Abstract
The dynamics of many cardiac arrhythmias, as well as the nature of transitions between different heart rhythms, have long been considered evidence of nonlinear phenomena playing a direct role in cardiac arrhythmogenesis. In most types of cardiac disease, the pathology develops slowly and gradually, often over many years. In contrast, arrhythmias often occur suddenly. In nonlinear systems, sudden changes in qualitative dynamics can, counter-intuitively, result from a gradual change in a system parameter –this is known as a bifurcation. Here, we review how nonlinearities in cardiac electrophysiology influence normal and abnormal rhythms and how bifurcations change the dynamics. In particular, we focus on the many recent developments in computational modeling at the cellular level focused on intracellular calcium dynamics. We discuss two areas where recent experimental and modeling work have suggested the importance of nonlinearities in calcium dynamics: repolarization alternans and pacemaker cell automaticity.
Keywords: alternans, calcium, bifurcation, model, pacemaking
1. INTRODUCTION
The systematic application of nonlinear dynamics as a means to describe and understand cardiac arrhythmias has grown increasingly popular over the last thirty or so years. The system that gives rise to the electrical activity in cardiac myocytes is highly complex: it consists of many interconnected parts such as ionic channels and transporters that often have a nonlinear dependence on one or more variables, such as the transmembrane potential or the concentration of an ionic species. To further complicate matters, different variables feed back on one another, through loops that may be positive or negative.
Unlike linear equations, nonlinear systems do not necessarily exhibit a direct proportionality between input and output. Indeed, such dependencies may exhibit threshold effects, biphasic relationships, and other nonlinearities. In addition, complex systems often exhibit emergent properties that are not easily predicted from the characteristics of the individual parts. Therefore, to analyze the dynamics of a complex system, and to predict the effects of a perturbation (e.g., the application of a drug), it is often necessary and almost always useful to employ mathematical models to simulate the resulting behavior. Mathematical models of electrophysiological dynamics in cardiac myocytes, consisting of coupled, nonlinear differential equations, have been developed with increasing complexity and specificity for almost 50 years (1). These models have inarguably helped increase our understanding of cellular electrophysiology and cardiac arrhythmias. In this article, we will review a range of areas where recent advances exemplify such advancement.
2. NONLINEAR DYNAMICS IN CARDIAC ELECTROPHYSIOLOGY
In cardiac electrophysiology, as in many other biological systems, nonlinear dynamics occur on multiple spatial scales: subcellular (e.g., at the single-channel level), cellular, and whole-heart. In the following, we review some of the nonlinearities in cardiac electrophysiological dynamics, and discuss their roles in normal and pathological rhythm dynamics.
2.1 Nonlinearities in action potential generation and morphology
The cardiac action potential is generated by a well-orchestrated flux of ions across the cell membrane through specialized ionic channels and transporters (Fig. 1A). Inward currents such as the sodium current (INa) and the L-type calcium current (ICa,L) depolarize the membrane, while a number of potassium currents repolarize the membrane back to its resting value (Fig. 1B).
Fig. 1.

Schematic illustration of ionic currents and action potential in a ventricular myocyte. a: The major channels and transporters involved in generating the cardiac action potential. Also shown is a rudimentary schematic of the intracellular calcium dynamics, which will be discussed more in Chapter 3. b: The fast upstroke of the action potential is generated by a large inward (negative) total current carried by sodium ions (off scale; goes to about −200 pA/pF in this model (158)). The slowly changing plateau phase is caused by a near-balance between ICa,L (inward) and IKs and IKr (outward). The rapid relaxation back to the resting membrane potential is due to IK1. Abbreviations: INa: sodium current; Ib,Na: background sodium current; ICa,L: L-type calcium current; Ib,Ca: background calcium current; IK,p: plateau potassium current; Ito: transient outward current; IKs: slow delayed rectifier current; IKr: rapid delayed rectifier current; IK1: inward rectifier current; INaK: sodium/potassium pump current; INaCa: sodium/calcium exchange current; IpCa: calcium pump current.
The currents through the different ionic channels and transporters are almost always a nonlinear function of voltage. Many of these nonlinearities play clear functional roles. Consider for example the inward rectifier current (IK1), whose conductance is large for a range of voltages around the resting membrane potential, but drops to zero for depolarized potentials. Hence, relatively small perturbations to the resting membrane potential (depolarizing or repolarizing) elicit large current responses (outward or inward, respectively) and IK1 thus stabilizes and controls the membrane resting potential, without playing much of a role during depolarization. Had IK1 not been strongly rectifying, a large outward current would have been elicited during the action potential, necessitating a large counteracting inward current and greatly increasing the metabolic cost of maintaining ionic homeostasis.
Another classic example of a nonlinearity in action potential dynamics is the threshold-range effect seen in the generation of the action potential upstroke. In a linear system, the response is always directly proportional to the input. However, in cardiac myocytes (and other excitable cells), depolarizing stimulation results in one of two responses: an action potential of essentially fixed amplitude (for suprathreshold stimuli) or no action potential (for smaller-amplitude subthreshold stimuli). Mechanistically, this all-or-none response results from a positive feedback loop, called the Hodgkin cycle, involving activation of the sodium current with depolarization.
Finally, a number of nonlinearities are involved in controlling the action potential duration. As the conductances of the currents involved in generating the action potential change throughout its phases, so does the membrane resistance. Indeed, the resistance is low at the resting membrane potential (due primarily to the large IK1 conductance), but increases steeply during the plateau phase (2, 3). Thus, tiny current perturbations late in the plateau can cause substantial membrane potential deviations, resulting in changes to the action potential duration. Recently, Bányász et al. (4) showed that a nonlinear relationship between membrane current and action potential duration in canine ventricular myocytes may contribute to the phenomenon of reverse rate dependence, i.e., that drug-induced prolongation of the action potential duration is less at faster rates, where the action potential is of shorter duration.
2.2 Action potential duration restitution
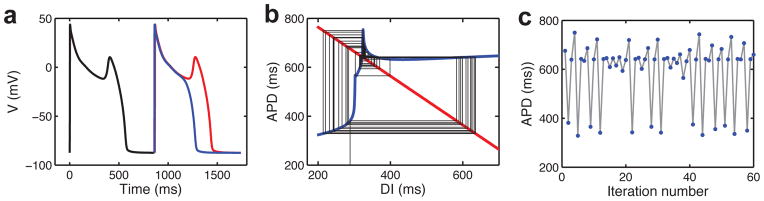
Shortening of the action potential during fast pacing is a critically important property of cardiac myocytes because it allows time for proper filling of the heart’s chambers between contractions. The action potential duration of a cardiac myocyte is reasonably well-described as a nonlinear function of the previous resting interval (the diastolic interval): fast pacing and extensive diastolic interval shortening cause more severe action potential shortening than intermediate pacing rates and diastolic interval shortening (Fig. 2A). This relationship is known as the restitution curve.
Fig. 2.
Alternans, higher-order periodic rhythms, and irregular dynamics. a: Action potential duration restitution curve obtained by applying premature stimuli in the Ten Tusscher and Panfilov model (158). b: Cobweb iterations of restitution map given by APDi+1=220–180exp(–DIi/60) (blue curve) and DIi=PCL–APDi (red line)(13), where APD is the action potential duration, DI is the diastolic interval, PCL is the constant pacing cycle length. Left: period-1 rhythm for PCL=400 ms. Right: alternans for PCL=220 ms; dashed line indicates the value of DI for which the slope of the restitution curve equals one. c: Bifurcation diagram of the restitution map (similar to that in Ref. (13)) with the condition that action potentials occur only for DI>5 ms. Numbers give stimuli:response values; ID indicate irregular dynamics, which is preceded by period-doubling bifurcations.
When submitted to fast pacing rates, cardiac cells and tissue often undergo a quantitative change from a period-1 rhythm, where each action potential has the same duration, to a period-2 rhythm exhibiting a beat-to-beat alternation in action potential duration, which has been putatively linked to the onset of cardiac arrhythmias (5–7). One of the early successes of applying nonlinear dynamics theory to cardiac electrophysiology was the demonstration of how such repolarization alternans may arise due to the nonlinear restitution relationship (8) as a consequence of a period-doubling bifurcation (9). With slow pacing, the operating point falls on the flat part of the curve, which is stable in the sense that the action potential duration changes little when the diastolic interval varies (Fig. 2B). Increasing the pacing rate shifts the operating point down the curve. When the slope of the restitution curve exceeds one, this stability is lost, a period-doubling bifurcation occurs, and the rhythm alternates (Fig. 2B). In smooth maps and models, this period-doubling is a smooth supercritical bifurcation. However, recent experimental and modeling work indicate that myocytes undergoing repolarization alternans may embody features of both a smooth bifurcation and a non-smooth border-collision bifurcation (10). It is not yet clear what physical features of the cellular dynamics would contribute to the discontinuity associated with border-collision bifurcations or to the seemingly hybrid bifurcation characteristics. We will return to a discussion of more recently described aspects of alternans mechanisms in Chapter 3.
Alternans represents just one of many higher-order period rhythms that can occur in paced cardiac cells or tissues. For rates faster than those required to induce alternans, a myriad of rhythms occur in some models and experimental preparations, an example from iteration of a restitution map is shown in Fig. 2C. Famously, in addition to periodic rhythms, nonlinear systems can also exhibit aperiodic dynamics with no phase-locking; such irregular dynamics highly suggestive of chaos have been observed in periodically paced cardiac tissue preparations (11, 12), as well as in models (reviewed in (13)).
2.3 Early afterdepolarizations
An early afterdepolarization (EAD) is a spontaneous extra depolarization prior to full repolarization of the membrane potential (Fig. 3A). EADs typically occur in purkinje fibers or ventricular myocytes when repolarizing potassium currents are reduced and/or depolarizing currents such as ICa,L and INaCa are increased. Because of the added depolarization event, EADs may sometimes trigger cardiac arrhythmias. The pattern by which EADs occur is often irregular, with only some action potentials exhibiting an EAD. Such irregularity may be due to intrinsic noise (e.g., random channel open/close kinetics) and/or deterministic chaos (14–17). Fully deterministic mathematical models are capable of producing irregular EAD dynamics when parameters are in the right range (15, 16, 18–20). Bifurcation analysis of such models has shown that the oscillatory EADs are associated with a Hopf bifurcation and a homoclinic bifurcation (19, 20), a bifurcation combination linked to the onset of chaos. By reducing the cell model to an iterative map of action potential duration and diastolic interval dynamics, Sato et al. (15) demonstrated how such a map, characterized by steep parts and sharp transitions, cause chaotic EAD dynamics (Fig. 3B and C).
Fig. 3.
Early afterdepolarization (EAD) dynamics. a: EADs in a mathematical model of a rabbit ventricular myocyte modified to simulate oxidative stress. The occurrence of EADs depends on a fine balance between inward and outward currents during the plateau of the action potential and is therefore sensitive to changes in stimulus timing. Here, a certain stimulus timing leads to a normal action potential (blue trace), while a stimulus applied 5 ms later causes an EAD (red trace). b: Iteration of a one-dimensional map based on the myocyte model leads to irregular (chaotic) dynamics, with aperiodic switching between short (no EAD) and long APD values (with EAD). c: APD values from iteration in (B) plotted vs. beat number. Modified from Ref. (15) with permission.
2.4 Nonlinearities in action potential propagation
During normal heart rhythms, action potentials propagate through the myocardium one wave at a time, activating the muscle cells and causing contraction. The propagating waves spread between neighboring cells, which are electrically coupled by gap junction proteins. The speed of propagation depends on the diastolic interval in the same way that action potential duration does: it is a nonlinear function that decreases steeply at short diastolic intervals and plateaus at long diastolic intervals.
The steepness of this dispersion curve means that during alternans, alternating beats may propagate with considerably different conduction velocities. A short action potential following a short diastolic interval will propagate at a slow speed, allowing the tissue at more distant locations to recover more (i.e., increase the diastolic interval). This prolongation of the diastolic interval in turn increases the action potential duration, such that the short action potential has become of long duration. Likewise, the alternate action potential can change duration dynamically in space from long to short. Thus, steep conduction velocity dispersion represents one way of forming a spatially heterogeneous pattern in a completely homogeneous tissue (21, 22). The theory of such pattern formation is a well-described part of nonlinear systems theory (23).
Some cardiac arrhythmias such as atrial flutter and monomorphic ventricular tachycardia can be maintained by waves repeatedly circulating an anatomical obstacle such as a valve or a scar. A simple way to simulate such reentry is to use a one-dimensional ring of virtual cardiac cells. In such a system, the properties of action potential restitution and dispersion control to a large extent important dynamics such as the transition to quasiperiodic dynamics for short ring sizes (24) and wave block (25–27).
In two- and three-dimensional tissue, propagation is also influenced by curvature such that stable spiral (two-dimensions) and scroll (three-dimensions) waves can form. Such spiral waves, which can arise in generic nonlinear, excitable systems such as chemical reactions (e.g., the Belousov-Zhabotinsky system), can underlie cardiac arrhythmias. Such spiral and scroll waves can undergo different bifurcations, which are hypothesized to underlie transitions between different cardiac arrhythmias (see Ref. (13) for a recent review).
2.5 Nonlinear dynamics and arrhythmias
Sudden cardiac death is typically caused by ventricular fibrillation, an arrhythmia where the ventricles of the heart are activated, presumably by scroll waves, in a manner so rapid and disorganized that pumping fails. Fibrillation is not a property of the individual cells or channel proteins; rather it is an emergent phenomenon of the tissue. In fact, during fibrillation, cells behave as they are expected to: they fire an action potential if recovered when activated. However, wave propagation through the tissue has become pathological: it is reentrant and irregular (28, 29), similar to simulated ventricular fibrillation displaying spatiotemporal chaos (30).
Among the first arrhythmias investigated systematically from a nonlinear dynamics point of view were Wenckeback rhythms and parasystole. In classic Wencheback rhythms, some waves block as they pass through the atrioventricular node, the anatomical passageway between the atria and the ventricles. The blocking occurs in particular phase-locked patterns [n+1:n, such that for every n+1 stimuli (atrial waves) there are only n responses (ventricular waves)], and can be understood theoretically from considerations of periodically stimulated nonlinear oscillators such as that underlying Fig. 2 (11, 31, 32). Parasystole is another example of entrainment, in this case as the interaction between the sinoatrial node (the natural pacemaker of the heart) and an ectopic pacemaker (a pathological additional pacemaker, often located in the ventricles of the heart), which feature patterns consistent with those occurring in two coupled nonlinear oscillators (33, 34).
2.6 Multistability
In situ, ventricular fibrillation is typically fatal, as it stops the pumping of blood. However, in an isolated, perfused heart, ventricular fibrillation can be long-lasting. The arrhythmia may be terminated by the application of a defibrillating shock. Fibrillation can also be initiated from normal (sinus) rhythm by an external perturbation, e.g., an electrical shock or a mechanical impact. Thus, a healthy heart is capable of (at least) two stable rhythms, with possible intermode switching by external stimuli. Such bistable behavior is consistent with a nonlinear system having two stable states (attractors), each reachable from different sets of initial conditions (basins of attraction).
Bistable behavior can also exist between different reentrant rhythms (35, 36), and between a stable fixed point, corresponding to a cell at rest, and at stable limit cycle, corresponding to pacemaking activity (18, 37) - we will return to this scenario in Chapter 4. Finally, bistability between different phase-locked rhythms of low periodicity [in particular between alternans (2:2) and regular beating (1:1) and between block (2:1) and regular beating] is recurrently observed in cardiac cells (38, 39), small tissues (11), whole hearts (39, 40), and mathematical cell (38, 41, 42) and tissue models (42, 43). At the level of the whole heart, bistability can also manifest between alternans with different spatiotemporal patterns (39). Indeed, in simulated tissue models, multistability between a range of different spatial alternans patterns can occur (42, 43), suggesting prudence should be taken when performing such simulations and interpreting their outcomes.
3. MODEL DYNAMICS AND ANALYSIS
Since the first Hodgkin-Huxley-type mathematical model of the cardiac sarcolemmal ionic dynamics was published in 1962 (44), new models have been developed frequently. Newly published cardiac cell models almost always build on existing models, but typically incorporate more detailed ionic current formalisms, more detailed intracellular calcium dynamics, and/or rely more heavily on species- and/or region-specific data. Model component recycling can span several model generations, which can make tracking and disentanglement of the individual components an arduous task (45). In order to lower model complexity and/or computational costs (in particular for three-dimensional tissue simulations), reduced models with fewer variables and parameters have also been developed, e.g., Refs. (46, 47).
The expansion of model development has resulted in the existence of multiple models for some species/cell-type combinations. This, in turn, has meant that models themselves have become the objects of comparative investigations, in terms of their structure, parameter values, parameter sensitivity, and simulated dynamics. Examples include models of human ventricular myocytes (3, 45, 46, 48), human atrial myocytes (49, 50), canine ventricular myocytes (51), rabbit ventricular myocytes (52), and rabbit sinoatrial node cells (53, 54). The goal of such analyses is not necessarily to determine if one model is better - such a task may not even be possible in the sense that different models may reflect variations in action potential morphology even for the same overall cell type. However, such comparisons do help clarify model differences.
As mentioned previously, cardiac myocytes and cardiac myocyte models typically exhibit a nonlinear dependence of action potential characteristics on system parameters. For example, increasing the conductance of the transient outward current (Ito) in ventricular myocytes or myocyte models induces a sharp transition in action potential morphology (55, 56). Varying two conductances simultaneously can have synergistic effects - a classic example is that of blocking two repolarizing currents, IKs and IKr, whichunder normal circumstances act as mutual reserves (57). Such parametric dependence is typically not investigated systematically during model development. Yet, many models do in fact capture experimentally observed behaviors such as various types of drug block, thus showcasing their predictive abilities.
Analyzing resulting behaviors upon systematic parameter variation, known as bifurcation analysis, is a much-employed tool in nonlinear dynamics. Bifurcation analysis has been widely used in theoretical neuroscience, e.g., to characterize different types of oscillations or bursting (58), but is less widespread in quantitative cardiac electrophysiology (we discuss recent examples in subsequent chapters). The strength of bifurcation analysis lies in its ability to provide insights into how abnormal dynamics arise with the change of (at most) a few parameters at a time. However, given the large number of parameters in modern models, it is a much too tedious technique to apply to all of them. Hence, in terms of analyzing parameter dependencies in cardiac electrophysiology models, other methods are often necessary. Additionally, novel approaches that systematically fit or optimize model parameters, replacing the traditional ad hoc determination based on individual electrophysiological recordings, have been used for model development. In this chapter, we will review some of these approaches.
3.1 Parameter estimation
Recent years have seen an increased effort in attempting more automated parameter estimation for cardiac electrophysiology models. A global parameter optimization, using a nonlinear least squares method applied to the Beeler-Reuter 1977 model (59), demonstrated that accurate reconstruction of the voltage waveform and the ionic currents is possible despite substantial deviation from default parameters (60). However, the Beeler-Reuter model has only 63 free parameters (60), while more modern models may have many more. In addition, the increased complexity of recent models may enlarge the number of local minima of the optimization objective function. Both of these effects contribute to the often prohibitive computational cost of global optimization approaches.
One approach to circumvent such lengthy computations is to focus on a limited number of parameters, typically those defining the maximal ionic conductances and the maximal flux of ions through sarcolemmal transporters and between intracellular compartments. Such a cutback is particularly useful when investigating cell-to-cell variability where, purportedly, the main source of variation is in the number of functional channels and transporters at their respective locations, while the parameters detailing the kinetics of individual channel/transporter proteins are largely conserved. By focusing only on the conductance parameters, genetic algorithms that repeatedly perturb parameters and select better fits have been used to reconstruct voltage wave forms from cardiac models and experimental data (61, 62).
Many of the newly developed cardiac myocyte models contain Markov models of one or more ionic currents, rather than the classic Hodgkin-Huxley formalism. While Markov models represent a more general description than Hodgkin-Huxley-type models, and may be able to simulate drug block better, they carry higher computational costs due to increased stiffness and/or a larger number of states (63). In addition, they typically contain many more parameters than Hodgkin-Huxley models. While parameter values in Markov models have often been hand-tuned, some modeling work has employed optimization of the parameters in a pre-defined Markov model structure to data from voltage clamp protocols using simulated annealing and simplex algorithms (64, 65). Recently, Menon et al. (66) provided proof-of-concept of optimizing model structure as well as parameters for a neuronal sodium current using a genetic algorithm.
3.2 Model analysis
The increasing complexity of the mathematical models of cardiac electrophysiology makes it difficult to pin-point causal relationship such as the effects of different ionic currents on action potential characteristics (67, 68), let alone on more complex phenomena such as alternans onset and alternans mechanism (68–70), without the use of sophisticated analysis tools. One such tool is the eigenmode analysis developed by Li and Otani (71), which quantifies the dependence of different behaviors (eigenmodes, e.g., alternans and various types of memory) on dynamical variables. Li and Otani further showed how this method can be used to eliminate alternans in a myocyte model, by applying stimuli with a particular timing that was predicted to kill the alternans eigenmode (72). In a tissue model, elimination of the alternans eigenmode can prevent spiral breakup (73). The eigenmode approach has also been used to quantify the relative contributions of voltage-dependent dynamics vs. intracellular calcium dependent dynamics to the onset of alternans (70), a problem we discuss more in Chapter 3.
Another approach to investigate the dependence of a model output to a parameter is to compute their mutual information. This method, applied to several ionic myocyte models and using parameter values sampled over all of parameter space, have demonstrated that most action potential characteristics are each controlled by very few parameters (74). This illustrates the fact that the normal action potential is overdetermined in cardiac myocyte models (3, 60, 74), however, parameters that are redundant during normal action potential generation may be important during simulations of drug application and/or pathophysiological conditions (3).
Despite the nonlinearities in cardiac electrophysiology models, multivariable linear regression analysis has turned out to be a useful approach to sensitivity analysis, at least in cases when the parameters of interest are not varied too far from their default values (68). Multivariable linear regression analysis has been used to characterize sensitivity of action potential markers (action potential duration, resting membrane potential, and overshoot potential) to input parameters (68). Importantly, this regression method can also work inversely to predict conductance parameter values based on system outputs such as action potential and calcium transient characteristics (75). The method is also capable of identifying model parameters influencing alternans occurrence, resulting in identification of both well-known alternans associated parameters (e.g., the conductances of ICa,L and IKr) as well as parameters not previously related to alternans and hence warranting further (experimental) examination (68).
4. INTRACELLULAR CALCIUM DYNAMICS AND CALCIUM TRANSIENT ALTERNANS
One aspect of cardiac myocyte function that has received particular attention in terms of model development in recent years is the intracellular calcium dynamics. Calcium differs from the other main ionic species involved in action potential generation (sodium and potassium) in that it takes part in numerous other processes. Calcium not only triggers mechanical contraction of the myocyte, it is also a ubiquitous signaling molecule (76). Hence, calcium is essential to cardiac myocytes; nevertheless, balance is key, as too much calcium is toxic (calcium overload is arrhythmogenic and severe overload causes cell death). Because of all these factors, calcium dynamics is tightly regulated.
Calcium enters ventricular myocytes primarily through the L-type calcium channel (LCC). LCCs open during the action potential upstroke, allowing an early, local rise in calcium concentration. The LCCs are localized in so-called transverse (t) tubules, invaginations in the sarcolemmal membrane (Fig. 4). This anatomical arrangement allows the LCCs to be in close proximity to another calcium channel: the ryanodine receptor (RyR), which spans the membrane of the sarcoplasmic reticulum (SR), an internal calcium store. The RyRs open in response to the local increase in calcium concentration, thus allowing efflux of calcium ions from the SR and further increase in calcium concentration. This calcium-induced calcium release forms a positive feedback loop with a gain of about 2.5 to 4 in most mammalian species, including humans (but can as large as 10 to 12 in mice and rats) (76), and plays an important functional role in excitation-contraction coupling.
Fig. 4.
Intracellular calcium handling. A ventricular myocyte consists of approximately 75 sarcomeres, each of length ~2 μm. Sarcomeres span the distance between adjacent t-tubules that contain sodium-calcium exchangers (NCX; blue) as well as L-type calcium-channels (LCC; cyan), which are colocated in functional calcium release units with Ryanodine receptors (RyR; green) in the junctional sarcoplasmic reticulum (JSR). A cell contains about 10,000–20,000 release units, or couplons, each of which in turn consists of 10–25 LCCs and 100–200 RyRs. The total calcium concentration in the cytosol, arising as a global contribution from all couplons, gives the calcium transient (CaT). Expanded from Ref. (114) with permission.
The increase in free cytosolic calcium, globally combined from all active LCC and RyR sites (“couplons” or “dyads”), is seen as the calcium transient (Fig. 4). The cytosolic calcium initiates mechanical contraction of the cell, and is then resequestered back into the SR by the sarcoplasmic-endoplasmic reticulum calcium ATPase (SERCA) pump or into the extracellular space by the sodium/calcium exchanger (Fig. 4), ending the cycle.
Efforts in modeling the intracellular calcium dynamics in ventricular myocytes date back to the 1977 Beeler-Reuter model (59), which used a single variable to describe the cytosolic calcium concentration. More modern models tend to include several subspaces with intercompartmental fluxes (1), in order to avoid using a partial differential equation formulation to capture the variations in calcium concentration throughout the cell. Such a multi-compartment approach was first used in the DiFranceso-Noble model (77), which also included a sarcolemmal sodium/calcium exchange current. Recently, some models have incorporated stochastic descriptions at the level of the couplon, in order to simulate the random activation of these individual units. Such modeling include work that has illustrated mechanistically how graded release at the level of the cell (i.e., total SR calcium release is a graded function of the amplitude of the L-type calcium current) may arise despite the positive feedback release at the couplon level (78–81) and work that has provided insights into the occurrence of intracellular calcium alternans (see below). Another line of modeling, in a nascent but rapidly progressing stage, is electromechanical modeling, in which a myofilament model of actin-myosin-based contraction is coupled to an ionic model (82).
4.1 Calcium transient alternans
As mentioned above (Chapter 1), cardiac myocytes often exhibit alternations in action potential duration when paced at a rapid rate - a pattern first explained by the occurrence of a period-doubling bifurcation when the slope of the restitution curve increases one (Fig. 2). While the onset of alternans do indeed correlate with this criterion in some studies (83–85), many instances have been described where alternans is absent when the restitution slope is greater than one, or where alternans does occur for slopes less than one (e.g., (86, 87) and references therein).
Such discrepancies may be partly due to the fact that the complex dynamics of the myocyte cannot be fully captured by a one-dimensional map such as the restitution curve. Indeed, the action potential duration of a given beat depends on more than just the prior diastolic interval, there is a dependence on the history of pacing – a memory effect. This deviation from a one-dimensional map is seen clearly in the so-called restitution portrait, obtained by sweeping through multiple pacing protocols (88). In some experiments, the onset of alternans has been shown to correlate with one of the slopes of the restitution portrait (89). In tissue models, alternans onset can be different from that of single cell models, in particular, electrotonic and conduction velocity restitution effects can suppress alternans (86).
Multiple lines of recent research have demonstrated that the limited predictability of the restitution hypothesis may also be due to the fact that action potential duration alternans (APD alternans) can be a consequence of alternans in the calcium transient, rather than arising as an instability in the sarcolemmal current and transmembrane potential system. Indeed, alternans in the calcium transient have been demonstrated in the absence of APD alternans, by applying an action potential voltage clamp to isolated myocytes (90). Because several of the sarcolemmal currents depend on calcium, alternans in the calcium concentration can induce secondary APD alternans. The dominant calcium dependent transmembrane currents are ICa,L and INaCa. A larger calcium transient will inactivate, and hence decrease, ICa,L, while increasing the efflux of calcium through the exchanger, thus increasing INaCa. Since both ICa,L and INaCa are depolarizing currents that help maintain the action potential plateau and prolong the action potential, the effect of a larger calcium transient on action potential duration depends on the particular balance of ICa,L and INaCa. The case where a larger calcium transient prolongs the concurrent action potential is called positive calcium-to-voltage coupling. Such positive coupling is predicted to result in electromechanically concordant alternans, where the long (short) action potential occurs with the large (small) calcium transient (91). In contrast, negative calcium-to-voltage coupling results when a larger calcium transient shortens the concurrent action potential. When alternans is caused primarily by a calcium cycling instability, negative calcium-to-voltage coupling is predicted to result in electromechanically discordant alternans, where the long (short) action potential occurs with the small (large) calcium transient (91). Both electromechanically concordant and discordant alternans have been seen experimentally, in different species and under different experimental conditions, presumably due to differences in the balance between of ICa,L and INaCa. However, concordance seems to be the more typical case (92).
Similarly to how APD alternans arises when there is insufficient time between beats for the sarcolemmal ion channels to fully recover, calcium transient alternans arises when there is insufficient time between each beat for the intracellular calcium handling system to cycle calcium completely. Considerable efforts have been put into elucidating the exact cellular mechanism underlying calcium transient alternans. The highly nonlinear relationship between SR load and calcium release from the SR has been suggested as key. A large SR load on a given beat will cause a large calcium release due to the steepness of the load-release curve at large loads. Such large release may deplete the SR, in particular if uptake of calcium into the SR is not sufficiently fast during rapid pacing, causing a decreased load on the subsequent beat and hence a decreased release, getting the system back to the large SR load state. Computational studies have shown that a steep load-release curve can indeed cause calcium transient alternans (91, 93, 94), especially when calcium uptake into the SR is slow (93, 94). Mathematical analysis of a piecewise-linear version of the Shiferaw et al. model (95), also showed explicitly how the onset of alternans shifts to faster pacing rates with increased SERCA pump uptake strength (96).
However, recent experimental findings in ventricular myocytes have shown that SR load alternations are not necessary for calcium transient alternans to occur (97). In this case, refractoriness of the RyRs may underlie the alternans if the pacing is too fast to allow full recovery of the channels between successive beats. The exact biophysical mechanism of such RyR refractoriness has not been fully determined (98), but one aspect involves binding and unbinding of the auxilary proteins triadin and junctin, regulated in turn by binding of calsequestrin, a calcium chelator whose free concentration depends on the SR calcium concentration. By incorporating these features of calsequestrin into a mathematical model of intracellular calcium dynamics, Restrepo et al. (80) showed how calcium transient alternans may occur due to RyR refractoriness, even in the absence of a steep load-release curve. Due to lack of recovery of RyR channels between beats, some dyads fire on odd beats, while a different number of other dyads fire on even beats (80). Using both a phenomenological model and a detailed biophysical model of diffusively coupled dyads, Cui et al. (99) and Rovetti et al. (100) demonstrated the dependence of alternans onset on couplon refractoriness as well as on parameters describing random and heterogeneous couplon firing, and couplon recruitment by activation of a neighboring couplon.
In short, much progress has been made in identifying mechanisms of alternans and determining whether alternans is driven primarily by alternations in action potential or in calcium transient morphology. That being said, attempting to attribute the cause of alternans to either sarcolemmal currents or intracellular calcium dynamics is probably not worthwhile given that due to the feedback between transmembrane potential and calcium cycling, both mechanisms are likely to contribute to alternans onset in a non-clamped cell (70, 101–103). Further, just as there are different ionic mechanisms underlying APD alternans under different conditions and in different cell types (Ref. (104) provides a recent review), a range of factors may contribute to calcium transient alternans.
4.2 Subcellular calcium alternans
Under some conditions, alternations in intracellular calcium present as subcellular alternans, where different regions of the myocyte alternate out of phase (Fig. 5A). Initially, such subcellular alternans were seen in atrial and ventricular myocytes under conditions of increased calcium release heterogeneity (105–107). More recently, subcellular alternans have been reported in calcium overloaded myocytes (108), and during fast pacing (109). Notably, subcellular alternans may also occur within myocytes in intact hearts during rapid pacing, as shown in a series of studies using confocal line scan imaging of individual myocytes in perfused hearts (110–112). Subcellular alternans may trigger intracellular calcium waves (105–108), which in turn might cause delayed and early afterdepolarizations (108, 113), transient depolarizations that can potentially initiate cardiac arrhythmias.
Fig. 5.
Subcellular calcium alternans. a: Subcellular calcium alternans during alternans control pacing in an isolated guinea pig ventricular cell. Modified from Ref. (115) with permission. b: Turing instability can occur with unstable calcium transient dynamics causing local growth of calcium transient alternans in combination with positive calcium-to-voltage coupling and negative voltage-to-calcium coupling to cause out-of-phase calcium transient alternans down the myocyte (C).
The formation of different out-of-phase regions within the myocyte may be due to fixed anatomical heterogeneity in calcium cycling properties, as recently hypothesized in modeling work (110). However, subcellular alternans can also arise in homogeneous cell models due to a pattern forming instability (114–116), similar to how spatially discordant APD alternans can arise in homogeneous tissue. Based on a theoretical analysis of coupled iterative maps of APD and calcium transient dynamics, Shiferaw and Karma (114) showed in seminal work that a diffusion-driven instability (i.e., a Turing instability) can cause subcellular alternans under certain conditions.
A Turing instability is a generic mechanism by which spatial gradients can arise in systems without underlying structural heterogeneity (117). The mechanism hinges on an autocatalytic process with limited diffusion giving rise to a more rapidly diffusing inhibitor. In the case of subcellular alternans, Shiferaw and Karma identified calcium alternans as the slowly diffusing activator and APD alternans as the faster inhibitor (114).
The particular conditions necessary for a Turing instability laid out by Shiferaw and Karma include negative calcium-to-voltage coupling when the myocyte is paced at a (rapid) constant rate. As mentioned above, negative calcium-to-voltage coupling causes calcium-driven whole-cell alternans to be electromechanically discordant. Electromechanical discordance has been observed experimentally, but electromechanical concordance may be more typical. Gaeta et al. (116) showed that the requirements for a Turing instability are also met in myocytes with positive calcium-to-voltage coupling, when the myocyte is paced by a particular pacing protocol used to eliminate APD alternans (Fig. 5B,C). In guinea pig ventricular myocytes, with whole-cell electromechanical concordance during static pacing, such APD alternans control pacing can indeed induce subcellular calcium alternans (Fig. 5A) (115). These findings were reproduced in a mathematical model without structural heterogeneity, emphasizing that anatomical inhomogeneity at the level of the cell is not a necessary requirement for the formation of subcellular alternans (115).
5. NONLINEAR DYNAMICS OF CARDIAC PACEMAKING
5.1 Bifurcation analysis
From a nonlinear dynamics point of view, spontaneous beating such as that occurring in cells in the sinoatrial node (the natural pacemaker of the heart), is associated with a stable limit cycle, i.e., a stable periodic orbit. Hence, the study of how spontaneous activity arises/ends can be approached by investigating how stable limit cycles gain/lose stability or arise/disappear in mathematical models, i.e., a bifurcation analysis. Different types of bifurcations are associated with different dynamics close to the bifurcation point. For example, in a Hopf bifurcation, a limit cycle is born with a certain (non-zero) frequency and infinitesimal amplitude, while in a homoclinic bifurcation, a limit cycle is born from a homoclinic orbit with infinitely long period and normal amplitude (Fig. 6). Such variations become important when limit cycle oscillators are perturbed away from the normal operating range by disease and/or drug application, but should also be considered when attempting to create biological pacemakers (118–120). In particular, some bifurcation sequences result in bistability between a limit cycle and a stable fixed point (Fig. 6). This is referred to as a “black hole” - perturbations that bring the trajectory sufficiently close to the stable fixed point, will result in abolishment of spontaneous activity (18, 37, 118, 121) – obviously a disastrous scenario for a pacemaker.
Fig. 6.

Abolishment of spontaneous oscillations. Simulations of the Irisawa-Noma model of a rabbit sinoatrial node cell (159) illustrating three ways in which spontaneous activity can stop (118). Left: Gradual decline in amplitude due to a supercritical Hopf bifurcation for decreasing calcium current conductance. Right: Skipped beats and increased period due to a homoclinic bifurcation occurring with increasing positive bias current injection. Top: Annihilation by injection of a brief stimulus current (arrow) due to bistability between a stable limit cycle and a stable fixed point occurring with zero pacemaker current and injected positive bias current. Such bistability can arise in different ways, e.g., a subcritical Hopf bifurcation followed by a saddle node bifurcation of limit cycles. Numbers on the ordinates give transmembrane potential in mV. See Ref. (118) for details on simulations.
When conductance parameters are varied in models of sinoatrial node cells, limit cycles typically arise from Hopf bifurcations (sub- and supercritical), homoclinic bifurcations, or saddle-node bifurcations of limit cycles (54, 118, 119, 122, 123). Bifurcation analysis also suggests that cells from the peripheral part of the sinoatrial node are more resilient to hyperpolarizing loads than those from the central part because of larger pacemaker (If) and sodium (INa) currents in the peripheral cells (54, 122). This is of importance because cells from the periphery of the sinoatrial node are subject to larger loads due to their coupling to atrial myocytes. This role of INa has also been shown in extensive tissue simulations and experiments (124).
As mentioned in Chapter 1, IK1 stabilizes the resting membrane potential of ventricular myoctes. By suppressing this current by viral gene transfer, biological pacemaker cells can be created (125). This phenomenon can be reproduced in ventricular cells models (120, 126–128). While modeling work showed that INaCa is the largest inward current during the pacemaking phase of the action potential (120, 128), bifurcation analysis revealed that 1) spontaneous activity was only abolished when INaCa was almost completely blocked; 2) spontaneous activity was only abolished because of secondary effects of INaCa on causing calcium overload, thus inactivating ICa,L (120). This example thus emphasizes the inherent difficulties in making arguments about cause and effect in complex, nonlinear systems.
Another strategy to create a biological pacemaker from an excitable cell is to overexpress the channel generating If (129). Modeling studies demonstrate that including If in the ventricular oscillators with suppressed IK1 moves the bifurcation point at which automaticity occurs to less-reduced IK1 conductance values (126, 127). In particular, Tong and Holden (127) used a two-parameter bifurcation analysis to show how a combination of IK1 reduction and If increase can result in stable oscillations far from bifurcation points. Overexpressing both If and IK1 in ventricular myocytes can speed up spontaneous firing compared to overexpression of If alone (130). Such synergy have not been explored in the models, but would probably need very substantial If increases. However, bifurcation analysis in one model with suppressed IK1 revealed a complex rate dependency on If and IK1, with the rate slowing down for increased IK1 in the absence of If, and a triphasic rate dependence on IK1 in the presence of If (126). Hence, at least for some IK1-range, increased IK1 does cause a speed up of spontaneous activity.
These modeling studies also illustrate model dependence of some of the results; for example, suppressing IK1 in the Luo-Rudy guinea pig ventricular myocyte model leads to a homoclinic bifurcation (127), while suppressing IK1 in a human ventricular myocyte model causes a saddle-node bifurcation of limit cycles (120). The extent to which these (and other) differences represent real substrate variation (e.g., species differences) may be limited, but remains to be tested.
5.2 Entrained oscillators as a mechanism of sinoatrial nodal cell pacemaking
The mathematical models discussed above rely on different ionic mechanisms to generate spontaneous beating. Typically, the net inward current that drives diastolic depolarization arises from a combination of factors: decay of outward potassium currents and onset of inward currents such as If, ICa,L, and INaCa. Similar to the increased recognition that intracellular calcium dynamics can play an important role in determining the duration of the action potential in cardiac myocytes, focus has been placed on calcium cycling as a modulator and/or controller of spontaneous beating in sinoatrial node cells. Although there are no t-tubules in sinoatrial node cells, ryanodine receptors are also clustered around L-type calcium channels in functional release units that may fire spontaneously (131). Calcium release from these units occurring late in diastolic depolarization triggers an efflux of calcium from the cell through the sodium/calcium exchanger, thus depolarizing the membrane.
Attempts to determine the relative roles of the sarcolemmal membrane currents (in particular If) and the intracellular calcium cycling in causing and rate-controlling spontaneous activity in sinoatrial node cells have lead to considerable controversy, but a consensus that both are important seems to have percolated recently (132, 133). Thus, it is possible that sinoatrial node cells possess both a “membrane clock” creating automaticity from sarcolemmal membrane currents and a “calcium clock” invoking INaCa late in diastolic depolarization. However, the calcium clock by itself may only generate sustained spontaneous activity under special conditions since it requires action potential generation (with inward calcium flux) for its perpetuation (132, 133).
Evidence for the importance of both If and calcium cycling includes findings that loss-of-function mutations in the channel that generates If are associated with sinus rhythm disturbances such as sinus bradycardia (134, 135), that block of If by ivabradine slows sinus rate in humans (134, 135), and that block of calcium release by ryanodine slows or abolishes spontaneous activity in isolated sinoatrial node cells (136). However, effects of ivabradine and ryanodine are very varied between different studies (as are effects of the calcium chelator BAPTA (137, 138)), which has contributed to the controversy. The interpretation of experimental data is also confounded by the fact that the total current during diastolic depolarization is very small, but is generated as the sum of much larger inward and outward currents. In addition, the membrane resistance in sinoatrial node cells is relatively large, such that small currents can have substantial effects on membrane potential. Finally, β-adrenergic and cholinergic stimulation regulate both clocks in the same direction through various mediators (cyclic AMP, protein kinase A, and Ca2+/calmodulin-dependent protein kinases II): β-adrenergic stimulation speeds up both clocks while cholinergic stimulation depresses the rate of both (132, 134).
Mathematical modeling of sinoatrial nodal cells suggests that the membrane current system and the calcium handling system can work synergistically: when the calcium clock is included, the coupled system is more robust and has a larger ability for the rate to change smoothly (139, 140). For example, adding the calcium clock decreases the value of the ICa,L conductance for which a bifurcation occurs that abolishes automaticity and shrinks/removes a range of calcium conductance values for which skipped beats occur. The coupling between the two clocks is highly complex through mutual dependencies on calcium concentration and fluxes and it remains to be seen if the sinoatrial cell is amenable to theoretical analyses based on separation of the two sub-systems, such as was done for theoretical investigations of the APD dynamics in ventricular myocytes (101, 114, 116).
6. CONCLUSIONS: THERAPY TRANSLATION AND FUTURE PERSPECTIVES
6.1 Nonlinear dynamics in cardiology: clinical applications
While it is clear that nonlinear dynamics and complex systems theory have been successful in providing mechanistic explanations of many aspects of cardiac arrhythmogenesis, it is less clear whether they can be applied practically to improve patient care and therapy. Indeed, translation of therapy predictions based on nonlinear systems analysis into clinical practise is almost non-existent. One area, which had fostered hope of such translation, is ventricular fibrillation termination based on chaos-control theory. The idea being that if ventricular fibrillation is deterministic chaos, then it should be terminable using stimuli smaller, less painful and/or damaging, than large defibrillation shocks. Unfortunately, while chaos-control efficacy has been demonstrated in experimental work and computer simulations (141, 142), it has not been successful in terminating fibrillation in the clinic. New strategies for unpinning and suppressing spiral and scroll waves based on theoretical and simulation work are under development (e.g., (143, 144), and some show promise in experiments (145, 146).
Other control methods, based on chaos control, have been employed to terminate repolarization alternans as a means to prevent alternans-induced ventricular tachycardia or fibrillation. While such alternans control works well in small pieces of tissue (147), its efficacy is considerably more limited in larger tissues due to wave-propagation dynamics, heterogeneities in repolarization, and the intrinsic wave nature of alternans (148–151). To date, only a single alternans control study has been performed in the clinic - a study that successfully controlled alternations in propagation time through the atrioventricular node, i.e., in a highly limited spatial region (152).
In general terms, many of the pacing strategies for alternans or spiral wave control fail at the level of the intact heart because of spatial heterogeneity, disordered anatomical structures, and/or other added spatiotemporal complexity. Paradoxically, other termination methods rely on intrinsic heterogeneity to create virtual electrodes close to the core of reentrant waves (143, 145, 146) – virtual electrodes are also key to classic defibrillation.
A different line of approach is drug development based on predictions from nonlinear dynamics; however, this too has proved arduous. Part of the challenge in bridging the gap between understanding the mechanistic bases of arrhythmias and applying such knowledge to improve therapy lies in the intricacy of developing drugs targeting a particular emergent property. For example, there is experimental evidence that flattening the action potential duration restitution curve is antiarrhythmic (153), however, current pharmaceuticals with this property also have deleterious primary effects.
6.2 Perspectives on nonlinear dynamics in a systems biology era
In many ways, there is a considerable overlap between physiology, nonlinear dynamics, complex systems theory, and systems biology (98, 154, 155). Emergent phenomena are ascribed systems dynamics; as mentioned here, cardiac electrophysiology is rich in emergent phenomena such as automaticity, complex rhythms, and fibrillation. Systems biology is often seen as a contrast to a reductionist approach. Indeed, many of the recent cardiac cell models certainly defy reductionism, in particular those that include quantitative descriptions of the biochemistry of intracellular signalling pathways (e.g., (156, 157)). In contrast, earlier models were much simpler, with a limited number of variables and parameters, which made them much more amenable to nonlinear dynamics analyses such as bifurcation analysis. Thus, in some ways, the approach of mathematical modeling in cardiac electrophysiology may be seen as moving from nonlinear dynamics to systems biology. Yet, nonlinear dynamics approaches, such as bifurcation analysis of select parameters, still provide mechanistic insights into electrophysiological problems (e.g., automaticity). Further, reducing complex model systems can allow for theoretical analyses such as that illustrating the Turing instability generating subcellular calcium alternans. Hence, we believe that different approaches can be corporative, and that the future of cardiac modeling, employing a range of models and methods, will continue to prove exciting and important.
Acknowledgments
This work has been supported by the National Institutes of Health grants R01HL101190 and R01HL094620. The authors thank Stephen A. Gaeta, Zhilin Qu, Byron N. Roberts, Daisuke Sato, and Yohannes Shiferaw for providing data and graphics used in the figures.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
References
- 1.Fink M, Niederer SA, Cherry EM, Fenton FH, Koivumäki JT, et al. Cardiac cell modelling: observations from the heart of the cardiac physiome project. Prog Biophys Mol Biol. 2011;104:2–21. doi: 10.1016/j.pbiomolbio.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Zaniboni M, Pollard AE, Yang L, Spitzer KW. Beat-to-beat repolarization variability in ventricular myocytes and its suppression by electrical coupling. Am J Physiol Heart Circ Physiol. 2000;278:H677–87. doi: 10.1152/ajpheart.2000.278.3.H677. [DOI] [PubMed] [Google Scholar]
- 3.Zaniboni M. 3D current-voltage-time surfaces unveil critical repolarization differences underlying similar cardiac action potentials: A model study. Mathematical Biosciences. 2011;233:98–110. doi: 10.1016/j.mbs.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Bányász T, Horváth B, Virág L, Bárándi L, Szentandrássy N, et al. Reverse rate dependency is an intrinsic property of canine cardiac preparations. Cardiovasc Res. 2009;84:237–44. doi: 10.1093/cvr/cvp213. [DOI] [PubMed] [Google Scholar]
- 5.Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation. 1999;99:1385–94. doi: 10.1161/01.cir.99.10.1385. [DOI] [PubMed] [Google Scholar]
- 6.Fox JJ, Riccio ML, Hua F, Bodenschatz E, Gilmour RF., Jr Spatiotemporal transition to conduction block in canine ventricle. Circ Res. 2002;90:289–96. doi: 10.1161/hh0302.104723. [DOI] [PubMed] [Google Scholar]
- 7.Chinushi M, Kozhevnikov D, Caref EB, Restivo M, El-Sherif N. Mechanism of discordant T wave alternans in the in vivo heart. J Cardiovasc Electrophysiol. 2003;14:632–8. doi: 10.1046/j.1540-8167.2003.03028.x. [DOI] [PubMed] [Google Scholar]
- 8.Nolasco JB, Dahlen RW. A graphic method for the study of alternation in cardiac action potentials. J Appl Physiol. 1968;25:191–6. doi: 10.1152/jappl.1968.25.2.191. [DOI] [PubMed] [Google Scholar]
- 9.Guevara MR, Ward G, Shrier A, Glass L. Electrical alternans and period-doubling bifurcations. IEEE Computers in Cardiology. 1984:167–70. [Google Scholar]
- 10.Berger CM, Zhao X, Schaeffer DG, Dobrovolny HM, Krassowska W, Gauthier DJ. Period-Doubling Bifurcation to Alternans in Paced Cardiac Tissue: Crossover from Smooth to Border-Collision Characteristics. Phys Rev Lett. 2007;99:058101. doi: 10.1103/PhysRevLett.99.058101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guevara MR, Glass L, Shrier A. Phase locking, period-doubling bifurcations, and irregular dynamics in periodically stimulated cardiac cells. Science. 1981;214:1350–3. doi: 10.1126/science.7313693. [DOI] [PubMed] [Google Scholar]
- 12.Chialvo DR, Gilmour RF, Jalife J. Low dimensional chaos in cardiac tissue. Nature. 1990;343:653–7. doi: 10.1038/343653a0. [DOI] [PubMed] [Google Scholar]
- 13.Qu Z. Chaos in the genesis and maintenance of cardiac arrhythmias. Prog Biophys Mol Biol. 2011;105:247–57. doi: 10.1016/j.pbiomolbio.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanskanen AJ, Greenstein JL, O’Rourke B, Winslow RL. The role of stochastic and modal gating of cardiac L-type Ca2+ channels on early after-depolarizations. Biophys J. 2005;88:85–95. doi: 10.1529/biophysj.104.051508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato D, Xie L-H, Sovari AA, Tran DX, Morita N, et al. Synchronization of chaotic early afterdepolarizations in the genesis of cardiac arrhythmias. Proc Natl Acad Sci USA. 2009;106:2983–8. doi: 10.1073/pnas.0809148106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato D, Xie L-H, Nguyen TP, Weiss JN, Qu Z. Irregularly Appearing Early Afterdepolarizations in Cardiac Myocytes: Random Fluctuations or Dynamical Chaos? Biophys J. 2010;99:765–73. doi: 10.1016/j.bpj.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lerma C, Krogh-Madsen T, Guevara M, Glass L. Stochastic Aspects of Cardiac Arrhythmias. J Stat Phys. 2007;128:347–74. [Google Scholar]
- 18.Chay TR, Lee YS. Impulse responses of automaticity in the Purkinje fiber. Biophys J. 1984;45:841–9. doi: 10.1016/S0006-3495(84)84228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chay TR, Lee YS. Phase resetting and bifurcation in the ventricular myocardium. Biophys J. 1985;47:641–51. doi: 10.1016/S0006-3495(85)83960-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran DX, Sato D, Yochelis A, Weiss JN, Garfinkel A, Qu Z. Bifurcation and Chaos in a Model of Cardiac Early Afterdepolarizations. Phys Rev Lett. 2009;102:1–4. doi: 10.1103/PhysRevLett.102.258103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qu Z, Garfinkel A, Chen PS, Weiss JN. Mechanisms of discordant alternans and induction of reentry in simulated cardiac tissue. Circulation. 2000;102:1664–70. doi: 10.1161/01.cir.102.14.1664. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe MA, Fenton FH, Evans SJ, Hastings HM, Karma A. Mechanisms for discordant alternans. J Cardiovasc Electrophysiol. 2001;12:196–206. doi: 10.1046/j.1540-8167.2001.00196.x. [DOI] [PubMed] [Google Scholar]
- 23.Karma A, Gilmour RF. Nonlinear Dynamics of Heart Rhythm Disorders. Physics Today. 2007;60:51. [Google Scholar]
- 24.Courtemanche M, Glass L, Keener J. Instabilities of a propagating pulse in a ring of excitable media. Phys Rev Lett. 1993;70:2182–85. doi: 10.1103/PhysRevLett.70.2182. [DOI] [PubMed] [Google Scholar]
- 25.Comtois P, Vinet A. Alternans amplification following a two-stimulus protocol in a one-dimensional cardiac ionic model of reentry: From annihilation to double-wave quasiperiodic reentry. Chaos. 2007;17:023125. doi: 10.1063/1.2740673. [DOI] [PubMed] [Google Scholar]
- 26.Otani NF. Theory of action potential wave block at-a-distance in the heart. Phys Rev E. 2007;75:1–17. doi: 10.1103/PhysRevE.75.021910. [DOI] [PubMed] [Google Scholar]
- 27.Krogh-Madsen T, Christini DJ. Pacing-induced spatiotemporal dynamics can be exploited to improve reentry termination efficacy. Phys Rev E. 2009;80:1–12. doi: 10.1103/PhysRevE.80.021924. [DOI] [PubMed] [Google Scholar]
- 28.Nash MP, Mourad A, Clayton RH, Sutton PM, Bradley CP, et al. Evidence for multiple mechanisms in human ventricular fibrillation. Circulation. 2006;114:536–42. doi: 10.1161/CIRCULATIONAHA.105.602870. [DOI] [PubMed] [Google Scholar]
- 29.Witkowski FX, Leon LJ, Penkoske PA, Giles WR, Spano ML, et al. Spatiotemporal evolution of ventricular fibrillation. Nature. 1998;392:78–82. doi: 10.1038/32170. [DOI] [PubMed] [Google Scholar]
- 30.Qu Z, Xie F, Garfinkel A, Weiss JN. Origins of spiral wave meander and breakup in a two-dimensional cardiac tissue model. Ann Biomed Eng. 2000;28:755–71. doi: 10.1114/1.1289474. [DOI] [PubMed] [Google Scholar]
- 31.Roberge FA, Nadeau RA. The nature of Wenckebach cycles. Can J Physiol Pharmacol. 1969;47:695–704. doi: 10.1139/y69-119. [DOI] [PubMed] [Google Scholar]
- 32.Guevara MR, Glass L. Phase locking, period doubling bifurcations and chaos in a mathematical model of a periodically driven oscillator: a theory for the entrainment of biological oscillators and the generation of cardiac dysrhythmias. J Math Biol. 1982;14:1–23. doi: 10.1007/BF02154750. [DOI] [PubMed] [Google Scholar]
- 33.Moe GK, Jalife J, Mueller WJ, Moe B. A mathematical model of parasystole and its application to clinical arrhythmias. Circulation. 1977;56:968–79. doi: 10.1161/01.cir.56.6.968. [DOI] [PubMed] [Google Scholar]
- 34.Lerma C, Lee CF, Glass L, Goldberger AL. The rule of bigeminy revisited: analysis in sudden cardiac death syndrome. J Electrocardiol. 2007;40:78–88. doi: 10.1016/j.jelectrocard.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Comtois P, Vinet A. Multistability of reentrant rhythms in an ionic model of a two-dimensional annulus of cardiac tissue. Phys Rev E. 2005;72:51927. doi: 10.1103/PhysRevE.72.051927. [DOI] [PubMed] [Google Scholar]
- 36.Hwang S-M, Kim TY, Lee KJ. Complex-periodic spiral waves in confluent cardiac cell cultures induced by localized inhomogeneities. Proc Natl Acad Sci USA. 2005;102:10363–8. doi: 10.1073/pnas.0501539102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jalife J, Antzelevitch C. Phase resetting and annihilation of pacemaker activity in cardiac tissue. Science. 1979;206:695–7. doi: 10.1126/science.493975. [DOI] [PubMed] [Google Scholar]
- 38.Yehia AR, Jeandupeux D, Alonso F, Guevara MR. Hysteresis and bistability in the direct transition from 1:1 to 2:1 rhythm in periodically driven single ventricular cells. Chaos. 1999;9:916–31. doi: 10.1063/1.166465. [DOI] [PubMed] [Google Scholar]
- 39.Walker ML, Wan X, Kirsch GE, Rosenbaum DS. Hysteresis effect implicates calcium cycling as a mechanism of repolarization alternans. Circulation. 2003;108:2704–9. doi: 10.1161/01.CIR.0000093276.10885.5B. [DOI] [PubMed] [Google Scholar]
- 40.Mines GR. On Dynamic Equilibrium in the Heart. J Physiol (Lond ) 1913;46:349–83. doi: 10.1113/jphysiol.1913.sp001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Surovyatkina E, Noble D, Gavaghan D, Sher A. Multistability property in cardiac ionic models of mammalian and human ventricular cells. Prog Biophys Mol Biol. 2010;103:131–41. doi: 10.1016/j.pbiomolbio.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Huang X, Qian Y, Zhang X, Hu G. Hysteresis and bistability in periodically paced cardiac tissue. Phys Rev E. 2010;81:51903. doi: 10.1103/PhysRevE.81.051903. [DOI] [PubMed] [Google Scholar]
- 43.Zhao X. Indeterminacy of spatiotemporal cardiac alternans. Phys Rev E. 2008;78:11902. doi: 10.1103/PhysRevE.78.011902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noble D. A modification of the Hodgkin--Huxley equations applicable to Purkinje fibre action and pace-maker potentials. J Physiol. 1962;160:317–52. doi: 10.1113/jphysiol.1962.sp006849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niederer SA, Fink M, Noble D, Smith NP. A meta-analysis of cardiac electrophysiology computational models. Expl Physiol. 2009;94:486–95. doi: 10.1113/expphysiol.2008.044610. [DOI] [PubMed] [Google Scholar]
- 46.Bueno-Orovio A, Cherry EM, Fenton FH. Minimal model for human ventricular action potentials in tissue. J Theor Biol. 2008;253:544–60. doi: 10.1016/j.jtbi.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 47.Biktashev VN, Suckley R, Elkin YE, Simitev RD. Asymptotic analysis and analytical solutions of a model of cardiac excitation. Bulletin Math Biol. 2008;70:517–54. doi: 10.1007/s11538-007-9267-0. [DOI] [PubMed] [Google Scholar]
- 48.Ten Tusscher KHWJ, Bernus O, Hren R, Panfilov AV. Comparison of electrophysiological models for human ventricular cells and tissues. Prog Biophys Mol Biol. 2006;90:326–45. doi: 10.1016/j.pbiomolbio.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 49.Cherry EM, Evans SJ. Properties of two human atrial cell models in tissue: restitution, memory, propagation, and reentry. J Theor Biol. 2008;254:674–90. doi: 10.1016/j.jtbi.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cherry EM, Hastings HM, Evans SJ. Dynamics of human atrial cell models: restitution, memory, and intracellular calcium dynamics in single cells. Prog Biophys Mol Biol. 2008;98:24–37. doi: 10.1016/j.pbiomolbio.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cherry EM, Fenton FH. A tale of two dogs: analyzing two models of canine ventricular electrophysiology. Am J Physiol Heart Circ Physiol. 2007;292:H43–55. doi: 10.1152/ajpheart.00955.2006. [DOI] [PubMed] [Google Scholar]
- 52.Romero L, Carbonell B, Trenor B, Rodríguez B, Saiz J, Ferrero JM. Systematic characterization of the ionic basis of rabbit cellular electrophysiology using two ventricular models. Prog Biophys Mol Biol. 2011 doi: 10.1016/j.pbiomolbio.2011.06.012. in press. [DOI] [PubMed] [Google Scholar]
- 53.Wilders R, Jongsma HJ, van Ginneken AC. Pacemaker activity of the rabbit sinoatrial node. A comparison of mathematical models. Biophys J. 1991;60:1202–16. doi: 10.1016/S0006-3495(91)82155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kurata Y, Matsuda H, Hisatome I, Shibamoto T. Regional difference in dynamical property of sinoatrial node pacemaking: role of na+ channel current. Biophys J. 2008;95:951–77. doi: 10.1529/biophysj.107.112854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greenstein JL, Wu R, Po S, Tomaselli GF, Winslow RL. Role of the calcium-independent transient outward current I(to1) in shaping action potential morphology and duration. Circ Res. 2000;87:1026–33. doi: 10.1161/01.res.87.11.1026. [DOI] [PubMed] [Google Scholar]
- 56.Maoz A, Krogh-Madsen T, Christini DJ. Instability in action potential morphology underlies phase 2 reentry: A mathematical modeling study. Heart Rhythm J. 2009;6:813–22. doi: 10.1016/j.hrthm.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 57.Roden DM. Taking the “idio” out of “idiosyncratic”: predicting torsades de pointes. Pacing clin electrophysiol: PACE. 1998;21:1029–34. doi: 10.1111/j.1540-8159.1998.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 58.Izhikevich E. Neural excitability, spiking and bursting. Int J Bifurcat Chaos. 2000;10:1171–266. [Google Scholar]
- 59.Beeler GW, Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J Physiol. 1977;268:177–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dokos S, Lovell NH. Parameter estimation in cardiac ionic models. Prog Biophys Mol Biol. 2004;85:407–31. doi: 10.1016/j.pbiomolbio.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 61.Kherlopian A, Ortega F, Christini DJ. Cardiac Myocyte Model Parameter Sensitivity Analysis and Model Transformation Using a Genetic Algorithm. 2011:1–4. [Google Scholar]
- 62.Syed Z, Vigmond E, Nattel S, Leon LJ. Atrial cell action potential parameter fitting using genetic algorithms. Med Biol Eng Comput. 2005;43:561–71. doi: 10.1007/BF02351029. [DOI] [PubMed] [Google Scholar]
- 63.Fink M, Noble D. Markov models for ion channels: versatility versus identifiability and speed. Philos Transact A Math Phys Eng Sci. 2009;367:2161–79. doi: 10.1098/rsta.2008.0301. [DOI] [PubMed] [Google Scholar]
- 64.Moreno JD, Zhu ZI, Yang P-C, Bankston JR, Jeng M-T, et al. A computational model to predict the effects of class I anti-arrhythmic drugs on ventricular rhythms. Science Transl Med. 2011;3:98ra83. doi: 10.1126/scitranslmed.3002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iyer V, Mazhari R, Winslow RL. A computational model of the human left-ventricular epicardial myocyte. Biophys J. 2004;87:1507–25. doi: 10.1529/biophysj.104.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Menon V, Spruston N, Kath WL. A state-mutating genetic algorithm to design ion-channel models. Proc Natl Acad Sci USA. 2009;106:16829–34. doi: 10.1073/pnas.0903766106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cha CY, Himeno Y, Shimayoshi T, Amano A, Noma A. A novel method to quantify contribution of channels and transporters to membrane potential dynamics. Biophys J. 2009;97:3086–94. doi: 10.1016/j.bpj.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sobie EA. Parameter sensitivity analysis in electrophysiological models using multivariable regression. Biophys J. 2009;96:1264–74. doi: 10.1016/j.bpj.2008.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Otani NF, Li M, Gilmour RF. What can nonlinear dynamics teach us about the development of ventricular tachycardia/ventricular fibrillation? Heart Rhythm J. 2005;2:1261–3. doi: 10.1016/j.hrthm.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 70.Jordan PN, Christini DJ. Characterizing the contribution of voltage- and calcium-dependent coupling to action potential stability: implications for repolarization alternans. Am J Physiol Heart Circ Physiol. 2007;293:H2109–18. doi: 10.1152/ajpheart.00609.2007. [DOI] [PubMed] [Google Scholar]
- 71.Li M, Otani NF. Ion channel basis for alternans and memory in cardiac myocytes. Ann Biomed Eng. 2003;31:1213–30. doi: 10.1114/1.1616930. [DOI] [PubMed] [Google Scholar]
- 72.Li M, Otani NF. Controlling alternans in cardiac cells. Ann Biomed Eng. 2004;32:784–92. doi: 10.1023/b:abme.0000030254.33176.f8. [DOI] [PubMed] [Google Scholar]
- 73.Allexandre D, Otani NF. Preventing alternans-induced spiral wave breakup in cardiac tissue: An ion-channel-based approach. Phys Rev E. 2004;70:61903. doi: 10.1103/PhysRevE.70.061903. [DOI] [PubMed] [Google Scholar]
- 74.Kharche S, Ludtke N, Panzeri S, Zhang H. A Global Sensitivity Index for Biophysically Detailed Cardiac Cell Models: A Computational Approach. Lecture Notes in Computer Science. 2009;5528:366–75. [Google Scholar]
- 75.Sarkar AX, Sobie EA. Regression analysis for constraining free parameters in electrophysiological models of cardiac cells. PLoS Comput Biol. 2010;6 doi: 10.1371/journal.pcbi.1000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 77.DiFrancesco D, Noble D. A model of cardiac electrical activity incorporating ionic pumps and concentration changes. Philos Trans R Soc Lond, B, Biol Sci. 1985;307:353–98. doi: 10.1098/rstb.1985.0001. [DOI] [PubMed] [Google Scholar]
- 78.Gaur N, Rudy Y. Multiscale modeling of calcium cycling in cardiac ventricular myocyte: macroscopic consequences of microscopic dyadic function. Biophys J. 2011;100:2904–12. doi: 10.1016/j.bpj.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Williams GSB, Huertas MA, Sobie EA, Jafri MS, Smith GD. Moment closure for local control models of calcium-induced calcium release in cardiac myocytes. Biophys J. 2008;95:1689–703. doi: 10.1529/biophysj.107.125948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Restrepo JG, Weiss JN, Karma A. Calsequestrin-mediated mechanism for cellular calcium transient alternans. Biophys J. 2008;95:3767–89. doi: 10.1529/biophysj.108.130419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stern MD, Song LS, Cheng H, Sham JS, Yang HT, et al. Local control models of cardiac excitation-contraction coupling. A possible role for allosteric interactions between ryanodine receptors. J Gen Physiol. 1999;113:469–89. doi: 10.1085/jgp.113.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trayanova NA, Rice JJ. Cardiac electromechanical models: from cell to organ. Front Physio. 2011;2:43. doi: 10.3389/fphys.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qu Z, Weiss JN, Garfinkel A. Cardiac electrical restitution properties and stability of reentrant spiral waves: a simulation study. Am J Physiol. 1999;276:H269–83. doi: 10.1152/ajpheart.1999.276.1.H269. [DOI] [PubMed] [Google Scholar]
- 84.Fenton F, Karma A. Vortex dynamics in three-dimensional continuous myocardium with fiber rotation: Filament instability and fibrillation. Chaos. 1998;8:20–47. doi: 10.1063/1.166311. [DOI] [PubMed] [Google Scholar]
- 85.Karma A. Electrical alternans and spiral wave breakup in cardiac tissue. Chaos. 1994;4:461. doi: 10.1063/1.166024. [DOI] [PubMed] [Google Scholar]
- 86.Cherry EM, Fenton FH. Suppression of alternans and conduction blocks despite steep APD restitution: electrotonic, memory, and conduction velocity restitution effects. Am J Physiol. 2004;286:H2332–41. doi: 10.1152/ajpheart.00747.2003. [DOI] [PubMed] [Google Scholar]
- 87.Shiferaw Y, Qu Z, Garfinkel A, Karma A, Weiss JN. Nonlinear dynamics of paced cardiac cells. Ann NY Acad Sci. 2006;1080:376–94. doi: 10.1196/annals.1380.028.x. [DOI] [PubMed] [Google Scholar]
- 88.Kalb SS, Dobrovolny HM, Tolkacheva EG, Idriss SF, Krassowska W, Gauthier DJ. The restitution portrait: a new method for investigating rate-dependent restitution. J Cardiovasc Electrophysiol. 2004;15:698–709. doi: 10.1046/j.1540-8167.2004.03550.x. [DOI] [PubMed] [Google Scholar]
- 89.Tolkacheva EG, Anumonwo JMB, Jalife J. Action potential duration restitution portraits of mammalian ventricular myocytes: role of calcium current. Biophys J. 2006;91:2735–45. doi: 10.1529/biophysj.106.083865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chudin E, Goldhaber J, Garfinkel A, Weiss J, Kogan B. Intracellular Ca2+ dynamics and the stability of ventricular tachycardia. Biophys J. 1999;77:2930–41. doi: 10.1016/S0006-3495(99)77126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shiferaw Y, Sato D, Karma A. Coupled dynamics of voltage and calcium in paced cardiac cells. Phys Rev E. 2005;71:021903. doi: 10.1103/PhysRevE.71.021903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Laurita KR, Rosenbaum DS. Cellular mechanisms of arrhythmogenic cardiac alternans. Prog Biophys Mol Biol. 2008;97:332–47. doi: 10.1016/j.pbiomolbio.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huertas MA, Smith GD, Györke S. Ca2+ alternans in a cardiac myocyte model that uses moment equations to represent heterogeneous junctional SR Ca2+ Biophys J. 2010;99:377–87. doi: 10.1016/j.bpj.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xie L-H, Sato D, Garfinkel A, Qu Z, Weiss JN. Intracellular Ca alternans: coordinated regulation by sarcoplasmic reticulum release, uptake, and leak. Biophys J. 2008;95:3100–10. doi: 10.1529/biophysj.108.130955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shiferaw Y, Watanabe MA, Garfinkel A, Weiss JN, Karma A. Model of intracellular calcium cycling in ventricular myocytes. Biophys J. 2003;85:3666–86. doi: 10.1016/S0006-3495(03)74784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thul R, Coombes S. Understanding cardiac alternans: a piecewise linear modeling framework. Chaos. 2010;20:045102. doi: 10.1063/1.3518362. [DOI] [PubMed] [Google Scholar]
- 97.Picht E, DeSantiago J, Blatter LA, Bers DM. Cardiac alternans do not rely on diastolic sarcoplasmic reticulum calcium content fluctuations. Circ Res. 2006;99:740–8. doi: 10.1161/01.RES.0000244002.88813.91. [DOI] [PubMed] [Google Scholar]
- 98.Weiss JN, Nivala M, Garfinkel A, Qu Z. Alternans and arrhythmias: from cell to heart. Circ Res. 2011;108:98–112. doi: 10.1161/CIRCRESAHA.110.223586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cui X, Rovetti RJ, Yang L, Garfinkel A, Weiss JN, Qu Z. Period-doubling bifurcation in an array of coupled stochastically excitable elements subjected to global periodic forcing. Phys Rev Lett. 2009;103:044102. doi: 10.1103/PhysRevLett.103.044102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rovetti R, Cui X, Garfinkel A, Weiss JN, Qu Z. Spark-induced sparks as a mechanism of intracellular calcium alternans in cardiac myocytes. Circ Res. 2010;106:1582–91. doi: 10.1161/CIRCRESAHA.109.213975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Qu Z, Shiferaw Y, Weiss JN. Nonlinear dynamics of cardiac excitation-contraction coupling: An iterated map study. Phys Rev E. 2007;75:011927. doi: 10.1103/PhysRevE.75.011927. [DOI] [PubMed] [Google Scholar]
- 102.Restrepo JG, Karma A. Spatiotemporal intracellular calcium dynamics during cardiac alternans. Chaos. 2009;19:7115. doi: 10.1063/1.3207835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jordan P, Christini D. Action Potential Morphology Influences Intracellular Calcium Handling Stability and the Occurrence of Alternans. Biophys J. 2006;90:672. doi: 10.1529/biophysj.105.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qu Z, Xie Y, Garfinkel A, Weiss JN. T-wave Alternans and Arrhythmogenesis in Cardiac Diseases. Front Physio. 2010;1:1–15. doi: 10.3389/fphys.2010.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Diaz ME, O’Neill SC, Eisner DA. Sarcoplasmic reticulum calcium content fluctuation is the key to cardiac alternans. Circ Res. 2004;94:650–6. doi: 10.1161/01.RES.0000119923.64774.72. [DOI] [PubMed] [Google Scholar]
- 106.Kockskamper J, Blatter LA. Subcellular Ca2+ alternans represents a novel mechanism for the generation of arrhythmogenic Ca2+ waves in cat atrial myocytes. J Physiol. 2002;545:65–79. doi: 10.1113/jphysiol.2002.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Diaz ME, Eisner DA, O’Neill SC. Depressed ryanodine receptor activity increases variability and duration of the systolic Ca2+ transient in rat ventricular myocytes. Circ Res. 2002;91:585–93. doi: 10.1161/01.res.0000035527.53514.c2. [DOI] [PubMed] [Google Scholar]
- 108.Xie L-H, Weiss JN. Arrhythmogenic consequences of intracellular calcium waves. Am J Physiol Heart Circ Physiol. 2009;297:H997–H1002. doi: 10.1152/ajpheart.00390.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cordeiro JM, Malone JE, Di Diego JM, Scornik FS, Aistrup GL, et al. Cellular and subcellular alternans in the canine left ventricle. Am J Physiol Heart Circ Physiol. 2007;293:H3506–16. doi: 10.1152/ajpheart.00757.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aistrup GL, Shiferaw Y, Kapur S, Kadish AH, Wasserstrom JA. Mechanisms underlying the formation and dynamics of subcellular calcium alternans in the intact rat heart. Circ Res. 2009;104:639–49. doi: 10.1161/CIRCRESAHA.108.181909. [DOI] [PubMed] [Google Scholar]
- 111.Aistrup GL, Kelly JE, Kapur S, Kowalczyk M, Sysman-Wolpin I, et al. Pacing-induced heterogeneities in intracellular Ca2+ signaling, cardiac alternans, and ventricular arrhythmias in intact rat heart. Circ Res. 2006;99:e65–73. doi: 10.1161/01.RES.0000244087.36230.bf. [DOI] [PubMed] [Google Scholar]
- 112.Kapur S, Wasserstrom JA, Kelly JE, Kadish AH, Aistrup GL. Acidosis and ischemia increase cellular Ca2+ transient alternans and repolarization alternans susceptibility in the intact rat heart. Am J Physiol Heart Circ Physiol. 2009;296:H1491–512. doi: 10.1152/ajpheart.00539.2008. [DOI] [PubMed] [Google Scholar]
- 113.Chen W, Aistrup G, Wasserstrom JA, Shiferaw Y. A mathematical model of spontaneous calcium release in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2011;300:H1794–805. doi: 10.1152/ajpheart.01121.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shiferaw Y, Karma A. Turing instability mediated by voltage and calcium diffusion in paced cardiac cells. Proc Natl Acad Sci USA. 2006;103:5670–5. doi: 10.1073/pnas.0511061103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gaeta SA, Bub G, Abbott GW, Christini DJ. Dynamical mechanism for subcellular alternans in cardiac myocytes. Circ Res. 2009;105:335–42. doi: 10.1161/CIRCRESAHA.109.197590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gaeta SA, Krogh-Madsen T, Christini DJ. Feedback-control induced pattern formation in cardiac myocytes: A mathematical modeling study. J Theor Biol. 2010 doi: 10.1016/j.jtbi.2010.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Turing AM. The chemical basis of morphogenesis. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1952;237:37–72. doi: 10.1098/rstb.2014.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guevara MR, Jongsma HJ. Three ways of abolishing automaticity in sinoatrial node: ionic modeling and nonlinear dynamics. Am J Physiol. 1992;262:H1268–86. doi: 10.1152/ajpheart.1992.262.4.H1268. [DOI] [PubMed] [Google Scholar]
- 119.Kurata Y, Hisatome I, Imanishi S, Shibamoto T. Roles of L-type Ca2+ and delayed-rectifier K+ currents in sinoatrial node pacemaking: insights from stability and bifurcation analyses of a mathematical model. Am J Physiol Heart Circ Physiol. 2003;285:H2804–19. doi: 10.1152/ajpheart.01050.2002. [DOI] [PubMed] [Google Scholar]
- 120.Kurata Y, Hisatome I, Matsuda H, Shibamoto T. Dynamical mechanisms of pacemaker generation in IK1-downregulated human ventricular myocytes: insights from bifurcation analyses of a mathematical model. Biophys J. 2005;89:2865–87. doi: 10.1529/biophysj.105.060830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Winfree AT. The Geometry of Biological Time. New York: Springer; 2000. [Google Scholar]
- 122.Kurata Y, Matsuda H, Hisatome I, Shibamoto T. Roles of hyperpolarization-activated current If in sinoatrial node pacemaking: insights from bifurcation analysis of mathematical models. Am J Physiol Heart Circ Physiol. 2010;298:H1748–60. doi: 10.1152/ajpheart.00729.2009. [DOI] [PubMed] [Google Scholar]
- 123.Pan Z, Yamaguchi R, Doi S. Bifurcation analysis and effects of changing ionic conductances on pacemaker rhythm in a sinoatrial node cell model. BioSystems. 2011;106:9–18. doi: 10.1016/j.biosystems.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 124.Butters TD, Aslanidi OV, Inada S, Boyett MR, Hancox JC, et al. Mechanistic Links Between Na+ Channel (SCN5A) Mutations and Impaired Cardiac Pacemaking in Sick Sinus Syndrome. Circ Res. 2010;107:126–37. doi: 10.1161/CIRCRESAHA.110.219949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Miake J, Marbán E, Nuss HB. Biological pacemaker created by gene transfer. Nature. 2002;419:132–3. doi: 10.1038/419132b. [DOI] [PubMed] [Google Scholar]
- 126.Kurata Y, Matsuda H, Hisatome I, Shibamoto T. Effects of pacemaker currents on creation and modulation of human ventricular pacemaker: theoretical study with application to biological pacemaker engineering. Am J Physiol Heart Circ Physiol. 2007;292:H701–18. doi: 10.1152/ajpheart.00426.2006. [DOI] [PubMed] [Google Scholar]
- 127.Tong WC, Holden AV. Bifurcation analysis of genetically engineered pacemaking in mammalian heart. J Biol Phys. 2006;32:169–72. doi: 10.1007/s10867-006-9004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Silva J, Rudy Y. Mechanism of pacemaking in I(K1)-downregulated myocytes. Circ Res. 2003;92:261–3. doi: 10.1161/01.RES.0000057996.20414.C6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qu J, Plotnikov AN, Danilo P, Shlapakova I, Cohen IS, et al. Expression and function of a biological pacemaker in canine heart. Circulation. 2003;107:1106–9. doi: 10.1161/01.cir.0000059939.97249.2c. [DOI] [PubMed] [Google Scholar]
- 130.Chan Y-C, Siu C-W, Lau Y-M, Lau C-P, Li RA, Tse H-F. Synergistic effects of inward rectifier (I) and pacemaker (I) currents on the induction of bioengineered cardiac automaticity. J Cardiovasc Electrophysiol. 2009;20:1048–54. doi: 10.1111/j.1540-8167.2009.01475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Maltsev AV, Maltsev VA, Mikheev M, Maltseva LA, Sirenko SG, et al. Synchronization of stochastic Ca2(+) release units creates a rhythmic Ca2(+) clock in cardiac pacemaker cells. Biophys J. 2011;100:271–83. doi: 10.1016/j.bpj.2010.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lakatta EG, Maltsev VA, Vinogradova TM. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart’s pacemaker. Circ Res. 2010;106:659–73. doi: 10.1161/CIRCRESAHA.109.206078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Noble D, Noble PJ, Fink M. Competing oscillators in cardiac pacemaking: historical background. Circ Res. 2010;106:1791–7. doi: 10.1161/CIRCRESAHA.110.218875. [DOI] [PubMed] [Google Scholar]
- 134.DiFrancesco D. The role of the funny current in pacemaker activity. Circ Res. 2010;106:434–46. doi: 10.1161/CIRCRESAHA.109.208041. [DOI] [PubMed] [Google Scholar]
- 135.Baruscotti M, Barbuti A, Bucchi A. The cardiac pacemaker current. J Mol Cell Cardiol. 2010;48:55–64. doi: 10.1016/j.yjmcc.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 136.Lakatta EG. A paradigm shift for the heart’s pacemaker. Heart Rhythm J. 2010;7:559–64. doi: 10.1016/j.hrthm.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vinogradova TM, Zhou YY, Bogdanov KY, Yang D, Kuschel M, et al. Sinoatrial node pacemaker activity requires Ca(2+)/calmodulin-dependent protein kinase II activation. Circ Res. 2000;87:760–7. doi: 10.1161/01.res.87.9.760. [DOI] [PubMed] [Google Scholar]
- 138.Himeno Y, Toyoda F, Satoh H, Amano A, Cha CY, et al. Minor contribution of cytosolic Ca2+ transients to the pacemaker rhythm in guinea pig sinoatrial node cells. Am J Physiol Heart Circ Physiol. 2011;300:H251–61. doi: 10.1152/ajpheart.00764.2010. [DOI] [PubMed] [Google Scholar]
- 139.Maltsev VA, Lakatta EG. Synergism of coupled subsarcolemmal Ca2+ clocks and sarcolemmal voltage clocks confers robust and flexible pacemaker function in a novel pacemaker cell model. Am J Physiol Heart Circ Physiol. 2009;296:H594–615. doi: 10.1152/ajpheart.01118.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang H, Joung B, Shinohara T, Mei X, Chen P-S, Lin S-F. Synergistic dual automaticity in sinoatrial node cell and tissue models. Circ J. 2010;74:2079–88. doi: 10.1253/circj.cj-10-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Garfinkel A, Spano ML, Ditto WL, Weiss JN. Controlling Cardiac Chaos. Science. 1992;257:1230. doi: 10.1126/science.1519060. [DOI] [PubMed] [Google Scholar]
- 142.Sinha S, Sridhar S. Controlling Spatiotemporal Chaos and Spiral Turbulence in Excitable Media. In: Schöll E, Schuster HG, editors. Handbook of Chaos Control. Weinheim, Germany: Wiley-VCH; 2007. [Google Scholar]
- 143.Pumir A, Nikolski V, Hörning M, Isomura A, Agladze K, et al. Wave emission from heterogeneities opens a way to controlling chaos in the heart. Phys Rev Lett. 2007;99:208101. doi: 10.1103/PhysRevLett.99.208101. [DOI] [PubMed] [Google Scholar]
- 144.Morgan SW, Biktasheva IV, Biktashev VN. Control of scroll-wave turbulence using resonant perturbations. Phys Rev E. 2008;78:046207. doi: 10.1103/PhysRevE.78.046207. [DOI] [PubMed] [Google Scholar]
- 145.Luther S, Fenton FH, Kornreich BG, Squires A, Bittihn P, et al. Low-energy control of electrical turbulence in the heart. Nature. 2011;475:235–39. doi: 10.1038/nature10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ripplinger CM, Krinsky VI, Nikolski VP, Efimov IR. Mechanisms of unpinning and termination of ventricular tachycardia. Am J Physiol Heart Circ Physiol. 2006;291:H184–92. doi: 10.1152/ajpheart.01300.2005. [DOI] [PubMed] [Google Scholar]
- 147.Hall GM, Gauthier DJ. Experimental control of cardiac muscle alternans. Phys Rev Lett. 2002;88:198102. doi: 10.1103/PhysRevLett.88.198102. [DOI] [PubMed] [Google Scholar]
- 148.Krogh-Madsen T, Karma A, Riccio ML, Jordan PN, Christini DJ, Gilmour RF. Off-site control of repolarization alternans in cardiac fibers. Phys Rev E. 2010;81:1–7. doi: 10.1103/PhysRevE.81.011915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Christini DJ, Riccio ML, Culianu CA, Fox JJ, Karma A, Gilmour RF. Control of Electrical Alternans in Canine Cardiac Purkinje Fibers. Phys Rev Lett. 2006;96:1–4. doi: 10.1103/PhysRevLett.96.104101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rappel W-J, Fenton F, Karma A. Spatiotemporal Control of Wave Instabilities in Cardiac Tissue. Phys Rev Lett. 1999;83:456. [Google Scholar]
- 151.Echebarria B, Karma A. Spatiotemporal control of cardiac alternans. Chaos. 2002;12:923–30. doi: 10.1063/1.1501544. [DOI] [PubMed] [Google Scholar]
- 152.Christini DJ, Stein KM, Markowitz SM, Mittal S, Slotwiner DJ, et al. Nonlinear-dynamical arrhythmia control in humans. Proc Natl Acad Sci U S A. 2001;98:5827–32. doi: 10.1073/pnas.091553398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Riccio ML, Koller ML, Gilmour RF. Electrical restitution and spatiotemporal organization during ventricular fibrillation. Circ Res. 1999;84:955–63. doi: 10.1161/01.res.84.8.955. [DOI] [PubMed] [Google Scholar]
- 154.Noble D. Systems biology and the heart. BioSystems. 2006;83:75–80. doi: 10.1016/j.biosystems.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 155.Lusis AJ, Weiss JN. Cardiovascular networks: systems-based approaches to cardiovascular disease. Circulation. 2010;121:157–70. doi: 10.1161/CIRCULATIONAHA.108.847699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hund TJ, Rudy Y. Rate dependence and regulation of action potential and calcium transient in a canine cardiac ventricular cell model. Circulation. 2004;110:3168–74. doi: 10.1161/01.CIR.0000147231.69595.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Saucerman JJ, Brunton LL, Michailova AP, McCulloch AD. Modeling beta-adrenergic control of cardiac myocyte contractility in silico. J Biol Chem. 2003;278:47997–8003. doi: 10.1074/jbc.M308362200. [DOI] [PubMed] [Google Scholar]
- 158.ten Tusscher KHWJ, Panfilov AV. Alternans and spiral breakup in a human ventricular tissue model. Am J Physiol Heart Circ Physiol. 2006;291:H1088–100. doi: 10.1152/ajpheart.00109.2006. [DOI] [PubMed] [Google Scholar]
- 159.Irisawa H, Noma A. Pacemaker mechanisms of rabbit sinoatrial node cells. In: Bouman LN, Jongsma HJ, editors. Cardiac Rate and Rhythm. The Hague: Nijhoff; 1982. [Google Scholar]





