Abstract
Parkinson's disease is usually characterized as a movement disorder; however cognitive abilities that are dependent on the prefrontal cortex decline at an early stage of the disease in most patients. The changes that underlie cognitive deficits in Parkinson’s disease are not well understood. We hypothesize that reduced dopamine signaling in the prefrontal cortex in Parkinson’s disease is a harbinger of detrimental synaptic changes in pyramidal neurons in the prefrontal cortex whose function is necessary for normal cognition. Our previous data showed that monkeys exposed to the neurotoxin, MPTP, but not exhibiting overt motor deficits (motor-asymptomatic), displayed cognitive deficits in prefrontal cortex-dependent tasks. The present results demonstrate that motor-asymptomatic MPTP-treated monkeys have a reduced dopamine concentration and a substantially lower number (50%) of asymmetric (excitatory) spine synapses in layer II/III, but not layer V, of the dorsolateral prefrontal cortex, compared with controls. In contrast, neither dopamine concentration nor asymmetric synapse number was altered in the entorhinal cortex of MPTP-treated monkeys. Together these findings suggest that the number of asymmetric spine synapses on dendrites in the prefrontal cortex is dopamine-dependent and that the loss of synapses may be a morphological substrate of the cognitive deficits induced by a reduction in dopamine neurotransmission in this region. Modulation of asymmetric spine synapse number in prefrontal cortex represents a novel neuroplastic function for dopamine.
Keywords: dopamine, Parkinson’ disease, prefrontal cortex, dendritic spine, synapse
Introduction
The 1-methyl-4-tetrahydropyridine (MPTP)-treated monkey has been used to model the loss of striatal dopamine (DA) innervation and motor abnormalities that typify Parkinson’s disease. However, we and others (Fernandez-Ruiz et al., 1995; Schneider and Kovelowski, 1990; Taylor et al., 1990) have noted that in monkeys exposed to low doses of MPTP such that they retain overtly normal motor faculties (termed motor-asymptomatic), there are cognitive deficits on tasks requiring normal prefrontal cortex function for over 12 months after treatment. These cognitive deficits are similar to those observed at an early stage in Parkinson’s disease (Dubois and Pillon, 1997; Lees and Smith, 1983). Paralleling the observations in the MPTP-treated monkey, a group of patients who inadvertently ingested MPTP were found to have a pattern of cognitive changes similar to those in patients with idiopathic Parkinson's disease (Stern et al., 1990). Furthermore, each of these MPTP-exposed patients, while having some subtle parkinsonian features, had in no case sufficient motor signs for a diagnosis of Parkinson’s disease, consistent with the hypothesis that cognitive changes can occur in the presence of few, or none, of the motor manifestations of Parkinsonism (Stern et al., 1990).
Prefrontal cortex activity and function is regulated by the action of several neuromodulators within the region and also by neuronal connections with other regions (Alexander et al., 1986; Fuster, 2008). Notably, the primate dorsolateral prefrontal cortex (DLPFC, area 46), which has a particularly important role in executive functions, receives a relatively dense dopaminergic input (Berger et al., 1991; Lewis and Sesack, 1997; Williams and Goldman-Rakic, 1998), and dysregulation of DA signaling in this region has been implicated in cognitive deficits (Brozoski et al., 1979; Jentsch et al., 1999) and in a variety of neuropsychiatric disorders (Carlsson et al., 2001; Goldman-Rakic, 1995; Schultz, 2002). Several studies have highlighted the need for DA-glutamate coactivation for normal prefrontal cortex function (Jay, 2003; Miller, 2000; Tseng and O'Donnell, 2004; Volk and Lewis, 2010). However, there is not a thorough understanding of the basis of DA-glutamate interactions that control the activity of pyramidal cells, which are the principal output neurons of the prefrontal cortex.
Dendritic spines are as the postsynaptic location for the vast majority of excitatory synapses, and as spines can change in shape, size and number over time, they play an important role in neural plasticity (Blanpied and Ehlers, 2004; Calabrese et al., 2006). Dendritic spines are the most common targets of dopaminergic terminals in the DLPFC, where they invariably form part of a three-way synaptic complex in which the dendritic spine of a pyramidal (glutamatergic) neuron is the target of both a DA-positive symmetric and an unlabeled asymmetric (excitatory) bouton (Goldman-Rakic et al., 1989; Smiley et al., 1992). This arrangement allows for strategic dopaminergic modulation of the overall excitability of cortical projection neurons by altering local spine responses to excitatory inputs. We propose that in the primate prefrontal cortex, dopaminergic control of glutamate transmission can occur by regulating the number of excitatory synapses on spines of pyramidal output neurons.
Our previous studies demonstrated that repeated phencyclidine (PCP) treatment of adult male monkeys, using a paradigm that causes cognitive deficits and decreased DA tone in the DLPFC, leads to significant reduction in the number of asymmetric spine synapses in layer II/III and layer V of DLPFC (Elsworth et al., 2011). These data suggest that dopaminergic tone is necessary for the maintenance of asymmetric spine synapses in DLPFC. However, a more critical test of this hypothesis is to examine the relationship between DA levels and asymmetric synapse number in DLPFC of MPTP-treated monkeys, as this drug has a more specific and lasting effect on DA neurons compared with PCP.
Thus, in the current study we examined the DLPFC of motor-asymptomatic MPTP-treated monkeys, to determine whether a significant loss of both DA concentration and the number of asymmetric spine synapses occurs. Such changes might be responsible for, or contribute to, the impairment in cognitive function that occurs in this model and in Parkinson’s disease.
Method
Animal treatment and tissue sampling
Young adult male African green monkeys (Chlorocebus sabaeus) in this study were housed and studied at the St Kitts Biomedical Research Foundation animal facility (AAALAC-accredited, St. Kitts, West Indies). Experiments were approved by the institutional animal care and use committees for Yale University and for St. Kitts Biomedical Research Foundation. MPTP-treated monkeys were injected intramuscularly with 2.25 mg/kg of the drug (Sigma-Aldrich, St Louis, MO) given in divided doses over 5 consecutive days (Taylor et al., 1990). It is well known that after exposure to MPTP, individual monkeys will exhibit different degrees of striatal DA loss and motor abnormalities (reviewed in Fox and Brotchie, 2010). Thus, although the main focus of this study is MPTP-treated monkeys that are motor asymptomatic, animals with more motor parkinsonian signs are included, to gauge whether there is a correspondence between the impact of MPTP on DLPFC (assessed by DA levels and asymmetric spine synapse number) and its effect on striatum (assessed by DA levels and motor performance). The extent of parkinsonian motor deficits correlates strongly with striatal DA concentration (Elsworth et al., 2000), and motor behavior is routinely measured at the animal facility following MPTP treatment in order to assign animals to specific studies. Cognitive deficits were not measured in the current study as it has been well established that they occur following MPTP (see Introduction), and because the relationship between DA and asymmetric synapse number in DLPFC was the focus of this study.
The motor behavior of MPTP-treated monkeys in the month after treatment was scored by blinded, trained observers using a quantitative time-sampling method (Taylor et al., 1994, 1997) and monkeys were assigned to a motor severity category, either asymptomatic or symptomatic (those with moderate/severe motor abnormalities). Monkeys were euthanized by an injection of sodium pentobarbital given at 1 month to 1 year after MPTP; the interval did not correlate significantly with DA levels or asymmetric synapse number, either within the motor behavior subgroups or with all MPTP-treated animals. Brains were perfused with saline containing heparin (1.0 liter with 1000 units) followed by a fixative (1.5–2 liters) consisting of 4% paraformaldehyde and 0.1% glutaraldehyde (prepared from catalog number 16300, Electron Microscopy Sciences, Hatfield, PA) in 0.1 M phosphate buffer (pH 7.4). Subsequently, brains were stored overnight in glutaraldehyde-free fixative, and then transferred to phosphate buffer containing 0.1% sodium azide. In some instances (for 2 controls and 2 MPTP-treated monkeys that were processed together) brains were not fixed during perfusion; instead coronal sections were post-fixed at refrigerator temperature in a series of increasing concentrations of fixative, each for at least 4 hours, starting with 1% paraformaldehyde with 0.05% glutaraldehyde, followed by 2% paraformaldehyde with 0.1% glutaraldehyde, 3% paraformaldehyde with 0.15% glutaraldehyde, 4% paraformaldehyde with 0.2% glutaraldehyde and finally 4% paraformaldehyde with 1% glutaraldehyde. We had previously tested variations of the post-fixation procedure in order to optimize it, based on tissue fixation and morphology at the electron microscope (EM) level, and to confirm that treatments that alter synapse number produced the same quantitative effect with both fixation methods. The MPTP loss measured in post-fixed tissue differed by less than 1% from that found in perfusion-fixed tissue.
Number of asymmetric synapses
The numbers of asymmetric spine synapses in layer II/III of DLPFC and entorhinal cortex, and layer V of DLPFC, were calculated using an unbiased electron microscopic stereological approach, as published previously (Elsworth et al., 2011; Leranth et al., 2008). Briefly, serial sections (200 micron) were cut in the coronal plane throughout the region on a vibratome, and systematically sorted into 10 sets. One randomly selected set of sections was post-fixed in 1% osmium tetroxide, dehydrated in 70% ethanol containing 1% uranyl acetate and flat embedded in Durcupan (Electron Microscopy Sciences, Fort Washington, PA). The volume of sampling areas was estimated using the Cavalieri Estimator module of the Stereo Investigator™ system (MicroBrightField Inc, Villiston, VT). The boundaries of Walker’s area 46 were determined according to the description of Leranth et al. (2008). Thereafter, 20 sampling sites for electron microscopic analysis were localized in the sampling area using a systematic-random approach, as described earlier (Leranth et al., 2008). Blocks were assembled for ultracutting, trimmed, and approximately four 75 nm thick consecutive ultrasections were cut from each sampling site. Digitized electron micrographs were taken for the physical disector at a final magnification of 11,000×. The disector technique requires picture pairs depicting identical regions in adjacent ultrasections, these identical regions being identified by landmarks, such as myelinated fibers, which do not change significantly between adjacent ultrasections due to their size. Prior to synapse counting, the pictures were coded for blind analysis. This sampling technique provided 20 dissectors for each region. Asymmetric spine synapses were counted according to the rules of the disector technique (Leranth et al., 2008) within an unbiased counting frame superimposed onto each electron micrograph. Synapsing spines were identified by the presence of postsynaptic densities, as well as by the absence of mitochondria, microtubules, and synaptic vesicles. The average volumetric density (synapse/µm3) of spine synapses was then determined by dividing the sum of spine synapses counted in all samples taken from that particular sampling area by the disector volume. The volumetric density of spine synapses was multiplied by the volume of the sampling area, determined earlier, to arrive at the total number of spine synapses. The number of spine synapses was calculated independently by two different investigators, and the results were cross-checked to preclude systematic analytical errors.
DA assay
DA concentration was measured in tissue dissected from the DLPFC and entorhinal cortex. The method used for determination of DA concentration is described in detail elsewhere (Morrow et al., 2011). Briefly, frozen tissues were first sonicated in cold perchloric acid containing dihydroxybenzylamine as internal standard. After centrifugation, catechols in the supernatant were adsorbed on an alumina column at pH 8.2, then eluted in dilute acid and finally separated by HPLC. DA was detected electrochemically, and quantified with respect to internal and external standards. The centrifuged tissue pellet was digested with sodium hydroxide, and its protein concentration measured using the Lowry method, so that DA concentration in tissue samples could be expressed as ng per mg protein. Some tissues for HPLC analysis used in this study were collected from monkeys involved in other projects over the past 10 years. These tissues were kept frozen at −70 degrees until assay, and the tissue content of DA was analyzed within a few months of collection. We have determined though that such samples are stable for many years when frozen at this temperature.
Statistics
Data were analyzed by 1-way or 2-way ANOVA, or by unpaired 2-tailed t-test, as appropriate, with p< 0.05 being regarded as significant (Prism 5.0d, GraphPad Software, La Jolla, CA). Variance in group means are given as ± standard error of the mean. Values of DA in DLPFC were log-transformed for statistical analysis as Bartlett’s test was statistically significant when using the raw data, but not when transformed.
Results
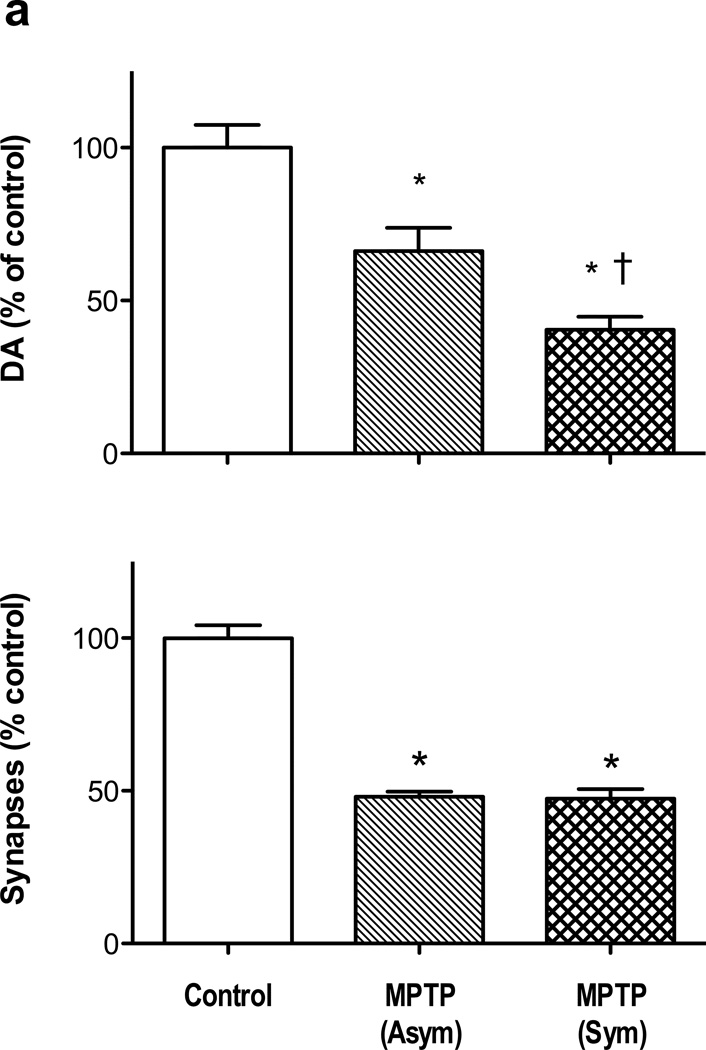
MPTP reduced the DA concentration in DLPFC in both motor severity groups. 1-way ANOVA revealed that there was a significant difference among the means [F(2,52) = 27.3, p< 0.0001], and Newman-Keul’s test indicated a significant difference of each MPTP group from controls [asymptomatic, p < 0.05; symptomatic < 0.001], and a difference between the MPTP-treated groups [p > 0.01] (Fig 1a). The extent of DA depletion in DLPFC was compared with the loss of DA in the striatal sub-region that is most affected by this regimen MPTP treatment in this species of monkey, the dorsolateral caudate nucleus (Elsworth et al., 2000). The asymptomatic motor group exhibited a 34% loss of DA concentration in DLPFC, compared with 95% loss of DA in dorsolateral caudate nucleus in the same animals, while the symptomatic motor group incurred a 59% loss of DA concentration in DLPFC compared with a over a 99% loss of DA in dorsolateral caudate nucleus in the same animals.
Figure 1.
Loss of DA concentration in DLPFC (panel a) and asymmetric synapses in layer II/III (panel b) of DLPFC in MPTP monkeys. * indicates significant difference from untreated controls, and † indicates a significant difference between MPTP-treated groups (see text for statistics). Numbers of animals for DA analysis: untreated 32, MPTP (Asym) 8, MPTP (Sym) 15. Number of animals for synapse counts: untreated 7, MPTP (Asym) 3, MPTP (Sym) 3. Data are expressed as percent of control values in DLPFC (DA, 0.74± 0.06 ng/mg protein; asymmetric synapses in layer II/III, 26.0 ± 1.1 × 109). Abbreviations: Asym, motor-asymptomatic; Sym, moderate to severe motor abnormalities.
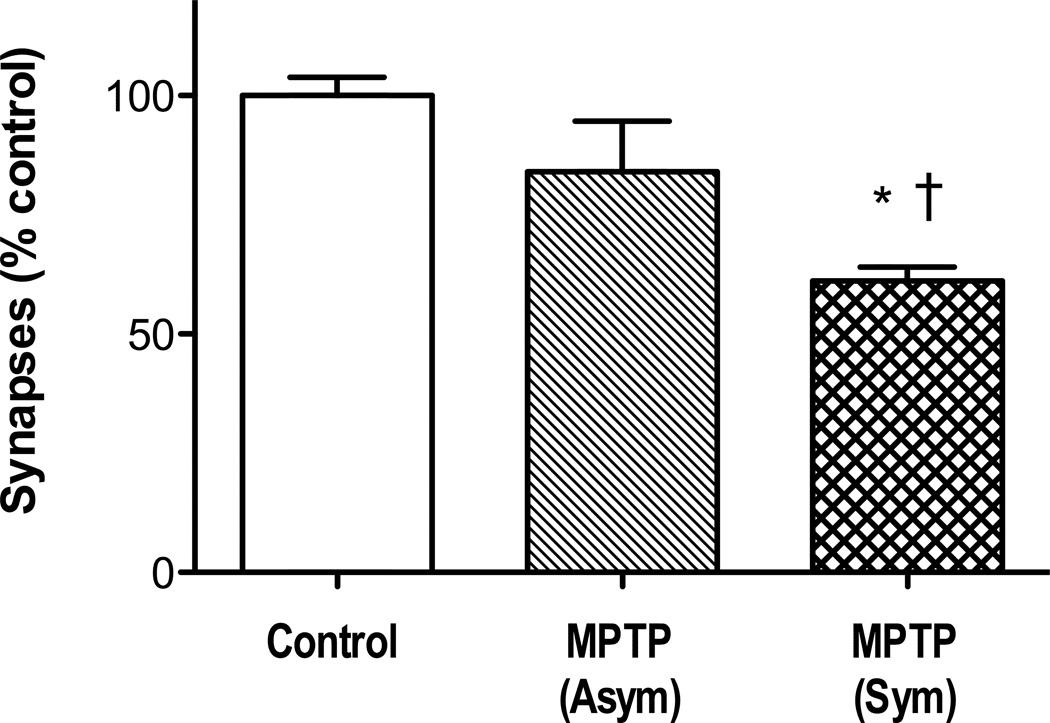
The number of asymmetric spine synapses in layer II/III of DLPFC from control and MPTP-treated monkeys were compared by 1-way ANOVA. The analysis showed that there was a significant difference among the means [F(2,10) = 53.0, p< 0.0001], and Newman-Keul’s test indicated a significant difference for each MPTP group from controls [p < 0.001], and no difference between the MPTP-treated groups (Fig 1b). Representative electron micrographs of DLPFC from control and MPTP-treated monkeys are shown in Figs 2a and 2b. The impact of MPTP on asymmetric spine synapses was different in layer V of DLPFC compared with layer II/III (Fig 3). Thus, 1-way ANOVA for number of synapses in layer V of control and MPTP groups was significant [F(2,9) = 11.7, p<0.005], but Newman-Keul’s test revealed that only the symptomatic MPTP monkeys had a significant loss of asymmetric synapses compared with controls (p<0.01). In addition, there was a significant difference in the number of asymmetric spine synapses in layer V between the asymptomatic and symptomatic MPTP groups (p<0.05).
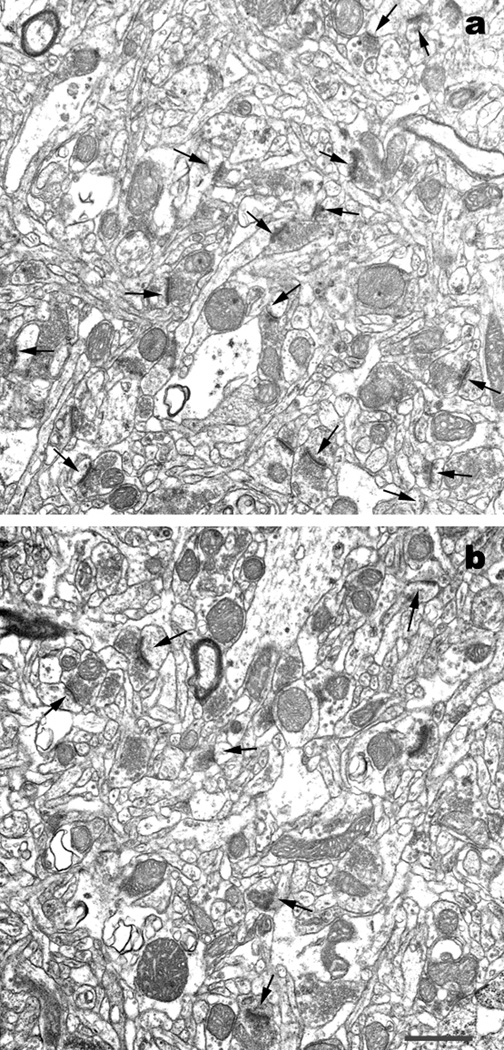
Figure 2.
EM image showing loss of asymmetric spine synapses (arrows) in DLPFC of an MPTP-treated monkey (panel b) compared with a control monkey (panel a). Scale bar represents 1 micrometer.
Figure 3.
Loss of asymmetric spine synapses in layer V of DLPFC in motor-symptomatic, but not motor-asymptomatic, MPTP monkeys. * indicates significant difference from untreated controls, and † indicates a significant difference between MPTP-treated groups (see text for statistics). Number of animals: untreated 6, MPTP (Asym) 3, MPTP (Sym) 3. Data are expressed as percent of control values for layer V of DLPFC (27.0 ± 1.0 × 109). Abbreviations: as in Figure 1.
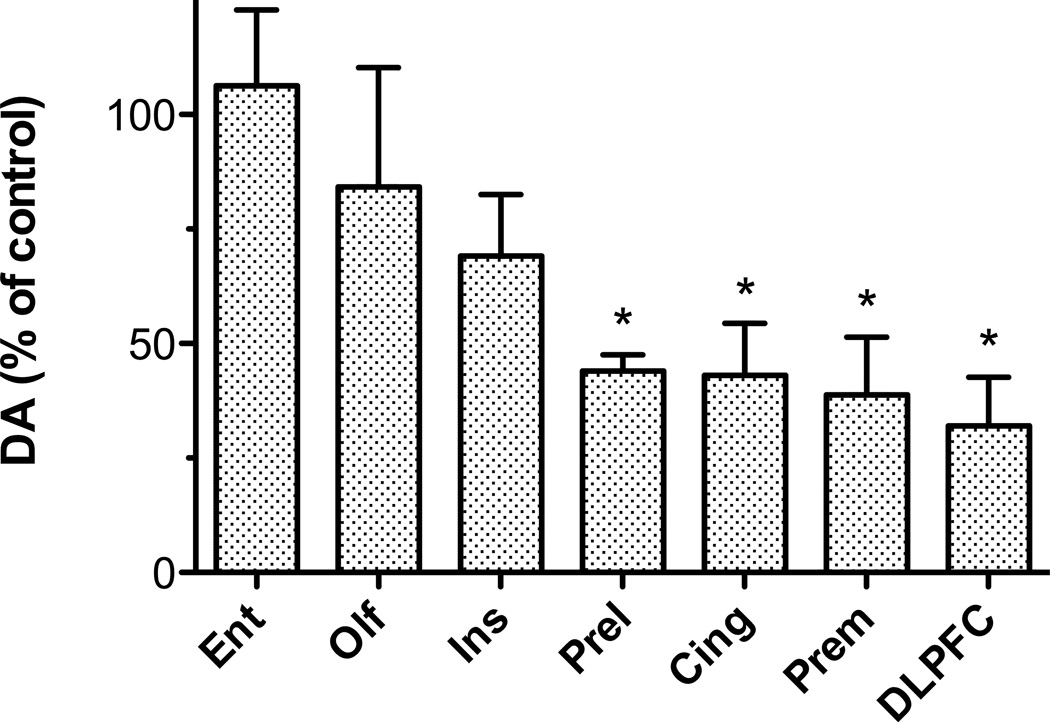
In view of the significant MPTP-induced changes in DA concentration and asymmetric synapse number observed in DLPFC, it was decided to analyze a control brain region in order to assess whether there were changes in asymmetric synapse number in a region with no MPTP-induced change in DA level. The entorhinal cortex was selected for analysis, as earlier unpublished data (Fig 4) indicated that this region was unaffected by MPTP.
Figure 4.
Differential loss of DA in cortical regions of MPTP-treated monkeys. Data are from a study with 4 MPTP-treated and 4 control monkeys. A 2-way ANOVA of DA values was significant for drug treatment (i.e., mean control and MPTP levels for each region) [F(1, 42) = 4.6, p < 0.05] and region [F(6,42) = 11.8, p < 0.0001]. The effect of MPTP in each region was subsequently analyzed by 2-tailed unpaired t-tests (* indicates significance at between p< 0.05 and p< 0.01). Abbreviations: Ento, entorhinal cortex; Olf, olfactory cortex; Ins, insular cortex; Prel, prelimbic cortex; Cing, cingulate cortex; Prem, premotor cortex.
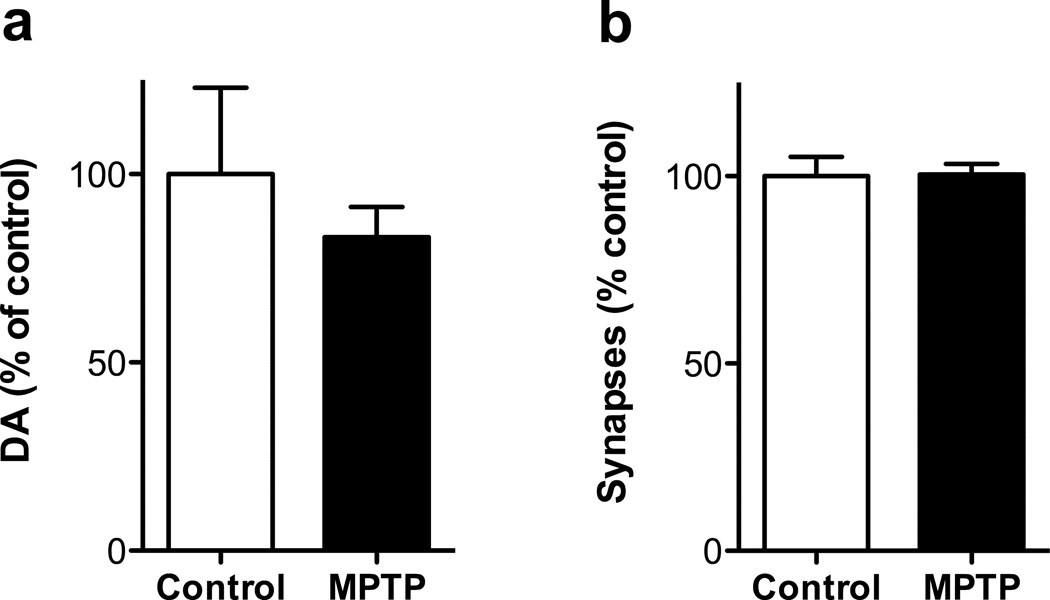
DA concentration in additional samples of entorhinal cortex from MPTP-treated monkeys and controls were analyzed (Fig 5a), which confirmed the lack of effect of the drug on DA in this region [t(16) = 0.83]. No significant effect of MPTP was detected on asymmetric synapse number (Fig 5b) [t(8) = 0.06]. Thus, in entorhinal cortex, a region in which MPTP does not induce a loss of DA concentration, there was no MPTP-induced loss of asymmetric spine synapses.
Figure 5.
No loss of DA concentration (panel a) or asymmetric synapses (panel b) in entorhinal cortex of MPTP-treated. Numbers of animals for DA analysis: untreated 7, MPTP 11. Number of animals for synapse counts: untreated 7, MPTP 3. Data are expressed as percent of control values in DLPFC (DA, 0.30 ± 0.07 ng/mg protein; asymmetric synapses, 36.1 ± 1.9 × 109). Abbreviations: as in Fig 1.
Discussion
While many studies have shown that the dopaminergic input to striatum and to some non-striatal regions are susceptible to MPTP-induced toxicity, the current data demonstrate that the dopaminergic innervation to the DLPFC, a region critical for cognitive control, is also impacted by the protoxin. There is no previous report of a significant MPTP-induced DA loss in the DPLFC, although Scatton et al. (1983) found DA depletion in frontal cortical regions in Parkinson’s disease. Furthermore, this study revealed that the number of asymmetric (glutamatergic) spine synapses was markedly reduced in layer II/III of the DLPFC by MPTP treatment. As signal transmission through cortical pyramidal (glutamatergic) neurons is critical for cognitive function (Miller, 2000), the current data support the hypothesis that DA is an important regulator of asymmetric spine synapse number in DLPFC, and that this property of DA contributes its role in prefrontal cortex-dependent cognition.
The loss of DA induced by MPTP was less in DLPFC than in dorsolateral caudate nucleus, the striatal sub-region that is most impacted by the toxicity. Thus, in those monkeys with asymptomatic motor Parkinsonism, the decrease in DA content in DLPFC was about 34% of control, while the dorsolateral caudate nucleus loss in these monkeys was 95%. The reason for the difference in vulnerability of these dopaminergic projections to MPTP corresponds to their different origins. The dopaminergic innervation of DLPFC arises primarily from the A9 cells dorsal to the SN pars compacta (Williams and Goldman-Rakic, 1998), a population that is less susceptible to MPTP than the more ventrally located DA neurons and which are mainly responsible for innervating the striatum (German et al., 1988). An important distinction between the populations of DA neurons innervating the DLPFC and striatum is the extent of loss that can be incurred before functional consequences are invoked. Thus, it is well known that the motor signs of Parkinsonism do not arise in animals until about 90–95% loss of striatal DA concentration occurs because compensatory mechanisms in the basal ganglia circuitry can maintain extracellular DA levels in the normal range until this threshold is crossed (Elsworth et al., 2000; Zigmond et al., 1989). In contrast, only moderate loss of DA terminals in prefrontal cortex is sufficient to reduce extracellular DA concentrations in this brain region (Bean and Roth, 1991; Venator et al., 1999). Furthermore, it appears that approximately 50% loss of tissue DA content in prefrontal cortex of rats or monkeys is sufficient to impair performance of behaviors dependent on the prefrontal cortex (Clinton et al., 2006; Roberts et al., 1994; Stam et al., 1989). Even a partial loss of DA tone in the prefrontal cortex (rather than a lesion of DA neurons innervating the prefrontal cortex) can induce cognitive dysfunction. Thus, following repeated PCP treatment there is a reduction in DA turnover (30–50%) in the DLPFC of monkeys and in DA turnover (25–35%) and efflux (40%) in the prefrontal cortex of rats, with these decreases being associated with deficits in performance of prefrontal cortex-dependent behavioral tasks (Jentsch et al., 1998; Jentsch et al., 1997a; Jentsch et al., 1997b). These findings indicate that a moderate loss of DA in the prefrontal cortex affects the cognitive functions of that region, whereas a moderate loss of striatal DA concentration does not overtly impact motor performance. This conclusion provides a likely explanation for deficits observed MPTP-treated monkeys performing prefrontal cortex-dependent cognitive tasks while displaying no or only mild motor abnormalities.
Previous data have identified a loss of asymmetric synapses on medium spiny neurons in striatum following a lesion of the nigrostriatal DA pathway (Day et al., 2006; Ingham et al., 1998). The present data are the first to associate DA with regulation of the number of asymmetric synapses on spines of pyramidal neurons in cortex, although it is known that damage to the dopaminergic innervation of rat prefrontal cortex results in a loss of dendritic spines (Wang and Deutch, 2008). With a 90% or greater decrease in striatal DA concentration induced by 6-hydroxydopamine in the rat, the loss of asymmetric synapses in striatum was estimated to be 19% (Ingham et al., 1998), or greater than 50% selectively in the striatopallidal projecting population of striatal neurons (Day et al., 2006). It is interesting that in the present study we obtained an approximate 50% loss of asymmetric synapses with far less depletion of DA than in the studies linking asymmetric synapses and DA loss in striatum. The reason for the discrepancy between DLPFC and striatum in terms of the extent of asymmetric synapse loss that is induced in response to a DA lesion could be linked to the apparent lack of equivalent compensatory mechanisms in DA neurons innervating DLPFC (see above paragraph), or a possible alternative explanation is that asymmetric synapses in DLPFC are more sensitive than those in striatum to synaptic DA levels.
A decrease in DA neurotransmission in DLPFC concomitant with a loss of asymmetric synapses and cognitive dysfunction in the motor-asymptomatic MPTP-treated monkey is reminiscent of our recent observations following PCP administration in the monkey (Elsworth et al., 2011). It is noteworthy that the parallel between the 2 models extends to the effect of the drugs on laminar distribution of spine synapses, as in both cases there was a greater loss in layer II/III than in layer V. The proposed relationship between a reduction in DA-mediated signaling and loss of asymmetric synapses in DLPFC is also supported by 2 separate studies in aged monkeys; the first demonstrating a decrease in DA concentration in the prefrontal cortex (Goldman-Rakic and Brown, 1981) and the second showing a loss of asymmetric synapses in layer II/III of DLPFC that strongly correlated with degree of impairment on a prefrontal cortex-dependent cognitive task (Peters et al., 2008). Thus, these data cited above from PCP-treated monkeys and aged monkeys support the present results, and strengthen the hypothesis that DA is a regulator of asymmetric spine synapse number in DLPFC. Thus, the motor-asymptomatic MPTP-treated monkey presents an ideal opportunity to test whether strategies that reverse DA loss specifically in DLPFC will restore both synapse number in that region and cognitive performance. Such research could provide new treatments for the cognitive deficits in PD and possibly also the cognitive decline associated with aging.
Acknowledgements
This work was supported by NIH grants NS064129 from NINDS to JDE and MH57483 from NIMH to RHR. We thank Feng-Pei Chen and Klara Szigeti-Buck for their excellent technical work at Yale University, and the following members of the St Kitts Biomedical Research Foundation staff for their invaluable assistance in animal treatments and excellent care of the animals: Alexis Nisbett, Dr. Milton C. Whittaker, Ernell Nisbett, and Clive Wilson.
Footnotes
Statement of Interest
None
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Bean AJ, Roth RH. Effects of haloperidol administration on in vivo extracellular dopamine in striatum and prefrontal cortex after partial dopamine lesions. Brain Research. 1991;549:155–158. doi: 10.1016/0006-8993(91)90613-z. [DOI] [PubMed] [Google Scholar]
- Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends in Neurosciences. 1991;14:21–27. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Ehlers MD. Microanatomy of dendritic spines: emerging principles of synaptic pathology in psychiatric and neurological disease. Biological Psychiatry. 2004;55:1121–1127. doi: 10.1016/j.biopsych.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Calabrese B, Wilson MS, Halpain S. Development and regulation of dendritic spine synapses. Physiology (Bethesda) 2006;21:38–47. doi: 10.1152/physiol.00042.2005. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Holm-Waters S, Tedroff J, et al. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annual Review of Pharmacology and Toxicology. 2001;41:237–260. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- Clinton SM, Sucharski IL, Finlay JM. Desipramine attenuates working memory impairments induced by partial loss of catecholamines in the rat medial prefrontal cortex. Psychopharmacology. 2006;183:404–412. doi: 10.1007/s00213-005-0221-2. [DOI] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nature Neuroscience. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- Dubois B, Pillon B. Cognitive deficits in Parkinson's disease. Journal of Neurology. 1997;244:2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Hajszan T, Leranth C, Roth RH. Loss of asymmetric spine synapses in dorsolateral prefrontal cortex of cognitively impaired phencyclidine-treated monkeys. Int J Neuropsychopharmacol. 2011:1–5. doi: 10.1017/S1461145711000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsworth JD, Taylor JR, Sladek JR, Jr, Collier TJ, et al. Striatal dopaminergic correlates of stable parkinsonism and degree of recovery in old-world primates one year after MPTP treatment. Neuroscience. 2000;95:399–408. doi: 10.1016/s0306-4522(99)00437-6. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Doudet DJ, Aigner TG. Long-term cognitive impairment in MPTP-treated rhesus monkeys. Neuroreport. 1995;7:102–104. [PubMed] [Google Scholar]
- Fox SH, Brotchie JM. The MPTP-lesioned non-human primate models of Parkinson's disease. Past, present, and future. Progress in Brain Research. 2010;184:133–157. doi: 10.1016/S0079-6123(10)84007-5. [DOI] [PubMed] [Google Scholar]
- Fuster J. The Prefrontal Cortex. London: Academic Press; 2008. [Google Scholar]
- German DC, Dubach M, Askari S, Speciale SG, et al. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonian syndrome in Macaca fascicularis: which midbrain dopaminergic neurons are lost? Neuroscience. 1988;24:161–174. doi: 10.1016/0306-4522(88)90320-x. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Brown RM. Regional changes of monoamines in cerebral cortex and subcortical structures of aging rhesus monkeys. Neuroscience. 1981;6:177–187. doi: 10.1016/0306-4522(81)90053-1. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Leranth C, Williams SM, Mons N, et al. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:9015–9019. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, Taggart P, Arbuthnott GW. Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. Journal of Neuroscience. 1998;18:4732–4743. doi: 10.1523/JNEUROSCI.18-12-04732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Progress in Neurobiology. 2003;69:375–390. doi: 10.1016/s0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Dazzi L, Chhatwal JP, Verrico CD, et al. Reduced prefrontal cortical dopamine, but not acetylcholine, release in vivo after repeated, intermittent phencyclidine administration to rats. Neuroscience Letters. 1998;258:175–178. doi: 10.1016/s0304-3940(98)00879-9. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Redmond DE, Jr, Elsworth JD, Taylor JR, et al. Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science. 1997a;277:953–955. doi: 10.1126/science.277.5328.953. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR, Elsworth JD, Redmond DE, Jr, et al. Altered frontal cortical dopaminergic transmission in monkeys after subchronic phencyclidine exposure: involvement in frontostriatal cognitive deficits. Neuroscience. 1999;90:823–832. doi: 10.1016/s0306-4522(98)00481-3. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Tran A, Le D, Youngren KD, et al. Subchronic phencyclidine administration reduces mesoprefrontal dopamine utilization and impairs prefrontal cortical-dependent cognition in the rat. Neuropsychopharmacology. 1997b;17:92–99. doi: 10.1016/S0893-133X(97)00034-1. [DOI] [PubMed] [Google Scholar]
- Lees AJ, Smith E. Cognitive deficits in the early stages of Parkinson's disease. Brain. 1983;106:257–270. doi: 10.1093/brain/106.2.257. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, Szigeti-Buck K, Bober J, et al. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14187–14191. doi: 10.1073/pnas.0806139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D, Sesack S. Dopamine Systems in the Primate Brain. In: Bloom FE, Bjorklund A, Hokfelt T, editors. The Primate Nervous System. Amsterdam: Elsevier; 1997. pp. 263–375. [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Roth RH, Redmond DE, Elsworth JD. Impact of methamphetamine on dopamine neurons in primates is dependent on age: implications for development of Parkinson's disease. Neuroscience. 2011;189:277–285. doi: 10.1016/j.neuroscience.2011.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Sethares C, Luebke JI. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience. 2008;152:970–981. doi: 10.1016/j.neuroscience.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AC, DeSalvia MA, Wilkinson LS, Collins P, et al. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analogue of the Wisconsin Card Sort Test: Possible interactions with subcortical dopamine. Journal of Neuroscience. 1994;14:2531–2544. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scatton B, Javoy-Agid F, Rouquier L, Dubois B, et al. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson's disease. Brain Research. 1983;275:321–328. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Kovelowski CJ. Chronic exposure to low doses of MPTP: I. Cognitive deficits in motor asymptomatic monkeys. Brain Research. 1990;519:122–128. doi: 10.1016/0006-8993(90)90069-n. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Williams SM, Szigeti K, Goldman-Rakic PS. Light and electron microscopic characterization of dopamine-immunoreactive axons in human cerebral cortex. Journal of Comparative Neurology. 1992;321:325–335. doi: 10.1002/cne.903210302. [DOI] [PubMed] [Google Scholar]
- Stam CJ, de Bruin JP, van Haelst AM, van der Gugten J, et al. Influence of the mesocortical dopaminergic system on activity, food hoarding, social-agonistic behavior, and spatial delayed alternation in male rats. Behavioral Neuroscience. 1989;103:24–35. doi: 10.1037//0735-7044.103.1.24. [DOI] [PubMed] [Google Scholar]
- Stern Y, Tetrud JW, Martin WR, Kutner SJ, et al. Cognitive change following MPTP exposure. Neurology. 1990;40:261–264. doi: 10.1212/wnl.40.2.261. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Elsworth JD, Roth RH, Sladek JR, Jr, et al. Cognitive and motor deficits in the acquisition of an object retrieval/detour task in MPTP-treated monkeys. Brain. 1990;113:617–637. doi: 10.1093/brain/113.3.617. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Elsworth JD, Roth RH, Sladek JR, Jr, et al. Behavioral effects of MPTP administration in the vervet monkey: a primate model of Parkinson's disease. In: Woodruff ML, Nonneman AJ, editors. Toxin-induced models of neurological disorders. New York: Plenum Press; 1994. pp. 139–174. [Google Scholar]
- Taylor JR, Elsworth JD, Roth RH, Sladek JR, Jr, et al. Severe long-term 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in the vervet monkey (Cercopithecus aethiops sabaeus) Neuroscience. 1997;81:745–755. doi: 10.1016/s0306-4522(97)00214-5. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. Journal of Neuroscience. 2004;24:5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venator DK, Lewis DA, Finlay JM. Effects of partial dopamine loss in the medial prefrontal cortex on local baseline and stress-evoked extracellular dopamine concentrations. Neuroscience. 1999;93:497–505. doi: 10.1016/s0306-4522(99)00131-1. [DOI] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. Prefrontal cortical circuits in schizophrenia. Curr Top Behav Neurosci. 2010;4:485–508. doi: 10.1007/7854_2010_44. [DOI] [PubMed] [Google Scholar]
- Wang HD, Deutch AY. Dopamine depletion of the prefrontal cortex induces dendritic spine loss: reversal by atypical antipsychotic drug treatment. Neuropsychopharmacology. 2008;33:1276–1286. doi: 10.1038/sj.npp.1301521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cerebral Cortex. 1998;8:321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Berger TW, Grace AA, Stricker EM. Compensatory responses to nigrostriatal bundle injury. Studies with 6-hydroxydopamine in an animal model of parkinsonism. Molecular and Chemical Neuropathology. 1989;10:185–200. doi: 10.1007/BF03159728. [DOI] [PubMed] [Google Scholar]