Abstract
Melanoma is the most aggressive form of skin cancer whose worldwide incidence is rising faster than any other cancer. Few treatment options are available to patients with metastatic disease, and standard chemotherapeutic agents are generally ineffective. Cytokines such as IFN-α or IL-2 can promote immune recognition of melanoma, occasionally inducing dramatic and durable clinical responses. Here, we discuss several immunomodulatory agents, the safety of which are being evaluated in clinical trials. Challenges include an incomplete understanding of signaling pathways, appropriate clinical dose and route, and systemic immunosuppression in advanced melanoma patients. We consider how targeted cytokine therapy will integrate into the clinical arena, as well as the low likelihood of success of single cytokine therapies. Evidence supports a synergy between cytokine immunotherapy and other therapeutic approaches in melanoma, and strengthening this area of research will improve our understanding of how to use cytokine therapy better.
Keywords: aldesleukin, cytokine, denenicokin, iboctadekin, IFN-α, IL-2, IL-12, IL-18, IL-21, immunotherapy, melanoma
Malignant melanoma
Melanoma is the most aggressive form of skin cancer and represents a significant health problem. The worldwide incidence of malignant melanoma is rising faster than any other cancer, and in the USA alone, over 68,000 new cases of melanoma were diagnosed in the year 2009 [1]. While thin primary melanomas are highly curable with surgery, the prognosis for patients with advanced disease is poor. Over 70% of patients with primary melanomas thicker than 4 mm or metastasis to the regional lymph nodes die of disseminated disease within 5 years of diagnosis [2]. The median survival of patients with metastatic disease is on the order of 10 months even with aggressive therapy [3]. Few treatment options are available to patients with metastatic disease, and standard chemotherapeutic agents are generally ineffective. Several lines of evidence suggest that melanoma represents an immunologically reactive tumor. Spontaneous regressions of malignant melanoma lesions have been observed and histologic evaluations have revealed that this process is mediated by activated lymphocytes [3,4]. Indeed, infiltration of T cells within melanoma tumors can be associated with a better prognosis [5]. Consequently, melanoma represents a form of cancer that may be relevant for studying the mechanisms in which the immune system regulates the development and outgrowth of malignant lesions. Other evidence also supports the concept that melanoma is a tumor that may be amenable to immune-based therapy. For example, melanoma incidence is increased in patients who are immunosuppressed [6] and circulating, tumor antigen-specific T cells are detectable in patients with melanoma [7]. Finally, in those melanoma patients who respond to immune-based therapies, vitiligo and autoimmune phenomena are often observed [8,9]. However, it is presumed that various mechanisms of immune evasion limit the ability of these T cells to mount effective and durable antitumor immune responses [10,11].
Role of cytokines in melanoma therapy
Cytokines are hormones produced endogenously in the body that serve to regulate immune function. These soluble factors can act in a somewhat paradoxical fashion to protect the host against disease or to contribute to inflammatory processes that exacerbate disease. Over the past several decades, recombinant forms of these cytokine mediators have been synthesized in a purified form and explored as a means of treating a variety of malignancies including melanoma. In theory, immunotherapy with cytokines is an advantageous approach for treatment of melanoma, because it has the potential to activate host immune cells with specificity for malignant cells, while sparing normal cells. This is in contrast to radiation-based or chemotherapeutic regimens that target actively dividing cells or exert a broad cytotoxic effect. Historically, clinical responses to cytokine immunotherapy have produced striking results in a subset of patients with melanoma for over three decades. Nonetheless, the cellular mechanisms that differentiate responding patients from nonresponders are poorly not understood. Experience has taught us that single-agent administration of any one cytokine is unlikely the best approach for all patients with melanoma, or with any other malignancy, for that matter. Important information has also been reported with regard to dosing, schedule and potential for maximizing the therapeutic potential of cytokines when used safely and responsibly in combination with other agents [12,13]. In particular, melanoma is a solid tumor in which targeted therapy is gaining momentum. Therefore, it may be desirable to explore how targeted agents may be combined with cytokines to achieve the maximum clinical benefit. A renewed interest in cytokine therapy is clearly emerging in the clinical oncology community [14]. In fact, a US National Cancer Institute-sponsored Immunotherapy Agent Workshop identified cytokines, including IL-12, IL-15 and various other immune response modifiers, as agents with high potential for use in treating cancer [15]. This article is focused on providing an overview of cytokine therapy in the treatment of melanoma. As such, in the subsequent sections we have chosen to highlight cytokines that have been actively tested and have shown promise in Phase I/II trials. The role of US FDA-approved cytokines including IFN-α and IL-2 as therapeutic agents for melanoma will be discussed. In addition, data will be presented on selected cytokines in earlier phases of evaluation (IL-12, IL-15, IL-18 and IL-21) that have produced promising results in clinical trials.
IFN-α
IFN-α is a cytokine that is produced endogenously in response to viral infection or dsRNA. The gene family encoding type I interferons (IFNs) consists of 13 IFN-α members, and a single member each of IFN-β, IFN-κ, IFN-ω and IFN-ε [16,17,201]. The discovery of this cytokine first stemmed from early experiments in 1957 conducted by Isaacs and Lindenmann investigating why cells were resistant to viral infection if previously infected by another virus [18,19]. The potential for IFN as an antitumor agent was soon realized based on its growth inhibitory and immunomodulatory properties [20,21]. This cytokine was later purified to homogeneity in solution [22] and produced in recombinant form following the cloning of the first IFN gene [23]. A majority of clinical studies in melanoma to date have utilized the IFN-α2 isoform, although clinical experience with other IFN family members including IFN-β has been noted in hepatitis B, multiple sclerosis and cancer [24–26].
The receptor for IFN-α is expressed on most cells, including stromal components, immune effector cells and on malignant melanoma cells (Figures 1 & 2; Table 1) [27]. This receptor is comprised of two transmembrane proteins (IFNAR1 and 2) which associate with the Janus family of kinases, Jak1 and tyrosine kinase 2 (Tyk2). Upon binding to its receptor, IFN-α initiates phosphorylation of Jak1 and Tyk2, which induce tyrosine phosphorylation upon the cytoplasmic tail of each receptor chain (IFNAR1 and 2). These phosphotyrosine residues provide a docking site for signal transducer and activator of transcription (STAT) proteins that can reside within the cytoplasm in their nonphosphorylated form [28–30]. Once the STAT proteins are phosphorylated, they can dimerize with other STATs, translocate to the nucleus and initiate transcription of IFN-responsive genes [31]. IFNs can also induce other post-translational modifications of STAT proteins, including arginine methylation, acetylation or ubiquitination that also regulate their biological function. The role for these additional post-translational modifications in mediating the biological response and antitumor effects of IFN-α remains an active area of research, and is reviewed in detail in [32,33]. IFN-α can also signal in a manner that is independent of or diverges from the Jak–STAT pathway. For example, IFN-α has been shown to activate the PI3K signaling pathway in a STAT1-independent manner [34,35]. A member of the CRK family of adaptor proteins, CRKL, can interact with Tyk2 and is phosphorylated following IFN-α stimulation. This can initiate a series of downstream events leading to RAP1 activation and subsequent MAPK pathway and STAT5 activation [36–39].
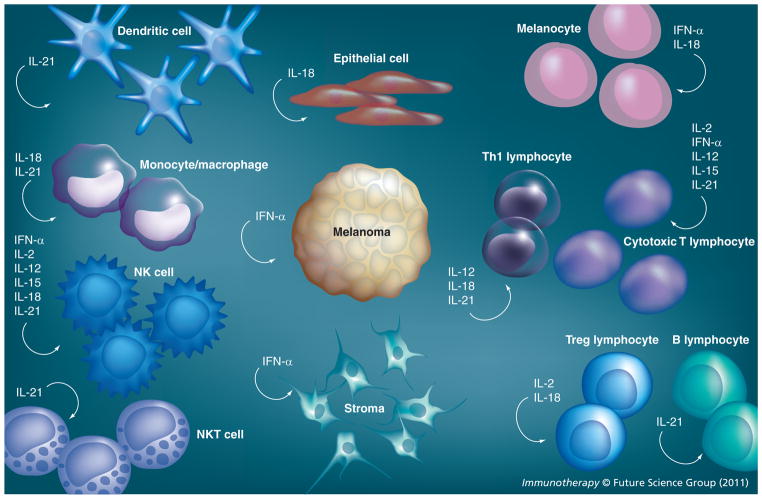
Figure 1.
Cytokine influence on immune, stromal and malignant cells present within the tumor microenvironment.
Figure 2. Representative signal transduction pathways of immunomodulatory cytokines.
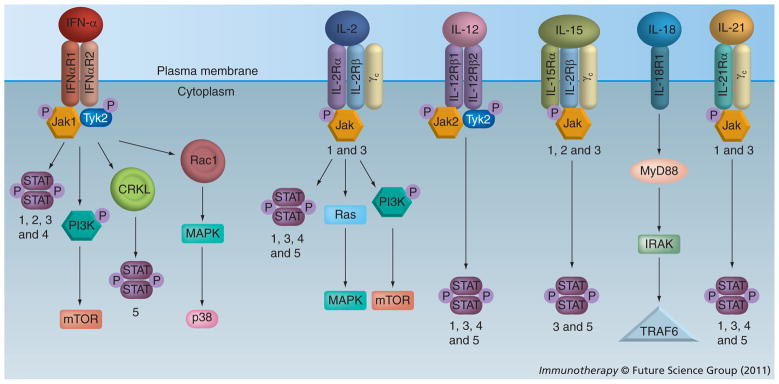
The numbers refer to the specific STAT and Jak molecules that are activated in that particular pathway.
IL-2R: IL-2 receptor; STAT: Signal transducer and activator of transcription; Tyk2: Tyrosine kinase 2.
Table 1.
Immunomodulatory cytokines, their target cells and subsequent effects.
| Cytokine | Target cells/receptor expression | Immunomodulatory effects | Ref. |
|---|---|---|---|
| IFN-α | Most somatic cells | Promotes proliferation and activation of NK and cytotoxic T cells | [44,46] |
| IL-2 | T lymphocytes, NK cells | Promotes proliferation and activation of cytotoxic T cells; survival of Tregs | [73,74] |
| IL-12 | T lymphocytes, NK cells | Promotes proliferation and activation of NK and cytotoxic T cells; secretion of IFN-γ | [101,103] |
| IL-15 | Cytotoxic and memory T lymphocytes, NK cells | Promotes survival of CD4+, CD8+ and memory T lymphocytes and NK cells | [114] |
| IL-18 | B, T lymph; NK cells, MΦ, fibroblasts, melanocytes, endothelial, epithelial cells | Promotes proliferation and activation of NK and cytotoxic T cells; secretion of IFN-γ; inhibits proliferation of Tregs | [119,126,127,130] |
| IL-21 | NK, NKT, DC, MΦ, B lymphocytes, T helper and cytotoxic T lymphocytes | Promotes proliferation of activated B cells, activated NK and NKT cells, and cytotoxic T lymphocytes, and differentiation of plasma cells | [150,152] |
DC: Dendritic cell; MΦ: Macrophage.
IFN-α can act directly upon melanoma cells to inhibit cellular growth, angiogenesis and promote apoptosis [40–43]. However, a number of studies have provided data indicating that the immunomodulatory effects of this cytokine are most important for mediating its antitumor effects [44,45]. Central to the antitumor activity of IFN-α is its ability to promote NK cell-mediated cytotoxicity and proliferation [46]. This cytokine also stimulates the proliferation, generation and activation of existing memory CD8+ cytotoxic T lymphocytes (CTLs) [44,46]. IFN-α has also been shown to modify the expression of surface molecules on tumor cells that increase their overall immunogenicity. For example, IFN-α upregulates tumor cell expression of MHC class I and II antigens, ICAM-1 and L-selectin [47,48]. Recent data also suggest that IFN-α may promote antitumor activity via inducing the expression of chemokines that allow for tumor antigen-specific T cells to migrate into tumors [49]. Clinical evidence has suggested that patients who develop autoimmune sequelae following IFN-α therapy are less likely to experience disease recurrence [8]. Together, these data support a role for modulation of immunity as a primary mechanism by which IFN-α might work in a subset of patients.
IFN-α in its recombinant form has been utilized in patients with melanoma since the 1980s [50]. This cytokine was initially used as a therapy for patients who presented with metastatic disease. In early Phase I and II clinical trials, high-dose regimens were used regularly and doses across individual trials were variable ranging from 3 to 100 MU/m2. These differences in dosing and the inherent variability of patients produced response rates ranging from 5 to 30% [51]. Complete and durable responses were usually encountered at higher dose levels, although even low-dose IFN-α can produce clinical responses in some patients. In addition, the route of administration has not emerged as a critical factor for clinical responses, as complete responses have been reported following either intravenous or intramuscular administration in sequential trials conducted at the same institution [50]. Indeed, more recent reviews of trials employing IFN-α confirm that this cytokine has activity in melanoma patients with metastatic disease [52]. Recent studies by Eggermont suggest that patients with ulcerated tumors demonstrate significantly greater benefit from adjuvant IFN-α compared with patients with nonulcerated tumors [53].
More controversial is the use of IFN-α as an adjuvant therapy following surgical resection of high-risk lesions. A number of studies indicate that IFN-α, regardless of the dose or formulation (PEGylated or not) has demonstrated a consistent disease-free survival benefit for patients with clinically lymph node-negative melanoma in clinical trials [54]. Long-term follow-up of patients treated with adjuvant high-dose IFN-α has shown no improvement in overall survival [55]. Combined with the toxicity profile associated with its administration, and recent data regarding a remarkable deterioration in quality-of-life scores of patients receiving adjuvant IFN-α, enthusiasm for use of IFN-α in the adjuvant setting is waning [56]. Nonetheless, this cytokine remains the only FDA-approved therapeutic option for patients following resection of high-risk lesions, and its use will probably continue in patients who have few other options and are tolerant of the toxicity profile. Recent studies have suggested that careful dose adjustment based on molecular markers of IFN-α activity may represent a means by which this cytokine can be administered more effectively on a patient-by-patient basis [13]. In addition, a greater understanding of the genetic, molecular or other biomarkers that differentiate responders from nonresponders could allow for great strides to be made in selecting patients most likely to benefit from this somewhat controversial regimen.
IL-2
IL-2 is a 15-kDa glycoprotein that functions as a growth factor for T cells and NK cells (Figure 1 & Table 1) [57–59]. This cytokine is a member of the common γ-chain (γc) cytokines (which includes IL-2, IL-4, IL-7, Il-9, IL-15 and IL-21). Upon binding to its receptor (IL-2 receptor [IL-2R]), IL-2 activates Jak1 and 3, which in turn, associate with the cytoplasmic domains of the IL-2Rβ and IL-2Rγc subunits (Figure 2). IL-2Rβ subsequently undergoes tyrosine phosphorylation, thereby creating docking sites for several intracellular proteins. IL-2 activates multiple signaling pathways in immune effectors upon binding to its receptor, including the Ras–MAPK pathway, the Jak-signal transducer and activator of transcription (STAT) pathway, and the PI3K pathway [60–62]. Although the precise functional role of each of these signaling pathways in mediating the antitumor effects of IL-2 remains under investigation, IL-2 induced activation of the Ras–MAPK pathway is known to promote cell survival and proliferation [63]. Similarly, STAT5 plays a crucial role in normal immune function [64–66]. STAT5 is required for IL-2 induced cell-cycle progression in T cells and for NK cell-mediated proliferation and cytolytic activity [67–69]. Both STAT5a and STAT5b are required for antigen-induced T-cell recruitment into tumor tissue [70]. STAT5 is known to mediate antiapoptotic signals, and inhibition of STAT5a/STAT5b promotes the apoptosis of IL-2-responsive primary and tumor-derived lymphoid cells [64,71,72].
Previous studies have suggested that the clinical response to IL-2 immunotherapy is mediated through the expansion and activation of cytotoxic lymphocytes within host tissues [73], although the precise signaling pathways responsible for mediating these effects are not known. Finally, the fact that IL-2 administration can produce such profound antitumor activity in select patients is somewhat contrary to what is known regarding certain cellular targets of this cytokine. For example, IL-2 can serve as a factor that promotes the survival and proliferation of CD4+CD25+Foxp3+ Tregs, which can suppress T-cell function and antitumor immune responses [74]. In addition, prolonged stimulation of T cells with IL-2 can promote activation-induced cell death, which can serve to limit the duration and magnitude of T-cell responses [75]. Together, these complexities show the importance of continued research into the mechanisms by which this and other cytokines exert their antitumor effect in humans.
Recombinant IL-2 (aldesleukin) is used as a therapeutic option for patients with metastatic malignant melanoma who have good performance status. It can induce complete or partial responses in approximately 10–20% of patients, where approximately a third of these individuals will experience a durable complete response [76]. The exact mechanism of action of IL-2 is not known. For instance, it is unknown whether clinical responses are a result of mechanisms set in motion during treatment, or a result of ongoing immunological events following completion of treatment. It is thought that the immune system plays a principal role in mediating the anti-tumor activity of IL-2. In support of this notion is the observation that autoimmune sequelae are frequently reported in patients who respond to IL-2 therapy [77–79]. Although approved by the FDA in this setting, toxicity involving multiple organ systems can occur following the administration of high-dose IL-2, and therefore patients must be carefully selected [80,81]. Aldesleukin received FDA approval in 1992 based on data derived from 255 patients with renal cell carcinoma (RCC) who were enrolled in seven separate Phase II clinical trials [82,83]. In these studies, patients received 600,000 or 720,000 IU/kg of recombinant IL-2 by 15-min infusion every 8 h during two 5-day courses at 8–12-week intervals. Objective responses were observed in 37 (15%) of the 255 RCC patients, including 17 durable complete responses (7%) and 20 partial responses (8%) [84]. Successful examples of treatment in melanoma include a high-dose bolus of IL-2 via intravenous infusion, for up to 14 cycles over a 28-week period. In this study of 26 patients with metastatic disease, four experienced complete response, up to 41 months or greater [85].
A majority of available data suggests that bolus intravenous administration of high-dose IL-2 is the most effective approach to dosing this cytokine as a single-agent immunotherapy. However, the appropriate dose and schedule of IL-2 administration remains under investigation. Numerous studies have also evaluated lower doses of aldesleukin administered sub cutaneously alone, or in combination with IFN-α [86–88]. These nonrandomized Phase II trials produced similar response rates and survival, but the responses appeared to be less durable than those seen with high-dose aldesleukin alone. A Phase III study of high-dose aldesleukin versus low-dose sub cutaneous aldesleukin plus IFN-α2b published by the Cytokine Working Group (CWG) further demonstrated that patients receiving high-dose aldesleukin had both a greater overall response rate and more durable complete responses [80]. Finally, data from the National Cancer Institute randomized three-arm study of high-dose intravenous bolus aldesleukin, low-dose intravenous bolus aldesleukin and subcutaneous moderate-dose aldesleukin indicated a superior response rate in patients receiving high-dose aldesleukin as compared with both the moderate- and low-dose arms [73].
A greater understanding of immunological parameters and systemic toxicity following IL-2 administration could potentially guide our efforts to identify and test rational combination approaches with this cytokine. Ideally, this cytokine could be safely administered and used in combination with other immune-based therapies. Administration of IL-2 has accompanied adoptive T-cell therapy with the goal of promoting survival and proliferation of transplanted autologous T cells [89]. Historically, this approach has produced dramatic clinical responses in some patients and has highlighted the promise for immune-based therapy in melanoma. IL-2 has also been explored in combination with gp100 (a melanocyte-specific antigen) [90] in the context of three Phase II CWG trials, but has not reported activity that was superior to high-dose IL-2 alone [91]. More recently, the CWG has also evaluated how IL-2 may be used in combination with GM-CSF following lymphodepleting chemotherapy with cyclophosphamide. This combination displayed an acceptable toxicity profile, but unfortunately, produced no remarkable difference in antitumor activity compared with standard high-dose IL-2 alone [92]. Similarly, recent Phase III studies from the Eastern Cooperative Oncology Group have tested IL-2 in combination with conventional chemotherapy, and have reported no effects on overall survival [93]. In contrast with the results from the CWG, an abstract presented at the American Society of Clinical Oncology (ASCO) annual meeting in 2009 reported the results of a two-arm Phase III trial in which patients with stage IV or locally advanced stage III melanoma received either high-dose IL-2 alone or gp100 peptide followed by high-dose IL-2. A total of 86 patients receiving gp100 peptide followed by high-dose IL-2 saw a 12.4% improvement in response rate compared with high-dose IL-2 alone [94]. Other trials have tested IL-2 combined with ipilimumab (anti-CTLA4 antibody) or IL-2 and gp100 peptide. Studies from the National Cancer Institute presented at the ASCO 2010 annual meeting reported a 10% improvement in complete response rate in patients receiving IL-2 and ipilimumab (compared with patients receiving ipilimumab and gp100 peptide) [95]. These early-phase clinical studies are challenging based on the expected toxicity profile of high-dose IL-2 alone. However, they will provide critical information regarding which combinations may be suited for more stringent Phase II studies, focused on efficacy in melanoma. Finally, a recent publication demonstrated that high levels of VEGF and fibronectin in the circulation correlate with poor response to IL-2 therapy, and poor survival overall, in patients with melanoma [96].
IL-12
IL-12 is a cytokine that remains of clinical interest due to its powerful immunomodulatory effects as a single agent, and as an immunostimulatory adjuvant. IL-12 was discovered by two independent groups, almost concurrently in 1989–1990 [14,97,98]. This cytokine is a 70-kDa, heterodimeric molecule composed of p35 and p40 subunits that are covalently linked via di sulfide bonds. The IL-12 receptor consists of two subunits, IL-12Rβ1 and β2, which associate with Jak2 and Tyk2 (Figure 2). Upon binding its receptor, IL-12 induces signal transduction via phosphorylation of STAT proteins including STAT1, STAT3, STAT4 and STAT5 [99]. However, signaling mediated via the STAT4 transcription factor is responsible for a majority of the downstream effects of this cytokine [100]. STAT4 is typically bound to the cytoplasmic region of IL-12Rβ2 and translocates to the nucleus upon receptor ligation [101]. IL-12 production is mediated by monocytes, macrophages, dendritic cells and other antigen-presenting cells. Indeed, proliferation of both T and NK cells is enhanced in response to IL-12, and contributes to its robust immunomodulatory activity. One important property of IL-12 is its ability to induce production of IFN-γ from immune effector cells. This propensity for promoting IFN-γ production is a means by which IL-12 facilitates cellular interactions that bridge innate and adaptive immune responses [101–103]. Specifically, IL-12-induced IFN-γ production promotes Th1-mediated cytotoxic immune responses and upregulates various extracellular molecules that aid in antigen presentation (e.g., MHC class I and II molecules) or cellular motility (e.g., ICAM-1). Importantly, IL-12 has been shown to induce the production of various anti-angiogenic chemokines in an IFN-γ-dependent manner, including IP-10 and MIG. These downstream effector molecules are thought to be one of the major means by which IL-12 exerts its antitumor activity (Figure 1 & Table 1) [104,105].
Clinical studies in patients with malignant melanoma have been conducted using recombinant IL-12 primarily as either a single agent or as a vaccine adjuvant administered in combination with various immunogenic melanoma peptides. A more limited number of studies have explored the use of IL-12 in combination with other cytokines or immunomodulators such as IFN-α, IL-2 or melanoma antigen vaccines [51,106–110]. Early trials examining the systemic administration of IL-12 produced disappointing results due to severe concerns with systemic toxicity [104,108]. An early Phase I study conducted in patients with cancer of multiple histologies (including melanoma) indicated that intravenous administration of 500 ng/kg of rhIL-12 was the maximum tolerated dose [111] and induced both a partial and a complete response in two separate patients on this trial. Subsequent Phase II studies using intravenous administration of rhIL-12 at this dose produced remarkable toxicity and, unfortunately, fatal toxicity [108]. These results reduced enthusiasm for single-agent IL-12 in the setting of melanoma and other solid tumors for a prolonged period of time. Upon further investigation, it was later revealed that this unexpected toxicity was probably due to a slight difference in the scheduling of IL-12 administration in the Phase II study. Namely, a single injection of rhIL-12 that was included in the Phase I study, but not in the schedule of administration in the Phase II study had a profound abrogating effect on IL-12-induced production of IFN-γ and toxicity [112].
As greater experience was gained throughout the medical oncology community in administering immunomodulatory therapies, it became clear that biologics usually possess a remarkably different dosing profile compared with standard cytotoxic agents. Arguably, our understanding of how biologic therapies are most appropriately dosed is in its infancy. In addition to the use of recombinant IL-12 protein, intratumoral administration of a plasmid encoding IL-12 has been demonstrated in a human trial to be effective in melanoma. Nine patients were assigned to one of three doses of plasmid DNA; two of nine experienced stable disease and one experienced complete remission [113]. Later trials using IL-12 via subcutaneous injection represent interesting examples of this, as this cytokine was well tolerated via this route of administration. Although limited efficacy was noted, subcutaneous administration of rhIL-12 appeared to increase the frequency in which memory or cytolytic CD8+ T cells infiltrated biopsied metastatic melanoma lesions [104,108]. One of the most interesting aspects of subcutaneous IL-12 administration lies in its ability to serve as an adjuvant for T-cell responses to melanoma vaccines. A series of clinical studies employing subcutaneous administration of IL-12 demonstrate that it has been well tolerated and has enhanced antigen-specific immune responses that were induced in response to vaccination with peptides that encode antigenic epitopes present on melanoma cells [109,110].
Despite these encouraging immunomodulatory properties of IL-12, clinical responses with this agent either alone or in combination with other immune-based therapies have been limited. These data are not entirely surprising due to a number of factors that could limit host responses to IL-12 administration. First, the immune-suppressive microenvironment in patients with advanced melanoma; second, individual polymorphisms that influence IFN-γ production or angiogenesis; and third, the fact that many patients have been heavily pretreated, which could alter the quality and magnitude of immune response. This cytokine may still hold promise in the context of combination regimens; however, a major roadblock to its continued evaluation is its limited supply. Indeed, the development of IL-12 has been halted and is only currently available in limited quantities through the National Cancer Institute for a small focused series of clinical trials.
IL-15
IL-15 is another promising cytokine that may have utility for treatment in melanoma owing to its powerful immunomodulatory properties. As such, in 1997, the National Cancer Institute released a ranked list of biological agents that showed the greatest promise in immunotherapy against cancer. IL-15 was ranked highest in this list (which included vaccine adjuvants, inhibitors of immunosuppressive pathways, as well as IL-12) [15]. IL-15 belongs to the common γc family of cytokines, which includes IL-2, IL-7 and IL-21. The IL-15 cytokine utilizes the same α and β receptor chains that are shared by IL-2, but signals through its own unique γc receptor (Figure 2) [15]. IL-15 is produced by DCs, macrophages and stromal cells. It serves as a T-cell growth factor, and acts on CD4+ T cells, CD8+ T cells, memory cells and NK cells (Figure 1) [114]. In contrast to IL-2, IL-15 prevents activation-induced cell death [15], and IL-15 is required for survival of CD8+ memory T cells [115]. Early experiments in mice have revealed that IL-15 serves to attract T lymphocytes to the injection site, and also mediates inflammatory cell infiltrate in arthritis models [116]. IL-15 was tested in early experiments and found to be effective at expanding patient tumor-infiltrating T lymphocytes (TILs) in vitro [117].
Although IL-15 has shown promise as a cancer therapy and vaccine adjuvant, no human trials have been completed to date. The first Phase I trial in refractory metastatic malignant melanoma opened in 2009, and although it was scheduled to close in January 2011, is still actively recruiting participants. This trial will determine the safety, toxicity and MTD of intravenous administration of this agent in 12 treatments, ranging from 0.3 to 25 μg/kg/day [301].
IL-18
IL-18 is one of 11 members of the IL-1 cytokine superfamily. This cytokine has attracted attention as an immunostimulatory molecule, as it promotes production of IFN-γ and activation of NK cells and CTLs (Figure 1 & Table 1). Endogenous IL-18 is translated as a 24-kDa inactive precursor protein and is later cleaved to an active 18-kDa moiety in the cytoplasm by a complex known as the inflammasome, through the activity of IL-1β-converting enzyme/caspase-1 [118,119]. This cytokine activates multiple signaling cascades, including both the MyD88–IR AK–NFκB pathway and the TR AF6-p38–MAPK pathway (Figur e 2) [119–121]. IL-18 modulates immune cell responses in part through its induction of IFN-γ. Downstream modulation of IL-18-induced gene expression can occur via dual mechanisms. First, it can activate the NF-κB and MAPK signal transduction pathways. Second, IL-18 directly promotes occupation of the AP-1-binding site in the IFN-γ promoter [122]. These proteins are part of a larger feedback loop, as it is known that NF-κB and IFN-γ, themselves, can (directly or indirectly) regulate IL-18 expression [123,124].
IL-18-binding protein (IL-18BP) is a soluble protein and a potent inhibitor of IL-18. Interestingly, expression of IL-18BP is constitutive, but increases in response to IFN-γ [121,125], thereby creating a mechanism of negative-feedback control. In addition to increased expression of IFN-γ, IL-18 appears to upregulate VCAM and ICAM in fibroblasts and endothelial cells [123].
IL-18 exerts its effects on cells of both the innate and adaptive immune system. This cytokine is expressed by macrophages, dendritic cells and keratinocytes [119]. It was originally identified as a protein that stimulated the production of IFN-γ in B and T lymphocytes, NK cells and macrophages [121,126–128]. However, the expression of the IL-18 receptor (IL-18R) has also been identified on endothelial and epithelial cells, fibroblasts and melanocytes [129]. One feature that makes IL-18 an attractive agent for cancer immunotherapy is its ability to augment the cytolytic potential of NK cells and CTLs [119,130,131]. In cooperation with IL-12, IL-18 does promote IFN-γ production in Th1-type CD4+ T cells [132,133] and NK cells [134]. Finally, expression of the IL-18R is also present on Tregs, and IL-18 is known to inhibit proliferation and function of Tregs [119,130]. Together, these immunomodulatory effects suggest that IL-18 may have unique potential for treatment of malignant melanoma. Despite these potentially advantageous properties, overexpression of IL-18 from tumor cells has also been demonstrated in a diverse set of cancers including esophageal, head and neck, breast, prostate and pancreatic cancer, as well as metastatic lung cancer [129,135,136]. Furthermore, in the setting of ovarian cancer, intratumoral and serum IL-18 expression was also correlated with disease progression [129,137]. Similarly, IL-18 produced endogenously by murine B16F10 melanoma cells has been shown to promote immune escape [138]. To our knowledge, the possibility that human melanoma cells may also produce IL-18 has not been explored in detail.
As a result of several clinical trials (summarized in Table 2), there is emerging clinical evidence that IL-18 remains an attractive immunotherapeutic cytokine with potential antitumor activity in melanoma. The first Phase I trial using recombinant IL-18 (SB-485232 [iboctadekin]; GlaxoSmithKline) as a single agent was performed in patients with refractory solid tumors or recurring lymphoma. Biological observations of patients in this trial included transient neutropenia, lymphopenia and thrombo cytopenia, and elevated serum levels of IFN-γ and IL-18BP. In this trial, a maximum tolerated dose was not reached. Of interest, at 14 days following the first dose, anti-IL-18 antibodies were detected in the serum in ten out of 26 patients, but there were no differences in toxicity or biological effects noted in these patients. Of the nine melanoma patients treated on this protocol, one patient with meta-static disease, who received 100 μg/kg intravenously, responded with 69% regression of target lesions [121,139]. A subsequent Phase I trial in melanoma and RCC patients was then conducted with the objective of testing alternative dosing schedules of rhIL-18. Of those patients who completed at least 12 cycles of treatment, 70% experienced stable disease and one patient with melanoma experienced a complete clinical response when evaluated at 15 months after completion of treatment. The treatment was again well tolerated, and a maximum tolerated dose was not reached [140]. Finally, a Phase II study examined the efficacy of IL-18 in 64 metastatic melanoma patients. IL-18 was well tolerated, while one patient achieved a partial response and four patients experienced stable disease for 6 months. Despite these data, the study was closed following completion of stage 1 owing to limited efficacy by the RECIST criteria [141,142]. In light of the fact that there is some variability in the clinical response to IL-18 treatment in melanoma patients with metastatic disease, the use of IL-18 in future combination therapy regimens warrants further investigation.
Table 2.
Current and completed Phase I and II trials in IL-12, IL-18 and IL-21 immunomodulatory therapy.
| Patient population | Trade name/sponsor | Phase | Trial status | Route | MTD | Ref. |
|---|---|---|---|---|---|---|
| IL-12 | ||||||
| Renal cancer, melanoma and colon cancer | rhIL-12 | I | Completed | iv. | 500 ng/kg | [111] |
| Various metastatic (including melanoma) | rhIL-12 and IFN-α2b | I | Completed | iv. | Not reached | [107] |
| Metastatic melanoma | rhIL-12 (Hoffman-LaRoche) | – | Completed | sc. | – | [175] |
| Metastatic melanoma | rhIL-12 | I | Completed | sc. | – | [176] |
| Melanoma | rhIL-12 and IFN-α2b | I | Completed | sc. | 500 ng/kg | [106] |
| Melanoma | rhIL-12 and Melan-A peptide | I | Completed | sc., iv. | Not reached | [109] |
| Melanoma | rhIL-12 and Melan-A-pulsed peripheral blood cells | II | Completed | sc. | Not reached | [110] |
| IL-18 | ||||||
| Advanced stage melanoma, renal cancer and lymphoma | Iboctadekin/SB-485232 (GlaxoSmithKline) | I | Completed | iv. | Not reached | [139] |
| Advanced stage melanoma and renal cancer | Iboctadekin/SB-485232 (GlaxoSmithKline) | I | Completed | iv. | Not reached | [140] |
| Metastatic melanoma | Iboctadekin/SB-485232 (GlaxoSmithKline) | II | Completed | iv. | Not reached | [142] |
| IL-21 | ||||||
| Metastatic melanoma | rhIL-21 (denenicokin; Novo Nordisk/ ZymoGenetics) | I | Completed | iv. | 30 μg/kg | [159] |
| Metastatic melanoma | rhIL-21 (denenicokin; Novo Nordisk/ ZymoGenetics) | I | Completed | iv. | 30 μg/kg | [160] |
| Metastatic melanoma | rhIL-21 (denenicokin; Novo Nordisk/ ZymoGenetics) | I | Completed | sc. | 200 μg/kg | [161] |
| Metastatic or recurrent melanoma | rhIL-21 | II | Ongoing | iv. | – | [162] |
| Metastatic or recurrent melanoma | rhIL-21 vs dacarbazine | II | Ongoing | – | – | [302] |
| Metastatic malignant melanoma | rhIL-21 | II | Completed | – | – | [303] |
| Metastatic malignant melanoma | rhIL-21 | II | Closed | – | – | [304] |
iv.: Intravenous; MTD: Maximum tolerated dose; rhIL-12: Recombinant human IL-12; rhIL-21: Recombinant human IL-21; sc.: Subcutaneous.
IL-21
IL-21 belongs to the common γc receptor (CD132) family of cytokines, which includes IL-2, IL-4, IL-7, IL-9 and IL-15 [143,144]. IL-21 is expressed by activated CD4+ T cells and NKT cells [143,145]. It binds to a heterodimeric receptor present on various innate and adaptive immune cells, including NK and NKT cells, dendritic cells, macrophages, B lymphocytes, helper and CTLs [143,144–147]. IL-21 binds the heterodimeric IL-21α/γc receptor and promotes the autophosphorylation of Jak1 and Jak3 (Figure 2). These signal transduction events result in the phosphorylation and activation of the transcription factors STAT1, STAT3, and to a lesser extent, STAT4 and STAT5 [61,143]. Interestingly, expression of IL-21 is mediated by STAT3, implying a positive feedback loop is operative in regulating the effects of this cytokine [148].
IL-21 exerts a wide range of biological effects on several cell types and has been shown to elicit antitumor activity in preclinical models of melanoma (also see Figure 1 & Table 1) [149]. It promotes proliferation and antibody iso-type switching in B lymphocytes, which have been preactivated through CD40 ligands [150], but induces apoptosis in naive B cells and in those that have been activated through Toll-like receptor (TLR)4 or TLR9 ligands [151]. Consistent with its proliferative effects on CD40-activated B cells, IL-21 promotes differentiation of mature antibody-producing plasma cells [152]. By contrast, IL-21 inhibits proliferation and promotes differentiation of NK cells and CTLs, as evidenced by increased expression of IFN-γ, perforin and granzyme B, and inhibits proliferation of Treg lymphocytes [146,153,154]. Unlike IL-2, IL-21 renders CD4+ T cells resistant to Treg suppression and does not enhance proliferation of Treg cells [155]. Rather, IL-21 is sufficient to increase the response of activated CTLs, but suppress the differentiation of naive T cells into CTLs [156]. In combination with CD25-mediated depletion of Treg lymphocytes, IL-21 has been shown to enrich the production of antigen-specific CTLs in vitro [157]. IL-21 has also been shown to activate a unique subset of NKT cells, resulting in expression of additional IL-21, among other cytokines [145,146]. Similar to other interleukins, the actions of IL-21 are complex. In addition to the features previously mentioned, IL-21 is sufficient to induce the differentiation of naive T helper cells into Th17 cells [158]. As the role of Th17 cells in cancer is not yet completely understood, this should also be considered when determining the use of IL-21 in human patients.
A number of Phase I studies (summarized in Table 2) have been completed using rhIL-21 (Denenicokin; Novo Nordisk A/S and ZymoGenetics) in patients with melanoma. In an initial Phase I trial, the MTD was determined to be 30 μg/kg, based on evidence of thrombocytopenia/neutropenia and elevated liver enzymes in patients receiving three intravenous doses per week for 6 weeks [159]. Overall, 29 patients were treated on this trial. One patient experienced a complete response at 3 months following treatment; while nine patients had stable disease. In a subsequent Phase I trial in 43 total patients with disease of multiple organ sites, a MTD of 30 μg/kg was achieved when IL-21 was administered in two 5-day cycles over the course of 20 days. Of the 24 patients with meta-static malignant melanoma, one subject (whom had received no prior therapies) had a complete response, while there were 11 melanoma patients with stable disease [160]. To investigate an alternative method of delivery, a Phase I trial has been conducted in Europe in which patients with metastatic melanoma received subcutaneous IL-21, and has determined the MTD of subcutaneous delivery to be approximately 200 μg/kg. Of 13 melanoma patients, one experienced a partial response and six experienced stable disease after 8 or 16 weeks of treatment [161]. Several additional Phase II multicenter trials, testing intravenous administration of the cytokine to patients with metastatic melanoma, are either ongoing or were recently closed [162,163]. Other reports of the effects of IL-21 in ongoing trials have been recently presented at ASCO, showing modest benefit in patients with meta-static disease undergoing intravenous treatment with IL-21. In this study, one patient with complete response at week 8 experienced progressive disease at week 11 [164]. Although these results are preliminary, data from these studies will provide important insights into the role for IL-21 as a treatment for malignant melanoma. Indeed, IL-21 has unique potential as an agent for use in combination therapeutic regimens. For example, IL-21 may be useful as a cytokine that can promote T-cell expansion ex vivo [165], a property that could be advantageous for adoptive therapy approaches. Finally, other preclinical data have shown that IL-21 may also be a useful immunomodulator to promote antibody-mediated tumor regression [166].
Barriers to effective cytokine therapy
In melanoma patients, numerous factors are operative that limit the efficacy of immunotherapeutic cytokines. The molecular targets that influence the clinical response to cytokine therapy have not been entirely defined, and the genetic or other host factors that differentiate responders and treatment failures are largely unknown. Therapy with select cytokines has been limited historically by frequent and often severe toxicity. Thus, further research is needed to identify factors that could spare putative ‘non-responders’ unnecessary treatment and toxicity or to identify immunomodulatory cytokines with a favorable toxicity profile. Interestingly, prior reports have suggested that various autoimmune sequelae are more prominent in patients that respond to cytokine immunotherapy [8,9]. The true prognostic potential of these and other parameters are being actively investigated in the context of multiple clinical trials involving cytokines in melanoma patients and will provide important information for gaining the maximum clinical benefit.
On a fundamental level, it is also apparent that there is room for improvement in identifying the most effective and individualized dosing schedules of cytokines. As with many biological therapies, higher doses do not necessarily translate into increased biological effects or clinical efficacy. For example, alternative dosing strategies have been investigated for melanoma patients receiving adjuvant IFN-α therapy and demonstrated that lower doses of IFN-α elicit an equivalent magnitude of downstream signal transduction compared with standard doses [13]. In principle, this approach could preserve patient compliance, reduce toxicity and potentially increase the likelihood of delivering a clinically effective, yet nontoxic dose of cytokine.
Multiple layers of immunosuppression have also been documented in patients with advanced melanoma and other cancers. For example, cellular mediators of suppression including Tregs, M2 macrophages and myeloid-derived suppressor cells are elevated in melanoma patients compared with healthy donors [74,167,168]. Soluble mediators of immunosuppression are also operative in the systemic circulation and in the local tumor microenvironment (Figure 3). These suppressive factors are numerous and include immuno modulatory cytokines (IL-10, IL-6, TGF-β, M-CSF and G-CSF), metabolic factors (arginase I, indoleamine 2,3-dioxygenase), components of oxidative stress (nitric oxide and free radicals), and other proteins involved in immune modulation and support of tumor growth (COX-2 and VEGF) [10,169–173]. As the biology of cytokine therapy is complex, it is important to note that even cytokines effective in activating cytotoxic immune cells can also support the development of suppressive cells. For example, IL-21 has been shown to induce differentiation of Th17 cells, whose role in cancer is still controversial [158]. Further complicating matters is the observation that melanoma cells can display reduced expression of cell surface molecules necessary for effective antigen presentation, including HLA proteins or other costimulatory molecules [11]. Thus, it could be argued that targeting mediators of immunosuppression is a necessary strategy for augmenting the antitumor activity of cytokines and other immunotherapeutic approaches.
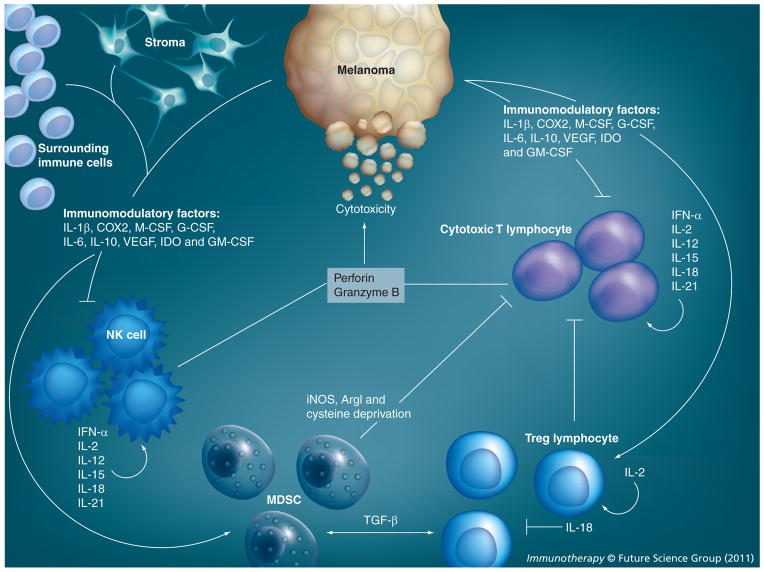
Figure 3. Effect of cytokines on immune cells in the tumor environment.
While cytotoxic immune cells can be present in the tumor environment, tumor and surrounding stroma also have the capability of secreting immunomodulatory cytokines that can support immunosuppressive cells such as regulatory T lymphocytes and myeloid-derived suppressor cells. These cells inactivate NK and cytotoxic T cells, subsequently establishing a protumorigenic environment.
ArgI: Arginase I; IDO: Indoleamine 2,3-dioxygenase.
Conclusion
There remains a great need for a further understanding of the mechanism by which cytokines elicit antitumor responses in patients with melanoma. Administration of these agents represents one of the few therapeutic approaches that can induce complete and durable responses in patients with advanced melanoma. Owing to this tremendous but unpredictable potential, it could be argued that there is opportunity for great advances to be made through clinical trials that test novel combinations of cytokines with various immune-stimulating agents or those that target the multiple soluble or cellular sources of immunosuppression in patients with melanoma. For example, it would be of particular interest to combine melanoma-specific peptides and agents that target Tregs with IL-2, IL-15 or IL-18. Tyrosine kinase inhibitors such as sunitinib that target myeloid-derived suppressor cells may also have promise in combination with multiple immunostimulatory cytokines as evidenced in cancers such as RCC [174]. Finally, cytokines that promote T-cell survival, such as IL-15, may be of interest in combination with adoptive transfer of melanoma antigen-specific T cells. Certainly, combination approaches will be challenging from a standpoint of appropriately attributing toxicity and response. This is particularly true with cytokines such as IL-2 and IFN-α that are thought to be most effective at high doses. However, a greater understanding of effective dosing and scheduling of cytokine administration is an area that could have an important and beneficial impact.
Future perspective
It is unlikely that therapy with high doses of single-agent cytokines will provide future advances for a majority of patients with melanoma. However, the full potential of cytokines as components of combination therapy has yet to be harnessed, provided they can be administered without excessive toxicity. Quite conceivably, cytokines will continue to play a viable role for a number of years as effective agents to be administered together with vaccines, small molecule inhibitors, monoclonal antibodies or other agents that reverse immunosuppression within the tumor-bearing host.
Executive summary.
Use of immunomodulatory agents in the treatment of melanoma
Melanoma is the most deadly skin cancer; the worldwide incidence of melanoma is increasing faster than any other malignancy.
The 5-year survival rate of patients with metastatic disease is <30%, due, largely, to the ineffectiveness of traditional radiation and chemotherapy.
Immunomodulatory proteins, known as cytokines, can stimulate the host’s normal immune system to attack tumors.
Immunomodulatory therapy such as IFN-α and IL-2 have shown efficacy as single agents in a limited number of melanoma patients.
This suggests that other immunomodulators may be useful, as single agents, adjuvant or combinatorial therapies, in melanoma.
Mechanism of immunomodulatory agents against melanoma
Cytokines stimulate NK, dendritic and cytotoxic T cells to destroy melanoma, in part through the secretion of cytolytic proteins such as perforin and granzyme B.
Cytokines such as IFN-α, IL-2, -12, -18 and -21 stimulate NK cells to secrete secondary immunomodulatory proteins (e.g., IFN-γ), thereby amplifying activation of the immune system.
A select subset of cytokines (IL-21 and IL-18) promotes the differentiation of antibody-producing plasma cells and suppresses the proliferation of inhibitory regulatory T lymphocytes.
Barriers to effective immunomodulatory therapy
Advanced melanoma patients demonstrate systemic immunosuppression, which may inhibit the effective utilization of exogenous cytokines for antitumor immune responses.
Cell populations such as regulatory T lymphocytes and myeloid-derived suppressor cells can also infiltrate tumors and inhibit the activity of cytotoxic T cells and NK cells, thereby creating a protumorigenic environment.
Conclusion
As single agents, immunomodulatory cytokines have shown efficacy in the treatment of a subgroup of patients with malignant and metastatic melanoma. However, the role for these cytokines as components of combination therapy regimens holds great promise.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
Gregory B Lesinski is supported by NIH Grants K22CA134551 and The Valvano Foundation for Cancer Research. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM. Biologic therapy. In: Balch CM, Houghton A, Sober A, Soong S-J, editors. Cutaneous Melanoma. 3. Quality Medical Publishing; St Louis, MO, USA: 1998. pp. 419–436. [Google Scholar]
- 3.Atkins MB. Immunotherapy and experimental approaches for metastatic melanoma. Hematol Oncol Clin North Am. 1998;12(4):877–902. doi: 10.1016/s0889-8588(05)70029-0. [DOI] [PubMed] [Google Scholar]
- 4.Saleh FH, Crotty KA, Hersey P, Menzies SW, Rahman W. Autonomous histopathological regression of primary tumours associated with specific immune responses to cancer antigens. J Pathol. 2003;200(3):383–395. doi: 10.1002/path.1369. [DOI] [PubMed] [Google Scholar]
- 5.Thor Straten P, Becker JC, Guldberg P, Zeuthen J. In situ T cells in melanoma. Cancer Immunol Immunother. 1999;48(7):386–395. doi: 10.1007/s002620050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6▪.Vajdic CM, Van Leeuwen MT, Webster AC, et al. Cutaneous melanoma is related to immune suppression in kidney transplant recipients. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2297–2303. doi: 10.1158/1055-9965.EPI-09-0278. Recent survey of the incidence of melanoma in 8000 immunosuppressed kidney transplant patients. [DOI] [PubMed] [Google Scholar]
- 7▪▪.Kawakami Y, Eliyahu S, Delgado CH, et al. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci USA. 1994;91(14):6458–6462. doi: 10.1073/pnas.91.14.6458. Seminal work that demonstrates that T lymphocytes can infiltrate into the tumor and are immunoreactive to melanoma-associated tumor antigens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8▪▪.Gogas H, Ioannovich J, Dafni U, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354(7):709–718. doi: 10.1056/NEJMoa053007. Describes results from a clinical trial of IFN-α in melanoma patients, showing a correlation between favorable outcome and the development of autoimmunity. [DOI] [PubMed] [Google Scholar]
- 9.Bouwhuis MG, Ten Hagen TL, Suciu S, Eggermont AM. Autoimmunity and treatment outcome in melanoma. Curr Opin Oncol. 2010 doi: 10.1097/CCO.0b013e328341edff. [DOI] [PubMed] [Google Scholar]
- 10.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11▪▪.Kageshita T, Hirai S, Ono T, Hicklin DJ, Ferrone S. Down-regulation of HLA class I antigen-processing molecules in malignant melanoma: association with disease progression. Am J Pathol. 1999;154(3):745–754. doi: 10.1016/S0002-9440(10)65321-7. Illustrates an alternative mode by which melanoma can evade immune recognition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parkinson DR, Talpaz M, Lee KH, et al. Interleukin-2 alone and in combination with other cytokines in melanoma: the investigational approach at the University of Texas MD Anderson Cancer Center. Cancer Treat Rev. 1989;16(Suppl A):39–48. doi: 10.1016/0305-7372(89)90021-2. [DOI] [PubMed] [Google Scholar]
- 13.Zimmerer JM, Lehman AM, Ruppert AS, et al. IFN-α-2b-induced signal transduction and gene regulation in patient peripheral blood mononuclear cells is not enhanced by a dose increase from 5 to 10 megaunits/m2. Clin Cancer Res. 2008;14(5):1438–1445. doi: 10.1158/1078-0432.CCR-07-4178. [DOI] [PubMed] [Google Scholar]
- 14.Yoshimoto T, Morishima N, Okumura M, Chiba Y, Xu M, Mizuguchi J. Interleukins and cancer immunotherapy. Immunotherapy. 2009;1(5):825–844. doi: 10.2217/imt.09.46. [DOI] [PubMed] [Google Scholar]
- 15▪.Cheever MA, Schlom J, Weiner LM, et al. Translational Research Working Group developmental pathway for immune response modifiers. Clin Cancer Res. 2008;14(18):5692–5699. doi: 10.1158/1078-0432.CCR-08-1266. Report from the Immunotherapeutic Agent Workshop, sponsored by the US National Cancer Institute, highlighting the efficacy of IL-12 and IL-15, among other cytokines, in the treatment of various cancers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen G, Diaz MO. Nomenclature of the human interferon proteins. J Interferon Cytokine Res. 1996;16(2):181–184. doi: 10.1089/jir.1996.16.181. [DOI] [PubMed] [Google Scholar]
- 17.Lafleur Dw, Nardelli B, Tsareva T, et al. Interferon-κ, a novel type I interferon expressed in human keratinocytes. J Biol Chem. 2001;276(43):39765–39771. doi: 10.1074/jbc.M102502200. [DOI] [PubMed] [Google Scholar]
- 18.Isaacs A, Lindenmann J. Virus interference I: the interferon. Proc R Soc Lond Ser B. 1957;147:258–267. [PubMed] [Google Scholar]
- 19.Isaacs A, Lindenmann J, Valentine RC. Virus interference, II: some properties of interferon. Proc R Soc Lond. 1957;147B:268–273. [PubMed] [Google Scholar]
- 20.Gresser I, Bourali C. Exogenous interferon and inducers of interferon in the treatment Balb-C mice inoculated with RC19 tumour cells. Nature. 1969;223(208):844–845. doi: 10.1038/223844a0. [DOI] [PubMed] [Google Scholar]
- 21.Paucker K, Cantell K, Henle W. Quantitative studies on viral interference in suspended L cells, III: effect of interfering viruses and interferon on the growth rate of cells. Virology. 1962;17:324–334. doi: 10.1016/0042-6822(62)90123-x. [DOI] [PubMed] [Google Scholar]
- 22.Rubinstein M, Rubinstein S, Familletti PC, et al. Human leukocyte interferon purified to homogeneity. Science. 1978;202(4374):1289–1290. doi: 10.1126/science.725605. [DOI] [PubMed] [Google Scholar]
- 23.Taniguchi T, Fujii-Kuriyama Y, Muramatsu M. Construction and identification of a bacterial plasmid containing the human fibroblast interferon gene sequences. Proc Japan Acad. 1979;55B:464–469. [Google Scholar]
- 24.Carpi A, Nicolini A, Antonelli A, Ferrari P, Rossi G. Cytokines in the management of high risk or advanced breast cancer: an update and expectation. Curr Cancer Drug Targets. 2009;9(8):888–903. doi: 10.2174/156800909790192392. [DOI] [PubMed] [Google Scholar]
- 25.Plosker G. Interferon-β-1b: a review of its use in multiple sclerosis. CNS Drugs. 2011;25(1):67–88. doi: 10.2165/11206430-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Sonneveld MJ, Rijckborst V, Boucher CA, Hansen BE, Janssen HL. Prediction of sustained response to peginterferon α-2b for hepatitis B e antigen-positive chronic hepatitis B using on-treatment hepatitis B surface antigen decline. Hepatology. 2010;52(4):1251–1257. doi: 10.1002/hep.23844. [DOI] [PubMed] [Google Scholar]
- 27.Kim Sh, Cohen B, Novick D, Rubinstein M. Mammalian type I interferon receptors consists of two subunits: IFNaR1 and IFNaR2. Gene. 1997;196(1–2):279–286. doi: 10.1016/s0378-1119(97)00240-0. [DOI] [PubMed] [Google Scholar]
- 28.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 29.Haque SJ, Williams BR. Signal transduction in the interferon system. Semin Oncol. 1998;25(1 Suppl 1):14–22. [PubMed] [Google Scholar]
- 30.Ransohoff RM. Cellular responses to interferons and other cytokines: the JAK-STAT paradigm. N Engl J Med. 1998;338(9):616–618. doi: 10.1056/NEJM199802263380911. [DOI] [PubMed] [Google Scholar]
- 31.Levy DE, Kessler DS, Pine R, Reich N, Darnell JE., Jr Interferon-induced nuclear factors that bind a shared promoter element correlate with positive and negative transcriptional control. Genes Dev. 1988;2(4):383–393. doi: 10.1101/gad.2.4.383. [DOI] [PubMed] [Google Scholar]
- 32.Bibeau-Poirier A, Servant MJ. Roles of ubiquitination in pattern-recognition receptors and type I interferon receptor signaling. Cytokine. 2008;43(3):359–367. doi: 10.1016/j.cyto.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Kramer OH, Knauer SK, Greiner G, et al. A phosphorylation-acetylation switch regulates STAT1 signaling. Genes Dev. 2009;23(2):223–235. doi: 10.1101/gad.479209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uddin S, Lekmine F, Sharma N, et al. The Rac1/p38 mitogen-activated protein kinase pathway is required for interferon α-dependent transcriptional activation but not serine phosphorylation of Stat proteins. J Biol Chem. 2000;275(36):27634–27640. doi: 10.1074/jbc.M003170200. [DOI] [PubMed] [Google Scholar]
- 35.Uddin S, Majchrzak B, Woodson J, et al. Activation of the p38 mitogen-activated protein kinase by type I interferons. J Biol Chem. 1999;274(42):30127–30131. doi: 10.1074/jbc.274.42.30127. [DOI] [PubMed] [Google Scholar]
- 36.Bos JL, De Rooij J, Reedquist KA. Rap1 signalling: adhering to new models. Nat Rev Mol Cell Biol. 2001;2(5):369–377. doi: 10.1038/35073073. [DOI] [PubMed] [Google Scholar]
- 37.Fish EN, Uddin S, Korkmaz M, Majchrzak B, Druker BJ, Platanias LC. Activation of a CrkL-stat5 signaling complex by type I interferons. J Biol Chem. 1999;274(2):571–573. doi: 10.1074/jbc.274.2.571. [DOI] [PubMed] [Google Scholar]
- 38.Feller SM. Crk family adaptors-signalling complex formation and biological roles. Oncogene. 2001;20(44):6348–6371. doi: 10.1038/sj.onc.1204779. [DOI] [PubMed] [Google Scholar]
- 39.Stork PJ. Does Rap1 deserve a bad Rap? Trends Biochem Sci. 2003;28(5):267–275. doi: 10.1016/S0968-0004(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 40.Chin YE, Kitagawa M, Kuida K, Flavell RA, Fu XY. Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol Cell Biol. 1997;17(9):5328–5337. doi: 10.1128/mcb.17.9.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang S, Bucana CD, Van Arsdall M, Fidler IJ. Stat1 negatively regulates angiogenesis, tumorigenicity and metastasis of tumor cells. Oncogene. 2002;21(16):2504–2512. doi: 10.1038/sj.onc.1205341. [DOI] [PubMed] [Google Scholar]
- 42.Selleri C, Sato T, Del Vecchio L, et al. Involvement of Fas-mediated apoptosis in the inhibitory effects of interferon-α in chronic myelogenous leukemia. Blood. 1997;89(3):957–964. [PubMed] [Google Scholar]
- 43.Baker PK, Pettitt AR, Slupsky JR, et al. Response of hairy cells to IFN-α involves induction of apoptosis through autocrine TNF-α and protection by adhesion. Blood. 2002;100(2):647–653. doi: 10.1182/blood.v100.2.647. [DOI] [PubMed] [Google Scholar]
- 44.Brassard DL, Grace MJ, Bordens RW. Interferon-α as an immunotherapeutic protein. J Leukoc Biol. 2002;71(4):565–581. [PubMed] [Google Scholar]
- 45.Lesinski GB, Anghelina M, Zimmerer J, et al. The anti-tumor effects of interferon-α are abrogated in a STAT1-deficient mouse. J Clin Invest. 2003;112(2):170–180. doi: 10.1172/JCI16603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Biron CA. Interferons α and β as immune regulators – a new look. Immunity. 2001;14(6):661–664. doi: 10.1016/s1074-7613(01)00154-6. [DOI] [PubMed] [Google Scholar]
- 47.Martin-Henao GA, Quiroga R, Sureda A, Gonzalez JR, Moreno V, Garcia J. L-selectin expression is low on CD34+ cells from patients with chronic myeloid leukemia and interferon-a up-regulates this expression. Haematologica. 2000;85(2):139–146. [PubMed] [Google Scholar]
- 48.Von Stamm U, Brocker EB, Von Depka Prondzinski M, et al. Effects of systemic interferon-α (IFN-α) on the antigenic phenotype of melanoma metastases. EORTC melanoma group cooperative study No. 18852. Mel Res. 1993;3(3):173–180. doi: 10.1097/00008390-199306000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Dengel LT, Norrod AG, Gregory BL, et al. Interferons induce CXCR3-cognate chemokine production by human metastatic melanoma. J Immunother. 2010;33(9):965–974. doi: 10.1097/CJI.0b013e3181fb045d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kirkwood JM, Ernstoff MS, Davis CA, Reiss M, Ferraresi R, Rudnick SA. Comparison of intramuscular and intravenous recombinant α-2 interferon in melanoma and other cancers. Ann Intern Med. 1985;103(1):32–36. doi: 10.7326/0003-4819-103-1-32. [DOI] [PubMed] [Google Scholar]
- 51.Balch CM. In: Cutaneous Melanoma. 3. Balch CM, Houghton AN, Sober AT, Soong SJ, editors. Quality Medical Publishing; St Louis, MO, USA: 1998. [Google Scholar]
- 52.Agarwala S. Improving survival in patients with high-risk and metastatic melanoma: immunotherapy leads the way. Am J Clin Dermatol. 2003;4(5):333–346. doi: 10.2165/00128071-200304050-00004. [DOI] [PubMed] [Google Scholar]
- 53.Eggermont A. Ulceration of primary melanoma and responsiveness to adjuvant interferon therapy: Analysis of the adjuvant trials EORTC18952 and EORTC18991 in 2,644 patients. J Clin Oncol. 2009;27(Suppl 15):Abstract 9007. [Google Scholar]
- 54.Hauschild A, Weichenthal M, Rass K, et al. Efficacy of low-dose interferon α2a 18 versus 60 months of treatment in patients with primary melanoma of >= 1.5 mm tumor thickness: results of a randomized Phase III DeCOG trial. J Clin Oncol. 2010;28(5):841–846. doi: 10.1200/JCO.2009.23.1704. [DOI] [PubMed] [Google Scholar]
- 55.Janku F, Kurzrock R. Adjuvant interferon in high-risk melanoma: end of the era? J Clin Oncol. 2010;28(2):E15–E16. doi: 10.1200/JCO.2009.24.9326. author reply E17–E18. [DOI] [PubMed] [Google Scholar]
- 56.Bottomley A, Coens C, Suciu S, et al. Adjuvant therapy with pegylated interferon α-2b versus observation in resected stage III melanoma: a Phase III randomized controlled trial of health-related quality of life and symptoms by the European Organisation for Research and Treatment of Cancer Melanoma Group. J Clin Oncol. 2009;27(18):2916–2923. doi: 10.1200/JCO.2008.20.2069. [DOI] [PubMed] [Google Scholar]
- 57.Baker PE, Gillis S, Smith KA. Monoclonal cytolytic T-cell lines. J Exp Med. 1979;149(1):273–278. doi: 10.1084/jem.149.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lotze MT, Grimm EA, Mazumder A, Strausser JL, Rosenberg SA. Lysis of fresh and cultured autologous tumor by human lymphocytes cultured in T-cell growth factor. Cancer Res. 1981;41(11 Pt 1):4420–4425. [PubMed] [Google Scholar]
- 60.Gesbert F, Delespine-Carmagnat M, Bertoglio J. Recent advances in the understanding of interleukin-2 signal transduction. J Clin Immunol. 1998;18(5):307–320. doi: 10.1023/a:1023223614407. [DOI] [PubMed] [Google Scholar]
- 61.Lin JX, Leonard WJ. The role of Stat5a and Stat5b in signaling by IL-2 family cytokines. Oncogene. 2000;19(21):2566–2576. doi: 10.1038/sj.onc.1203523. [DOI] [PubMed] [Google Scholar]
- 62.Theze J, Alzari PM, Bertoglio J. Interleukin 2 and its receptors: recent advances and new immunological functions. Immunol Today. 1996;17(10):481–486. doi: 10.1016/0167-5699(96)10057-c. [DOI] [PubMed] [Google Scholar]
- 63.Kondadasula SV, Varker KA, Lesinski GB, et al. Activation of extracellular signaling regulated kinase in natural killer cells and monocytes following IL-2 stimulation in vitro and in patients undergoing IL-2 immunotherapy: analysis via dual parameter flow-cytometric assay. Cancer Immunol Immunother. 2008;57(8):1137–1149. doi: 10.1007/s00262-007-0444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Behbod F, Nagy ZS, Stepkowski SM, et al. Specific inhibition of Stat5a/b promotes apoptosis of IL-2-responsive primary and tumor-derived lymphoid cells. J Immunol. 2003;171(8):3919–3927. doi: 10.4049/jimmunol.171.8.3919. [DOI] [PubMed] [Google Scholar]
- 65.Fung MM, Rohwer F, Mcguire KL. IL-2 activation of a PI3K-dependent STAT3 serine phosphorylation pathway in primary human T cells. Cell Signal. 2003;15(6):625–636. doi: 10.1016/s0898-6568(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 66.Yu TK, Caudell EG, Smid C, Grimm EA. IL-2 activation of NK cells: involvement of MKK1/2/ERK but not p38 kinase pathway. J Immunol. 2000;164(12):6244–6251. doi: 10.4049/jimmunol.164.12.6244. [DOI] [PubMed] [Google Scholar]
- 67.Imada K, Bloom ET, Nakajima H, et al. Stat5b is essential for natural killer cell-mediated proliferation and cytolytic activity. J Exp Med. 1998;188(11):2067–2074. doi: 10.1084/jem.188.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moriggl R, Sexl V, Piekorz R, Topham D, Ihle JN. Stat5 activation is uniquely associated with cytokine signaling in peripheral T cells. Immunity. 1999;11(2):225–230. doi: 10.1016/s1074-7613(00)80097-7. [DOI] [PubMed] [Google Scholar]
- 69.Moriggl R, Topham DJ, Teglund S, et al. Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity. 1999;10(2):249–259. doi: 10.1016/s1074-7613(00)80025-4. [DOI] [PubMed] [Google Scholar]
- 70.Kagami S, Nakajima H, Kumano K, et al. Both STAT5a and STAT5b are required for antigen-induced eosinophil and T-cell recruitment into the tissue. Blood. 2000;95(4):1370–1377. [PubMed] [Google Scholar]
- 71.Battle TE, Frank DA. The role of STATs in apoptosis. Curr Mol Med. 2002;2(4):381–392. doi: 10.2174/1566524023362456. [DOI] [PubMed] [Google Scholar]
- 72.Zamorano J, Wang HY, Wang R, Shi Y, Longmore GD, Keegan AD. Regulation of cell growth by IL-2: role of STAT5 in protection from apoptosis but not in cell cycle progression. J Immunol. 1998;160(7):3502–3512. [PubMed] [Google Scholar]
- 73.Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21(16):3127–3132. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74▪.Ilkovitch D, Lopez DM. Immune modulation by melanoma-derived factors. Exp Dermatol. 2008;17(12):977–985. doi: 10.1111/j.1600-0625.2008.00779.x. Describes the duality of the immunomodulatory effects of IL-2, as an activator of both CD8+ cytotoxic T lymphocytes and regulatory T lymphocytes. [DOI] [PubMed] [Google Scholar]
- 75.Wang R, Ciardelli TL, Russell JH. Partial signaling by cytokines: cytokine regulation of cell cycle and Fas-dependent, activation-induced death in CD4+ subsets. Cell Immunol. 1997;182(2):152–160. doi: 10.1006/cimm.1997.1220. [DOI] [PubMed] [Google Scholar]
- 76.Atkins MB. Interleukin-2 in metastatic melanoma: what is the current role? Cancer J Sci Am. 2000;6(Suppl 1):S8–S10. [PubMed] [Google Scholar]
- 77.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 78.Moschos SJ, Mandic M, Kirkwood JM, Storkus WJ, Lotze MT. Focus on FOCIS: interleukin 2 treatment associated autoimmunity. Clin Immunol. 2008;127(2):123–129. doi: 10.1016/j.clim.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 79.Waithman J, Gebhardt T, Davey GM, Heath WR, Carbone FR. Cutting edge: enhanced IL-2 signaling can convert self-specific T cell response from tolerance to autoimmunity. J Immunol. 2008;180(9):5789–5793. doi: 10.4049/jimmunol.180.9.5789. [DOI] [PubMed] [Google Scholar]
- 80.Mcdermott DF, Regan MM, Clark JI, et al. Randomized Phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23(1):133–141. doi: 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 81.Lissoni P, Bordin V, Vaghi M, et al. Ten-year survival results in metastatic renal cell cancer patients treated with monoimmunotherapy with subcutaneous low-dose interleukin-2. Anticancer Res. 2002;22(2B):1061–1064. [PubMed] [Google Scholar]
- 82.Fisher RI, Rosenberg SA, Fyfe G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am. 2000;6(Suppl 1):S55–S57. [PubMed] [Google Scholar]
- 83.Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13(3):688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 84.Mcdermott DF, Atkins MB. Application of IL-2 and other cytokines in renal cancer. Expert Opin Biol Ther. 2004;4(4):455–468. doi: 10.1517/14712598.4.4.455. [DOI] [PubMed] [Google Scholar]
- 85.Tarhini AA, Kirkwood JM, Gooding WE, Cai C, Agarwala SS. Durable complete responses with high-dose bolus interleukin-2 in patients with metastatic melanoma who have experienced progression after biochemotherapy. J Clin Oncol. 2007;25(25):3802–3807. doi: 10.1200/JCO.2006.10.2822. [DOI] [PubMed] [Google Scholar]
- 86.Dutcher JP, Atkins M, Fisher R, et al. Interleukin-2-based therapy for metastatic renal cell cancer: the Cytokine Working Group experience, 1989–1997. Cancer J Sci Am. 1997;3(Suppl 1):S73–S78. [PubMed] [Google Scholar]
- 87.Dutcher JP, Fisher RI, Weiss G, et al. Outpatient subcutaneous interleukin-2 and interferon-α for metastatic renal cell cancer: five-year follow-up of the Cytokine Working Group Study. Cancer J Sci Am. 1997;3(3):157–162. [PubMed] [Google Scholar]
- 88.Dutcher JP, Logan T, Gordon M, et al. Phase II trial of interleukin 2, interferon α, and 5-fluorouracil in metastatic renal cell cancer: a cytokine working group study. Clin Cancer Res. 2000;6(9):3442–3450. [PubMed] [Google Scholar]
- 89.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21(2):233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bakker AB, Schreurs MW, De Boer AJ, et al. Melanocyte lineage-specific antigen gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J Exp Med. 1994;179(3):1005–1009. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sosman JA, Carrillo C, Urba WJ, et al. Three Phase II cytokine working group trials of gp100 (210M) peptide plus high-dose interleukin-2 in patients with HLA-A2-positive advanced melanoma. J Clin Oncol. 2008;26(14):2292–2298. doi: 10.1200/JCO.2007.13.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gunturu KS, Meehan KR, Mackenzie TA, et al. Cytokine working group study of lymphodepleting chemotherapy, interleukin-2, and granulocyte-macrophage colony-stimulating factor in patients with metastatic melanoma: clinical outcomes and peripheral-blood cell recovery. J Clin Oncol. 2010;28(7):1196–1202. doi: 10.1200/JCO.2009.24.8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Atkins MB, Hsu J, Lee S, et al. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon α-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2008;26(35):5748–5754. doi: 10.1200/JCO.2008.17.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schwartzentruber DJ. A Phase III multi-institutional randomized study of immunization with the gp100:209–217(210M) peptide followed by high-dose IL-2 compared with high-dose IL-2 alone in patients with metastatic melanoma. J Clin Oncol. 2009;27(Suppl 18):Abstract CR A9011. [Google Scholar]
- 95.Prieto PA. Cytotoxic T lymphocyte-associated antigen 4 blockade with ipilimumab: Long-term follow-up of 179 patients with metastatic melanoma. J Clin Oncol. 2010;28(Suppl 15):Abstract 8544. [Google Scholar]
- 96.Sabatino M, Kim-Schulze S, Panelli MC, et al. Serum vascular endothelial growth factor and fibronectin predict clinical response to high-dose interleukin-2 therapy. J Clin Oncol. 2009;27(16):2645–2652. doi: 10.1200/JCO.2008.19.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kobayashi M, Fitz L, Ryan M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170(3):827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stern AS, Podlaski FJ, Hulmes JD, et al. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc Natl Acad Sci USA. 1990;87(17):6808–6812. doi: 10.1073/pnas.87.17.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O’shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139–156. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 100.Thierfelder WE, Van Deursen JM, Yamamoto K, et al. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382(6587):171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 101.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3(2):133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 102.Brunda MJ, Luistro L, Warrier RR, et al. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993;178(4):1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 104.Nagai H, Oniki S, Fujiwara S, Yoshimoto T, Nishigori C. Antimelanoma immunotherapy: clinical and preclinical applications of IL-12 family members. Immunotherapy. 2010;2(5):697–709. doi: 10.2217/imt.10.46. [DOI] [PubMed] [Google Scholar]
- 105.Voest EE, Kenyon BM, O’reilly MS, Truitt G, D’amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 1995;87(8):581–586. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- 106.Alatrash G, Hutson TE, Molto L, et al. Clinical and immunologic effects of subcutaneously administered interleukin-12 and interferon α-2b: Phase I trial of patients with metastatic renal cell carcinoma or malignant melanoma. J Clin Oncol. 2004;22(14):2891–2900. doi: 10.1200/JCO.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 107.Eisenbeis CF, Lesinski GB, Anghelina M, et al. Phase I study of the sequential combination of interleukin-12 and interferon α-2b in advanced cancer: evidence for modulation of interferon signaling pathways by interleukin-12. J Clin Oncol. 2005;23(34):8835–8844. doi: 10.1200/JCO.2005.02.1691. [DOI] [PubMed] [Google Scholar]
- 108.Del Vecchio M, Bajetta E, Canova S, et al. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13(16):4677–4685. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 109.Cebon J, Jager E, Shackleton MJ, et al. Two Phase I studies of low dose recombinant human IL-12 with Melan-A and influenza peptides in subjects with advanced malignant melanoma. Cancer Immun. 2003;3:7. [PubMed] [Google Scholar]
- 110.Peterson AC, Harlin H, Gajewski TF. Immunization with Melan-A peptide-pulsed peripheral blood mononuclear cells plus recombinant human interleukin-12 induces clinical activity and T-cell responses in advanced melanoma. J Clin Oncol. 2003;21(12):2342–2348. doi: 10.1200/JCO.2003.12.144. [DOI] [PubMed] [Google Scholar]
- 111.Atkins MB, Robertson MJ, Gordon M, et al. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin Cancer Res. 1997;3(3):409–417. [PubMed] [Google Scholar]
- 112.Leonard JP, Sherman ML, Fisher GL, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-γ production. Blood. 1997;90(7):2541–2548. [PubMed] [Google Scholar]
- 113.Heinzerling L, Burg G, Dummer R, et al. Intratumoral injection of DNA encoding human interleukin 12 into patients with metastatic melanoma: clinical efficacy. Hum Gene Ther. 2005;16(1):35–48. doi: 10.1089/hum.2005.16.35. [DOI] [PubMed] [Google Scholar]
- 114.Fewkes NM, Mackall CL. Novel γ-chain cytokines as candidate immune modulators in immune therapies for cancer. Cancer J. 2010;16(4):392–398. doi: 10.1097/PPO.0b013e3181eacbc4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8+ T cells. J Exp Med. 2002;196(7):935–946. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 117.Lewko WM, Smith TL, Bowman DJ, Good RW, Oldham RK. Interleukin-15 and the growth of tumor derived activated T-cells. Cancer Biother. 1995;10(1):13–20. doi: 10.1089/cbr.1995.10.13. [DOI] [PubMed] [Google Scholar]
- 118.Gu Y, Kuida K, Tsutsui H, et al. Activation of interferon-γ inducing factor mediated by interleukin-1β converting enzyme. Science. 1997;275(5297):206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 119.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10(2):89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 120.Park S, Cheon S, Cho D. The dual effects of interleukin-18 in tumor progression. Cell Mol Immunol. 2007;4(5):329–335. [PubMed] [Google Scholar]
- 121.Srivastava S, Salim N, Robertson MJ. Interleukin-18: biology and role in the immunotherapy of cancer. Curr Med Chem. 2010;17(29):3353–3357. doi: 10.2174/092986710793176348. [DOI] [PubMed] [Google Scholar]
- 122.Barbulescu K, Becker C, Schlaak JF, Schmitt E, Meyer Zum Buschenfelde KH, Neurath MF. IL-12 and IL-18 differentially regulate the transcriptional activity of the human IFN-γ promoter in primary CD4+ T lymphocytes. J Immunol. 1998;160(8):3642–3647. [PubMed] [Google Scholar]
- 123.Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol. 2003;73(2):213–224. doi: 10.1189/jlb.0602313. [DOI] [PubMed] [Google Scholar]
- 124.Kim YM, Im JY, Han SH, Kang HS, Choi I. IFN-γ up-regulates IL-18 gene expression via IFN consensus sequence-binding protein and activator protein-1 elements in macrophages. J Immunol. 2000;165(6):3198–3205. doi: 10.4049/jimmunol.165.6.3198. [DOI] [PubMed] [Google Scholar]
- 125.Moller B, Paulukat J, Nold M, et al. Interferon-γ induces expression of interleukin-18 binding protein in fibroblast-like synoviocytes. Rheumatology (Oxford) 2003;42(3):442–445. doi: 10.1093/rheumatology/keg146. [DOI] [PubMed] [Google Scholar]
- 126.Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378(6552):88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 127.Tominaga K, Yoshimoto T, Torigoe K, et al. IL-12 synergizes with IL-18 or IL-1β for IFN-γ production from human T cells. Int Immunol. 2000;12(2):151–160. doi: 10.1093/intimm/12.2.151. [DOI] [PubMed] [Google Scholar]
- 128.Ahn HJ, Maruo S, Tomura M, et al. A mechanism underlying synergy between IL-12 and IFN-γ-inducing factor in enhanced production of IFN-γ. J Immunol. 1997;159(5):2125–2131. [PubMed] [Google Scholar]
- 129.Vidal-Vanaclocha F, Mendoza L, Telleria N, et al. Clinical and experimental approaches to the pathophysiology of interleukin-18 in cancer progression. Cancer Metastasis Rev. 2006;25(3):417–434. doi: 10.1007/s10555-006-9013-3. [DOI] [PubMed] [Google Scholar]
- 130.Carroll RG, Carpenito C, Shan X, et al. Distinct effects of IL-18 on the engraftment and function of human effector CD8 T cells and regulatory T cells. PLoS One. 2008;3(9):E3289. doi: 10.1371/journal.pone.0003289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 132.Guo L, Wei G, Zhu J, et al. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci USA. 2009;106(32):13463–13468. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hata H, Yoshimoto T, Hayashi N, Hada T, Nakanishi K. IL-18 together with anti-CD3 antibody induces human Th1 cells to produce Th1- and Th2-cytokines and IL-8. Int Immunol. 2004;16(12):1733–1739. doi: 10.1093/intimm/dxh174. [DOI] [PubMed] [Google Scholar]
- 134.Chaix J, Tessmer MS, Hoebe K, et al. Cutting edge: Priming of NK cells by IL-18. J Immunol. 2008;181(3):1627–1631. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lissoni P, Brivio F, Rovelli F, et al. Serum concentrations of interleukin-18 in early and advanced cancer patients: enhanced secretion in metastatic disease. J Biol Regul Homeost Agents. 2000;14(4):275–277. [PubMed] [Google Scholar]
- 136.Tsuboi K, Miyazaki T, Nakajima M, et al. Serum interleukin-12 and interleukin-18 levels as a tumor marker in patients with esophageal carcinoma. Cancer Lett. 2004;205(2):207–214. doi: 10.1016/j.canlet.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 137.Akahiro J, Konno R, Ito K, Okamura K, Yaegashi N. Impact of serum interleukin-18 level as a prognostic indicator in patients with epithelial ovarian carcinoma. Int J Clin Oncol. 2004;9(1):42–46. doi: 10.1007/s10147-003-0360-6. [DOI] [PubMed] [Google Scholar]
- 138.Cho D, Song H, Kim YM, et al. Endogenous interleukin-18 modulates immune escape of murine melanoma cells by regulating the expression of Fas ligand and reactive oxygen intermediates. Cancer Res. 2000;60(10):2703–2709. [PubMed] [Google Scholar]
- 139.Robertson MJ, Mier JW, Logan T, et al. Clinical and biological effects of recombinant human interleukin-18 administered by intravenous infusion to patients with advanced cancer. Clin Cancer Res. 2006;12(14 Pt 1):4265–4273. doi: 10.1158/1078-0432.CCR-06-0121. [DOI] [PubMed] [Google Scholar]
- 140.Robertson MJ, Kirkwood JM, Logan TF, et al. A dose-escalation study of recombinant human interleukin-18 using two different schedules of administration in patients with cancer. Clin Cancer Res. 2008;14(11):3462–3469. doi: 10.1158/1078-0432.CCR-07-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Golab J, Stoklosa T. Technology evaluation: SB-485232, GlaxoSmithKline. Curr Opin Mol Ther. 2005;7(1):85–93. [PubMed] [Google Scholar]
- 142.Tarhini AA, Millward M, Mainwaring P, et al. A Phase 2, randomized study of SB-485232, rhIL-18, in patients with previously untreated metastatic melanoma. Cancer. 2009;115(4):859–868. doi: 10.1002/cncr.24100. [DOI] [PubMed] [Google Scholar]
- 143.Davis ID, Skak K, Smyth MJ, Kristjansen PE, Miller DM, Sivakumar PV. Interleukin-21 signaling: functions in cancer and autoimmunity. Clin Cancer Res. 2007;13(23):6926–6932. doi: 10.1158/1078-0432.CCR-07-1238. [DOI] [PubMed] [Google Scholar]
- 144.Parrish-Novak J, Dillon SR, Nelson A, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408(6808):57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 145.Coquet JM, Kyparissoudis K, Pellicci DG, et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178(5):2827–2834. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- 146.Andorsky DJ, Timmerman JM. Interleukin-21: biology and application to cancer therapy. Expert Opin Biol Ther. 2008;8(9):1295–1307. doi: 10.1517/14712598.8.9.1295. [DOI] [PubMed] [Google Scholar]
- 147.Skak K, Kragh M, Hausman D, Smyth MJ, Sivakumar PV. Interleukin 21: combination strategies for cancer therapy. Nat Rev Drug Discov. 2008;7(3):231–240. doi: 10.1038/nrd2482. [DOI] [PubMed] [Google Scholar]
- 148.Nurieva R, Yang XO, Martinez G, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448(7152):480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 149.Petersen CC, Diernaes JE, Skovbo A, Hvid M, Deleuran B, Hokland M. Interleukin-21 restrains tumor growth and induces a substantial increase in the number of circulating tumor-specific T cells in a murine model of malignant melanoma. Cytokine. 2010;49(1):80–88. doi: 10.1016/j.cyto.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 150.Pene J, Gauchat JF, Lecart S, et al. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J Immunol. 2004;172(9):5154–5157. doi: 10.4049/jimmunol.172.9.5154. [DOI] [PubMed] [Google Scholar]
- 151.Mehta DS, Wurster AL, Whitters MJ, Young DA, Collins M, Grusby MJ. IL-21 induces the apoptosis of resting and activated primary B cells. J Immunol. 2003;170(8):4111–4118. doi: 10.4049/jimmunol.170.8.4111. [DOI] [PubMed] [Google Scholar]
- 152.Ozaki K, Spolski R, Ettinger R, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173(9):5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 153.Sivori S, Cantoni C, Parolini S, et al. IL-21 induces both rapid maturation of human CD34+ cell precursors towards NK cells and acquisition of surface killer Ig-like receptors. Eur J Immunol. 2003;33(12):3439–3447. doi: 10.1002/eji.200324533. [DOI] [PubMed] [Google Scholar]
- 154.Strengell M, Matikainen S, Siren J, et al. IL-21 in synergy with IL-15 or IL-18 enhances IFN-γ production in human NK and T cells. J Immunol. 2003;170(11):5464–5469. doi: 10.4049/jimmunol.170.11.5464. [DOI] [PubMed] [Google Scholar]
- 155.Peluso I, Fantini MC, Fina D, et al. IL-21 counteracts the regulatory T cell-mediated suppression of human CD4+ T lymphocytes. J Immunol. 2007;178(2):732–739. doi: 10.4049/jimmunol.178.2.732. [DOI] [PubMed] [Google Scholar]
- 156.Hinrichs CS, Spolski R, Paulos CM, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111(11):5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li Y, Yee C. IL-21 mediated Foxp3 suppression leads to enhanced generation of antigen-specific CD8+ cytotoxic T lymphocytes. Blood. 2008;111(1):229–235. doi: 10.1182/blood-2007-05-089375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(h)17 cells. Nature. 2007;448(7152):484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Davis ID, Skrumsager BK, Cebon J, et al. An open-label, two-arm, Phase I trial of recombinant human interleukin-21 in patients with metastatic melanoma. Clin Cancer Res. 2007;13(12):3630–3636. doi: 10.1158/1078-0432.CCR-07-0410. [DOI] [PubMed] [Google Scholar]
- 160.Thompson JA, Curti BD, Redman BG, et al. Phase I study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinoma. J Clin Oncol. 2008;26(12):2034–2039. doi: 10.1200/JCO.2007.14.5193. [DOI] [PubMed] [Google Scholar]
- 161.Schmidt H, Brown J, Mouritzen U, et al. Safety and clinical effect of subcutaneous human interleukin-21 in patients with metastatic melanoma or renal cell carcinoma: a Phase I trial. Clin Cancer Res. 2010;16(21):5312–5319. doi: 10.1158/1078-0432.CCR-10-1809. [DOI] [PubMed] [Google Scholar]
- 162.Hashmi MH, Van Veldhuizen PJ. Interleukin-21: updated review of Phase I and II clinical trials in metastatic renal cell carcinoma, metastatic melanoma and relapsed/refractory indolent non-Hodgkin’s lymphoma. Expert Opin Biol Ther. 2010;10(5):807–817. doi: 10.1517/14712598.2010.480971. [DOI] [PubMed] [Google Scholar]
- 163.Petrella TM. Phase II study of interleukin in patients with recurrent or metastatic melanoma. Presented at: The 7th World Congress on Melanoma; Vienna, Austria. 12–16 May 2009. [Google Scholar]
- 164.Davis I. Activity of recombinant human interleukin-21 (rIL-21) in patients (pts) with stage IV malignant melanoma (MM) without prior treatment: clinical data from a Phase IIa study. J Clin Oncol. 2008;26(Suppl 20):Abstract 3042. [Google Scholar]
- 165.Huarte E, Fisher J, Turk MJ, et al. Ex vivo expansion of tumor specific lymphocytes with IL-15 and IL-21 for adoptive immunotherapy in melanoma. Cancer Lett. 2009;285(1):80–88. doi: 10.1016/j.canlet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jaime-Ramirez AC, Mundy-Bosse BL, Kondadasula SV, et al. Interleukin-12 enhances the anti-tumor actions of trastuzumab via NK cell IFN-γ production. J Immunol. 2011 doi: 10.4049/jimmunol.1000328. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cote AL, Usherwood EJ, Turk MJ. Tumor-specific T-cell memory: clearing the regulatory T-cell hurdle. Cancer Res. 2008;68(6):1614–1617. doi: 10.1158/0008-5472.CAN-07-6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Filipazzi P, Valenti R, Huber V, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25(18):2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 169.Brody JR, Costantino CL, Berger AC, et al. Expression of indoleamine 2,3-dioxygenase in metastatic malignant melanoma recruits regulatory T cells to avoid immune detection and affects survival. Cell Cycle. 2009;8(12):1930–1934. doi: 10.4161/cc.8.12.8745. [DOI] [PubMed] [Google Scholar]
- 170.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR−/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70(11):4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 171.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185(4):2273–2284. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Toomey D, Conroy H, Jarnicki AG, Higgins SC, Sutton C, Mills KH. Therapeutic vaccination with dendritic cells pulsed with tumor-derived Hsp70 and a COX-2 inhibitor induces protective immunity against B16 melanoma. Vaccine. 2008;26(27–28):3540–3549. doi: 10.1016/j.vaccine.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 173.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2(10):1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 174.Finke JH, Rini B, Ireland J, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14(20):6674–6682. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
- 175.Mortarini R, Borri A, Tragni G, et al. Peripheral burst of tumor-specific cytotoxic T lymphocytes and infiltration of metastatic lesions by memory CD8+ T cells in melanoma patients receiving interleukin 12. Cancer Res. 2000;60(13):3559–3568. [PubMed] [Google Scholar]
- 176.Bajetta E, Del Vecchio M, Mortarini R, et al. Pilot study of subcutaneous recombinant human interleukin 12 in metastatic melanoma. Clin Cancer Res. 1998;4(1):75–85. [PubMed] [Google Scholar]
Patent
- 201.Conklin DC, Grant FJ, Rixon MW, Kindsvogel W. US6329175. Interferon-ε. 2002
Websites
- 301.A Phase I Study of Intravenous Recombinant Human IL-15 in Adults With Refractory Metastatic Malignant Melanoma and Metastatic Renal Cell Cancer ( NCT01021059) http://clinicaltrials.gov/ct2/show/NCT01021059?term=NCT01021059&rank=1.
- 302.A randomized Phase II study of interleukin-21 (rIL-21) versus dacarbazine (DTIC) in patients with metastatic or recurrent melanoma ( NCT01152788) http://clinicaltrials.gov/ct2/show/NCT01152788?term=NCT01152788&rank=1.
- 303.Interleukin-21 in treating patients with metastatic or recurrent malignant melanoma ( NCT00514085) http://clinicaltrials.gov/ct2/show/NCT00514085?term=NCT00514085&rank=1.
- 304.Efficacy study of IL-21 to treat metastatic melanoma ( NCT00336986) http://clinicaltrials.gov/ct2/show/NCT00336986?term=NCT00336986&rank=1.