Abstract
PARP-1 is rapidly activated by DNA strand breaks, which finally leads to the modulation of multiple protein activities in DNA replication, DNA repair and checkpoint control. PARP-1 may be involved in homologous recombination, and poly(ADP-ribosyl)ation of p53 represents one possible mechanism that activates p53 as a recombination surveillance factor. Here, we examined the influence of PARP-1 on homology-directed double-strand break (DSB) repair by use of a fluorescence- and I-SceI- meganuclease-based assay with either episomal or chromosomally integrated DNA substrates. Surprisingly, the transient expression of both full-length PARP-1 and of a dominant negative mutant, retaining the DNA-binding but lacking the catalytic domain, down-regulated DSB repair in a dose-dependent manner. This effect was seen regardless of p53 status, however, with enhanced inhibition in the presence of wild-type p53. Taken together, our data reveal that PARP-1 overexpression counteracts DSB repair independently of its enzymatic activity and of poly(ADP-ribosyl)ation of p53 in particular, but synergizes with p53 in suppressing chromosomal rearrangements.
INTRODUCTION
Double-strand breaks (DSBs) represent the most detrimental DNA lesion and may be formed by ionizing radiation, oxygen radicals, inhibition of topoisomerase or DNA polymerase activity, or by specific endonucleases, such as the V(D)J recombinase (1,2). Error-prone DSB repair may lead to cancer-inducing genetic aberrations, including translocations, deletions, chromosome losses or gene amplification. Mammalian cells utilize two major DSB repair pathways, namely the mutagenic mechanism of non-homologous end joining (NHEJ) and the generally nonmutagenic process of homologous recombination (HR). Conservative HR, i.e. gene conversion or cross-over, depends on the strand transfer activity of Rad51 and auxiliary proteins of the Rad52 epistasis group (3). Nonconservative HR, i.e. single-strand annealing (SSA) or replication slippage, represents a Rad51-independent mechanism. The critical importance of HR for mammalian organisms was convincingly demonstrated by the embryonic lethal phenotypes of knockout mice with deficiencies in either Rad51, Rad50 or Nbs1 (4–7).
Activation of PARP-1 and p53 represents two of the earliest DNA damage responses that trigger various cellular functions involved in the maintenance of the genome stability (8). PARP-1 binds to DNA strand breaks with high affinity and subsequently poly(ADP-ribosyl)ates itself and other nuclear proteins involved in chromatin structure, DNA base excision repair and recombination (9,10). Automodification of PARP-1, which entails a massive increase in negative charges, may lead to the dissociation of PARP-1 from the strand break and thus give access to DNA repair enzymes in a controlled manner (11). In agreement with an involvement of PARP-1 in DSB repair, PARP-1 disruption in knockout mice or inhibition of poly(ADP-ribosyl)ation in cellular systems stimulate micronucleus formation, sister-chromatid exchange (SCE) and gene amplification (12–15). HR participates in SCEs and gene amplifications (16,17), suggesting an involvement of PARP-1 in HR. Moreover, PARP-1 was reported to co-immunoprecipitate with the Werner syndrome (WRN) protein, a RecQ helicase that suppresses error-prone Rad51-dependent recombination by branch migrating illegitimate recombination intermediates (18,19). However, recent observations indicated that PARP-1 is not required for the assembly of Rad51 foci in the nucleus and does not colocalize with Rad51, indicating that PARP-1 does not represent a component of the recombination machinery (20). So far, the results from recombination measurements have not provided a conclusive picture of the role of PARP-1 in HR. Waldman and colleagues (21,22) showed that in mouse fibroblasts, a competitive inhibitor of poly(ADP-ribosyl)ation does not affect extrachromosomal HR but stimulates intrachromosomal HR. In contrast, Semionov and colleagues (23) noticed that chemical inhibition of PARP-1 in the same cell type positively regulates extrachromosomal HR, while Schultz et al. (20) were unable to see any changes in HR frequencies with chromosomally integrated substrates, when using Chinese hamster cells.
PARP-1 was proposed to act upstream of p53 in the damage signalling pathway, either via physical interactions with and poly(ADP-ribosyl)ation of p53 or, indirectly, via stimulation of the DNA strand break sensing ATM kinase which phosphorylates p53 on serine 15 (24–32). Moreover, PARP-1 and p53 were demonstrated to cooperate in stabilizing the genome (10), suggesting a functional connection in DNA repair. In addition to a role of p53 in checkpoint control, numerous studies showed a close association between p53 and DNA repair (33). In particular, compelling evidence indicates that wild-type p53 (wt-p53) down-regulates spontaneous and DSB-induced HR, independently of its role in transcriptional transactivation, cell cycle control and apoptosis (34–37). Concerning the mechanism by which p53 directly counteracts HR, important clues have come from the identification of p53–Rad51 complexes, the characterization of the physical interactions between p53 and strand exchange intermediates, and from studies on the DNA substrate dependence (33,34,37–39). From these results, it is conceivable that p53 abrogates continued strand exchange by the recombinase Rad51 after specifically recognizing erroneous intermediates of HR. Phosphorylation by ATM and the interaction with Bloom’s syndrome (BLM) protein, another RecQ DNA helicase which may counteract defective processing of DNA replication intermediates, seem to be important in stabilizing p53 contacts in the vicinity of the DSB repair machinery (40).
In the present work, we evaluated the precise role of PARP-1 in homology-directed DSB repair. Previous studies utilized recombination assay systems that rely on colony outgrowth under drug selection, but conflicting data were obtained (20–23). Here, we applied our fluorescence-based DSB repair assay that scores the fraction of EGFP-positive cells in a population of nonfluorescent cells (37). To discriminate between PARP-1 functions in recombinative versus alternative DNA repair pathways, we applied a test system which has been designed in such a way as to trigger recombinative DNA exchange processes by DSB formation in vivo (37). Trans-dominant inhibition of poly(ADP- ribosyl)ation by overexpressing the DNA-binding domain of PARP-1 (PARP-DBD) has previously been exploited as a powerful tool to examine the role of PARP-1 (41–43). We therefore compared the consequences of ectopically expressing PARP-DBD and PARP-1 on HR within extra- or intrachromosomal DNA substrates. To understand whether recombination regulation by PARP-1 involves direct protein–protein interactions or poly(ADP-ribosyl)ation of p53, we extended our studies on DSB repair to cellular systems with differing p53 status.
MATERIALS AND METHODS
Plasmid constructs
pGC-PARP-DBD and pGC-PARP-1 were created by transferring the SmaI fragment, encompassing the region of cDNA encoding truncated and full-length PARP-1, from the vector pPARP6 and pPARP31, respectively, into the EcoRV- restriction site of pGC (44). DNA-modifying enzymes were purchased from Roche and New England Biolabs.
Cell culture and establishment of transgenic rhesus monkey cell lines
The parental lines were LLC-MK2 from rhesus monkey (Macaca mulatta) kidney and the human chronic myelogenous leukaemia cell line K562. Concerning the p53 status, LLC-MK2 cells endogenously express p53(Δ237–239), which displays a Pab240 reactive conformation, and is inactive with regards to transcriptional transactivation, cell cycle control or recombination regulatory activities (38). In K562, the p53 gene is homozygously deleted (37).
LLC-MK2(neo), LLC-MK2(p53her), LMV and newly established transgenic clones were maintained at 37°C in DMEM supplemented with 10% FCS. FCS had been stripped by stirring 1 litre with 10 g charcoal (Norit A, Merck) and 1 g Dextran 40 (Merck) for 30 min and subsequent centrifugation at 13 000 g (35,38). LMV-PARP, LMV-p53her and LMV-p53her/PARP cells were established after co-transfection of LMV cells with pGC-PARP-1 (8 µg) and/or pSV53her (8 µg) (45) and phyg (2 µg) (35). Antibiotic selection was performed by the addition of 0.2 mg/ml hygromycin B (Roche). Six to 12 hygromycin-resistant clones were analysed each for PARP-1 and/or p53her expression by western analysis of total homogenates.
Clones derived from K562 cells were maintained in phenol red-free RPMI with 12% charcoal-stripped FCS (37). K562 cells expressing the transcription factor Gal4ERVP (KMV) were utilized for estradiol-inducible expression of I-SceI meganuclease in transient electroporation assays. KMV subclones with stably integrated HR-EGFP/3′EGFP recombination plasmid and KMV(p53her) cells with HR-EGFP/3′EGFP were subjected to the analysis of DSB repair on cellular chromosomes.
The cell cultures used in this work were free from Mycoplasma contamination.
Cell cycle analysis and detection of apoptosis by fluorescence microscopy
Cell cycle profiles were determined after preparation of cells essentially as described (46). Briefly, log-phase cells were transferred to medium containing 1 µM β-estradiol (Sigma). Twenty-four, 48 and 72 h later, the cells were collected by trypsinization and centrifugation, fixed, stained with propidium iodide and analysed in a FACS® Calibur flow cytometer (Becton Dickinson). Generation times were determined as described before (35).
For fluorescence microscopy, cells were grown overnight on coverslips in six-well plates followed by addition of 1 µM β-estradiol to the medium and incubation for 4 h. Subsequently, the cells were γ-irradiated with 5 Gy from a 137Cs source. After further incubation for 12 h in fresh culture medium, the slides were washed with PBS and fixed with 3.7% formaldehyde in PBS for 10 min at room temperature. Fixed slides were permeabilized with 0.3% Triton X-100 for 3 min. The preparations were stained with 0.1 µg/ml 4′,6-diamidino-2-phenylindole (DAPI) in PBS for 10 min in the dark, washed with PBS and H2O, and then mounted. Images were taken with a Leica epifluorescence microscope equipped with objective lenses 63×/1.4 and 40×/1.0 and a digital camera (Spot Model 1.3.0).
Immunoblot analysis
For western blotting, monkey cells were either left untreated or incubated with estradiol and/or transfected with expression plasmids. γ-Irradiation was performed as above. At the times indicated, the cells were harvested, washed with PBS and total cell homogenates obtained by scraping the cells from the culture dish with a rubber policeman; 1–2 × 106 cells were resuspended in 100 µl 3× SDS sample buffer (65 mM Tris–HCl, pH 6.8; 10% glycerol; 2.3% SDS; 5% β-mercaptoethanol; bromophenol blue) and incubated at 99°C for 8 min. K562-derived cells were harvested by centrifugation and processed as described for monkey cells. Homogenates from 2–3 × 105 cells were electrophoresed on 8–15% PAGE and transferred to Immobilon-P membranes (Millipore). Proteins were immunodetected by the following primary antibodies: DO1 directed against the N-terminal amino acids 21–25 of human p53 (Oncogene/Calbiochem), the IgG fraction of sheep serum raised against human p53 (Roche Diagnostics), mAb F-I-23 directed against the DNA binding domain of PARP-1 (kind gift of G.G. Poirier) or goat polyclonal IgG N-20 raised against the PARP-1 N-terminus (Santa Cruz), Ab-1 against human p21 (Oncogene/Calbiochem) and 2D2 against Bax (Biocarta). For western analyses of actin, we applied affinity-purified goat polyclonal antibody I-19 (Santa Cruz), for α- and β-tubulin mAbs DM1A+DM1B (Biocarta). Affinity purified, peroxidase-conjugated secondary IgGs were obtained from Biomol. Visualization of the immunocomplexed bands was achieved by Super-Signal West Dura Extended Duration Substrate chemiluminescence enhancement (Pierce).
Recombination assays
For the determination of recombination frequencies with plasmid substrates, LLC-MK2(neo), LLC-MK2(p53her), LMV, LMV-p53her, LMV-PARP and LMV-p53her/PARP cells were grown overnight in six-well plates, thereby reaching 50–80% confluency. The cells were then subjected to lipofection with a total amount of 2 µg plasmid DNA [recombination plasmid, I-SceI expression vector pCMV-I-SceI, and PARP expression or pBlue Script II KS (+/–) (pBs) control plasmid] and 4 µl FUGENE 6 Transfection Reagent according to the manufacturer’s instructions (Roche). Subsequently, transfected cultures were incubated in the presence of 1 µM estradiol until FACS® analysis. Poly(ADP-ribosyl)ation was inhibited by the inclusion of 1 mM 3-aminobenzamide (Sigma), caspase-mediated cleavage was antagonized by the caspase inhibitor Z-DEVD-FMK (Sigma) at a concentration of 50 µM for 24 h, followed by flow cytometric quantification of recombination frequencies 48 h after removal of Z-DEVD-FMK.
For the determination of recombination frequencies with chromosomally integrated substrates, 0.4 × 107 KMV or KMV(p53her) cells, carrying chromosomally integrated HR-EGFP/3′EGFP plasmid, were electroporated at 240 V and 1050 mF with a total amount of 20 µg plasmid DNA (pCMV-I-SceI and PARP expression plasmid or pBS) and subsequently cultivated in fresh tissue culture medium with 200 nM β-estradiol.
For recombination measurements 48–72 h after transfection, 0.1–1 × 107 cells were harvested, washed in 1–5 ml PBS supplemented with 0.2% EDTA (PBS–EDTA) and resuspended at a density of 2–5 × 106 cells/ml in PBS–EDTA. To determine the EGFP reconstitution frequency from the fraction of green fluorescent cells within a population of nonfluorescent cells, the cells were analysed flow-cytometrically in a FACS® (Becton Dickinson) as described previously (37). Each measurement was paralleled by the same cotransfection including a control plasmid, which carried wt-EGFP at the acceptor position. The fraction of EGFP-positive cells in these controls was determined and was used to normalize each single recombination frequency, to exclude rate deviations related to growth regulatory effects. Each experiment was carried out at least two times with one to three independent measurements for each cell clone or transfection set-up, to calculate mean values and SEM.
RESULTS
PARP-1 down-regulates DSB repair independently of its poly(ADP-ribosyl)ation activity
PARP-1 has been linked to DNA recombination, since PARP-1 gene disruption or inhibition of poly(ADP-ribosyl)ation stimulate SCE and gene amplification (12–15). However, from their chemical inhibitor studies, Schultz et al. concluded that poly(ADP-ribosyl)ation does not play an important role in homology-directed DSB-repair (20).
To clarify the role of PARP-1 in HR, we modulated PARP-1 function by ectopically expressing either full-length PARP-1 or the mere DNA binding domain (DBD), which leads to trans-dominant inhibition of poly(ADP-ribosyl)ation, followed by quantitative analysis of homology-directed DSB repair. We applied an EGFP-based assay, in which recombinative DNA-exchange processes are triggered by targeted DSB formation in living cells (37). When the rare-cutting meganuclease I-SceI is expressed, it will cleave the recombination substrate at the recognition sequence within the acceptor EGFP gene variant (EJ-EGFP, HR-EGFP or Δ-EGFP; Fig. 1A). Subsequently, HR between the acceptor and the 5′ truncated donor EGFP sequence (3′EGFP), located downstream on the same or on the sister chromatid, may restore a functional EGFP gene. Accordingly, gene conversion, cross-over, SSA and replication slippage may all give rise to a population of green fluorescent cells. To quantify recombination frequencies, the ratios between green fluorescent cells and the total number of cells in the population was determined by flow cytometry. In order to exclude rate deviations caused by growth regulatory effects with the individual cell type used, a cotransfection with a control plasmid carrying a wild-type copy of EGFP at the acceptor position was performed in parallel with each recombination experiment and was used to normalize recombination frequency.
Figure 1.
Effect of PARP-1 and PARP-DBD expression on DSB repair with episomal DNA substrates. (A) Structure of the recombination substrates to assay homology-directed DSB repair. The EGFP-based recombination plasmids EJ-EGFP/3′EGFP, HR-EGFP/3′EGFP or Δ-EGFP/3′EGFP (37) contain the following elements: a puromycin resistance cassette, an acceptor EGFP gene under the control of the CMV promoter and containing a cleavage site (filled triangle) for the rare-cutting I-SceI meganuclease, a spacer region with a hygromycin resistance cassette and the donor 3′EGFP gene, which carries two truncating stop codons in place of the start codon (black cross). The acceptor genes EJ-EGFP, HR-EGFP and Δ-EGFP are characterized by the duplication of 5 bp, or the removal of 4 bp or 46 bp from the EGFP sequence, thereby creating either a sufficient (199 bp, 191 bp) or limiting (164 bp) stretch of homology between the acceptor and the donor genes 5′ to the I-SceI recognition sequence. After the expression of I-SceI and subsequent cleavage within the acceptor gene, unilateral transfer of genetic information (gene conversion), SSA, cross-over or replication slippage within the same chromatid or between sister chromatids can restore a functional EGFP gene at the acceptor position. Recombination frequencies were determined as the fraction of green fluorescent cells within the population of nonfluorescent cells. Transfection efficiencies were individually determined in triplicates after electroporation with a wt-EGFP control plasmid and used to normalize each single recombination frequency. (B) Relative recombination frequencies. Recombination assays were performed by lipofecting LLC-MK2(neo) cells devoid of wt-p53 or LLC-MK2(p53her) cells carrying wt-p53 with recombination plasmid Δ-EGFP/3′EGFP and pCMV-I-SceI for I-SceI-mediated substrate cleavage. For overexpression of PARP-DBD and PARP-1, we cotransfected the cells with plasmids pPARP6 and pPARP31, respectively. The control cells received pBS instead. Subsequently, cultivation was continued for 72 h in the presence of estradiol at a final concentration of 1 µM, to fully activate the p53 moiety within the p53her fusion protein. Recombination frequencies in pBS transfected LLC-MK2(neo) cells were taken as 100% (29 × 10–2) and the relative recombination frequencies calculated. The mean values and SEM from three recombination measurements are shown graphically. (C) PARP expression. Western blot analysis of total cellular homogenates was performed 72 h after transfection and cultivation in the presence of 1 µM estradiol with the plasmids pBS, pPARP6 (PARP-DBD) and pPARP31 (PARP-1). For immunodetection, we applied goat polyclonal IgG N-20. The positions of full-length PARP-1 (113 kDa) and the truncated PARP-DBD (38 kDa) are marked by an arrow. The filters were reprobed with anti-actin antibody to control for loading differences.
To study HR as a function of PARP-1 activity, we transfected LLC-MK2(neo) cells (38) with plasmids pPARP6 or pPARP31 (41,47). These plasmids direct the overexpression of PARP-DBD and PARP-1, respectively, as was confirmed by western blotting (Fig. 1C). The recombination vector Δ-EGFP/3′EGFP as well as the expression construct for I-SceI meganuclease were introduced concomitantly and FACS® analysis was performed 72 h later. The absolute recombination frequency for the pBS-transfected control cells was 29 × 10–2. LLC-MK2(neo) cells ectopically expressing PARP-DBD or PARP-1 showed recombination frequencies that were reduced by 42% and 19%, respectively (Fig. 1B).
Previous reports indicated that PARP-1 poly(ADP-ribosyl)ates p53 (25,26), another recombination-regulatory molecule (34–38). To investigate the consequences of a change in the PARP-1 activity status in a wt-p53 background, we replaced the LLC-MK2(neo) line, endogenously expressing the functionally inactive mutant p53(Δ237–239), by LLC-MK2(p53her) (38). LLC-MK2(p53her) cells are a LLC-MK2(neo) derivative expressing p53her, i.e. human wt-p53 fused to the human estrogen receptor hormone-binding domain, which represents bona fide wt-p53 in the presence of estradiol as verified by functional studies (38,45). p53her expression resulted in a 49% decrease in HR measured by EGFP reconstitution among successfully transfected cells (Fig. 1B). When we subjected LLC-MK2(p53her) cells to cotransfections with pPARP6 or pPARP31, we monitored a 58% and 55% reduction in the LLC-MK2(neo) control recombination frequency due to the combined expression of exogenous p53her/PARP-DBD and p53her/PARP-1 proteins, respectively.
To examine the DSB repair phenotype of cells after chemical inhibition of PARP-1 enzyme activity, we determined the effect of the poly(ADP-ribosyl)ation inhibitor, 3-aminobenzamide (3-AB) (reviewed in 9,10). After a 24 h treatment with 3-AB LLC-MK2(neo) cells exhibited DNA exchange frequencies that were decreased by 16 ± 5%, PARP-1 overexpressing LLC-MK2(neo) cells showed a 28 ± 9% reduction due to drug treatment, i.e. both overexpression of the trans-dominant-negative PARP-DBD and enzymatic inactivation of PARP-1 caused down-regulation of HR. Taken together, in our experiments with an extrachromosomal recombination plasmid, suppression of HR by PARP-1 did not depend on its enzymatic activity.
Overexpression of either PARP-DBD or PARP-1 suppress HR between chromosomally integrated DNA substrates
PARP-1 has been shown to poly(ADP-ribosyl)ate several architectural components of the eukaryotic chromatin including histones H1 and H2B (9). To ask the question whether chromatin packaging of the DNA exchange substrates may influence the regulatory effect of PARP-1 on DSB repair, we chose to modify the PARP-1 activity status in cell lines carrying recombination substrates in the cellular chromosomes. For that purpose, we utilized K562-derived KMV cell clones, which carry a stably integrated HR-EGFP/3′EGFP substrate (37; Fig. 1A). In our previous study, we demonstrated by genomic PCR analyses of the recombination products from the chromosomes of sorted green fluorescing cells that green fluorescence indeed resulted from the reconstitution of a wt-EGFP gene by homology-directed DSB repair.
To introduce a DSB at the position of the I-SceI recognition sequence within the chromosome, we electroporated the tester strain with the I-SceI expression plasmid pCMV-I-SceI. To alter PARP-1 activities, we simultaneously introduced either pPARP6 or pPARP31. In control experiments with pBS, we recorded an absolute recombination frequency of 8 × 10–4 for the p53-negative KMV line. Strikingly, almost complete recombination suppression was caused by the ectopic expression of either PARP-DBD or PARP-1 (Fig. 2A). To be able to detect cooperativity between PARP-1 and p53 in the combined analysis, expression constructs for low-level expression were purposefully chosen such that only small effects were achieved with PARP-DBD or PARP-1 alone (Fig. 2B). For that purpose we utilized the pGC-vector, which produces ∼100-fold lower protein amounts as compared with the pCMV-vector (37; Fig. 2C). Consequently, no significant recombination suppression was noted in KMV cells upon transfection with either pGC-PARP-DBD or pGC-PARP-1 (Fig. 2B). To examine the effect of p53, we utilized KMV cells stably transfected with both the HR-EGFP/3′EGFP recombination plasmid and with pSV53her, the latter providing stable expression of p53her (37). In KMV(p53her) cells HR between HR-EGFP and 3′EGFP was reduced by 58%, as compared with the KMV control. In contrast to the situation in KMV cells, transfection of KMV(p53her) cells with either pGC-PARP-DBD or pGC-PARP-1 caused a dramatic decrease in the recombination frequencies down to background values. In conclusion, recombination interference by PARP-DBD and PARP-1 became apparent both with DNA substrates that were presented on transiently transfected plasmid DNA and on the chromosome and was dose-dependent.
Figure 2.
Effect of PARP-1 and PARP-DBD expression on DSB repair with chromosomal recombination substrates. (A) Homology-directed DSB repair after high-level expression of PARP-DBD and PARP-1. The parental line is the p53-negative line KMV containing the recombination substrate HR-EGFP/3′EGFP in the cellular chromosomes (37). Test cells were subjected to electroporation with pCMV-I-SceI and further cultivated for 48–72 h. For the comparative analysis of PARP-dependent recombination regulatory effects, the cells were coelectroporated with pBS, pPARP6 (PARP-DBD) and pPARP31 (PARP-1), respectively. For the determination of relative recombination frequencies, frequencies in control cells with pBS were taken as 100% (8 × 10–4). Mean values and SEM from two independent experiments with one to three recombination measurements each are shown. (B) DSB repair after low-level expression of PARP-DBD and PARP-1. Relative recombination frequencies were determined from three to six independent measurements after electroporation of KMV and KMV(p53her) cells carrying chromosomally integrated HR-EGFP/3′EGFP with pCMV-I-SceI together with pBS, pGC-PARP-DBD or pGC-PARP-1, and after further cultivation for 72 h in the presence of the inducing agent β-estradiol (200 nM). Note that estradiol treatment led to transcriptional activation of the pGC-based expression constructs in transfected KMV and KMV(p53her) cells and in addition enabled activation of p53 in KMV(p53her) cells only. (C) Western blot analysis. Expression of PARP-1 and PARP-DBD was analysed after electroporation of KMV cells carrying HR-EGFP/3′EGFP with the plasmids pGC-PARP-DBD, pGC-PARP-1, pBS (control), pPARP6 and pPARP31 as in Figure 1C. Note the weak signal specific for PARP-DBD after pGC-PARP-DBD electroporation as compared with the intense signal in pPARP6 electroporated cells. Correspondingly, an increase in PARP-1 expression above the endogenous expression level was undetectable after pGC-PARP-1 but noticeable after pPARP31 transfection. Equal loading was verified by reprobing with anti-tubulin antibodies.
Biological consequences of PARP-1 expression in stably transfected cells
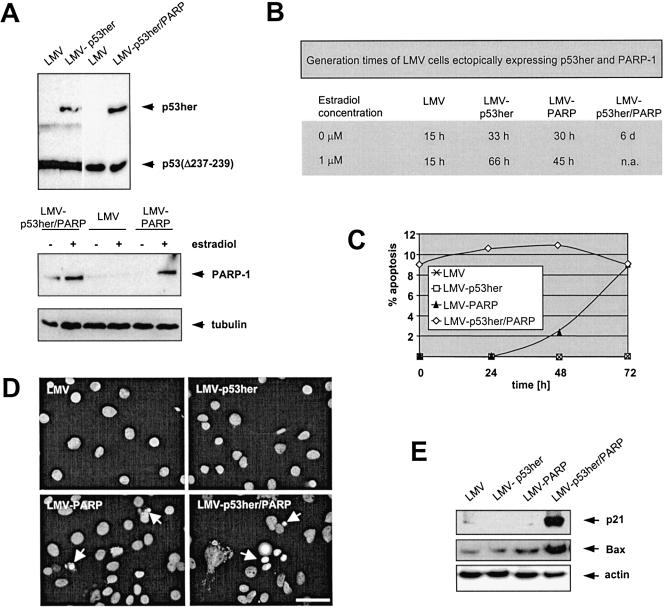
So far, our data from transient expression studies demonstrated that PARP-1 inhibits DSB repair. Next, we generated stable cell clones inducibly expressing PARP-1. As a parental line we chose LMV, i.e. LLC-MK2 cells stably expressing the transcription factor Gal4ERVP for estradiol-inducible transgene expression via pGC vectors (35). In our previous study, we demonstrated that the pGC vector system and the SV40 driven pSVp53her plasmid yield equivalent protein levels (37), thus enabling us to express comparable amounts of PARP-1 and p53her by pGC-PARP and pSVp53her and to induce both protein activities by estradiol. After cotransfection of LMV cells with phyg, which confers hygromycin resistance, and either pSVp53her, pGC-PARP-1 or pSVp53her plus pGC-PARP-1, hygromycin-resistant clones were isolated and screened by western blotting. Ectopic expression of either p53her or PARP-1 was detectable in various clones; however, only one PARP-1 positive clone was successfully established after pSVp53her plus pGC-PARP-1 cotransfection. p53her was constitutively produced and functionally stimulated after estradiol application (LMV-p53her, LMV-p53her/PARP), whereas PARP-1 expression was estradiol-inducible (LMV-PARP, LMV-p53her/PARP) (Fig. 3A,B). LMV-p53her and LMV-PARP cells were characterized by a significant growth retardation, as indicated by a 3- to 4-fold increase in the generation times in the presence of estradiol and a 2-fold increase even in its absence, probably due to residual amounts of estradiol-like substances in the stripped media (Fig. 3B). LMV-p53her/PARP proliferated at extremely retarded growth rates in the absence of estradiol, which again can be explained by basal levels of exogenous PARP-1 and residual p53her activation. Even more strikingly, cultivation in the presence of estradiol resulted in a daily 20% decrease in cell numbers rather than an increase.
Figure 3.
Characterization of cell lines stably expressing p53her and PARP-1. (A) Western blot analyses. Immunoblots showing the expression of the ectopically expressed p53her protein and the endogenous mutant p53(Δ237–239), as detected by use of the IgG fraction of sheep serum raised against human p53 (LMV-p53her) or mAb DO1 (LMV-p53her/PARP). PARP-1 was immunodetected by the mAb F-I-23 and the filter reprobed with anti-tubulin antibody. Total homogenates of 2 × 105 LMV-p53her, LMV-PARP, LMV-p53her/PARP and LMV control cells were loaded, respectively. Inducible PARP-1 expression was examined after cultivation in the absence (–) or presence (+) of estradiol for 16 h (2 µM). Note that endogenous PARP-1 was hardly detectable at the exposure period chosen. Arrows indicate the positions of p53her (90 kDa), p53(Δ237–239) (53 kDa), PARP-1 (113 kDa) and tubulin (α: 57 kDa, β: 54 kDa). (B) Generation times. Proliferation of LMV, LMV-p53her, LMV-PARP and LMV-p53her/PARP cells was analysed in the absence or presence of 1 µM estradiol. LMV-p53her/PARP cells displayed a 20% reduction in cell number per day when cultivated with estradiol (n.a., not applicable). (C) FACS® analyses of propidium iodide stained cells. During a time course of 72 h after estradiol application (1 µM), the appearance of a sub-G1 peak was followed flow- cytometrically for LMV, LMV-p53her, LMV-PARP and LMV-p53her/PARP cells. The time after estradiol induction is given at the x-axis in h, the y-axis shows the percentage of cells with a sub-G1 DNA content indicative of apoptosis. (D) Microscopic detection of apoptotic features. After culturing LMV, LMV-p53her, LMV-PARP and LMV-p53her/PARP for 4 h in estradiol-containing medium (1 µM), the cells were treated with a single dose of 5 Gy and cultivation continued for 12 h followed by staining with DAPI and fluorescence microscopy. Arrows denote apoptotic cell nuclei. The bar represents 50 µm. (E) p21 and Bax expression. Cells were estradiol-induced and irradiated as in (D). Subsequently, total homogenates of 3 × 105 LMV, LMV-p53her, LMV-PARP and LMV-p53her/PARP cells were subjected to western blotting and immunodetection by use of the anti-p21 and anti-Bax mAbs Ab-1 and 2D2, respectively, followed by reprobing with anti-actin antibody.
PARP-1 has been implicated in induction or execution of apoptosis, although contradictory opinions exist on the underlying mechanism with respect to the involvement of poly(ADP-ribosyl)ation and signalling to p53 (9,48,49). The unusual growth characteristics of LMV-p53her/PARP cells prompted us to analyse these cells for potential apoptotic features. Thus, we performed cell cycle analysis at different times after estradiol exposure by FACS® analysis of propidium iodide stained cells. As demonstrated in Figure 3C, the percentage of LMV-p53her/PARP cells with a sub-G1 DNA content, which is strongly indicative of apoptosis, represented ∼10% before and after estradiol treatment. Interestingly, a sub-G1 cell population also appeared in LMV-PARP cells around 48 h (2.5%) of hormone exposure with a further increase until 72 h (8.8%), and similar results were obtained after γ-irradiation with a dose of 5 Gy (data not shown). Under the same conditions, sub-G1 cells were absent in LMV-p53her and LMV cells. Consistently, when we inspected DAPI stained cell nuclei by fluorescence microscopy, LMV-p53her/PARP cells exhibited typical features of apoptosis (Fig. 3D). Using this method, highly condensed and fragmented nuclei were also found in the samples of estradiol-induced LMV-PARP cells as early as 12 h after irradiation, whereas unirradiated LMV-PARP or LMV-p53her and LMV cell nuclei remained intact. In order to understand whether PARP-1 is a positive regulator of p53-dependent transcriptional transactivation in LMV-p53her/PARP cells under these conditions (26,31), which may be related to the abnormal growth characteristics of these cells, we also investigated the expression of the two major target genes, p21 and Bax, by western blotting (Fig. 3E). As compared with the other LMV lines, LMV-p53her/PARP cells showed dramatically increased levels of p21 and Bax. Our results indicated that PARP-1 expression in cells derived from the rhesus monkey cell line LLC-MK2 is followed by growth retardation and programmed cell death, particularly in a wt-p53-positive background.
DSB repair in stably transfected cells expressing exogenous PARP-1
To investigate whether recombination interference by PARP-1 was also detectable in LMV-PARP and LMV-p53her/PARP cells, we transfected the newly generated stable cell clones with pCMV-I-SceI and either the recombination vector EJ-EGFP/3′EGFP, HR-EGFP/3′EGFP or Δ-EGFP/3′EGFP. EJ-EGFP at the acceptor position additionally supports in frame reconstitution of EGFP via NHEJ, HR- versus Δ-EGFP raises the length of homology with 3′EGFP neighbouring the cleavage site (37). Flow cytometric analysis was performed after 72 h of cultivation in the presence of estradiol and relative recombination frequencies were calculated (Fig. 4). In the parental LMV cells, we measured mean recombination frequencies of 20 × 10–2 for EJ-EGFP, 12 × 10–2 for HR-EGFP and 12 × 10–2 for Δ-EGFP. In LMV-p53her cells, 71–75% of the control values were measured with all three recombination substrates. The relative frequencies in LMV-PARP cells were similarly reduced to 69–81%, whereas in LMV-p53her/PARP cells, we recorded increased rather than decreased mean values (101–138%). Thus, unlike the results after transient PARP-1 expression (Figs 1 and 2), the down-regulatory effect of PARP-1 was abolished in stably transfected LMV-p53her/PARP cells coexpressing p53her.
Figure 4.
Homologous DSB repair in LMV-p53her, LMV-PARP and LMV-p53her/PARP cells. Homologous exchange was analysed between either EJ-EGFP, HR-EGFP or Δ-EGFP and 3′EGFP (upper panel map) after colipofection of LMV (– p53her, – PARP-1), LMV-p53her (+ p53her, – PARP-1), LMV-PARP (– p53her, + PARP-1) and LMV-p53her/PARP cells (+ p53her, + PARP-1) with the respective recombination construct and the meganuclease expression vector pCMV-I-SceI. Mean values including SEM of the relative recombination frequencies are diagrammatically shown for two to six independent experiments with two or three measurements each. The recombination frequencies in the LMV control cells (20 × 10–2 for EJ-EGFP, 12 × 10–2 for HR-EGFP, 12 × 10–2 for Δ-EGFP) were taken as 100%.
To examine whether the unexpectedly high DSB-repair activities in LMV-p53her/PARP cells were related to the apoptotic features of these cells (Fig. 3C,D), we introduced the recombination plasmid Δ-EGFP/3′EGFP into the same series of cell lines followed by a 24 h treatment with the irreversible caspase 3 inhibitor Z-DEVD-FMK, which also inhibits caspases 6, 7, 8 and 10 (50). FACS® analysis of EGFP-positivity was performed after a total cultivation period of 72 h. Importantly, control transfections served to normalize each single recombination frequency, so that we could rule out the possibility that rate deviations were simply caused by altered transfection efficiencies, by growth regulatory effects or differences in cell lethality with the individual cell line used. As shown in Figure 5, in LMV cells no changes in the HR frequencies were detectable in the presence of Z-DEVD-FMK. Similarly, only a minor drug-dependent reduction in the recombination frequencies was measured in LMV-p53her and LMV-PARP cells. However, caspase inhibition in LMV-p53her/PARP cells caused a significant down-regulation of HR (P = 0.036), resulting in the same relative recombination frequency of 78% as seen with LMV-p53her cells. For comparison, Z-DEVD-FMK treatment of LLC-MK2(neo) and LLC-MK2(p53her) cells upon transient transfection with pGC-PARP-1 did not cause a statistically significant reduction in the HR values (data not shown). These findings suggest that apoptotic processes induced by coexpression of PARP-1 and p53her in stably transfected cells may indirectly cause an apparent stimulation of DNA exchange processes that neutralize the anti-recombinogenic activities of PARP-1 and p53her.
Figure 5.
Caspase inhibition affects DSB repair in LMV-p53her/PARP cells. Homology-directed DSB repair was analysed and relative recombination frequencies calculated as described in the legend to Figure 4. After lipofection with the recombination plasmid Δ-EGFP/3′EGFP and with pCMV-I-SceI LMV (– p53her, – PARP-1), LMV-p53her (+ p53her, – PARP-1), LMV-PARP (– p53her, + PARP-1) and LMV-p53her/PARP (+ p53her, + PARP-1), cells were treated without (filled bars) and with the caspase inhibitor (open bars) for 24 h.
DISCUSSION
Numerous studies have indicated critical roles for both PARP-1 and p53 in the maintenance of genome integrity (reviewed in 8,33,51). PARP-1 and p53 are thought to promote base excision repair (BER) via physical interactions with the BER multiprotein complex (52–55). A large body of evidence also indicates that wt-p53 down-regulates spontaneous and DSB-induced HR (34–38). From our current knowledge, p53 may either block erroneous strand exchange by the recombinase Rad51 or exonucleolytically degrade mispaired intermediates of HR, thereby preventing detrimental rearrangements. However, the molecular mechanism that may underlie the putative anti-recombinogenic role of PARP-1 has remained elusive (11,20). Thus, PARP-1 may directly protect strand breaks from processing or recruit recombination surveillance proteins like WRN or p53. Alternatively, poly(ADP-ribosyl)ation of histones may alter the chromatin configuration and activate p53 functions in HR regulation. From recent reports, poly(ADP-ribosyl)ation may additionally modulate recombination indirectly, because NAD+ depletion inhibits the activity of the Sir2/SIRT1 deacetylase which also affects chromosome remodelling and p53 (56,57).
In the present work, we investigated the role of PARP-1 in DSB repair by use of the rare-cutting, site-specific I-SceI endonuclease, rather than by ionizing irradiation, which introduces base modifications in addition to strand breaks. Therefore, by focusing on targeted DSBs, we were able to exclude indirect effects on HR by DNA excision repair. For our analysis, we utilized two well-established isogenic cellular systems, each with and without wt-p53 (35,37,38). Using two experimental strategies to inactivate PARP-1 enzymatic activity, namely overexpression of PARP-DBD and chemical inhibition, we have observed decreased DSB repair activities in the wt-p53-negative background. Moreover, down- regulation of HR by wt-p53 was further enhanced after PARP-DBD overexpression. Although less markedly, the overexpression of full-length PARP-1 also exerted an inhibitory effect. These results largely excluded an involvement of poly(ADP-ribosyl)ation and p53 signalling in the anti-recombinative effect of PARP-1. Our data rather support the idea that tight binding of PARP-1 to DSBs prevents access for recombination enzymes (11). The less prominent effect by PARP-1 versus PARP-DBD may be explained by the fact that the interaction of PARP-1 with strand breaks is modulated by automodification, i.e. DNA-break sealing will be possible after release of poly(ADP-ribosyl)ated PARP-1 (10).
Our recent studies on the regulatory role of p53 in recombination had indicated an influence of the protein/DNA substrate ratio on the degree of recombination interference (37). In agreement with a similar dose-dependence, the effect of PARP-DBD and PARP-1 on HR was dramatic when the protein/DNA substrate ratio was high in experiments with high-level protein expression and less then 10 intrachromosomally located recombination substrates (37). Conversely, intermediate HR frequency changes were observed with high-level protein expression and extrachromosomal substrates at estimated copy numbers of 105 (58). Moreover, a dose-dependence also became apparent when we studied intrachromosomal HR in the presence of different PARP-DBD and PARP-1 protein levels. Thus, high-level expression of exogenous PARP-DBD or PARP-1 led to a complete suppression of HR as compared with a minor reduction with 100-fold lower protein amounts. High-level expression conditions generated PARP-DBD and PARP-1 levels, which, according to western blot analysis, were similar to endogenous PARP-1, i.e. amounted to ∼106 molecules per cell or one molecule per 1000 bp (59). Importantly, DSB concentrations in the order of the affinity constant of PARP-1 (10–10 M) were achieved in these KMV cells which carried approximately six I-SceI cleavable substrates after chromosomal integration (37). In conclusion, HR suppression by exogenous PARP-DBD and PARP-1 can be explained by direct interactions with DNA breaks.
Interestingly, the small influence of low levels of PARP-DBD and PARP-1 on intrachromosomal recombination was dramatically enhanced in a wt-p53 background. This finding suggested a synergistic effect by p53 and both PARP-DBD and PARP-1, although in experiments with extrachromosomal substrates, the data rather indicated an additive effect. With the DNA-binding domain of PARP-1 being sufficient, this synergism cannot be explained by an involvement of PARP-1 enzymatic activity, thereby excluding p53 activation by poly(ADP-ribosyl)ation, Sir2/SIRT1 inhibition or chromatin opening via charge repulsion by poly(ADP-ribose) chains. The DNA binding domain of PARP-1 was proposed to participate in molecular complexes with proteins involved in genome integrity (43). However with respect to p53, mapping data of the physical interaction site on PARP-1 argue against a synergistic mechanism that would involve physical interactions of p53 within the PARP-DBD (27). Similar to PARP-1 and p53, synergistic activities in minimizing end-to-end fusions and other chromosomal aberrations were also reported for PARP-1 and Ku, another DNA-end binding protein (60), as well as for p53 and Ku (61). p53 has been shown to complex V(D)J recombination intermediates, to specifically recognize early strand exchange intermediates, particularly in trimeric complexes with Rad51, and to abrogate NHEJ and HR involving sequence divergences (38,39,62–64). Therefore, the combined protection of DNA breaks and repair intermediates by PARP-1 and p53 coupled to complementary interactions with repair enzymes of Rad51-independent and -dependent HR pathways may cause a synergistic inhibition particularly in highly organized repair complexes within the nuclear chromatin.
In accord with the results from transient expression studies, HR was down-regulated after PARP-1 expression in stably transfected LMV cells lacking wt-p53. In contrast, in cells stably coexpressing PARP-1 and p53her, indirect effects antagonized HR interference by PARP-1 and wt-p53. Importantly, in these LMV-p53her/PARP cells, a severe reduction in cell survival and a marked transactivation of two p53 target genes were observed in parallel. These results are consistent with two models involving apoptotic signalling of PARP-1 upstream of p53: First, poly(ADP-ribosyl)ation of p53 rapidly enhances accumulation of p53, activation of sequence-specific DNA binding and p53-dependent apoptotic DNA fragmentation (24,27,28,31). Second, prolonged PARP-1 activation and NAD+ depletion may inhibit Sir2/SIRT1, the NAD+-dependent deacetylase which suppresses p53-mediated transactivation and delays apoptosis (56,57). Yet, in the presence of the inducing agent, we also detected apoptotic features in LMV-PARP cells without wt-p53, although to a much lesser degree as compared with LMV-p53her/PARP cells. From this, p53-independent functions of PARP-1 in apoptosis induction may contribute to the phenotype of LMV-p53her/PARP cells. Our recombination experiments performed in the presence of the caspase inhibitor supported the idea that apoptosis induction in LMV-p53her/PARP cells was underlying the failure of exogenous PARP-1 and p53her to down-regulate HR. Previous observations have led to the suggestion that apoptosis positively regulates gene rearrangements; however, the precise mechanism remains to be determined (50,65). One possibility is that the formation of DNA strand breaks which is mediated by apoptotic nucleases may trigger DNA rearrangements.
Our findings on the anti-recombinogenic role of PARP-1 are consistent with previous reports that documented negative regulation of micronuclei formation, SCEs, chromosome fusions and gene amplification after genotoxic treatment by endogenous and ectopically expressed PARP-1 (14,66,67). They also support the conclusions drawn from double knockout studies indicating a cooperation between p53 and PARP-1 by suppressing chromosome aberrations such as fragmentations, end-to-end fusions and abnormally long and heterogeneous telomeres (9,10). Our data are also in accord with a previous report which demonstrated that resealing of irradiation-induced DSBs is slowed down by PARP-DBD (68). Conversely, in another study PARP-DBD overexpression was shown to enhance gene amplification in response to MNNG treatment (12). At first glance, our data also seem to contradict the results from studies using chemical inhibitors of poly(ADP-ribosyl)ation. Thus, 3-AB stimulated SCEs but not translocations (69), 3-methoxybenzamide potentiated intra- but not extrachromosomal HR (21,22) and 1,5-isoquinolinediol increased the frequency of extrachromosomal HR (23) but did not significantly influence HR on meganuclease-cleaved chromosomally integrated substrates (20). One possible explanation for the different outcomes may be that the known effects of PARP-1 inhibition on DNA base-excision repair and the consequent (indirect) activation of the HR pathway may override to some extent the direct inhibitory effect on HR reported here. Additionally, PARP-1-mediated growth regulation may affect HR measurements as determined by colony formation assays. Taken together, differences in the repair trigger, in the PARP-1/DNA substrate ratio, in the p53-status and cytotoxic effects may have influenced previous studies on the role of PARP-1 in genome stabilization, and this may resolve some of the contradictions. In this work, we largely excluded rate deviations related to indirect effects by targeted DSB formation to trigger HR, by use of a fluorescence-based HR assay with a fast readout and by use of various cellular systems differing in PARP-1 status.
In summary, we have established that PARP-1 is a negative regulator of DSB repair in a manner that is dose-dependent and apparently does not involve the covalent modification of target proteins like p53 with poly(ADP-ribose) or NAD+ depletion. The present study further demonstrated cooperative functions of PARP-1 and p53 in suppressing DSB-repair on cellular chromosomes. PARP-1 null mice exhibit high genomic instabilities with a further increase seen in PARP-1 and p53 double knockouts, which are characterized by chromosome aberrations, indicative of deregulated HR (9,10). Moreover, after PARP-1 inactivation in p53–/– mice, tumorigenesis is enhanced and the tumour spectrum widened. Consistently, our results suggest a synergistic role between PARP-1 and p53 in controlling DSB repair processes, thereby synergistically minimizing aberrant chromosomal rearrangements and suppressing tumorigenesis.
Acknowledgments
ACKNOWLEDGEMENTS
Our special thanks go to Nuray Akyüz for introduction to FACS® analysis. We thank Prof. G.G. Poirier, Québec, Canada, for antibody F-I-23, Marion Kurth for initial help and Evelyn Bendrat for experimental assistance during clonal establishment. This work was supported by the Deutsche Forschungsgemeinschaft, grants Wi 1376/1-5 and Wi 1376/3-1 and by the Land Baden-Württemberg, Forschungs schwerpunktprogramm: Fehlregulation von Apoptose als Grundlage für Krankheit.
REFERENCES
- 1.Pierce A.J., Stark,J.M., Araujo,F.D., Moynahan,M.E., Berwick,M. and Jasin,M. (2001) Double-strand breaks and tumorigenesis. Trends Cell Biol., 11, S52–S59. [DOI] [PubMed] [Google Scholar]
- 2.Rothkamm K., Krüger,I., Thompson,L.T. and Löbrich,M. (2003) Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol. Cell. Biol., 23, 5706–5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambert S., Saintigny,Y., Delacote,F., Amiot,F., Chaput,B., Lecomte,M., Huck,S., Bertrand,P. and Lopez,B.S. (1999) Analysis of intrachromosomal homologous recombination in mammalian cell, using tandem repeat sequences. Mutat. Res., 433, 159–168. [DOI] [PubMed] [Google Scholar]
- 4.Lim D.-S. and Hasty,P. (1996) A mutation in mouse rad51 results in an early embryonic lethal that is suppressed by a p53 mutation. Mol. Cell. Biol., 16, 7133–7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuzuki T., Fujii,Y., Sakumi,K., Tominaga,Y., Nakao,K., Sekiguchi,M., Matsushiro,A., Yoshimura,Y. and Morita,T. (1996) Targeted disruption of the Rad51 gene leads to lethality in embryonic mice. Proc. Natl Acad. Sci. USA, 93, 6236–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo G., Yao,M.S., Bender,C.F., Mills,M., Bladl,A.R., Bradley,A. and Petrini,J.H. (1999) Disruption of mRad50 causes embryonic stem cell lethality, abnormal embryonic development and sensitivity to ionizing radiation. Proc. Natl Acad. Sci. USA, 96, 7376–7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tauchi H., Kobayashi,J., Morishima,K., van Gent,D.C., Shiraishi,T., Verkaik,N.S., van Heems,D., Ito,E., Nakamura,A., Sonoda,E., Takata,M., Takeda,S., Matsuura,S. and Komatsu,K. (2002) Nbs1 is essential for DNA repair by homologous recombination in higher vertebrate cells. Nature, 420, 93–98. [DOI] [PubMed] [Google Scholar]
- 8.Bürkle A. (2001) Physiology and pathophysiology of poly(ADP-ribosyl)ation. Bioessays, 23, 795–806. [DOI] [PubMed] [Google Scholar]
- 9.deMurcia G. and Shall,S. (2000) Poly (ADP-ribosylation) Reactions: From DNA Damage and Stress Signalling to Cell Death. Oxford University Press, Oxford, UK. [Google Scholar]
- 10.Tong W.-M., Cortes,U. and Wang,Z.-Q. (2001) Poly(ADP-ribose) polymerase: a guardian angel protecting the genome and suppressing tumorigenesis. Biochim. Biophys. Acta, 1552, 27–37. [DOI] [PubMed] [Google Scholar]
- 11.Lindahl T., Satoh,M.S., Poirier,G.G. and Klungland,A. (1995) Post-translational modification of poly(ADP-ribose) polymerase induced by DNA strand breaks. Trends Biol. Sci., 20, 405–411. [DOI] [PubMed] [Google Scholar]
- 12.Küpper J.-H., Müller,M. and Bürkle,A. (1996) Trans-dominant inhibition of poly(ADP-ribosyl)ation potentiates carcinogen-induced gene amplification in SV40-transformed Chinese hamster cells. Cancer Res., 56, 2715–2717. [PubMed] [Google Scholar]
- 13.Menissier deMurcia J., Niedergang,C., Trucco,C., Ricoul,M., Dutrillaux,B., Mark,M., Oliver,F.J., Masson,M., Dierich,A., LeMeur,M., Walztinger,C., Chambon,P. and de Murcia,G. (1997) Requirement of poly (ADP-ribose)polymerase in recovery from DNA damage in mice and in cells. Proc. Natl Acad. Sci. USA, 94, 7303–7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z.Q., Stingl,L., Morrison,C., Jantsch,M., Los,M., Schulze-Osthoff,K. and Wagner,E.F. (1997) PARP is important for genomic stability but dispensable in apoptosis. Genes Dev., 11, 2347–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simbulan-Rosenthal C.M., Haddad,B.R., Rosenthal,D.S., Weaver,Z., Coleman,A., Luo,R., Young,H.M., Wang,Z.Q., Ried,T. and Smulson,M.E. (1999) Chromosomal aberrations in PARP(–/–) mice: genome stabilization in immortalized cells by reintroduction of poly(ADP-ribose) polymerase cDNA. Proc. Natl Acad. Sci. USA, 96, 13191–13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schimke R.T. (1988) Gene amplification in cultured cells. J. Biol. Chem., 263, 5989–5992. [PubMed] [Google Scholar]
- 17.Lambert S. and Lopez,B.S. (2001) Role of RAD51 in sister-chromatid exchanges in mammalian cells. Oncogene, 20, 6627–6631. [DOI] [PubMed] [Google Scholar]
- 18.Saintigny Y., Makienko,K., Swanson,C., Emond,M.J. and Monnat,J.,Jr (2002) Homologous recombination resolution defect in Werner Syndrome. Mol. Cell. Biol., 22, 6971–6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lebel M., Lavoie,J., Gaudreault,I., Bronsard,M. and Drouin,R. (2003) Genetic cooperation between the Werner Syndrome protein and poly(ADP-ribose) polymerase-1 in preventing chromatid breaks, complex chromosomal rearrangements and cancer in mice. Am. J. Pathol., 162, 1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schultz N., Lopez,E., Saleh-Gohari,N. and Helleday,T. (2003) Poly(ADP-ribose) polymerase (PARP-1) has a controlling role in homologous recombination. Nucleic Acids Res., 31, 4959–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waldman B.C. and Waldman,A.S. (1990) Illegitimate and homologous recombination in mammalian cells: differential sensitivity to an inhibitor of poly(ADP-ribosylation). Nucleic Acids Res., 19, 5943–5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waldman B.C. and Waldman,A.S. (1991) Stimulation of intrachromosomal homologous recombination in mammalian cells by an inhibitor of poly(ADP-ribosylation). Nucleic Acids Res., 18, 5981–5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Semionov A., Cournoyer,D. and Chow,T.Y. (1999) Inhibition of poly(ADP-ribose)polymerase stimulates extrachromosomal homologous recombination in mouse Ltk-fibroblasts. Nucleic Acids Res., 27, 4526–4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitacre C.M., Hashimoto,H., Tsai,M.L., Chatterjee,S., Berger,S.J. and Berger,N.A. (1995) Involvement of NAD-poly(ADP-ribose) metabolism in p53 regulation and its consequences. Cancer Res., 55, 3697–3701. [PubMed] [Google Scholar]
- 25.Wesierska-Gadek J., Bugajska-Schretter,A. and Cerni,C. (1996) ADP-ribosylation of p53 tumor suppressor protein: mutant but not wild-type p53 is modified. J. Cell. Biochem., 62, 90–101. [DOI] [PubMed] [Google Scholar]
- 26.Vaziri H., West,M.D., Allsopp,R.C., Davison,T.S., Wu,Y.S., Arrowsmith,C.H., Poirier,G.G. and Benchimol,S. (1997) ATM-dependent telomere loss in aging human diploid fibroblasts and DNA damage lead to the post-translational activation of p53 protein involving poly(ADP-ribose) polymerase. EMBO J., 16, 6018–6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumari S.R., Mendoza-Alvarez,H. and Alvarez-Gonzalez,R. (1998) Functional interactions of p53 with poly(ADP-ribose) polymerase (PARP) during apoptosis following DNA damage: covalent poly(ADP-ribosyl)ation of p53 by exogenous PARP and noncovalent binding of p53 to the Mr 85,000 proteolytic fragment. Cancer Res., 58, 5075–5078. [PubMed] [Google Scholar]
- 28.Wang X., Ohnishi,K., Takahashi,A. and Ohnishi,T. (1998) Poly(ADP-ribosyl)ation is required for p53-dependent signal transduction induced by radiation. Oncogene, 17, 2819–2825. [DOI] [PubMed] [Google Scholar]
- 29.Pleschke J.M., Kleczkowska,H.E., Strohm,M. and Althaus,F.R. (2000) Poly(ADP-ribose) binds to specific domains in DNA damage checkpoint proteins. J. Biol. Chem., 275, 40974–40980. [DOI] [PubMed] [Google Scholar]
- 30.Valenzuela M.T., Guerrero,R., Nunez,M.I., Ruiz De Almodovar,J.M., Sarker,M., de Murcia,G. and Oliver,F.J. (2002) PARP-1 modifies the effectiveness of p53-mediated DNA damage response. Oncogene, 21, 1108–1116. [DOI] [PubMed] [Google Scholar]
- 31.Wieler S., Gagne,J.-P., Vaziri,H., Poirier,G.G. and Benchimol,S. (2003) Poly(ADP-ribose) polymerase-1 is a positive regulator of the p53-mediated G1 arrest response following ionizing radiation. J. Biol. Chem., 278, 18914–18921. [DOI] [PubMed] [Google Scholar]
- 32.Ishizuka S., Martin,K., Booth,C., Potten,C.S., de Murcia,G., Bürkle,A. and Kirkwood,T.B.L. (2003) Poly(ADP-ribose) polymerase-1 is a survival factor for radiation-exposed intestinal epithelial stem cells in vivo. Nucleic Acids Res., 31, 6198–6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albrechtsen N., Dornreiter,I., Grosse,F., Kim,E., Wiesmüller,L. and Deppert,W. (1999) Maintenance of genomic integrity by p53: complementary roles for activated and non-activated p53. Oncogene, 18, 7706–7717. [DOI] [PubMed] [Google Scholar]
- 34.Saintigny Y., Rouillard,D., Chaput,B., Soussi,T. and Lopez,B.S. (1999) Mutant p53 proteins stimulate spontaneous and radiation-induced intrachromosomal homologous recombination independently of the alteration of the transactivation activity and of the G1 checkpoint. Oncogene, 18, 3553–3565. [DOI] [PubMed] [Google Scholar]
- 35.Dudenhöffer C., Kurth,M., Janus,F., Deppert,W. and Wiesmüller,L. (1999) Dissociation of the recombination control and the sequence-specific transactivation function of p53. Oncogene, 18, 5773–5784. [DOI] [PubMed] [Google Scholar]
- 36.Willers H., McCarthy,E.E., Wu,B., Wunsch,H., Tang,W., Taghian,D.G., Xia,F. and Powell,S.N. (2000) Dissociation of p53-mediated suppression of homologous recombination from G1/S cell-cycle checkpoint control. Oncogene, 19, 632–639. [DOI] [PubMed] [Google Scholar]
- 37.Akyüz N., Boehden,G.S., Süsse,S., Rimek,A., Preuss,U., Scheidtmann,K.-H. and Wiesmüller,L. (2002) DNA substrate dependence of the p53-mediated regulation of double-strand break repair. Mol. Cell. Biol., 22, 6306–6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dudenhöffer C., Rohaly,G., Will,K., Deppert,W. and Wiesmüller,L. (1998) Specific mismatch recognition in heteroduplex intermediates by p53 suggests a role in fidelity control of homologous recombination. Mol. Cell. Biol., 18, 5332–5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Süsse S., Janz,C., Janus,F., Deppert,W. and Wiesmüller,L. (2000) Role of heteroduplex joints in the functional interactions between human Rad51 and wild-type p53. Oncogene, 19, 4500–4512. [DOI] [PubMed] [Google Scholar]
- 40.Sengupta S., Linke,S.P., Pedeux,R., Yang,Q., Farnsworth,J., Garfield,S.H., Valerie,K., Shay,J.W., Ellis,N.A., Wasylyk,B. and Harris,C.C. (2003) BLM helicase-dependent transport of p53 to sites of stalled DNA replication forks modulates homologous recombination. EMBO J., 22, 1210–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Küpper J.H., de Murcia,G. and Bürkle,A. (1990) Inhibition of poly(ADPribosyl)ation by overexpressing the poly(ADP-ribose) polymerase DNA-binding domain in mammalian cells. J. Biol. Chem., 265, 18721–18724. [PubMed] [Google Scholar]
- 42.Schreiber V., Hunting,D., Trucco,C., Gowans,B., Grunwald,D., de Murcia,G. and Menissier de Murcia,J.M. (1995) A dominant-negative mutant of human poly(ADP-ribose)polymerase affects cell recovery, apoptosis and sister chromatid exchange following DNA damage. Proc. Natl Acad. Sci. USA, 92, 4753–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cayuela M.L., Carrillo,A., Ramirez,P., Parrilla,P. and Yelamos,J. (2001) Genomic instability in a PARP-1(–/–) cell line expressing PARP-1 DNA-binding domain. Biochem. Biophys. Res. Commun., 13, 285, 289–294. [DOI] [PubMed] [Google Scholar]
- 44.Braselmann S., Graninger,P. and Busslinger,M. (1993) A selective transcriptional induction system for mammalian cells based on Gal4-estrogen receptor fusion proteins. Proc. Natl Acad. Sci. USA, 90, 1657–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roemer K. and Friedmann,T. (1993) Modulation of cell proliferation and gene expression by a p53-estrogen receptor hybrid protein. Proc. Natl Acad. Sci. USA, 90, 9252–9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zink D., Mayr,C., Janz,C. and Wiesmüller,L. (2002) Association of p53 and MSH2 with recombinative repair complexes during S-phase. Oncogene, 21, 4788–4800. [DOI] [PubMed] [Google Scholar]
- 47.Bernges F., Bürkle,A., Küpper,J.H. and Zeller,J. (1997) Functional overexpression of human poly(ADP-ribose) polymerase in transfected rat tumor cells. Carcinogenesis, 18, 663–668. [DOI] [PubMed] [Google Scholar]
- 48.Yu S.-W., Wang,H., Poitras,M.F., Coombs,C., Bowers,W.J., Federoff,H.J., Poirier,G.G., Dawson,T.M. and Dawson,V.L. (2002) Mediation of poly(ADP-ribose)polymerase-1-dependent cell death by apoptosis-inducing factor. Science, 297, 259–263. [DOI] [PubMed] [Google Scholar]
- 49.Saldeen J., Tillmar,L., Karlsson,E. and Welsh,N. (2003) Nicotinamide- and caspase-mediated inhibition of poly(ADP-ribose) polymerase are associated with p53-independent cell cycle (G2) arrest and apoptosis. Mol. Cell. Biochem., 243, 113–122. [DOI] [PubMed] [Google Scholar]
- 50.Sim S.-P. and Liu,L.F. (2001) Nucleolytic cleavage of the Mixed Lineage Leukemia breakpoint cluster region during apoptosis. J. Biol. Chem., 34, 31590–31595. [DOI] [PubMed] [Google Scholar]
- 51.Dantzer F., Schreiber,V., Niedergang,C., Trucco,C., Flatter,E., De La Rubia,G., Oliver,J., Rolli,V., Menissier-de Murcia,J. and de Murcia,G. (1999) Involvement of poly(ADP-ribose) polymerase in base excision repair. Biochimie, 81, 69–75. [DOI] [PubMed] [Google Scholar]
- 52.Masson M., Niedergang,C., Schreiber,V., Muller,S., Menissier-de Murcia,J. and de Murcia,G. (1998) XRCC1 is specifically associated with poly(ADP-ribose) polymerase and negatively regulates its activity following DNA damage. Mol. Cell. Biol., 18, 3563–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beneke R., Geisen,C., Zevnik,B., Bauch,T., Müller,W.-U., Küpper,J.-H. and Möröy,T. (2000) DNA excision repair and DNA damage-induced apoptosis are linked to poly(ADP-ribosyl)ation but have different requirements for p53. Mol. Cell. Biol., 20, 6695–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Offer H., Milyavsky,M., Erez,N., Matas,D., Zurer,I., Harris,C.C. and Rotter,V. (2001) Structural and functional involvement of p53 in BER in vitro and in vivo. Oncogene, 20, 581–589. [DOI] [PubMed] [Google Scholar]
- 55.Zhou J., Ahn,J., Wilson,S.H. and Prives,C. (2001) A role for p53 in base excision repair. EMBO J., 20, 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howitz K., Bitterman,K.J., Cohen,H.Y., Lamming,D.W., Lavu,S., Wood,J.G., Zipkin,R.E., Chung,P., Kisielewski,A., Zhang,L.-L., Scherer,B. and Sinclair,D.A. (2003) Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature, 425, 1–5. [DOI] [PubMed] [Google Scholar]
- 57.Zhang J. (2003) Are poly(ADP-ribosyl)ation by PARP-1 and deacetylation by Sir2 linked? Bioessays, 25, 808–814. [DOI] [PubMed] [Google Scholar]
- 58.Tseng W.-C., Haselton,F.R. and Giorgio,T.D. (1997) Transfection by cationic liposomes using simultaneous single cell measurements of plasmid delivery and transgene expression. J. Biol. Chem., 272, 25641–25647. [DOI] [PubMed] [Google Scholar]
- 59.Tong W.-M., Galendo,D. and Wang,Z.-Q. (2000) Role of DNA break-sensing molecule poly(ADP-ribose) polymerase (PARP) in cellular function and radiation toxicity. Cold Spring Harb. Symp. Quant. Biol., 65, 583–591. [DOI] [PubMed] [Google Scholar]
- 60.Tong W.-M., Cortes,U., Hande,M.P., Ohgaki,H., Cavalli,L.R., Lansdorp,P.M., Haddad,B.R. and Wang,Z.-Q. (2002) Synergistic role of Ku80 and poly(ADP-ribose) polymerase in suppressing chromosomal aberrations and liver cancer formation. Cancer Res., 62, 6990–6996. [PubMed] [Google Scholar]
- 61.Difilippantonio M.J., Zhu,J., Chen,H.T., Meffre,E., Nussenzweig,M.C., Max,E.E., Ried,T. and Nussenzweig,A. (2000) DNA repair protein Ku80 suppresses chromosomal aberrations and malignant transformation. Nature, 404, 510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gebow D., Miselis,N. and Liber,H.L. (2000) Homologous and nonhomologous recombination resulting in deletion: effects of p53 status, microhomology and repetitive DNA length and orientation. Mol. Cell. Biol., 20, 4028–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Janz C., Süsse,S. and Wiesmüller,L. (2002) p53 and recombination intermediates: role of tetramerization at DNA junctions in complex formation and exonucleolytic degradation. Oncogene, 21, 2130–2140. [DOI] [PubMed] [Google Scholar]
- 64.Perkins E.J., Nair,A., Cowley,D.O., Dyke,T.V., Chang,Y. and Ramsden,D.A. (2002) Sensing of intermediates in V(D)J recombination by ATM. Genes Dev., 16, 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stanulla M., Wang,J., Chervinsky,D.S., Thandla,S. and Aplan,P.D. (1997) DNA cleavage within the MLL breakpoint cluster region is a specific event which occurs as part of higher-order chromatin fragmentation during the initial stages of apoptosis. Mol. Cell. Biol., 17, 4070–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.D'Adda di Fagagna F., Hande,M.P., Tong,W.-M., Lansdorp,P.M., Wang,Z.-Q. and Jackson,S.P. (1999) Functions of poly(ADP-ribose) polymerase in controlling telomere length and chromosomal stability. Nature Genet., 23, 76–80. [DOI] [PubMed] [Google Scholar]
- 67.Meyer R., Müller,M., Beneke,S., Küpper,J.H. and Bürkle,A. (2000) Negative regulation of alkylation-induced sister-chromatid exchange by poly(ADP-ribose) polymerase-1 activity. Int. J. Cancer, 88, 351–355. [DOI] [PubMed] [Google Scholar]
- 68.Rudat V., Bachmann,N., Küpper,J.H. and Weber,K.J. (2001) Overexpression of the DNA-binding domain of poly(ADP-ribose) polymerase inhibits rejoining of ionizing radiation-induced DNA double-strand breaks. Int. J. Radiat. Biol., 77, 303–307. [DOI] [PubMed] [Google Scholar]
- 69.Dominguez I., Mateos,S. and Cortes,F. (2000) Yield of SCEs and translocations produced by 3 aminobenzamide in cultured Chinese hamster cells. Mutat. Res., 448, 29–34. [DOI] [PubMed] [Google Scholar]