INTRODUCTION
Highly active antiretroviral therapy (HAART) has resulted in improvements in morbidity and mortality for HIV-infected individuals but is associated with lipodystrophy characterized by body fat redistribution and metabolic changes. The characteristic body fat changes include the loss of subcutaneous fat in the limbs, face and buttocks (lipoatrophy) and /or a relative increase or conservation of fat in the trunk, breast, neck and abdomen (lipohypertrophy).1 The predominant metabolic changes include dyslipidemia with increased triglyceride levels and disorders of glucose homeostasis including insulin resistance. Lipodystrophy can be a psychologically devastating consequence of HAART and can result in poor compliance or reluctance to initiate HAART. There is no proven, effective therapy for lipoatrophy.
The pathogenesis of these abnormalities remains unclear. Proposed mechanisms for the role of antiretroviral agents initially focused on the impact of protease inhibitors in increasing lipolysis2 and decreasing lipogenesis3. One hypothesis implicates decreased activation of peroxisome proliferator-activated receptor gamma (PPAR-γ), an important regulator of adipocyte differentiation and proliferation, in patients on protease inhibitors (PI).4 As this enzyme is preferentially expressed in peripheral fat, this may account for the fat redistribution seen in the syndrome. PPAR-γ stimulation also improves insulin sensitivity and hyperlipidemia, and its decreased activity in the setting of PI, could account for the metabolic changes.5,6
An alternative hypothesis for the lipoatrophic changes implicates the nucleoside analogue reverse transcriptase inhibitors (NRTIs).7 The thymidine analogues stavudine (d4T) and zidovudine (AZT) have been thought to play a key role both on the basis of in vitro inhibition of mitochondrial DNA polymerase gamma,8 decreased transcription of mitochondrial RNA and changes in oxidation phosphorylation9 and multiple clinical trials showing higher rates of lipoatrophy with NRTI-based therapy.10,11,12,13
Rosiglitazone and pioglitazone are both agents in the class of thiazolidinediones (TZDs) and are agonists for PPAR-γ activation and influence the transcription of genes that regulate adipogenesis, glucose and lipid metabolism. In vitro, TZDs promote adipogenesis. In HIV uninfected populations, TZDs have been used to treat type 2 diabetes and are known to improve insulin resistance, and increase limb fat. In congenital lipodystrophy syndromes, troglitazone increased peripheral fat, decreased visceral fat and improved glycemic abnormalities.14,15,16 TZDs may also dose-dependently increase mRNA expression and secretion of adiponectin, which could impact on insulin resistance and fat redistribution. In a meta-analysis of randomized trials of TZDs in diabetic patients, pioglitazone and rosiglitazone were shown to have different impacts on lipid levels.17
Given these observations, six randomized trials were conducted to compare changes in body fat or insulin among HIV positive individuals randomized to TZD or placebo.18,19,20,21,22,23 The results have been mixed. Rosiglitazone was associated with a significant increase in total body fat and subcutaneous leg fat area in one study22 and with increased leg fat in another.21 Other trials failed to show any significant effects on limb fat.18,19,20 As thymidine analogues could counteract the impact of TZDs on PPAR-γ, subset analyses of individual trials have hypothesized that rosiglitazone may be useful to increase limb fat in patients not receiving a thymidine analogue.18 The single placebo-controlled trial of pioglitazone demonstrated that pioglitazone marginally improved limb fat mass, particularly among patients not receiving d4T.23
This meta-analysis of the six randomized trials was undertaken to better determine the overall effect of TZD therapy on peripheral fat in HIV positive adults, to compare the effects of rosiglitazone and pioglitazone and to conduct subset analyses defined by the use at baseline of a thymidine analogue or a protease inhibitor.
METHODS
Randomized, placebo-controlled trials of a thiazolidinedione in HIV positive individuals which were published at the time of the analysis and had an outcome measure of body fat were identified through PubMed and consultation with experts in the field. Principle investigators of the identified trials were contacted and all agreed to participate in the meta-analysis.
The meta-analysis was conducted with the use of the original, individual-patient data from the six participating, randomized, placebo-controlled trials investigating the effects of rosiglitazone (n=5) or pioglitazone (n=1) on changes in body fat and insulin resistance in HIV positive patients (Table 1). 18,19,20,21,22,23 From the study with a factorial design in which patients were randomly assigned to receive either metformin or placebo and to receive either rosiglitazone or placebo,22 only patients randomized to either active rosiglitazone and metformin placebo (n=26) or to the dual placebo arm (n=27) were included in the meta-analysis.
Table 1.
Summary of Studies Included in Meta-Analysis
| Australia18 (n=108) |
USA-Boston21 (n=28) |
USA-ACTG22 (n=53) |
Finland19 (n=30) |
Canada20 (n=78) |
France23 (n=130) |
|
|---|---|---|---|---|---|---|
| Inclusion Criteria | ||||||
| Lipoatrophy | < 20% limb tissue or limb fat % at least 10% less than truncal fat % |
Clinically diagnosed |
waist-hip ratio > 0.95 for men or > 0.85 for women or a waist circumference > 100 cm |
Clinically diagnosed |
Clinically diagnosed | Clinically diagnosed |
| Antiretroviral therapy | Stable ≥12 weeks | Stable ≥12 weeks | Stable > 60 days | >>18 months | Stable ≥12 weeks | Stable > 6 months |
| Insulin resistance | Not required | Yes | Yes | Not required | Not required | Not required |
| HIV viral load | - | - | < 10,000 copies/mL | - | - | <400 copies/mL |
| CD4 Count | - | - | - | - | - | > 200 cells/mm3 |
| Study Characteristics | ||||||
| Duration of follow-up | 48 weeks | 12 weeks | 16 weeks | 24 weeks | 24 weeks | 48 weeks |
| Timing of DXA measurements | weeks 0, 24, 48 | weeks 0, 12 | weeks 0, 16 | - | weeks 0, 24 | weeks 0, 48 |
| Timing of Lipid Measurements | weeks 0, 8, 16, 24, 32, 40, 48 |
weeks 0, 12 | weeks 0, 8, 16 | weeks 0, 6, 12, 18, 24 |
weeks 0, 12, 24 | weeks 0, 48 |
| Active Drug | rosiglitazone 4mg bid |
rosiglitazone 4mg qd |
rosiglitazone 4mg qd | rosiglitazone 8mg qd |
rosiglitazone 4mg qd | pioglitazone 30mg qd |
| Primary Outcome | limb fat | insulin sensitivity | change in fasting insulin | subcutaneous fat | percentage change in limb fat |
limb fat |
| Patient Characteristics | ||||||
| Male | 106 (98%) | 21 (75%) | 34 (64%) | 25 (83%) | 76 (97%) | 106 (82%) |
| Age (years) | 45 (40, 50) | 45 (39, 50) | 45 (41, 50) | 42 (39, 48) | 47 (41, 53) | 44 (39, 50) |
| CD4 count (cells/mm3) | 542 (428.5, 724) | 432 (239.5, 617) | 641 (400, 922) | 526 (329, 707) | 439 (364, 641) | 582 (404, 735) |
| Viral Load < 50 copies/ml | 58 (54%) | 20 (74%) | 39 (74%) | 20 (67%) | 59 (76%) | 85 (66%) |
| Protease inhibitor use | 66 (61%) | 18 (64%) | 35 (66%) | 22 (73%) | 76 (97%) | 63 (48%) |
| AZT or d4T | 53 (49%) | 19 (68%) | 34 (64%) | 28 (93%) | 55 (70%) | 79 (61%) |
| Limb fat (kg) | 2.3 (1.7, 3.4) | 3.9 (2.4, 5.7) | 6.4 (3.6, 10.6) | - | 3.2 (2.5,4.3) | 2.2 (1.6, 3.5) |
| Body mass index (kg/m2) | 22.9 (21.3,24.6) | 24.3 (22.5, 28.4) | 28.2 (26.1, 32.0) | 23.6 (21.3,25.7) | 24.3 (22.3, 26.6) | 21.6 (20.1,23.4) |
| Glucose (mmol/L) | 5.1 (4.9, 5.5) | 5.3 (4.7, 6.0) | 5.4 (5.1, 5.8) | 5.3 (4.8, 5.8) | 5.0 (4.5, 5.4) | 5.1 (4.6, 5.5) |
| Insulin (mlU/L) | 8.3 (5.6, 11.5) | 16.2 (10.3,24.7) | 17 (14, 26.5) | 9 (6, 14) | 9.6 (7.2, 15.7) | 7.6 (5.6, 11.4) |
| Cholesterol (mmol/L) | 6.0 (5.1, 6.9) | 5.3 (4.5, 6.5) | 5.6 (4.8, 6.6) | 5.9 (5.2, 6.5) | 5.5 (4.7, 6.5) | 5.8 (4.7,6.8) |
| Triglycerides (mmol/L) | 2.9 (1.9,4.2) | 2.8 (1.9, 6.6) | 2.9 (2.0, 5.1) | 2.8 (1.9,4.3) | 3.2 (1.9,4.3) | 2.5 (1.5, 3.9) |
DXA = dual energy X-ray absorptiometry
All lipid measurements were done on fasting samples
The primary outcome of this meta-analysis was the impact of the TZD relative to placebo on changes in limb fat mass as determined by dual energy X-ray absorptiometry (DXA) scan analysis.
Statistical Methods
All data were combined into a single dataset. Baseline characteristics were summarized by treatment arm with counts and percentages for the categorical variables and with medians and interquartile ranges (IQR) for the continuous variables. Categorical variables were compared between treatment groups with chi square tests or Fisher’s exact tests, as appropriate. Continuous variables were compared between treatment groups with Wilcoxon rank sum tests.
Generalized Estimating Equation (GEE) models were used to estimate the effect of TZD therapy on changes in limb fat while adjusting for covariates and correlation among multiple observations within individuals.24 The exchangeable correlation structure was assumed. This methodology allows the number and timing of observations to vary within individuals. Covariates which had a p value of less than 0.10 in the univariate models or those believed to be possible confounding factors were candidates for inclusion in the multivariable regression model. Prespecified interaction terms between the use of d4T or AZT at baseline, the use of PIs at baseline, and treatment variables (TZD vs placebo, and dummy variables for rosiglitazone and pioglitazone compared to placebo) were tested for significance in the multivariate model.
At each follow-up visit, patients were classified as limb fat “responders” if they had an increase in limb fat mass from baseline of at least 10%; otherwise they were classified as “non-responders”. GEE logistic regression models were used to identify predictors of a significant limb fat response by this definition.
The outcome variables of change in limb fat mass and increase in limb fat mass from baseline of at least 10% were chosen to adjust for possible imbalances in baseline limb fat between treatment groups.
Data were analyzed with SAS Version 9.1.
RESULTS
Participant Characteristics
Four hundred and twenty-seven patients were included in the meta-analysis. In total, 213 (50%) patients were randomized to the TZD therapy and 214 (50%) were randomized to placebo. Of the patients allocated to TZD therapy, 149 (70%) received rosiglitazone and 64 (30%) received pioglitazone.
Table 2 describes the baseline characteristics of the patients by treatment arm and for the whole population. Patients randomized to TZD therapy had marginally higher limb fat at baseline than patients randomized to placebo (3.1 vs 2.7 kg, p=.06). There was no significant difference between the TZD and placebo groups in any of other characteristics tested.
Table 2.
Baseline Demographic and Clinical Characteristics by Treatment Group
| Total (N=427) |
Placebo (N=214) |
TZD (N=213) |
p value | ||||
|---|---|---|---|---|---|---|---|
| Study Characteristics | |||||||
| Clinical Center | Canada | 78 (18%) | 39 (18%) | 39 (18%) | 0.99 | ||
| Finland | 30 (7%) | 15 (7%) | 15 (7%) | ||||
| USA-ACTG | 53 (12%) | 27 (13%) | 26 (12%) | ||||
| USA-Boston | 28 (7%) | 12 (6%) | 16 (8%) | ||||
| France | 130 (30%) | 66 (31%) | 64 (30%) | ||||
| Australia | 108 (25%) | 55 (26%) | 53 (25%) | ||||
| Demographic Characteristics | |||||||
| Male | 368 (86%) | 185 (86%) | 183 (86%) | 0.87 | |||
| Age (years) | 45 (40-51) | 45 (40-51) | 44 (39-50) | 0.63 | |||
| Caucasian | 151 (80%) | 78 (84%) | 73 (77%) | 0.23 | |||
| Clinical Characteristics | |||||||
| AIDS Defining Illness | 131 (31%) | 61 (29%) | 70 (33%) | 0.31 | |||
| Smoking Status | Current | 138 (33%) | 66 (31%) | 72 (34%) | 0.79 | ||
| Past | 107 (25%) | 55 (26%) | 52 (25%) | ||||
| Never | 179 (42%) | 92 (43%) | 87 (41%) | ||||
| CD4 cells/mm3 | 542 (383-724) | 542 (384-747) | 542 (382-721) | 0.81 | |||
| Viral Load (copies/mL) | 50 (50-200) | 50 (50-200) | 50 (50-200) | 0.94 | |||
| Viral Load < 50 copies/mL | 281 (66%) | 141 (66%) | 140 (66%) | 0.97 | |||
| Baseline statin use | 39 (10%) | 16 (8%) | 23 (12%) | 0.21 | |||
| Baseline Body Fat | |||||||
| Body Mass Index (kg/m2) | 23.2(21.3-25.7) | 23.0 (21.1-25.2) | 23.4 (21.5-26.4) | 0.18 | |||
| Arm Fat (kg) | 1.0 (0.7-1.7) | 132 | 1.0 (0.7-1.6) | 133 | 1.0 (0.7-1.8) | 0.54 | |
| Leg Fat (kg) | 2.0 (1.4-3.1) | 132 | 1.9 (1.3-2.9) | 134 | 2.1 (1.4-3.4) | 0.13 | |
| Limb Fat (kg) | 2.9 (1.9-4.4) | 197 | 2.7 (1.8-4.1) | 196 | 3.1 (2.1-4.7) | 0.06 | |
| Total Body Fat (kg) | 11.5 (8.2-17.0) | 147 | 11.1 (8.0-16.9) | 148 | 11.9 (8.5-17.4) | 0.34 | |
| Current Antiretroviral Use | |||||||
| AZT or d4T Use | 268 (63%) | 137 (64%) | 131 (62%) | 0.55 | |||
| AZT Use | 105 (25%) | 57 (27%) | 48 (23%) | 0.26 | |||
| d4T Use | 163 (38%) | 80 (37%) | 83 (39%) | 0.81 | |||
| NRTI Use | 404 (95%) | 202 (95%) | 202 (95%) | 0.99 | |||
| NNRTI Use | 192 (45%) | 90 (42%) | 102 (48%) | 0.23 | |||
| EFV use | 103 (24%) | 51 (24%) | 52 (24%) | 0.89 | |||
| PI Use | Boosted PI | 156 (37%) | 78 (36%) | 78 (37%) | 0.99 | ||
| Unboosted PI | 124 (29%) | 62 (29%) | 62 (29%) | ||||
| Not Used | 147 (34%) | 74 (35%) | 73 (34%) | ||||
| Years on ARV | 6.9 (5.0-9.9) | 213 | 6.8 (5.0-9.7) | 212 | 7.0 (5.0-10.0) | 0.50 | |
| Years on HAART | 5.0 (3.4-6.3) | 192 | 4.9 (3.6-6.3) | 200 | 5.0 (3.2-6.3) | 0.65 | |
| Changes to ARVs during study | 88 (30%) | 39 (26%) | 49 (33%) | 0.22 | |||
| Baseline laboratory values | |||||||
| Cholesterol (mmol/L) | 5.7 (4.9-6.7) | 203 | 5.8 (4.9-6.7) | 208 | 5.7 (4.8-6.8) | 0.90 | |
| Triglycerides (mmol/L) | 2.8 (1.8-4.3) | 205 | 2.9 (1.8-4.3) | 208 | 2.8 (1.7-4.2) | 0.81 | |
| LDL (mmol/L) | 3.2 (2.6-4.0) | 166 | 3.3 (2.6-4.1) | 181 | 3.2 (2.5-3.9) | 0.28 | |
| HDL (mmol/L) | 1.1 (0.9-1.3) | 200 | 1.1 (0.9-1.3) | 204 | 1.1 (0.9-1.3) | 0.70 | |
| Glucose (mmol/L) | 5.1 (4.7-5.6) | 212 | 5.1 (4.7-5.6) | 211 | 5.1 (4.7-5.6) | 0.33 | |
| Insulin (mIU/L) | 9.6 (6.4-15.7) | 200 | 9.7 (6.5-15.6) | 203 | 9.6 (6.2-15.9) | 0.60 | |
| HOMA | 2.2 (1.5-3.7) | 200 | 2.2 (1.5-3.6) | 202 | 2.1 (1.4-3.7) | 0.45 | |
| ALT (U/L) | 32 (23-48) | 211 | 33 (23-49) | 210 | 32 (23-47) | 0.66 | |
| AST (U/L) | 30 (23-39) | 210 | 30 (23-38) | 209 | 29 (23-40) | 0.93 | |
Values are presented as N (%) or median (interquartile range).
TZD = thiazolidinediones, ACTG = AIDS Clinical Trials Group, VL = viral load, PI = protease inhibitor, NNRTI = non nucleoside reverse transcriptase inhibitor, AZT = zidovudine, d4T = stavudine, ARV = antiretroviral therapy, HOMA = homeostasis model assessment, LDL = low density lipoprotein, HDL = high density lipoprotein, ALT = alanine transaminase, AST= aspartate transaminases
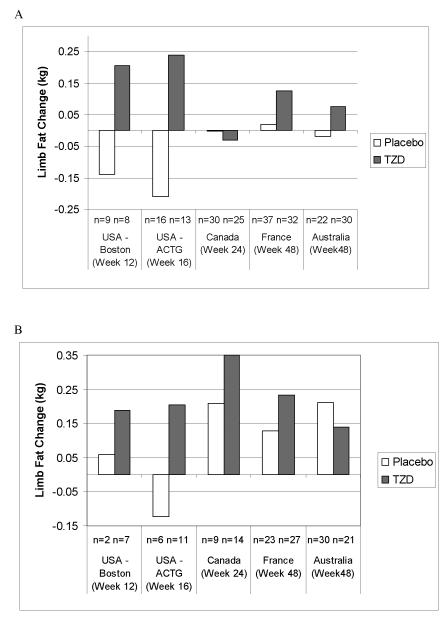
Limb Fat Changes
Measures of limb fat from DXA scans were available for 393 patients from five trials at baseline and for 373 patients at both baseline and at least one follow-up visit.18,20,21,22,23 Figure 1 shows the median limb fat change from baseline by study center, treatment group and baseline use of AZT or d4T. The median limb fat change was not statistically significantly different between TZD and placebo for any of the subgroups in Figure 1.
Figure 1.
Median limb fat mass change from baseline by study in (A) Patients on AZT or d4T, (B) Patients not on AZT or d4T
Table 3 describes the results of univariate GEE models for limb fat changes from baseline. Smoking status, use of d4T or AZT at baseline and use of d4T at baseline were associated with lower limb fat mass change while weeks of follow-up and NNRTI use were associated with higher limb fat mass change. The overall effect of TZD therapy was statistically significant (GEE regression coefficient = 0.14 kg, p=.04). Patients receiving pioglitazone had significantly higher changes in limb fat mass compared to patients receiving placebo (coeff=0.33 kg, p<.01) while patients on rosiglitazone had changes in limb fat mass that were not significantly greater than those in patients receiving placebo (coeff=0.07 kg, p=0.36).
Table 3.
Univariate Generalized Estimating Equation (GEE) Regression Models
| GEE Linear Regression model of Change in Limb Fat Mass |
GEE Logistic Regression model of 10% Increase in Limb Fat from Baseline |
|||||
|---|---|---|---|---|---|---|
|
| ||||||
| Covariates | Estimate | 95% CI | p value | OR | 95% CI | |
| Study Characteristics | ||||||
| Week of follow-up | 0.004 | (0.002, 0.007) | 0.0001 | 1.01 | (1.01, 1.02) | <.0001 |
| TZD vs. Placebo | 0.14 | (0.006, 0.27) | 0.04 | 1.38 | (0.95, 2.00) | 0.09 |
| Treatment | ||||||
| Pioglitazone | 0.33 | (0.11, 0.55) | 0.004 | 2.19 | (1.23, 3.89) | 0.007 |
| Rosiglitazone | 0.07 | (−0.08, 0.21) | 0.36 | 1.19 | (0.79, 1.77) | 0.40 |
| Placebo (ref.) | . | . | . | |||
| Center | ||||||
| France | −0.02 | (−0.18, 0.15) | 0.85 | 1.07 | (0.68, 1.68) | 0.76 |
| Canada | −0.20 | (−0.35, −0.05) | 0.009 | 0.51 | (0.28, 0.92) | 0.03 |
| USA-ACTG | −0.37 | (−0.68, −0.07) | 0.02 | 0.46 | (0.22, 0.97) | 0.04 |
| USA-Boston | −0.19 | (−0.43, 0.05) | 0.12 | 0.47 | (0.18, 1.19) | 0.11 |
| Australia (ref.) | . | . | ||||
| Demographic Characteristics | ||||||
| Male | 0.03 | (−0.30, 0.37) | 0.84 | 1.32 | (0.67, 2.62) | 0.42 |
| Age (per 10 years) | −0.007 | (−0.10, 0.08) | 0.88 | 0.97 | (0.77, 1.21) | 0.79 |
| Caucasian | 0.16 | (−0.13, 0.46) | 0.28 | 2.56 | (0.83, 7.86) | 0.10 |
| Clinical Characteristics | ||||||
| AIDS Defining Illness | −0.003 | (−0.15, 0.14) | 0.97 | 1.10 | (0.73, 1.66) | 0.64 |
| Baseline CD4 per 100 cells/mm3 | 0.01 | (−0.02, 0.04) | 0.40 | 1.03 | (0.96, 1.09) | 0.43 |
| Baseline CD4 < 500 cells/mm3 | −0.07 | (−0.21, 0.06) | 0.27 | 0.87 | (0.60, 1.26) | 0.47 |
| Baseline VL < 50 copies/mL | 0.11 | (−0.04, 0.26) | 0.15 | 1.15 | (0.79, 1.67) | 0.48 |
| Ever Smoked | −0.14 | (−0.27, 0.002) | 0.05 | 1.06 | (0.73, 1.54) | 0.77 |
| Baseline Statin Use | −0.04 | (−0.24, 0.16) | 0.67 | 0.85 | (0.43, 1.67) | 0.63 |
| Current Antiretroviral use | ||||||
| PI Use | ||||||
| Boosted PI | −0.08 | (−0.24, 0.07) | 0.30 | 0.73 | (0.48, 1.12) | 0.15 |
| Unboosted PI | −0.11 | (−0.30, 0.08) | 0.26 | 0.94 | (0.58, 1.54) | 0.82 |
| No PI (ref.) | . | . | . | |||
| NNRTI use | ||||||
| Efavirenz | 0.31 | (0.16, 0.46) | <0.0001 | 1.89 | (1.24, 2.89) | .003 |
| Other | 0.08 | (−0.10, 0.26) | 0.39 | 0.94 | (0.58, 1.52) | .80 |
| NNRTI Use | 0.20 | (0.06, 0.33) | 0.005 | 1.31 | (0.91, 1.90) | 0.15 |
| AZT or d4T Use | −0.25 | (−0.38, −0.12) | <.001 | 0.66 | (0.45, 0.96) | 0.03 |
| AZT Use Only | −0.12 | (−0.29, 0.05) | 0.18 | 0.95 | (0.60, 1.49) | 0.81 |
| d4T Use Only | −0.17 | (−0.31, −0.03) | 0.01 | 0.67 | (0.46, 0.98) | 0.04 |
| Baseline Body Fat | ||||||
| BMI (kg/m2) | −0.01 | (−0.04, 0.01) | 0.30 | 0.93 | (0.88, 0.98) | <.01 |
| Limb Fat | −0.02 | (−0.06, 0.02) | 0.24 | 0.92 | (0.85, 0.99) | 0.02 |
| Limb Fat >15% | −0.006 | (−0.25, 0.24) | 0.96 | 0.69 | (0.41, 1.16) | 0.16 |
| Baseline HOMA | 0.007 | (−0.006, 0.02) | 0.30 | 1.03 | (1.00, 1.07) | 0.08 |
| log10 Baseline Insulin | −0.09 | (−0.35,0.16) | 0.48 | 1.06 | (0.55, 2.04) | 0.86 |
TZD = thiazolidinediones, VL = viral load, PI = protease inhibitor, NNRTI = non nucleoside reverse transcriptase inhibitor, AZT = zidovudine, d4T = stavudine, BMI = body mass index, HOMA = homeostasis model assessment
In a multivariable GEE model controlling for study, week of follow-up, NNRTI use and thymidine analogue use, there was a benefit of TZDs relative to placebo on limb fat mass gains (coeff=.13, p=.05) but no difference in the efficacy of TZDs according to thymidine analogue use in a separate model with an interaction term (p=0.64).
In a multivariable GEE model examining the effects of each TZD separately after adjusting for study, NNRTI use and d4T use (Table 4), patients receiving pioglitazone had higher changes in limb fat mass (coeff=0.35 kg, p<0.01) than patients receiving placebo while patients receiving rosiglitazone had similar changes in limb fat mass as patients receiving placebo (coeff = 0.05 kg, p=0.48). Interactions between TZD use and use of AZT or d4T, between rosiglitazone and pioglitazone and use of AZT or d4T, between TZD use and NNRTI use and between rosiglitazone and pioglitazone and PI use were not statistically significant, indicating that the effectiveness of TZDs generally and rosiglitazone and pioglitazone specifically did not vary according to use of thymidine analogues or PIs.
Table 4.
Multivariable Generalized Estimating Equation (GEE) Regression Models
| GEE Linear Regression model of Change in Limb Fat Mass |
GEE Logistic Regression model of 10% Increase in Limb Fat from Baseline |
|||||
|---|---|---|---|---|---|---|
|
| ||||||
| Covariates | Estimate | 95% CI | p value | OR | 95% CI | |
| Week of follow-up | 0.004 | (0.001, 0.006) | 0.0016 | 1.01 | (1.00, 1.02) | 0.0017 |
| Treatment | ||||||
| Pioglitazone | 0.35 | (0.11, 0.58) | 0.004 | 2.28 | (1.07, 4.88) | 0.03 |
| Rosiglitazone | 0.05 | (−0.10, 0.21) | 0.48 | 1.11 | (0.72, 1.72) | 0.63 |
| Placebo (ref.) | . | . | . | |||
| Center | ||||||
| France | −0.18 | (−0.37, 0.01) | 0.06 | 0.73 | (0.37, 1.45) | 0.37 |
| Canada | −0.07 | (−0.22, 0.08) | 0.34 | 0.72 | (0.37, 1.40) | 0.33 |
| USA-ACTG | −0.28 | (−0.58, 0.03) | 0.07 | 0.64 | (0.28, 1.49) | 0.30 |
| USA-Boston | −0.09 | (−0.34, 0.16) | 0.47 | 0.65 | (0.23, 1.81) | 0.41 |
| Australia (ref.) | . | . | . | |||
| NNRTI Use - efavirenz | 0.31 | (0.17, 0.46) | <0.0001 | 1.94 | (1.26, 2.99) | 0.003 |
| NNRTI use – other | 0.004 | (−0.18, 0.19) | 0.97 | 0.79 | (0.48, 1.30) | 0.36 |
| Stavudine use | −0.15 | (−0.28, −0.02) | 0.02 | |||
TZD = thiazolidinediones, NNRTI = non nucleoside reverse transcriptase inhibitor
Limb Fat Response
One hundred and fifty-three patients (41%) had an increase of at least 10% in limb fat mass at least once during follow-up. Table 3 shows the results of univariate GEE logistic regression models of the probability of a 10% increase in limb fat mass from baseline. A multivariate GEE logistic regression model to identify predictors of limb fat mass response demonstrated that patients receiving pioglitazone were significantly more likely to achieve a 10% increase in limb fat at a given visit (odds ratio [OR] = 2.28, p=0.03) than patients receiving placebo while patients receiving rosiglitazone had similar likelihood of a 10% increase in limb fat mass as patients receiving placebo (OR=1.11, p=0.63) after controlling for study, length of follow-up, use of d4T and NNRTI use.
Adverse Events
Six patients on TZD therapy and seven patients on placebo discontinued the study drug because of adverse events. Among patients on rosiglitazone, reasons for discontinuation included elevated triglycerides (n=2),18,19 abdominal and parotid swellings (n=1),18 diarrhea (n=1),22 nausea (n=1),22 and requirement for prohibited medication (n=1).22 Among patients on placebo, reasons for discontinuation of study drug included portal hypertension with possible venous thrombosis (n=1)18, elevated lactate (n=2),22 elevated liver function tests (n=2),11,22 nausea (n=1)22 and a combination of toxicities (n=1).22
Grade 3 or 4 levels of ALT were reported in only one patient on rosiglitazone.18 Grade 3 or 4 levels of AST were reported in seven patients: two on rosiglitazone, one on pioglitazone and four on placebo during the study periods. As noted above, two patients on placebo stopped study medication because of elevated AST.
Rosiglitazone increased the risk of hypercholesterolemia18 and hypertriglyceridemia18,19 in individual studies. In contrast, pioglitazone increased HDL cholesterol and had no deleterious effects on triglycerides, total, or LDL cholesterol.23 One patient on pioglitazone died suddenly from an unknown cause. There was no case of new or increasing congestive heart failure or documented myocardial infarction.
DISCUSSION
This meta-analysis of individual-patient data from six randomized, placebo-controlled trials of TZD from five countries forms the largest assessment of the effect of TZD therapy on limb fat among HIV-positive individuals. Overall, there was a small impact of TZD on limb fat gains in the combined dataset, unlikely to be clinically meaningful. Sub-analysis demonstrated a significant effect of pioglitazone but not of rosiglitazone on increases in limb fat relative to placebo. There was no difference in limb fat response or in the effectiveness of TZDs according to whether or not patients were receiving protease inhibitors.
In this meta analysis, while the use of thymidine analogues was associated with poorer limb fat outcomes, the effectiveness of TZD therapy did not vary by thymidine analogue use. Our data contrast those recently presented in abstract form which showed rosiglitazone to be effective at increasing limb fat among HIV positive individuals with mild limb fat loss on thymidine-sparing regimens.25 However, while the mean change in limb fat to week 48 was significantly higher in the rosiglitazone group than the placebo group (911g +/− 1215 vs 253g +/− 1039, p=.018), the difference between treatment groups in median changes in limb fat was more modest although still similar in magnitude to prior switch studies off of thymidine analogue NRTIs (448 vs 153, p=.02) (personal communication, Grace McComsey, August 6, 2009). Since there was no comparison group of patients on thymidine analogues in the study, it is difficult to determine if the effectiveness of rosiglitazone was due to the absence of thymidine analogues or to differences in the patient population with regard to baseline line levels of limb fat, racial background or other factors. At this time, the data from this trial is not available for inclusion in the meta-analysis.
Our data are consistent with previous studies demonstrating that patients receiving d4T or AZT have increased risks of lipoatrophy over time.26 Since regimens containing abacavir (ABC)/lamivudine (3TC) or tenofovir (TDF)/emtricitabine (FTC) are associated with lower risks of lipoatrophy, and have been shown in vitro to have less mitochondrial toxicity, regimens excluding d4T and AZT are now recommended among patients initiating antiretroviral therapy.13,27 Among patients with established lipoatrophy, switching drug regimens from those containing d4T or AZT to either ABC or TDF may result in peripheral fat gain.28,29,30,31,32 However, since only 15 patients in the combined meta-analysis stopped d4T or AZT during the study period, this phenomenon is unlikely to have impacted changes in limb fat in our study.
Differences in the efficacy of TZD therapy among trials may have occurred because of disparities in the study populations at enrolment with respect to age, gender, baseline BMI, use of protease inhibitors, d4T and AZT and other ARVs, duration of ARV use, the presence or degree of insulin resistance or because of differences in changes in limb fat in the placebo group. In the two trials in which rosiglitazone increased limb fat, insulin resistance was an eligibility criterion21,22 as it was in a smaller, open-label study in which rosiglitazone increased limb fat in patients with HIV-associated lipoatrophy.33
In a sub-study in the Australian cohort, PPAR-γ expression in fat biopsies was not increased in subjects on thymidine analogues randomized to rosiglitazone either at week 2 or 48 but was increased in subjects not on thymidine analogues at week 2 in those on rosiglitazone and in both rosiglitazone and placebo groups at week 48.34 This would suggest that intact mitochondrial function could be required for TZD-induced stimulation of PPAR-γ expression in human adipose tissue.
The differential efficacy between pioglitazone and rosiglitazone could be due to in part to differences in dosing, the fact that the pioglitazone study was conducted several years later than the rosiglitazone studies, unmeasured differences in the patients enrolled in the pioglitazone study or biologic differences between the agents. For example, differential impacts of rosiglitazone and pioglitazone on adiponectin could have been associated with different limb fat outcomes. However, we were not able to assess this in our meta-analysis.
Rosiglitazone was associated with increases in triglycerides18,19 and total cholesterol18,19,21, in several of the studies in the meta-analysis. In another study, rosiglitazone increased LDL cholesterol and decreased HDL cholesterol22 while pioglitazone has been shown to improve HDL cholesterol.23 There were few documented increases in liver transaminases among participants of these studies. In a meta-analysis of randomized trials of rosiglitazone for the treatment of type 2 diabetes in non HIV populations, a significant increase in the risk of myocardial infarction and a trend towards an increase in deaths due to cardiovascular causes was demonstrated.35 While we did not observe an increased risk of cardiovascular disease in this meta-analysis, many studies excluded patients at higher cardiovascular risk, the follow up period was short and our sample size was not large enough to rule out the possibility of this adverse event.
While use of d4T and AZT is still common in resource limited settings, the incidence of HIV lipoatrophy in the developed world is decreasing due to improved knowledge of strategies to avoid the development of this syndrome among patients on HAART. To date, trials in antiretroviral-naïve patients have shown a lower incidence of lipoatrophy with the use of abacavir or tenofovir but more long term follow up is necessary to determine whether they prevent, or simply slow the development of the problem.36 While d4T and AZT might be avoided in first-line regimens, it may be necessary to use one of these drugs later in disease due to toxicity, virologic failure or drug resistance. If this is the case, the development of lipoatrophy may just be deferred to a later stage of disease.
The relative contributions of other agents in the HAART combination are also unclear. In ACTG 5005s, initiation of efavirenz was associated with relative less loss of limb fat, compared with nelfinavir.12 In ACTG 5142, a randomized trial of nucleoside, non-nucleoside and protease inhibitor sparing regimens for initial HIV treatment, the risk of lipoatrophy was greater in those randomized to efavirenz than those randomized to lopinavir/r even after controlling for NRTI use.11 In our analysis, fat gains were greater in patients on efavirenz over time. Patients in the meta-analysis were treatment experienced, on stable ARV regimens with higher CD4 counts, lower viral load and lower baseline limb fat mass than patients in ACTG 5142. Patients in the meta-analysis were attempting to reverse loss of limb fat mass whereas patients in ACTG 5142 were experiencing initial loss of limb fat mass, which may explain the difference in findings. Further, our results must be interpreted with caution, as the effect of efavirenz was not an a priori hypothesis of interest, patients were not randomized to ARV treatments and the significant association between efavirenz and limb fat mass gain may be due to an unmeasured confounder.
Strengths of our study include the large sample size which increased the statistical power to test for interactions and conduct subset analyses, the availability of individual patient data, repeated measures of limb fat over time, inclusion of trials with different doses of TZD and a broader range of patient baseline characteristics than in any single trial.
These data and concerns about cardiovascular risk from studies in non HIV-infected populations37 suggest that the use of rosiglitazone for peripheral lipoatrophy is not justified. Further research on the use of pioglitazone is justified, as it may have a stronger and more significant impact on lipoatrophy, more favorable effects on lipids and no increased risk of cardiovascular events.38 More data are needed on the safety of this compound and investigation of populations that might benefit most from this treatment strategy. Moreover, further research is needed as to the cardiovascular effects of these insulin-sensitizing strategies, independent of effects on fat atrophy.
ACKNOWLEDGEMENTS
Drs. Raboud and Walmsley acknowledge Career Scientist Award support from the Ontario HIV Treatment Network. Andrew Carr is a recipient of a Practitioner Fellowship from the Australian National Health and Medical Research Council.
Pharmaceutical support for the Canadian study was provided by GlaxoSmithKline Inc.
Funding for the ACTG study: This study was partly supported by the Adult AIDS Clinical Trials Group funded by the National Institute of Allergy and Infectious Diseases (AI38858, AI38855, AI27663, AI25924, AI27664, AI27659) and by General Clinical Research Center Units funded by the National Center for Research Resources (RR RR000750, RR00096, RR00052, RR00083). Pharmaceutical support was provided by Bristol-Myers Squibb Company and GlaxoSmithKline, Inc.
Funding for the study by Hadigan et al: National Institutes of Health grants RO1 DK59535, K23 DK 02844, M01-RR30088 and M01-RR02635.
Funding for the ANRS 113 Lipiot study: The French National Agency for AIDS and Viral Hepatitis Research (ANRS) sponsored the trial.
Funding for the Australian study: Bristol-Myers Squibb. Study drug was supplied by GlaxoSmithKline.
GLITAZONE LIPOATROPHY META-ANALYSIS WORKING GROUP MEMBERS
Toronto, Canada – Sandy Shen, DeSheng Su; Sydney, Australia – Sean Emery; ACTG: David Wininger, Susan Koletar; Paris, France - E Lanoy, L Slama.
Footnotes
Conflicts of Interest
Andrew Carr has received research funding from Abbott, Merck and Roche; consultancy fees from Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck and Roche; lecture sponsorships from Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck and Roche; and has served on advisory boards for Abbott, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck and Roche.
Dominique Costagliola has received travel grants, consultancy fees and honoraria from Abbott, BMS, Boehringer-Ingelheim, Gilead, Janssen, Merck, GSK and Roche.
Steven Grinspoon has previously served as a consultant and received research funding from Theratechnologies and Serono and receives research support from Bristol-Meyers Squibb.
Kathleen Mulligan has served on advisory boards for Abbott, Theratechnologies, and Serono; has consulted for Amylin and Amgen; and has received research support from Theratechnologies.
Jussi Sutinen has received lecture sponsorships from Abbott, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Roche and Tibotec; has consulted for GlaxoSmithKline; and served on advisory board for Tibotec.
Sharon Walmsley has received lecture sponsorships and has served on advisory boards for GlaxoSmithKline, Boehringer-Ingelheim, Bristol-Meyers Squibb, Abbott, Roche, Merck, Tibotec, Gilead and Phizer.
References
- 1.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. New Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 2.Mynarcik D, Wei LX, Komaroff E, Ferris R, McNurlan M, Gelato M. Chronic loss of subcutaneous adipose tissue in HIV-associated lipodystrophy may not be associated with accelerated apoptosis. JAIDS. 2005;38:367–71. [PubMed] [Google Scholar]
- 3.Roche R, Poizot-Martin I, Martin-El Yazidi C, Compe E, Gastaut J-A, Torresani J, Planells R. Effects of antiretroviral drug combinations on the differentiation of adipocytes. AIDS. 2002;16:13–20. doi: 10.1097/00002030-200201040-00003. [DOI] [PubMed] [Google Scholar]
- 4.Serghides L, Nathoo S, Walmsley S, Kain KC. CD36 deficiency induced by antiretroviral therapy. AIDS. 2002;16:353–8. doi: 10.1097/00002030-200202150-00006. [DOI] [PubMed] [Google Scholar]
- 5.Miyaoka K, Kuwasako T, Hirano K, et al. CD36 deficiency associated with insulin resistance. Lancet. 2001;357:686–7. doi: 10.1016/s0140-6736(00)04138-6. [DOI] [PubMed] [Google Scholar]
- 6.Aitman TJ. CD36, insulin resistance, and coronary heart disease. Lancet. 2001;357:651–2. doi: 10.1016/S0140-6736(00)04149-0. [DOI] [PubMed] [Google Scholar]
- 7.Brinkman K, Smeitink JA, Romijn JA, Reiss P. Mitochondrial toxicity induced by nucleoside-anologue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet. 1999;354:1112–5. doi: 10.1016/S0140-6736(99)06102-4. [DOI] [PubMed] [Google Scholar]
- 8.Birkus G, Hitchcock MJ, Cihlar T. Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother. 2002;46:716–23. doi: 10.1128/AAC.46.3.716-723.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mallon PW, Unemori P, Sedwell R, Morey A, Rafferty M, Williams K, Chisholm D, Samaras K, Emery S, Kelleher A, Cooper DA, Carr A, SAMA Investigators In vivo, nucleoside reverse-transcriptase inhibitors alter expression of both mitochondrial and lipid metabolism genes in the absence of depletion of mitochondrial DNA. J Infect Dis. 2005;191:1686–96. doi: 10.1086/429697. [DOI] [PubMed] [Google Scholar]
- 10.Mallon PW, Miller J, Cooper DA, Carr A. Prospective evaluation of the effects of antiretroviral therapy on body composition in HIV-1 infected men starting therapy. AIDS. 2003;17:971–9. doi: 10.1097/00002030-200305020-00005. [DOI] [PubMed] [Google Scholar]
- 11.Haubrich RH, Riddler SA, DiRienzo AG, Komarow L, Powderly WG, Klingman K, Garren KW, Butcher DL, Rooney JF, Haas DW, Mellors JW, Havlir DV, AIDS Clinical Trials Group (ACTG) A5142 Study Team Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS. 2009;23(9):1109–18. doi: 10.1097/QAD.0b013e32832b4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dube MP, Parker RA, Tebas P, Grinspoon SK, Zackin RA, Robbins GK, Roubenoff R, Shafer RW, Wininger DA, Meyer WA, III, Snyder SW, Mulligan K. Glucose metabolism, lipid disorders, and body fat changes in antiretroviral-naïve subjects randomized to nelfinavir or efavirenz plus dual nucleosides. AIDS. 2005;19:1807–1818. doi: 10.1097/01.aids.0000183629.20041.bb. [DOI] [PubMed] [Google Scholar]
- 13.Podzamczer D, Ferrer E, Sanchez P, et al. Less lipoatrophy and better lipid profile with abacavir as compared to stavudine: 96 week results of a randomized study. JAIDS. 2007;44:139–47. doi: 10.1097/QAI.0b013e31802bf122. [DOI] [PubMed] [Google Scholar]
- 14.Arighu E, Duncan-Morin J, Sebring N, et al. Efficacy and Safety of troglitazone in the treatment of lipodystrophy syndromes. Ann Intern Med. 2000;133:263–274. doi: 10.7326/0003-4819-133-4-200008150-00009. [DOI] [PubMed] [Google Scholar]
- 15.Mori Y, Murakawa Y, Okada K, Horikoshi H, Yokoyama J, Tajia N, et al. Effect of troglitazone on body fat distribution in type 2 diabetic patients. Diabetes Care. 1999;22:908–912. doi: 10.2337/diacare.22.6.908. [DOI] [PubMed] [Google Scholar]
- 16.Kawai T, Takei I, Oguma Y, Ohashi N, Tokui M, Oguchi S, et al. Effects of troglitazone on fat distribution in the treatment of male type 2 diabetes. Metabolism. 1999;48:1102–1107. doi: 10.1016/s0026-0495(99)90122-1. [DOI] [PubMed] [Google Scholar]
- 17.Chiquette E, Ramirez G, DeFronzo R. A Meta-Analysis Comparing the Effect of Thiazolidinediones on Cardiovascular Risk Factors. Arch Internal Med. 2004;164:2097–2104. doi: 10.1001/archinte.164.19.2097. [DOI] [PubMed] [Google Scholar]
- 18.Carr A, Workman C, Carey D, et al. No effect of rosiglitazone for treatment of HIV-1 lipoatrophy: randomized, double-blind, placebo-controlled trial. Lancet. 2004;363:429–438. doi: 10.1016/S0140-6736(04)15489-5. [DOI] [PubMed] [Google Scholar]
- 19.Sutinen J, Häkkinen AM, Westerbacka J, et al. Rosiglitazone in the treatment of HAART-associated lipodystrophy: a randomized double-blind placebo-controlled study. Vol. 8. International Medical Press; 2003. pp. 199–207. [PubMed] [Google Scholar]
- 20.Cavalcanti R, Raboud JM, Shen S, Kain K, Cheung A, Walmsley S. A randomized, placebo-controlled trial of rosiglitazone for HIV-related lipoatrophy. JID. 2007;195(12):1754–61. doi: 10.1086/518005. [DOI] [PubMed] [Google Scholar]
- 21.Hadigan C, Yawetz S, Thomas A, Havers F, Sax F, Grinspoon S. Metabolic effects of rosiglitazone in HIV lipodystrophy: A randomized, controlled trial. Annals of Internal Medicine. 2006;140:786–795. doi: 10.7326/0003-4819-140-10-200405180-00008. [DOI] [PubMed] [Google Scholar]
- 22.Mulligan K, Yang Y, Wininger D, et al. Effects of metformin and rosiglitazone in HIV-infected patients with hyperinsulinemia and elevated waist/hip ratio. AIDS. 2007;21:47–57. doi: 10.1097/QAD.0b013e328011220e. [DOI] [PubMed] [Google Scholar]
- 23.Slama L, Lanoy E, Valanin MA, Bastard JP, Chermak A, Boutekatjirt A, William-Faltaos D, Billaud E, Molina JM, Capeau J, Costagliola D, Rozenbaum W. Effect of pioglitazone on HIV-1 related lipodystrophy: a randomized double-blind placebo-controlled trial (ANRS 113) Antiviral Therapy. 2008;13:67–76. [PubMed] [Google Scholar]
- 24.Zeger SL, Liang KY. Longitudinal data analysis of discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 25.El Bejjani D, Tungsiripat M, Rizk N, O’Riordan M, Ross A, Hilemann C, Storer N, Harrill D, McComsey G. Rosiglitazone Improves Lipoatrophy in Patients receiving Thymidine-Sparing Regimens; 16th Conference on Retroviruses and Opportunistic Infections; 2009; Abstract 42LB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saint-Marc T, Partisani M, Poizot-Martin I, et al. A syndrome of peripheral fat wasting (lipodystrophy) in patients receiving long-term nucleoside analogue therapy. AIDS. 1999;13:1659–1667. doi: 10.1097/00002030-199909100-00009. [DOI] [PubMed] [Google Scholar]
- 27.Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [Accessed May 1, 2008]. Jan 29, 2008. pp. 1–128. Available http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Table 18, page 83. [Google Scholar]
- 28.Moyle G, Savin CA, Cartledge J, Johnson M, Wilkins E, Churchill D, Hay P, Fakoya A, Murphy M, Scullard G, Leen C, Reilly G, RAVE (Randomized Abacavir versus Viread Evaluation) Group UK A randomized comparative trial of tenofovir DF or abacavir as replacement for a thymidine analogue in persons with lipoatrophy. AIDS. 2006;20:2043–50. doi: 10.1097/01.aids.0000247574.33998.03. [DOI] [PubMed] [Google Scholar]
- 29.Carr A, Workman C, Smith DE, Hoy J, Hudson J, Doong N, et al. Abacavir substitution for nucleoside analogs in patients with HIV lipoatrophy: a randomized trial. JAMA. 2002;288:207–215. doi: 10.1001/jama.288.2.207. [DOI] [PubMed] [Google Scholar]
- 30.Martin A, Smith DE, Carr A, Ringland C, Amin J, Emery S, et al. Reversibility of lipoatrophy in HIV-infected patients 2 years after switching from a thymidine analogue to Abacavir: the MITOX Extension Study. AIDS. 2004;18:1029–36. doi: 10.1097/00002030-200404300-00011. [DOI] [PubMed] [Google Scholar]
- 31.John M, McKinnon EJ, James IR, Nolan DA, Herrmann SE, Moore CB, White AJ, Mallal SA. Randomized, controlled, 48-week study of switching and/or protease inhibitors to combivir/Abacavir to prevent or reverse lipoatrophy in HIV-infected patients. JAIDS. 2003;33:29–33. doi: 10.1097/00126334-200305010-00005. [DOI] [PubMed] [Google Scholar]
- 32.McComsey GA, Ward DJ, Hessenthaler SM, Sension MG, et al. Improvement in lipoatrophy associated with highly active antiretroviral therapy in human immunodeficiency virus-infected patients switched from stavudine to abacavir or zidovudine: the results of the TARHEEL study. Clin Infect Dis. 2004;38:263–270. doi: 10.1086/380790. [DOI] [PubMed] [Google Scholar]
- 33.Gelato MC, Mynarcik DC, Quick JL, Steigbigel RT, Fuhrer J, Brathwaite CE, et al. Improved insulin sensitivity and body fat distribution in HIV-infected patients treated with rosiglitazone: a pilot study. J Acquir Immune Defic Syndr. 2002;31:163–170. doi: 10.1097/00126334-200210010-00006. [DOI] [PubMed] [Google Scholar]
- 34.Mallon PWG, Sedwell R, Rogers G, Nolan D, Unemori P, Hoy J, Samaras K, Kelleher A, Emery S, Cooper DA, Carr A, for the Rosey Investigators Adipose Tissue is Limited by Antiretroviral Drug-Induced Mitochondrial Dysfunction. JID. 2008;198:1794–803. doi: 10.1086/593179. [DOI] [PubMed] [Google Scholar]
- 35.Nissen SE, Wolski K. Effect of Rosiglitazone on the Risk of Myocardial Infarction and Death from Cardiovascular Causes. N Engl J Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 36.Pozniak AL, Gallant JE, DeJesus E, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz versus fixed-dose zidovudine/lamivudine and efavirenz in antiretroviral-naïve patients: virologic, immunologic and morphologic changes – a 96 week analysis. JAIDS. 2006;43:535–40. doi: 10.1097/01.qai.0000245886.51262.67. [DOI] [PubMed] [Google Scholar]
- 37.Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta analysis. JAMA. 2007;298:1189–95. doi: 10.1001/jama.298.10.1189. [DOI] [PubMed] [Google Scholar]
- 38.Nagajothi N, Adigopula S, Balamuthusamy S, et al. Pioglitazone and the risk of myocardial infarction and other major adverse cardiac events: a meta-analysis of randomized, controlled trials. Am J Ther. 2008;15(6):506–11. doi: 10.1097/MJT.0b013e318167180c. [DOI] [PubMed] [Google Scholar]