Abstract
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) causes severe hemorrhagic necrotizing pneumonia associated with high mortality. Exotoxins have been implicated in the pathogenesis of this infection; however, the cellular mechanisms responsible remain largely undefined. Because platelet-neutrophil aggregates (PNAs) can dysregulate inflammatory responses and contribute to tissue destruction, we investigated whether exotoxins from MRSA could stimulate formation of PNAs in human whole blood. Strong PNA formation was stimulated by toxins from stationary phase but not log phase CA-MRSA, and α-hemolysin was singularly identified as the mediator of this activity. MRSA exotoxins also caused neutrophil (polymorphonuclear leukocyte) activation, as measured by increased CD11b expression, although platelet binding was not driven by this mechanism; rather, α-hemolysin–induced PNA formation was solely platelet P-selectin dependent. These findings suggest a role for S. aureus α-hemolysin–induced PNA formation in alveolar capillary destruction in hemorrhagic/necrotizing pneumonia caused by CA-MRSA and offer novel targets for intervention.
Keywords: Staphylococcus aureus, α-hemolysin, platelet-neutrophil aggregates, necrotizing pneumonia
Staphylococcus aureus has long been a major cause of healthcare-associated pneumonia. Recently, hemorrhagic necrotizing pneumonia caused by community-associated methicillin-resistant S. aureus (CA-MRSA) has emerged worldwide [1, 2] and carries a mortality rate of 50%–80%. Intra-alveolar hemorrhage and extensive lung necrosis characterize this infection, and when present, these features portend poor outcomes [3].
Massive polymorphonuclear leukocyte (PMNL) influx into lung parenchyma is also characteristic of S. aureus pneumonia [4]. Activated, hyperadherent PMNLs and inappropriate triggering of degranulation can damage or destroy the microvascular endothelium and adjacent tissues—a process mediated by the neutrophil complement receptor type III (CR3; CD11b/CD18) [5]. Platelets also mediate inflammatory reactions [6, 7], and platelet-neutrophil aggregates (PNAs) contribute to organ failure [8] and soft-tissue necrosis [9, 10] in some life-threatening infections by reducing microvascular perfusion and by direct injury to the endothelium. In addition, animal studies have shown that blockade of PNA formation prevented acid- and lipopolysaccharide-induced experimental lung injury [11]. Together, these findings suggest that toxin-mediated neutrophil activation and PNA-mediated disruption/occlusion of the pulmonary microcirculation may also contribute to the pathogenesis of MRSA necrotizing pneumonia.
In the present study, we demonstrate that exotoxins from stationary phase CA-MRSA induced both PNA formation and PMNL activation in human whole blood and that these activities are independent of Panton-Valentine leukocidin (PVL). Using both a classic biochemical approach and analysis of toxin-deficient isogenic mutants, we definitively demonstrate that S. aureus α-hemolysin is the sole exotoxin responsible for PNA formation. Finally, we show that α-hemolysin–induced PNA formation is mediated by platelet P-selectin. Together, these results suggest a unique mechanism by which S. aureus α-hemolysin may contribute to alveolar capillary leakage, hemorrhage, and lung destruction during MRSA necrotizing pneumonia and offer novel targets for intervention.
MATERIALS AND METHODS
Bacterial Strains and Exotoxin Preparation
Five clinical CA-MRSA strains, previously characterized by our laboratory [12] and others [13], were used (Table 1). These strains were obtained from patients with infections of varying severity and produced variable amounts of PVL [12]. In addition, a wild-type USA300 clinical MRSA isolate called “LAC” (for Los Angeles County) and its isogenic PVL-deficient mutant (LACΔpvl) were kindly provided by Dr Frank R. DeLeo (Laboratory of Human Bacterial Pathogenesis, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Hamilton, MT) [13]. Last, CA-MRSA USA300 JE2 strain, derived from USA300 LAC and cured of 3 small plasmids, was obtained from the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA; Chantilly, VA). All CA-MRSA isolates carried the SCCmec Type IV cassette and the genes for LukS and LukF but produced variable amounts of PVL [12]. To prepare exotoxins, overnight cultures of S. aureus in Mueller-Hinton II (MHII) broth were washed, diluted to 1–3 × 106colony-forming units/mL in fresh MHII, and grown at 37°C in 5% CO2 with shaking (0.85 × g) for 4 hours (mid-log phase) or 24 hours (stationary phase). Culture supernatants (CSN) were cleared of bacteria by centrifugation and filter sterilization (0.22 μm) and stored frozen at −70°C.
Table 1.
Characteristics and Functional Activity of Methicillin-Resistant Staphylococcus aureus (MRSA) Strains Used in This Study
| Agonist | Site of Isolation | PVL Gene Probe | Hemolytic Activity, HU/mL | PNA Formation,a%, MFI ± SD | CD42b, MFI ± SD | CD11b, MFI ± SD |
|---|---|---|---|---|---|---|
| Media | NA | NA | 0 | 8.3 ± 0.3 | 0.5 ± 0.01 | 37.9 ± 9.9 |
| MRSA 3801 | Blood | + | 256 | 62.0 ± 4.4 | 3.9 ± 0.3 | 86.9 ± 2.7 |
| FPR3757 | Skin abscess | + | 540 | 90.9 ± 2.9 | 2.9 ± 0.7 | 92.3 ± 2.3 |
| MRSA 2349 | Wound | + | 128 | 38.2 ± 0.6 | 3.1 ± 0.2 | 77.6 ± 12.7 |
| MRSA 2241 | Wound | + | 128 | 65.8 ± 3.0 | 2.9 ± 0.3 | 83.0 ± 17.6 |
| MRSA 1740 | Skin abscess | − | 288 | 76.5 ± 5.7 | 2.9 ± 0.5 | 77.9 ± 7.4 |
| LAC | Skin abscess | + | 256 | 53.6 ± 2.6 | 1.71 ± 0.03 | 94.9 ± 2.3 |
| LACΔpvl | NA | − | 256 | 57.4 ± 4.5 | 1.7 ± 0.3 | 88.7 ± 2.2 |
| JE2 | NA | + | 160 | 46.7 ± 8.9 | 2.8 ± 0.02 | 124.9 ± 2.3 |
| JE2Δhla | NA | + | 30 | 12.5 ± 1.3 | 2.3 ± 0.1 | 100.6 ± 4.1 |
| JE2Δplc | NA | + | 160 | 51.9 ± 10.6 | 2.8 ± 0.6 | 117.1 ± 4.5 |
Stationary phase culture supernatants (40 µL) from the indicated strains were added to 100 µL of heparinized human blood for 10 minutes at 37°C. Hemolytic activity was determined by rabbit erythrocyte lysis assay. Platelet-neutrophil aggregate (PNA) formation and the mean fluorescence intensity (MFI) of the platelet CD42b and polymorphonuclear leukocyte CD11b markers were measured by flow cytometry, as described in Methods. All clinical isolates were kindly provided by Dr Francoise Perdreau-Remington (University of California, San Francisco) in 2003. The correlation coefficient (r2) between hemolytic activity and PNA formation percentage, hemolytic activity and CD11b MFI, and CD11b and formation percentage were 0.75, 0.08, and 0.13, respectively. Data are mean ± SD, unless otherwise indicated, and represent duplicate experiments from 4 independent blood donors.
Abbreviations: HU, hemolytic units; NA, not applicable; PVL, Panton-Valentine leukocidin.
a Defined as the percentage of CD11b+ cells that were positive for CD42b.
PNA Formation, Platelets, and PMNL Activation
Work involving humans was approved by the Boise VAMC's institutional review board (IRB) of record (the VA Puget Sound Health Care System IRB; Seattle, WA), and the Boise VAMC's Research and Development Committee. All volunteers gave written informed consent prior to participation. Peripheral blood was drawn from healthy, nonsmoking adults (3 men and 5 women; age, 20–55 years) who had not taken any prescription or over-the-counter medication in the 10 days prior to participation and had abstained from caffeine consumption 24 hours prior to the blood donation. Female participants were not pregnant and were denied oral contraceptive use. Heparinized (10 U/mL) whole blood was collected without the aid of a tourniquet, and PNA formation was assessed as previously described [9]. Briefly, blood (100 µL) was incubated at 37°C for 10 minutes with 10 µL of fluorescein isothiocyanate–conjugated anti-human CD42b (clone HIP1, BD Biosciences, San Diego, CA) to identify platelets and phycoerythrin-conjugated anti-human CD11b (clone Bear1, Beckman Coulter Immunotech, Fullerton, CA) to identify PMNLs. Blood was stimulated for an additional 5 minutes with 10 µL of either (1) Dulbecco's phosphate-buffered saline (DPBS; Sigma) supplemented to contain 2 mM calcium and 100 µM zinc (negative control), (2) 0.124 U of recombinant phospholipase C (rPLC) from Clostridium perfringens (positive control) [14], (3) native toxins from log or stationary phase CA-MRSA [12], or (4) recombinant S. aureus α-hemolysin or phosphatidylinositol-specific phospholipase C (PI-PLC; prepared as described below). It should be noted that C. perfringens PLC preferentially cleaves phosphatidylcholine moieties in cell membranes, whereas the MRSA PI-PLC targets phosphatidylinositol-containing lipids. The C. perfringens PLC is also a sphingomyelinase. After erythrocyte lysis, samples were fixed with paraformaldehyde and analyzed using a FACS Epic XL flow cytometer. Granulocytes were gated on the basis of forward- and side-scatter profiles. PNA formation was defined as the percentage of CD11b+ cells that were positive for CD42b. The relative number of platelets/PMNL was given by the CD42b mean fluorescence intensity (MFI) of these dual-positive events. PMNL activation was indicated by an increased CD11b MFI. Some experiments used isolated human platelets and neutrophils obtained as we have previously described [15]. Tests were run in triplicate, and data are expressed as the mean ± SD for 4 independent experiments, with each experiment using blood from a different donor.
Isolation and Identification of Toxin(s) Mediating PNA Formation
Stationary phase culture supernatants from the LACΔpvl was fractionated by 2 rounds of isoelectric focusing (pH 3–10). Sterile, uninoculated MHII media was similarly treated and used as a negative control. Resultant fractions were tested for their ability to induce PNA formation and/or PMNL activation as described above.
Proteins in fractions with the highest level of PNA activity were precipitated with trichloroacetic acid, resolved by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and visualized with Bio-Safe Coomassie blue (Bio-Rad), and resultant bands were excised from the gel for identification by Orbitrap liquid chromatography tandem mass spectrometry (MS/MS) (Proteomics and Mass Spectrometry Core Facility, Oklahoma State University). MS/MS profiles were analyzed using Mascot (Matrix Science, London, UK) and X! Tandem (The GPM, version 2007.01.01.1). Scaffold Proteome Software 3.0 (Proteome Software, Portland, OR) was used to validate MS/MS-based peptide and protein identifications. Peptide and protein identifications were accepted if they could be established at >80% and >99% probability, respectively, as specified by the Peptide Prophet algorithm [16]. Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony.
Plasmid Constructs
Work involving recombinant DNA was approved by the Boise VAMC's institutional biosafety committee of record (the VA Puget Sound Health Care System Recombinant DNA Committee). Cloning of the S. aureus α-hemolysin (hla) and phosphatidylinositol-specific phospholipase C (plc) genes was performed using standard molecular cloning procedures. Chromosomal DNA from S. aureus strain FPR3757 (USA 300) was used as a template for hla and plc gene amplification in a PerkinElmer GeneAmp polymerase chain reaction (PCR) System 2400 thermocycler. The hla forward (5′-CTCGAGGCAGATTCTGATATTAATATTAAAACC-3′) and hla reverse (5′-CCCTTTTTCTTCTTTACTGTTTAATTCCTAGG-3′) primers were used to amplify mature α-hemolysin, as previously described by Eichstaedt et al [17], with appended XhoI and BamHI restriction sites (underlined). The plc forward (5′-CATATGAGTGGTTGGTATCATTCAG-3′) and reverse (5′-GCATTCACTTTAATATCTATCATTATTTATTGAGCTC-3′) primers were used to amplify the plc gene, with appended NdeI and XhoI restriction sites (underlined). Following purification, hla and plc PCR products were digested and cloned into the pET14b expression vector (Invitrogen, Carlsbad, CA), containing an N-terminal His tag. Plasmid sequences were verified through a commercial sequencing service (Gene Gateway, Hayward, CA).
Recombinant Protein Expression and Purification
BL21 (DE3) bacteria harboring either the pET14b-hla or pET14b-plc plasmid were grown in LB medium containing carbenicillin (75 µg/mL). Overnight cultures were diluted 1:100 in 300 mL of fresh LB medium and incubated at 37°C. At an OD600 of 0.5, gene expression was induced with 1 mM IPTG, and cultures were grown for an additional 2.5 hours. Bacteria were harvested by centrifugation, and pellets were resuspended in BugBuster protein extraction reagent (Novagen, EMD Chemicals, Gibbstown, NJ) supplemented with protease inhibitors (1 mM AEBSF, 2.0 µg/mL aprotinin, 10 µM bestatin, 10 mM E-64, 100 μM leupeptin, and 1 μM pepstatin), benzonase nuclease (25 U/mL; Novagen), and lysozyme (1 kU/mL; Sigma). Lysates were cleared by centrifugation (13 000 × g; 20 minutes), and the resulting supernatant was loaded on a 5-mL HisTrap HP column (GE Healthcare), followed by 15 column-volumes of wash buffer (20 mM sodium phosphate, 0.5 M sodium chloride, and 40 mM imidazole, pH 7.4), and bound proteins eluted with 2 column-volumes of elution buffer (20 mM sodium phosphate, 0.5 M sodium chloride, and 500 mM imidazole). Buffer exchange into DPBS was performed on a Sephadex G-25 column. Protein concentration was determined by use of a bicinchoninic acid protein assay reagent (Pierce, Rockford, IL), and the purity of the proteins was assessed using SDS-PAGE analysis with silver staining. Recombinant proteins were filter sterilized and stored at −70°C.
Recombinant Protein Assays
α-hemolysin activity was assayed by a standard rabbit erythrocyte lysis assay [18]. Recombinant α-hemolysin contained 5120 HU/mL of activity and 0.125 mg/mL total protein. S. aureus phosphatidylinositol-specific phospholipase C (Sa-PI-PLC) activity was qualitatively assessed using a plate-based enzymatic assay described by Wei et al [19] with minor modifications. Briefly, recombinant Sa-PI-PLC (0.005–1 µg in 20 µL of 10 mM HEPES) or PI-PLC from Bacillus cereus (Sigma) was added to individual wells punched in BBL CHROMagar Listeria plates (BD Biosciences, Franklin Lakes, NJ), which contain phosphatidylinositol bisphosphate (PIP2) as substrate. After overnight incubation at 37°C, PI-PLC activity was detected as an opaque zone of insoluble diacylglycerol created by enzymatic cleavage of PIP2.
Construction of hla- and plc-Deficient MRSA
CA-MRSA USA300 JE2 strain, cured of plasmids containing tetracycline and erythromycin resistance genes, was used for construction of toxin-deficient MRSA. Standard molecular procedures were used for DNA manipulation, plasmid isolation, purification, and transformation. An in-frame gene deletion procedure for gram-positive pathogens has previously been described by our laboratory [20]. Briefly, deletion constructs targeting replacement of the entire coding sequence of either the hla or plc gene with the kanamycin antibiotic selectable marker (kan) were constructed. Regions flanking the hla or plc targets were amplified using chromosomal DNA from S. aureus strain FPR3757 and ligated to the kanamycin gene in the pMAD vector, which contains an erythromycin marker, a thermosensitive replication origin, and constitutively expressed β-galactosidase [21]. Primers for amplifying the hla upstream/downstream fragments were as follows: hlaUPf1, 5′-GAGGATCCTCCATACAAAATCCGCATCA-3′; hlaUPr1, 5′-AAATGTAATTAAACAGTAAAGAAAACAGCTGAG-3′; hlaDOWNf1, 5′-GAGTCGACTGTTTTCATTTTCATCATCCT-3′; and hlaDOWNr1, 5′-ACCATCATCAACTGGCTTACGACGTCAG-3′. Primers for amplifying the plc upstream/downstream fragments were as follows: plcUPf1, 5′-GAGGATCCAGGCTCAGAAGGGTGTATTTTGT-3′; plcUPr1, 5′-GTAAAATCATCACTACTCACCAACCCAGCTGAG -3′; plcDOWNf1, 5′-GAGTCGGACAGGATTCAATAATGATATTAAGACGAG-3′; and plcDOWNr1, 5′-AGTTAGGGCCTTTTTCTCTCGACGTCAG-3′. The restriction sites were BamHI, SalI, and PstI (underlined). Primers for kan were as follows: 5′-GAGTCGACCCCAGCGAACCATTTGAGGTGAT-3′ and 5′-GACCTACTTAACAAAATCQATGGATCTCAGCTGAG-3′, with appended SalI as a restriction site (underlined). The resulting hla and plc targeting vectors were conditioned by passage through S. aureus strain RN4220 and introduced into S. aureus JE2 by phage transduction. Transductants were selected at 30°C on tryptic soy agar in the presence of erythromycin (10 µg/mL), kanamycin 300 µg/mL, and X-Gal (50 µg/mL). Integration of the kan cassette into the JE2 chromosome at the hla or plc locus was achieved through temperature shift. Double-crossover events were identified by replica plating for susceptibility to erythromycin. PCR, restriction analysis, and Southern blot hybridization confirmed successful integration of a single targeting construct for hla and plc, yielding the mutant JE2Δhla and JE2Δplc strains. Growth curves of the mutants were compared to that of the parental JE2 strain, and α-hemolysin activity and PI-PLC activity were assayed as described above. Total secretory proteins from the parental and mutant strains were resolved in 12% SDS-PAGE gel and visualized by silver staining.
Complementation Analysis
A genetic complementation method was designed for hla in strain JE2Δhla. Briefly, the wild-type hla gene and endogenous promoter region were PCR amplified and cloned into the pMAD shuttle vector, using the following designated primers: 5′-GTCGACTAGCAATACTTTATTGTCCCATGA-3′ and (5′-CTTAAGGTACAACAGTTTGGGAAAGGTACC-3′, with appended SalI and NcoI as restriction sites. The pMAD(hla) construct was introduced into S. aureus RN4220 prior to phage transduction into JE2Δhla. PCR was used to detect hla expression in JE2Δhla /hla+ complemented transductants. Stationary phase culture supernatants from these constructs were assayed for hemolytic activity by using the rabbit erythrocyte lysis assay to verify production of functional α-hemolysin by this construct.
Statistical Analysis
Data sets were analyzed using Stata/SE (StataCorp 2003, College Station, TX). Significant differences among groups were determined by implementing a 2-tailed, unequal variance Student t test. Statistical significance was defined as a P value of < .05.
RESULTS
MRSA Exotoxin(s) Promote PNA Formation
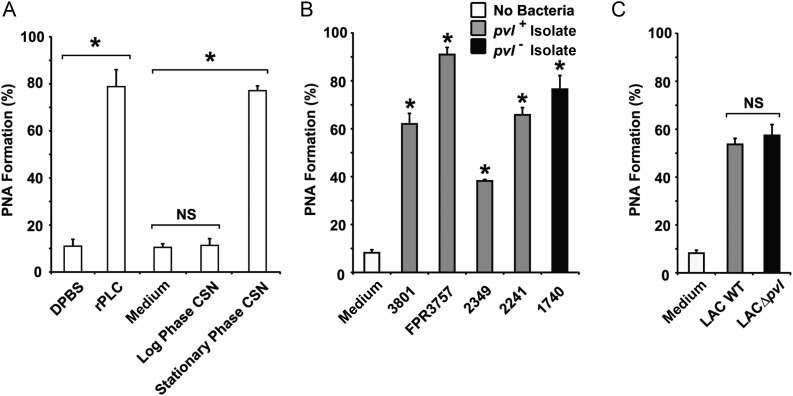
Exotoxins from log or stationary phase cultures of CA-MRSA strain FPR3757 were tested for their ability to induce PNA formation in human whole blood. The FPR3757 strain belongs to the most widespread CA-MRSA clone associated with invasive infections, USA300, and was the first such strain to undergo complete genome sequencing [22]. In negative control samples, few PMNLs had adherent platelets (mean [±SD], 9% ± 1.2% CD11b/CD42b dual-positive events; Figure 1A). A similar finding was observed in whole blood treated with log phase culture supernatants (Figure 1A). In contrast, stationary phase exotoxins induced high-level PNA aggregate formation (a mean [±SD] of 79% ± 3.2% of granulocytes were CD42b positive; Figure 1A) comparable to the known PNA agonist, C. perfringens PLC [23]. These findings demonstrate that at least 1 stationary phase–specific exotoxin from USA300 MRSA strongly elicits heterotypic platelet-neutrophil aggregation.
Figure 1.
Methicillin-resistant Staphylococcus aureus (MRSA) exotoxin(s) promote platelet-neutrophil aggregate (PNA) formation independently of Panton-Valentine Leukocidin (PVL). Formation of PNA in human whole blood was quantitated by dual-color flow cytometry. A, Blood samples (100 µL) were preincubated with phycoerythrin-CD11b and fluorescein isothiocyanate–CD42b and then stimulated with 40 µL of bacteria-free log or stationary phase culture supernatant (CSN) from MRSA strain FPR3757 or an appropriate negative control (medium). Clostridium perfringens phospholipase C (r-PLC) served as a positive control. B and C, Stationary phase CSN from various pvl +(grey bars) or a pvl – (black bar) community-acquired MRSA clinical isolates (B) or stationary phase CSN from wild-type (WT) MRSA strain LAC (grey bar) and its isogenic pvl deficient mutant (LACΔpvl; black bar. The percentage of dual-positive CD11b/CD42b polymorphonuclear leukocytes was calculated using SYSTEM II flow cytometry software. Data represent the mean (±SD) of 4 samples from 2 independent blood donors. *P < .05, by the Student t test. Abbreviation: NS, not statistically significant.
Formation of PNA Is Independent of PVL
The PNA activity of culture supernatants from 5 additional CA-MRSA clinical isolates was evaluated. These isolates produce widely variable amounts of PVL (0–796 ng/mL) [12]. All culture supernatants induced significant PNA formation, although no correlation was observed between the level of PNA activity and the presence or quantity of PVL produced (Figure 1B), suggesting that PVL is not involved in this process. This notion was confirmed by studies of stationary phase toxins from wild-type CA-MRSA strain LAC and its isogenic pvl-deficient mutant (LACΔpvl), in which comparable PNA-inducing activity was observed (Figure 1C).
Identification of the S. aureus PNA–Inducing Factor
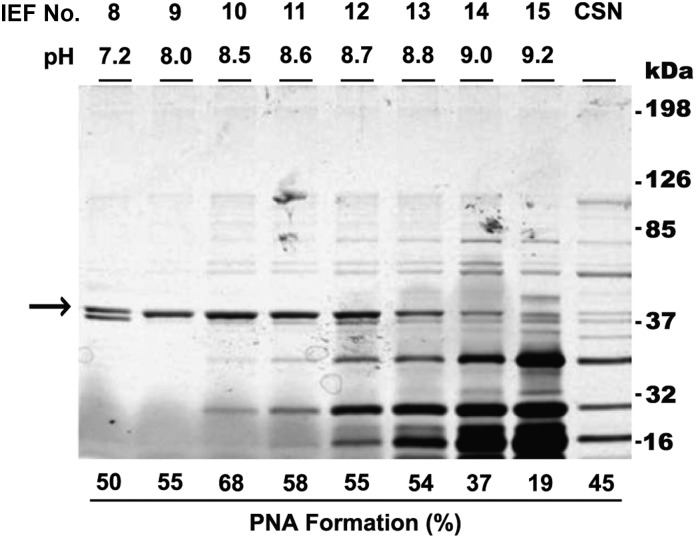
To identify the exotoxin(s) causing PNA formation, stationary phase culture supernatants from the LACΔpvl strain were fractionated by isoelectric focusing, and fractions were tested for PNA-inducing activity. Eight fractions (pH range, 7.2–9.0) induced PNA formation, with maximal PNA activity (mean [±SD], 67.9% ± 7.2%) observed in fraction 10 (pH 8.5; Figure 2). PNA activity correlated with the presence of an 37-kDa protein band (Figure 2). Mass spectrometry analysis of this band identified 2 S. aureus proteins: α-hemolysin (Sa-AH; molecular weight, 36 kDa) and phosphatidylinositol-specific phospholipase C (Sa-PI-PLC; molecular weight, 35 kDa).
Figure 2.
Presence of a 37-kDa protein band coincides with platelet-neutrophil aggregate (PNA) activity. Proteins from LACΔpvl stationary phase culture supernatant (CSN) were fractionated by isoelectric focusing (IEF), resolved by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and visualized by silver staining. Fraction numbers and their corresponding pHs are given above each lane. Unfractionated CSN from the original LACΔpvl strain is also shown. PNA activity is given below each lane, and molecular weight markers are on the right. The intensity of an approximately 37-kDa protein band (arrow) appears to correlate with PNA-inducing activity. Data are representative of 2 independent experiments.
S. aureus α-Hemolysin Promotes PNA Formation
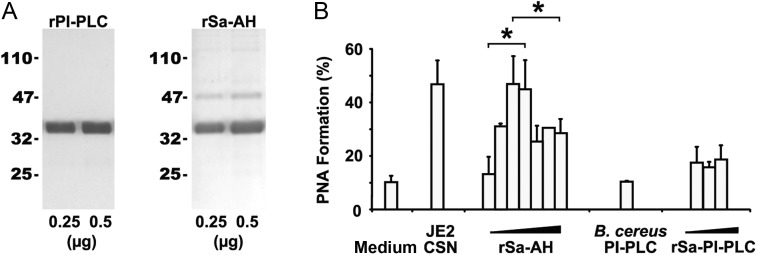
To elucidate which of these proteins stimulated PNA activity, recombinant proteins (Figure 3A) were generated. Both recombinant α-hemolysin (rSa-AH) and recombinant PI-PLC (rPI-PLC) were enzymatically active, and each was independently tested for the ability to promote PNA formation. Low amounts of rSa-AH (range, 0.015–0.125 µg) dose-dependently stimulated PNA formation (Figure 3B); higher doses (range, 2.5–5 µg) were inhibitory (Figure 3B), possibly because of α-hemolysin–induced platelet lysis [24]. α-hemolysin is heat sensitive [25], and treatment of rSa-AH or culture supernatants at 60°C for 30 minutes ablated PNA activity (data not shown). In contrast to rSa-AH, neither rSa-PI-PLC (range, 0.5–10 µg) nor a commercial preparation of B. cereus PI-PLC (range, 0.5–1 U) promoted PNA formation (Figure 3B). Furthermore, a positive correlation was found between the amount of α-hemolysin activity in culture supernatants and the magnitude of the PNA response (r2 = 0.75; Table 1).
Figure 3.
Staphylococcus aureus α-hemolysin, not phosphatidylinositol-specific phospholipase C (PI-PLC), promotes PNA formation. A, Purified recombinant S. aureus α-hemolysin (rSa-AH) and PI-PLC (rSa-PI-PLC) proteins were resolved by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and visualized by silver staining. B, Increasing concentrations of rSa-AH (range, 0.015–5 µg) and rSa-PI-PLC (range, 0.5–10 µg) were assessed for PNA-inducing activity, using human blood as described in Figure 1. Data are representative of 2 independent experiments from 2 separate blood donors. *P < .05, by the Student t test. Abbreviation: B. cereus, Bacillus cereus.
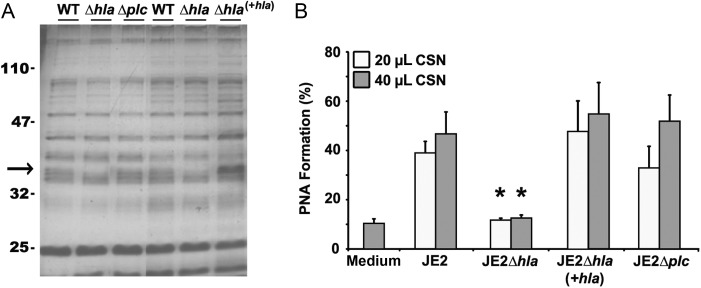
As further confirmation, 2 toxin-deficient mutants (hla deficient or plc deficient) were created in the MRSA USA300 strain JE2 background. Southern blot analysis confirmed insertion of a kanamycin antibiotic gene cassette replacing either hla or plc gene in the JE2 genome (data not shown). The hla (JE2Δhla)– and plc (JE2Δplc)–deficient mutant strains maintained growth kinetics similar to that of the parental JE2 strain. In both strains, protein banding patterns in stationary phase culture supernatants appeared to be identical to that for the parental JE2 strain, except for the marked absence of an approximately 37-kDa molecular weight band (Figure 4A). Unlike the plc-deficient mutant, the JE2Δhla strain displayed a significant (81%) reduction in hemolytic activity (Table 1); residual hemolytic activity is likely attributable to γ-hemolysin and/or the LukED variant since both lyse rabbit erythrocytes [26, 27] and both are commonly found in S. aureus clinical isolates [26], including strain LAC (Dr Frank DeLeo, personal communication). Furthermore, only the stationary phase culture supernatants from the α-hemolysin–deficient JE2Δhla strain failed to promote PNA formation (Figure 4B). Complementation of the hla gene in the JE2Δhla mutant restored PNA activity (Figure 4B). Taken together, these data strongly suggest that α-hemolysin is the sole MRSA exotoxin that stimulates heterotypic platelet-neutrophil coaggregation in human whole blood.
Figure 4.
α-hemolysin is the sole mediator of exotoxin-induced platelet-neutrophil aggregate (PNA) formation. A, Proteins from stationary phase culture supernatant of the parental JE2 strain and its isogenic hla (JE2Δhla)– and plc (JE2Δplc)–deficient mutant strains were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and visualized with silver staining. Both mutants lack an approximately 37-kDa band (arrow), which was restored only in the hla-complemented strain (JE2Δhla/hla+). B, Stationary phase culture supernatant (CSN) from wild-type (WT) methicillin-resistant Staphylococcus aureus strain JE2, its isogenic hla-deficient and hla-complemented strains (JE2Δhla and JE2Δhla/hla+, respectively), and its isogenic plc-deletion mutant (JE2Δplc) were assessed for PNA-inducing activity as described in Figure 1. Data are representative of 2 independent experiments from 2 blood donors. *P < .05, by the Student t test, versus JE2 wild-type CSN.
Platelet P-selectin Mediates α-Hemolysin–Induced PNA Formation
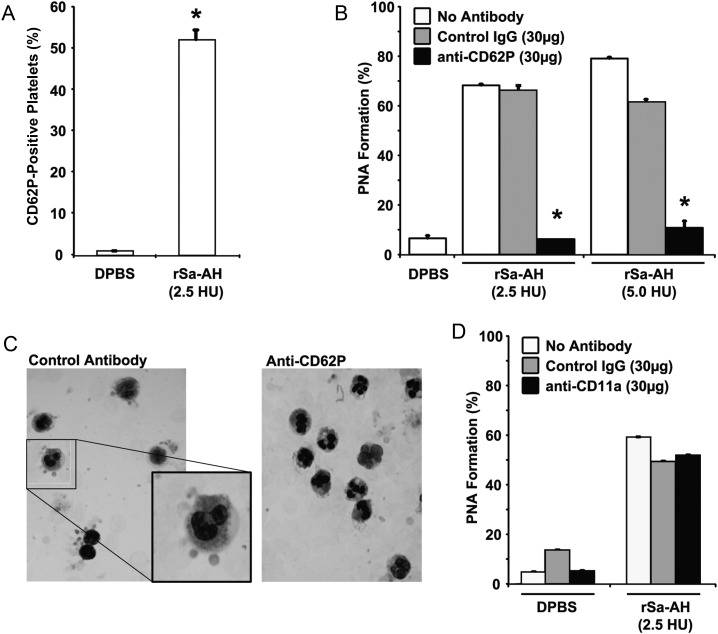
The relative number of platelets bound per PMNL was assessed using the MFI of the CD42b signal. Despite the α-hemolysin–induced increase in the percentage of PMNLs that were positive for the platelet marker (Figure 1A and 1B), the number of platelets bound per PMNL was relatively low (MFI, 1.7–3.9; Table 1). This suggested that α-hemolysin causes platelets to bind PMNL individually or in a rosette-type pattern. Such a pattern is largely mediated by platelet P-selectin (CD62P) [28]. Since our data clearly show that rSa-AH directly increases CD62P on isolated platelets (mean CD62P positivity [±SD], 52.15% ± 2.4%; MFI [±SD], 2.07 ± 0.1; Figure 5A), we next tested whether α-hemolysin–induced PNA formation could be inhibited by blocking this adhesin. Anti-CD62P specifically reduced α-hemolysin–induced PNA formation by a mean (±SD) of 90% ± 2.5% (Figure 5B). This reduction was confirmed by light microscopy (Figure 5C). Similarly, anti-CD62P reduced PNA formation induced by culture supernatants from JE2 MRSA by 86% (data not shown). Antibody blockade of CD11a was without effect (Figure 5D).
Figure 5.
P-selectin mediates α-hemolysin–mediated platelet-neutrophil aggregate (PNA) formation. A, Recombinant Staphylococcus aureus α-hemolysin (rSa-AH)–induced P-selectin expression on isolated gel-filtered platelets was assessed by flow cytometry, using fluorescein isothiocyanate–CD62P antibody (Santa Cruz Biotechnology, Santa Cruz, CA). A total of 2.5 hemolytic units (HU) of rSa-AH equates to 0.06 µg of protein. Data are given as the percentage of platelets expressing CD62P. B, rSa-AH-induced PNA formation was assessed by flow cytometry in the presence of a blocking anti-CD62P antibody, an equal amount of isotype-matched control antibody, or vehicle control (ie, no antibody). C, A Wright's stained cytospin preparation of α-hemolysin–treated whole blood samples from panel B demonstrating a rosette-type pattern of platelet adherence to polymorphonuclear leukocytes in the presence of the control antibody (left; and enlarged inset); rosetting was prevented by anti-CD62P treatment (right). D, rSa-AH–induced PNA formation was assessed in the presence of 30 µg anti-LFA-1 blocking antibody, an isotype-matched control antibody (both from Santa Cruz Biotechnology, Santa Cruz, CA), or vehicle control (ie, no antibody). Data are given as the percentage of dual-positive events and are representative of 2 independent experiments from 2 blood donors. *P < .05, by the Student t test. Abbreviations: DPBS, Dulbecco's phosphate-buffered saline; IgG, immunoglobulin.
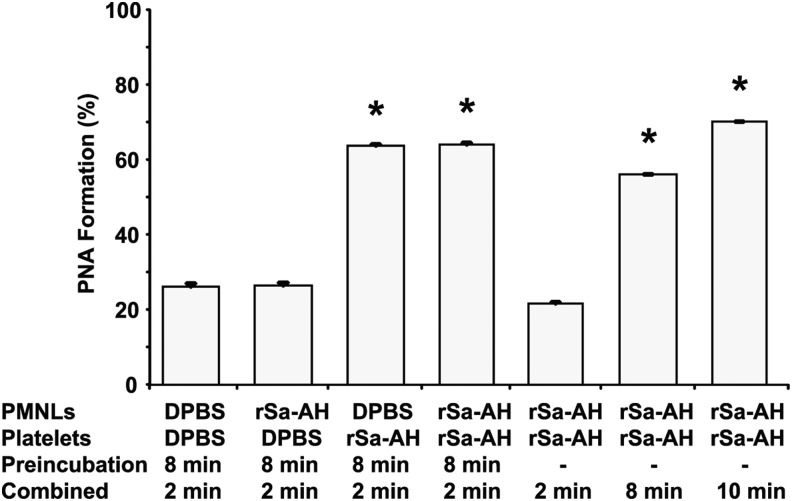
To examine the relative contribution of each cell type to PNA formation, isolated, gel-filtered platelets were exposed to rSa-HA for 8 minutes and then combined with unstimulated isolated PMNLs for 2 minutes, or vice versa, and PNA formation was assessed as before. PNA formation only occurred when platelets were preactivated with α-hemolysin (Figure 6); preactivation of PMNLs with rSa-HA for 8 minutes did not promote adherence of resting platelets (Figure 6). These findings are consistent with our data demonstrating that CD11b intensity did not correlate with α-hemolysin concentration (r2 = 0.08; Table 1) and that increased CD11b intensity did not correlate with increased PNA formation (r2 = 0.13; Table 1), and they agree with previous reports demonstrating that human PMNLs are largely resistant to α-hemolysin [29].
Figure 6.
Activated platelets are essential for recombinant Staphylococcus aureus α-hemolysin (rSa-AH)–mediated platelet-neutrophil aggregate (PNA) formation. To examine the relative contribution of each cell type to PNA formation, isolated, gel-filtered platelets were exposed to rSa-HA (2.5 hemolytic units [HU]) for 8 minutes and then combined with unstimulated isolated polymorphonuclear leukocytes (PMNLs) for 2 min, or vice versa, and PNA formation was assessed as described in Figure 1. As controls, isolated cells were incubated together in the presence of rSa-HA for the indicated times. PNA formation above baseline only occurred when platelets were preactivated with α-hemolysin; preactivation of PMNLs with rSa-HA for 8 minutes did not stimulate aggregate formation. *P < .05, by the Student t test. Abbreviation: DPBS, Dulbecco's phosphate-buffered saline.
Taken together, these findings suggest that direct toxin-induced upregulation of platelet P-selectin, and not the gpIIbIIIa-fibrinogen-CD11b axis, plays the critical role in α-hemolysin–induced PNA formation.
DISCUSSION
The recent emergence of CA-MRSA hemorrhagic necrotizing pneumonia over the past decade is associated with a severe morbidity and high mortality [1]. Understanding the host response to bacterial toxins is crucial to devising strategies for therapeutic intervention, particularly when antibiotic selection may be limited due to bacterial resistance. Previous studies have shown that S. aureus exotoxins, including PVL and α-hemolysin, contribute to the pathology of CA-MRSA pneumonia through activation of alveolar macrophages/monocytes and by excessive PMNL recruitment [30–34]. Other studies have shown that α-hemolysin enhances neutrophil adherence to endothelial cells [35, 36] and triggers NLRP3-inflammasome signaling in human monocytes, leading to the release of interleukin 1β and interleukin 18 [34]—processes that play major roles in the pathogenesis of acute respiratory distress syndrome [37]. Our findings extend these observations to include α-hemolysin–induced platelet-neutrophil coaggregation as another plausible cellular mechanism whereby the host response to toxin production could contribute to destruction of the lung microvasculature in hemorrhagic necrotizing MRSA pneumonia. Furthermore, we identify platelet P-selectin as the principal ligand supporting such coaggregation. Although controversy remains regarding the relative importance of individual staphylococcal exotoxins to the pathogenesis of necrotizing pneumonia, we conclusively demonstrate here that PVL has no role in this mechanism. Thus, our findings add to a growing body of evidence supporting a critical role for α-hemolysin in hemorrhagic necrotizing pneumonia [33, 34, 38].
We and others have clearly implicated a role for PNA formation in the pathogenesis of tissue destruction in other infectious diseases, including clostridial and streptococcal myonecrosis [9, 10, 15, 23], sepsis/septic shock [39, 40], hemolytic uremic syndrome [41], nephritis [42], and myocardial infarction [43]. P-selectin–dependent PNA formation has been shown to enhance neutrophil oxidative activity [44] and thereby cause increased vascular permeability and pulmonary dysfunction in mice with sickle cell disease [45]. Thus, targeting host response molecules for therapeutic intervention offers a novel alternative to traditional antimicrobial treatment strategies. Results presented here further demonstrate that platelet activation is essential for α-hemolysin–induced PNA formation and that heterotypic cellular aggregation is P-selectin dependent.
Although the signaling mechanism by which α-hemolysin activates platelets has yet to be identified, several possibilities exist. For instance, platelets express Toll-like receptors [46] and disintegrin/metalloproteinase (the ADAM family) receptors, of which Toll-like receptor 2 and ADAM10 recognize α-hemolysin directly [47, 48]. Engagement of these receptors could activate Src family kinases [48, 49], signaling molecules common in α-granule degranulation and P-selectin translocation in platelets. In addition, α-hemolysin could indirectly activate platelets through stimulation of leukocytes or endothelial cells, facilitating the release of platelet agonists such as platelet-activating factor [50], prostacyclin, collagen, adenosine diphosphate, or thromboxane-A2. Last, changes in shear stress that may occur in the pulmonary microvasculature during CA-MRSA pneumonia may further stimulate platelet activation and augment α-hemolysin–induced PNA formation.
In summary, α-hemolysin, not PVL, mediates PNA formation in a P-selectin–dependent manner. This suggests that blockade of the P-selectin/PSGL-1 receptor-ligand interaction may limit α-hemolysin–induced lung destruction in MRSA pneumonia.
Notes
Acknowledgments. We thank Dr Frank R. DeLeo (NIAID, NIH), for providing the CA-MRSA LAC and its isogenic LACΔpvl strain; Dr Francoise Perdreau-Remington (University of California, San Francisco), for providing clinical CA-MRSA strains; and the NARSA, for the MRSA JE2 strain.
Financial support. This work was supported in part by the US Department of Veterans Affairs, Office of Research and Development, Biomedical Laboratory Research Program (grants to D. L. S. and A. E. B.), and by the Mountain States Tumor Medical Research Institute, Boise, Idaho (grant to T. P.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bradley SF. Staphylococcus aureus pneumonia: emergence of MRSA in the community. Seminars in Respiratory and Critical Care Medicine. 2005;26:643–9. doi: 10.1055/s-2005-925528. [DOI] [PubMed] [Google Scholar]
- 2.Pan E, Diep B, Charlebois E, et al. Population dynamics of nasal strains of methicillin resistant Staphylococcus aureus and their relation to community associated disease activity. The Journal of Infectious Diseases. 2005;192:811–8. doi: 10.1086/432072. [DOI] [PubMed] [Google Scholar]
- 3.Gillet Y, Vanhems P, Lina G, et al. Factors predicting mortality in necrotizing community-acquired pneumonia caused by Staphylococcus aureus containing Panton-Valentine leukocidin. Clin Infect Dis. 2007;45:315–21. doi: 10.1086/519263. [DOI] [PubMed] [Google Scholar]
- 4.Adem PV, Montgomery CP, Husain AN, et al. Staphylococcus aureus sepsis and the waterhouse-friderichsen syndrome in children. N Engl J Med. 2005;353:1245–51. doi: 10.1056/NEJMoa044194. [DOI] [PubMed] [Google Scholar]
- 5.Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000;80:617–53. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 6.Zarbock A, Polanowska-Grabowska RK, Ley K. Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Reviews. 2007;21:99–111. doi: 10.1016/j.blre.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Zarbock A, Ley K. The role of platelets in acute lung injury (ALI) Front Biosci. 2009;14:150–8. doi: 10.2741/3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters MJ, Heyderman RS, Faust S, Dixon GLJ, Inwald DP, Klein NJ. Severe meningococcal disease is characterized by early neutrophil but not platelet activation and increased formation and consumption of platelet-neutrophil complexes. J Leukoc Biol. 2003;73:722–30. doi: 10.1189/jlb.1002509. [DOI] [PubMed] [Google Scholar]
- 9.Bryant AE, Chen RYZ, Nagata Y, et al. Clostridial gas gangrene. II. phospholipase C-induced activation of platelet gpIIbIIIa mediates vascular occlusion and myonecrosis in clostridium perfringens gas gangrene. The Journal of Infectious Diseases. 2000;182:808–15. doi: 10.1086/315757. [DOI] [PubMed] [Google Scholar]
- 10.Bryant AE, Bayer CR, Chen RYZ, Guth PH, Wallace RJ, Stevens DL. Vascular dysfunction and ischemic destruction of tissue in streptococcus pyogenes infection: the role of streptolysin O-induced platelet/neutrophil complexes. The Journal of Infectious Diseases. 2005;192:1014–22. doi: 10.1086/432729. [DOI] [PubMed] [Google Scholar]
- 11.Zarbock A. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. The Journal of Clinical Investigation. 2006;116:3211–9. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton SM, Bryant AE, Carroll KC, et al. In vitro production of Panton-Valentine leukocidin among strains of methicillin-resistant Staphylococcus aureus causing diverse infections. Clinical Infectious Diseases. 2007;45:1550–8. doi: 10.1086/523581. [DOI] [PubMed] [Google Scholar]
- 13.Voyich JM, Otto M, Mathema B, et al. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? The Journal of Infectious Diseases. 2006;194:1761–70. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- 14.Titball RW, Leslie DL, Harvey S, Kelly D. Hemolytic and sphingomyelinase activities of Clostridium perfringens alpha-toxin are dependent on a domain homologous to that of an enzyme from the human arachidonic acid pathway. Infect Immun. 1991;59:1872–4. doi: 10.1128/iai.59.5.1872-1874.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryant AE, Bayer CR, Aldape MJ, Wallace RJ, Titball RW, Stevens DL. Clostridium perfringens phospholipase C-induced platelet/leukocyte interactions impede neutrophil diapedesis. J Med Microbiol. 2006;55:495–504. doi: 10.1099/jmm.0.46390-0. [DOI] [PubMed] [Google Scholar]
- 16.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Analytical Chemistry. 2002;74:5383–92. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 17.Eichstaedt S, Gabler K, Below S, et al. Effects of Staphylococcus aureus-hemolysin A on calcium signalling in immortalized human airway epithelial cells. Cell Calcium. 2009;45:165–76. doi: 10.1016/j.ceca.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Bernheimer AW, Schwartz LL. Isolation and composition of staphylococcal alpha toxin. Journal of General Microbiology. 1963;30:455–68. doi: 10.1099/00221287-30-3-455. [DOI] [PubMed] [Google Scholar]
- 19.Wei Z, Zenewicz LA, Goldfine H. Listeria monocytogenes phosphatidylinositol-specific phospholipase C has evolved for virulence by greatly reduced activity on GPI anchors. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12927–31. doi: 10.1073/pnas.0501725102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Y, Bryant AE, Salmi DB, McIndoo E, Stevens DL. vfr, a novel locus affecting cysteine protease production in streptococcus pyogenes. J Bacteriol. 2009;191:3189–94. doi: 10.1128/JB.01771-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnaud M, Chastanet A, Debarbouille M. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Appl Environ Microbiol. 2004;70:6887–91. doi: 10.1128/AEM.70.11.6887-6891.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diep BA, Gill SR, Chang RF, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired methicillin-resistant Staphylococcus aureus. The Lancet. 2006;367:731–9. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 23.Bryant AE, Chen RYZ, Nagata Y, et al. Clostridial gas gangrene. I. Cellular and molecular mechanisms of microvascular dysfunction induced by exotoxins of clostridium perfringens. The Journal of Infectious Diseases. 2000;182:799–807. doi: 10.1086/315756. [DOI] [PubMed] [Google Scholar]
- 24.Bayer AS, Ramos MD, Menzies BE, Yeaman MR, Shen AJ, Cheung AL. Hyperproduction of alpha-toxin by Staphylococcus aureus results in paradoxically reduced virulence in experimental endocarditis: a host defense role for platelet microbicidal proteins. Infection and Immunity. 1997;65:4652–60. doi: 10.1128/iai.65.11.4652-4660.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gemmell CG, Peterson PK, Townsend K, Quie PG, Kim Y. Biological effects of the interaction of staphylococcal alpha-toxin with human serum. Infection and Immunity. 1982;38:981–5. doi: 10.1128/iai.38.3.981-985.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morinaga N, Kaihou Y, Noda M. Purification, cloning and characterization of variant LukE-LukD with strong leukocidal activity of staphylococcal bi-component leukotoxin family. Microbiol Immunol. 2003;47:81–90. doi: 10.1111/j.1348-0421.2003.tb02789.x. [DOI] [PubMed] [Google Scholar]
- 27.Kaneko J, Kamio Y. Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: structures, pore-forming mechanism, and organization of the genes. Bioscience, Biotechnology, and Biochemistry. 2004;68:981–1003. doi: 10.1271/bbb.68.981. [DOI] [PubMed] [Google Scholar]
- 28.Abou-Saleh H, Thaorat JF, Yacoub D, Merhi Y. Neutrophil P-selectin-glycoprotein-ligand-1 binding to platelet P-selectin enhances metalloproteinase 2 secretion and platelet-neutrophil aggregation. Thrombosis and Haemostasis. 2005;94:1230–5. doi: 10.1160/TH05-05-0344. [DOI] [PubMed] [Google Scholar]
- 29.Vandenesch F, Lina G, Henry T. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: a redundant arsenal of membrane-damaging virulence factors? Front Cell Infect Microbiol. 2012;2:12. doi: 10.3389/fcimb.2012.00012. 2013;2:2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diep BA, Chan L, Tattevin P, et al. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proceedings of the National Academy of Sciences. 2010;107:5587–92. doi: 10.1073/pnas.0912403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labandeira-Rey M, Couzon F, Boisset S, et al. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007;315:1130–3. doi: 10.1126/science.1137165. [DOI] [PubMed] [Google Scholar]
- 32.Wardenburg JB, Patel RJ, Schneewind O. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun. 2007;75:1040–4. doi: 10.1128/IAI.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wardenburg JB, Bae T, Otto M, DeLeo FR, Schneewind O. Poring over pores: [alpha]-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med. 2007;13:1405–6. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 34.Craven RR, Gao X, Allen IC, et al. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS ONE. 2009;4:e7446. doi: 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grandel U, Reutemann M, Kiss L, et al. Staphylococcal alpha-toxin provokes neutrophil-dependent cardiac dysfunction: role of ICAM-1 and cys-leukotrienes. American Journal of Physiology - Heart and Circulatory Physiology. 2002;282:H1157–65. doi: 10.1152/ajpheart.00165.2001. [DOI] [PubMed] [Google Scholar]
- 36.Krull M, Dold C, Hippenstiel S, Rosseau S, Lohmeyer J, Suttorp N. Escherichia coli hemolysin and Staphylococcus aureus alpha-toxin potently induce neutrophil adhesion to cultured human endothelial cells. The Journal of Immunology. 1996;157:4133–40. [PubMed] [Google Scholar]
- 37.Dolinay T, Kim YS, Howrylak J, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. American Journal of Respiratory and Critical Care Medicine. 2012;185:1225–34. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartlett AH, Foster TJ, Hayashida A, Park PW. Alpha-toxin facilitates the generation of CXC chemokine gradients and stimulates neutrophil homing in Staphylococcus aureus pneumonia. The Journal of Infectious Diseases. 2008;198:1529–35. doi: 10.1086/592758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark SR, Ma AC, Tavener SA, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–9. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 40.McDonald B, Urrutia R, Yipp B, Jenne C, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host & Microbe. 2012;12:324–33. doi: 10.1016/j.chom.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Stahl AL, Sartz L, Nelsson A, Bekassy ZD, Karpman D. Shiga toxin and lipopolysaccharide induce platelet-leukocyte aggregates and tissue factor release, a thrombotic mechanism in hemolytic uremic syndrome. PLoS One. 2009;4:e6990. doi: 10.1371/journal.pone.0006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson RJ, Alpers CE, Pritzl P, et al. Platelets mediate neutrophil-dependent immune complex nephritis in the rat. The Journal of Clinical Investigation. 1988;82:1225–35. doi: 10.1172/JCI113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nijm J, Wikby A, Tompa A, Olsson AG, Jonasson L. Circulating levels of proinflammatory cytokines and neutrophil-platelet aggregates in patients with coronary artery disease. The American Journal of Cardiology. 2005;95:452–6. doi: 10.1016/j.amjcard.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 44.Nagata K, Tsuji T, Todoroki N, et al. Activated platelets induce superoxide anion release by monocytes and neutrophils through P-selectin (CD62) The Journal of Immunology. 1993;151:3267–73. [PubMed] [Google Scholar]
- 45.Polanowska-Grabowska R, Wallace K, Field JJ, et al. P-selectin mediated platelet-neutrophil aggregate formation activates neutrophils in muse and human sickle cell disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:2392–9. doi: 10.1161/ATVBAHA.110.211615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blair P, Rex S, Vitseva O, et al. Stimulation of toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circulation Research. 2009;104:346–54. doi: 10.1161/CIRCRESAHA.108.185785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maslinska D, Laure-Kamionowska M, Maslinski S. Toll-like receptors in rat brains injured by hypoxic-ischemia or exposed to staphylococcal alpha-toxin. Folia Neuropathol. 2004;42:125–32. [PubMed] [Google Scholar]
- 48.Wilke GA, Wardenburg JB. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proceedings of the National Academy of Sciences. 2010;107:13473–8. doi: 10.1073/pnas.1001815107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z, Zhang G, Liu J, et al. An important role of the Src family kinase Lyn in stimulating platelet granule secretion. Journal of Biological Chemistry. 2010;285:12559–70. doi: 10.1074/jbc.M109.098756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suttorp N, Buerke M, Tannert-Otto S. Stimulation of PAF-synthesis in pulmonary artery endothelial cells by Staphylococcus aureus alpha-toxin. Thrombosis Research. 1992;67:243–52. doi: 10.1016/0049-3848(92)90143-x. [DOI] [PubMed] [Google Scholar]