Abstract
The most common forms of dystonia are those that develop in adults and affect a relatively isolated region of the body. Although these adult-onset focal dystonias are most prevalent, knowledge of their etiologies and pathogenesis has lagged behind some of the rarer generalized dystonias, where the identification of genetic defects has facilitated both basic and clinical research. This summary provides a brief review of the clinical manifestations of the adult-onset focal dystonias, focussing attention on less well-understood clinical manifestations that need further study. It also provides a simple conceptual model for the similarities and differences among the different adult-onset focal dystonias, as a rationale for lumping them together as a class of disorders while at the same time splitting them into subtypes. The concluding section outlines some of the most important research questions for the future. Answers to these questions are critical for advancing our understanding of this group of disorders, and for developing novel therapeutics.
INTRODUCTION
The dystonias are a group of disorders with many different clinical manifestations, but certain features in common.1–3 The shared features are involuntary sustained or intermittent muscle contractions causing abnormal postures and/or repetitive movements. Dystonic movements often are patterned or twisting, but a tremor-like movement occasionally predominates. The most common forms are the adult-onset focal dystonias (AOFD), involving the neck (cervical dystonia, CD), upper face (blepharospasm, BL) mouth and jaw (oromandibular, OMD), larynx (LD), or a limb (e.g. writer’s cramp and other focal hand dystonias).
Many prior reviews have addressed typical clinical aspects and treatment of AOFD.4–15 In this summary we focus instead on some of the less well-characterized features and important unanswered questions for research. The first part addresses issues relevant to specific AOFD. The second part addresses themes relevant to all, including a conceptual framework for interpreting similarities and differences. The final part addresses the most pressing research questions that have emerged from a series of international meetings.
ADULT-ONSET FOCAL DYSTONIAS
Cervical Dystonia (CD)
Clinical features
CD is characterized by excessive involuntary activity of neck muscles leading to abnormal movements of the head and neck pain. Several overlapping manifestations have been described.16–18 The head may turn to the right or left (torticollis), it may tilt to one side (laterocollis), or it may tilt upwards (retrocollis) or downwards (anterocollis). In addition to abnormal head positions, there may be tremulous movements or spasmodic jerking of the head.19–25
Etiology & Pathogenesis
There is increasing evidence that CD is etiologically heterogeneous.26, 27 Early clues for this heterogeneity came from recognition that certain manifestations are associated with specific diseases. For example, predominant anterocollis or retrocollis seem more common in Parkinson’s disease or other parkinsonian syndromes than in idiopathic CD, although formal studies comparing different populations are lacking.28, 29 Similarly, fixed manifestations are uncommon for idiopathic CD and more often are due to syrinx or psychogenic causes.30–32
Genetic etiologies have received considerable attention because of the existence of rare families with multiple affected members. Additionally, CD may occur among patients carrying defects in genes more commonly associated with generalized dystonia such as the TOR1A gene at the DYT1 locus33, 34 or the THAP1 gene at the DYT6 locus.35 Three new candidate genes for CD recently have been identified by exome sequencing: CIZ1,36 GNAL,37, 38 and ANO3.39 The last gene appears to be associated with tremulous CD, illustrating the importance of clinical subtypes. Although these genes account for relatively few cases, a genetic basis may explain many sporadic cases, as 10–20% of patients have at least one family member with AOFD.40–42
There are also important non-genetic causes. For example, exposure to dopamine receptor antagonists causes both acute dystonic reactions and tardive syndromes with prominent dystonia.43, 44 There also are associations with trauma45, 46 or autoimmune disease,47 although causal links have not been established. Finally, CD may result from focal lesions.48 These apparently acquired forms of CD may result from non-genetic mechanisms, or they may reflect combination of an acquired insult and genetic predisposition.8, 49
The exact areas of the nervous system responsible for causing CD remain uncertain.50–53 CD may be secondary to local brain injury, and lesions have been identified in many regions.48 In idiopathic CD where focal lesions are absent, voxel-based morphometry (VBM) also has revealed abnormalities in many regions.54–58 Functional imaging of regional blood flow with positron-emission tomography (PET) or metabolic mapping with fluorodeoxyglucose PET have pointed to different regions as well.59–61
Among the regions identified by imaging, most studies have revealed no obvious histopathological abnormalities.62, 63 However, the numbers of brains studied has been small, and the methods applied not suitable for detecting subtle abnormalities. Two recent studies revealed subtle abnormalities in the cerebellum. One revealed low Purkinje neuron densities in CD,64 and another revealed Purkinje neuron loss and torpedo bodies to be more prominent in CD with tremor compared to tremor alone.65
Therapeutic challenges
Injections of botulinum toxin provide relief from abnormal movements and pain.13, 66–68 Resistance may develop with long term use, but appears uncommon with modern preparations of the toxin.69, 70 The main drawbacks include the requirement for injections every 3–4 months, and occasional transient side effects such as dysphagia or head drop. There also is increasing recognition of the need for higher doses over time and low levels of satisfaction among some patients.71, 72
Although it usually is possible to achieve good therapeutic outcomes with botulinum toxin, a few subtypes seem more challenging. Patients with predominant anterocollis are more difficult to treat,28, 29, 73 possibly due to contraction of the deep pre-vertebral paraspinal muscles that cannot be reached without radiographic guidance.74, 75 Even with guidance, many patients with anterocollis still fail to respond, so the reasons for poor outcomes in this subgroup remain unclear.
The use of botulinum toxins has substantially reduced the numbers of patients undergoing surgical procedures such as peripheral denervation, although surgery is still useful in some cases.76 Deep brain stimulation (DBS) has gained increasingly popularity because of proven and long-term efficacy in generalized primary dystonias, and some secondary dystonias.77–80 It is gaining increasing popularity for AOFD,81, 82 although patient selection criteria are not well established.
The potential benefits of physical therapy are not well characterized.68 Many patients request it, and many providers recommend it, because it seems intuitively useful. However, optimal methods have not been systematically studied, with different therapists each promoting different strategies and techniques based on personal experience.83–86 There are no large-scale blinded studies comparing strategies or demonstrating efficacy. In fact, some of the most objective studies have failed to demonstrate benefit.84 Whether this reflects the futility of physical therapy, the use of inappropriate methods, or insensitive outcome measures remains unknown.
Blepharospasm (BL)
Clinical features
BL is characterized by involuntary spasms of the orbicularis oculi muscles. There are several overlapping phenotypes.87–90 The most characteristic is prolonged spasms of eye closure. Another manifestation is frequent blinking, which may be isolated or associated with spasms. Increased blinking often precedes the development of tonic spasms, but isolated increased blinking also may be a distinct disorder.91 Discriminating normal blinks from blinks of blepharospasm is challenging because criteria discriminating a blink versus a short spasm have not been established.
Another phenotype is failure to voluntarily open the eyes with no apparent spasm of the orbicularis oculi. This phenomenon is sometimes called “apraxia” of eyelid opening. It may occur in patients who have had more obvious orbicularis oculi spasms, it may occur with other disorders, or it may occur in isolation. In some cases, it may be a manifestation of dystonia due to contraction of the pretarsal portion of the orbicularis oculi, antagonizing eyelid opening.92, 93 However, “apraxia” is more properly due to failure of levator contraction, and therefore it is not considered dystonic.94
Etiology & Pathogenesis
The etiology and pathogenesis of BL is multifactorial. Epidemiological studies suggest a genetic component.8 BL may occur in the setting of generalized or segmental dystonia associated with some of genes described above for CD, but no genes for isolated BL have been found. Since there is considerable phenotypic overlap between familial and sporadic BL, many cases of sporadic BL may have a genetic influence.95
Any model for pathogenesis should take into account several non-genetic factors. BL is associated with focal lesions in the nervous system,96 exposure to certain drugs,43, 97 and degenerative disorders.98 Eye disorders, particularly dry eye, commonly precede BL.99, 100 Coffee appears to have a protective effect, although a mechanistic explanation has not been established.99
Another common feature of BL is photophobia, which might also be called photo-oculodynia since it refers to pain induced by light. Some wavelengths of light are worse than others.101, 102 The intrinsically photosensitive retinal ganglion cells which contain melanopsin, a light-sensitive pigment, might be the mediator.103 Melanopsin has a peak absorption in the blue area, and may explain observations that blue light is most uncomfortable. Photophobia has been reported in blind patients, so it may not depend on the pathway that leads to conscious vision. A portion of the melanopsin cells project directly to the thalamic nuclei (posterior, lateral posterior, intergeniculate) that are responsive to pain.104 A potentially relevant finding is one 18-fluorodeoxyglucose PET study showing that BL patients with photophobia compared to patients without photophobia had hypermetabolism of the ventral anterior and ventral lateral thalamus.105 Whether the melanopsin pathway is affected in BL remains to be established.
The functional anatomy of BL also remains unclear.50 BL rarely can result from focal lesions, and presumably causative lesions have been identified in several areas.106 Several regions also have been identified by VBM, PET, and functional MRI (fMRI).58, 105, 107–115
Physiological studies have shown hyperexcitability of the blink reflex in BL. More recently, an increase blink reflex plasticity has been found. Pairing high frequency stimulation of the supraorbital nerve with the R2 component of the blink reflex leads to an effect that resembles long term potentiation, and this has been reported to be exaggerated in one study116 and normal in another.117 Additionally, the temporal discrimination threshold is elevated in BL patients.118, 119 All of these features are found also in other AOFD.
Therapeutic challenges
Spasms and blinking generally respond to injections of botulinum toxin.13, 66, 67 The main limitations include the need for injections approximately every 3 months and transient side effects such as ptosis, diplopia, or dry eyes. The phenotype of “apraxia” of eyelid opening is more difficult to treat.120 Some patients respond to injections of botulinum toxin into the pre-tarsal orbicularis oculi,92 and some respond to myectomy.121 Other surgical approaches use methods developed for ptosis repair, including levator shortening and removal of redundant lid tissue. An intriguing possibility involves local injection of bupivacaine into the levator, causing muscle damage, followed by regeneration that might produce muscle shortening and stiffening (A. Scott, personal communication). This strategy did not work for ptosis in patients with chronic progressive external ophthalmoplegia, but may have been due to insufficient doses.122
DBS is another surgical method sometimes used in the treatment of BL.79 This approach is offered when medical therapy fails or when BL is accompanied by dystonia elsewhere. BL responds in some, but not others.82, 123 Curiously, orbicularis oculi spasms and apparent apraxia can be induced by DBS in some patients, where they did not previously exist.124 The reasons for this phenomenon are unclear. For all surgical procedures there are many unresolved questions including patient selection criteria, and safety and durability of benefits.77–80
Another area of therapeutics that has not received much attention involves eyeglasses. Although many patients report benefit from wearing dark glasses, there are few relevant studies. If the melanopsin pathways play an important role, FL-41 tinted glasses (rose colored) that block blue light might be most effective.125
Laryngeal dystonia (LD)
Clinical features
LD encompasses several overlapping clinical phenotypes characterized by abnormal activity of the muscles of the larynx.126–129 The most common subtypes are adductor or abductor spasmodic dysphonia (SD), while less common subtypes include laryngeal breathing dystonia (also known as dystonic respiratory stridor)130–132 and singer’s dystonia.133 In SD, spasms of laryngeal muscles cause intermittent voice breaks. SD is a task-specific dystonia where spasms typically occur during speaking but abate with singing, shouting, crying or whispering. Adductor SD affects 90% of cases and is caused by overactivity of vocal fold adductors, with intermittent hyperadduction leading to an intermittently strained and strangled voice. Spasms tend to occur with vowels, particularly during glottal stops between vowels. Abductor SD is less common, and is caused by overactivity of the abductor muscles, with excessive opening of the vocal folds and prolonged breathy voice breaks. Spasms are most prominent with voiceless consonants (p, t, k, h, s, f) before vowels. Rarely, adductor and abductor SD occur together.
LD may occur with vocal tremor, which is characterized by more regular oscillations of multiple laryngeal muscles.129, 134, 135 SD and tremor may be difficult to discriminate because apparently regular voice breaks in SD may resemble the rhythmic oscillations of tremor. LD is readily confused with another disorder, muscle tension dysphonia (MTD), where there is a general constriction of multiple laryngeal muscles leading to a strained voice that does not vary with different parts of speech.136–138 Isolated MTD has many features atypical for dystonia and can be reversed with voice therapy. However, many patients with LD may have features of MTD, and the exact relationship between LD and MTD remains unclear. MTD may not be a manifestation of dystonia, but rather a behavioral compensation for voice weakness or instability.
Uncertainties regarding diagnostic criteria and distinctions among the subtypes of LD are important challenges.128 SD and other forms of LD are poorly recognized by providers not familiar with the disorder, in part because of the lack of diagnostic criteria. In addition, there are no validated severity scales for LD.
Etiology and pathogenesis
The etiology and pathogenesis of LD is heterogeneous.128 Several observations point to a genetic contribution, although no genes responsible for isolated LD have been found. LD may be a particularly prominent or isolated manifestation of mutations more commonly associated with generalized dystonia, especially the THAP1 gene.139, 140 Recently, mutations in the TUBB4a gene have emerged as a potential cause in some families where generalized dystonia includes prominent laryngeal and lingual dystonia.141–143 The “whispering dysphonia” in these patients is atypical for sporadic SD and may reflect a compensatory adaptation to laryngeal spasms. These families emphasize the importance of phenotypic subtypes within LD. There also is strong epidemiological evidence for non-genetic contributions. Many patients report developing LD following an upper respiratory infection, laryngeal trauma, exposure to a neuroleptic, or periods of high stress.129, 144, 145
The neuroanatomical basis for LD also remains an open question. Any model for pathogenesis should account for difficulties with speaking while sparing closely related tasks of singing, whispering, shouting and crying. PET studies have revealed both increases and decreases in blood flow to several regions of the cerebral cortex in adductor SD.146 Studies involving fMRI during symptomatic and asymptomatic tasks also have pointed to abnormal activation of the cerebral cortex and cerebellum,147, 148 while diffusion tensor imaging (DTI) has revealed white matter defects in or near the basal ganglia, cerebellum and thalamus.149 A VBM study revealed changes in cerbral cortex and cerebellum.150
Treatment challenges
Botulinum toxins are routinely used for treating adductor SD, despite frequent transient adverse effects such as vocal weakness, breathiness, and choking with liquids.126, 151–153 Satisfactory responses are more difficult to achieve in abductor SD. There is only one small, single-site, double-blinded study of botulinum toxins for adductor SD,153, 154 but none for other forms of LD.
Several different surgical approaches also are offered to patients with SD including myectomy targeting the thyroarytenoids, thyroplasty to alter the cartilaginous structure of the larynx, or denervation-reinnervation procedures that cut branches of the recurrent laryngeal nerve to the thyroarytenoid muscles and suture the stump to the ansa cervicalis.128, 155 However, there are no controlled trials addressing safety and efficacy for these procedures.
Limb dystonias
Clinical features
Isolated limb dystonia can occur in adults, most commonly an upper limb. Most are task-specific, where the limb has been used for a repetitive activity for long periods.156 Dystonia can arise with virtually any task. Dystonia may remain task-specific, or it may lose specificity over time. One common form is writer’s cramp. Another is musician’s dystonia among patients who play wind or string instruments, or piano.157, 158 Similar to other AOFD, tremor is common and may predominate.80 Isolated leg dystonias are less common in adults. They may be sporadic,159 or associated with repetitive activities such running marathons.160 Leg dystonia may also be a presenting manifestation of Parkinson disease, emerging before tremor an bradykinesia become apparent.
In addition to repetitive activity, there may be a history of trauma. A controversial entity is the complex regional pain syndrome (CRPS), where approximately half of the patients have relatively fixed dystonic postures. Patients with fixed dystonia are more likely to be psychogenic,31 and many CRPS cases may be psychogenic.30, 46, 161
Etiology and pathogenesis
The cause of most limb dystonias is multifactorial, with environmental factors of repetitive activity and trauma interacting with an inherent predisposition. One predisposing substrate may be a loss of inhibitory processes in the nervous system, along with abnormal plasticity.162 Another important factor is the interaction between the task and the mechanical ability of the limb. If the demand exceeds the ability, compensatory adaptations may result in abnormal motor behavior. This is particularly important in musicians where task demands may be extreme.163
Imaging studies have pointed to different brain regions underlying focal hand dystonias.50 In VBM studies, one frequent finding is an abnormality in the hand area of the sensorimotor cortex.164 In fMRI studies, there frequently are increases in activity of the sensorimotor cortex and decreases in the supplementary motor area, but there also are changes in basal ganglia and cerebellum.
Treatment challenges
Oral drugs are seldom of value. Botulinum toxin injections are useful, but results are variable because of the complex patterns of hand movements, the numbers of muscles sometimes involved, and difficulties in discriminating abnormal movements from compensations.165 Deciding where and how much to inject can be challenging, particularly in musicians where there is a need for exquisitely good motor control.
Various forms of occupational therapy are reported to be of benefit in small but usually uncontrolled trials.166, 167 Results are inconsistent and short-lived, so these methods are not widely used. Limb dystonias respond well to DBS of the globus pallidus. Additionally, there reports of success with thalamotomy or thalamic DBS, a target not often used for other dystonias.168 Whether this means the ideal target for limb dystonias is different from other dystonias is not known.
Less well-studied AOFD
Oromandibular dystonia (OMD)
Dystonic movements may be limited to lower facial muscles, jaw, or tongue. These regions may be affected in isolation or together. When they occur together with BL, the combination is sometimes called Brueghel or Meige syndrome, although these eponyms have been questioned.169
Involvement of the lower face causes grimacing, lip pursing, and other facial contortions. Involvement of jaw muscles leads to sustained, repetitive or action-induced jaw opening or closing, protrusion or retraction, or deviation. Symmetric contractions of jaw closers (medial pterygoids, masseters and temporalis muscles) leads to jaw closing dystonia or dystonic bruxism. Symmetric contraction of jaw openers (lateral pterygoids and digastrics) leads to jaw opening or protrusion. Asymmetric contraction of openers or closers leads to lateral deviations.170 In addition, simultaneous or alternating movements of openers and closers may result in jaw tremor.171
Tongue movements can be sustained, episodic, or action-induced.172–176 Lingual dystonia may be isolated and idiopathic,90, 177, 178 or a feature of a more complex dystonia syndrome.179
OMD often is aggravated by talking or chewing, causing disability related to speaking or eating, and sometimes causing serious weight loss.180, 181 Jaw pain can be prominent, leading to misdiagnosis as the temporomandibular joint syndrome.182 Musicians who play wind instruments may develop embouchure dystonia involving lip, jaw, and tongue muscles.183 It presumably occurs because of repetitive practice, but may spread to involve activities besides music. OMD sometimes follows dental work, although a causal relationship has not been established.
OMD does not respond well to oral medications. Some cases respond to botulinum toxins, but treatment outcomes seem less predictable than other focal dystonias.
Axial dystonia
Axial dystonia, apart from the neck, is uncommon in adults.184 Truncal dystonia may occur in isolation, or as a feature of Parkinson’s disease and related conditions.185 Patients may bend forward (camptocormia), backwards (opisthotonus), or sideways (Pisa syndrome). Movements can be fixed or spasmodic. Dystonia is not the cause for all abnormal truncal postures. For example, scoliosis may be a manifestation of truncal dystonia, but scoliosis also may have other causes.186, 187 Exposure to dopamine receptor antagonists may lead to truncal dystonia, where bending is more often backwards.44
Treatment with oral medications usually is ineffective. Botulinum toxin injections into the paraspinal and abdominal muscles may help, but results usually are modest because of broad involvement of large truncal muscles.188, 189 Excellent results have been reported with DBS,190 but few patients have been studied.
FOCAL DYSTONIAS: TO LUMP OR SPLIT?
The case for splitting
The preceding summary highlights obvious differences in the overt clinical features of the AOFD. Even within the major subtypes there are distinct phenotypes for each, with important treatment implications. In addition to differences in clinical manifestations, other differences are clear from epidemiological studies. For example, most AOFD are more common in women than men, except for limb dystonias (Table 1). AOFD also differ in age at onset. A meta-analysis encompassing 5057 patients across 83 different studies191 revealed significant differences in mean age at onset for writer’s cramp (38.4 years), CD (40.8 years), SD (43.0 years) and BL/OMD (55.7 years). The risk of spread also varies.192, 193 BL is the most likely to spread, with approximately 50% risk of spread beyond the orbicularis oculi over 5 years. In comparison, for CD and SD the risk of spread is only 15% over 5 years. Another difference among the AOFD is that some emerge only with specific tasks, while others lack obvious task specificity.156, 194–196 Finally, there are different epidemiological associations such as trauma for CD, dry eye for BL, laryngitis for LD, and repetitive use for limb dystonias.
Table 1.
Sex Differences Among Adult-Onset Focal Dystonias
| Focal dystonia | Total cases | F:M ratio |
|---|---|---|
| CD | 2634 | 1.5 |
| SD | 1411 | 2.0 |
| BL | 739 | 2.0 |
| UL | 296 | 0.6 |
| OMD | 37 | 3.1 |
There also are important etiological differences among the AOFD. These differences are most obvious for inherited AOFD, where some genes are linked preferentially with one subtype.49, 197 These many differences lead to obvious questions regarding why AOFD are lumped together as a group.
The case for lumping
Shared genes
Historically, many AOFD were considered to be distinct entities under the category of “occupational cramps” or spasms. In 1976 Marsden proposed grouping them as “formes fruste” of generalized dystonia.198 The evidence for combining them included observations that AOFD phenotypically resemble more generalized dystonias, observations that one AOFD sometimes spreads to include another, and observations that patients with one AOFD often had family members with different AOFD. A review of published reports of 13 families revealed 5 with a single type of AOFD, suggesting a familial influence for specific types.8 However, 8 families had multiple different types of AOFD, suggesting a common influence for different subtypes. A combined analysis of 4 families where first degree relatives were examined directly suggested 54% had concordant phenotypes while 46% had mixed or discordant phenotypes.8 These discordant families provide indirect evidence for shared etiological factors.8
More direct evidence for genetic factors comes from genes responsible for early-onset generalized dystonias. The common GAG deletion in TOR1A responsible for generalized DYT1 dystonia may be associated with a phenotype resembling AOFD. A survey of 3216 patients in 17 studies of TOR1A mutations listed 21 cases with non-generalized syndromes including focal, segmental and multifocal patterns.33 Another study with 3028 patients across 17 studies listed 25 cases with non-generalized syndromes.34
AOFD are more frequently associated with the THAP1 gene, where the classical phenotype is generalized dystonia that begins in the neck or arm during adolescence or early adulthood.139 A review of 106 cases revealed 19 (18%) with focal and 30 (28%) with segmental distributions.199 Another review of 130 published cases revealed 21 (16.2%) with focal and 45 (34.6%) with segmental distributions.35
A similar overlap among AOFD is evident for the newly discovered genes. GNAL mutations were associated with CD, BL, LD, and limb dystonia.37, 38 ANO3 mutations were associated with CD, but several cases also had LD or limb dystonia.39
Shared molecular and cellular pathways
At present, delineating shared pathways for AOFD is challenging because only a few genes are known.197, 200 These genes play roles in different molecular and cellular pathways. The TOR1A gene encodes a molecular chaperone, THAP1 a transcription factor, CIZ1 a DNA replication enzyme, ANO3 an ion channel, TUBB4a a microtubule-associated protein, and GNAL a G-protein involved in intracellular signaling. It is not yet clear how, or if, these pathways intersect.
However, several shared pathways have been recognized among the many different mixed dystonia syndromes where many causes have been identified.201, 202 One of the earliest themes recognized involves dopamine signaling.14, 203, 204 Dystonia is a feature of several inherited defects that affect dopamine synthesis directly such as DOPA-responsive dystonia,205 or indirectly, such as Lesch-Nyhan disease.206, 207 The dopamine theme is not limited to inherited defects, since drugs that block dopamine transmission can induce acute dystonic reactions or tardive dystonia in the absence of a genetic defect.43 Many patients with early-onset Parkinson’s disease present with dystonia of one limb as the dominant clinical feature.208, 209 Imaging114, 115, 210 and postmortem studies211 also have revealed subtle abnormalities of dopamine systems in both inherited and sporadic primary dystonias. Defects in dopamine signaling also are linked with dystonia in animal models.212–214 These observations suggest that dysfunction of dopamine signaling is a shared theme for several types of dystonia.
Another shared theme identified via studies of animals with drug-induced215–217 or inherited218, 219 dystonia involves defects in ion channels. Inherited defects in the CACNA1A gene that encodes a calcium channel have been linked with a variety of neurological disorders in humans, including CD and writer’s cramp.220 Defects in the KCNMA1 gene encoding a potassium channel underlie some paroxysmal dyskinesias,221 where dystonia may be a prominent feature.222 The ANO3 gene described above is thought to encode a calcium-activated chloride channel.39
Another shared molecular theme involves mitochondrial dysfunction.223–225 Dystonia occurs in mitochondrial disorders such as Leber’s optic neuropathy, Leigh’s syndrome, and the Mohr-Tranebjaerg dystonia-deafness syndrome.226, 227 In some cases, dystonia can be the dominant neurological problem.228, 229 In other cases it may be limited to focal or segmental patterns.226, 229–231 The mitochondrial theme is not restricted to inherited defects. Dystonia occurs among children232 and non-human primates233 exposed to the 3-nitropropionic acid, a mitochondrial poison. Mitochondrial defects also have been associated with sporadic AOFD.234, 235 Thus mitochondrial dysfunction is a shared feature of certain dystonias.
Shared anatomical circuitry
Historically, dystonia has been attributed to dysfunction of the basal ganglia. The evidence supporting a role for the basal ganglia is strong and has been reviewed several times.236–238 However, there has been increasing appreciation that other brain regions also may be involved, particularly the cerebellum. These studies also have been reviewed.50, 51, 239–242 Dystonia now is viewed as a disorder where the basal ganglia play a role as one node in a broader network that includes the cerebellum and other regions.
Certain subtypes of dystonia may share a causal pathology in the basal ganglia, while others may arise primarily from dysfunction of the cerebellum. It also is possible that combined dysfunction of two nodes is related to the expression of dystonia, the “two hit hypothesis”.243, 244 Alternatively, dystonia may arise from defective communication among different nodes in the network.216, 245 Although more work needs to be done to determine which of these models for anatomical pathogenesis is most appropriate for different subtypes of dystonia, it is clear that there is a shared anatomical network for many types of dystonia.
Shared physiological substrates
Three common themes have arisen from physiological studies.162 The first theme involves loss of inhibitory processes, which have been found in different types of dystonia and at multiple levels of the neuraxis including the spinal cord, brainstem, and cortex.238
The second theme involves defects in sensorimotor integration.246 Although patients with AOFD do not have overt sensory deficits, they have consistently higher spatial and temporal somatosensory discrimination thresholds.119, 247 Altered sensory thresholds have been reported for many types of dystonia, and can be measured in both affected and unaffected body parts, even among unaffected family members in large pedigrees where other members are affected.119, 247
The third theme involves maladaptive neural plasticity, which also has been reported for many different types of dystonia.196, 204, 248 It is striking that these three themes have arisen so many times across different types of dystonia including the AOFD, inherited generalized syndromes, and acquired dystonias. These observations support the concept that AOFD share certain physiological defects.
A conceptual model for lumpers & splitters
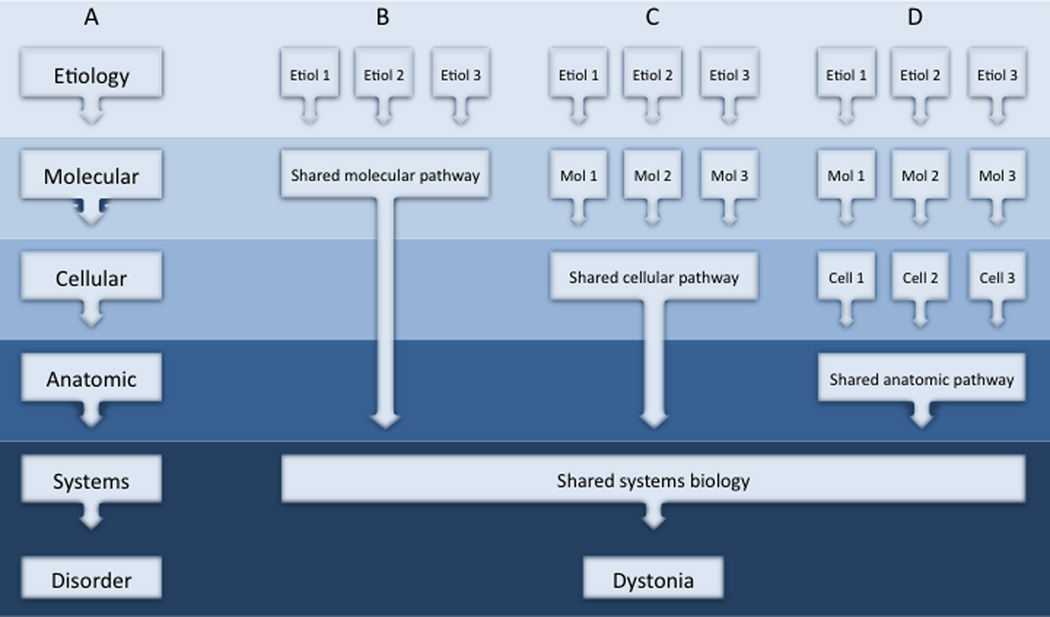
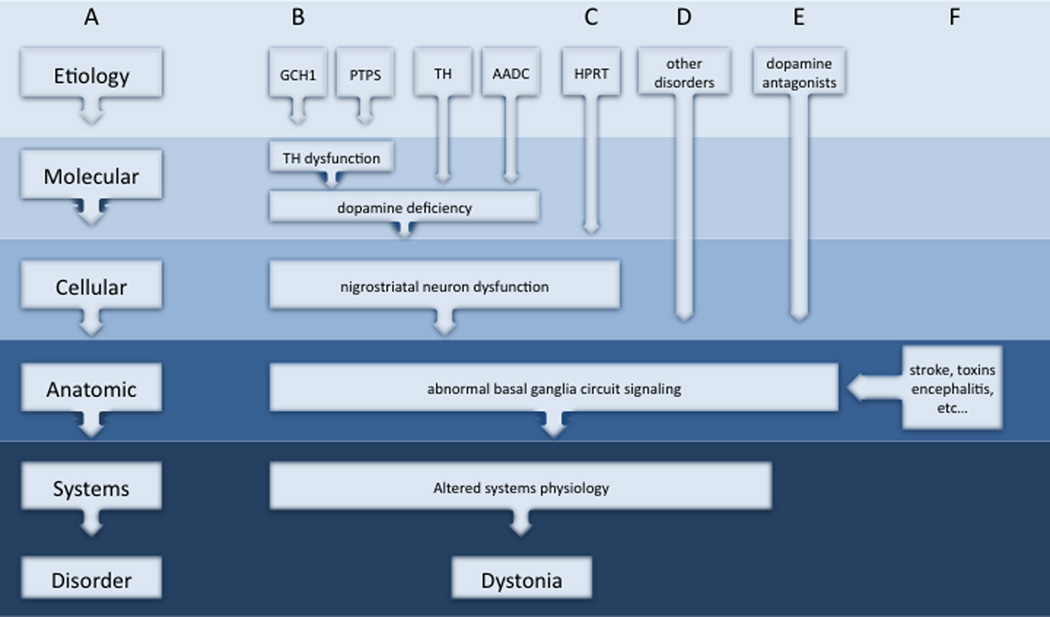
The differences and similarities among AOFD can be accommodated by a conceptual model that focuses on different biological levels.202 Like other disorders, the pathogenesis of dystonia can be viewed as a multi-step process where some original insult triggers a series of abnormalities at the molecular, cellular, anatomical, and physiological levels (Figure 1A). While there may be many different triggers, specific subgroups of dystonia may share some downstream mechanisms of pathogenesis. Interactions among molecular and cellular pathways may occur for specific subgroups of dystonia (Figure 1B), but it seems naïve to assume that all will intersect at one common molecular pathway. Instead, pathogenesis may converge at the systems level, by affecting the same brain region, or by causing the same physiological substrate (Figure 1C–D). This model for pathogenesis can be constructed for some themes in dystonia, such as dopaminergic dysfunction and basal ganglia defects (Figure 2). Further studies are needed to understand how other dystonias should be grouped.
Figure 1.
Conceptual model for similarities and differences among different dystonias. A simplified scheme for pathogenesis begins with a specific etiology and proceeds through a series of downstream processes in the pathogenesis of dystonia (A). Etiologically different types of dystonia may share a similar molecular pathway and similar subsequent pathogenesis (B). Alternatively, etiologically different types of dystonia may differ at the molecular level, yet disrupt the same neuronal population (C). Etiologically different types of dystonia instead may disrupt a common anatomical circuit (D). Ultimately, all may share some similar biological defect at the systems level to result in the patterns of muscle activity that define dystonia.
Figure 2.
Example model for shared mechanisms of pathogenesis at different biological levels. A simplified scheme for pathogenesis begins with a specific etiology and proceeds through a series of downstream processes in the pathogenesis of dystonia (A). Some disorders are known to disrupt a common molecular pathway involving dopamine synthesis via different molecular mechanisms (B). Two gene defects (GCH1 and PTPS) affect dopamine synthesis by disrupting biopterin metabolism, while two other gene defects (TH and AADC) affect other steps in dopamine synthesis.323 All 4 of these disorders disrupt nigrostriatal dopamine neuron function, signaling in basal ganglia circuits, and alter systems physiology to produce dystonia. Nigrostriatal dopamine neurons may be affected via other genetic mechanisms (C: HPRT)207 or exposure to dopamine receptor antagonists(D)43 to cause dystonia. Damage may also occur to basal ganglia circuits more directly, as in some mitochondrial disorders, glutaric aciduria, biotin-responsive basal ganglia disease, 3-nitropropionic acid exposure (E).14
This model has important implications for experimental therapeutics.202 Interventions targeting “upstream” pathways may be useful for preventing the cascade of events that leads to specific types of dystonia, such as targeting the TOR1A gene by RNAi for DYT1 dystonia.249 However, this intervention seems unlikely to have any therapeutic impact on other forms of dystonia that do not involve this molecular pathway. On the other hand, targeting “downstream” pathways may be useful for interrupting the cascade of events in broader groups of dystonias. For example, DBS of the globus pallidus has proven effective for many etiologically unrelated forms of dystonia, most likely because it alters common signaling pathways.79 The botulinum toxins are effective in an even broader group of unrelated dystonias, because they target the final common defect involving overactive muscles.
FUTURE PROSPECTS
In the past few years, there have been enormous strides in delineating the many clinical manifestations of dystonia, discovering underlying etiologies, and elucidating mechanisms of pathogenesis. At the same time, many new questions have arisen. This section summarizes some of the important unsolved questions, organized according to different research disciplines. These questions emerged from a series of recent workshops focussing on research priorities (Table 4).
Table 4.
Research Priorities for AOFD
| Research discipline | Research priorities |
|---|---|
| Molecular & cellular | Identify novel genes |
| Elucidate shared molecular pathways | |
| Develop cell models to study molecular pathogenesis | |
| Develop animal models to study pathogenesis | |
| Anatomical | Distinguish cause from consequence |
| Elucidate reasons for inconsistent findings | |
| Utilize animal models for understanding circuitry | |
| Conduct more precise autopsy studies | |
| Physiological | Distinguish cause from consequence |
| Explore molecular and anatomical basis | |
| Explore physiological mechanisms of sensory tricks | |
| Utilize animal models for understanding circuitry | |
| Clinical research | Refine appreciation of clinical subtypes |
| Explore relationship to tremor | |
| Explore relevance of non-motor features | |
| Improve education and awareness | |
| Clinical trials | Refine or develop rating scales |
| Develop trial designs for novel drugs | |
| Conduct trials of recently discovered agents |
This table provides partial a list of some of the important research questions in AOFD, listed according to research discipline rather than priority. These items were included on the basis of a series of workshops focused on delineating research priorities in each of the areas.
Clinical research
Historically, there has been a tendency to lump different AOFD together for studies. This strategy has been helpful for increasing sample sizes, but underlying etiological heterogeneity may introduce unknown variables and thereby mask findings relevant to specific subtypes. Until these variables are better understood, it seems safer to study phenotypically homogeneous or etiologically defined patient populations separately, before combining data across groups. Detailed clinical information therefore is essential for generating defined populations for studies. Merely listing cases as having one of the AOFD no longer seems sufficient.
There also are a number of other important clinical issues to address. The relationship between dystonia and tremor has become increasingly murky.19–21, 24, 250 The nosological relationships between dystonia and several related disorders also needs attention. Some examples include athetosis, mirror movements, “apraxia” of eyelid opening, MTD, paroxysmal dyskinesias, and pseudo-dystonia.15 The significance of non-motor features and impact on quality of life also needs to be explored, as there is growing suspicion that they may have greater impact than the movement disorder itself.251–253
Finally, there seems a need for better awareness of the many clinical manifestations of dystonia. Although dystonia is readily recognized by experts in movement disorders, there is poor recognition among primary care givers and general neurologists22, 254, 255 with several years often elapsing between symptom onset and diagnosis.256, 257
Molecular & cellular basis
While several genes for AOFD recently have been reported, there are many more families for which genes are not yet known, and even more sporadic cases where genetic contributions are an open question.49 The relevance of the newly discovered genes to broader populations also must be explored by examining large numbers of AOFD. The identification of additional genes is important, as it may facilitate diagnostic testing and identification of shared pathways that can become the targets of rational drug design.202
While many genes responsible for mixed dystonia syndromes often are fully penetrant,14 all genes so far discovered for isolated dystonia syndromes appear to be partially penetrant. The partial penetrance may suggest dystonia is a polygenic disorder or one that requires an additional environmental trigger.49 The mechanisms of partial penetrance have so far received little attention, yet seem important for providing clues to pathogenesis.
Anatomical circuitry
Recent information has led to a shift in thinking away from the basal ganglia as the sole cause of dystonia to a network model that includes other motor systems.50, 51, 241 This shift has raised numerous questions for research. Exactly which regions of the network are most important and how the network is disrupted to cause dystonia remain to be determined.
Two major challenges emerged from recent reviews of imaging studies in dystonia.50, 51 One is a lack of consistent findings across studies. While some inconsistencies may reflect differences among AOFD or imaging methods, there are inconsistencies even within a single imaging modality of the same AOFD. For example, many studies identify the basal ganglia as being abnormal in CD, but different subregions are affected in different studies, occasionally with opposing changes in the same brain region using the same imaging modalities.258 Resolving these inconsistencies is important for establishing reliability and may facilitate recognition of common patterns in different AOFD.
Another major challenge is that imaging studies are correlational, and it is difficult to discriminate which brain abnormalities cause dystonia from those that reflect secondary adaptations. Motor pathways of the brain are interconnected, so abnormalities in one region influence another. A related difficulty is that movement itself changes the brain. Highly trained individuals, such as musicians and golfers, normally develop changes in brain structure and function that can be measured by modern imaging methods.259, 260 Even non-professionals show measurable changes when learning how to juggle over a period as short as one week.260 These observations raise concern that many abnormalities observed in imaging studies might not be the cause of dystonia, but rather a consequence of the abnormal movements. There are similar concerns regarding the secondary effects of sensory feedback on brain structure and function. Disentangling cause from effect in dystonia will require the application of novel strategies.261
Additional histopathological studies are needed to further explore recent findings of subtle abnormalities among cerebellar Purkinje neurons in patients with CD.64, 65 Studies in animal models may reveal anomalies that can be subsequent targets of human investigations.214, 262, 263 Animal models also may be valuable in testing hypotheses regarding cause and effect.264–266 Unfortunately, while there are many animal models for generalized dystonias,264, 266, 267 there are few for AOFD. There are no accepted animal models for LD. There is one rat model for BL.243 For CD, some rodent268, 269 or primate models270–272 have been proposed. However, few of these models have been replicated by more than one laboratory, and none have been adequately validated. Developing and validating animal models for AOFD remains a high priority. Until better AOFD models are developed, insights from animal studies must rely on extrapolations of results from models of generalized dystonias.
Human & animal physiology
Physiological studies of humans repeatedly have identified three common themes of loss of inhibition, abnormal sensorimotor integration, and maladaptive plasticity.162 How these themes may be linked remains unclear. While these defects have been attributed to dysfunction of the basal ganglia,204, 237, 238 direct evidence for the responsible anatomical circuitry is lacking, and the possibility that they arise instead from dysfunction of the cerebellum has been raised.50, 53, 273, 274
Evidence that these physiological defects play a causal role in dystonia also is lacking, and the possibility remains that some may be secondary to the movement disorder instead.162 Two related pieces of evidence have been cited as evidence for causality. One is that these abnormalities can be detected for body regions unaffected by dystonia, and the other is that they can be detected from both sides of the brain, even when symptoms are unilateral. These findings may imply a predisposing endophenotype. However, it is widely known that “unaffected” body regions are often not normal in dystonic patients, and physiological stimuli applied to one side of the body can be detected on both sides of the brain. Additionally, the occurrence of some physiological abnormalities in psychogenic dystonia supports the view that they may not be causal.275 Thus further direct evidence for a causal role seems important to establish. Here again, animal models could be valuable in discriminating cause from consequence.
Sensory tricks also appear to be common among many of the AOFD, and their clinical features have been characterized in detail.4, 276 However, the physiological mechanisms responsible for their effectiveness has received less attention, and may provide clues towards pathogenesis.
Clinical trials & experimental therapeutics
Discoveries from basic sciences have pointed to some novel targets for rational drug development. Several drug-screening assays have been devised.277, 278 Additionally, there are several small animal models suitable for empirical testing of drugs.267 These models also have been used to identify promising new agents. There is additional interest in the possibility of using drugs that suppress dyskinesias in Parkinson’s disease, because some dyskinesias have a dystonic quality.279
Clinical studies and trials require validated measurement tools that are sensitive to severity. Several rating scales have been used for years, but each has some limitations.280, 281 Validated rating scales are lacking for some AOFD, and none have been validated for children. In addition, there is increased appreciation for the impact of non-motor features, but how they relate to overall quality of life remains unexplored.282–284
A major barrier in testing candidate drugs in clinical trials is limited experience with trial designs in dystonia. Effective designs have been developed for trials involving botulinum toxins13, 66 or DBS,285, 286 but there are no widely accepted designs for drugs. Large, double-blinded, placebo-controlled trials present challenges for rare diseases, and they are financially unattractive to industry. Similar to Parkinson’s disease, it is likely that trial designs for symptomatic therapies will differ from those of disease-modifying therapies. Finally, any trial in AOFD must incorporate a means for addressing the cyclical swings in severity associated with treatment with botulinum toxins. Addressing these issues will be important for translating the many novel scientific discoveries and candidate drugs into meaningful new treatments for AOFD.
Table 2.
Familial Clustering of Adult-Onset Focal Dystonias
| Source | Type of dystonia |
|---|---|
| Bhidayasiri et al, 2005291 | UL |
| Brancati et al, 2002292 | BL, CD, UL |
| Bressman et al, 1996 (A)293 | CD, UL |
| Bressman et al, 1996 (B)293 | CD |
| Cassetta et al, 1999294 | BL, CD OMD |
| Defazio et al, 2003a (1)295 | BL |
| Defazio et al, 2003a (2)295 | BL |
| Defazio et al, 2003b (3)296 | BL, CD |
| Defazio et al, 2003b (4)296 | BL |
| Defazio et al, 2003b (5)296 | CD, UL |
| Defazio et al, 2003b (6)296 | BL, UL |
| Defazio et al, 2003b (10)296 | BL, CD, OMD |
| Gasser et al, 1998297 | UL |
| Jimenez-Jimenez et al, 2002298 | BL, CD, OMD |
| Leube et al, 1996299 | CD, LD, UL |
| Leube et al, 199742 | CD, LD, UL |
| Micheli et al, 1994300 | CD, UL |
| Munchau et al, 2000301 | BL, CD, UL, OMD |
| Norgren et al, 2011302 | BL, CD, G, S, UL |
| O’Riordan et al, 2004186 | CD, UL |
| Puschmann et al, 2011303 | BL, CD, OMD, LL, UL |
| Schmidt et al, 2006 (A)304 | UL |
| Schmidt et al, 2006 (B)304 | UL |
| Schmidt et al, 2006 (C)304 | UL |
| Uitti et al, 1993305 | CD |
| Winter et al, 2012306 | BL, CD, LD, OMD, UL |
This table was modified and extended from a prior review.8 It includes individual families with AOFD with at least 3 affected and subjects were directly examined. In some cases, clinical details were limited and only presenting features were provided. Reports containing more than one family show families separately.
In other Abbreviations: BL=blepharospasm; CD=cervical dystonia; G=generalized; LD=laryngeal dystonia; OMD=oromandibular dystonia; S=segmental; UL=upper limb dystonia.
Table 3.
THAP1 Sequence Variants & Focal Dystonias
| Source | Total cases |
Focal dystonias |
Type(s) of focal dystonia |
|---|---|---|---|
| Blanchard et al, 2011199 | 178 | 1 | CD/UL (1) |
| Bonetti et al, 2009307 | 158 | 1 | CD/FHD(1) |
| Bressman et al, 2009308 | 104 | 5 | FHD(4), LD(1) |
| Cheng et al, 2011309 | 111 | 2 | CFD/CD(1), CFD/CD/LD(1) |
| Cheng et al, 2012310 | 102 | 7 | CD(2), CD/BL(2), CD/BL/trunk(1), CD/OMD(1), CD/trunk(1) |
| Clot et al, 2010311 | 113 | 2 | CD(1), CFD/CD/LD/UE(1) |
| Djarmati et al, 2009312 | 320 | 2 | CD/FHD(2) |
| Dobricic et al, 2012313 | 281 | 4 | UL(1), LD(1), CD/LD(1), CD/UL(1) |
| Fuchs et al, 2009139 | 180 | 11 | UL(4), LD(1), UL/LL(1), CD/UL(2), OMD/CFD(1), LD/CD/CFD(1) CFD/OMD(1) |
| Groen et al, 2010314 | 455 | 4 | CD(1), OMD/UE/CD(1), OMD/UE/CD/LD (1), CD/UE/Trunk(1) |
| Groen et al, 2011315 | 109 | 1 | LD/OMD(1) |
| Houlden et al, 2010316 | 390 | 5 | CFD/OMD (1), CD/UL (3), OMD/UL(1) |
| LeDoux et al, 201235 | 750 | 3 | CD/UL(1), SD/CD/UL/CFD/OMD(2) |
| Lohmann et al, 2012317 | 567 | 3 | CD(1), UL+S(2) |
| Paison-Ruiz et al, 2009318 | 24 | 2 | UL/LL(1), UL/CFD(1) |
| Prudente et al, 201364 | 6 | 4 | CD(2), CD/BL(1), CD/BL/FD(1) |
| Sohn et al, 2010319 | 610 | 5 | CD (4), CD/OMD/LD (1) |
| Song et al, 2011320 | 231 | 2 | CD(1), CD/trunk (1) |
| Van Gerpen et al, 2010321 | 1 | 1 | UL/LL(1) |
| Xiao et al, 2010140 | 1114 | 17 | CD(6), LD(5), BL(2), OMD(1), UL(1), BL/LD/CFD(1), UL/CD/OMD(1) |
| Xiromerisiou et al, 2012322 | 150 | 1 | CD(1) |
This table includes a summary of THAP1 sequence variants among patients with primary dystonia, where focal or segmental patterns were found. The distributions were inferred from information provided in the original reports, which sometimes was limited. The numbers in parentheses indicate the numbers of cases for type. In many cases, the pathogenicity of the sequence variant has not been established.
Abbreviations: BL=blepharospasm; CD=cervical dystonia; CFD=craniofacial dystonia; LD=laryngeal dystonia; LL=ower limb dystonia; OMD=oromandibular dystonia; S=segmental dystonia (regions not specified); UL=upper limb dystonia.
ACKNOWLEDGMENTS
This summary was developed in part from a series of meetings addressing the AOFD sponsored by a grant to the Dystonia Coalition from the Office of Rare Diseases Research in the National Center for Advancing Translational Sciences and the National Institute of Neurological Disorders and Stroke at the NIH (U54 NS065701). Some support also came from private foundations (American Dystonia Association, Beat Dystonia, The Bachmann-Strauss Dystonia & Parkinson Foundation, Benign Essential Blepharospasm Research Foundation, Dystonia Europe, Dystonia Medical Research Foundation, Foundation for Dystonia Research, National Spasmodic Dysphonia Association, and National Spasmodic Torticollis Association) and Industry (Allergan Inc., Ipsen Ltd., Medtronics Inc., and Merz Pharmaceuticals).
Footnotes
DISCLOSURES
A. Berardelli has received grants from the Italian Ministry of University, from the Benign Essential Blepharospasm Research Foundation and from Boehringer Ingelheim, Lundbeck, UCB, Allergan, and Merz Pharmaceuticals.
C. Comella. Has received research support from Allergan Inc., Merz Pharmaceuticals, Ipsen Limited, NIH, and Parkinson Disease Foundation. She also has received consulting fees from Allergan Inc., Ipsen Ltd, Medronic Incl, Merz Pharmaceuticals, and Neupathe.
G. Defazio has received funds from the Italian Ministry of University and from the Benign Essential Blepharospasm Research Foundation. He also has received honoraria for lecturing from Glaxo Smith Kline, UCB pharma, Lundbeck and Allergan.
M.R. DeLong has received research grant support from the NIH and the American Parkinson Disease Association. He also has received consulting fees from Medtronic, Inc., Merck, Inc, and Merz Pharmaceutical, and compensation from the Dystonia Medical Research Foundation, for which he serves as Scientific Director. He services as a Board member for the American Parkinson Disease Foundation and the Bachman-Strauss Dystonia & Parkinson Foundation, Inc. (ex-officio).
S. Factor has received grant support from Teva, Ceregene, Ipsen, EMD Serono, Allergan, Medtronics, Michael J. Fox Foundation, Consolidated Anti-Aging Foundation and NIH. He has also received Honoraria from Scientiae for a CME program on dystonia, University of Florida speaker program, as a consultant for Chelsea Therapeutics, Merz, Ipsen, Adamas and for editorial service as section editor of Current Neurology and Neuroscience Reports. He has received Royalties from Demos and Blackwell Futura for textbooks.
W. Galpern has nothing to disclose.
H. A. Jinnah has received research grant support from the NIH, the Atlanta Clinical & Translational Science Institute, the Dystonia Medical Research Foundation, the Emory University Research Council, and the Bachmann-Strauss Dystonia & Parkinson’s Foundation. He also is principal investigator for the Dystonia Coalition, which receives the majority of its support through NIH grant NS065701 from the Office of Rare Diseases Research in the National Center for Advancing Translational Sciences and National Institute of Neurological Disorders and Stroke. The Dystonia Coalition receives additional material or administrative support from industry sponsors (Allergan Inc., Ipsen Biopharm, Medtronics Inc, and Merz Pharmaceuticals) as well as private foundations (The American Dystonia Society, The Bachmann-Strauss Dystonia and Parkinson Foundation, BeatDystonia, The Benign Essential Blepharospasm Foundation, Dystonia Europe, Dystonia Ireland, The Dystonia Medical Research Foundation, The Dystonia Society, The Foundation for Dystonia Research, The National Spasmodic Dysphonia Association, and The National Spasmodic Torticollis Association). Dr. Jinnah has served as a consultant for Psyadon Pharmaceuticals and Savient Pharmaceuticals. He also serves on the Scientific Advisory Boards for Cure Dystonia Now, the Dystonia Medical Research foundation, Tyler's Hope for a Dystonia Cure, the Lesch-Nyhan Syndrome Children’s Research Foundation, and Lesch-Nyhan Action France.
C. L. Ludlow has received research grant support from the NIH. She has served as a consultant for Passy Muir, Inc. She also serves on the Scientific Advisory Boards for the National Spasmodic Dysphonia Association and the National Foundation of Swallowing Disorders.
J. Perlmutter has received research grant support from the NIH (TH000448, NS41509, and NS075321). He also has received support from the American Academy of Neurology, the Murphy Fund, the American Parkinson Disease Association (APDA) Center for Advanced PD Reesearch at Washiington University; the Greater St. Louis Chapter of the APDA, the McDonnel Center for Higher Brain Function, and the Barnes-Jewish Hospital Foundation.
A. Rosen has nothing to disclose.
AUTHOR ROLES
A. Berardelli: Manuscript review and critique.
C. Comella: Manuscript design, review and critique.
G DeFazio: Collection of source data, writing portions of 1st draft, review and critique.
M.R. DeLong: Manuscript review and critique.
S. Factor: Collection of source data, writing portions of 1st draft, review and critique.
W. Galpern: Manuscript review and critique.
M. Hallett: Collection of source data, writing portions of 1st draft, review and critique.
H. A. Jinnah: Design and organization, collection of source data, writing 1st draft, organizing final version.
C. L. Ludlow: Manuscript design, review and critique.
J. Perlmutter: Manuscript design, review and critique.
A. Rosen: Collection of source data, writing portions of 1st draft, review and critique.
BIBLIOGRAPHY
- 1.Fahn S. Concept and classification of dystonia. Adv Neurol. 1988 Jan 8;50 [PubMed] [Google Scholar]
- 2.Fahn S. The varied clinical expressions of dystonia. Neurol Clinics. 1984;2:541–554. [PubMed] [Google Scholar]
- 3.Fahn S. Clinical variants of idiopathic torsion dystonia. J Neurol Neurosurg Psychiatry. 1989;(Suppl):96–100. doi: 10.1136/jnnp.52.suppl.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evatt ML, Freeman A, Factor S. Adult-onset dystonia. Handb Clin Neurol. 2011;100:481–511. doi: 10.1016/B978-0-444-52014-2.00037-9. [DOI] [PubMed] [Google Scholar]
- 5.Jankovic J. Dystonic disorders. In: Jankovic J, Tolosa E, editors. Parkinson's disease and movement disorders. 5th ed. Philadelphia: Lippincott, Williams & Wilkins; 2007. pp. 319–347. [Google Scholar]
- 6.Tarsy D, Simon DK. Dystonia. N Engl J Med. 2006;355:818–829. doi: 10.1056/NEJMra055549. [DOI] [PubMed] [Google Scholar]
- 7.Geyer HL, Bressman SB. The diagnosis of dystonia. Lancet Neurol. 2006;5:780–790. doi: 10.1016/S1474-4422(06)70547-6. [DOI] [PubMed] [Google Scholar]
- 8.Defazio G, Berardelli A, Hallett M. Do primary adult-onset focal dystonias share aetiological factors? Brain. 2007;130:1183–1193. doi: 10.1093/brain/awl355. [DOI] [PubMed] [Google Scholar]
- 9.Defazio G. Epidemiology of primary and secondary dystonia. In: Stacey ME, editor. Handbook of Dystonia. New York: Informa Healthcare USA, Inc; 2007. pp. 11–20. [Google Scholar]
- 10.Adam OR, Jankovic J. Treatment of dystonia. Parkinsonism Relat Disord. 2007;13(Suppl 3):S362–S368. doi: 10.1016/S1353-8020(08)70031-2. [DOI] [PubMed] [Google Scholar]
- 11.Jankovic J. Treatment of dystonia. Lancet Neurol. 2006;5:864–872. doi: 10.1016/S1474-4422(06)70574-9. [DOI] [PubMed] [Google Scholar]
- 12.Bhidayasiri R, Tarsy D. Treatment of dystonia. Expert Rev Neurother. 2006;6:863–886. doi: 10.1586/14737175.6.6.863. [DOI] [PubMed] [Google Scholar]
- 13.Albanese A, Barnes MP, Bhatia KP, et al. A systematic review on the diagnosis and treatment of primary (idiopathic) dystonia and dystonia plus syndromes: repor of an EFNS/MDS-ES task force. Eur J Neurol. 2006;13:433–444. doi: 10.1111/j.1468-1331.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- 14.Fung VS, Jinnah HA, Bhatia K, Vidailhet M. Assessment of the patient with dystonia: An update on dystonia syndromes. Mov Disord. 2013 doi: 10.1002/mds.25549. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: A consensus update. Mov Disord. 2013 doi: 10.1002/mds.25475. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan J, Brin MF, Fahn S. Idiopathic cervical dystonia: clinical characteristics. Mov Disord. 1991;6:119–126. doi: 10.1002/mds.870060206. [DOI] [PubMed] [Google Scholar]
- 17.Jankovic J, Leader S, Warner D, Schwartz K. Cervical dystonia: clinical findings and associated movement disorders. Neurology. 1991;41:1088–1091. doi: 10.1212/wnl.41.7.1088. [DOI] [PubMed] [Google Scholar]
- 18.Rondot P, Marchand MP, Dellatorlas G. Spasmodic torticollis-review of 220 patients. Can J Neurol Sci. 1991;18:143–151. doi: 10.1017/s0317167100031619. [DOI] [PubMed] [Google Scholar]
- 19.Elble RJ. Defining dystonic tremor. Curr Neuropharmacol. 2013;11:48–52. doi: 10.2174/157015913804999478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiebler S, Schmidt A, Zittel S, et al. Arm tremor in cervical dystonia-Is it a manifestation of dystonia or essential tremor? Mov Disord. 2011 doi: 10.1002/mds.23837. [DOI] [PubMed] [Google Scholar]
- 21.Quinn NP, Schneider SA, Schwingenschuh P, Bhatia KP. Tremor - some controversial aspects. Mov Disord. 2011;26(1):18–23. doi: 10.1002/mds.23289. [DOI] [PubMed] [Google Scholar]
- 22.Lalli S, Albanese A. The diagnostic challenge of primary dystonia: evidence from misdiagnosis. Mov Disord. 2010;25:1619–1626. doi: 10.1002/mds.23137. [DOI] [PubMed] [Google Scholar]
- 23.Pal PK, Samii A, Schulzer M, Mak E, Tsui JK. Head tremor in cervical dystonia. Can J Neurol Sci. 2000;27:137–142. [PubMed] [Google Scholar]
- 24.Deuschl G, Bain P, Brin M. Consensus statement of the movement disorder society on tremor. Mov Disord. 1998;13(Suppl. 3):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 25.Rivest J, Marsden CD. Trunk and head tremor as isolated manifestations of dystonia. Mov Disord. 1990;5:60–65. doi: 10.1002/mds.870050115. [DOI] [PubMed] [Google Scholar]
- 26.Dauer WT, Burke RE, Greene P, Fahn S. Current concepts on the clinical features, aetiology and management of idiopathic cervical dystonia. Brain. 1998;121:547–560. doi: 10.1093/brain/121.4.547. [DOI] [PubMed] [Google Scholar]
- 27.Singer C, Velickovic M. Cervical dystonia: Etiology and pathophysiology. Neurol Clin. 2008;26(Suppl 1):9–22. doi: 10.1016/s0733-8619(08)80002-3. [DOI] [PubMed] [Google Scholar]
- 28.Rivest J, Quinn N, Marsden CD. Dystonia in Parkinson's disease, multiple system atrophy, and progressive supranuclear palsy. Neurology. 1990;40:1571–1578. doi: 10.1212/wnl.40.10.1571. [DOI] [PubMed] [Google Scholar]
- 29.Revuelta GJ, Benatar M, Freeman A, et al. Clinical subtypes of anterocollis in parkinsonian syndromes. J Neurol Sci. 2011;315:100–103. doi: 10.1016/j.jns.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawley JS, Weiner WJ. Psychogenic dystonia and peripheral trauma. Neurology. 2011;77:496–502. doi: 10.1212/WNL.0b013e3182287aaf. [DOI] [PubMed] [Google Scholar]
- 31.Ibrahim NM, Martino D, van de Warrenburg BP, et al. The prognosis of fixed dystonia: A follow-up study. Parkinsonism Relat Disord. 2009 doi: 10.1016/j.parkreldis.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Schrag A, Trimble M, Quinn N, Bhatia K. The syndrome of fixed dystonia: an evaluation of 103 patients. Brain. 2004;127:2360–2372. doi: 10.1093/brain/awh262. [DOI] [PubMed] [Google Scholar]
- 33.Kabakci K, Hedrich K, Leung JC, et al. Mutations in DYT1: extension of the phenotypic and mutational spectrum. Neurology. 2004;62:395–400. doi: 10.1212/01.wnl.0000113024.84178.f7. [DOI] [PubMed] [Google Scholar]
- 34.Xiao J, Bastian RW, Perlmutter JS, et al. High-throughput mutational analysis of TOR1A in primary dystonia. BMC Med Genet. 2009;10:24. doi: 10.1186/1471-2350-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LeDoux MS, Xiao J, Rudzinska M, et al. Genotype-phenotype correlations in THAP1 dystonia: molecular foundations and description of new cases. Parkinsonism Relat Disord. 2012;18:414–425. doi: 10.1016/j.parkreldis.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao J, Uitti RJ, Zhao Y, et al. Mutations in CIZ1 cause adult onset primary cervical dystonia. Ann Neurol. 2012;71:458–469. doi: 10.1002/ana.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuchs T, Saunders-Pullman R, Masuho I, et al. Mutations in GNAL cause primary torsion dystonia. Nat Genet. 2013;45:88–92. doi: 10.1038/ng.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vemula SR, Puschmann A, Xiao J, et al. Role of G-alpha(olf) in familial and sporadic adult-onset primary dystonia. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt102. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Charlesworth G, Plagnol V, Holmstrom KM, et al. Mutations in ANO3 Cause Dominant Craniocervical Dystonia: Ion Channel Implicated in Pathogenesis. Am J Hum Genet. 2012;91:1041–1050. doi: 10.1016/j.ajhg.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waddy HM, Fletcher NA, Harding AE, Marsden CD. A genetic study of idiopathic focal dystonias. Ann Neurol. 1991;29:320–324. doi: 10.1002/ana.410290315. [DOI] [PubMed] [Google Scholar]
- 41.Stojanovic M, Cvetkovic D, Kostic VS. A genetic study of idiopathic focal dystonias. J Neurol. 1995;242(8):508–511. doi: 10.1007/BF00867421. [DOI] [PubMed] [Google Scholar]
- 42.Leube B, Kessler KR, Goecke T, Auburger G, Benecke R. Frequency of familial inheritance among 488 index patients with idiopathic focal dystonia and clinical variability in a large family. Mov Disord. 1997;12:1000–1006. doi: 10.1002/mds.870120625. [DOI] [PubMed] [Google Scholar]
- 43.Cardoso F. Drug-induced dystonia. In: Stacey MA, editor. Handbook of dystonia. New York: Informa Healthcare, Inc; 2008. pp. 267–276. [Google Scholar]
- 44.Molho ES, Feustel PJ, Factor SA. Clinical comparison of tardive and idiopathic cervical dystonia. Mov Disord. 1998;13(3):486–489. doi: 10.1002/mds.870130319. [DOI] [PubMed] [Google Scholar]
- 45.O'Riordan S, Hutchinson M. Cervical dystonia following peripheral trauma--a case-control study. J Neurol. 2004;251:150–155. doi: 10.1007/s00415-004-0291-9. [DOI] [PubMed] [Google Scholar]
- 46.van Rooijen DE, Geraedts EJ, Marinus J, Jankovic J, van Hilten JJ. Peripheral trauma and movement disorders: a systematic review of reported cases. J Neurol Neurosurg Psychiatry. 2011;82(8):892–898. doi: 10.1136/jnnp.2010.232504. [DOI] [PubMed] [Google Scholar]
- 47.Baizabal-Carvallo JF, Jankovic J. Movement disorders in autoimmune diseases. Mov Disord. 2012;27:935–946. doi: 10.1002/mds.25011. [DOI] [PubMed] [Google Scholar]
- 48.LeDoux MS, Brady KA. Secondary cervical dystonia associated with structural lesions of the central nervous system. Mov Disord. 2003;18:60–69. doi: 10.1002/mds.10301. [DOI] [PubMed] [Google Scholar]
- 49.Lohmann K, Klein C. Hereditary dystonia: What's new? What's next? Mov Disord. 2013 doi: 10.1002/mds.25536. this issue. [DOI] [PubMed] [Google Scholar]
- 50.Neychev VK, Gross R, Lehericy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiol Dis. 2011;42:185–201. doi: 10.1016/j.nbd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zoons E, Booij J, Nederveen AJ, Dijk JM, Tijssen MA. Structural, functional and molecular imaging of the brain in primary focal dystonia--a review. Neuroimage. 2011;56:1011–1020. doi: 10.1016/j.neuroimage.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 52.Sadnicka A, Hoffland BS, Bhatia KP, van de Warrenburg BP, Edwards MJ. The cerebellum in dystonia - Help or hindrance? Clin Neurophysiol. 2012;123:65–70. doi: 10.1016/j.clinph.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 53.Avanzino L, Abbruzzese G. How does the cerebellum contribute to the pathophysiology of dystonia. Basal Ganglia. 2012 in press. [Google Scholar]
- 54.Draganski B, Schneider SA, Fiorio M, et al. Genotype-phenotype interactions in primary dystonias revealed by differential changes in brain structure. Neuroimage. 2009;47:1141–1147. doi: 10.1016/j.neuroimage.2009.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Draganski B, Thun-Hohnstein C, Bogdahn U, Windler J, May A. Motor circuit gray matter changes in idiopathic cervical dystonia. Neurology. 2003;61:1228–1231. doi: 10.1212/01.wnl.0000094240.93745.83. [DOI] [PubMed] [Google Scholar]
- 56.Pantano P, Totaro P, Fabbrini G, et al. A transverse and longitudinal MR imaging voxel-based morphometry study in patients with primary cervical dystonia. AJNR Am J Neuroradiol. 2010;32:81–84. doi: 10.3174/ajnr.A2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Egger K, Mueller J, Schocke M, et al. Voxel based morphometry reveals specific gray matter changes in primary dystonia. Mov Disord. 2007;22:1538–1542. doi: 10.1002/mds.21619. [DOI] [PubMed] [Google Scholar]
- 58.Obermann M, Yaldizli Z, De Greiff A, et al. Morphometric changes of sensorimotor structures in focal dystonia. Mov Disord. 2007;22:1117–1123. doi: 10.1002/mds.21495. [DOI] [PubMed] [Google Scholar]
- 59.Galardi G, Perani D, Grassi F, et al. Basal ganglia and thalamo-cortical hypermetabolism in patients with spasmodic torticollis. Acta Neurol Scand. 1996;94:172–176. doi: 10.1111/j.1600-0404.1996.tb07049.x. [DOI] [PubMed] [Google Scholar]
- 60.Magyar-Lehman S, Antonini A, Roelcke U, et al. Cerebral glucose metabolism in patients with spasmodic torticollis. Mov Disord. 1997;12:704–708. doi: 10.1002/mds.870120513. [DOI] [PubMed] [Google Scholar]
- 61.Naumann M, Magyar-Lehmann S, Reiners K, Erbguth F, Leenders KL. Sensory tricks in cervical dystonia: perceptual dysbalance of parietal cortex modulates frontal motor programming. Ann Neurol. 2000;47(3):322–328. [PubMed] [Google Scholar]
- 62.Standaert DG. Update on the pathology of dystonia. Neurobiol Dis. 2011;42:148–151. doi: 10.1016/j.nbd.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holton JL, Schneider SA, Ganesharajah T, et al. Neuropathology of primary adult-onset dystonia. Neurology. 2008;70:695–699. doi: 10.1212/01.wnl.0000302175.76229.f0. [DOI] [PubMed] [Google Scholar]
- 64.Prudente CN, Pardo CA, Xiao J, et al. Neuropathology of cervical dystonia. Exp Neurol. 2012;241:95–104. doi: 10.1016/j.expneurol.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma K, Babij R, Cortes E, Vonsattel JP, Louis ED. Cerebellar pathology of a dual clinical diagnosis: Patients with essential tremor and dystonia. Tremor Other Hyperkinet Mov. 2012;2:1–6. doi: 10.7916/D8JD4VJ5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simpson DM, Blitzer A, Brashear A, et al. Assessment: Botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70:1699–1706. doi: 10.1212/01.wnl.0000311389.26145.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hallett M, Benecke R, Blitzer A, Comella CL. Treatment of focal dystonias with botulinum neurotoxin. Toxicon. 2009;54:628–633. doi: 10.1016/j.toxicon.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jankovic J. Medical treatment of dystonia. Mov Disord. 2013 doi: 10.1002/mds.25552. this issue. [DOI] [PubMed] [Google Scholar]
- 69.Brin MF, Comella CL, Jankovic J, Lai F, Naumann M. Long-term treatment with botulinum toxin type A in cervical dystonia has low immunogenicity by mouse protection assay. Mov Disord. 2008;23:1353–1360. doi: 10.1002/mds.22157. [DOI] [PubMed] [Google Scholar]
- 70.Ruiz PJ, Castrillo JC, Burguera JA, et al. Evolution of dose and response to botulinum toxin A in cervical dystonia: a multicenter study. J Neurol. 2011;258:1055–1057. doi: 10.1007/s00415-010-5880-1. [DOI] [PubMed] [Google Scholar]
- 71.Skogseid IM, Kerty E. The course of cervical dystonia and patient satisfaction with long-term botulinum toxin A treatment. Eur J Neurol. 2005;12:163–170. doi: 10.1111/j.1468-1331.2004.01053.x. [DOI] [PubMed] [Google Scholar]
- 72.Sethi KD, Rodriguez R, Olayinka B. Satisfaction with botulinum toxin treatment: a cross-sectional survey of patients with cervical dystonia. J Med Econ. 2012;15:419–423. doi: 10.3111/13696998.2011.653726. [DOI] [PubMed] [Google Scholar]
- 73.Waln O, Ledoux MS. Blepharospasm plus cervical dystonia with predominant anterocollis: A distinctive subphenotype of segmental craniocervical dystonia? Tremor Other Hyperkinet Mov. 2011;2011(1) doi: 10.7916/D8SQ8Z4T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Glass GA, Ku S, Ostrem JL, Heath S, Larson PS. Fluoroscopic, EMG-guided injection of botulinum toxin into the longus colli for the treatment of anterocollis. Parkinsonism Relat Disord. 2009;15:610–613. doi: 10.1016/j.parkreldis.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 75.Bhidayasiri R. Treatment of complex cervical dystonia with botulinum toxin: involvement of deep-cervical muscles may contribute to suboptimal responses. Parkinsonism Relat Disord. 2011;17(Suppl 1):S20–S24. doi: 10.1016/j.parkreldis.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 76.Arce CA. Selective denervation in cervical dystonia. In: Stacey MA, editor. Handbook of dystonia. New York: Informa Healthcare USA, Inc; 2007. pp. 381–392. [Google Scholar]
- 77.Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci. 2006;29:229–257. doi: 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marks WJJ. Brain surgery for dystonia. In: Stacey MA, editor. Handbook of dystonia. New York: Informa Healtcare USA, Inc; 2007. pp. 393–406. [Google Scholar]
- 79.Moro E, Gross RE, Krauss JK. What's new in surgical treatments for dystonia? Mov Disord. 2013 doi: 10.1002/mds.25550. This issue. [DOI] [PubMed] [Google Scholar]
- 80.Vidailhet M, Jutras MF, Grabli D, Roze E. Deep brain stimulation for dystonia. J Neurol Neurosurg Psychiatry. 2012 doi: 10.1136/jnnp-2011-301714. [DOI] [PubMed] [Google Scholar]
- 81.Kiss ZH, Doig-Beyaert K, Eliasziw M, Tsui J, Haffenden A, Suchowersky O. The Canadian multicentre study of deep brain stimulation for cervical dystonia. Brain. 2007;130:2879–2886. doi: 10.1093/brain/awm229. [DOI] [PubMed] [Google Scholar]
- 82.Limotai N, Go C, Oyama G, et al. Mixed results for GPi-DBS in the treatment of cranio-facial and cranio-cervical dystonia symptoms. J Neurol. 2011;258:2069–2074. doi: 10.1007/s00415-011-6075-0. [DOI] [PubMed] [Google Scholar]
- 83.Zetterberg L, Halvorsen K, Farnstrand C, Aquilonius SM, Lindmark B. Physiotherapy in cervical dystonia: six experimental single-case studies. Physiother Theory Pract. 2008;24:275–290. doi: 10.1080/09593980701884816. [DOI] [PubMed] [Google Scholar]
- 84.Boyce MJ, Canning CG, Mahant N, Morris J, Latimer J, Fung VS. Active exercise for individuals with cervical dystonia: a pilot randomized controlled trial. Clin Rehabil. 2012;27:226–235. doi: 10.1177/0269215512456221. [DOI] [PubMed] [Google Scholar]
- 85.Delnooz CC, Horstink MW, Tijssen MA, van de Warrenburg BP. Paramedical treatment in primary dystonia: a systematic review. Mov Disord. 2009;24:2187–2198. doi: 10.1002/mds.22608. [DOI] [PubMed] [Google Scholar]
- 86.Tassorelli C, Mancini F, Balloni L, et al. Botulinum toxin and neuromotor rehabilitation: An integrated approach to idiopathic cervical dystonia. Mov Disord. 2006;21:2240–2243. doi: 10.1002/mds.21145. [DOI] [PubMed] [Google Scholar]
- 87.Grandas F, Elston J, Quinn N, Marsden CD. Blepharospasm: a review of 264 patients. J Neurol Neurosurg Psychiatry. 1988;51:767–772. doi: 10.1136/jnnp.51.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peckham EL, Lopez G, Shamim EA, et al. Clinical features of patients with blepharospasm: a report of 240 patients. Eur J Neurol. 2011;18:382–386. doi: 10.1111/j.1468-1331.2010.03161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jankovic J, Ford J. Blepharospasm and orofacial-cervical dystonia: clinical and pharmacological findings in 100 patients. Ann Neurol. 1983;13:402–411. doi: 10.1002/ana.410130406. [DOI] [PubMed] [Google Scholar]
- 90.Tolosa E, Marti MJ. Blepharospasm-oromandibular dystonia syndrome (Meige's syndrome): clinical aspects. Adv Neurol. 1988;49:73–84. [PubMed] [Google Scholar]
- 91.Conte A, Defazio G, Ferrazzano G, et al. Is increased blinking a form of blepharospasm? Neurol. 2013 doi: 10.1212/WNL.0b013e318296e99d. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aramideh M, Ongerboer de Visser BW, Brans JW, Koelman JH, Speelman JD. Pretarsal application of botulinum toxin for treatment of blepharospasm. J Neurol Neurosurg Psychiatry. 1995;59:309–311. doi: 10.1136/jnnp.59.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Inoue K, Rogers JD. Botulinum toxin injection into Riolan's muscle: somatosensory 'trick'. Eur Neurol. 2007;58:138–141. doi: 10.1159/000104713. [DOI] [PubMed] [Google Scholar]
- 94.Aramideh M, Ongerboer de Visser BW, Devriese PP, Bour LJ, Speelman JD. Electromyographic features of levator palpebrae superioris and orbicularis oculi muscles in blepharospasm. Brain. 1994;117:27–38. doi: 10.1093/brain/117.1.27. [DOI] [PubMed] [Google Scholar]
- 95.Defazio G, Abbruzzese G, Girlanda P, et al. Phenotypic overlap in familial and sporadic primary adult-onset extracranial dystonia. J Neurol. 2012 doi: 10.1007/s00415-012-6514-6. [DOI] [PubMed] [Google Scholar]
- 96.Obeso JA, Gimenez-Roldan S. Clinicopathologic correlation in symptomatic dystonia. Adv Neurol. 1988;50:113–122. [PubMed] [Google Scholar]
- 97.Skidmore F, Reich SG. Tardive dystonia. Curr Treat Options Neurol. 2005;7:231–236. doi: 10.1007/s11940-005-0016-0. [DOI] [PubMed] [Google Scholar]
- 98.Rana AQ, Kabir A, Dogu O, Patel A, Khondker S. Prevalence of blepharospasm and apraxia of eyelid opening in patients with parkinsonism, cervical dystonia and essential tremor. Eur Neurol. 2012;68:318–321. doi: 10.1159/000341621. [DOI] [PubMed] [Google Scholar]
- 99.Defazio G, Abbruzzese G, Aniello MS, et al. Environmental risk factors and clinical phenotype in familial and sporadic primary blepharospasm. Neurology. 2011;77:631–637. doi: 10.1212/WNL.0b013e3182299e13. [DOI] [PubMed] [Google Scholar]
- 100.Martino D, Defazio G, Alessio G, et al. Relationship between eye symptoms and blepharospasm: a multicenter case-control study. Mov Disord. 2005;20:1564–1570. doi: 10.1002/mds.20635. [DOI] [PubMed] [Google Scholar]
- 101.Herz NL, Yen MT. Modulation of sensory photophobia in essential blepharospasm with chromatic lenses. Ophthalmology. 2005;112:2208–2211. doi: 10.1016/j.ophtha.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 102.Adams WH, Digre KB, Patel BC, Anderson RL, Warner JE, Katz BJ. The evaluation of light sensitivity in benign essential blepharospasm. Am J Ophthalmol. 2006;142:82–87. doi: 10.1016/j.ajo.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 103.Digre KB, Brennan KC. Shedding light on photophobia. J Neuroophthalmol. 2012;32:68–81. doi: 10.1097/WNO.0b013e3182474548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Noseda R, Constandil L, Bourgeais L, Chalus M, Villanueva L. Changes of meningeal excitability mediated by corticotrigeminal networks: a link for the endogenous modulation of migraine pain. J Neurosci. 2010;30:14420–14429. doi: 10.1523/JNEUROSCI.3025-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Emoto H, Suzuki Y, Wakakura M, et al. Photophobia in essential blepharospasm--a positron emission tomographic study. Mov Disord. 2010;25:433–439. doi: 10.1002/mds.22916. [DOI] [PubMed] [Google Scholar]
- 106.Khooshnoodi MA, Factor SA, Jinnah HA. Secondary blepharospasm associated with structural lesions of the brain. J Neurol Sci. 2013 doi: 10.1016/j.jns.2013.05.022. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Etgen T, Muhlau M, Gaser C, Sander D. Bilateral grey-matter increase in the putamen in primary blepharspasm. J Neurol Neurosurg Psychiat. 2006;77:1017–1020. doi: 10.1136/jnnp.2005.087148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Horovitz SG, Ford A, Ali Najee-ullah M, Ostuni JL, Hallett M. Anatomical correlates of blepharospasm. Translational Neurodegeneration. 2012;1:12. doi: 10.1186/2047-9158-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dresel C, Haslinger B, Castrop F, Wohlschlaeger AM, Ceballos-Baumann AO. Silent event-related fMRI reveals deficient motor and enhanced somatosensory activation in orofacial dystonia. Brain. 2006;129:36–46. doi: 10.1093/brain/awh665. [DOI] [PubMed] [Google Scholar]
- 110.Schmidt KE, Linden DE, Goebel R, Zanella FE, Lanfermann H, Zubcov AA. Striatal activation during blepharospasm revealed by fMRI. Neurology. 2003;60:1738–1743. doi: 10.1212/01.wnl.0000063306.67984.8c. [DOI] [PubMed] [Google Scholar]
- 111.Kerrison JB, Lancaster JL, Zamarripa FE, et al. Positron emission tomography scanning in essential blepharospasm. Am J Ophthalmol. 2003;136:846–852. doi: 10.1016/s0002-9394(03)00895-x. [DOI] [PubMed] [Google Scholar]
- 112.Suzuki Y, Mizoguchi S, Kiyosawa M, et al. Glucose hypermetabolism in the thalamus of patients with essential blepharospasm. J Neurol. 2007;254:890–896. doi: 10.1007/s00415-006-0468-5. [DOI] [PubMed] [Google Scholar]
- 113.Hutchinson M, Nakamura T, Moeller JR, et al. The metabolic topography of esential blepharospasm. Neurology. 2000;55:673–677. doi: 10.1212/wnl.55.5.673. [DOI] [PubMed] [Google Scholar]
- 114.Perlmutter JS, Stambuk MK, Markham J, et al. Decreased [18F]spiperone binding in putamen in idiopathic focal dystonia. J Neurosci. 1997;17:843–850. doi: 10.1523/JNEUROSCI.17-02-00843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Karimi M, Moerlein SM, Videen TO, et al. Decreased striatal dopamine receptor binding in primary focal dystonia: a D2 or D3 defect? Mov Disord. 2011;26:100–106. doi: 10.1002/mds.23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Quartarone A, Sant'Angelo A, Battaglia F, et al. Enhanced long-term potentiation-like plasticity of the trigeminal blink reflex circuit in blepharospasm. J Neurosci. 2006;26:716–721. doi: 10.1523/JNEUROSCI.3948-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zeuner KE, Knutzen A, Al-Ali A, et al. Associative stimulation of the supraorbital nerve fails to induce timing-specific plasticity in the human blink reflex. PLoS One. 2010;5:e13602. doi: 10.1371/journal.pone.0013602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fiorio M, Tinazzi M, Scontrini A, et al. Tactile temporal discrimination in patients with blepharospasm. J Neurol Neurosurg Psychiatry. 2008;79:796–798. doi: 10.1136/jnnp.2007.131524. [DOI] [PubMed] [Google Scholar]
- 119.Bradley D, Whelan R, Kimmich O, et al. Temporal discrimination thresholds in adult-onset primary torsion dystonia: an analysis by task type and by dystonia phenotype. J Neurol. 2012;259:77–82. doi: 10.1007/s00415-011-6125-7. [DOI] [PubMed] [Google Scholar]
- 120.Rana AQ, Shah R. Combination of blepharospasm and apraxia of eyelid opening: a condition resistant to treatment. Acta Neurol Belg. 2012;112:95–96. doi: 10.1007/s13760-012-0019-z. [DOI] [PubMed] [Google Scholar]
- 121.Georgescu D, Vagefi MR, McMullan TF, McCann JD, Anderson RL. Upper eyelid myectomy in blepharospasm with associated apraxia of lid opening. Am J Ophthalmol. 2008;145:541–547. doi: 10.1016/j.ajo.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 122.Andrews RM, Griffiths PG, Chinnery PF, Turnbull DM. Evaluation of bupivacaine-induced muscle regeneration in the treatment of ptosis in patients with chronic progressive external ophthalmoplegia and Kearns-Sayre syndrome. Eye (Lond) 1999;13:769–772. doi: 10.1038/eye.1999.225. [DOI] [PubMed] [Google Scholar]
- 123.Reese R, Gruber D, Schoenecker T, et al. Long-term clinical outcome in meige syndrome treated with internal pallidum deep brain stimulation. Mov Disord. 2011;26:691–698. doi: 10.1002/mds.23549. [DOI] [PubMed] [Google Scholar]
- 124.Weiss D, Wachter T, Breit S, et al. Involuntary eyelid closure after STN-DBS: evidence for different pathophysiological entities. J Neurol Neurosurg Psychiatry. 2010;81:1002–1007. doi: 10.1136/jnnp.2009.196691. [DOI] [PubMed] [Google Scholar]
- 125.Blackburn MK, Lamb RD, Digre KB, et al. FL-41 tint improves blink frequency, light sensitivity, and functional limitations in patients with benign essential blepharospasm. Ophthalmology. 2009;116:997–1001. doi: 10.1016/j.ophtha.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Blitzer A. Spasmodic dysphonia and botulinum toxin: experience from the largest treatment series. Eur J Neurol. 2010;17(Suppl 1):28–30. doi: 10.1111/j.1468-1331.2010.03047.x. [DOI] [PubMed] [Google Scholar]
- 127.Brin MF, Blitzer A, Stewart C. Laryngeal dystonia (spasmodic dysphonia): observations of 901 patients and treatment with botulinum toxin. Adv Neurol. 1998;78:237–252. [PubMed] [Google Scholar]
- 128.Ludlow CL, Adler CH, Berke GS, et al. Research priorities in spasmodic dysphonia. Oto Head Neck Surg. 2008;139:495–505. doi: 10.1016/j.otohns.2008.05.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tisch SHD, HM B, Law M, IE C, Darveniza P. Spasmodic dysphonia: clinical features and effects of botulinum toxin therapy in 169 patients - an Australian experience. J Clin Neurosci. 2003;10:434–438. doi: 10.1016/s0967-5868(03)00020-1. [DOI] [PubMed] [Google Scholar]
- 130.Zwirner P, Dressler D, Kruse E. Spasmodic laryngeal dyspnea: a rare manifestation of laryngeal dystonia. Eur Arch Otorhinolaryngol. 1997;254:242–245. doi: 10.1007/BF00874096. [DOI] [PubMed] [Google Scholar]
- 131.Grillone GA, Blitzer A, Brin MF, Annino DJ, Saint-Hilaire MH. Treatment of adductor laryngeal breathing dystonia with botulinum toxin type A. Laryngoscope. 1994;104:30–32. doi: 10.1288/00005537-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 132.Marion MH, Klap P, Perrin A, Cohen M. Stridor and focal laryngeal dystonia. Lancet. 1992;339:457–458. doi: 10.1016/0140-6736(92)91060-l. [DOI] [PubMed] [Google Scholar]
- 133.Chitkara A, Meyer T, Keidar A, Blitzer A. Singer's dystonia: first report of a variant of spasmodic dysphonia. Ann Otol Rhinol Laryngol. 2006;115:89–92. doi: 10.1177/000348940611500201. [DOI] [PubMed] [Google Scholar]
- 134.White LJ, Klein AM, Hapner ER, et al. Coprevalence of tremor with spasmodic dysphonia: a case-control study. Laryngoscope. 2011;121:1752–1755. doi: 10.1002/lary.21872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wolraich D, Vasile Marchis-Crisan C, Redding N, Khella SL, Mirza N. Laryngeal tremor: co-occurrence with other movement disorders. ORL J Otorhinolaryngol Relat Spec. 2010;72:291–294. doi: 10.1159/000317032. [DOI] [PubMed] [Google Scholar]
- 136.Roy N. Functional dysphonia. Curr Opin Otolaryngol Head Neck Surg. 2003;11:144–148. doi: 10.1097/00020840-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 137.Altman KW, Atkinson C, Lazarus C. Current and emerging concepts in muscle tension dysphonia: a 30-month review. J Voice. 2005;19:261–267. doi: 10.1016/j.jvoice.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 138.Morrison MD, Rammage LA, Belisle GM, Pullan CB, Nichol H. Muscular tension dysphonia. J Otolaryngol. 1983;12:302–306. [PubMed] [Google Scholar]
- 139.Fuchs T, Gavarini S, Saunders-Pullman R, et al. Mutations in the THAP1 gene are responsible for DYT6 primary torsion dystonia. Nat Genet. 2009 doi: 10.1038/ng.304. [DOI] [PubMed] [Google Scholar]
- 140.Xiao J, Zhao Y, Bastian RW, et al. Novel THAP1 sequence variants in primary dystonia. Neurology. 2010;74:229–238. doi: 10.1212/WNL.0b013e3181ca00ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hersheson J, Mencacci NE, Davis M, et al. Mutations in the autoregulatory domain of beta-tubulin 4a cause hereditary dystonia. Ann Neurol. 2012 doi: 10.1002/ana.23832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wilcox RA, Winkler S, Lohmann K, Klein C. Whispering dysphonia in an Australian family (DYT4): a clinical and genetic reappraisal. Mov Disord. 2011;26:2404–2408. doi: 10.1002/mds.23866. [DOI] [PubMed] [Google Scholar]
- 143.Lohmann K, Wilcox RA, Winkler S, et al. Whispering dysphonia (DYT4 dystonia) is caused by a mutation in the TUBB4 gene. Ann Neurol. 2012 doi: 10.1002/ana.23829. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schweinfurth JM, Billante M, Courey MS. Risk factors and demographics in patients with spasmodic dysphonia. Laryngoscope. 2002;112:220–223. doi: 10.1097/00005537-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 145.Tanner K, Roy N, Merrill RM, Sauder C, Houtz DR, Smith ME. Case-control study of risk factors for spasmodic dysphonia: A comparison with other voice disorders. Laryngoscope. 2012;122:1082–1092. doi: 10.1002/lary.22471. [DOI] [PubMed] [Google Scholar]
- 146.Ali SO, Thomassen M, Schulz GM, et al. Alterations in CNS activity induced by botulinum toxin treatment in spasmodic dysphonia: an H215O PET study. J Speech Lang Hear Res. 2006;49:1127–1146. doi: 10.1044/1092-4388(2006/081). [DOI] [PubMed] [Google Scholar]
- 147.Haslinger B, Erhard P, Dresel C, Castrop F, Roettinger M, Ceballos-Baumann AO. "Silent event-related" fMRI reveals reduced sensorimotor activation in laryngeal dystonia. Neurology. 2005;65:1562–1569. doi: 10.1212/01.wnl.0000184478.59063.db. [DOI] [PubMed] [Google Scholar]
- 148.Simonyan K, Ludlow CL. Abnormal activation of the primary somatosensory cortex in spasmodic dysphonia: An fMRI study. Cereb Cortex. 2010;20:2749–2759. doi: 10.1093/cercor/bhq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Simonyan K, Tovar-Moll F, Ostuni J, et al. Focal white matter changes in spasmodic dysphonia: a combined diffusion tensor imaging and neuropathological study. Brain. 2008;131:447–459. doi: 10.1093/brain/awm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Simonyan K, Ludlow CL. Abnormal structure-function relationship in spasmodic dysphonia. Cereb Cortex. 2012;22:417–425. doi: 10.1093/cercor/bhr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Blitzer A, Brin MF, Stewart CF. Botulinum toxin management of spasmodic dysphonia (laryngeal dystonia): a 12-year experience in more than 900 patients. Laryngoscope. 1998;108:1435–1441. doi: 10.1097/00005537-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 152.Watts CC, Whurr R, Nye C. Botulinum toxin injections for the treatment of spasmodic dysphonia. Cochrane Database Syst Rev. 2004;3:CD004327. doi: 10.1002/14651858.CD004327.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Whurr R, Nye C, Lorch M. Meta-analysis of botulinum toxin treatment of spasmodic dysphonia: a review of 22 studies. Int J Lang Commun Disord. 1998;33(Suppl):327–329. doi: 10.3109/13682829809179445. [DOI] [PubMed] [Google Scholar]
- 154.Truong DD, Rontal M, Rolnick M, AEA KM. Double-blind controlled study of botulinum toxin in adductor spasmodic dysphonia. Laryngoscope. 1991;101:630–634. doi: 10.1288/00005537-199106000-00010. [DOI] [PubMed] [Google Scholar]
- 155.Ludlow CL. Treatment for spasmodic dysphonia: limitations of current approaches. Curr Opin Otolaryngol Head Neck Surg. 2009;17:160–165. doi: 10.1097/MOO.0b013e32832aef6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Torres-Russotto D, Perlmutter JS. Task-specific dystonias: a review. Ann N Y Acad Sci. 2008;1142:179–199. doi: 10.1196/annals.1444.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Altenmuller E, Baur V, Hofmann A, Lim VK, Jabusch HC. Musician's cramp as manifestation of maladaptive brain plasticity: arguments from instrumental differences. Ann N Y Acad Sci. 2012;1252:259–265. doi: 10.1111/j.1749-6632.2012.06456.x. [DOI] [PubMed] [Google Scholar]
- 158.Conti AM, Pullman S, Frucht SJ. The hand that has forgotten its cunning--lessons from musicians' hand dystonia. Mov Disord. 2008;23:1398–1406. doi: 10.1002/mds.21976. [DOI] [PubMed] [Google Scholar]
- 159.Schneider SA, Edwards MJ, Grill SE, et al. Adult-onset primary lower limb dystonia. Mov Disord. 2006;21:767–771. doi: 10.1002/mds.20794. [DOI] [PubMed] [Google Scholar]
- 160.Wu LJ, Jankovic J. Runner's dystonia. Journal of the neurological sciences. 2006;251(1–2):73–76. doi: 10.1016/j.jns.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 161.Munts AG, Mugge W, Meurs TS, et al. Fixed dystonia in complex regional pain syndrome: a descriptive and computational modeling approach. BMC Neurol. 2011;11:53. doi: 10.1186/1471-2377-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Quartarone A, Hallett M. Emerging concepts in the physiological basis of dystonia. Mov Disord. 2013 doi: 10.1002/mds.25532. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Leijnse JN, Hallett M. Etiological musculo-skeletal factor in focal dystonia in a musician's hand: A case study of the right hand of a guitarist. Mov Disord. 2007;22:1803–1808. doi: 10.1002/mds.21636. [DOI] [PubMed] [Google Scholar]
- 164.Garraux G, Bauer A, Hanakawa T, Wu T, Kansaku K, Hallett M. Changes in brain anatomy in focal hand dystonia. Ann Neurol. 2004;55:736–739. doi: 10.1002/ana.20113. [DOI] [PubMed] [Google Scholar]
- 165.Lungu C, Karp BI, Alter K, Zolbrod R, Hallett M. Long-term follow-up of botulinum toxin therapy for focal hand dystonia: outcome at 10 years or more. Mov Disord. 2011;26:750–753. doi: 10.1002/mds.23504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zeuner KE, Shill HA, Sohn YH, et al. Motor training as treatment in focal hand dystonia. Mov Disord. 2005;20:335–341. doi: 10.1002/mds.20314. [DOI] [PubMed] [Google Scholar]
- 167.Zeuner KE, Bara-Jimenez W, Noguchi PS, Goldstein SR, Dambrosia JM, Hallett M. Sensory training for patients with focal hand dystonia. Ann Neurol. 2002;51:593–598. doi: 10.1002/ana.10174. [DOI] [PubMed] [Google Scholar]
- 168.Horisawa S, Taira T, Goto S, Ochiai T, Nakajima T. Long-term improvement of musician's dystonia after stereotactic ventrooralthalamotomy. Ann Neurol. 2013 doi: 10.1002/ana.23877. in press. [DOI] [PubMed] [Google Scholar]
- 169.LeDoux MS. Meige syndrome: what's in a name? Parkinsonism Relat Disord. 2009;15:483–489. doi: 10.1016/j.parkreldis.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Thompson PD, Obeso JA, Delgado G, Gallego J, Marsden CD. Focal dystonia of the jaw and the differential diagnosis of unilateral jaw and masticatory spasm. J Neurol Neurosurg Psychiatry. 1986;49:651–656. doi: 10.1136/jnnp.49.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schneider SA, Bhatia KP. The entity of jaw tremor and dystonia. Mov Disord. 2007;22:1491–1495. doi: 10.1002/mds.21531. [DOI] [PubMed] [Google Scholar]
- 172.Marsden CD. Blepharospasm-oromandibular dystonia syndrome (Brueghel's syndrome) A variant of adult-onset torsion dystonia? J Neurol Neurosurg Psychiatry. 1976;39:1204–1209. doi: 10.1136/jnnp.39.12.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tan EK, Chan LL. Sensory tricks and treatment in primary lingual dystonia. Mov Disord. 2005;20:388. doi: 10.1002/mds.20388. [DOI] [PubMed] [Google Scholar]
- 174.Ishii K, Tamaoka A, Shoji S. A case of primary focal lingual dystonia induced by speaking. Eur J Neurol. 2001;8:507. doi: 10.1046/j.1468-1331.2001.00276.x. [DOI] [PubMed] [Google Scholar]
- 175.Baik JS, Park JH, Kim JY. Primary lingual dystonia induced by speaking. Mov Disord. 2004;19:1251–1252. doi: 10.1002/mds.20157. [DOI] [PubMed] [Google Scholar]
- 176.Papapetropoulos S, Singer C. Primary focal lingual dystonia. Mov Disord. 2006;21:429–430. doi: 10.1002/mds.20790. [DOI] [PubMed] [Google Scholar]
- 177.Esper CD, Freeman A, Factor SA. Lingual protrusion dystonia: frequency, etiology and botulinum toxin therapy. Parkinsonism Relat Disord. 2010;16:438–441. doi: 10.1016/j.parkreldis.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 178.Charles PD, Davis TL, Shannon KM, Hook MA, Warner JS. Tongue protrusion dystonia: treatment with botulinum toxin. South Med J. 1997;90:522–525. doi: 10.1097/00007611-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 179.Schneider SA, Aggarwal A, Bhatt MH, et al. Severe tongue protrusion dystonia: clinical syndromes and possible treatment. Neurology. 2006;67:940–943. doi: 10.1212/01.wnl.0000237446.06971.72. [DOI] [PubMed] [Google Scholar]
- 180.Papapetropoulos S, Singer C. Eating dysfunction associated with oromandibular dystonia: clinical characteristics and treatment considerations. Head Face Med. 2006;2:47. doi: 10.1186/1746-160X-2-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Singer C, Papapetropoulos S. A comparison of jaw-closing and jaw-opening idiopathic oromandibular dystonia. Parkinsonism Relat Disord. 2006;12:115–118. doi: 10.1016/j.parkreldis.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 182.Raudino F. Is temporomandibular dysfunction a cranial dystonia? An electrophysiological study. Headache. 1994;34:471–475. doi: 10.1111/j.1526-4610.1994.hed3408471.x. [DOI] [PubMed] [Google Scholar]
- 183.Frucht SJ, Fahn S, Greene PE, et al. The natural history of embouchure dystonia. Mov Disord. 2001;16:899–906. doi: 10.1002/mds.1167. [DOI] [PubMed] [Google Scholar]
- 184.Bhatia KP, Quinn NP, Marsden CD. Clinical features and natural history of axial predominant adult onset primary dystonia. J Neurol Neurosurg Psychiatry. 1997;63:788–791. doi: 10.1136/jnnp.63.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Doherty KM, van de Warrenburg BP, Peralta MC, et al. Postural deformities in Parkinson's disease. Lancet Neurol. 2011;10:538–549. doi: 10.1016/S1474-4422(11)70067-9. [DOI] [PubMed] [Google Scholar]
- 186.O'Riordan S, Lynch T, Hutchinson M. Familial adolescent-onset scoliosis and later segmental dystonia in an Irish family. J Neurol. 2004;251:845–848. doi: 10.1007/s00415-004-0444-x. [DOI] [PubMed] [Google Scholar]
- 187.Domenech J, Tormos JM, Barrios C, Pascual-Leone A. Motor cortical hyperexcitability in idiopathic scoliosis: could focal dystonia be a subclinical etiological factor? Eur Spine J. 2010;19:223–230. doi: 10.1007/s00586-009-1243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Benecke R, Dressler D. Botulinum toxin treatment of axial and cervical dystonia. Disabil Rehabil. 2007;29:1769–1777. doi: 10.1080/01421590701568262. [DOI] [PubMed] [Google Scholar]
- 189.Comella CL, Shannon KM, Jaglin J. Extensor truncal dystonia: successful treatment with botulinum toxin injections. Mov Disord. 1998;13:552–555. doi: 10.1002/mds.870130330. [DOI] [PubMed] [Google Scholar]
- 190.Zittel S, Moll CK, Hamel W, et al. Successful GPi deep brain stimulation in a patient with adult onset primary axial dystonia. J Neurol Neurosurg Psychiatry. 2009;80:811–812. doi: 10.1136/jnnp.2008.153262. [DOI] [PubMed] [Google Scholar]
- 191.O'Riordan S, Raymond D, Lynch T, et al. Age at onset as a factor in determining the phenotype of primary torsion dystonia. Neurology. 2004;63:1423–1426. doi: 10.1212/01.wnl.0000142035.26034.c2. [DOI] [PubMed] [Google Scholar]
- 192.Weiss EM, Hershey T, Karimi M, et al. Relative risk of spread of symptoms among the focal onset primary dystonias. Mov Disord. 2006;21:1175–1181. doi: 10.1002/mds.20919. [DOI] [PubMed] [Google Scholar]
- 193.Martino D, Berardelli A, Abbruzzese G, et al. Age at onset and symptom spread in primary adult-onset blepharospasm and cervical dystonia. Mov Disord. 2012;27:1447–1450. doi: 10.1002/mds.25088. [DOI] [PubMed] [Google Scholar]
- 194.Shamim EA, Chu J, Scheider LH, Savitt J, Jinnah HA, Hallett M. Extreme task specificity in writer's cramp. Mov Disord. 2011;26:2107–2109. doi: 10.1002/mds.23827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lin PT, Hallett M. The pathophysiology of focal hand dystonia. J Hand Ther. 2009;22:109–113. doi: 10.1016/j.jht.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Quartarone A, Siebner HR, Rothwell JC. Task-specific hand dystonia: can too much plasticity be bad for you? Trends Neurosci. 2006;29:192–199. doi: 10.1016/j.tins.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 197.LeDoux MS, Dauer WT, Warner T. Emerging molecular pathways for dystonia. Mov Disord. 2013 doi: 10.1002/mds.25547. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Marsden CD. The problem of adult-onset idiopathic torsion dystonia and other isolated dyskinesias in adult life (including blepharospasm, oromandibular dystonia, dystonic writer's cramp, and torticollis, or axial dystonia) Adv Neurol. 1976;14:259–276. [PubMed] [Google Scholar]
- 199.Blanchard A, Ea V, Roubertie A, et al. DYT6 dystonia: review of the literature and creation of the UMD Locus-Specific Database (LSDB) for mutations in the THAP1 gene. Hum Mutat. 2011;32:1213–1224. doi: 10.1002/humu.21564. [DOI] [PubMed] [Google Scholar]
- 200.Bragg DC, Armata IA, Nery FC, Breakefield XO, Sharma N. Molecular pathways in dystonia. Neurobiol Dis. 2011;42:136–147. doi: 10.1016/j.nbd.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Thompson VB, Jinnah HA, Hess EJ. Convergent mechanisms in etiologically-diverse dystonias. Expert Opin Ther Targets. 2011;15:1387–1403. doi: 10.1517/14728222.2011.641533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jinnah HA, Hess EJ. Experimental therapeutics for dystonia. Neurotherapeutics. 2008;5:198–209. doi: 10.1016/j.nurt.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Perlmutter JS, Mink JW. Dysfunction of dopaminergic pathways in dystonia. Adv Neurol. 2004;94:163–170. [PubMed] [Google Scholar]
- 204.Peterson DA, Sejnowski TJ, Poizner H. Convergent evidence for abnormal striatal synaptic plasticity in dystonia. Neurobiol Dis. 2010;37:558–573. doi: 10.1016/j.nbd.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Segawa M, Nomura Y, Nishiyama N. Dopa-responsive dystonia. In: Stacey MA, editor. Handbook of dystonia. Yew York: Informa Healthcare, Inc; 2008. pp. 219–244. [Google Scholar]
- 206.Jinnah HA, Visser JE, Harris JC, et al. Delineation of the motor disorder of Lesch-Nyhan disease. Brain. 2006;129:1201–1217. doi: 10.1093/brain/awl056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Visser JE, Baer PR, Jinnah HA. Lesch-Nyhan syndrome and the basal ganglia. Brain Res Rev. 2000;32:449–475. doi: 10.1016/s0165-0173(99)00094-6. [DOI] [PubMed] [Google Scholar]
- 208.Tolosa E, Compta Y. Dystonia in Parkinson's disease. J Neurol. 2006;253(Suppl. 7):7–13. doi: 10.1007/s00415-006-7003-6. [DOI] [PubMed] [Google Scholar]
- 209.Jankovic J, Tintner R. Dystonia and parkinsonism. Parkinsonism & Rel Disord. 2001;8:109–121. doi: 10.1016/s1353-8020(01)00025-6. [DOI] [PubMed] [Google Scholar]
- 210.Troiano AR, Stoessl AJ. Neuroimaging in dystonia. In: Stacey MA, editor. Handbook of dystonia. New York: Informa Healthcare, Inc; 2008. pp. 93–106. [Google Scholar]
- 211.Augood SJ, Hollingsworth Z, Albers DS, et al. Dopamine transmission in DYT1 dystonia. Adv Neurol. 2004;94:53–60. [PubMed] [Google Scholar]
- 212.Boyce S, Clarke CE, Luguin R, et al. Induction of chorea and dystonia in Parkinsonian primates. Mov Disord. 1990;5:3–7. doi: 10.1002/mds.870050103. [DOI] [PubMed] [Google Scholar]
- 213.Perlmutter JS, Tempel LW, Black KJ, Parkinson D, Todd RD. MPTP induces dystonia and parkinsonism. Clues to the pathophysiology of dystonia. Neurology. 1997;49:1432–1438. doi: 10.1212/wnl.49.5.1432. [DOI] [PubMed] [Google Scholar]
- 214.Song CH, Fan X, Exeter CJ, Hess EJ, Jinnah HA. Functional analysis of dopaminergic systems in a DYT1 knock-in mouse model of dystonia. Neurobiol Dis. 2012;48:66–78. doi: 10.1016/j.nbd.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jinnah HA, Sepkuty JP, Ho T, et al. Calcium channel agonists and dystonia in the mouse. Mov Disord. 2000;15:542–551. doi: 10.1002/1531-8257(200005)15:3<542::AID-MDS1019>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 216.Neychev V, Fan X, Mitev VI, Hess EJ, Jinnah HA. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain. 2008;131:2499–2509. doi: 10.1093/brain/awn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pizoli CE, Jinnah HA, Billingsley ML, Hess EJ. Abnormal cerebellar signaling induces dystonia in mice. J Neurosci. 2002;22:7825–7833. doi: 10.1523/JNEUROSCI.22-17-07825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shirley TL, Rao LM, Hess EJ, Jinnah HA. Paroxysmal dyskinesias in mice. Mov Disord. 2008;23:259–264. doi: 10.1002/mds.21829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Campbell DB, Hess EJ. L-type calcium channels contribute to the tottering mouse dystonic episodes. Mol Pharmacol. 1999;55:23–31. doi: 10.1124/mol.55.1.23. [DOI] [PubMed] [Google Scholar]
- 220.Hess EJ, Jen JC, Jinnah HA. Neuronal voltage-gated calcium channels: brief overview of their function and clinical implications in neurology. Neurology. 2010;75:937–938. doi: 10.1212/WNL.0b013e3181eee9e8. [DOI] [PubMed] [Google Scholar]
- 221.Du W, Bautista JF, Yang H, et al. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nature Genet. 2005;37:733–738. doi: 10.1038/ng1585. [DOI] [PubMed] [Google Scholar]
- 222.Demirkiran M, Jankovic J. Paroxysmal dyskinesias: clinical features and classification. Ann Neurol. 1995;38:571–579. doi: 10.1002/ana.410380405. [DOI] [PubMed] [Google Scholar]
- 223.Nemeth AH. The genetics of primary dystonias and related disorders. Brain. 2002;125:695–721. doi: 10.1093/brain/awf090. [DOI] [PubMed] [Google Scholar]
- 224.Moustris A, Edwards MJ, Bhatia KP. Movement disorders and mitochondrial disease. Handb Clin Neurol. 2011;100:173–192. doi: 10.1016/B978-0-444-52014-2.00010-0. [DOI] [PubMed] [Google Scholar]
- 225.Wallace DC, Murdock DG. Mitochondria and dystonia: the movement disorder connection? Proc Natl Acad Sci U S A. 1999;96:1817–1819. doi: 10.1073/pnas.96.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Swerdlow RH, Wooten GF. A novel deafness/dystonia peptide gene mutation that causes dystonia in female carriers of Mohr-Tranebjaerg syndrome. Ann Neurol. 2001;50:537–540. doi: 10.1002/ana.1160. [DOI] [PubMed] [Google Scholar]
- 227.Swerdlow NR, Juel VC, Wooten GF. Dystonia with and without deafness is caused by TIMM8A mutation. Adv Neurol. 2004;94:147–153. [PubMed] [Google Scholar]
- 228.McFarland R, Chinnery PF, Blakely EL, et al. Homoplasmy, heteroplasmy, and mitochondrial dystonia. Neurology. 2007;69:911–916. doi: 10.1212/01.wnl.0000267843.10977.4a. [DOI] [PubMed] [Google Scholar]
- 229.Simon DK, Friedman J, Breakefield XO, et al. A heteroplasmic mitochondrial complex I gene mutation in adult-onset dystonia. Neurogenetics. 2003;4:199–205. doi: 10.1007/s10048-003-0150-3. [DOI] [PubMed] [Google Scholar]
- 230.Kim HT, Edwards MJ, Tyson J, Quinn NP, Bintner-Glindzicz M, Bhatia KP. Blepharospasm and limb dystonia caused by Mohr-Tranebjaerg syndrome with a novel splice site mutation in the deafness/dystonia peptide gene. Mov Disord. 2007;22:1328–1331. doi: 10.1002/mds.21351. [DOI] [PubMed] [Google Scholar]
- 231.Muller-Vahl KR, Kolbe H, Egensperger R, Dengler R. Mitochondriopathy, blepharospasm, and treatment with botulinum toxin. Muscle Nerve. 2000;23:647–648. doi: 10.1002/(sici)1097-4598(200004)23:4<647::aid-mus27>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 232.He F, Zhang S, Qian G, Zhang C. Delayed dystonia with striatal CT lucencies induced by a mycotoxin (3-nitropropionic acid) Neurology. 1995;45:2178–2183. doi: 10.1212/wnl.45.12.2178. [DOI] [PubMed] [Google Scholar]
- 233.Palfi S, Leventhal L, Goetz CG, et al. Delayed onset of progressive dystonia following subacute 3-nitropropionic acid treatment in Cebus apella monkeys. Mov Disord. 2000;15:524–530. [PubMed] [Google Scholar]
- 234.Schapira AH, Warner T, Gash MT, Cleeter MW, Marinho CF, Cooper JM. Complex I function in familial and sporadic dystonia. Ann Neurol. 1997;41:556–559. doi: 10.1002/ana.410410421. [DOI] [PubMed] [Google Scholar]
- 235.Benecke R, Strumper P, Weiss H. Electron transfer complex I defect in idiopathic dystonia. Ann Neurol. 1992;32:683–686. doi: 10.1002/ana.410320512. [DOI] [PubMed] [Google Scholar]
- 236.Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. The pathophysiology of primary dystonia. Brain. 1998;121:1195–1212. doi: 10.1093/brain/121.7.1195. [DOI] [PubMed] [Google Scholar]
- 237.Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–234. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- 238.Hallett M. Pathophysiology of dystonia. J Neural Transm Suppl. 2006;70:485–488. doi: 10.1007/978-3-211-45295-0_72. [DOI] [PubMed] [Google Scholar]
- 239.Jinnah HA, Hess EJ. A new twist on the anatomy of dystonia: the basal ganglia and the cerebellum. Neurology. 2006;67:1740–1741. doi: 10.1212/01.wnl.0000246112.19504.61. [DOI] [PubMed] [Google Scholar]
- 240.Niethammer M, Carbon-Correll M, Argyelan M, Eidelberg D. Hereditary dystonia as a neurodevelopmental circuit disorder: Evidence from neuroimaging. Neurobiol Dis. 2010;42:202–209. doi: 10.1016/j.nbd.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lehericy S. A current view of the anatomic basis of dystonia. Mov Disord. 2013 doi: 10.1002/mds.25527. This issue. [DOI] [PubMed] [Google Scholar]
- 242.Zoons E, Tijssen MAJ. Pathological changes in the brain in cervical dystonia pre- and post-mortem: a commentary with a special focus on the cerebellum. Exp Neurol. 2013 doi: 10.1016/j.expneurol.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 243.Schicatano EJ, Basso MA, Evinger C. Animal model explains the origins of the cranial dystonia benign essential blepharospasm. J Neurophysiol. 1997;77:2842–2846. doi: 10.1152/jn.1997.77.5.2842. [DOI] [PubMed] [Google Scholar]
- 244.Argyelan M, Carbon M, Niethammer M, et al. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J Neurosci. 2009;29:9740–9747. doi: 10.1523/JNEUROSCI.2300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Calderon DP, Fremont R, Kraenzlin F, Khodakhah K. The neural substrates of rapid-onset dystonia-parkinsonism. Nat Neurosci. 2011;14:357–365. doi: 10.1038/nn.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Tinazzi M, Fiorio M, Fiaschi A, Rothwell JC, Bhatia KP. Sensory functions in dystonia: insights from behavioral studies. Mov Disord. 2009;24:1427–1436. doi: 10.1002/mds.22490. [DOI] [PubMed] [Google Scholar]
- 247.Bradley D, Whelan R, Walsh R, et al. Temporal discrimination threshold: VBM evidence for an endophenotype in adult onset primary torsion dystonia. Brain. 2009;132:2327–2335. doi: 10.1093/brain/awp156. [DOI] [PubMed] [Google Scholar]
- 248.Rothwell JC, Huang YZ. Systems-level studies of movement disorders in dystonia and Parkinson's disease. Curr Opin Neurobiol. 2003;13:691–695. doi: 10.1016/j.conb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 249.Gonzalez-Alegre P, Bode N, Davidson BL, Paulson HL. Silencing primary dystonia: lentiviral-mediated RNA interference therapy for DYT1 dystonia. J Neurosci. 2005;25:10502–10509. doi: 10.1523/JNEUROSCI.3016-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Shaikh AG, Jinnah HA, Tripp RM, et al. Irregularity distinguishes limb tremor in cervical dystonia from essential tremor. J Neurol Neurosurg Psychiat. 2008;79:187–189. doi: 10.1136/jnnp.2007.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Stamelou M, Edwards MJ, Hallett M, Bhatia KP. The non-motor syndrome of primary dystonia: clinical and pathophysiological implications. Brain. 2012;135:1668–1681. doi: 10.1093/brain/awr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kuyper DJ, Parra V, Aerts S, Okun MS, Kluger BM. Nonmotor manifestations of dystonia: a systematic review. Mov Disord. 2011;26:1206–1217. doi: 10.1002/mds.23709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zurowski M, Marsh L, McDonald W. Psychiatric comorbidities in dystonia: Emerging concepts. Mov Disord. 2013 doi: 10.1002/mds.25501. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Logroscino G, Livrea P, Anaclerio D, et al. Agreement among neurologists on the clinical diagnosis of dystonia at different body sites. J Neurol Neurosurg Psychiatry. 2003;74:348–350. doi: 10.1136/jnnp.74.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Albanese A, Lalli S. Is this dystonia? Mov Disord. 2009;24:1725–1731. doi: 10.1002/mds.22597. [DOI] [PubMed] [Google Scholar]
- 256.Bertram KL, Williams DP. Diagnostic Delay in CD. Movement Disorders. 2012;27(Suppl 1):S333. [Google Scholar]
- 257.Jog M, Chouinard S, Hobson D, et al. Causes for treatment delays in dystonia and hemifacial spasm: a Canadian survey. Can J Neurol Sci. 2011;38:704–711. doi: 10.1017/s0317167100012270. [DOI] [PubMed] [Google Scholar]
- 258.Zoons E, Tijssen MAJ. Pathological changes in the brain in cervical dystonia pre- and post-mortem: a commentary with a special focus on the cerebellum. Exp Neurol. doi: 10.1016/j.expneurol.2013.04.005. in press. [DOI] [PubMed] [Google Scholar]
- 259.Draganski B, May A. Training-induced structural changes in the adult human brain. Behav Brain Res. 2008;192:137–142. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 260.Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 262.Song CH, Bernhard D, Bolarinwa C, Hess EJ, Smith Y, Jinnah HA. Subtle microstructural changes of the striatum in a DYT1 knock-in mouse model of dystonia. Neurobiol Dis. 2013 doi: 10.1016/j.nbd.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zhang L, Yokoi F, Jin YH, et al. Altered dendritic morphology of Purkinje cells in Dyt1 DeltaGAG knock-in and purkinje cell-specific Dyt1 conditional knockout mice. PLoS One. 2011;6:e18357. doi: 10.1371/journal.pone.0018357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Oleas J, Yokoi F, MP D, Pisani A, Li Y. Engineering animal models for dystonia: What have we learned? Mov Disord. 2013 doi: 10.1002/mds.25583. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Jinnah HA, Hess EJ, LeDoux MS, Sharma N, Baxter MG, DeLong MR. Rodent models for dystonia research: characteristics, evaluation, and utility. Mov Disord. 2005;20:283–292. doi: 10.1002/mds.20364. [DOI] [PubMed] [Google Scholar]
- 266.Hess EJ. Symptomatic animal models for dystonia. Mov Disord. 2013 doi: 10.1002/mds.25526. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Jinnah HA, Richter A, Mink JW, et al. Animal models for drug discovery in dystonia. Expert Opin Drug Discovery. 2008;3:83–97. doi: 10.1517/17460441.3.1.83. [DOI] [PubMed] [Google Scholar]
- 268.Raike RS, Pizoli CE, Weisz C, van den Maagdenberg AM, Jinnah HA, Hess EJ. Limited regional cerebellar dysfunction induces focal dystonia in mice. Neurobiol Dis. 2012 doi: 10.1016/j.nbd.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Nakazawa M, Kobayashi T, Matsuno K, Mita S. Possible involvement of a o-receptor subtype in the neck dystonia in rats. Pharmacol Biochem Behav. 1999;62:123–126. doi: 10.1016/s0091-3057(98)00146-4. [DOI] [PubMed] [Google Scholar]
- 270.Klier EM, Wang H, Constantin AG, Crawford JD. Midbrain control of three-dimensional head orientation. Science. 2002;295:1314–1316. doi: 10.1126/science.1067300. [DOI] [PubMed] [Google Scholar]
- 271.Holmes AL, Forcelli PA, Desjardin JT, et al. Superior colliculus mediates cervical dystonia evoked by inhibition of the substantia nigra pars reticulata. J Neurosci. 2012:32. doi: 10.1523/JNEUROSCI.2295-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Guehl D, Cuny E, Ghorayeb I, Michelet T, Bioulac B, Burbaud P. Primate models of dystonia. Prog Neurobiol. 2009;87:118–131. doi: 10.1016/j.pneurobio.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 273.Perlmutter JS, Thach WT. Writer's cramp: questions of causation. Neurology. 2007;69:331–332. doi: 10.1212/01.wnl.0000269330.95232.7c. [DOI] [PubMed] [Google Scholar]
- 274.Popa T, Velayudhan B, Hubsch C, et al. Cerebellar processing of sensory inputs primes motor cortex plasticity. Cereb Cortex. 2013;23:305–314. doi: 10.1093/cercor/bhs016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Espay AJ, Morgante F, Purzner J, Gunraj CA, Lang AE, Chen R. Cortical and spinal abnormalities in psychogenic dystonia. Ann Neurol. 2006;59:825–834. doi: 10.1002/ana.20837. [DOI] [PubMed] [Google Scholar]
- 276.Martino D, Liuzzi D, Macerollo A, Aniello MS, Livrea P, Defazio G. The phenomenology of the geste antagoniste in primary blepharospasm and cervical dystonia. Mov Disord. 2010;25:407–412. doi: 10.1002/mds.23011. [DOI] [PubMed] [Google Scholar]
- 277.Cao S, Hewett JW, Yokoi F, et al. Chemical enhancement of torsinA function in cell and animal models of torsion dystonia. Dis Model Mech. 2010;3:386–396. doi: 10.1242/dmm.003715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Hewett JW, Tannous B, Niland BP, et al. Mutant torsinA interferes with protein processing through the secretory pathway in DYT1 dystonia cells. Proc Natl Acad Sci USA. 2007;104:7271–7276. doi: 10.1073/pnas.0701185104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Jankovic J. Motor fluctuations and dyskinesias in Parkinson's disease: clinical manifestations. Mov Disord. 2005;20(Suppl 11):S11–S16. doi: 10.1002/mds.20458. [DOI] [PubMed] [Google Scholar]
- 280.Comella CL, Leurgans S, Wuu J, Stebbins GT, Chmura T. Rating scales for dystonia: a multicenter trial. Mov Disord. 2003;18:303–312. doi: 10.1002/mds.10377. [DOI] [PubMed] [Google Scholar]
- 281.Wabbels B, Jost WH, Roggenkamper P. Difficulties with differentiating botulinum toxin treatment effects in essential blepharospasm. J Neural Transm. 2011;118:925–943. doi: 10.1007/s00702-010-0546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Pekmezovic T, Svetel M, Ivanovic N, et al. Quality of life in patients with focal dystonia. Clin Neurol Neurosurg. 2009;111:161–164. doi: 10.1016/j.clineuro.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 283.Slawek J, Friedman A, Potulska A, et al. Factors affecting the health-related quality of life of patients with cervical dystonia and the impact of botulinum toxin type A injections. Funct Neurol. 2007;22:95–100. [PubMed] [Google Scholar]
- 284.Hall TA, McGwin G, Jr, Searcey K, et al. Health-related quality of life and psychosocial characteristics of patients with benign essential blepharospasm. Arch Ophthalmol. 2006;124:116–119. doi: 10.1001/archopht.124.1.116. [DOI] [PubMed] [Google Scholar]
- 285.Kupsch A, Benecke R, Muller J, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006;355:1978–1990. doi: 10.1056/NEJMoa063618. [DOI] [PubMed] [Google Scholar]
- 286.Vidailhet M, Vercueil L, Houeto JL, et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med. 2005;352:459–467. doi: 10.1056/NEJMoa042187. [DOI] [PubMed] [Google Scholar]
- 287.Soland VL, Bhatia KP, Volonte MA, Marsden CD. Focal task-specific tremors. Mov Disord. 1996;11:665–670. doi: 10.1002/mds.870110611. [DOI] [PubMed] [Google Scholar]
- 288.Adler CH, Edwards BW, Bansberg SF. Female predominance in spasmodic dysphonia. J Neurol Neurosurg Psychiat. 1997;63:688. doi: 10.1136/jnnp.63.5.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Konkiewitz EC, Trender-Gerhard I, Kamm C, et al. Service-based survey of dystonia in Munich. Neuroepidemiol. 2002;21:202–206. doi: 10.1159/000059525. [DOI] [PubMed] [Google Scholar]
- 290.Warner TT. Sex-related influences on the frequency and age of onset of primary dystonia. Neurology. 1999;53:1871–1873. doi: 10.1212/wnl.53.8.1871. [DOI] [PubMed] [Google Scholar]
- 291.Bhidayasiri R, Jen JC, Baloh RW. Three brothers with a very-late-onset writer's cramp. Mov Disord. 2005;20:1375–1377. doi: 10.1002/mds.20568. [DOI] [PubMed] [Google Scholar]
- 292.Brancati F, Defazio G, Caputo V, et al. Novel Italian family supports clinical and genetic heterogeneity of primary adult-onset torsion dystonia. Mov Disord. 2002;17:392–397. doi: 10.1002/mds.10077. [DOI] [PubMed] [Google Scholar]
- 293.Bressman SB, Warner TT, Almasy L, et al. Exclusion of the DYT1 locus in familial torticollis. Ann Neurol. 1996;40:681–684. doi: 10.1002/ana.410400421. [DOI] [PubMed] [Google Scholar]
- 294.Cassetta E, Del Grosso N, Bentivoglio AR, Valente EM, Frontali M, Albanese A. Italian family with cranial cervical dystonia: clinical and genetic study. Mov Disord. 1999;14:820–825. doi: 10.1002/1531-8257(199909)14:5<820::aid-mds1015>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 295.Defazio G, Brancati F, Valente EM, et al. Familial blepharospasm is inherited as an autosomal dominant trait and relates to a novel unassigned gene. Mov Disord. 2003;18:207–212. doi: 10.1002/mds.10314. [DOI] [PubMed] [Google Scholar]
- 296.Defazio G, Aniello MS, Masi G, Lucchese V, De Candia D, Martino D. Frequency of familial aggregation in primary adult-onset cranial cervical dystonia. Neurol Sci. 2003;24:168–169. doi: 10.1007/s10072-003-0113-3. [DOI] [PubMed] [Google Scholar]
- 297.Gasser T, Windgassen K, Bereznai B, Kabus C, Ludolph AC. Phenotypic expression of the DYT1 mutation: a family with writer's cramp of juvenile onset. Ann Neurol. 1998;44:126–128. doi: 10.1002/ana.410440119. [DOI] [PubMed] [Google Scholar]
- 298.Jimenez-Jimenez F, Martinez-Castrillo JC, Baron-Rubio M, et al. Familial focal dystonia. Eur Neurol. 2002;48:232–234. doi: 10.1159/000066171. [DOI] [PubMed] [Google Scholar]
- 299.Leube B, Rudnicki D, Ratzlaff T, Kessler KR, Benecke R, Auburger G. Idiopathic torsion dystonia: assignment of a gene to chromosome 18p in a German family with adult onset, autosomal dominant inheritance and purely focal distribution. Hum Mol Genet. 1996;5:1673–1677. doi: 10.1093/hmg/5.10.1673. [DOI] [PubMed] [Google Scholar]
- 300.Micheli S, Fernandez-Pardal M, Quesada P, Brannan T, Obeso JA. Variable onset of adult inherited focal dystonia: a problem for genetic studies. Mov Disord. 1994;9:64–68. doi: 10.1002/mds.870090110. [DOI] [PubMed] [Google Scholar]
- 301.Munchau A, Valente EM, Davis MB, et al. A Yorkshire family with adult-onset cranio-cervical primary torsion dystonia. Mov Disord. 2000;15:954–959. doi: 10.1002/1531-8257(200009)15:5<954::aid-mds1028>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 302.Norgren N, Mattson E, Forsgren L, Holmberg M. A high-penetrance form of late-onset torsion dystonia maps to a novel locus (DYT21) on chromosome 2q14.3-q21.3. Neurogenetics. 2011;12:137–143. doi: 10.1007/s10048-011-0274-9. [DOI] [PubMed] [Google Scholar]
- 303.Puschmann A, Xiao J, Bastian RW, Searcy JA, LeDoux MS, Wszolek ZK. An African-American family with dystonia. Parkinsonism Relat Disord. 2011;17:547–550. doi: 10.1016/j.parkreldis.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Schmidt A, Jabusch HC, Altenmuller E, et al. Dominantly transmitted focal dystonia in families of patients with musician's cramp. Neurology. 2006;67:691–693. doi: 10.1212/01.wnl.0000230148.00035.f9. [DOI] [PubMed] [Google Scholar]
- 305.Uitti RJ, Maraganore DM. Adult onset familial cervical dystonia: report of a family including monozygotic twins. Mov Disord. 1993;8:489–494. doi: 10.1002/mds.870080413. [DOI] [PubMed] [Google Scholar]
- 306.Winter P, Kamm C, Biskup S, et al. DYT7 gene locus for cervical dystonia on chromosome 18p is questionable. Mov Disord. 2012;27:1819–1821. doi: 10.1002/mds.25219. [DOI] [PubMed] [Google Scholar]
- 307.Bonetti M, Barzaghi C, Brancati F, et al. Mutation screening of the DYT6/THAP1 gene in Italy. Mov Disord. 2009;24:2424–2427. doi: 10.1002/mds.22861. [DOI] [PubMed] [Google Scholar]
- 308.Bressman SB, Raymond D, Fuchs T, Heiman GA, Ozelius LJ, Saunders-Pullman R. Mutations in THAP1 (DYT6) in early-onset dystonia: a genetic screening study. Lancet Neurol. 2009;8:441–446. doi: 10.1016/S1474-4422(09)70081-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Cheng FB, Wan XH, Feng JC, Wang L, Yang YM, Cui LY. Clinical and genetic evaluation of DYT1 and DYT6 primary dystonia in China. Eur J Neurol. 2011;18:497–503. doi: 10.1111/j.1468-1331.2010.03192.x. [DOI] [PubMed] [Google Scholar]
- 310.Cheng FB, Ozelius LJ, Wan XH, et al. THAP1/DYT6 sequence variants in non-DYT1 early-onset primary dystonia in China and their effects on RNA expression. J Neurol. 2012;259:342–347. doi: 10.1007/s00415-011-6196-5. [DOI] [PubMed] [Google Scholar]
- 311.Clot F, Grabli D, Burbaud P, et al. Screening of the THAP1 gene in patients with early-onset dystonia: myoclonic jerks are part of the dystonia 6 phenotype. Neurogenetics. 2011;12:87–89. doi: 10.1007/s10048-010-0264-3. [DOI] [PubMed] [Google Scholar]
- 312.Djarmati A, Schneider SA, Lohmann K, et al. Mutations in THAP1 (DYT6) and generalised dystonia with prominent spasmodic dysphonia: a genetic screening study. Lancet Neurol. 2009;8:447–452. doi: 10.1016/S1474-4422(09)70083-3. [DOI] [PubMed] [Google Scholar]
- 313.Dobricic VS, Kresojevic ND, Svetel MV, et al. Mutation screening of the DYT6/THAP1 gene in Serbian patients with primary dystonia. J Neurol. 2012 doi: 10.1007/s00415-012-6753-6. [DOI] [PubMed] [Google Scholar]
- 314.Groen JL, Ritz K, Contarino MF, et al. DYT6 dystonia: mutation screening, phenotype, and response to deep brain stimulation. Mov Disord. 2010;25:2420–2427. doi: 10.1002/mds.23285. [DOI] [PubMed] [Google Scholar]
- 315.Groen JL, Yildirim E, Ritz K, et al. THAP1 mutations are infrequent in spasmodic dysphonia. Mov Disord. 2011;26:1952–1954. doi: 10.1002/mds.23682. [DOI] [PubMed] [Google Scholar]
- 316.Houlden H, Schneider SA, Paudel R, et al. THAP1 mutations (DYT6) are an additional cause of early-onset dystonia. Neurology. 2010;74:846–850. doi: 10.1212/WNL.0b013e3181d5276d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Lohmann K, Uflacker N, Erogullari A, et al. Identification and functional analysis of novel THAP1 mutations. Eur J Hum Genet. 2012;20:171–175. doi: 10.1038/ejhg.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Paisan-Ruiz C, Ruiz-Martinez J, Ruibal M, et al. Identification of a novel THAP1 mutation at R29 amino-acid residue in sporadic patients with early-onset dystonia. Mov Disord. 2009;24:2428–2429. doi: 10.1002/mds.22849. [DOI] [PubMed] [Google Scholar]
- 319.Sohn AS, Glockle N, Doetzer AD, et al. Prevalence of THAP1 sequence variants in German patients with primary dystonia. Mov Disord. 2010;25:1982–1986. doi: 10.1002/mds.23207. [DOI] [PubMed] [Google Scholar]
- 320.Song W, Chen Y, Huang R, et al. Novel THAP1 gene mutations in patients with primary dystonia from southwest China. J Neurol Sci. 2011;309:63–67. doi: 10.1016/j.jns.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 321.Van Gerpen JA, Ledoux MS, Wszolek ZK. Adult-onset leg dystonia due to a missense mutation in THAP1. Mov Disord. 2010;25:1306–1307. doi: 10.1002/mds.23086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Xiromerisiou G, Houlden H, Scarmeas N, et al. THAP1 mutations and dystonia phenotypes: genotype phenotype correlations. Mov Disord. 2012;27:1290–1294. doi: 10.1002/mds.25146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Kurian MA, Gissen P, Smith M, Heales S, Jr, Clayton PT. The monoamine neurotransmitter disorders: an expanding range of neurological syndromes. Lancet Neurol. 2011;10:721–733. doi: 10.1016/S1474-4422(11)70141-7. [DOI] [PubMed] [Google Scholar]