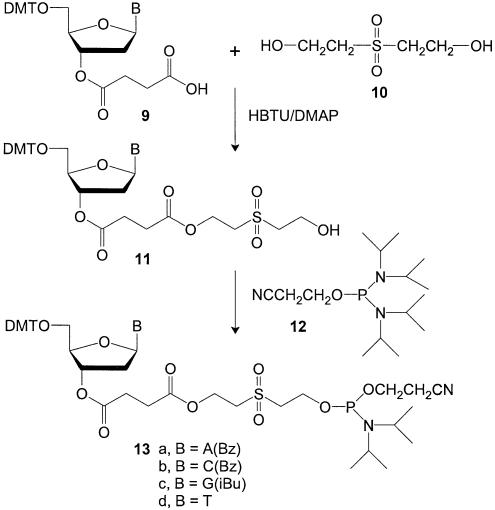
In initial communications (18–20), we demonstrated the validity of this approach by synthesizing three sets of linker phosphoramidites containing either succinic acid, diglycolic acid, or hydroquinone-O,O′-diacetic acid and an ethylene glycol spacer (6–8) (see Figure 1). In this article we describe the synthesis and properties of an improved set of reagents (13a–d) containing succinic acid and a 2,2′-sulfonyldiethanol (10) spacer. These new phosphoramidite reagents retain the desirable coupling properties of conventional phosphoramidites but also allow rapid release of the oligonucleotide product after synthesis through β-elimination of the sulfonyl diethanol spacer. Additionally, relatively expensive universal supports, based on the earlier diol type linkers, can now be replaced with much less expensive, underivatized amino or hydroxyl supports. The 2,2′-sulfonyldiethanol containing linker phosphoramidites are expected to provide significant advantages in the ease, speed and cost of preparing large numbers of oligonucleotides.
MATERIALS AND METHODS
General methods
Underivatized long chain alkylamine CPG (LCAA-CPG) supports were obtained from CPG Inc. (Lincoln Park, NJ), phosphoramidite reagents from Transgenomic (Omaha, NE) or Glen Research (Sterling, VA), and other reagents from Sigma-Aldrich. Nucleosides with the hydroquinone-O,O′-diacetic acid linker or Q-Linker (5,21) and phosphoramidites 6–8 (19) were prepared as previously described. Two molar triethylammonium phosphate was prepared by stirring 85% H3PO4 (272 ml), triethylamine (836 ml) and water (892 ml) in an ice bath (∼2 h) until a homogenous solution forms. Oligonucleotide synthesis was performed on either a four-column, eight base position Applied Biosystems 394 (Foster City, CA) or a dual 96-well plate, 10 base position MerMade IV (BioAutomation, Plano, TX) synthesizer. Quantitative dimethoxytrityl colorimetric analysis was performed as previously described (22) to determine nucleoside loadings. Amino groups were determined by dimethoxytrityl derivatization (21,23) and then colorimetric analysis. Oligonucleotide mass spectra were obtained by MALDI-TOF on a Voyager DE-STR instrument using a 3-hydoxypicolinic acid matrix. Nucleoside LCMS were obtained on a Bruker Daltronics esquire 3000. Capillary gel electrophoresis (CGE) analysis was performed on an Agilent 3D CE instrument using PVA coated capillaries and a polyethylene glycol matrix according to Agilent applications note 5988-4303EN.
Preparation of nucleoside-3′-O-succinates (9)
A solution of 5′-dimethoxytrityl-N-protected 2′-deoxyribonucleoside (10 mmol), succinic anhydride (15 mmol, 1.5 g) and triethylamine (30 mmol, 4.2 ml) in dichloromethane (100 ml) was stirred at room temperature (∼5 h or more) until TLC (5% MeOH/CHCl3) showed complete conversion of the starting nucleoside. The reaction was then poured into 0.5 M triethylammonium phosphate buffer (prepared from 2 M stock solution) and the mixture extracted with CH2Cl2. The combined CH2Cl2 extracts were evaporated to dryness to yield the triethylammonium salts of the nucleoside-3′-O-succinates (9) as white foams, which were used without further purification.
Preparation of nucleoside 11
An aqueous solution of 65% 2,2′-sulfonyldiethanol (50 mmol, 9.6 ml) was co-evaporated to dryness with anhydrous acetonitrile (2 × 50 ml) and pyridine (2 × 30 ml). The residue was redissolved in pyridine (80 ml) and nucleoside-3′-O-succinate triethylammonium salt (9) (10 mmol), 4-dimethylaminopyridine (13 mmol, 1.59 g), O-benzotriazol-1-yl-N,N,N′N′-tetramethyluronium hexafluorophosphate (13 mmol, 4.93 g), and diisopropylethylamine (13 mmol, 2.3 ml) were added. The solution was stirred at room temperature (10 min) and checked by TLC (5% MeOH/CHCl3) for complete reaction. The pyridine was partially removed by evaporation to produce a slurry, which was then redissolved in CHCl3 (250 ml). The solution was washed with water (4 × 250 ml) and then evaporated to dryness. Silica gel column chromatography was performed using successive elution with 0–3% or 0–5% CHCl3/MeOH, depending upon the nucleoside. After purification, the pure product 11 was evaporated under vacuum to yield a solid foam with yields of ∼80–90%. MS analysis: 11a, 916, C46H48N5O12S + Na+; 11b, 892, C45H47N3O13S + Na+; 11c, 875, C43H49N5O13S + Na+; 11d, 803, C39H44N2O13S + Na+.
Preparation of phosphoramidite 13
Nucleoside 11 (5.0 mmol) was dissolved in anhydrous acetonitrile (60 ml) inside a septum sealed flask. Diisopropylamine (6 mmol, 0.82 ml) followed by 2-cyanoethyl tetraisopropylphosphorodiamidite (12) (7.5 mmol, 2.4 ml) were added with stirring. Then, a solution of 0.45 M tetrazole (12.2 ml, 5.5 mmol) in anhydrous acetonitrile was added and the reaction stirred at room temperature (2.5 h). When complete, CH2Cl2 (150 ml) was added to the reaction mixture and the resulting solution was washed with 5% aq. NaHCO3 (1 × 200 ml), aq. NaCl (2 × 200 ml), and water. The organic phase was evaporated to dryness. Silica gel column chromatography was performed by using 42:52:6 CH2Cl2/hexanes/triethylamine to pack a column (∼6 cm diameter × 10 cm high). The crude product was dissolved in the same solvent (8 ml), applied to the column and eluted with the same solvent (1000 ml). Then, elution was performed with 5% triethylamine/CHCl3. The purified product was evaporated to dryness under vacuum to obtain a white foam in 70–85% yield. 31P-NMR (CDCl3): 13a, 150.85 p.p.m.; 13b, 150.88 p.p.m.; 13c, 150.97 and 151.04 p.p.m.; 13d, 150.33 p.p.m. MS analysis: 13a, 1094, C55H65N7O13SP + H+; 13b, 1092, C54H64N5O14SP + Na+; 13c, 1098, C52H66N7O14SP + Na+; 13d, 981, C48H61N4O14SP + H+. UV (EtOH): 13a, 234.5 and 280.5 nm; 13b, 236.5, 260 and 305.5 nm; 13c, 237 and 276 nm; 13d, 267 nm.
Oligonucleotide synthesis in columns
Underivatized amino LCAA-CPG support (∼10 mg for a 0.2 µmol scale or ∼40 mg for a 1 µmol scale) was accurately weighed into ABI synthesis columns. Linker phosphoramidite solutions 13 were installed on spare base positions 5–8. Sequences were entered with an additional ‘fake’ 3′-base to work around the software’s expectation of prederivatized columns. Synthesis was performed without further modifications from standard methods. The amount of nucleoside added to the support was determined by quantitative colorimetric analysis of the dimethoxytrityl cation released from the first detritylation step. After synthesis a custom end procedure with a shorter NH4OH cleavage step (5 min) was used to release the products. Base deprotection with NH4OH (16 h, 55°C) and desalting on a Sephadex G-25 were performed as usually done. MALDI-TOF data for representative syntheses: 13a, dAGC GGA TAA CAA TTT CAC ACA GGA, calc. 7377.81, obs. 7370.65; 13b, dAAC TAG TGG ATC CCC CGG GCT GC, calc. 7024.53, obs. 7021.97; 13c, dCGA GGT CGA CGG TAT CG, calc. 5250.40, obs. 5251.66; 13d, dGTA AAA CGA CGG CCA GT, calc. 5227.41, obs. 5229.27.
5′-Phosphorylation was evaluated by preparing 5′-p-dGTA AAA CGA CGG CCA GT using either 13d or commercial 5′-phosphorylating phosphoramidite 14 for the last phosphoramidite addition. MALDI-TOF data: for sample made with 13d, calc. 5307.39, obs, 5306.10; for sample made with 14, calc. 5307.39, obs. 5308.82.
Oligonucleotide synthesis in 96-well plates
Underivatized amino LCAA-CPG support (either ∼2 or ∼4 mg) was dispensed into 96-well synthesis plates (OF1100; Orochem Technologies, Westmont, IL) by aspirating dry CPG into a 10 µl aerosol filter pipet tip (VWR 53510-031), which had been trimmed to ∼5–10 mm long so it would deliver the desired amount of CPG. A Drummond Pipet-Aid (VWR 53498-001) with both suction and positive air pressure allowed rapid transfer of the CPG into each synthesis well. Up to two synthesis plates were installed in the MerMade IV synthesizer. Linker phosphoramidites in either 30 or 60 ml screw-capped bottles were installed on any of the six available spare base positions. 5-Ethylthiotetrazole (0.25 M) was used as activator. Addition of the first phosphoramidite was performed using a ‘single coupling’ cycle using the amounts and concentration of 13 described in Table 5. Subsequent chain extensions were performed using a ‘double coupling’ to ensure high quality. After synthesis, products were cleaved from the support using NH4OH (5 min, 2 × 200 µl) and transferred by centrifugation to collection plates, which were then sealed and deprotected (16 h, 55°C).
Table 5. Summary of 96-well plate synthesis results.
| 13 | Base concentration | Nanomoles recovered (CV%) | Nanomoles recovered (CV%) | ||||
|---|---|---|---|---|---|---|---|
| Volume added (µl) | 4.4 mg of CPG | 2.3 mg of CPG | Volume added (µl) | 4.4 mg of CPG | 2.3 mg of CPG | ||
| 0.1 M | dA | 40 | 74 (23%) | 59 (10%) | 20 | 38 (11%) | 27 (18%) |
| 0.1 M | dC | 40 | 87 (12%) | 61 (19%) | 20 | 37 (21%) | 36 (26%) |
| 0.1 M | dG | 40 | 81 (22%) | 40 (36%) | 20 | 59 (20%) | 45 (24%) |
| 0.1 M | T | 40 | 94 (22%) | 94 (27%) | 20 | 70 (13%) | 42 (17%) |
| 0.05 M | dA | 40 | 53 (21%) | 38 (23%) | 20 | 31 (17%) | 21 (14%) |
| 0.05 M | dC | 40 | 68 (14%) | 46 (31%) | 20 | 37 (16%) | 24 (22%) |
| 0.05 M | dG | 40 | 52 (13%) | 30 (26%) | 20 | 49 (14%) | 27 (11%) |
| 0.05 M | T | 40 | 57 (27%) | 55 (19%) | 20 | 57 (12%) | 41 (13%) |
| 0.025 M | dA | 40 | 36 (15%) | 23 (13%) | 20 | 26 (13%) | 16 (13%) |
| 0.025 M | dC | 40 | 46 (18%) | 27 (17%) | 20 | 29 (14%) | 18 (11%) |
| 0.025 M | dG | 40 | 41 (12%) | 24 (16%) | 20 | 34 (7%) | 22 (8%) |
| 0.025 M | T | 40 | 37 (10%) | 32 (18%) | 20 | 39 (11%) | 29 (18%) |
| Ctrl-CPGa | dA | n/a | 67 (15%) | 49 (22%) | |||
| Ctrl-CPGa | dC | n/a | 74 (19%) | 53 (10%) | |||
| Ctrl-CPGa | dG | n/a | 100 (17%) | 60 (10%) | |||
| Ctrl-CPGa | T | n/a | 82 (10%) | 56 (7%) | |||
aPrederivatized P1 with 4 with ∼30 µmol/g loading.
RESULTS AND DISCUSSION
A number of phosphoramidite reagents have been developed to incorporate various nucleoside and non-nucleoside residues into oligonucleotides (24,25). However, there have only been a few reagents reported with cleavable linkages. Mostly these have been intended for oligonucleotide end modifications (26–29) and not for coupling to the solid-phase support. Only phosphoramidite 5 with a cleavable 3′-ester linkage (30) has been described for the synthesis of an oligonucleotide on a solid-phase support. Although 5 was the first example of a cleavable linker phosphoramidite with an ester linkage, its use was restricted to the synthesis of oligonucleotides immobilized on glass chips for DNA microarrays.
We sought a simple and inexpensive set of linker phosphoramidites which could be used for general oligonucleotide synthesis. In our first attempt (19), we added ethylene glycol to a nucleoside-3′-O-succinate (9) and then converted it into phosphoramidite 6. We also prepared reagents 7 and 8, which had more easily cleavable diglycolic and hydroquinone-O,O′-diacetic acid linker arms (19). All three reagents 6–8 satisfactorily allowed oligonucleotide synthesis on either amino or hydroxyl supports. As expected, release of the oligonucleotide products was fastest (∼95% cleavage in 2 min) when 8 was used. Unfortunately, the nucleoside loadings obtained from 8 were less than obtained from 6 or 7. Additionally, 8 was unsatisfactory because of the cost of hydroquinone-O,O′-diacetic acid and the low overall yield of its synthesis.
Therefore, a better linker phosphoramidite reagent was required. This reagent needed to provide the rapid cleavage of the hydroquinone-O,O′-diacetic acid linker, but possess greater coupling efficiency and allow a cheaper and more efficient synthesis. These requirements lead us to investigate the synthetic route shown in Scheme 1. In this scheme, the readily available and inexpensive nucleoside-3′-O-succinates (9) were the starting materials. However, a different spacer arm, 2,2′-sulfonyldiethanol (10) was used. Esterification of 10 to the terminal COOH group of 9 using HBTU and DMAP rapidly produced 11 in 80–90% yield. Phosphitylation with 12 produced the linker phosphoramidites 13a–d in high yield.
Scheme 1. Synthesis of the improved linker phosphoramidite reagent 13.
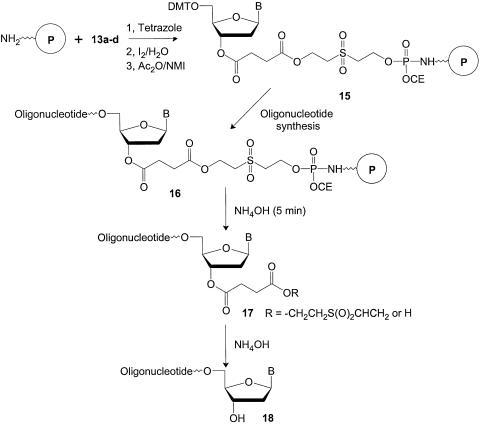
The diol spacer 10 has been used as part of a common reagent 14 (see Figure 2) to introduce terminal monophosphate groups on the 5′ ends of oligonucleotides (31). However, it has occasionally been used to anchor the 3′ end of oligonucleotides on supports and glass microarrays (26,32–34) when release of 3′-terminally phosphorylated oligonucleotides was acceptable. When 10 was used as part of the linker phosphoramidites, oligonucleotide synthesis proceeded as shown in Scheme 2.
Figure 2.
Structure of 5′-phosphorylation reagent 14.
Scheme 2. Oligonucleotide synthesis on universal amino supports using linker phosphoramidite reagents.
In Scheme 2, the simplest possible type of universal support, an inexpensive amino derivatized support, was used in every synthesis column or well of a multi-well synthesis plate. Linker phosphoramidite 13 was then added using a standard phosphoramidite coupling cycle to produce a phosphoramidate diester linkage to the support 15. These linkages have been well characterized and are known to be stable in the conditions of solid-phase oligonucleotide synthesis (35–37). Unreacted amino groups on the support were acetylated and prevented from further reaction by the acetic anhydride/N-methylimidazole capping step included in the coupling cycle. The remainder of the synthesis was then performed using conventional reagents (1) to produce an oligonucleotide attached to the support 16. A brief treatment of 16 with ammonium hydroxide released oligonucleotide 17 from the support by β-elimination. The phosphoramidate residue remaining on the support was discarded with the used support. Hydrolysis of the 3′-succinate residue of 17 to produce the product with the desired 3′-terminal OH group (18) was performed off the support at the same time as base deprotection.
The rapid β-elimination of the sulfonyl diethanol spacer allowed quick and easy isolation of the synthetic products from the synthesis plates or columns. Significantly, however, no additional processing time, steps or reagents were required because deprotection of the 3′-OH group occurred at the same time and under the same conditions as removal of the base protecting groups. No 3′-dephosphorylation was required and all of the previous problems associated with the dephosphorylation step in previous universal support strategies were eliminated. Also, no changes to the phosphoramidite coupling cycles were required to use the linker phosphoramidite reagents. A DNA synthesizer with four additional base positions was needed to accommodate reagents 13a–d. However, since many instruments now hold eight or more different phosphoramidites, this was not a serious limitation.
The validity of Scheme 2 was confirmed by preparing oligonucleotide sequences of between 17 and 24 bases using reagents 13a–d and support P2. These syntheses were performed using an ABI 394 DNA synthesizer with eight base positions and without any modifications to our regular synthesis and deprotection protocols. The completely deprotected products were the same as control sequences made on conventional prederivatized supports and analysis by MALDI-TOF mass spectrometry confirmed the complete removal of all protecting groups and linker arms.
The utility of 13d as a 5′-phosphorylating reagent was also confirmed by preparing p-GTAAAACGACGGCCAGT using both 13d and 14 as the phosphoramidite for the 5′-phosphorylation step. Both reagents produced identical products, as confirmed by CGE and MALDI-TOF analysis. Thus, the β-elimination in 13 was very similar to that of 14. Therefore, linker phosphoramidite reagents can also be used to introduce 5′-phosphate groups, although a single nucleoside will be added to the crude product mixture. While this nucleoside is an easily removable impurity, we don’t expect 13 to replace 14 as a 5′-phosphorylating agent. Instead, 13 allows multiple oligonucleotides to be produced in a single tandem synthesis (18,38). In a tandem synthesis, a string of multiple oligonucleotides can be prepared as a single synthesis by using multiple insertions of phosphoramidite 13. Each time 13 is inserted, a new oligonucleotide with a 3′-terminal OH group is started and a 5′-terminal phosphate group is added onto the preceding sequence (20). Tandem oligonucleotide synthesis has significant potential to make sets of oligonucleotides, such as PCR primers, which are always used together. However, full details on the use of linker phosphoramidites in tandem synthesis will be published separately.
The rate of oligonucleotide release from the solid-phase supports was compared by collecting ammonium hydroxide aliquots at 1 min intervals and, after deprotection, the amount of A260 material recovered was plotted versus time. These results showed linkers 13d and 14 were quickly cleaved with 95% of the oligonucleotide released in ∼5 min. The fast release from the support is especially convenient for synthesis in multi-well plates where the cleavage and transfer of the oligonucleotides is usually performed manually.
Both the quality and quantity of synthetic oligonucleotides depend upon the properties of the solid-phase support used. Generally, small scale synthesis is performed on supports derivatized to ∼30–40 µmol/g of nucleoside. However, lower nucleoside loadings (∼10 µmol/g) are sometimes preferred for long oligonucleotides and higher loadings (75–200 µmol/g) are preferred for large-scale synthesis. We were interested in determining how well linker phosphoramidite 13 would work on different types of supports and so we evaluated oligonucleotide synthesis on LCAA-CPG supports in three categories: low-loaded (<50 µmol/g of NH2 groups); conventionally loaded (∼100 µmol/g of NH2 groups); and high-loaded (>150 µmol/g of NH2 groups). The mean pore sizes of these supports ranged from 313 to 1984 Å with amino group loadings between 23 and 440 µmol/g (Table 1).
Table 1. Comparison of different loading methods on the LCAA-CPG supports evaluated.
| Support | Mean pore size (Å) | Surface loading (µmol/g) | ||||||
|---|---|---|---|---|---|---|---|---|
| Manufacturer’s NH2 assay | DMT-ClO4 assay | Coupling with 1a | Coupling with 6a | Coupling with 13a | ||||
| P1 | 585 | 125 | 48 | 45 | 43 | 44 | ||
| P2 | 574 | 101 | 52 | 44 | 43 | |||
| P3 | 1088 | 78 | 43 | 38 | 39 | 47 | ||
| P4 | 1984 | 48 | 33 | 18 | 20 | 20 | ||
| P5 | 1918 | 23 | 23 | 18 | 18 | 18 | ||
| P6 | 505 | 145 | 82 | 70 | 82b | 56 | 75b | |
| P7 | 503 | 162 | 58 | 59 | 70b | 62 | 76b | |
| P8 | 525 | 170 | 65 | 72 | 87b | 50 | 76b | |
| P9 | 525 | 183 | 62 | 68 | 77b | 51 | 67b | |
| P10 | 313 | 440 | 57 | 55 | 62b | 29 | 56b | |
aDetermined by DMT analysis after coupling with 0.1 M 1 (B = T), except as noted below.
bLoadings obtained with 0.2 M 1 (B = T).
The amino surface loadings determined by the manufacturer are not a good indication of the potential nucleoside loading because many of the amino groups are inaccessible for oligonucleotide synthesis. Therefore, Table 1 also includes the results obtained from a dimethoxytrityl perchlorate derivatization assay (21,23), which provides a better idea of the useful support loading. Additionally, we compared the amount of nucleoside which could be attached by direct reaction of phosphoramidites 1, 6 and 13. The loadings obtained with either linker phosphoramidites 6 or 13 and conventional phosphoramidites (1) were similar for the low- and medium-loaded CPGs (P1–P5), but 1 produced slightly higher loadings on the high-loaded CPGs (P6–P10).
Low-loaded CPG was evaluated because one trend is towards synthesis on smaller scales. It is possible that fewer amino groups on the surface might lead to syntheses of higher quality due to either greater spacing among the amino groups or the absence of side reactions caused by excess amino groups. The low-loaded, wide-pore supports P4–P5 were used to prepare the 24mer dCACGGATAACAATTTCAC ACAGCT using either 0.05 or 0.1 M solutions of 13d (Table 2). The same sequence was also prepared on conventional 4 made on P1 for comparison. Analysis of the crude material produced by CGE showed that the amount of full-length material in the crude product was slightly improved on the lower loaded supports, but at the cost of reducing the amount of product by one-third to one-half.
Table 2. Synthesis of dCACGGATAACAATTTCACACAGCT on low-loaded CPG using 13d.
| Support | Concentration of 13d (M) | First nucleoside loading (µmol/g) | % of NH2 groups occupieda | Crude product (A260 units/10 mg CPG) | Purityb (%) |
|---|---|---|---|---|---|
| P4 | 0.05 | 15 | 65 | 24 | 79.2 |
| P4 | 0.10 | 18 | 78 | 27 | 80.3 |
| P5 | 0.05 | 14 | 29 | 19 | 76.9 |
| P5 | 0.10 | 20 | 42 | 25 | 75.2 |
| 4c | n/a | 30 | 24 | 52 | 74.2 |
aBased on the manufacturer’s amino group determination.
bAmount of full-length product determined by CGE.
cPrederivatized support prepared using P1.
Linker phosphoramidites (13) were tested on high-loading CPG since large-scale synthesis is important for producing oligonucleotides for pharmaceutical applications. Supports P6–P10 (30–35 mg/synthesis) were used to produce the 20mer dACCTTATGTATCATACACAT on an ABI 394 synthesizer under two conditions. Synthesis was performed using a 1 µmol scale cycle and either 0.1 or 0.2 M phosphoramidites. The crude products were quantitated by UV (260 nm) and then analyzed by CGE as shown in Table 3.
Table 3. Synthesis of dACCTTATGTATCATACACAT on high-loading CPGs using 13d.
| Support | 0.1 M phosphoramiditesa | 0.2 M phosphoramiditesa | ||||
|---|---|---|---|---|---|---|
| Crude product (A260 units/10 mg CPG) | Overall yieldb (%) | Average coupling yieldb (%) | Crude product (A260 units/10 mg CPG) | Overall yieldb (%) | Average coupling yieldb (%) | |
| P6 | 77 | 87.9 | 99.3 | 97 | 87.8 | 99.3 |
| P7 | 64 | 88.1 | 99.3 | 82 | 85.6 | 99.2 |
| P8 | 68 | 86.9 | 99.3 | 87 | 87.7 | 99.3 |
| P9 | 73 | 92.7 | 99.6 | 89 | 89.3 | 99.4 |
| P10 | 19 | 32.6 | 94.3 | 40 | 32.2 | 94.2 |
aConcentration of 13 and conventional phosphoramidites.
bDetermined by CGE.
Good results were obtained for every synthesis, except for the two on P10, which had poor coupling efficiencies (∼94%), presumably because of the small 313 Å pores (see Table 1). For supports P6–P9, use of 0.1 M phosphoramidites produced nucleoside loadings between 50 and 60 µmol/g (Table 1) and yielded ∼60–80 A260 units of product per 10 mg of CPG (Table 3). Doubling the phosphoramidite concentration only increased the nucleoside loading and the amount of product by 20–50% (Tables 1 and 3). This indicated that the maximum usable loading of 500 Å LCAA-CPG was ∼75 µmol/g. Significantly, however, the quality of all of the products made with 13d on supports P6–P9 was very good, with >85% full-length product. Thus, the linker phosphoramidites may find application in larger scale oligonucleotide syntheses on high-loaded supports.
The majority of oligonucleotide syntheses are performed on LCAA-CPG supports with amino group loadings of ∼100 µmol/g. After our initial tests, we examined the effect of different concentrations of linker phosphoramidites on the synthesis scale using such supports. Increasing or decreasing the concentration of 13, while using common synthesis columns containing the same amount of LCAA-CPG, can allow an increase or decrease in the amount of oligonucleotide produced. This was demonstrated using synthesis columns containing ∼10 mg of P1 and an unmodified 0.2 µmol scale synthesis cycle (ABI 394 synthesizer).
The 17mer dACCTTATGTATCCACAT was prepared using 13d at concentrations of 0.025, 0.1 and 0.2 M (the concentration of 1 remained at 0.1 M). The initial nucleoside loadings obtained were determined to be 17, 40 and 51 µmol/g, respectively, and the amount of crude material recovered (normalized to 10 mg of CPG) was 16, 32 and 61 A260 units, respectively. The 17mers made using 0.025, 0.10 and 0.20 M 13d were analyzed by CGE and the amount of full-length product in each was, respectively, 86, 90 and 89%. This showed that the synthesis scale could be adjusted by controlling the concentration of the linker phosphoramidite without affecting the product quality.
To further confirm the versatility of this approach, all four reagents 13a–d were used to make the 20mer, dACCTTATGTATCATACACAN (N = A, C, G, or T) with the concentration of 13a–d varied from 0.025 to 0.15 M. The nucleoside loadings obtained and the amounts of crude product produced are shown in Table 4. Again, the purities of the crude products, as determined by CGE, were all similar (∼70–80% of full-length product). As expected, the amount of nucleoside added in the first coupling step and the amount of final product obtained was related to the concentration of linker phosphoramidite used. When the concentration of 13 was lower, less nucleoside was added and less product was obtained. Generally, the 0.025 and 0.05 M solutions of 13 produced only approximately one-third to two-thirds the amount of crude product as a 0.1 M solution. Use of a 0.15 M solution of 13 on P1 sometimes produced a bit more product, but not enough to justify the higher reagent consumption. For many applications, the reduced oligonucleotide quantities produced by the 0.025 or 0.05 M solutions of 13 would be quite satisfactory. This approach reduces the number of types of synthesis columns required to be kept in inventory from at least eight (four bases, each at two or more different scales) to only one.
Table 4. Synthesis of dACCTTATGTATCATACACAN on P1 using 13a–d for N (ABI 394).
| Reagenta | Nucleoside loadingb (µmol/g) | Crude product (A260 units/10 mg CPG) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.025 M | 0.05 M | 0.10 M | 0.15 M | 0.025 M | 0.05 M | 0.10 M | 0.15 M | |
| 13a | 17 | 21 | 46 | 40 | 15 | 22 | 21 | 36 |
| 13b | 28 | 27 | 39 | 43 | 13 | 29 | 43 | 40 |
| 13c | 18 | 19 | 37 | 36 | 19 | 15 | 32 | 36 |
| 13d | 19 | 21 | 51 | 47 | 17 | 23 | 52 | 43 |
a13a–d were used at the concentrations shown in the table. All other phosphoramidites were used at 0.1 M in an unmodified 0.2 µmol scale synthesis.
bDetermined by quantitative trityl analysis after addition of 13a–d.
However, the majority of small scale oligonucleotides are now being produced in high-throughput synthesizers using a multi-well plate format and not prepacked columns. In this format, the cost of making the oligonucleotides, especially for commercial producers, is a critical concern. We were especially interested in how the linker phosphoramidites would perform in this synthesis format with respect to both chemical (oligonucleotide quantity and quality) and economic (cost savings) performance. Therefore, we examined how the amount of oligonucleotide produced varied when the amounts of CPG support and linker phosphoramidite (both concentration and volume) were varied, since optimizing consumption of these materials is important for maximum cost efficiency.
A MerMade IV synthesizer with a two 96-well plate capacity was used along with support P1. A ‘dry loading’ protocol [% coefficient of variation (i.e. % coefficient of variation = standard deviation / mean × 100%) ∼ 10%] dispensed 2.3 or 4.4 mg of support into the plate one well at a time. Support derivatization was performed using either a single addition of 20 or 40 µl of linker phosphoramidite in the first coupling cycle. Reagents 13a–d were tested at concentrations of 0.025, 0.05 and 0.1 M. Each condition was performed on between 12 and 48 wells, so we could examine the variability of the method. A row of 12 wells with conventionally derivatized support 4 served as a control. The octanucleotide test sequences dTTTTTTTN (N = A, C, G or T) were prepared. After synthesis and deprotection, UV quantitation of the crude products was used to determine the number of nanomoles produced.
The average number of nanomoles produced from each test condition, along with the %CVs, are shown in Table 5. These conditions produced amounts ranging from ∼20 to 100 nmol. As expected, syntheses performed with more CPG or with more linker phosphoramidite always produced more material than those with less, but the synthesis scale could be adjusted by changing any one of the three parameters (amount of CPG, concentration of 13 and volume of 13). There was also significant well-to-well variation (%CV = 8–37%) in the amount of product produced by these test conditions. However, most (32 of 48) of the experiments had %CVs of between 10 and 20% and only 12 of 48 experiments had %CVs between 21 and 30%. The amount of product produced from the control syntheses on prederivatized supports (4) was also quite variable, with %CVs of between 7 and 22%.
The variable product amounts could have been caused by either variation in the CPG dispensing, synthesizer operation, or the linker phosphoramidite coupling performance. Generally, it is believed that synthesis in multi-well plate format is less consistent than synthesis in individual columns and a 20% CV for synthesis in 384-well plates has been reported (11), similar to our observations.
Guidelines for the amounts of linker phosphoramidite and LCAA-CPG required to produce oligonucleotides on various scales are provided in Table 6. These guidelines indicate that between 500 and 2000 nmol of 13 are required for each synthesis. Significantly, this reagent consumption is much less than the amount of phosphoramidite 1 consumed during regular coupling cycles. For example, in our 50 nmol scale 96-well plate syntheses, a double addition (2 × 65 µl) of 0.1 M phosphoramidite 1 (13 000 nmol) is used in each coupling cycle. In terms of coupling reactions per gram of phosphoramidite, 13 yields 500–2000 couplings/g, while regular phosphoramidites (1) yield <200 couplings/g. The phosphoramidite consumption for the first nucleoside addition can be much less than the consumption for subsequent chain extension cycles because quantitative conversion of all of the amino sites into nucleosides is not required. This is different from subsequent cycles where coupling efficiencies close to 100% are required.
Table 6. Guidelines for synthesis on various scales using 13 on the MerMade synthesizer.
| Synthesis scale (nmol) | Concentration (M) | Volume (µl) | Weight of CPG (mg) | Phosphoramidite 13 consumption (nmol) | |
|---|---|---|---|---|---|
| 1 | 10–20 | 0.025 | 20 | 2.3 | 500 |
| 2 | 20–30 | 0.025 | 20 | 4.4 | 500 |
| 3 | 20–30 | 0.025 | 40 | 2.3 | 1000 |
| 4 | 30–40 | 0.025 | 40 | 4.4 | 1000 |
| 5 | 30–40 | 0.05 | 20 | 4.4 | 1000 |
| 6 | 40–50 | 0.05 | 40 | 4.4 | 2000 |
| 7 | 40–50 | 0.10 | 20 | 4.4 | 2000 |
The lability of the sulfonyl diethanol linker in 13 provides a significant advantage by reducing the time required for cleavage from the support. However, the stability of 13 was a concern and so we monitored the stability of acetonitrile solutions of 13 over time. Solutions of 13 (0.05 M) were installed and the 20mer dACCTTATGTATCCACAN was synthesized repeatedly over 2 weeks. The crude material produced was quantitated by UV and analyzed by CGE. These results showed that 0.05 M solutions of 13 (dA-Bz, dC-Bz and T) could be kept for up to 2 weeks on the synthesizer with only a small decrease (∼10–20%) in the amount of crude product produced. The lifetime of the dG reagent was shorter, with only approximately three-quarters as much product being produced after only 5 days. Fortunately, CGE analyses of the products showed that the age of 13 had no effect on the product quality, since the amount of full-length oligonucleotide in all the crude products was very similar. Based on these observations, we recommend that solutions of dA, dC and T (13a, b and d) be used within 1–2 weeks and that dG (13c) solutions be used within only 3–5 days.
In conclusion, linker phosphoramidites are a useful new class of reagents which allow the first nucleoside of a sequence to be attached to an amino support using a regular phosphoramidite coupling cycle. Cleavage of the linker between the phosphoramidate and the nucleoside releases products with only 3′-OH terminal ends and no extra cleavage or 3′-dephosphorylation steps are required. Advantageously, these phosphoramidites can be used at lower concentrations and in lower amounts than regular phosphoramidites to both modulate the amount of oligonucleotide produced and to lower reagent costs. Synthesis of multiple oligonucleotides in a single tandem synthesis is also possible with these reagents and details on this will be presented in a subsequent article.
SUPPLEMENTARY MATERIAL
MALDI-TOF results for representative oligonucleotides prepared using 13a–d and 5′-phosphorylated oligonucleotides is available. Analytical results from capillary gel electrophoresis for representative oligonucleotides and the results of the rate of cleavage experiment using ammonium hydroxide are also available as Supplementary Material at NAR Online.
Acknowledgments
ACKNOWLEDGEMENTS
We would like to thank CPG Inc. and Transgenomic for supplying CPG supports and phosphoramidite reagents. We thank Fady Girgis, Marko Markicevic, Maria Loskot, Heidi Huettler and Bernd Kalisch for technical assistance and the Alberta Heritage Foundation for Medical Research for financial support.
REFERENCES
- 1.Sanghvi Y.S., Ravikumar,V.T., Scozzari,A.N. and Cole,D.L. (2001) Application of green chemistry in the manufacture of oligonucleotide drugs. Pure Appl. Chem., 73, 175–180. [Google Scholar]
- 2.Pon R.T. (2000) Solid-phase supports for oligonucleotide synthesis. In Beaucage,S.L., Glick,G.D., Bergstrom,D.E. and Jones,R.A. (eds), Current Protocols in Nucleic Acid Chemistry. John Wiley & Sons, New York, NY, pp. 3.1.1–3.1.28.
- 3.Song Q.L. and Sanghvi,Y.S. (2001) Unexpected results and recourse in process optimization of nucleoside 3′-O-succinates. Nucl. Nucl. Nucleic Acids, 20, 1267–1270. [DOI] [PubMed] [Google Scholar]
- 4.Mullah B. and Andrus,A. (1997) Automated synthesis of double dye-labeled oligonucleotides using tetramethylrhodamine (TAMRA) solid supports. Tetrahedron Lett., 38, 5751–5754. [Google Scholar]
- 5.Pon R.T. and Yu,S. (1997) Hydroquinone-O,O′-diacetic acid (‘Q-linker’) as a replacement for succinyl and oxalyl linker arms in solid phase oligonucleotide synthesis. Nucleic Acids Res., 25, 3629–3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pon R.T., Yu,S., Guo,Z., Deshmukh,R. and Sanghvi,Y.S. (2001) Reusable solid-phase supports for oligonucleotides and antisense therapeutics. J. Chem. Soc. Perkin Trans., 1, 2638–2643. [Google Scholar]
- 7.Pon R.T. and Yu,S. (1999) Efficient and rapid coupling of nucleosides to amino derivatized solid-phase supports. Syn. Lett., 1778–1780. [Google Scholar]
- 8.Pon R.T., Yu,S.Y. and Sanghvi,Y.S. (1999) Rapid esterification of nucleosides to solid-phase supports for oligonucleotide synthesis using uronium and phosphonium coupling reagents. Bioconjug. Chem., 10, 1051–1057. [DOI] [PubMed] [Google Scholar]
- 9.Lashkari D.A., Hunickesmith,S.P., Norgren,R.M., Davis,R.W. and Brennan,T. (1995) An automated multiplex oligonucleotide synthesizer: development of high-throughput, low-cost DNA synthesis. Proc. Natl Acad. Sci. USA, 92, 7912–7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rayner S., Brignac,S., Bumeister,R., Belosludtsev,Y., Ward,T., Grant,O., OBrien,K., Evans,G.A. and Garner,H.R. (1998) MerMade: an oligodeoxyribonucleotide synthesizer for high throughput oligonucleotide production in dual 96-well plates. Genome Res., 8, 741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng J.Y., Chen,H.H., Kao,Y.S., Kao,W.C. and Peck,K. (2002) High throughput parallel synthesis of oligonucleotides with 1536 channel synthesizer. Nucleic Acids Res., 30, e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gough G.R., Brunden,M.J. and Gilham,P.T. (1983) 2′(3′)-O-Benzoyluridine 5′ linked to glass: an all purpose support for solid phase synthesis of oligodeoxyribonucleotides. Tetrahedron Lett., 24, 5321–5324. [Google Scholar]
- 13.Scott S., Hardy,P., Sheppard,R.C. and McLean,M.J. (1994) A universal support for oligonucleotide synthesis. In Epton,R. (ed.), Innovation and Perspectives in Solid-Phase Synthesis. Peptides, Proteins and Nucleic Acids, Biological and Biomedical Applications. Mayflower Worldwide Ltd., Birmingham, pp. 115–124.
- 14.Scheuerlarsen C., Rosenbohm,C., Jorgensen,T.J.D. and Wengel,J. (1997) Introduction of a universal solid support for oligonucleotide synthesis. Nucl. Nucl., 16, 67–80. [Google Scholar]
- 15.Nelson P.S., Muthini,S., Vierra,M., Acosta,L. and Smith,T.H. (1997) Rainbow™ universal CPG: a versatile solid support for oligonucleotide synthesis. Biotechniques, 22, 752–756. [DOI] [PubMed] [Google Scholar]
- 16.Azhayev A.V. and Antopolsky,M.L. (2001) Amide group assisted 3′-dephosphorylation of oligonucleotides synthesized on universal A-supports. Tetrahedron, 57, 4977–4986. [Google Scholar]
- 17.Guzaev A.P. and Manoharan,M. (2003) A conformationally preorganized universal solid support for efficient oligonucleotide synthesis. J. Am. Chem. Soc., 125, 2380–2381. [DOI] [PubMed] [Google Scholar]
- 18.Pon R.T., Yu,S. and Sanghvi,Y.S. (2001) Multiple oligonucleotide synthesis in tandem on solid-phase supports for small and large scale synthesis. Nucl. Nucl. Nucleic Acids, 20, 985–989. [DOI] [PubMed] [Google Scholar]
- 19.Pon R.T. and Yu,S. (2001) Linker phosphoramidite reagents for oligonucleotide synthesis on underivatized solid-phase supports. Tetrahedron Lett., 43, 8943–8947. [Google Scholar]
- 20.Pon R.T. and Yu,S. (2002) Tandem oligonucleotide synthesis using linker (First Base™) phosphoramidites. Nucleic Acids Res. Suppl., 2, 1–2. [DOI] [PubMed] [Google Scholar]
- 21.Pon R.T. (2000) Attachment of nucleosides to solid-phase supports. In Beaucage,S.L., Glick,G.D., Bergstrom,D.E. and Jones,R.A. (eds), Current Protocols in Nucleic Acids Chemistry. John Wiley & Sons, New York, NY, pp. 3.2.1–3.2.23.
- 22.Pon R.T., Usman,N. and Ogilvie,K.K. (1988) Derivatization of controlled pore glass beads for solid phase oligonucleotide synthesis. Biotechniques, 6, 768–775. [PubMed] [Google Scholar]
- 23.Reddy M.P., Rampal,J.B. and Beaucage,S.L. (1987) An efficient procedure for the solid phase tritylation of nucleosides and nucleotides. Tetrahedron Lett., 28, 23–26. [Google Scholar]
- 24.Beaucage S.L. and Iyer,R.P. (1993) The synthesis of modified oligonucleotides by the phosphoramidite appproach and their applications. Tetrahedron, 49, 6123–6194. [Google Scholar]
- 25.Beaucage S.L. and Iyer,R.P. (1992) Advances in the synthesis of oligonucleotides by the phosphoramidite approach. Tetrahedron, 48, 2223–2311. [Google Scholar]
- 26.Shchepinov M.S., Case-Green,S.C. and Southern,E.M. (1997) Steric factors influencing hybridisation of nucleic acids to oligonucleotide arrays. Nucleic Acids Res., 25, 1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang S.Y. and Bergstrom,D.E. (2003) Reversible biotinylation phosphoramidite for 5′-end-labeling, phosphorylation and affinity purification of synthetic Oligonucleotides. Bioconjug. Chem., 14, 80–85. [DOI] [PubMed] [Google Scholar]
- 28.Fang S.Y. and Bergstrom,D.E. (2003) Fluoride-cleavable biotinylation phosphoramidite for 5′-end-labeling and affinity purification of synthetic oligonucleotides. Nucleic Acids Res., 31, 708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skrzypczynski Z. and Wayland,S. (2003) New reagents for the introduction of reactive functional groups into chemically synthesized DNA probes. Bioconjug. Chem., 14, 642–652. [DOI] [PubMed] [Google Scholar]
- 30.Kwiatkowski M., Fredriksson,S., Isaksson,A., Nilsson,M. and Landegren,U. (1999) Inversion of in situ synthesized oligonucleotides: improved reagents for hybridization and primer extension in DNA microarrays. Nucleic Acids Res., 27, 4710–4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horn T. and Urdea,M.S. (1986) A chemical 5′-phosphorylation of oligodeoxyribonucleotides that can be monitored by trityl cation release. Tetrahedron Lett., 27, 4705–4708. [Google Scholar]
- 32.Gryaznov S.M. and Letsinger,R.L. (1993) Anchor for one step release of 3′-aminooligonucleotides from a solid support. Tetrahedron Lett., 34, 1261–1264. [Google Scholar]
- 33.Bader R., Hinz,M., Schu,B. and Seliger,H. (1997) Oligonucleotide microsynthesis of a 200-mer and of one dimensional arrays on a surface of hydroxylated polypropylene tape. Nucl. Nucl., 16, 829–833. [Google Scholar]
- 34.Glazer M., Fidanza,J., McGall,G. and Frank,C. (2001) Colloidal silica films for high-capacity DNA probe arrays. Chem. Mater., 13, 4773–4782. [Google Scholar]
- 35.Mag M. and Engels,J.W. (1989) Synthesis and selective cleavage of oligodeoxyribonucleotides containing non-chiral internucleotide phosphoramidate linkages. Nucleic Acids Res., 17, 5973–5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seliger H., Bader,R., Hinz,M., Rotte,B., Astriab,A., Markiewicz,M. and Markiewicz,W.T. (1997) Synthetic oligonucleotide combinatorial libraries—tools for studying nucleic acid interactions. Nucl. Nucl., 16, 703–710. [Google Scholar]
- 37.Gryaznov S.M., Lloyd,D.H., Chen,J.K., Schulz,R.G., Dedionisio,L.A., Ratmeyer,L. and Wilson,W.D. (1995) Oligonucleotide N3′→P5′ phosphoramidates. Proc. Natl Acad. Sci. USA, 92, 5798–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pon R.T., Yu,S. and Sanghvi,Y.S. (2002) Tandem oligonucleotide synthesis on solid-phase supports for the production of multiple oligonucleotides. J. Org. Chem., 67, 856–864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.