Abstract
There are well-established patterns of structural brain changes associated with aging. The change in brain volume with age and with the diseases of aging presents a particular challenge for MRI studies in the elderly. Structural MRI is important for studies in normal aging, late-life depression, dementia, Alzheimer disease and other cognitive disorders to examine how age-associated changes in neuroanatomy are associated with specific age-related changes in brain function. Functional MRI has been a major advance for the fields of cognitive and affective neuroscience by allowing investigators to test theories of the underlying neural pathways controlling cognitive and emotional processes. In this chapter, we will review the contribution of MRI studies to late-life mood and anxiety disorders: major depression, bipolar disorder and anxiety disorders in late-life.
Keywords: Structural MRI, Age-related brain changes, Functional MRI, Methodological challenges in late-life MRI, Late-life mood disorders
Over the last decade there has been a rapid increase in the availability of MR imaging. It is likely that the increase in accessibility, as well as the decrease in scanning costs, will continue to increase the use of MRI. Neuroimaging may offer not only insights into the neurobiology of late-life mental disorders, but may also contribute to the effort of personalizing existing treatments and discover new, more efficacious ones. In this chapter, we will review the contribution of MRI studies to late-life mood and anxiety disorders: major depression, bipolar disorder, and anxiety disorders in late-life.
1 Methodologic Challenges of MRI in Late-Life
1.1 The Influence of Brain Morphometric Changes on fMRI
There are well-established patterns of structural brain changes associated with aging. With increasing age, the brain decreases in overall volume, the cortical gyri become smaller, and the sulci and ventricles become larger. These changes in brain volume vary across individuals and occur even in individuals who are otherwise apparently healthy. The changes have been described in a number of studies (e.g., Raz et al. 1997; Resnick et al. 2000) and seem to vary across the brain with most prominent decrease in volume reported in the frontal cortex.
The change in brain volume with age and with the diseases of aging presents a particular challenge for functional MRI studies in these populations: how should these structural changes be accounted for when comparing the functional signal? In a standard fMRI analysis plan the functional images from all the subjects in a study are lined-up with each other (alignment, cross-registration, warping, normalization). If the brains have significantly different shapes and sizes then the brain alignment may bias the results by contributing more CSF (due to the larger sulci and ventricles) of the more atrophic brains as compared to more gray matter from the less atrophic brains. The standard alignment algorithms vary in their ability to account for the variability in brain structure (Wu et al. 2006). Some investigators have addressed this problem by using a larger smoothing kernel in studies of aging subjects (e.g., 10 mm instead of standard 6 mm or 8 mm full-width half-maximum Gaussian). This approach recognizes that the alignment may be worse in the elderly population, and corrects for it by making the images blurrier. This allows the statistical voxel-wise comparison to find group differences even if there is some discrepancy in the spatial co-localization. An alternative approach that other investigators have used involves avoiding the registration problems altogether, by focusing on a region-of-interest (ROI)-based analysis (Aizenstein et al. 2011), using ROIs defined in the acquired fMRI space, rather than relying on normalizing the images.
1.2 The BOLD Hemodynamic Response in Healthy Aging
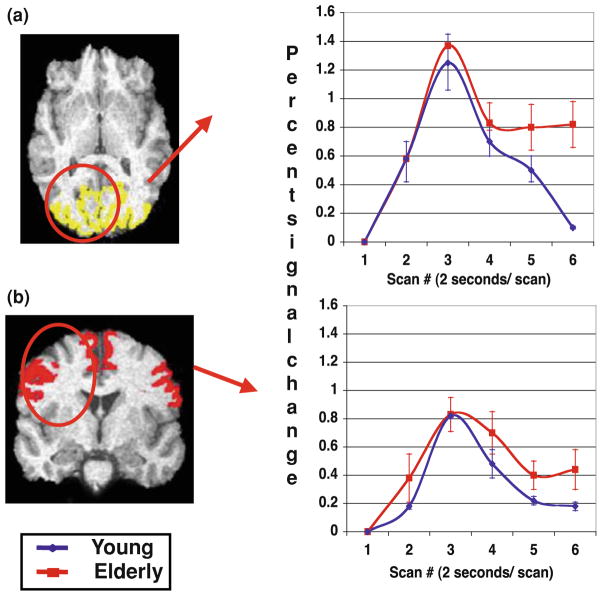
Functional MRI depends on an intact BOLD hemodynamic response function (HRF), i.e., the cascade of neurophysiologic events that leads from neural activation to a change in the measured T2* MR signal. Aging is associated with cerebrovascular changes, so one would expect that it might also alter the BOLD signal. This is of critical importance in interpreting whether the signal identified in an fMRI study of aging reflects changes in neural activity (as is often presumed) or whether the changes are due to the age-related changes in the coupling of the neural activity to the fMRI signal (i.e., the BOLD HRF). To examine the BOLD HRF in aging we compared healthy college-age subjects and healthy elderly control subjects while they performed a simple visual and motor task (e.g., tapping with their index finger in response to the word TAP in the center of the screen). The resulting fMRI time series (see Fig. 1) show a similar peak for both the young and the elderly subjects in both the visual and motor regions. This suggests that by focusing the analysis on the peak of the HRF the difference in signal observed on fMRI will likely reflect differences in neural activation.
Fig. 1.
Time series for a visual and b motor ROIs. Blue line represents mean young percent signal change from baseline; red line is elderly percent signal change. Error bars represent ±1 SEM
2 MRI Methods for Studying Late-Life Mood Disorders
2.1 Structural Imaging
Structural MRI methods can be used to identify and quantify patterns of changes in volumetric neuroimaging studies. The various structural MRI sequences enable the identification of structural alterations such as (a) volume in gray matter, white matter, and cerebrospinal fluid from high resolution T1-weighted images (Raz et al. 2005, 1998; Rosano et al. 2005) (b) white matter hyperintensities (WMH) from FLAIR images (Gunning-Dixon and Raz 2000; Soderlund et al. 2003) (c) white matter integrity from diffusion weighted imaging (Pfefferbaum et al. 2005; Salat et al. 2005) (d) myelination from magnetization transfer imaging (van Es et al. 2006). Advanced neuroimaging sequences like diffusion spectrum imaging (DSI) and Q-ball imagings are currently being used for studying the white matter tracts (Schmahmann et al. 2007; Fig. 2).
Fig. 2.
T1, T2, FLAIR, DTI, and T2* images from 3T scanner
Structural MRI is useful for studying the patterns of neuroanatomical changes in geriatric research. Structural MRI is important for studies in normal aging, late-life depression, dementia, Alzheimer disease, and other cognitive disorders to examine how age-associated changes in neuroanatomy are associated with specific age-related changes in brain function, such as the changes that may be in cognition. Structural MRI allows for identification of both macrostructural and microstructural neuropathologic changes, including atrophy, cerebrovascular changes, demyelination, and changes in membrane integrity.
2.2 BOLD Functional MRI
In the early 1990s a number of investigators showed that not only could MR be used to visualize neuroanatomy and structural pathology but, by tuning the MR contrast appropriately, MR could be used to visualize the dynamic changes in blood oxygenation across the brain; this was the beginning of functional MRI (Schmahmann et al. 2007). Over the subsequent years, a number of studies have shown that this Blood Oxygenation Level Dependent (BOLD) signal could be used to map brain activity on a variety of cognitive and affective tasks.
Functional MRI has been a major advance for the fields of cognitive and affective neuroscience by allowing investigators to test theories of the underlying neural pathways controlling cognitive and emotional processes. This approach is often referred to as ‘human brain mapping.’ In addition to studying ‘normal’ human brain function, fMRI can also be used to characterize the functional activation patterns in patient groups. This area of clinical fMRI research has recently led to a number of new insights into the nature of psychopathology and treatment— including the description of a dorsal versus ventral processing imbalance in depression (Phillips et al. 2003), overlap in response patterns with placebo and with medication (Mayberg et al. 2005), and paradoxical nonlinear activation patterns in mild cognitive impairment (Wierenga and Bondi 2007), suggesting a compensatory stage prior to the onset of dementia.
Functional MRI has utilized either resting state paradigms or activation paradigms involving various emotional or cognitive tasks.
2.2.1 Resting State
Over the last decade fMRI has been adapted to examine the connectivity of the Default-Mode Network, an organized functional network of several brain regions active during resting state and inhibited during the performance of active tasks (Raichle et al. 2001). Analysis of resting state activity may enhance the understanding of the biological underpinning of mental illnesses pathophysiology. A primary component of the resting-state network, is the default-mode network, a functionally connected network, which includes as core nodes the posterior cingulate cortex and the medial frontal cortex. Activity of the default-mode network, is believed to reflect self-referential thought, that is suppressed with goal-directed task activity. Activity in the default-mode network is affected in Alzheimer’s disease, Major Depressive Disorder (Greicius et al. 2007; Sheline et al. 2009) and anxiety disorders (Zhao et al. 2007; Fig. 3).
Fig. 3.
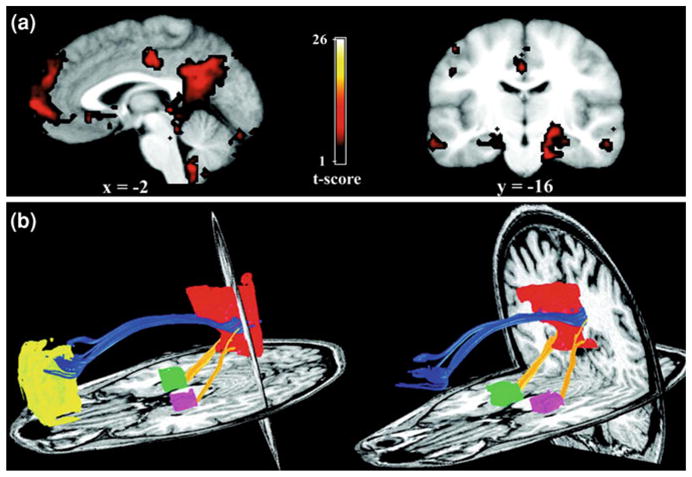
Functional connectivity reflects structural connectivity in the DMN. a Task-free, functional connectivity in the DMN is shown in a group of six subjects. The PCC/RSC and MPFC clusters are best appreciated on the sagittal view. Prominent bilateral MTL clusters are seen on the coronal image (left side of image corresponds to left side of brain). b DTI fiber tractography in a single subject demonstrates the cingulum bundle (blue tracts) connecting the PCC/RSC to the MPFC. The yellow tracts connect the bilateral MTL to the PCC/RSC. Note that generally the tracts from the MPFC enter the more rostral aspect of the PCC/RSC ROI corresponding to the PCC proper, whereas the tracts from MTL enter the more caudal aspect of the PCC/RSC ROI corresponding to the RSC proper. Left and right columns show slightly different views of the same tracts to highlight the distinct entry points into the PCC/RSC. There were no tracts connecting the MPFC to the MTL. (from Greicius et al. 2008). For permissions, please e-mail:journals.permissions@oxfordjournals.org
2.2.2 fMRI Studies of Affective Processing in Late-Life Mood Disorders
Several computer-administered paradigms for measuring affect processing are amenable to functional MRI. These include having subjects respond to emotional faces (e.g., Ekman faces, (Ekman and Friesen 1971), images (e.g., the International Affective Picture System, (Lang and Bradley 1997) words, and stories. Studies with these paradigms have identified an affect processing circuit, which includes bilateral medial ventral structures including the amygdala, the ventral striatum, the orbitofrontal cortex, and the pre-and sub-genual anterior cingulate cortex. These ventral regions seem to show increased activation corresponding to the peak of the ‘emotional’ experience, whether it has positive or negative valence. There has also been a strong association of these regions with activity in dorsal (described as less affective and more cognitive) structures including the dorsal anterior cingulate cortex and the dorsolateral prefrontal cortex.
Several investigators have integrated these findings into models of affective processing (Mayberg 1997; Phillips et al. 2003; Siegle et al. 2002). The key feature of these models is that the dorsal information processing circuit is more specific for the cognitive elements and regulates the affective activation and processing that occurs in the ventral structures. Thus, for instance, in the fMRI study by Ochsner (2002) the amygdala is activated by negatively valenced emotional stimuli. With cognitive reappraisal of the negative stimuli, the dorsal prefrontal cortex becomes active and the amygdala shows decreased activation, apparently secondary to modulation by the dorsal PFC-mediated reappraisal.
Models of dorsal and ventral cognitive and affective processing have been specifically applied as a framework for studying mid-life depression (Mayberg 1997; Phillips et al. 2003). Both of these models describe mid-life depression as resulting from impaired cognitive and affective processing in these circuits. These models have not yet been applied to LLD. However, the notion of disconnection between the dorsal and ventral circuits would seem to apply even more in late-life as compared to mid-life depression, since in LLD there is more evidence of microstructural changes in the PFC white matter tracts that connect these regions (Alexopoulos et al. 2002; Taylor et al. 2004). Future studies are needed to test this hypothesis.
2.2.3 fMRI Studies of Cognitive Processing in Late-Life Mood Disorders
Several functional neuroimaging studies of late-life mood disorders have been conducted during cognitive activation (e.g., de Asis et al. 2001). Bilateral deficits in dACC and hippocampus were observed during word generation task. These cognitive probes are used for studying the basis of cognitive changes in late-life mood disorders, and for engaging key structures implicated in these disorders (e.g., ACC, dlPFC, hippocampus). Functional imaging performed during controlled cognitive tasks standardizes behavior and therefore decreases variability in brain response.
2.3 Perfusion Functional MRI
A significant limitation of BOLD fMRI is concern that the BOLD hemodynamic response is inherently relative. That is the raw BOLD signal does not provide a reliable estimate of regional blood flow in a region. Rather it is contrast of the BOLD signal on alternating experimental versus control tasks that provide the meaningful signal. In contrast to this limitation, PET imaging with an O-15 radioligand is capable of providing quantitative blood flow measures. In MR imaging, a technique analogous to O-15 PET is also available, and is referred to as Arterial Spin Label (ASL) imaging, or perfusion imaging (Detre et al. 1992; Aguirre et al. 2005). In perfusion MR imaging the MR excitation signal is inverted to provide a ‘tagged’ signal, which is alternated with an ‘untagged’ image. Comparing the tagged and untagged provides a quantitative measure the perfusion of the region. Full-brain voxel-wise perfusion images provide a quantitative image of the perfusion across the brain. Investigators have recently used perfusion imaging to demonstrate similar findings as with PET blood flow studies, e.g., decreased parietal-temporal resting perfusion with Alzheimer’s disease (Alsop et al. 2010). In addition to providing quantitative resting perfusion, ASL has also recently been used for investigating the blood flow changes associated with tasks (Fernández-Seara et al. 2007). Perfusion fMRI, however, is limited due to slow acquisition time, reduced coverage, and lower SNR compared to BOLD fMRI. The two primary methods for perfusion imaging are referred to as Continuous Arterial Spin Labeling (CASL) and Pulsed Arterial Spin Labeling (PASL). CASL is believed to provide better signal quality, but generally requires special hardware for providing the continuous tagging pulse.
3 MRI Changes in Specific Late-Life Mental Disorders
Throughout its history, DSM architects have struggled with the seemingly fundamental, but complex question of how to define a mental disorder. Current proposals indicate that a spectrum model of mental illness will be embraced in DSM-5, prompting renewed concern and debate about pathologizing normal behavior (Pierre 2010).
Recently the NIMH launched the Research Domain Criteria (RDoC) project to create a framework for research on pathophysiology, especially for genomics and neuroscience. The RDoC project is intended to be the next step in the process of ensuring valid and reliable diagnosis (Insel et al. 2010). Thus, the project intends to classify mental disorders based on dimensions of observable behavior and neurobiological measures, dimensions such as fear and its extinction, response to stress, impulsive behavior, executive function, and working memory. Increasing evidence suggests that abnormality in one dimension frequently occurs in multiple diagnoses of mental disorders. Cutting across traditional diagnostic categories, RDoC will encompass multiple levels of analysis, from genes to neural circuits to behaviors and will be developed for the research community to help break out of diagnostic formulations that may have more reliability than validity.
We will present in this chapter MRI changes in DSM-IVTR disorders, while keeping in mind that an increasing body of the literature focuses on emotion regulation in late-life, executive function and working memory.
3.1 Late-Life Major Depression (LLD)
Depression in the elderly causes significant distress, disability, and loss of life. The importance of considering late-life depression separately from mid-life depression follows from an extensive literature that has identified biological, psychological, and social factors specific for late-life depression. Loss of function and loss of social support are common psychosocial factors in the presentation of depression in the elderly. However, biological factors are also prevalent in LLD. Two key features that distinguish the brain in elderly versus young subjects are cerebrovascular disease and neurodegeneration, and both are known risk factors for depression [reviewed in Lavretsky and Small (2004)]. However, as these processes (cerebrovascular disease and neurodegeneration) exist on a continuum, it is likely that even subsyndromal disease (e.g., cerebrovascular disease without overt strokes and pre-morbid Alzheimer’s disease) could also contribute to the depressive syndrome in elderly.
3.1.1 Structural Neuroimaging in Late-Life Depression
The neuroimaging findings in LLD overlap with other diseases of aging, including Alzheimer’s disease and cerebrovascular disease. Central and cortical atrophy have been widely reported on both CT [reviewed by Morris and Rapoport (1990)] and MRI (Ballmaier et al. 2004a; Pantel et al. 1997; Rabins et al. 1991). LLD is also associated with reduced frontal lobe volume in general (Kumar et al. 2000), and in particular, the orbitofrontal cortex (Ballmaier et al. 2004b; Lai et al. 2000; Lee et al. 2003) as well as the gyrus rectus and anterior cingulate (Ballmaier et al. 2004b). There also are basal ganglia lesions (Rabins et al. 1991; Steffens et al. 1998; Tupler et al. 2002), especially in the caudate (Krishnan et al. 1992), and the putamen (Steffens et al. 1998; Tupler et al. 2002), that may be worse among late-onset patients. Finally, there is an association between chronic, treatment-resistant depression in groups of mixed ages and right, frontostriatal atrophy (Shah et al. 2002) and reduced volume of the left temporal cortex including the hippocampus (Shah et al. 2002). Recently volumetric studies have identified differences between early versus late-onset LLD (Ballmaier et al. 2004a, b; Andreescu et al. 2011), with the late onset showing less frontal and more temporal and parietal atrophy.
The hippocampus and amygdala appear to be especially sensitive to the effects of major depression. In a study in which the subjects ranged in age from 23 to 86 years of age, both the hippocampus bilaterally and the amygdala core nuclei bilaterally showed reduced volume in depressed subjects relative to controls (Sheline et al. 1999). Reduced hippocampal volume is particularly, but not exclusively, related to later age-of-onset (Steffens et al. 2002), and hippocampal volume was inversely related to conversion to dementia (Steffens et al. 2002). Moreover, the lifetime duration of depression (measured either as years since first episode or total number of days spent depressed) is very closely associated with hippocampal volume (Bell-McGinty et al. 2002; Sheline et al. 1999).
In addition to studies of regional volume there have also been a number of reports of differences in the MR signal within the white matter of individuals with LLD. Several studies using semi-quantitative ratings (Butters et al. 2004; Greenwald et al. 1998) and semi-automated measures (Taylor et al. 2003) have found increased presence of white matter hyperintensities in periventricular and subcortical regions. Salloway et al. (1996) found the periventricular and subcortical hyperintensities to be most severe in those LLD subjects with late-onset depression. More recently, diffusion tensor imaging has been used to more specifically study the white matter tracts, with results showing decreased fractional anisotropy (a measure of diffusion orientation which is used as a marker of white matter integrity) in prefrontal white matter (Alexopoulos et al. 2002; Taylor et al. 2004). While some studies have found that the disturbances in the white matter are associated with poor treatment response (Alexopoulos et al. 2002, 2008) others have not found this to be the case (Salloway et al. 2002).
3.1.2 Functional Imaging in Late-Life Depression
To date, most of the functional neuroimaging studies reported on LLD have focused on the resting state and have identified changes in baseline (i.e., resting) cerebral activity between patients and controls (reviewed in Table 1). One of the earliest studies (Sackeim et al. 1990) demonstrated global decreased CBF using the xenon inhalation technique. A decrease in global brain metabolism in LLD was also found with PET (Kumar et al. 2000). Baxter et al. (1989) and Bench et al. (1993) using PET have found the decreased blood flow and metabolism in depression, in samples with age ranges extending from mid-life through late-life, to be most prominent in the frontal cortex. Other specific areas with reported decreases in LLD versus controls in PET studies include the medial temporal lobe (Grön et al. 2002) and the caudal ACC (de Asis et al. 2001).
Table 1.
Review of functional neuroimaging findings in late-life depression
| Reference | Imaging modality | Subjects | Primary finding |
|---|---|---|---|
| Sackeim et al. (1990) | SPECT (rCBF using 133Xe inhalation) | 30 LLD; 30 EC | Reduced global rCBF |
| Kumar et al. (1993) | PET (Glucose-15) | 8 LLD; 8 EC | Reduced global cerebral metabolism |
| Lesser et al. (1994) | SPECT (rCBF using 133Xe & Technetium-99 m- HMPAO) | 39 LLD; 20 EC | Reduced global rCBF |
| Smith et al. (1999) | PET (Glucose-15) | 6 LLD; 6 EC | Increased activity in right ACC pre-treatment, which decreased with treatment |
| de Asis et al. (2001) | [150]H20 PET (paced word generation) | 6 LLD; 5 EC | Decreased activation b/l in dorsal ACC & Hippocampus |
| Gron et al. (2002) | Block-design FMRI (declarative memory) | 12 LLD; 12 EC | Increased vlpfc, decreased hippocampus |
| Aizenstein et al. (2005) | Event-related BOLD fMRI (sequence learning) | 11 LLD; 12 EC | Decreased b/l PFC and increased R striatum |
| Aizenstein et al. (2006) | Event-related BOLD fMRI (cognitive control task) | 14 LLD; 15 EC | Decreased PFC and decreased ACC |
| Brassen et al. (2008) | Block-design fMRI (emotion reactivity) pre-and post-treatment | 13 LLD; 12 EC | Decreased response to negative stimuli in the vmPFC, correlated with symptoms severity and attenuated by symptom improvement |
| Smith et al. (2009) | PET study | 16 LLD; 13 EC | Increased cortical glucose metabolism in brain regions with cerebral atrophy (compensatory response) |
| Andreescu et al. (2009a) | Event-related BOLD fMRI (cognitive control task) | 8 LLD | Sustained activation in the dorsal ACC in subjects with LLD and increased anxiety |
| Kenny et al. (2010) | Resting-state fMRI | 16 LLD; 17 EC | Increased connectivity in the frontal, limbic, parietal, and temporal areas. |
Note SPECT single photon emission computerized tomography, PET positron emission tomography, BOLD blood oxygen level dependent, PFC prefrontal cortex, ACC anterior cingulate cortex
In a recent study exploring resting-state connectivity in the default-mode network in late-life depression, we reported that, compared with non-depressed elderly, depressed subjects pretreatment had decreased connectivity in the sub-genual anterior cingulate cortex and increased connectivity in the dorsomedial prefrontal cortex and the orbitofrontal cortex. The abnormal connectivity was significantly correlated with the white matter hyperintensity burden. Remitted elderly depressed subjects had improved functional connectivity compared to pretreatment, although alterations persisted in the anterior cingulate and the prefrontal cortex when remitted elderly depressed subjects were compared with non-depressed elderly. These results provide evidence for altered default-mode network connectivity in late-life depression and emphasizes the role of vascular changes in late-life depression etiopathogenesis (Wu et al. 2011).
Several functional neuroimaging studies of LLD have been conducted during cognitive activation (e.g., de Asis et al. 2001; Grön et al. 2002). The cognitive activation functional imaging studies conducted in LLD have replicated the general patterns of regional activity found during resting studies. In a study using a word generation task, de Asis et al. (2001) found reduced CBF bilaterally in the dorsal anterior cingulate and the hippocampus (as measured compared to controls), and on a verbal declarative memory task, Grön (2002) found decreased left VLPFC and hippocampal activation compared to elderly controls. Recently, on a cognitive control task comparing LLD to elderly controls (Aizenstein et al. 2005); and see Sect. 3 of this chapter) we found decreased BOLD activation in the DLPFC and ACC, and in a sequence learning task comparing LLD to elderly controls we found the depressed elderly to have decreased prefrontal activation and increased striatal activation. The increased striatal activation occurred during the trials that violated the predictive sequential pattern, and thus are consistent with reports in mid-life depression of increased negative reward activity in depression.
3.2 Late-Life Bipolar Disorder (LLBD)
Although the prevalence rates of Bipolar Disorder are relatively low among community-dwelling elderly (up to 0.1%), there is significantly higher prevalence (and higher morbidity) in institutional settings, such as personal care homes and nursing homes where prevalence rates may be as high as 10%. Bipolar disorder the elderly is probably heterogenous and its etiopathogenesis is complex.
Bipolar disorder may be divided into two distinct subtypes, the late-onset bipolar (LOB) and the early onset bipolar (EOB) groups. LOB patients tend to have a milder illness in terms of manic severity but they have higher medical and neurological burden. They also have lower familial burden of bipolar illness as compared to EOB patients. There is an increased risk of dementia and stroke in patients with late-life bipolar disorder (Vasudev and Thomas 2010).
Structural and functional neuroimaging data in LLBD is quite scarce. The few studies exploring the neurobiology of late-life bipolar disorder have reported that relative to elderly controls and EOB, late-onset bipolar subjects have increased hyperintense lesions on T2 images around the putamen, as well as in the deep white matter in frontal and parietal regions (Altshuler et al. 1995; Beyer et al. 2004). The authors concluded that their results provide empirical support to the link between vascular risk factors and late-onset BD. It is plausible that, as in the case of MDD, the T2 hyperintensities, which reflect ischemia, area long-term consequence rather than a cause of bipolar illness. One possibility is that people with BD have an excess of atherosclerotic risk factors that lead to microvascular pathology at an even earlier age than MDD. However, using a strict selection of elderly cases with BD and careful case–control matching for clinical and demographic variables, no volumetric differences were found between LLBD subjects in the hippocampus, amygdala, entorhinal, and anterior cingulate cortex, nor a higher degree of WMH, when compared with healthy individuals (Delaloye et al. 2009).
3.3 Late-Life Anxiety disorders
With an estimated community prevalence of 7.3%, late-life generalized anxiety disorder (GAD) is the most common anxiety disorder among the elderly (Wetherell et al. 2001; Wittchen and Hoyer 2001). Late-life GAD is associated with decreased quality of life (de BEURS et al. 1999; Wetherell et al. 2001), cognitive impairment (Mantella et al. 2007; Caudle et al. 2007), increased health care utilization (de BEURS et al. 1999), and poorer recovery after disabling medical events (de BEURS et al. 1999; Astrom 1996). Recent fMRI studies have reported that, when attempting to regulate their emotional responses, elderly anxious subjects failed to activate prefrontal regions involved in the down-regulation of negative emotions. These results, showing that elderly anxious subjects are not effectively engaging the PFC in suppressing worry, may be clinically relevant for developing personalized therapeutic strategies for the treatment of late-life GAD (Andreescu et al. 2011).
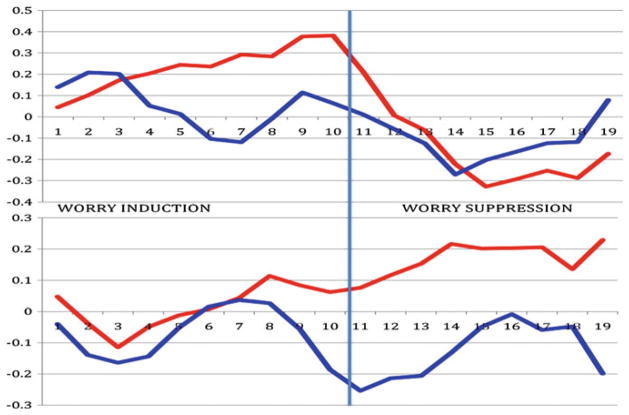
Moreover, time-series analysis of non-anxious subjects showed that amygdala and sACC activate in reverse synchronicity during phases of worry modulation (see Fig. 4). In contrast, elderly GAD subjects displayed same direction activation of the sACC and the amygdala during worry induction. Moreover, time-series cross-correlation analysis showed a decrease correlation between the amygdala and sACC in elderly GAD (Fig. 5).
Fig. 4.
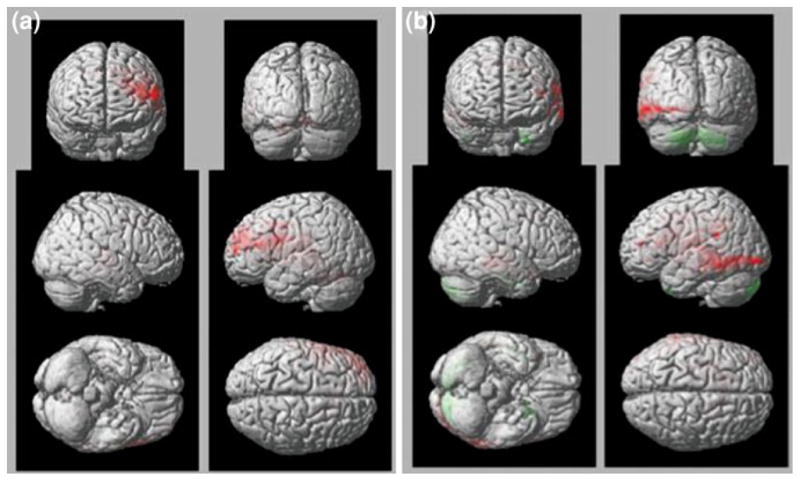
Elderly non-anxious subjects engage the PFC in suppressing worry (a). Elderly GAD subjects (b) are not effective in engaging the PFC and maintained an increased activation the posterior areas (temporo-occipital) (b)
Fig. 5.
Amygdala (red)- sACC (blue) functional connectivity during worry induction and worry suppression in elderly controls (up) and elderly GAD subjects (down)
These results suggest a possible age-related inability of the regulatory regions such as sACC to modulate the worry process in late-life GAD (Andreescu et al. 2009b).
4 Future Directions
MRI has revolutionized clinical neuroscience research and has led to a more sophisticated understanding of the neural substrates of mental disorders (Kumar and Ajilore 2008). There has been tremendous increase in the use of MRI (functional and structural) in studying the brain. These neuroimaging studies have provided deep insight of how the brain works in terms of brain development, function, aging, and other diseases. The use of neuroimaging is important for studying the aging brain as it provides a platform for non-invasive studies of structure and function to increase our understanding of the cognitive aging (Reuter-Lorenz and Lustig 2005) and other age-related changes in the brain.
Applying MRI approaches to the identification of predictors of treatment response may allow us early in the course of therapeutic interventions to identify sub-groups of subjects with difficultly in treating mood disorders. In the future, such subjects may be selected, based on their MRI profile, for more aggressive interventions. Markers of white matter pathology may identify elderly patients for whom the risk of antidepressant treatment may not be balanced by a high probabability of treatment response (Kumar and Ajilore 2008; Alexopoulos et al. 2008). In a more personalized medicine era, advances in neuroimaging and genomics could provide a personalized database that may help tailor and guide treatment choices (Kumar and Ajilore 2008)
A potential new treatment is real-time fMRI (de Charms 2008), that has been recently introduced as a method to directly control activation of localized brain regions to affect neurophysiological mechanisms that mediate behavior and cognition (de Charms 2007). Positive results have been reported in modulating pain perception (de Charms et al. 2005), and more recently in the down-modulation of the sACC (real-time fMRI neurofeedback) (Paul Hamilton et al. HBM 2011). Incorporating real-time fMRI in the future offers the promise of clinical translation in which neuroimaging may also be used for clinical interventional purposes.
References
- Aguirre GK, John AD, Wang J. In Neuroimaging, Part A. Vol. 66. Academic Press; 2005. Perfusion fMRI for functional neuroimaging; pp. 213–236. http://www.sciencedirect.com/science/article/B7CV0-4HX9TNB-7/2/5cf4567f9e04a1d0150f4e642a68fd1a. [DOI] [PubMed] [Google Scholar]
- Aizenstein HJ, Butters MA, Figurski JL, Stenger VA, Reynolds CF, III, Carter CS. Prefrontal and striatal activation during sequence learning in geriatric depression. Biol Psychiatry. 2005;58(4):290–296. doi: 10.1016/j.biopsych.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Aizenstein HJ, Butters MA, Clark KA, Figurski JL, Stenger VA, Nebes RD, et al. Prefrontal and striatal activation in elderly subjects during concurrent implicit and explicit sequence learning. Neurobiol Aging. 2006;27(5):741–751. doi: 10.1016/j.neurobiolaging.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Aizenstein HJ, Clark KA, Butters MA, Cochran J, Stenger VA, Meltzer CC, Reynolds CF, Carter CS. The BOLD hemodynamic response in healthy aging. J Cogn Neurosci. 2011;16(5):786–793. doi: 10.1162/089892904970681. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Choi SJ, Murphy CF, Lim KO. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry. 2002;159(11):1929–1932. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Latoussakis V, Kanellopoulos D, Klimstra S, Lim KO, Hoptman MJ. Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry. 2008;165(2):238–244. doi: 10.1176/appi.ajp.2007.07050744. [DOI] [PubMed] [Google Scholar]
- Alsop DC, Dai W, Grossman M, Detre JA. Arterial spin labeling blood flow MRI: its role in the early characterization of Alzheimer’s disease. J Alzheimers Dis. 2010;20(3):871–880. doi: 10.3233/JAD-2010-091699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler LL, Curran JG, Hauser P, Mintz J, Denicoff K, Post R. T2 hyperintensities in bipolar disorder: magnetic resonance imaging comparison and literature meta-analysis. Am J Psychiatry. 1995;152(8):1139–1144. doi: 10.1176/ajp.152.8.1139. [DOI] [PubMed] [Google Scholar]
- Andreescu C, Butters M, Lenze EJ, Venkatraman VK, Nable M, Reynolds CF, III, et al. fMRI activation in late-life anxious depression: a potential biomarker. Int J Geriatr Psychiatry. 2009a;24(8):820–828. doi: 10.1002/gps.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreescu C, Wu M, Siegle Greg J, Thompson W, Aizenstein H. Worry modulation in late-life generalized anxiety disorder. Presented at the ACNP 48 annual meeting; Hollywood, Florida. 2009b. [Google Scholar]
- Andreescu C, Gross JJ, Lenze E, Edelman KD, Snyder S, Tanase C, Aizenstein H. Altered cerebral blood flow patterns associated with pathologic worry in the elderly. Depression and Anxiety. 2011;28(3):202–209. doi: 10.1002/da.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrom M. Generalized anxiety disorder in stroke patients: a 3-year longitudinal study. Stroke. 1996;27(2):270–275. doi: 10.1161/01.str.27.2.270. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Kumar A, Thompson PM, Narr KL, Lavretsky H, Estanol L, DeLuca H, Toga AW. Localizinggraymatter deficits in late-onset depression using computational cortical pattern matching methods. Am J Psychiatry. 2004a;161(11):2091–2099. doi: 10.1176/appi.ajp.161.11.2091. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Toga AW, Blanton RE, Sowell ER, Lavretsky H, Peterson J, Pham D, Kumar A. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. Am J Psychiatry. 2004b;161(1):99–108. doi: 10.1176/appi.ajp.161.1.99. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, Gerner RH, Sumida RM. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry. 1989;46(3):243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- Bell-McGinty S, Butters MA, Meltzer CC, Greer PJ, Reynolds CF, Becker JT. Brain morphometric abnormalities in geriatric depression: long-term neurobiological effects of illness duration. Am J Psychiatry. 2002;159(8):1424–1427. doi: 10.1176/appi.ajp.159.8.1424. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Friston KJ, Brown RG, Frackowiak RSJ, Dolan RJ. Regional cerebral blood flow in depression measured by positron emission tomography: the relationship with clinical dimensions. Psychol Med. 1993;23(03):579–590. doi: 10.1017/S0033291700025368. [DOI] [PubMed] [Google Scholar]
- Beyer JL, Kuchibhatla M, Payne ME, Moo-Young M, Cassidy F, Macfall KJ, Krishnan RR. Hippocampal volume measurement in older adults with bipolar disorder. AmJ Geriatr Psychiatr Off J Am Assoc Geriatric Psychiatry. 2004;12(6):613–620. doi: 10.1176/appi.ajgp.12.6.613. [DOI] [PubMed] [Google Scholar]
- Brassen S, Kalisch R, Weber-Fahr W, Braus DF, Buchel C. Ventromedial prefrontal cortex processing during emotional evaluation in late-life depression: a longitudinal functional magnetic resonance imaging study. Biol Psychiatry. 2008;64(4):349–355. doi: 10.1016/j.biopsych.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, Zmuda MD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatr. 2004;61(6):587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- Caudle DD, Senior AC, Wetherell JL, Rhoades HM, Beck JG, Kunik ME, Lynn Snow A, Wilson NL, Stanley MA. Cognitive errors, symptom severity, and response to cognitive behavior therapy in older adults with generalized anxiety disorder. American J Geriatr Psychiatr. 2007;15(8):680–689. doi: 10.1097/JGP.0b013e31803c550d. [DOI] [PubMed] [Google Scholar]
- de Asis JM, Stern E, Alexopoulos GS, Pan H, Van Gorp W, Blumberg H, et al. Hippocampal and anterior cingulate activation deficits in patients with geriatric depression. Am J Psychiatry. 2001;158(8):1321–1323. doi: 10.1176/appi.ajp.158.8.1321. [DOI] [PubMed] [Google Scholar]
- de Beurs E, Beekman ATF, van Balkom AJLM, Deeg DJH, van Dyck R, van Tilburg W. Consequences of anxiety in older persons: its effect on disability, well-being and use of health services. Psychol Med. 1999;29(03):583–593. doi: 10.1017/s0033291799008351. [DOI] [PubMed] [Google Scholar]
- de Charms RC. Reading and controlling human brain activation using real-time functional magnetic resonance imaging. Trends Cognit Sci. 2007;11(11):473–481. doi: 10.1016/j.tics.2007.08.014. [DOI] [PubMed] [Google Scholar]
- de Charms RC. Applications of real-time fMRI. Nat Rev Neurosci. 2008;9(9):720–729. doi: 10.1038/nrn2414. [DOI] [PubMed] [Google Scholar]
- de Charms RC, Maeda F, Glover GH, Ludlow D, Pauly JM, Soneji D, Gabrieli JDE, Mackey SC, Raichle ME. Control over brain activation and pain learned by using real-time functional MRI. Proc Natl Acad Sci USA. 2005;102(51):18626–18631. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaloye C, de Bilbao F, Moy G, Baudois S, Weber K, Canuto A, Campos L, et al. Neuroanatomical and neuropsychological features of euthymic patients with bipolar disorder. Am J Geriatr Psychiatr Off J Am Assoc Geriatric Psychiatry. 2009;17(12):1012–1021. doi: 10.1097/JGP.0b013e3181b7f0e2. [DOI] [PubMed] [Google Scholar]
- Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23(1):37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Constants across cultures in the face and emotion. J Pers Soc Psychol. 1971;17(2):124–129. doi: 10.1037/h0030377. [DOI] [PubMed] [Google Scholar]
- Fernández-Seara MA, Wang J, Wang Z, Korczykowski M, Guenther M, Feinberg DA, Detre JA. Imaging mesial temporal lobe activation during scene encoding: comparison of fMRI using BOLD and arterial spin labeling. Hum Brain Mapp. 2007;28(12):1391–1400. doi: 10.1002/hbm.20366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald BS, Kramer-Ginsberg E, Krishnan KR, Ashtari M, Auerbach C, Patel M. Neuroanatomic localization of magnetic resonance imaging signal hyperintensities in geriatric depression. Stroke: a J Cereb Circul. 1998;29(3):613–617. doi: 10.1161/01.str.29.3.613. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62(5):429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, et al. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cerebral Cortex. 2008;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gron G, Bittner D, Schmitz B, Wunderlich AP, Riepe MW. Subjective memory complaints: objective neural markers in patients with Alzheimer’s disease and major depressive disorder. Ann Neurol. 2002;51(4):491–498. doi: 10.1002/ana.10157. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14(2):224–232. doi: 10.1037/0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Glover GH, Hsu JJ, Johnson RF, Gotlib IH. Modulation of subgenual anterior cingulate cortex activity with real-time neurofeedback. Hum Brain Mapp. 2011;32(1):22–31. doi: 10.1002/hbm.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Kenny ER, O’Brien JT, Cousins DA, Richardson J, Thomas AJ, Firbank MJ, et al. Functional connectivity in late-life depression using resting-state functional magnetic resonance imaging. Am J Geriatr Psychiatry. 2010;18(7):643–651. doi: 10.1097/JGP.0b013e3181cabd0e. [DOI] [PubMed] [Google Scholar]
- Krishnan KRR, McDonald WM, Escalona PR, Doraiswamy PM, Chul Na, Husain MM, Figiel GS, Boyko OB, Ellinwood EH, Nemeroff CB. Magnetic resonance imaging of the caudate nuclei in depression: preliminary observations. Arch Gen Psychiatry. 1992;49(7):553–557. doi: 10.1001/archpsyc.1992.01820070047007. [DOI] [PubMed] [Google Scholar]
- Kumar A, Newberg A, Alavi A, Berlin J, Smith R, Reivich M. Regional cerebral glucose metabolism in late-life depression and Alzheimer disease: a preliminary positron emission tomography study. Proc Natl Acad Sci U S A. 1993;90(15):7019–7023. doi: 10.1073/pnas.90.15.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Ajilore O. Magnetic resonance imaging and late-life depression: potential biomarkers in the era of personalized medicine. Am J Psychiatry. 2008;165(2):166–168. doi: 10.1176/appi.ajp.2007.07111771. [DOI] [PubMed] [Google Scholar]
- Kumar A, Bilker W, Jin Z, Udupa J. Atrophy and high intensity lesions: complementary neurobiological mechanisms in late-life major depression. Neuropsychopharmacology. 2000;22(3):264–274. doi: 10.1016/S0893-133X(99)00124-4. [DOI] [PubMed] [Google Scholar]
- Lai TJ, Payne ME, Byrum CE, Steffens DC, Ranga K, Krishnan R. Reduction of orbital frontal cortex volume in geriatric depression. Biol Psychiatry. 2000;48(10):971–975. doi: 10.1016/S0006-3223(00)01042-8. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM. International affective picture systems (iaps): technical manual and affective ratings. University of Florida; Gainesville: 1997. [Google Scholar]
- Lavretsky H, Small GW. Late-Life Depression. Oxford University Press; New York: 2004. Mixed cognitive and depressive symptoms. [Google Scholar]
- Lee S-H, Payne ME, Steffens DC, McQuoid DR, Lai T-J, Provenzale JM, Krishnan KRR. Subcortical lesion severity and orbitofrontal cortex volume in geriatric depression. Biol Psychiatry. 2003;54(5):529–533. doi: 10.1016/S0006-3223(03)00063-5. [DOI] [PubMed] [Google Scholar]
- Lesser IM, Mena I, Boone KB, Miller BL, Mehringer CM, Wohl M. Reduction of cerebral blood flow in older depressed patients. Arch Gen Psychiatry. 1994;51(9):677–686. doi: 10.1001/archpsyc.1994.03950090009002. [DOI] [PubMed] [Google Scholar]
- Mantella RC, Butters MA, Dew MA, Mulsant BH, Begley AE, Tracey MB, Shear K, Reynolds CF, Lenze EJ. Cognitive impairment in late-life generalized anxiety disorder. Am J Geriatr Psychiatr. 2007;15(8):673–679. doi: 10.1097/JGP.0b013e31803111f2. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9(3):471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–656. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Morris P, Rapoport SI. Neuroimaging and affective disorder in late life: a review. Canadian Can J Psychiatry Revue Canadienne De Psychiatrie. 1990;35(4):347–354. doi: 10.1177/070674379003500415. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. J Cognit Neurosci. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Pantel J, Schröder J, Essig M, Popp D, Dech H, Knopp MV, Schad LR, Eysenbach K, Backenstra M, Friedlinger M. Quantitative magnetic resonance imaging in geriatric depression and primary degenerative dementia. J Affect Disord. 1997;42(1):69–83. doi: 10.1016/S0165-0327(96)00105-X. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. NeuroImage. 2005;26(3):891–899. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54(5):515–528. doi: 10.1016/S0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Pierre JM. The borders of mental disorder in psychiatry and the dsm. J Psychiatr Pract. 2010;16(6):375–386. doi: 10.1097/01.pra.0000390756.37754.68. [DOI] [PubMed] [Google Scholar]
- Rabins PV, Pearlson GD, Aylward E, Kumar AJ, Dowell K. Cortical magnetic resonance imaging changes in elderly inpatients with major depression. Am J Psychiatry. 1991;148(5):617–620. doi: 10.1176/ajp.148.5.617. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7(3):268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: Evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12(1):95–114. doi: 10.1037/0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, Bryan RN, Zonderman AB. One-year age changes inMRI brain volumes in older adults. Cereb Cortex. 2000;10(5):464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005;15(2):245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Rosano C, Becker J, Lopez O, Lopez-Garcia P, Carter CS, Newman A, Kuller L, Aizenstein H. Morphometric analysis of gray matter volume in demented older adults: exploratory analysis of the cardiovascular health study brain mri database. Neuroepidemiology. 2005;24(4):221–229. doi: 10.1159/000085140. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Prohovnik I, Moeller JR, Brown RP, Apter S, Prudic J, et al. Regional cerebral blood flow in mood disorders: i. comparison of major depressives and normal controls at rest. Arch Gen Psychiatry. 1990;47(1):60–70. doi: 10.1001/archpsyc.1990.01810130062009. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DN, Greve AJ, van der Kouwe W, Hevelone ND, Zaleta AK, Rosen BR, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobio Aging. 2005;26(8):1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Salloway S, Malloy P, Kohn R, Gillard E, Duffy J, Rogg J, Tung G, Richardson E, Thomas C, Westlake R. MRI and neuropsychological differences in early-and late-life-onset geriatric depression. Neurology. 1996;46(6):1567–1574. doi: 10.1212/wnl.46.6.1567. [DOI] [PubMed] [Google Scholar]
- Salloway S, Boyle PA, Correia S, Malloy PF, Cahn-Weiner DA, Lon Schneider K, Krishnan RR, Nakra R. The relationship of MRI subcortical hyperintensities to treatment response in a trial of sertraline in geriatric depressed outpatients. Am J Geriatr Psychiatr Off J Am Assoc Geriatric Psychiatry. 2002;10(1):107–111. [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D’Arceuil HE, de Crespigny JA, van Wedeen J. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130(3):630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Shah PJ, Glabus MF, Goodwin GM, Ebmeier KP. Chronic, treatment-resistant depression and right fronto-striatal atrophy. British J Psychiatr. 2002;180(5):434–440. doi: 10.1192/bjp.180.5.434. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. In: Proceedings of theNational Academy ofSciences. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. Journal Neurosci. 1999;19(12):5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51(9):693–707. doi: 10.1016/S0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Smith GS, Reynolds CF, III, Pollock B, Derbyshire S, Nofzinger E, Dew MA, et al. Cerebral glucose metabolic response to combined total sleep deprivation and antidepressant treatment in geriatric depression. Am J Psychiatry. 1999;156(5):683–689. doi: 10.1176/ajp.156.5.683. [DOI] [PubMed] [Google Scholar]
- Smith GS, Kramer E, Ma Y, Kingsley P, Dhawan V, Chaly T, et al. The functional neuroanatomy of geriatric depression. Int J Geriatr Psychiatry. 2009;24(8):798–808. doi: 10.1002/gps.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund H, Nyberg L, Adolfsson R, Nilsson L, Launer L. High prevalence of white matter hyperintensities in normal aging: relation to blood pressure and cognition. Cortex. 2003;39(4–5):1093–1105. doi: 10.1016/S0010-9452(08)70879-7. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Krishnan KRR. Structural neuroimaging and mood disorders: recent findings, implications for classification, and future directions. Biol Psychiatry. 1998;43(10):705–712. doi: 10.1016/S0006-3223(98)00084-5. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Payne ME, Greenberg DL, Byrum CE, Welsh-Bohmer KA, Ryan Wagner H, MacFall JR. Hippocampal volume and incident dementia in geriatric depression. Am J Geriatr Psychiatr Off J Am Assoc Geriatric Psychiatry. 2002;10(1):62–71. [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Steffens DC, Payne ME, Provenzale JM, Ranga Rama Krishnan K. Localization of age-associated white matter hyperintensities in late-life depression. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(3):539–544. doi: 10.1016/S0278-5846(02)00358-5. [DOI] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Payne Martha E, McQuoid DR, Provenzale JM, Steffens DC, Krishnan KRR. Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. Am J Psychiatry. 2004;161(7):1293–1296. doi: 10.1176/appi.ajp.161.7.1293. [DOI] [PubMed] [Google Scholar]
- Tupler LA, Ranga K, Krishnan R, McDonald WM, Dombeck CB, D’Souza S, Steffens DC. Anatomic location and laterality of MRI signal hyperintensities in late-life depression. J Psychosom Res. 2002;53(2):665–676. doi: 10.1016/S0022-3999(02)00425-7. [DOI] [PubMed] [Google Scholar]
- van Es ACGM, van der Flier WM, Admiraal-Behloul F, Olofsen H, Bollen ELEM, Middelkoop HAM, Weverling-Rijnsburger AWE, Westendorp RGJ, van Buchem MA. Magnetization transfer imaging of gray and white matter in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2006;27(12):1757–1762. doi: 10.1016/j.neurobiolaging.2005.09.042. [DOI] [PubMed] [Google Scholar]
- Vasudev A, Thomas A. Bipolar disorder’ in the elderly: what’s in a name? Maturitas. 2010;66(3):231–235. doi: 10.1016/j.maturitas.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Wetherell JL, Gatz M, Pedersen NL. A longitudinal analysis of anxiety and depressive symptoms. Psychol Aging. 2001;16(2):187–195. doi: 10.1037//0882-7974.16.2.187. [DOI] [PubMed] [Google Scholar]
- Wierenga CE, Bondi MW. Use of functional magnetic resonance imaging in the early identification of Alzheimer’s disease. Neuropsychol Rev. 2007;17(2):127–143. doi: 10.1007/s11065-007-9025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen HU, Hoyer J. Generalized anxiety disorder: nature and course. J Clin Psychiatry. 2001;62(Suppl 11):15–19. discussion 20-21. [PubMed] [Google Scholar]
- Wu M, Andreescu C, Butters MA, Tamburo R, Reynolds CF, III, Aizenstein H. Default-mode network connectivity and white matter burden in late-life depression. Psychiatry Res. 2011;194(1):39–46. doi: 10.1016/j.pscychresns.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Carmichael O, Lopez-Garcia P, Carter CS, Aizenstein HJ. Quantitative comparison of AIR, SPM, and the fully deformable model for atlas-based segmentation of functional and structural MR images. Hum Brain Mapp. 2006;27(9):747–754. doi: 10.1002/hbm.20216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X-H, Wang P-J, Li C-B, Hu Z-H, Xi Q, Wu W-Y, Tang X-W. Altered default mode network activity in patient with anxiety disorders: an fMRI study. Eur J Radiol. 2007;63(3):373–378. doi: 10.1016/j.ejrad.2007.02.006. [DOI] [PubMed] [Google Scholar]