Abstract
Daily intravenous (IV) busulfan is increasingly being used in hematopoietic cell transplantation (HCT) conditioning regimens. Intravenous busulfan doses administered at the traditional frequency of every 6 hours can be targeted (TBu) to a patient-specific concentration at steady state (Css) using therapeutic drug monitoring. In this report, we describe our experiences with TDM of daily IV busulfan in an adult population, with the specific aims of 1) evaluating covariates associated with busulfan clearance; 2) assessing the feasibility of therapeutic drug monitoring for outpatient administration of daily TBu with pharmacokinetic sampling over 6 hours. A retrospective pharmacokinetic analysis was conducted in 87 adults receiving daily TBu as part of cyclophosphamide followed by TBU (CY/TBU), fludarabine monophosphate (fludarabine) followed by TBU, or TBU concurrent with fludarabine conditioning. The desired Css was achieved in 85% of patients receiving daily IV busulfan. Busulfan clearance was not associated with gender or age, but was associated with the day of dosing and conditioning regimen (p=0.0016). In patients receiving CY/TBU, no differences in clearance were found between dosing days (p>0.36); however, clearance decreased significantly in patients receiving fludarabine-based regimens (p=0.0016). Busulfan clearance and Css estimates from pharmacokinetic sampling over 8, 11, or 24 hours were comparable (p>0.4). However, pharmacokinetic modeling of individual patient concentration-time data over 6 hours could not reliably estimate busulfan clearance or Css.
Keywords: Busulfan, pharmacokinetics, hematopoietic cell transplant, personalized medicine, therapeutic drug monitoring
INTRODUCTION
Several hematopoietic stem cell transplant (HCT) conditioning regimens include high-dose busulfan. A variety of clinical outcomes, including both toxicity and lack of efficacy, are associated with the systemic exposure of busulfan. Such outcomes are expressed as area under the plasma concentration-time curve (AUC) or concentration at steady state (Css). These pharmacodynamic associations of busulfan are affected by other components such as the conditioning regimen, the recipient’s age, and the underlying disease (see previous reviews1, 2). Over the past decade, there have been an increasing number of HCT centers that target busulfan (TBU) doses to achieve the patient-specific busulfan exposure using therapeutic drug monitoring.2–5 Dosing of TBU with therapeutic drug monitoring is conducted by obtaining blood samples after a dose based on body weight or body surface area, quantitating the plasma concentrations, and then modeling the individual concentration-time data to estimate the individual patient’s busulfan exposure and clearance. Using that individual’s busulfan clearance, subsequent doses are adjusted to achieve the desired busulfan exposure because clearance equals the dose divided by AUC and Css equals AUC divided by dosing interval.
The recent trend in administering busulfan every 24 hours (i.e., daily) from the traditional approach of every 6 hour administration has lead to the potential for IV busulfan to be administered within an ambulatory clinic. Daily IV busulfan is typically administered as a 3-hour infusion every 24 hours, for a total of 4 doses. Although less frequent dosing of IV busulfan offers the potential advantage of outpatient administration, it has the potential disadvantage of fewer doses to conduct therapeutic drug monitoring and thus fewer opportunities to achieve the desired busulfan exposure. When our center initiated using daily IV busulfan, targeting of these doses was necessary to allow for historic comparisons with our TBU after oral administration. We sought to identify the optimal initial weight-based dose of daily IV busulfan to rapidly achieve the desired Css and design a pharmacokinetic sampling schema that allows for accurate daily busulfan dose targeting within an outpatient setting. A logistically feasible outpatient pharmacokinetic sampling schedule during the first 6 hours after the start of the daily 3-hour IV busulfan infusion may reduce the need for clinical resources (i.e., nursing and laboratory staff time), increase patient convenience, and potentially result in significant cost savings. Thus, the objectives of this retrospective analysis in adults receiving daily IV busulfan are to: 1) summarize our experience with therapeutic drug monitoring to TBU; 2) evaluate covariates associated with busulfan clearance; 3) assess the feasibility of therapeutic drug monitoring in the outpatient setting with pharmacokinetic sampling over 6 hours.
METHODS
Study population
This was a retrospective study of patients who received HCT conditioning with daily IV busulfan and therapeutic drug monitoring at the Seattle Cancer Care Alliance from September 2004-November 2009 under the aegis of protocols approved by the Fred Hutchinson Cancer Research Center Institutional Review Board. All patients received TBU personalized using therapeutic drug monitoring to achieve a patient-specific desired average steady-state busulfan plasma concentration (Css). Records were examined for demographic data (age, sex, height, weight, body surface area) and clinical data (disease, conditioning regimen). Standard practice for prophylaxis of busulfan-induced seizures was phenytoin.
Conditioning regimen
One of the following conditioning regimens was administered: 1) cyclosphosphamide followed by TBU (cyclophosphamide 60 mg/kg/day IV on days −7 and −6, TBU on days −5 to −2); 2) fludarabine monophosphate (abbreviated as fludarabine) followed by TBU (fludarabine 30 mg/m2/day IV on days −9 through −6, TBU on days −5 to −2, and rabbit antithymocyte globulin (rATG, Thymoglobulin®) 0.5 mg/kg IV on day −3, 2.5 mg/kg on day −2, and 3 mg/kg on day −1); and 3) TBU concurrent with fludarabine (fludarabine 50 mg/m2/day IV on days −6 through −2, TBU on days −5 to −2, and rATG 0.5 mg/kg IV on day −3, 2.5 mg/kg on day −2, and 3 mg/kg on day −1). All patients received TBU for 4 days for a total of 4 doses.
Daily IV busulfan dosing
Administration of daily IV busulfan doses was standardized regarding the time of administration (5 am), duration of busulfan infusion (3 hours), and delivery of saline flush to clear the infusion line of busulfan for consistency of daily IV busulfan administration and pharmacokinetic sampling. The first busulfan dose (dose 1) was weight-based as determined by the treatment protocol. Busulfan dose 1 was 3.2 mg/kg in the first cohort of patients, and was subsequently increased to 4 mg/kg based on the average clearance in that initial cohort. Busulfan dose 1 was calculated using actual body weight if it was less than ideal body weight, or adjusted ideal body weight (AIBW, which equals 0.25 (actual weight – ideal weight) + ideal weight) if actual body weight was greater than ideal body weight.6 The ideal body weight in adults was calculated: for males = 50 kg + (2.3 kg for each inch over 5 feet); for females = 45.5 kg + (2.3 kg for each inch over 5 feet).
All subsequent busulfan doses were personalized to achieve the desired patient-specific Css, which was based on the treatment protocol and could be changed by the attending physician.
Pharmacokinetic sampling and analysis
Blood samples (3 mL/sample) were collected in sodium heparin tubes after busulfan doses 1, 2, and 3. These samples were drawn at the end of the 3-hour infusion, and at 3.25, 4.5, 6, 8, 11, and 24 hours (i.e., prior to subsequent dose) after the beginning of the infusion. The first sample was drawn at the end of the 3-hour infusion and no samples were drawn during the infusion. Samples were stored on wet-ice or refrigerated, and transported to the laboratory. Plasma busulfan concentrations were analyzed by gas chromatography with mass selective detection as previously described.3 The dynamic range was from 62 to 4500 ng/mL and the intraday and interday coefficient of variations were less than 5% and 8%, respectively.
After quantitation of busulfan samples, the individual patient’s concentration-time data were fit using WinNonlin (version 5.2) via noncompartmental and compartmental modeling. The model selected was determined based on visual inspection of the model fit compared to the individual concentration-time data. The AUC from time 0 to infinity (AUC0 to ∞) was calculated after each dose. In order to provide same day results, AUC0 to ∞ and its estimated corresponding clearance were determined using concentration-time data measured through 11 hours (first 22 patients) and 8 hours (subsequent 65 patients) after the beginning of infusion. Clearance and Css were calculated based on the following equations: clearance = dose divided by AUC and Css = AUC divided by dosing interval. All clearances are reported based on AIBW, which is the optimal body metric for IV busulfan over a population of underweight to obese patients.7, 8 Css was calculated as AUC0 to ∞ multiplied by busulfan molecular weight (246.3 g/mol) divided by the dosing interval (24 hours). After calculation of the patient’s clearance, the target dose for subsequent doses was calculated linearly to achieve the target Css. Successful TBU dosing was confirmed after doses 2 and 3, with further dose adjustments as needed.
In order to compare the body metrics of AIBW, body surface area (BSA), and actual body weight for determination of the initial busulfan dose, the amount of busulfan administered for dose 1 was normalized by AIBW (mg/kg), actual body weight (mg/kg), and BSA (mg/m2). Each patient’s clearance was recalculated using AIBW (mL/min/kg), actual body weight (mL/min/kg), and BSA (mL/min/m2). According to body mass index (BMI), four weight categories were defined: underweight (BMI<18 kg/m2), normal (BMI 18–26.9 kg/m2), obese (BMI 27–35 kg/m2) and severely obese (BMI>35 kg/m2).7, 9 BMI was calculated as weight (kg)/height2 (m2). Body metric-normalized clearances were compared by one-way ANOVA.
For this retrospective analysis, all concentration-time data were re-analyzed in WinNonlin to evaluate the bias and precision of our current clinical practice (i.e., sampling over 8 hours) for daily IV TBU. For patients targeted to a concentration of 900 ng/mL, for the purpose of this analysis 900 ± 5% was considered to be within the target range.
Statistical methods
Descriptive statistics were used to summarize our experience with therapeutic drug monitoring of daily IV TBU. Each participant’s average busulfan clearance based on AIBW was calculated from busulfan clearance after dose 1, 2 and 3. The data were normally distributed.
The difference in busulfan clearance between doses was evaluated using the mixed procedure linear regression model, taking into account repeated measurements within subjects, with sex, age, dose number, and conditioning regimen as covariates. The interdose variability was calculated by determining the percent (%) change in busulfan clearance. For example, the difference between busulfan clearance from dose 1 to dose 2 was calculated as follows:
To calculate the shortest duration of pharmacokinetic sampling, the percent difference in busulfan Css was calculated using the pharmacokinetic sampling over 24 hours as the reference value. For example, the difference between busulfan Css estimated from 8 hours (i.e., our current clinical practice) to busulfan Css estimated from 24 hours was calculated as follows:
To evaluate effect of sampling duration on clearance, a separate mixed procedure linear regression model taking into account repeated measurements was used. This analysis was conducted with 1-compartmental and noncompartmental model data, including sampling duration (i.e., 24, 11, 8, and 6 hours) as an additional covariate, with sampling over 24 hours as the reference value.
All statistical analyses were performed using SAS 9.2 (Cary, NC). A p-value of less than 0.05 was considered significant for all evaluations.
RESULTS
Patient population
Patient pre-transplant demographics and HCT characteristics are described in Table 1. All patients had hematologic diseases or malignancies. The mean age was 50.5 ± 11.0 years (range: 19.1 – 65.5). The actual body weight was 80.3 ± 20.2 kg (range: 45.2 – 170.7), and AIBW was 68.2 ± 10.9 kg (range: 47.8 – 103.5). Sixty percent (52 of 87) of the patients were male. The majority received either TBU and fludarabine ± rATG (51.7%) or cyclophosphamide followed by TBU (48.3%) as HCT conditioning. Fludarabine was followed by TBU in 29 patients and TBU was given concurrently with fludarabine in 16 patients. No other antineoplastic agents or irradiation was given immediately before or concomitantly with TBU. All patients received daily IV busulfan, which was targeted to various Css. All patients had a narrow desired busulfan Css range, between 800 to 1000 ng/mL (Table 1).
Table 1.
Description of patient populationa
Legend: TBU = targeted busulfan; rATG = rabbit anti-thymocyte globulin; Css = concentration at steady state.
| Characteristic | |
|---|---|
| Number | 87 |
| Male | 52 (59.8%) |
| Age (years) | 50.5±11.0 |
| Actual body weight (kg) | 80.3±20.2 |
| Adjusted ideal body weight (kg) | 68.2±10.9 |
| Conditioning regimen | |
| Cyclophosphamide/TBU | 42 (48.3%) |
| TBU+ fludarabine ± rATG | |
| Fludarabine followed by TBU | 29 (33.3%) |
| TBU concurrent with fludarabine | 16 (18.4%) |
| Desired Css (ng/mL) | |
| 800–900 | 43 (49.4%) |
| 800–1000 | 38 (43.7%) |
| To 900 | 5 (5.7%) |
| 950–1000 | 1 (1.1%) |
| Diagnosis | |
| Acute myeloid leukemia | 22 (25.3%) |
| Myelofibrosis | 22 (25.3%) |
| Myelodysplastic syndrome | 21 (24.1%) |
| Chronic myelomonocytic leukemia | 9 (10.3%) |
| Acute myeloid leukemia/myelodysplastic syndrome | 7 (8.0%) |
| Chronic myeloid leukemia | 2 (2.3%) |
| Polycythemia vera | 2 (2.3%) |
| Agnogenic myeloid metaplasia | 1 (1.1%) |
| Chronic lymphocytic leukemia | 1 (1.1%) |
Data shown in n (%) or mean + standard deviation (SD).
Initial dosing weight and success with achieving desired busulfan Css
Initially, a daily IV busulfan dose of 3.2 mg/kg was administered for dose 1. An interim analysis demonstrated that few patients achieved a busulfan Css of 800–1000 ng/ml with this dose and the first daily IV busulfan dose was raised to 4 mg/kg. This higher dose of 4 mg/kg has been shown to achieve a busulfan Css of 800–1000 ng/ml in a larger percentage of patients.2 Sixty-two of the 87 patients received 4 mg/kg for dose 1. The ability to achieve the desired Css with this higher dose 1 and TBU using therapeutic drug monitoring is reported in Table 2. After dose 1, the desired Css was achieved in 22.6% (14 of 62); 37.1% (23 of 62) were below their desired Css; and 40.3% (25 of 62) above their desired Css. No patients required a dose change of greater than 50% to attain the target Css.
Table 2.
Success of targeting of daily IV busulfan with pharmacokinetic sampling over 8 hours after dose 1, 2, and 3.
Legend: AIBW = adjusted ideal body weight; Css = concentration at steady-state.
| Dose 1 | Dose 2 | Dose 3 | |
|---|---|---|---|
| Dose based on | 4 mg/kg AIBW | Dose 1 clearance | Dose 2 clearance |
| Number evaluable | 62 | 61 | 61 |
| Average Css (ng/mL) | 903 ± 169 | 873 ± 110 | 872 ± 111 |
| Coefficient of variation in Css | 18.7% | 12.6% | 12.8% |
| Success of targeting, as number (%) of patients achieving desired Css: | |||
| Overall population | |||
| Achieved targeta | 14 (22.6%) | 45 (73.8%) | 52 (85.2%) |
| Below targeta | 23 (37.1%) | 6 (9.8%) | 3 (4.9%) |
| Above targeta | 25 (40.3%) | 10 (16.4%) | 6 (9.8%) |
| 800–900 ng/mL | 43 | 42 | 42 |
| Achieved target | 7 (16.3%) | 29 (69.0%) | 37 (88.1%) |
| Below target | 16 (37.2%) | 6 (14.3%) | 2 (4.8%) |
| Above target | 20 (46.5%) | 7 (16.7%) | 3 (7.1%) |
| 800–1000 ng/mL | 15 | 15 | 15 |
| Achieved target | 7 (46.7%) | 13 (86.7%) | 13 (86.7%) |
| Below target | 5 (33.3%) | 0 | 0 |
| Above target | 3 (20.0%) | 2 (13.3%) | 2 (13.3%) |
| To 900 ng/mL (±5%) | 4 | 4 | 4 |
| Achieved target | 0 | 3 (75.0%) | 2 (50.0%) |
| Below target | 2 (50.0%) | 0 | 1 (25.0%) |
| Above target | 2 (50.0%) | 1 (25.0%) | 1 (25.0%) |
Data shown in number (%) or mean ± standard deviation.
We also compared using the different body metrics of AIBW, actual body weight, and BSA to calculate the initial daily IV busulfan dose. In the 62 patients receiving 4 mg/kg by AIBW for dose 1, the median (range) amount of busulfan administered for dose 1 was 3.6 (range: 2.3 – 4.0) mg/kg by actual body weight and 143 (range: 122 – 159) mg/m2 by BSA. Initial busulfan clearances at dose 1 by different weight metrics and body mass index-based weight category are shown in Table 3. Clearance was significantly different between the different BMI weight categories when normalized by actual body weight (p=0.001), but not when normalized by AIBW (p=0.236) or BSA (p=0.822).
Table 3.
Initial busulfan clearance at dose 1 by different weight metrics and body mass index-based weight category
| Underweight | Normal | Obese | Severely Obese | p-value | |
|---|---|---|---|---|---|
| Number, N (%) | 0 (0) | 49 (56.3) | 32 (36.8) | 6 (6.9) | --- |
| mL/min/kg AIBWa | ND | 3.18 ±0.68 (1.85–5.23) |
3.19 ±0.55 (2.17–4.30) |
3.64 ± 0.60 (2.70–4.48) |
0.236 |
| mL/min/kg ABWa | ND | 3.01 ±0.62 (1.86–4.55) |
2.62 ± 0.46 (1.60–3.76) |
2.35 ± 0.46 (1.71 –2.98) |
0.001 |
| mL/min/m2 BSAa | ND | 114 ±24 (69–177) |
113 ± 19 (71 –158) |
119 ± 23 (85–142) |
0.822 |
Legend: Data shown as mean ± SD (range); AIBW = adjusted ideal body weight; ABW = actual body weight; BSA = body surface area; ND = no data
Therapeutic drug monitoring after dose 1 substantially increased the number of patients within target range, both in the overall population with various desired Css and by the specific Css ranges. For example, in the overall population, therapeutic drug monitoring after dose 1 led to an additional 51.2% of patients achieving the desired Css, with 22.6% within target after dose 1 (i.e., 4 mg/kg) and 73.8% within target after dose 2 (i.e., daily IV TBU based on therapeutic drug monitoring after dose 1). Therapeutic drug monitoring after dose 3 further increased the number of patients within target range, with the target Css being achieved in 85.2% of the population.
Busulfan pharmacokinetics
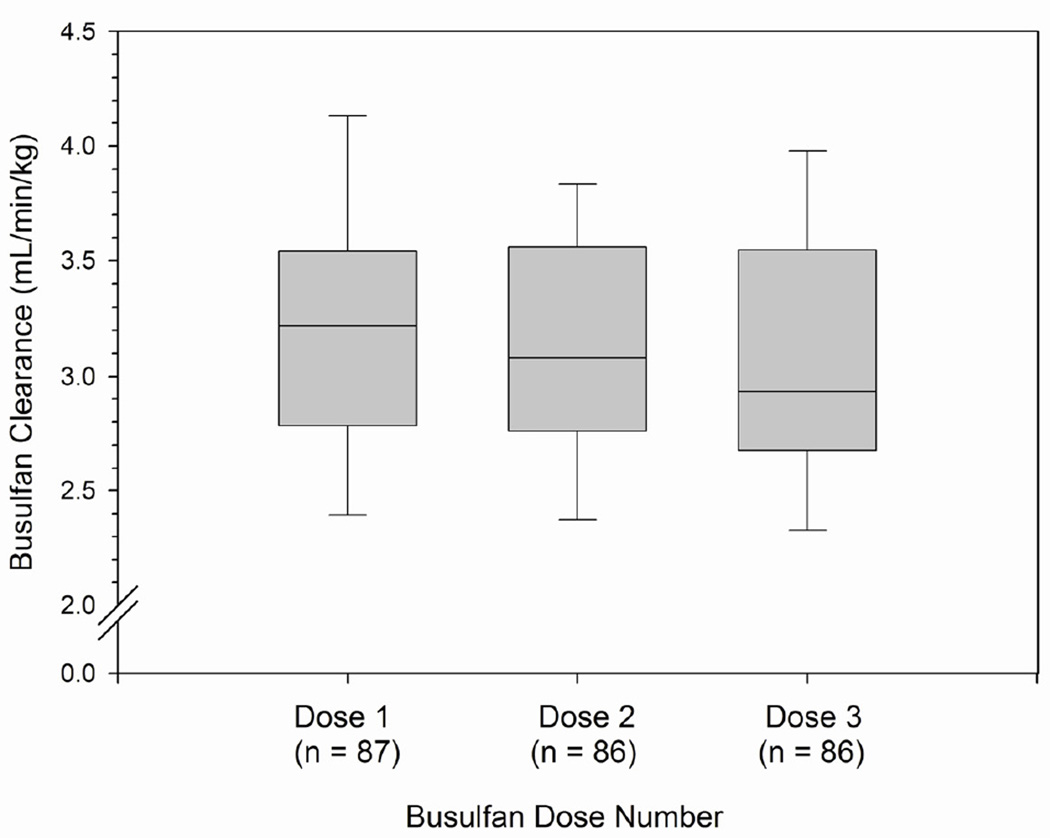
After dose 1, 2 and 3, the average (± SD, range) clearance was 3.22 ± 0.63 (range: 1.85 – 5.23), 3.12 ± 0.57 (range: 1.71 – 4.49), and 3.07 ± 0.60 (range: 1.82 – 4.63), respectively. The data are also presented as box and whisker plots of busulfan clearance (ml/min/kg of AIBW) after doses 1, 2, and 3 in Figure 1. The interpatient variability in busulfan clearance, represented by coefficient of variation (SD/mean), ranged from 18.2% to 19.6%.
Figure 1.
Median daily IV busulfan clearance (ml/min/kg of AIBW) after doses 1, 2, and 3. Legend: Box and whiskers represent 25th and 75th percentiles (interquartile range) and 10th and 90th percentiles, respectively. AIBW = adjusted ideal body weight.
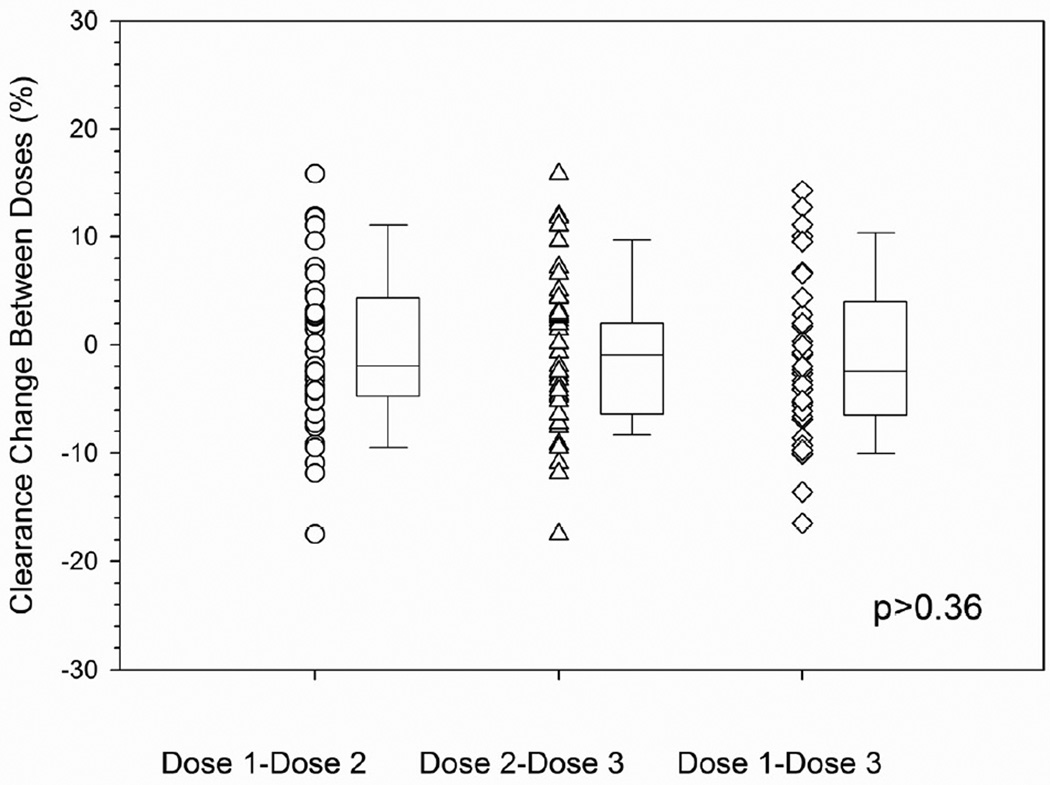
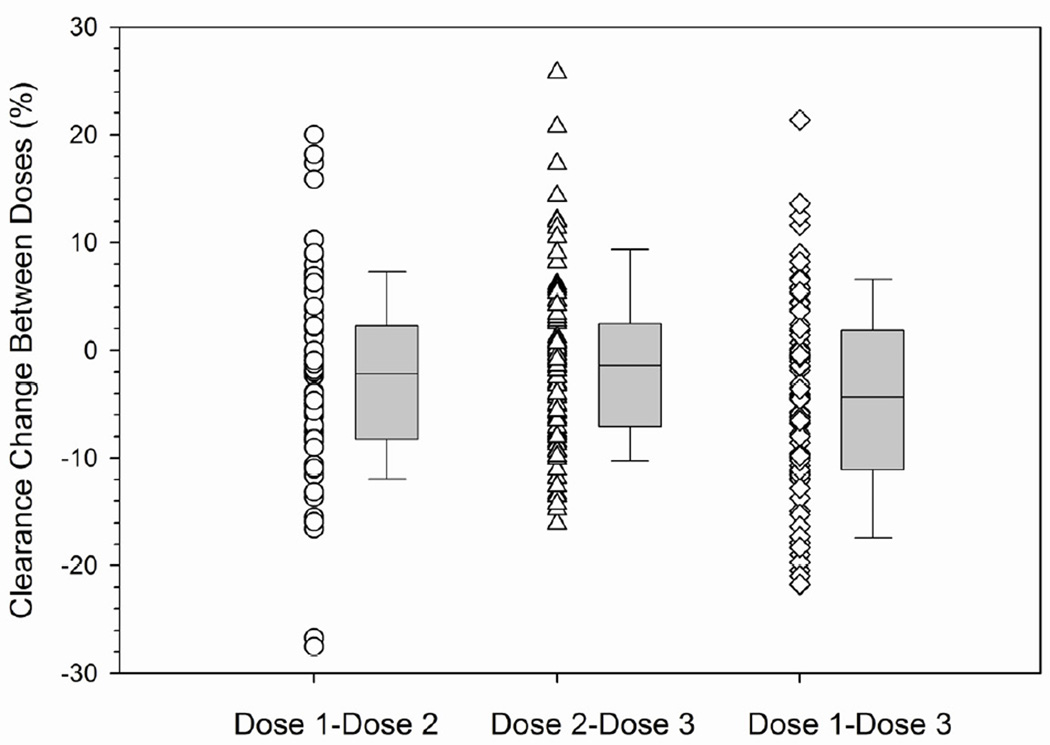
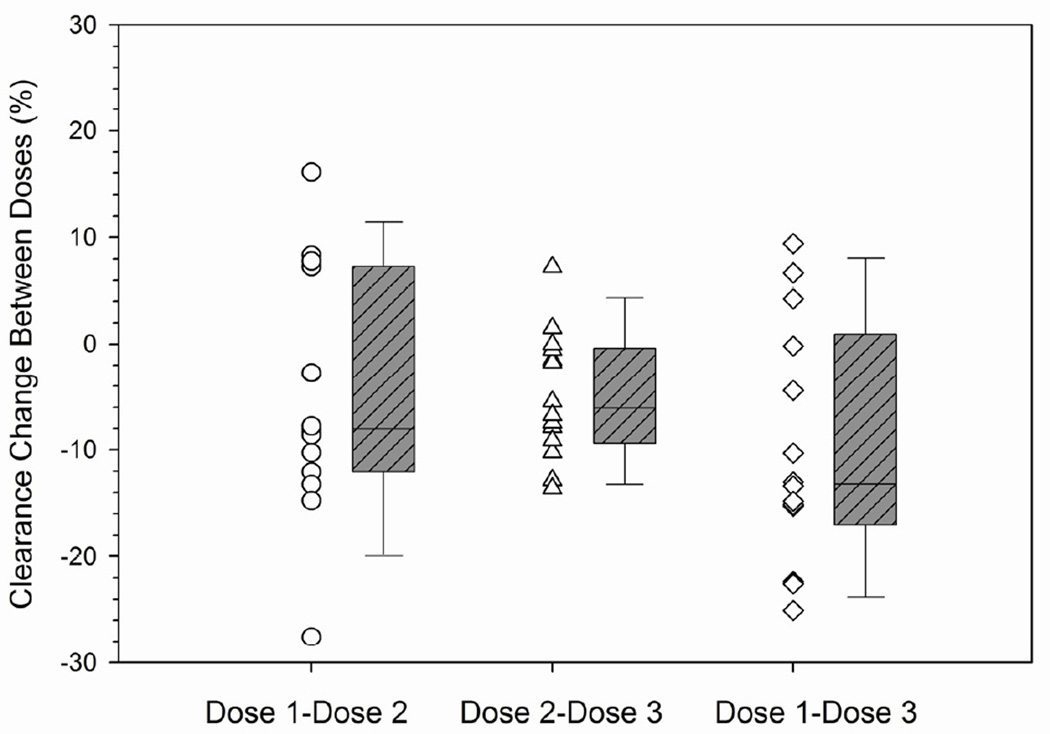
The interdose or within-patient variability was also characterized to help guide how many days of therapeutic drug monitoring are needed for daily IV TBU in future studies. Therapeutic drug monitoring was not conducted after dose 2 in 3 patients and after dose 3 in 11 patients. Therefore, interdose variability of busulfan clearance could be calculated in 78 patients. Busulfan clearance was calculated using 1-compartment modeling of pharmacokinetic data obtained over 24 hours. The interdose coefficient of variation in busulfan clearance was an average 6.0% (range: 1.3% – 17.6%). The change in busulfan clearance between doses is shown in Table 4 and Figure 2. The majority of patients (i.e., 49 of 76, 64.5%) had a minimal (i.e., ≤10%) change in clearance from dose 1 to dose 3. A greater than 10% change was chosen as potentially clinically significant in consideration of the narrow target Css of this patient population. Twenty-one patients (27.6%) had a >10% decrease in busulfan clearance and 6 patients (7.9%) had a >10% increase in busulfan clearance.
Table 4.
Dose-to-dose variability of daily IV busulfan clearance (CL)a,c
Legend: TBU = targeted busulfan; AIBW = adjusted ideal body weight.
| Dose 1 to 2 | Dose 2 to 3 | Dose 1 to 3 | |
|---|---|---|---|
| All patients | |||
| Number (N) | 84b | 76b | 76b |
| % Change in CL | −3.1 ±8.7 | −1.4 ±8.0 | −4.6 ± 9.4 |
| (−27.6 to+16.7%) | (−15.4 to+24.1%) | (−25.1 to+16.1%) | |
| >10% increase | 6 (7.1) | 6 (7.9) | 6 (7.9) |
| Minimal (≤10%) change | 61 (72.6) | 61 (80.3) | 49 (64.5) |
| >10% decrease | 17 (20.2) | 9 (11.8) | 21 (27.6) |
| Cyclophosphamide followed byTBU | |||
| N | 40 | 37 | 37 |
| % Change in CL, | −0.6 ±7.3 | −0.3 ±7.5 | −1.0 ±7.6 |
| mean ± SD (range) | (−17.5 to+15.9%) | (−10.2 to+24.1%) | (−16.5% to+14.2%) |
| >10% increase | 4 (10.0) | 4 (10.8) | 4 (10.8) |
| ≤10% change | 33 (82.5) | 31 (83.8) | 30 (81.1) |
| >10% decrease | 3 (7.5) | 2 (5.4) | 3 (8.1) |
| Fludarabine, then TBU | |||
| N | 29 | 25 | 25 |
| % Change in CL | −5.6 ±8.3 | −1.1 ±9.3 | −7.1 ±9.1 |
| (−24.4 to+16.7%) | (−15.4 to+20.5%) | (−22.1 to+16.1%) | |
| >10% increase | 1 (3.4%) | 2 (8.0%) | 2 (8.0%) |
| Minimal (≤10%) change | 19 (65.5%) | 19 (76.0%) | 14 (56.0%) |
| >10% decrease | 9 (31.0%) | 4 (16.0%) | 9 (36.0%) |
| Fludarabine concurrent withTBU | |||
| N | 15 | 14 | 14 |
| % Change in CL | −5.1 ±11.1 | −4.9 ±5.9 | −9.7 ±11.2 |
| (−27.6 to+16.1%) | (−13.6 to+7.2%) | (−25.1 to+9.4%) | |
| >10% increase | 1 (6.7%) | 0 | 0 |
| Minimal (≤10%) change | 9 (60.0%) | 11 (78.6%) | 5 (35.7%) |
| >10% decrease | 5 (33.3%) | 3(21.4%) | 9 (64.3%) |
Clearance as ml/min/kg of AIBW;
Data not available in 3 patients after dose 2 and 11 patients after dose 3;
Data presented as N (%) or mean ± SD (range).
Figure 2.
Minimal interdose variability in daily IV busulfan clearancea by conditioning regimen.
aInterdose busulfan clearance (ml/min/kg of AIBW) calculated as [(CLdose 2 – CLdose 1)/CLdose1] * 100% (see statistical methods for additional details). Box and whiskers represent 25th and 75th percentiles (interquartile range) and 10th and 90th percentiles, respectively. TBU = targeted busulfan; AIBW = adjusted ideal body weight.
A. Cyclophosphamide followed by TBU.
Legend: p>0.36 for all comparisons.
B. Fludarabine followed by TBU.
C. TBU concurrent with fludarabine.
We sought to identify if any clinically available factors were associated with busulfan clearance. Clearance was not associated with sex or gender, but was associated with dosing day and conditioning regimen (p=0.0016). For patients receiving cyclosphosphamide followed by TBU, there was minimal interdose variability in busulfan clearance with the majority of patients (30 of 37, or 81.1%) having ≤10% change in busulfan clearance. There was greater interdose variability in busulfan clearance in those receiving TBU with fludarabine. Specifically, a minimal (≤10%) interdose change in busulfan clearance was observed in 56% (14 of 25) and 35.7% (5 of 14) of patients conditioned with fludarabine followed by TBU or with TBU administered concurrently with fludarabine, respectively. Similar results were found using both the 1-compartment and noncompartmental model with sampling over 8 hours (data not shown).
Differences in busulfan Css and clearance estimates based on sampling duration
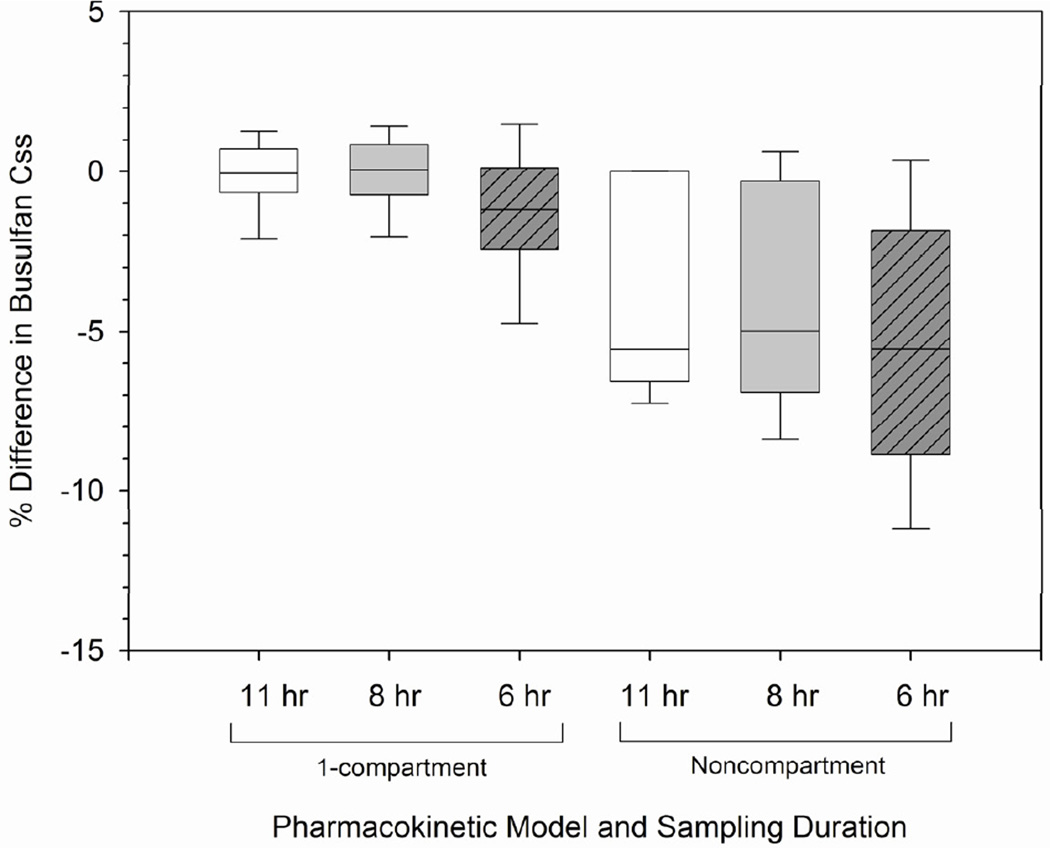
The differences in busulfan Css estimates based on sampling duration are shown in Figure 3. Using both 1-compartmental and noncompartmental modeling, sampling over 8, 11, or 24 hours were comparable (p>0.4), although 1-compartmental Css estimates from sampling over 8 and 11 hours were most similar to 24 hour sampling with mean ± SD differences of −0.18% ± 1.91% and −0.32% ± 1.80%, respectively. Decreased sampling duration over 6 hours was less reliable, with differences in Css estimates ranging from −12.4% to +9.0% (1-compartment; p<0.001) and - 25.7% to +11.8% (noncompartment; p<0.001) compared to sampling over 24 hours.
Figure 3.
Feasibility of shorter pharmacokinetic sampling intervals to estimate Css Legend: Css = concentration at steady-state. Percent difference in Css estimated from pharmacokinetic samples obtained over 24 hours (gold standard) compared to that from shorter time intervals (i.e., 11, 8, and 6 hours). First sample obtained at the end of a 3-hour infusion. Css estimated using either 1-compartment model or noncompartmental analysis. Box and whiskers represent 25th and 75th percentiles (interquartile range) and 10th and 90th percentiles, respectively.
To further evaluate if sampling duration was adequate, we also evaluated the effect of sampling duration on busulfan clearance estimates using time as a covariate. Using the 1-compartment model, differences in clearance estimates were similar to sampling over 11 and 299 8 (p=0.2534), but not 6 hours (p=0.03). Using noncompartmental modeling, clearance was significantly affected by sampling duration (p<0.0001), and sampling over 6 hours was significantly different compared to sampling durations of 8, 11, and 24 hours (p<0.01).
DISCUSSION
There are several key findings of this retrospective analysis of adults receiving daily IV busulfan as part of various HCT conditioning regimens. First, the desired Css can be achieved with therapeutic drug monitoring over a short enough time interval (i.e., 8 hours) to personalize the next dose of daily IV busulfan. Second, there is minimal dose-to-dose (i.e., interdose) variability of busulfan clearance, which could reduce the number of doses that need therapeutic drug monitoring relative to oral busulfan. Finally, 8 hours is the minimal sampling duration to accurately target busulfan Css with the current pharmacokinetic modeling technique of analyzing individual patient busulfan concentration-time data.
Rapid achievement of the desired busulfan Css is critical for improving clinical outcomes with therapeutic drug monitoring of busulfan.10 Therefore, it is important to choose the optimal initial daily IV busulfan dose which is based on AIBW. Dosing based on AIBW is optimal over a population with a wide range of BMI categories because busulfan clearance significantly varies among different BMI categories (i.e. underweight, normal, obese, and severely obese) when expressed relative to the actual body weight. The differences are minimized when busulfan clearance is normalized by AIBW and/or BSA.7, 8 Therefore, dosing IV busulfan by AIBW or BSA is optimal as they are associated with less interpatient variability in busulfan clearance, and in turn, as is the resulting busulfan Css.
In patients whose desired Css is 800–1000 ng/ml, the daily IV busulfan dose of 4 mg/kg led to a higher percentage of patients (63%) achieving the desired Css than those receiving 3.2 mg/kg (11%).2 Furthermore, fewer patients needed large (i.e., >50%) modifications in the daily IV busulfan dose when the initial dose was 4 mg/kg. The subsequent daily IV busulfan doses were targeted using therapeutic drug monitoring. The majority of the population achieved the desired Css with sampling over 8 hours using pharmacokinetic modeling of an individual patient’s concentration-time data. This allowed for dose 2, 3 and 4 to be personalized to achieve the desired Css. Daily IV busulfan dose targeting was conducted as part of studies administering cyclophosphamide (CY) before daily IV busulfan (i.e., CY/TBU)11 and administering daily IV BU combined with the nucleoside analog, fludarabine in hopes of continued improved outcomes with busulfan-containing regimens.12, 13 These desired Css ranges were chosen based on our historic experience targeting oral busulfan, with 800–900 ng/ml being the desired Css for CY/TBU14 and 800–1000 ng/ml being the desired Css for fludarabine/TBU.13
Interindividual variability of busulfan clearance was 19%, which agrees with previous reports ranging from 16% to 34%.2, 15–19 With daily IV busulfan administration, busulfan clearance decreased minimally (average of −4.6%) from dose 1 to dose 3. However, in the limited population of 15 patients who received TBU concomitant with fludarabine, busulfan clearance progressively decreased from dose 1 to 3 (average of −9.7%). This decreased clearance would lead to a slight increase in busulfan Css during a 4-day course of daily IV busulfan, with unclear clinical significance. Healthcare practitioners who conduct therapeutic drug monitoring in patients conditioned with TBU concomitant with fludarabine should target the daily IV busulfan dose to the lower portion of their desired Css range to compensate for this decreased clearance and its resultant higher Css.
Recent pharmacodynamic analyses suggest that a busulfan Css greater than 1026 ng/mL is associated with higher non-relapse mortality in patients receiving daily IV busulfan with total body irradiation and concomitant fludarabine.17 Many studies of fludarabine/ busulfan regimens incorporate therapeutic drug monitoring,5, 13 and thus daily IV TBU is expected to continue. Achieving a narrow desired busulfan Css with therapeutic drug monitoring should also be balanced with the expense of an inpatient admission, which is necessitated when the pharmacokinetic sampling duration is 8 hours or longer. In this analysis, clearance estimates were similar with pharmacokinetic sampling over 8, 11 and 24 hours from the beginning of the 3-hour infusion of daily IV busulfan. However, a 6-hour sampling duration resulted in more variable Css estimates with noncompartmental or 1-compartmental modeling of individual concentration-time data (Figure 3). Of note, no pharmacokinetic samples were drawn during the daily IV busulfan infusion. Accurate estimates of daily IV busulfan clearance can be achieved with a 6-hour sampling duration if post-Bayesian estimates are obtained by incorporating an individual patient’s concentration-time data into a population pharmacokinetic model.8 Bayesian pharmacokinetic modeling can be conducted using an individual patient’s concentration-time data with a population pharmacokinetic model to estimate that individual’s busulfan clearance.20, 21 This tool can reduce the sampling duration to 6 hours, but prospective validation is needed.8 A Bayesian approach has also successfully been used with therapeutic drug monitoring of cyclophosphamide in HCT recipients22, 23 and mycophenolate mofetil in renal transplant recipients.24
In summary, an initial daily IV busulfan dose of 4 mg/kg with therapeutic drug monitoring can successfully achieve the desired Css, even over narrow Css ranges. Therapeutic drug monitoring of daily IV BU should be conducted by sampling over a minimum of 8 hours after the start of infusion, following at least the initial dose due to minimal dose-to-dose variability of busulfan clearance in most patients. As more centers implement therapeutic drug monitoring of daily IV busulfan to improve outcomes, prospective validation of a Bayesian population pharmacokinetic approach should allow for a transition to a more convenient and economical outpatient sampling schedule.
Acknowledgments
Supported in part by grants HL036444, CA076930, and CA15704, NIH, Bethesda, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCune JS, Gibbs JP, Slattery JT. Plasma concentration monitoring of busulfan: does it improve clinical outcome? Clin Pharmacokinet. 2000;39(2):155–165. doi: 10.2165/00003088-200039020-00005. [DOI] [PubMed] [Google Scholar]
- 2.McCune JS, Holmberg LA. Busulfan in hematopoietic stem cell transplant setting. Expert Opin Drug Metab Toxicol. 2009;5(8):957–969. doi: 10.1517/17425250903107764. [DOI] [PubMed] [Google Scholar]
- 3.Slattery JT, Risler LJ. Therapeutic monitoring of busulfan in hematopoietic stem cell transplantation. Ther Drug Monit. 1998;20(5):543–549. doi: 10.1097/00007691-199810000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Kletzel M, Jacobsohn D, Duerst R. Pharmacokinetics of a test dose of intravenous busulfan guide dose modifications to achieve an optimal area under the curve of a single daily dose of intravenous busulfan in children undergoing a reduced-intensity conditioning regimen with hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12(4):472–479. doi: 10.1016/j.bbmt.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 5.Pidala J, Kim J, Anasetti C, et al. Pharmacokinetic targeting of intravenous busulfan reduces conditioning regimen related toxicity following allogeneic hematopoietic cell transplantation for acute myelogenous leukemia. J Hematol Oncol. 3:36. doi: 10.1186/1756-8722-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pai MP, Paloucek FP. The origin of the"ideal" body weight equations. Ann Pharmacother. 2000;34(9):1066–1069. doi: 10.1345/aph.19381. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen L, Leger F, Lennon S, Puozzo C. Intravenous busulfan in adults prior to haematopoietic stem cell transplantation: a population pharmacokinetic study. Cancer Chemother Pharmacol. 2006;57(2):191–198. doi: 10.1007/s00280-005-0029-0. [DOI] [PubMed] [Google Scholar]
- 8.Salinger DH, Vicini P, Blough DK, O'Donnell PV, Pawlikowski MA, McCune JS. Development of a population pharmacokinetics-based sampling schedule to target daily intravenous busulfan for outpatient clinic administration. J Clin Pharmacol. 2010;50(11):1292–1300. doi: 10.1177/0091270009357430. [DOI] [PubMed] [Google Scholar]
- 9.Gibbs JP, Gooley T, Corneau B, et al. The impact of obesity and disease on busulfan oral clearance in adults. Blood. 1999;93(12):4436–4440. [PubMed] [Google Scholar]
- 10.Dix SP, Wingard JR, Mullins RE, et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant. 1996;17(2):225–230. [PubMed] [Google Scholar]
- 11.Rezvani A, Storer B, McCune J, Batchelder A, McDonald G, Deeg H. Poster presentation at American Society of Hematology. New Orleans, LA: 2009. Low Toxicity and Non-Relapse Mortality with Reversed-Order Conditioning (Cyclophosphamide Followed by Busulfan) in Allogeneic Hematopoietic Cell Transplantation. 2009. p. Abstract #1175. [Google Scholar]
- 12.Russell JA, Tran HT, Quinlan D, et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002;8(9):468–476. doi: 10.1053/bbmt.2002.v8.pm12374451. [DOI] [PubMed] [Google Scholar]
- 13.Bornhauser M, Storer B, Slattery JT, et al. Conditioning with fludarabine and targeted busulfan for transplantation of allogeneic hematopoietic stem cells. Blood. 2003;102(3):820–826. doi: 10.1182/blood-2002-11-3567. [DOI] [PubMed] [Google Scholar]
- 14.Scott BL, Sandmaier BM, Storer B, et al. Myeloablative vs nonmyeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous leukemia with multilineage dysplasia: a retrospective analysis. Leukemia. 2006;20(1):128–135. doi: 10.1038/sj.leu.2404010. [DOI] [PubMed] [Google Scholar]
- 15.Andersson BS, Madden T, Tran HT, et al. Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as pretransplantation conditioning therapy: a phase I study. Biol Blood Marrow Transplant. 2000;6(5A):548–554. doi: 10.1016/s1083-8791(00)70064-4. [DOI] [PubMed] [Google Scholar]
- 16.Madden T, de Lima M, Thapar N, et al. Pharmacokinetics of once-daily IV busulfan as part of pretransplantation preparative regimens: a comparison with an every 6-hour dosing schedule. Biol Blood Marrow Transplant. 2007;13(1):56–64. doi: 10.1016/j.bbmt.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 17.Geddes M, Kangarloo SB, Naveed F, et al. High busulfan exposure is associated with worse outcomes in a daily i.v. busulfan and fludarabine allogeneic transplant regimen. Biol Blood Marrow Transplant. 2008;14(2):220–228. doi: 10.1016/j.bbmt.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Ryu SG, Lee JH, Choi SJ, et al. Randomized comparison of four-times-daily versus once426 daily intravenous busulfan in conditioning therapy for hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2007;13(9):1095–1105. doi: 10.1016/j.bbmt.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Almog S, Kurnik D, Shimoni A, et al. Linearity and stability of intravenous busulfan pharmacokinetics and the role of glutathione in busulfan elimination. Biol Blood Marrow Transplant. 2010 doi: 10.1016/j.bbmt.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Bleyzac N, Souillet G, Magron P, et al. Improved clinical outcome of paediatric bone marrow recipients using a test dose and Bayesian pharmacokinetic individualization of busulfan dosage regimens. Bone Marrow Transplant. 2001;28(8):743–751. doi: 10.1038/sj.bmt.1703207. [DOI] [PubMed] [Google Scholar]
- 21.Bertholle-Bonnet V, Bleyzac N, Galambrun C, et al. Influence of underlying disease on busulfan disposition in pediatric bone marrow transplant recipients: a nonparametric population pharmacokinetic study. Ther Drug Monit. 2007;29(2):177–184. doi: 10.1097/FTD.0b013e318039b478. [DOI] [PubMed] [Google Scholar]
- 22.Salinger DH, McCune JS, Ren AG, et al. Real-time dose adjustment of cyclophosphamide in a preparative regimen for hematopoietic cell transplant: a Bayesian pharmacokinetic approach. Clin Cancer Res. 2006;12(16):4888–4898. doi: 10.1158/1078-0432.CCR-05-2079. [DOI] [PubMed] [Google Scholar]
- 23.McCune JS, Batchelder A, Guthrie KA, et al. Personalized Dosing of Cyclophosphamide in the Total Body Irradiation-Cyclophosphamide Conditioning Regimen: A Phase II Trial in Patients With Hematologic Malignancy. Clin Pharmacol Ther. 2009;85(6):615–622. doi: 10.1038/clpt.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Meur Y, Buchler M, Thierry A, et al. Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am J Transplant. 2007;7(11):2496–2503. doi: 10.1111/j.1600-6143.2007.01983.x. [DOI] [PubMed] [Google Scholar]