Abstract
Previous studies showed noxious unilateral forepaw electrical stimulation surprisingly evoked negative blood-oxygenation-level-dependent (BOLD), cerebral blood flow (CBF), and cerebral blood volume (CBV) fMRI responses in the bilateral striatum whereas the local neuronal spike and c-Fos activities increased. These negative responses are associated with vasoconstriction and appeared to override the increased hemodynamic responses that typically accompanied with increased neural activity. The current study aimed to investigate the role of μ-opioid system in modulating vasoconstriction in the striatum associated with noxious stimulation on a 4.7-Tesla MRI scanner. Specifically, we investigated: i) how morphine (a μ-opioid receptor agonist) affects the vasoconstriction in the bilateral striatum associated with noxious electrical forepaw stimulation in rats, and ii) how naloxone (an opioid receptor antagonist) and eticlopride (a dopamine D2/D3 receptor antagonist) modulates the morphine effects onwards. Injection of morphine enhanced the negative striatal CBV responses to noxious stimulation. Sequential injection of naloxone in the same animals abolished the stimulus-evoked vasoconstriction. In a separate group of animals, injection of eticlopride following morphine also reduced the vasoconstriction. Our findings suggested that noxious stimulation endogenously activated opioid and dopamine receptors in the striatum and thus leading to vasoconstriction.
Keywords: CBV, Striatum, Morphine, Naloxone, Eticlopride, fMRI, Vasoconstriction
Introduction
Pain-evoked negative blood-oxygenation-level-dependent (BOLD), cerebral blood flow (CBF) or cerebral blood volume (CBV) responses in the striatum have been previously shown in conscious rats (Morrow et al., 1998) and rats under medetomidine (Zhao et al., 2008) and α-chloralose (Shih et al., 2009; Shih et al., 2011) anesthesia. In the noxious forepaw electrical stimulation model, the local neuronal spike and c-Fos activities were found to increase in the striatum (Shih et al., 2009). Such negative CBV responses in the striatum may be associated with neurotransmitter-induced vasoconstriction and were markedly attenuated by intravenous injection of 1 mg/kg eticlopride, a dopamine D2/D3 receptor antagonist (Shih et al., 2009), or lesion of a major dopaminergic afferent pathway in the substantia nigra (SN) by 6-hydroxydopamine (Chen et al., 2009). These findings were attributed to the effect of dopamine action which is known to have regional vascular effect. Stimulating the dopamine D1/D5 receptors causes vasodilation (Chen et al., 2005), whereas stimulating the D2/D3 receptors causes vasoconstriction (Choi et al., 2006). These findings suggest that endogenous dopamine function is associated with noxious stimulation-induced vasoconstriction, which overrides the positive hemodynamic fMRI responses typically accompanied with increased neural activity.
Opioid is involved in modulating nociception. Activation of the μ-opioid receptor produces analgesia (Sora et al., 1997). Direct activation of μ-opioid receptors by morphine causes sustained CBV decreases in the striatum (Liu et al., 2007). The relationship between the opioid and dopamine system is complex. Opioids modulate dopamine release in the striatum via hyperpolarization of GABAergic interneurons (Johnson and North, 1992). Most striatal neurons express both opioid and dopamine D2/D3 receptors (Choi et al., 2006; Tempel and Zukin, 1987). Opioid–dopamine system has been shown to play an important role in reward processes and associated with endogenous analgesia, drug addiction, and the placebo effect (de la Fuente-Fernandez et al., 2002). It is unknown how endogenous opioid neurotransmission affect the stimulus-evoked responses in the striatum associated with noxious stimulation.
The goal of this study was to investigate the role of μ-opioid system in modulating vasoconstriction in the striatum associated with noxious stimulation. Specifically, we analyzed: i) how morphine (a prototypical μ-opioid receptor agonist) affects the vasoconstriction in the bilateral striatum associated with noxious electrical forepaw stimulation in rats, ii) how naloxone (an opioid receptor antagonist) modulates the morphine effects, and iii) how eticlopride (a dopamine D2/D3 receptor antagonist, same dose as used in (Shih et al., 2009)) modulates the morphine effects. These experiments were done on a 4.7-T MRI scanner using an established blood-pool contrast agent, superparamagnetic iron oxide nanoparticles (SPIOs), as a measure of CBV changes (Mandeville et al., 1998). This technique is known to increase functional contrast. A high dose (30 mg Fe/kg) was used to reduce potential contamination from BOLD signals (Lu et al., 2007). We tested the hypothesis that activation of the μ-opioid and/or dopamine D2/D3 receptors is necessary to evoke vasoconstriction in the striatum during noxious stimulation. These findings could have strong implications in neuroscience and pain research as well as the underlying sources of the fMRI signals.
Materials and methods
Subjects
A total of 20 adult male Wistar rats (250–300 g; National Laboratory Animal Center, Taiwan) were used in the present study. Animals were housed in the vivarium (12:12-h light–dark cycle, controlled humidity and temperature) with free access to food and water. All experimental procedures were approved by the Institute of Animal Care and Utilization Committee at Academia Sinica, Taipei, Taiwan.
Animal preparation
The animal preparation was similar to that described previously (Shih et al., 2009). On the experiment day, each rat was initially anesthetized with 3% isoflurane. A PE-50 catheter was inserted into the right femoral vein for subsequent drug administration. Alpha-chloralose (70 mg/kg, i.v.) was used for anesthesia. The isoflurane was then discontinued and the rats were allowed to breathe spontaneously to keep the physiological autoregulation intact. The rat was firmly fixed in a custom-designed holder for pain studies with tapes to restrain the body (Shih et al., 2008; Shih et al., 2007). The body temperature was maintained by a warm-water blanket at 37 °C using an automatic feedback control system via a rectal temperature probe. Although it has been shown that body temperature may be altered by morphine injection (Clark, 1979; Ushijima et al., 1985), changes in rectal temperature did not exceed ±0.5 °C in the present study. The end-tidal CO2 concentration was continuously monitored (Capnomac Ultima, Datex-Ohmeda, Helsinki, Finland). The end-tidal CO2 concentration during the experiment was 3.0–3.5%. To avoid potential perturbation of animal physiology, arterial pCO2 and arterial blood pressure (BP) were measured in a separate group of animals (total n= 10, n=5 for each group) under identical experimental condition. The right femoral artery was cannulated with PE50 tubing. BP was continuously monitored via the arterial line by a small animal monitoring system using the AcqKnowledge software (BIOPAC Systems Inc., Santa Barbara, CA, USA) and arterial pCO2 was measured by withdrawing 0.1 ml arterial blood sample per measurement (ABL5, Radiometer America Inc., Westlake, OH, USA). Data are shown in Table 1. An established CBV fMRI technique was employed (Mandeville et al., 1998) by using SPIOs (Resovist, Schering, Berlin, Germany, 30 mg Fe/kg, i.v.). The Resovist is shown to provide stable CBV fMRI results without substantial signal drifts (Shih et al., 2009). During neuronal activation, increases in regional CBV would increase the amount of SPIOs, leading to a lower MR signal intensity and vice versa.
Table 1.
Arterial blood pressure and pCO2 changes.
| Control
|
Morphine
|
Naloxone
|
Eticlopride
|
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Stimulation | Baseline | Stimulation | Baseline | Stimulation | Baseline | Stimulation | |
| Arterial BP (mm Hg) | 99.2±10.7 | 108.2±10.8* | 81.4±12.8# | 103.5±8.9* | 99.8±17.6 | 107.5±18.7* | 97.7±13.7 | 107.9±13.3* |
| Arterial pCO2 (mm Hg) | 42.6±5.7 | 41.5±4.5 | 51.7±5.8# | 53.2±6.8 | 45±6.9 | 43±6.5 | 48.4±8.7 | 42±5.8 |
P<0.05, compared with pre-stimulation baseline period.
P<0.01, compared with baseline (control).
Forepaw electrical stimulation was then applied to the rat. Two needle electrodes were inserted under the skin of the right forepaw: one between the first and second digits and the other between the third and fourth digits. These electrodes were then fixed with surgical tape and the stimulation was confirmed by observing digit twitching. Electrical stimulation at 10 mA with a 3-Hz square wave and a 0.5-ms pulse duration was applied to the rats by a constant-current stimulator (AM system, model 2100, Carlsburg, WA, USA). The 10 mA stimulation is known to induce nociception (Lowe et al., 2007; Shih et al., 2009; Zhao et al., 2008).
fMRI data acquisition
MR images were captured using a 4.7-T Bruker Biospec 47/40 spectrometer with a 72-mm volume coil used as the RF transmitter and a quadrature surface coil placed on the head as the receiver. A T2-weighted pilot image was taken in the axial and mid-sagittal plane to localize the anatomical position by identifying the anterior commissure (bregma −0.8 mm). A FLASH sequence comprising 60 time frames was used for CBV fMRI experiments with a repetition time of 150 ms, echo time of 20 ms, flip angle of 22.5°, field of view of 2.56×2.56 cm2, slice thickness of 1.5 mm, acquisition matrix of 128×64 (zero-filled to 128×128), and temporal resolution of 9.6 s. An off–on–off paradigm was used to detect the responses to electrical stimulation, with the first and last 20 frames categorized as baseline, and the middle 20 frames collected during stimulation.
Pharmacological MRI
Morphine (5 mg/kg, i.v.) was used to study the effect of μ-opioid receptors in nociception-evoked vasoconstriction, whereas naloxone (0.7 mg/kg, i.v.) was used to antagonize the residual effect of morphine after 35 min of morphine treatment (n=5). A dopamine D2/D3 receptor antagonist, eticlopride (1 mg/kg, i.v.), was given in another group of rats (n=5) following about 15 min of morphine treatment to determine the contribution of opioid–dopamine neurotransmission on vasoconstriction (Fig. 1). The dosages of the drugs were chosen based on our previous studies (Shih et al., 2009; Sun et al., 2006).
Fig. 1.
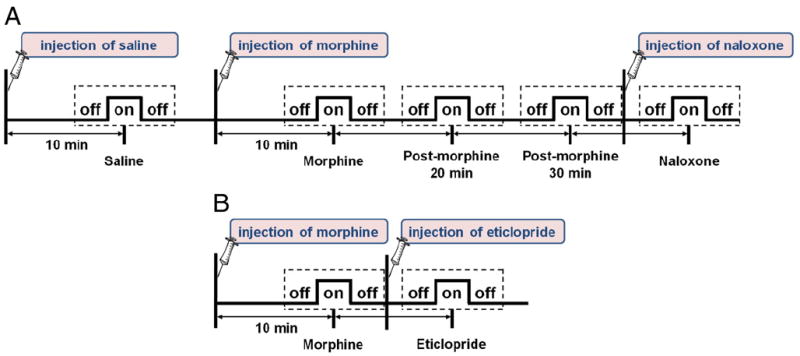
(A) fMRI paradigm of morphine–naloxone experiment. The off–on–off paradigm indicates the period of noxious forepaw electrical stimulation, whereas the dashed box represents the imaging period. (B) fMRI paradigm of morphine–eticlopride experiment. Paradigms in panels A and B correspond to the results in Fig. 3 and Fig. 4, respectively.
Data analysis
Images were analyzed using a custom-built image processing interface (Shih et al., 2007; Shih et al., 2008). In general, time series MRI data are co-registered by Statistical Parametric Mapping (SPM) if needed. Correlation coefficient (CC) analysis was performed on a pixel-by-pixel basis to correlate MR signal changes with electrical stimulation. These CC maps were then coregistered to a digitized rat brain atlas to obtain the averaged CC images by spatial averaging, where the CBV increases were shown blue–purple colors and CBV decreases were shown in red–yellow colors. The same transformation was applied to all images in the dataset for coregistration. We did not observe extreme responses in a particular subject.
The region of interests (ROIs) were defined via a digitized rat brain atlas using the method proposed previously to avoid bias to a particular activation map (Shih et al., 2008). The number of pixels with signal changes that differed significantly from the baseline values was quantified as the responsive area as described elsewhere (Shih et al., 2009). In brief, the signals were compared between before and during electrical stimulation using two-tailed paired t-tests, with P<0.05 considered indicative of a responsive pixel. Note that the P value here was not corrected for multiple comparisons, same as our previous study (Shih et al., 2009). The number of responsive pixels was only counted within the ROI (~400 pixels in the striatum and ~180 pixels in the cortex). The ΔR2* value, which approximately varies linearly with the CBV change, was calculated as follows:
where Spre and Sstim are the signal intensity before and during stimulation, respectively.
Both empirical data and extensive Monte Carlo modeling indicate an approximately linear relationship between ΔR2* and CBV fraction over the range that is physiologically relevant (see Wu et al., 2004 for review). The drug-induced relative basal CBV index was defined as the average of the MR signals during the “off” period before each noxious forepaw electrical stimulation epoch (i.e., the first “off” period after drug injection, see Fig. 1), whereby the signals in the superior sagittal sinus were used to calibrate the clearance of iron oxide. The data were normalized to the control group and expressed as the percent change relative to the control data.
For statistical analysis, repeated-measures ANOVA with Bonferroni post-hoc test was used to compare stimulus-evoked changes in responsive area, ΔR2* value, and relative basal CBV level modulated by different drug treatments. Homogeneity of the variances was assessed by Levene’s test and Games–Howell post-hoc test was employed only if unequal variance. The significance level was set at P<0.05.
Results
Mean arterial BP and arterial pCO2 was measured in a separate group of five animals under the same experimental conditions (Table 1). Noxious forepaw stimulation significantly increased arterial BP about 10–20 mm Hg, but has no effect on arterial pCO2 under control, morphine-, and naloxone-pretreatment. Morphine injection itself decreased arterial BP and increased arterial pCO2, while naloxone and eticlopride reversed the morphine effect.
Fig. 2 shows the effects of morphine and the sequential administration of naloxone or eticlopride on the relative basal CBV without forepaw stimulation. Morphine reduced basal CBV in the striatum relative to control (P<0.01). Naloxone and eticlopride both reversed the morphine effect on basal CBV, resulting in CBV increases in the striatum (P<0.05). Similar effects were observed in the contralateral primary somatosensory cortex (cS1).
Fig. 2.
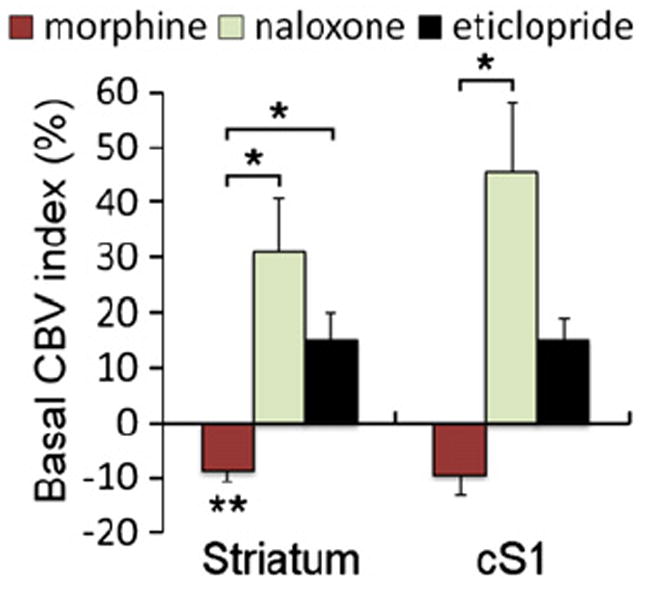
Comparison of the relative basal CBV (i.e., without forepaw stimulation) in different treatment groups. Ten rats were included in the morphine-treated groups, and two groups of five rats each were included in the naloxone- and eticlopride-treated groups, respectively. The basal CBV differences were defined as the average of the CBV signals during the “off” period before electrical stimulation (see Fig. 1), whereby the signals in the superior sagittal sinus were used to calibrate the clearance of iron oxide. All data were normalized and expressed as the percent change relative to control. *, **Statistically significant levels at P<0.05 and P<0.01 (different from control), respectively. Error bars are s.e.m. values.
Unilateral noxious forepaw stimulation increased CBV in the cS1 and decreased CBV in the bilateral striatum. Saline did not affect the stimulus-evoked CBV responses (Fig. 3). Morphine, a μ-opioid receptor agonist, attenuated the vasodilation in the cS1 and accentuated the vasoconstriction in the striatum. The responsive area and the magnitude of vasoconstriction in the striatum were larger after morphine compared to saline injection (P<0.05). The responsive area and magnitude of vasodilation in the cS1 were reduced after morphine (P<0.05). Negative CBV responses in the ipsilateral primary somatosensory cortex (iS1) were also detected (P<0.01).
Fig. 3.
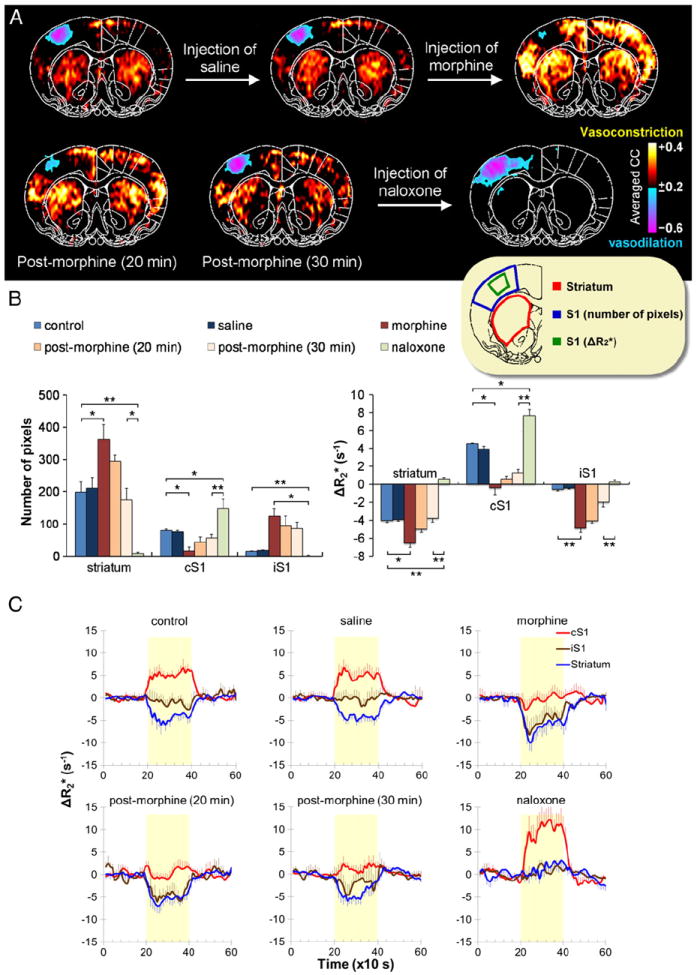
Effects of morphine and naloxone on nociception-evoked negative CBV fMRI signals (n=5). (A) A 10-mA electrical stimulation was applied to the rat’s right forepaw, and then CBV fMRI was conducted with data acquired at bregma +0.7 mm. Images were analyzed using a correlation method with spatial averaging after atlas-based coregistration, where vasodilation and vasoconstriction are indicated by blue–purple and red–yellow colors, respectively. Note that correlation analysis is not a measure of magnitude changes, but sensitive to the response pattern. Nociception-induced vasodilation was evident in the cS1. Vasoconstriction was evident in the striatum of both hemispheres, and a mild reduction of CBV was observed in the iS1. Preinjecting saline had no effect on images, whereas preinjecting morphine (5 mg/kg, i.v.) enhanced the nociception-induced vasoconstriction. The effect of morphine decreased with time, and the stimulation-evoked response reached close to control levels at 30 min postinjection. Thereafter, preinjecting naloxone (0.7 mg/kg, i.v.) resulted in a dramatic blockage of the entire vasoconstriction response in the observed brain section. (B) The responsive area and the magnitude CBV changes evoked by noxious stimulation under different drug treatments. The ROIs (inset) were defined via the anatomy to avoid bias to a particular activation map. The responsive area was quantified by the number of responsive pixels in the region, and the magnitude CBV changes was quantified by ΔR2* (s−1) values. A larger ROI was used for quantifying the responsive area in the S1. Note that for the responsive area, only vasoconstrictive pixels were counted for the striatum and iS1, and only vasodilative pixels were counted for the cS1. (C) Corresponding CBV fMRI time-courses. *, **Statistically significant levels at P<0.05 and P<0.01, respectively. Error bars are s.e.m. values.
The effects of morphine on CBV fMRI signal changes decreased gradually over time, returning close to pre-injection pattern by 30 min after morphine. The time-dependent effects of the morphine on striatal CBV fMRI responses were significant for both the responsive area (F2,12 = 7.332, P<0.01) and magnitude (F2,12 = 4.968, P<0.05).
Naloxone, administered 35 min after the morphine to antagonize its residual effect, abolished the vasoconstriction in the striatum (P<0.05, compared with that 30 min after morphine) while enhancing the responsive area and the magnitude of the vasodilation in the cS1 (P<0.01) (Fig. 3). Stimulus-evoked CBV responses increased slightly (instead of decreased) in the striatum and the iS1 after naloxone (P<0.01), indicating the endogenous neurotransmission-induced vasoconstriction overrode activation-induced vasodilation before naloxone.
To further investigate the source of the negative CBV fMRI signal, a dopamine D2/D3 receptor antagonist, eticlopride, was administered 15 min after morphine on a separate group of animals (Fig. 4). Morphine injection attenuated the vasodilation in the cortex and accentuated the vasoconstriction in the striatum. Eticlopride reduced the vasoconstriction in the striatum, but did not recover the vasodilation in the cS1 compared with control. Both the responsive area (P<0.01) and magnitude (P<0.05) were statistically different between morphine and eticlopride in the striatum. Note that the morphine–eticlopride group appeared to have weaker ΔR2* responses compared with morphine–naloxone group, but independent two-sample t-test revealed no difference in striatal ΔR2* responses to noxious stimuli between two groups (P=0.1139). This animal variation also did not change the fact that eticlopride markedly reduced stimulus-evoked vasoconstriction after morphine treatment.
Fig. 4.
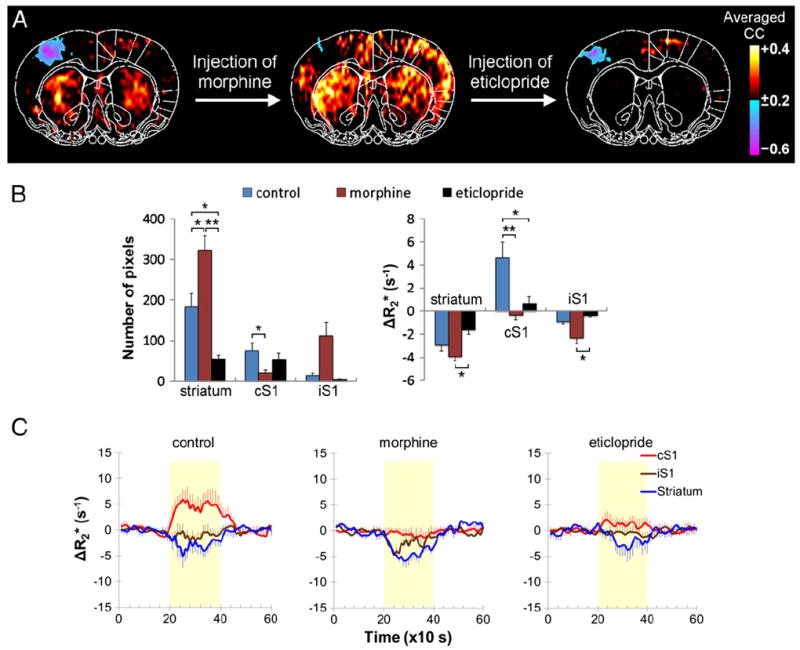
Morphine-enhanced stimulus-evoked vasoconstriction is mediated by the activation of dopamine D2/D3 receptors (n=5). (A) Morphine (5 mg/kg, i.v.) was given to enhance stimulus-evoked vasoconstriction. After 15 min of morphine treatment, injection of a dopamine D2/D3 receptor antagonist, eticlopride (1 mg/kg, i.v.), reduced the morphine effect. Vasodilation and vasoconstriction are indicated by blue–purple and red–yellow colors, respectively. (B) The responsive area and the magnitude CBV changes evoked by noxious stimulation under different drug treatments. (C) Corresponding CBV fMRI time-courses. *, **Statistically significant at P<0.05 and P<0.01, respectively. Error bars are s.e.m. values.
Discussion
This study demonstrates the pharmacological modulation of the striatal negative response under our experimental conditions. Morphine, a μ-opioid receptor agonist, markedly enhances vasoconstriction in the bilateral striatum. Naloxone, an opioid receptor antagonist, abolishes the vasoconstriction in the striatum after administration of morphine. Eticlopride, a dopamine D2/D3 receptor antagonist, markedly reduces the vasoconstriction in the striatum after administration of morphine. We conclude that noxious stimulation endogenously activates opioid and dopamine receptors, leading to vasoconstriction and thus the negative CBV fMRI responses in the striatum. Such endogenously induced vasoconstriction in the striatum appears to override the positive hemodynamic fMRI responses typically accompanied by increased neural activity. These findings also suggest cautions in interpreting hemodynamic-based fMRI signals as these negative CBV changes may confound fMRI studies of drug addiction and Parkinson’s disease, among others, that involve opioid–dopamine neurotransmission. These findings corroborate our hypothesis that activation of the μ-opioid and/or dopamine D2/D3 receptors evokes vasoconstriction in the striatum during noxious stimulation. These results highlight the importance of neurotransmission in modulating fMRI signals (Attwell and Iadecola, 2002; Choi et al., 2006; Shih et al., 2009) and could have implications in neuroscience and pain imaging research.
The BOLD contribution to iron oxide CBV fMRI signal could be significant at higher field and with low iron oxide dose (Lu et al., 2007). To minimize the BOLD contribution, our study was performed at 4.7 T and a higher iron oxide dose (30 mg/kg) was used compared to the 10–15 mg/kg used at other 4.7 T CBV fMRI studies (Mandeville et al., 1998; Shen et al., 2008). Correction of the BOLD contribution would require repeating the measurement of R2 during stimulation at different levels of blood susceptibility (Kennan et al., 1998). This was not performed. Nevertheless, our previous study showed that morphine decreased the positive BOLD signal changes evoked by noxious electrical stimulation at the sciatic nerve (Chang and Shyu, 2001), indicating that the morphine-enhanced vasoconstriction in the present study was unlikely contaminated by BOLD contribution.
Motion may induce false-positive or negative fMRI signal changes. The fMRI responses in the present study were unlikely attributed to motion because the rat head was firmly fixed in a custom-designed head holder and no significant motion was observed under our animal fMRI setup (Shih et al., 2009). Shift of the brain position usually causes strong positive/negative correlation at the edges, or spike in the time-course data. This was not observed in all trials. The enhanced vasoconstriction by morphine was also unlikely elicited by motion because morphine can suppress pain sensation and therefore reduce motion artifact.
Morphine has cardiovascular effects (such as decreases blood flow, BP, heart rate, and respiration rate) that could affect the fMRI signal changes (Fennessy and Rattray, 1971). Arterial pCO2 was increased after morphine injection, but we found decreased basal CBV in the striatum and some cortical regions, consistent with that reported previously (Liu et al., 2007). Previous study showed that BOLD signal increased 20 min after the onset of morphine injection (Shah et al., 2005). Our study showed significant CBV changes at 10 min, this may be due to better sensitivity of CBV fMRI compared with BOLD or differences in MRI sequence (RARE sequence was used for fMRI by Shah et al.). In addition, Shah et al. used halothane for anesthesia and the present study used α-chloralose. This is however unlikely the major cause because another CBV fMRI study showed that morphine significantly decreased striatal CBV and the responses peaked at 10 min after morphine injection under halothane anesthesia (Liu et al., 2007). The morphine cardiovascular effects may not enhance the stimulus-evoked striatal vasoconstriction because a lowered basal CBV level restricts an area showing further CBV decreases. In addition, noxious forepaw electrical stimuli were found to increase BP of 10–20 mm Hg. Such changes are lower than the threshold (>35 mm Hg) for inducing nonspecific changes in hemodynamic fMRI signals (Tuor et al., 2002) and we did not observe any nonspecific CBV increase after stimulus onset. Furthermore, activation of sympathetic nerves by noxious stimuli may reduce CBV. This does not contribute to the morphine-enhanced striatal vasoconstriction because the same stimulation intensity was applied for all conditions. The stimulation model used in the present study has been shown to produce sustained negative striatal BOLD, CBV, and CBF responses (Shih et al., 2011). The negative striatal CBV responses can be eliminated by injection of dopamine D2/D3 antagonist (Shih et al., 2009) or unilateral lesion of dopamine neurons by 6-hydroxydopamine in the SN (Chen et al., 2009), indicating that the signal origin is highly associated with neurotransmission but not BP effect. Therefore, the enhanced stimulus-evoked vasoconstriction in the striatum after morphine treatment was independent of the cardiovascular effects; rather it was associated with opioid and dopamine neurotransmission in the brain.
Our findings supported the notion that opioid and dopamine systems modulate the vascular responses in the striatum. Morphine markedly enhanced stimulus-evoked vasoconstriction in the bilateral striatum. A plausible explanation for the enhanced vasoconstriction following morphine injection is a rapid upregulation of D2/D3 receptors. Morphine administration causes rapid development of super-sensitive dopamine receptors without affecting the affinity of the dopamine receptors (Martin and Takemori, 1985). Acute upregulation of D2/D3, but not D1/D5 receptors was found to occur following administration of μ-opioid receptor agonist (Rooney et al., 1991) and activation of the D2/D3 receptors causes vasoconstriction (Choi et al., 2006). Positron-emission tomography studies using D2-like radioligands (11C-FLB 457 and 11C-raclopride) also support this notion by showing that administration of a μ-opioid receptor agonist significantly increased D2/D3 receptor binding in both the cortical and the striatal areas (Hagelberg et al., 2004a; Hagelberg et al., 2002).
The morphine-enhanced vasoconstriction in the bilateral striatum was abolished by naloxone, a competitive antagonist of morphine. This demonstrates unequivocally that endogenous opioids are involved in the generation of stimulus-evoked vasoconstriction in the bilateral striatum. Slight stimulus-evoked vasodilation was observed in both the striatum and the iS1 after naloxone treatment, which may simply reflect the local neuronal activity changes evoked by noxious stimulation. This provides a means that the neurovascular coupling could be hampered by the vascular actions of endogenous opioids.
The morphine-enhanced vasoconstriction in the bilateral striatum was reduced by eticlopride, a dopamine D2/D3 receptor antagonist. Eticlopride was injected 15 min after morphine treatment. This time point was selected in order to verify the involvement of dopamine D2/D3 receptor system in morphine-enhanced vasoconstriction. Our data suggested that the opioid and dopamine D2/D3 receptors both contribute to the striatal vasoconstriction in noxious stimulation. The effect of morphine is closely associated with the function of dopamine D2 receptors. Dopamine D2 receptor-knockout mice exhibit no rewarding effects of morphine (Elmer et al., 2002; Maldonado et al., 1997). These are also in good agreement with that morphine induced rapid upregulation of D2/D3 receptors and may lead to enhanced vasoconstriction in the striatum.
Nigrostriatal pathway has been implicated in pain modulation. SN has direct projections to the striatum and electrical stimulation of the SN reduces pain-associated behavior (Chudler and Dong, 1995). Noxious forepaw stimulation in rats with unilateral lesion of dopamine neurons by 6-hydroxydopamine in the SN (an animal model of Parkinson disease) showed significant reduction of the vasoconstriction in the ipsilateral striatum (Chen et al., 2009). Patients with Parkinson disease — a disease characterized by a deficiency in the formation or action of dopamine — show reduced threshold of pain sensation (Chudler and Dong, 1995; Hagelberg et al., 2004b). Animal models of Parkinson disease also showed reduced threshold of pain sensation (Lin et al., 1984). In addition, activation of dopamine D2/D3 receptors, but not D1/D5 receptors, alleviates pain, whereas micro-injection of eticlopride, a dopamine D2/D3 receptor antagonist, into the striatum enhances pain-associated behavior (Magnusson and Fisher, 2000). It has been shown that noxious forepaw electrical stimulation (50 mA, 0.5 ms, 5 Hz) induced significant increase of D2 receptor binding potential in the contralateral striatum/nucleus accumbens in cats using 11C-raclopride PET (Inoue et al., 2004). No significant change in D2 receptor binding potential was observed during innocuous electrical stimulation in cats and humans (Thobois et al., 2004). Our previous CBV fMRI study also demonstrated that bilateral striatal vasoconstriction was only evoked by noxious electrical stimulation, but not by innocuous stimulation (Shih et al., 2009; Shih et al., 2011) and those vasoconstriction responses can be blocked by D2/D3 receptor antagonist (Shih et al., 2009). Taken together, these findings support the important role of dopaminergic neurotransmission in the striatal vasoconstriction, which, we speculate, may play a role in the suppression of pain-related excitability.
Striatal responses to noxious stimuli in human studies are controversial, with some studies reporting strong (Bingel et al., 2004) and others reporting no significant responses (Casey, 1999). Nonetheless, no pain-induced negative BOLD fMRI response in the striatum has been reported in conscious humans to our knowledge, although the nucleus accumbens, which also have dense expression of dopamine D2/D3 receptors, showed negative BOLD response to noxious stimuli in humans (Becerra et al., 2001; Borras et al., 2004). This discrepancy in pain-induced negative striatal fMRI responses between humans and animals could be due to anesthesia confound in animal studies. This is however unlikely because stimulus-evoked striatal negative BOLD response has been detected in lightly-sedated rats using fMRI (Pawela et al., 2010; Zhao et al., 2008) and pain-related striatal CBF decrease has been observed in conscious rats using autoradiography (Morrow et al., 1998). Other possible reasons for such discrepancy could be due to differences in pain stimuli and/or species. Further studies are needed to resolve the apparent discrepancy in striatal fMRI responses between humans and rats.
In summary, the present study demonstrates that endogenously induced opioid–dopamine neurotransmission associated with noxious forepaw stimulation is responsible for the negative CBV fMRI signal changes in the striatum. These negative CBV changes apparently counteract the conventional positive fMRI responses associated with increased neural activity. We speculate that the striatal vasoconstriction in noxious stimulation may be associated with suppression of pain-related excitability. In addition, these findings suggest cautions in interpreting hemodynamic-based fMRI signals as they may confound fMRI studies of cognitive function, motor control, reward signaling, drug addiction, and neurological disorders (such as Parkinson’s disease) that involve opioid–dopamine neurotransmission. Future studies will attempt to dissect the effects of different opioid receptor subtypes on striatal fMRI responses and the effects of morphine on fMRI signals in Parkinsonian animal model.
Acknowledgments
The authors acknowledge technical support from the Functional and Micro-Magnetic Resonance Imaging Center supported by the National Research Program for Genomic Medicine, National Science Council, Taiwan, R.O.C. (NSC97-3112-B-001-013). Dr. Yen-Yu Ian Shih was supported by American Heart Association (10POST4290091), Clinical Translational Science Awards (CTSA 004A, 018A, and 150565IM00065, parent grant UL1RR025767), and San Antonio Area Foundation.
References
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Bingel U, Glascher J, Weiller C, Buchel C. Somatotopic representation of nociceptive information in the putamen: an event-related fMRI study. Cereb Cortex. 2004;14:1340–1345. doi: 10.1093/cercor/bhh094. [DOI] [PubMed] [Google Scholar]
- Borras MC, Becerra L, Ploghaus A, Gostic JM, DaSilva A, Gonzalez RG, Borsook D. fMRI measurement of CNS responses to naloxone infusion and subsequent mild noxious thermal stimuli in healthy volunteers. J Neurophysiol. 2004;91:2723–2733. doi: 10.1152/jn.00249.2003. [DOI] [PubMed] [Google Scholar]
- Casey KL. Forebrain mechanisms of nociception and pain: analysis through imaging. Proc Natl Acad Sci U S A. 1999;96:7668–7674. doi: 10.1073/pnas.96.14.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Shyu BC. A fMRI study of brain activations during non-noxious and noxious electrical stimulation of the sciatic nerve of rats. Brain Res. 2001;897:71–81. doi: 10.1016/s0006-8993(01)02094-7. [DOI] [PubMed] [Google Scholar]
- Chen YC, Choi JK, Andersen SL, Rosen BR, Jenkins BG. Mapping dopamine D2/D3 receptor function using pharmacological magnetic resonance imaging. Psychopharmacology (Berl) 2005;180:705–715. doi: 10.1007/s00213-004-2034-0. [DOI] [PubMed] [Google Scholar]
- Chen CC, Shih YY, Mo KC, Yao NW, Lin ZJ, Huang CH, Shyu BC, Chang C. Mapping dopaminergic denervation in Parkinson disease in vivo and in situ: the visualization of structural details by CBV-weighted fMRI. J Cereb Blood Flow Metab. 2009;29:S607–S608. [Google Scholar]
- Choi JK, Chen YI, Hamel E, Jenkins BG. Brain hemodynamic changes mediated by dopamine receptors: role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. NeuroImage. 2006;30:700–712. doi: 10.1016/j.neuroimage.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Chudler EH, Dong WK. The role of the basal ganglia in nociception and pain. Pain. 1995;60:3–38. doi: 10.1016/0304-3959(94)00172-B. [DOI] [PubMed] [Google Scholar]
- Clark WG. Influence of opioids on central thermoregulatory mechanisms. Pharmacol Biochem Behav. 1979;10:609–613. doi: 10.1016/0091-3057(79)90241-7. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Schulzer M, Stoessl AJ. The placebo effect in neurological disorders. Lancet Neurol. 2002;1:85–91. doi: 10.1016/s1474-4422(02)00038-8. [DOI] [PubMed] [Google Scholar]
- Elmer GI, Pieper JO, Rubinstein M, Low MJ, Grandy DK, Wise RA. Failure of intravenous morphine to serve as an effective instrumental reinforcer in dopamine D2 receptor knock-out mice. J Neurosci. 2002;22:RC224. doi: 10.1523/JNEUROSCI.22-10-j0004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennessy MR, Rattray JF. Cardiovascular effects of intravenous morphine in the anaesthetized rat. Eur J Pharmacol. 1971;14:1–8. doi: 10.1016/0014-2999(71)90116-6. [DOI] [PubMed] [Google Scholar]
- Hagelberg N, Kajander JK, Nagren K, Hinkka S, Hietala J, Scheinin H. Mu-receptor agonism with alfentanil increases striatal dopamine D2 receptor binding in man. Synapse. 2002;45:25–30. doi: 10.1002/syn.10078. [DOI] [PubMed] [Google Scholar]
- Hagelberg N, Aalto S, Kajander J, Oikonen V, Hinkka S, Nagren K, Hietala J, Scheinin H. Alfentanil increases cortical dopamine D2/D3 receptor binding in healthy subjects. Pain. 2004a;109:86–93. doi: 10.1016/j.pain.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Hagelberg N, Jaaskelainen SK, Martikainen IK, Mansikka H, Forssell H, Scheinin H, Hietala J, Pertovaara A. Striatal dopamine D2 receptors in modulation of pain in humans: a review. Eur J Pharmacol. 2004b;500:187–192. doi: 10.1016/j.ejphar.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Inoue M, Katsumi Y, Hayashi T, Mukai T, Ishizu K, Hashikawa K, Saji H, Fukuyama H. Sensory stimulation accelerates dopamine release in the basal ganglia. Brain Res. 2004;1026:179–184. doi: 10.1016/j.brainres.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennan RP, Scanley BE, Innis RB, Gore JC. Physiological basis for BOLD MR signal changes due to neuronal stimulation: separation of blood volume and magnetic susceptibility effects. Magn Reson Med. 1998;40:840–846. doi: 10.1002/mrm.1910400609. [DOI] [PubMed] [Google Scholar]
- Lin MT, Wu JJ, Tsay BL. Effects of kainic acid injections in the striatum on physiologic and behavioral functions in conscious rats. Exp Neurol. 1984;83:71–83. doi: 10.1016/0014-4886(84)90047-5. [DOI] [PubMed] [Google Scholar]
- Liu CH, Greve DN, Dai G, Marota JJ, Mandeville JB. Remifentanil administration reveals biphasic phMRI temporal responses in rat consistent with dynamic receptor regulation. NeuroImage. 2007;34:1042–1053. doi: 10.1016/j.neuroimage.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe AS, Beech JS, Williams SC. Small animal, whole brain fMRI: innocuous and nociceptive forepaw stimulation. NeuroImage. 2007;35:719–728. doi: 10.1016/j.neuroimage.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Lu H, Scholl CA, Zuo Y, Stein EA, Yang Y. Quantifying the blood oxygenation level dependent effect in cerebral blood volume-weighted functional MRI at 9.4 T. Magn Reson Med. 2007;58:616–621. doi: 10.1002/mrm.21354. [DOI] [PubMed] [Google Scholar]
- Magnusson JE, Fisher K. The involvement of dopamine in nociception: the role of D(1) and D(2) receptors in the dorsolateral striatum. Brain Res. 2000;855:260–266. doi: 10.1016/s0006-8993(99)02396-3. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Saiardi A, Valverde O, Samad TA, Roques BP, Borrelli E. Absence of opiate rewarding effects in mice lacking dopamine D2 receptors. Nature. 1997;388:586–589. doi: 10.1038/41567. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJ, Kosofsky BE, Keltner JR, Weissleder R, Rosen BR, Weisskoff RM. Dynamic functional imaging of relative cerebral blood volume during rat forepaw stimulation. Magn Reson Med. 1998;39:615–624. doi: 10.1002/mrm.1910390415. [DOI] [PubMed] [Google Scholar]
- Martin JR, Takemori AE. Increased sensitivity to dopamine agonists following a single dose of morphine or levorphanol in mice. Eur J Pharmacol. 1985;119:75–84. doi: 10.1016/0014-2999(85)90324-3. [DOI] [PubMed] [Google Scholar]
- Morrow TJ, Paulson PE, Danneman PJ, Casey KL. Regional changes in forebrain activation during the early and late phase of formalin nociception: analysis using cerebral blood flow in the rat. Pain. 1998;75:355–365. doi: 10.1016/s0304-3959(98)00016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawela CP, Biswal BB, Hudetz AG, Li R, Jones SR, Cho YR, Matloub HS, Hyde JS. Interhemispheric neuroplasticity following limb deafferentation detected by resting-state functional connectivity magnetic resonance imaging (fcMRI) and functional magnetic resonance imaging (fMRI) NeuroImage. 2010;49:2467–2478. doi: 10.1016/j.neuroimage.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney KF, Armstrong RA, Sewell RD. Increased dopamine receptor sensitivity in the rat following acute administration of sufentanil, U50,488H and D-Ala2-D-Leu5-enkephalin. Naunyn-Schmiedebergs Arch Pharmacol. 1991;343:458–462. doi: 10.1007/BF00169546. [DOI] [PubMed] [Google Scholar]
- Shah YB, Haynes L, Prior MJ, Marsden CA, Morris PG, Chapman V. Functional magnetic resonance imaging studies of opioid receptor-mediated modulation of noxious-evoked BOLD contrast in rats. Psychopharmacology (Berl) 2005;180:761–773. doi: 10.1007/s00213-005-2214-6. [DOI] [PubMed] [Google Scholar]
- Shen Q, Ren H, Duong TQ. CBF, BOLD, CBV, and CMRO(2) fMRI signal temporal dynamics at 500-msec resolution. J Magn Reson Imaging. 2008;27:599–606. doi: 10.1002/jmri.21203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih YY, Chen YY, Chen JC, Chang C, Jaw FS. ISPMER: integrated system for combined PET, MRI, and electrophysiological recording in somatosensory studies in rats. Nucl Instrum Meth A. 2007;580:938–943. [Google Scholar]
- Shih YY, Chen YY, Chen CC, Chen JC, Chang C, Jaw FS. Whole-brain functional magnetic resonance imaging mapping of acute nociceptive responses induced by formalin in rats using atlas registration-based event-related analysis. J Neurosci Res. 2008;86:1801–1811. doi: 10.1002/jnr.21638. [DOI] [PubMed] [Google Scholar]
- Shih YY, Chen CC, Shyu BC, Lin ZJ, Chiang YC, Jaw FS, Chen YY, Chang C. A new scenario for negative functional magnetic resonance imaging signals: endogenous neurotransmission. J Neurosci. 2009;29:3036–3044. doi: 10.1523/JNEUROSCI.3447-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih YY, Wey HY, De La Garza BH, Duong TQ. Striatal and cortical BOLD, blood flow, blood volume, oxygen consumption, and glucose consumption changes in noxious forepaw electrical stimulation. J Cereb Blood Flow Metab. 2011;31:832–841. doi: 10.1038/jcbfm.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Takahashi N, Funada M, Ujike H, Revay RS, Donovan DM, Miner LL, Uhl GR. Opiate receptor knockout mice define mu receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci U S A. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JJ, Yang JW, Shyu BC. Current source density analysis of laser heat-evoked intra-cortical field potentials in the primary somatosensory cortex of rats. Neuroscience. 2006;140:1321–1336. doi: 10.1016/j.neuroscience.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Tempel A, Zukin RS. Neuroanatomical patterns of the mu, delta, and kappa opioid receptors of rat brain as determined by quantitative in vitro autoradiography. Proc Natl Acad Sci U S A. 1987;84:4308–4312. doi: 10.1073/pnas.84.12.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thobois S, Hassoun W, Ginovart N, Garcia-Larrea L, Le Cavorsin M, Guillouet S, Bonnefoi F, Costes N, Lavenne F, Broussolle E, Leviel V. Effect of sensory stimulus on striatal dopamine release in humans and cats: a [(11)C]raclopride PET study. Neurosci Lett. 2004;368:46–51. doi: 10.1016/j.neulet.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Tuor UI, McKenzie E, Tomanek B. Functional magnetic resonance imaging of tonic pain and vasopressor effects in rats. Magn Reson Imaging. 2002;20:707–712. doi: 10.1016/s0730-725x(02)00599-4. [DOI] [PubMed] [Google Scholar]
- Ushijima I, Tanaka M, Tsuda A, Koga S, Nagasaki N. Differential effects of morphine on core temperature in stressed and non-stressed rats. Eur J Pharmacol. 1985;112:331–337. doi: 10.1016/0014-2999(85)90778-2. [DOI] [PubMed] [Google Scholar]
- Wu EX, Tang H, Jensen JH. Applications of ultrasmall superparamagnetic iron oxide contrast agents in the MR study of animal models. NMR Biomed. 2004;17:478–483. doi: 10.1002/nbm.923. [DOI] [PubMed] [Google Scholar]
- Zhao F, Zhao T, Zhou L, Wu Q, Hu X. BOLD study of stimulation-induced neural activity and resting-state connectivity in medetomidine-sedated rat. NeuroImage. 2008;39:248–260. doi: 10.1016/j.neuroimage.2007.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]


