Abstract
Background. DNA vaccines have been very poorly immunogenic in humans but have been an effective priming modality in prime-boost regimens. Methods to increase the immunogenicity of DNA vaccines are needed.
Methods. HIV Vaccine Trials Network (HVTN) studies 070 and 080 were multicenter, randomized, clinical trials. The human immunodeficiency virus type 1 (HIV-1) PENNVAX®-B DNA vaccine (PV) is a mixture of 3 expression plasmids encoding HIV-1 Clade B Env, Gag, and Pol. The interleukin 12 (IL-12) DNA plasmid expresses human IL-12 proteins p35 and p40. Study subjects were healthy HIV-1–uninfected adults 18–50 years old. Four intramuscular vaccinations were given in HVTN 070, and 3 intramuscular vaccinations were followed by electroporation in HVTN 080. Cellular immune responses were measured by intracellular cytokine staining after stimulation with HIV-1 peptide pools.
Results. Vaccination was safe and well tolerated. Administration of PV plus IL-12 with electroporation had a significant dose-sparing effect and provided immunogenicity superior to that observed in the trial without electroporation, despite fewer vaccinations. A total of 71.4% of individuals vaccinated with PV plus IL-12 plasmid with electroporation developed either a CD4+ or CD8+ T-cell response after the second vaccination, and 88.9% developed a CD4+ or CD8+ T-cell response after the third vaccination.
Conclusions. Use of electroporation after PV administration provided superior immunogenicity than delivery without electroporation. This study illustrates the power of combined DNA approaches to generate impressive immune responses in humans.
Keywords: vaccination, electroporation, plasmid cytokine adjuvant
DNA-based immunization offers several advantages [1]. DNA vaccines contain nonliving, nonreplicating, and nontransmissible material, providing an improved safety profile over live attenuated viral vectors. They do not elicit antivector immunity, retaining potency through multiple boost cycles. In theory, DNA vaccines are simple and relatively inexpensive to construct, readily produced in large quantities, easy to characterize, and stable and can be combined into complex formulations.
Despite the early enthusiasm from results of studies in small animals, DNA vaccines have not generated robust immune responses in humans [2–6]. The amount of antigen produced by each transfected cell is low because of the low transcription rate of antigen sequences being driven off the cytomegalovirus promoter [7–9]. One approach to augment the immunogenicity of DNA is to combine the DNA vaccine with a plasmid cytokine adjuvant [10–13]. Interleukin 12 (IL-12) is a key cytokine for the induction of cellular immune responses [14, 15]. Interleukin 15 (IL-15) is a member of the common cytokine receptor γ-chain family [16–18] that fosters development of long-lived memory T-cell responses [19–21].
A newer strategy for increasing immune potency has been to deliver the plasmids with in vivo electroporation. Electroporation enhances uptake of DNA into cells by temporarily generating an electrical field that increases the permeability of cell membranes and moves the macromolecules through the briefly open membrane pores. Clinical applications of electroporation have been tested, especially in cancer treatment and gene therapy [22–24]. Electroporation has elicited HIV-specific cellular immune responses in mice [25] and simian immunodeficiency virus–specific immune responses in macaques [26]. In macaque studies, genetic optimization, electroporation, and IL-12 plasmid adjuvant have improved the immunogenicity of DNA vaccines in vivo [26]. More recently, Vasan et al reported on a trial that showed the potential to increase vaccine-induced cellular responses to a DNA vaccine relative to intramuscular injection alone [27]. However, electroporation remains investigational [28] and has not been licensed by the Food and Drug Administration for clinical use. This is the first report on the combination of these approaches in humans. Here, we summarize the results of 2 trials of an HIV plasmid DNA vaccine, PENNVAX®-B (PV), one investigating HIV consensus clade B Gag, Pol, and Env with IL-12 or IL-15 plasmid cytokine adjuvants delivered by intramuscular injection without electroporation (HIV Vaccine Trials Network [HVTN] study 070) and the other investigating the same vaccine with plasmid IL-12 delivered intramuscularly with electroporation (HVTN study 080). The results illustrate the power of these combined DNA approaches to generate impressive immune responses in humans.
METHODS
Study Design
HVTN studies 070 and 080 were multicenter, randomized, placebo-controlled, double-blind phase I trials conducted in the United States by the HVTN, supported by the National Institute of Allergy and Infectious Diseases.
Participants
Participants were healthy HIV-1–uninfected adults (age, 18–50 years). For HVTN study 080, body mass index (BMI; calculated as the weight in kilograms divided by the height in meters squared) of >30 was exclusionary, although 1 person with a BMI of 32 was inadvertently enrolled. Participants were provided signed informed consent in their native language.
Ethics
The studies were reviewed by the Food and Drug Administration and the Recombinant DNA Advisory Committee and were approved by the institutional review boards and biosafety committees affiliated with the study sites. Both studies were registered at Clinicaltrials.gov (HVTN study 070, NCT00528489; HVTN study 080, NCT00991354).
Interventions
In HVTN study 070, participants were randomly assigned to receive PV (6 mg) alone (30 subjects), PV plus IL-15 (0.8 mg; 10 subjects), PV plus IL-15 (2 mg; 30 subjects), PV plus IL-12 (1.5 mg; 30 subjects), or placebo (20 subjects; 1:5 ratio of placebo recipients to vaccinees in each group). In HVTN study 080, participants were randomly assigned to receive PV (3 mg; 10 subjects), PV plus IL-12 (1 mg; 30 subjects), or placebo (8 subjects). Randomization was based on a sequence of computer-generated random numbers that was provided to site pharmacists by a central statistical and data monitoring center.
Vaccinations were given on days 0, 28, 84, and 168 in HVTN study 070 and on days 0, 28, and 84 in HVTN study 080. The higher dosages given in HVTN study 070 required intramuscular injections in both the right and left deltoid muscles. For the electroporation study, the study products were administered to one deltoid followed by in vivo electroporation with the CELLECTRA® Adaptive Constant Current Electroporation Device (Inovio Pharmaceuticals [formerly VGX Pharmaceuticals], Blue Bell, PA) with 3 pulses at 0.5 A constant current, with a 52-millisecond pulse length and a 1-second rest between pulses.
Study Agents
PV is a mixture of 3 expression plasmids encoding HIV-1 clade B protein Env (pEY2E1-B), Gag (gag02CAM), and Pol (pPK2C1). The plasmid backbone includes a eukaryotic gene expression unit that contains elements from the human cytomegalovirus immediate early promoter/enhancer and the bovine growth hormone polyadenylation signal, a chimeric kanamycin resistance gene, and a pUC bacterial origin of replication. The vaccine is formulated in 30 mM citrate buffer (pH 6.5) containing 150 mM NaCl, 0.01% ethylenediamine tetraacetic acid, and 0.25% bupivacaine- HCl.
The IL-12 DNA adjuvant is a dual promoter expression plasmid expressing the genes encoding human IL-12 proteins p35 and p40 under separate regulatory control [29]. The IL-15 DNA adjuvant encodes the human IL-15 gene [29].
PV and IL-15 DNA (intramuscular study) was developed by the lab of David Weiner and supplied by VGX (Inovio). The IL-12 DNA adjuvant was developed by the Weiner laboratory in collaboration with Wyeth and provided by Wyeth Vaccines Research (now Pfizer), and Profectus Biosciences. The same lots of PV and IL-12 DNA supplied both studies.
For both trials, the placebo was sodium chloride injection USP, 0.9%.
Safety/Tolerability Assessments
Local and systemic reactogenicity signs and symptoms were assessed 30 minutes after injections and daily for the following 3 days and graded on the basis of the DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (version 1.0, December 2004; clarification August 2009). HVTN study 080 participants also rated their injection site pain on a 10-point visual analog scale (VAS) immediately and 5 and 25 minutes following electroporation. Two weeks following each injection, HVTN study 080 participants completed a 2-item questionnaire that asked their willingness to undergo electroporation under different scenarios.
Intracellular Cytokine Staining
Cryopreserved peripheral blood mononuclear cells (PBMCs) were thawed and stimulated with 3 Env, 2 Gag, and 3 Pol potential T-cell epitope (PTE) peptide pools. 0.5% dimethyl sulfoxide was used as negative control. PBMCs stimulated with SEB or a cytomegalovirus peptide pool were used as positive controls. Intracellular cytokine staining (ICS) was performed using a cross-validated 10-color protocol [30]. Subjects with high background responses in the negative control (>0.1% of T cells expressing interferon γ [IFN-γ] and/or interleukin 2 [IL-2]) were excluded from analysis.
Humoral Assays
Serological tests for binding antibodies to consensus S Env, a group M consensus gp140 protein (provided by Drs Liao and Haynes) [31] and Gag p55 (HVTN study 080 only) antigens were assessed with a validated enzyme-linked immunosorbent assay, using a single serum dilution (1:20) [32, 33]. A positive response was defined as a difference between the ODs of antigen-containing and non–antigen-containing wells of >0.2 and a difference of ≥3 times the value on day 0 (prior to vaccination).
Neutralizing antibodies against HIV-1 were measured in a TZM-bl cell assay [34]. For HVTN study 070, neutralization against HIV-1 strains MN and SF162.LS was assessed; a titer of ≥25 was considered a positive response. For HVTN study 080, neutralization against HIV-1 strains BaL.26, MN.3, NW965.26, NP03.113, and SF162.LS were assessed; a titer of ≥10 was considered a positive response. Thresholds for a positive response were based on values observed in placebo recipients, which can vary depending on the virus envelopes used in each assay.
Statistical Methods
All participants were included in the analysis of safety data, and all with reliable assay data were included in immunogenicity analyses, regardless of the number of vaccinations received. Wilcoxon rank sum tests were used to test for differences between groups in the severity of reactogenicity, VAS scores, magnitudes of response to the ICS assay, and BMI distributions. Differences between arms for the electroporation acceptability questions and response rates were tested with Fisher exact tests. Tests were 2 sided, and differences were considered statistically significant if P < .05. For the ICS assay, positivity for a peptide pool was based on a 1-sided Fisher exact test comparing the percentage of T cells with positive staining for IL-2 and/or IFN-γ between the experimental and negative control wells, with a Bonferroni multiplicity adjustment for the 2 T-cell comparisons and 8 peptide pools. No other adjustments for multiple comparisons were made. If any peptide pool for a T-cell subset was positive, then the overall response was considered positive. The protein-specific magnitude of the response was the maximum for the protein-specific pools, with the overall magnitude being the sum of the protein magnitudes. Logistic regression modeling was used to assess the effect of demographic characteristics on response.
RESULTS
Trial Populations
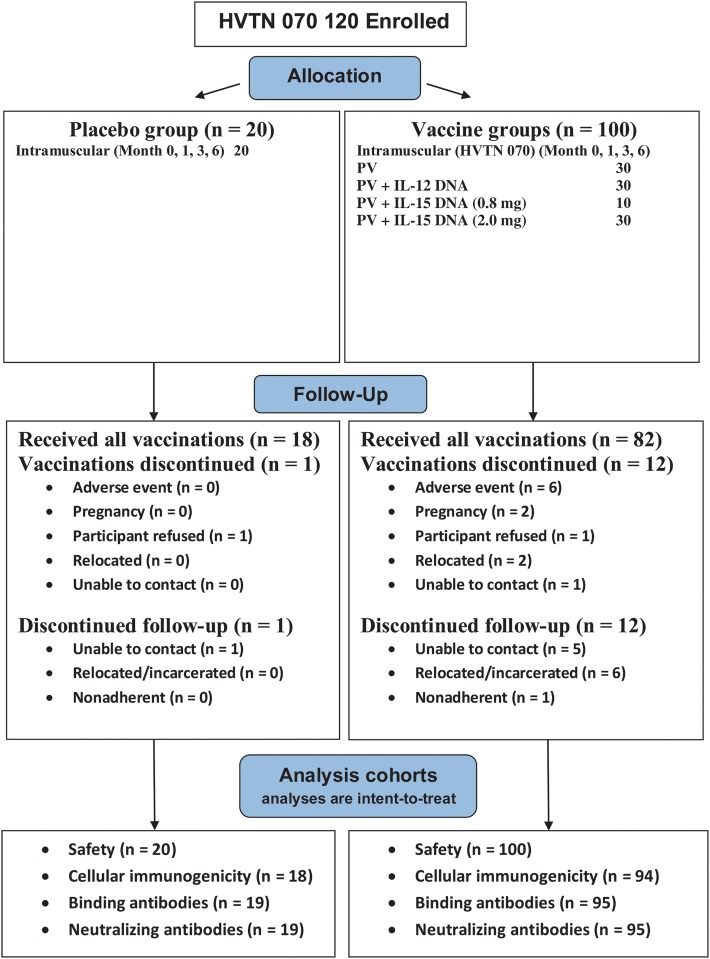
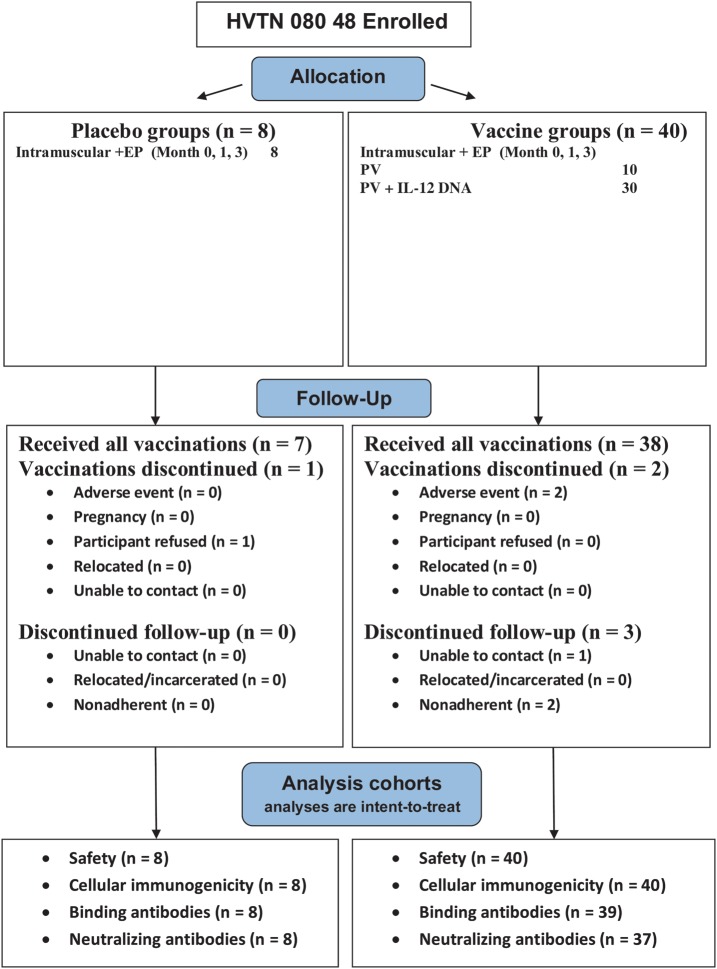
The protocol involving intramuscular administration (HVTN study 070) enrolled 120 participants between October 2007 and January 2009 at 7 US sites. The protocol involving intramuscular administration with electroporation (HVTN study 080) enrolled 48 participants between November 2009 and May 2010 at 3 US sites (Table 1 and Figure 1). Because of different eligibility criteria, participants associated with the intramuscular delivery plus electroporation had a lower BMI.
Table 1.
Participant Characteristics and Number of Vaccinations Received
| Characteristic | Intramuscular Control (n=20) | Intramuscular Vaccine (n = 100) | Intramuscular Plus EP Control (n = 8) | Intramuscular Plus EP Vaccine (n = 40) | Pa | Total (n = 168) |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 7 (35.0) | 54 (54.0) | 2 (25.0) | 19 (47.5) | .49 | 82 (48.8) |
| Female | 13 (65.0) | 46 (46.0) | 6 (75.0) | 21 (52.5) | 86 (51.2) | |
| Ethnicity/race | ||||||
| White, non-Hispanic | 16 (80.0) | 47 (47.0) | 8 (100.0) | 32 (80.0) | .002 | 103 (61.3) |
| African American, non-Hispanic | 3 (15.0) | 35 (35.0) | 0 (0) | 3 (7.5) | 41 (24.4) | |
| Hispanic | 1 (5.0) | 8 (8.0) | 0 (0) | 1 (2.5) | 10 (6.0) | |
| Other | 0 (0.0) | 10 (10.0) | 0 (0) | 4 (10.0) | 14 (8.3) | |
| Age, y | 30 (20–48) | 31 (18–50) | 25 (21–47) | 25 (19–41) | .002 | 29 (18–50) |
| Body mass indexb | 25.8 (17.4–39.2) | 26.3 (17.8–39.9) | 22.4 (20.5–28.6) | 24.8 (19.0–32.1) | .02 | 25.6 (17.4–39.9) |
| Vaccinations receivedc | ||||||
| First | 20 (100) | 100 (100) | 8 (100) | 40 (100) | 168 (100) | |
| Second | 19 (95.0) | 96 (96.0) | 7 (87.5) | 39 (97.5) | 161 (95.8) | |
| Third | 18 (90.0) | 91 (91.0) | 7 (87.5) | 38 (95.0) | 154 (91.7) | |
| Fourth | 18 (90.0) | 83 (83.0) | NA | NA | 101 (84.2) | |
| All | 18 (90.0) | 82 (82.0) | 7 (87.5) | 38 (95.0) | 145 (86.3) |
Data are no. (%) of subjects or median (range).
Abbreviations: EP, electroporation; NA, not applicable.
a For the comparison of the intramuscular vaccine group and the intramuscular vaccine plus EP group. Fisher exact tests are used for comparisons by sex and ethnicity race (white, non-Hispanic vs other). Wilcoxon rank sum tests are used for age and body mass index comparisons.
b Calculated as the weight in kilograms divided by the height in meters squared.
c Sixteen participants permanently discontinued vaccinations, and an additional 7 in intramuscular arms missed ≥1 vaccination.
Figure 1.
Allocation, follow-up, and analysis for HIV Vaccine Trials Network (HVTN) study 070 and HVTN study 080. Abbreviations: EP, electroporation; IL-12, interleukin 12; IL-15, interleukin 15; PV, PENNVAX-B DNA vaccine.
For the intramuscular protocol, 100 participants (83.3%) received all 4 vaccinations (Table 1). Thirteen (1 control and 12 vaccinees) permanently discontinued vaccinations early because of peripheral neuropathy possibly related to vaccination (1 subject), unrelated adverse events (AEs; 5 subjects), vaccine refusal (2 subjects), pregnancy (2 subjects), relocation (2 subjects), and loss to contact (1 subject). For the intramuscular delivery with electroporation protocol, all but 3 (1 control and 2 vaccinees) received all 3 vaccinations. Two participants discontinued because of injection site pain and tenderness, and 1 discontinued because of an unrelated AE (preexisting spinal stenosis). There were no statistical differences between the percentages of controls and vaccinees discontinuing vaccinations or study follow-up for either administration mode.
Tolerability and Reactogenicity
For the intramuscular delivery plus electroporation protocol, all participants experienced some pain immediately following each vaccination (median VAS, 5.0–5.4 across vaccinations; range 0.4–9.0), with no statistically significant differences between arms. VAS scores decreased at 5 minutes (median, 0.7–0.9; range, 0–6.9) and 25 minutes (median, 0.5–1.0; range, 0–6.0). PV recipients recorded higher VAS scores than PV plus IL-12 recipients 5 and 25 minutes after each vaccination (P < .001 at 5 minutes, and P<.04 at 25 minutes). Controls had more pain than PV plus IL-12 recipients 5 minutes after the second and third vaccinations (P = .02 for both comparisons).
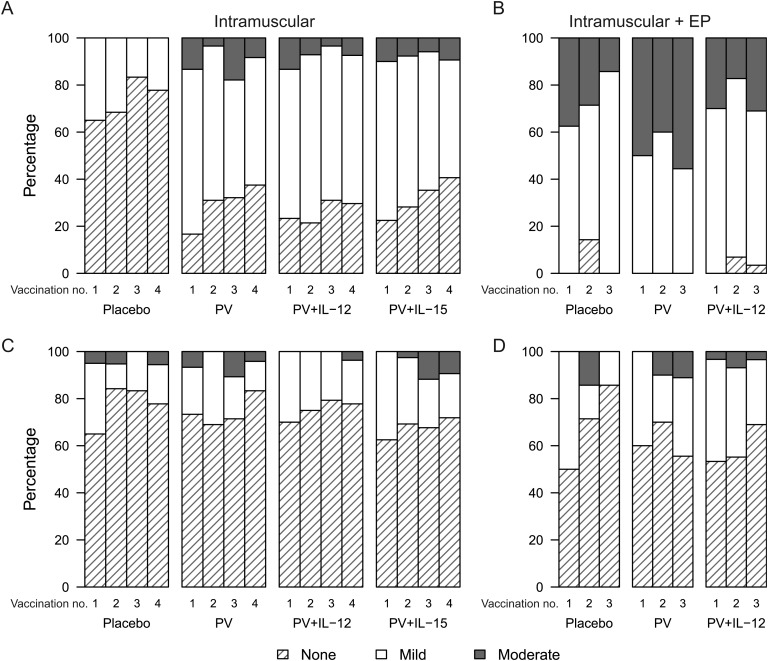
Vaccine administration was generally safe and well tolerated, with no severe systemic reactogenicity. The maximum severity of local pain or tenderness within 3 days following either type of administration was generally mild, with no increase in severity with subsequent vaccinations (Figure 2). No one reported severe injection site symptoms. The maximum severity of local pain or tenderness was greater in the group that underwent intramuscular delivery with electroporation than in the group that underwent intramuscular delivery among those receiving PV plus IL-12 (P = .046) and controls (P = .003), but not for the PV arms (P = .26). For intramuscular delivery plus electroporation, there was no difference in severity between the control, PV, and PV plus IL-12 arms.
Figure 2.
Reactogenicity symptoms. Maximum severity of local pain or tenderness (A and B) and of systemic symptoms (C and D) following each study injection. A and C, Intramuscular administration. B and D, Intramuscular administration with electroporation. No severe symptoms were reported. Onset of a reaction was within the first 3 days following a study injection. Reactions were followed to resolution to determine the maximum severity. Systemic reactions included malaise and/or fatigue, headache, chills, myalgia, arthralgia, nausea, and vomiting. For intramuscular administration, the interleukin 15 (IL-15) 0.8-mg and 2-mg dose groups are combined because there were no differences in the reactogenicity profiles. Abbreviations: EP, electroporation; IL-12, interleukin 12; PV, PENNVAX-B DNA vaccine.
Only 6 participants had AEs considered definitely or probably related to the study agents or mode of administration, and all were deemed of mild severity. These were injection site pruritus (2 subjects; intramuscular delivery), hematoma (2 subjects; intramuscular delivery), pain/induration (1 subject; intramuscular delivery plus electroporation), and vasovagal reaction (1 subject; intramuscular delivery plus electroporation). Eleven participants reported severe AEs, and all were judged unrelated or probably unrelated to vaccination. Four serious AEs were reported for the intramuscular study: an exacerbation of cervical radiculopathy with peripheral sensorimotor neuropathy, reported as possibly related to PV vaccination; a spontaneous abortion, probably unrelated to PV plus IL-12 vaccination 38 days earlier; and hospitalization for fever, flank pain, and abdominal lymphadenopathy, considered probably unrelated to PV plus IL-12 vaccination. One death occurred 6 months after PV vaccination, 1 week after the participant had completed study participation, with no new or active AEs reported. According to a relative, the death was from a drug overdose, but this could not be confirmed by the study site. The death was reported as unrelated by the study sponsor. No severe AEs were reported for the intramuscular delivery plus electroporation study. No one became HIV infected, died, or had life-threatening events while in either study.
Acceptability of Electroporation
All but 1 participant responded at each assessment time that they would be either definitely or probably willing to undergo electroporation in association with a new vaccine against a serious disease, at each assessment time; the exception involved a subject in the PV plus IL-12 group, who responded after the third vaccination that they would probably not be willing to undergo electroporation. The majority responded that they would definitely be willing to undergo electroporation (72.9% after the first vaccination, 69.6% after the second, and 65.9% after the third), with no statistically significant differences between arms. If electroporation was used to increase the effectiveness of an existing vaccine, the percentages of participants responding that they would definitely be willing to undergo electroporation were 33.3% after the first vaccination, 32.6% after the second, and 27.3% after the third.
Humoral Immunogenicity
Binding antibody responses to Env were minimal, with only 2 responses in 2 different study participants (Table 2). The intramuscular delivery plus electroporation arms had minimal antibody responses to Gag p55 antigen (not tested in the intramuscular study) following the third vaccination (10.0% [1/10] in the PV group and 22.2% [6/27] in the PV plus IL-12 group). There was no difference in neutralizing antibody responses to Tier 1 isolates between vaccine and placebo recipients following the third vaccination.
Table 2.
Humoral Responses
| Assay, Antigen, Time Point | Administration Method | Placebo | PV | PV Plus IL-12 | PV Plus IL-15a |
|---|---|---|---|---|---|
| Binding | |||||
| Consensus S Env | |||||
| After third dose | Intramuscular delivery | 0/19 (0) | 0/27 (0) | 1/30 (3.3) | 0/27 (0) |
| After fourth dose | Intramuscular delivery | 0/17 (0) | 0/27 (0) | 0/28 (0) | 0/23 (0) |
| After second dose | Intramuscular delivery with EP | 0/8 (0) | 0/10 (0) | 0/29 (0) | … |
| After third dose | Intramuscular delivery with EP | 0/8 (0) | 1/10 (10.0) | 0/27 (0) | … |
| P55 | |||||
| After second dose | Intramuscular delivery with EP | 0/8 (0) | 0/10 (0) | 1/29 (3.4) | … |
| After third dose | Intramuscular delivery with EP | 0/8 (0) | 1/10 (10.0) | 6/27 (22.2) | … |
| Neutralization | |||||
| MN | |||||
| After third dose | Intramuscular deliveryb | 3/19 (15.8) | 1/27 (3.7) | 6/30 (20.0) | 7/27 (25.9) |
| After fourth dose | Intramuscular deliveryb | 2/17 (11.8) | 0/27 (0) | 1/28 (3.6) | 2/23 (8.7) |
| SF162.LS | |||||
| After third dose | Intramuscular deliveryb | 1/19 (5.3) | 0/27 (0) | 0/30 (0) | 0/27 (0) |
| After fourth dose | Intramuscular deliveryb | 0/17 (0) | 0/27 (0) | 0/28 (0) | 0/23 (0) |
| MW965.26 | |||||
| After second dose | Intramuscular delivery with EPc | 0/8 (0) | 6/10 (60.0) | 2/27 (7.4) | … |
| After third dose | Intramuscular delivery with EPc | 1/8 (12.5) | 2/10 (20.0) | 3/27 (11.1) | … |
Data are no. of subjects with a humoral response/no. tested (%).
Abbreviations: EP, electroporation; IL-12, interleukin 12; IL-15, interleukin 15; PV, PENNVAX-B DNA vaccine.
a Data for the PV plus IL-15 group are for the 2.0-mg dose of IL-15. No responses were observed at the lower, 0.8-mg dose (n = 10).
b Among the positive responders to intramuscular delivery, the neutralization titer range was 26–54, with a titer of ≥25 being the criterion for a positive response.
c For intramuscular delivery with EP, no responses were observed in any arm for the Bal.26, MN.3, NPO3.13, or SF162.LS viruses. Among positive responders, the neutralization titer range was 10–20, with a titer of ≥10 being the criterion for a positive response.
Cellular Immunogenicity
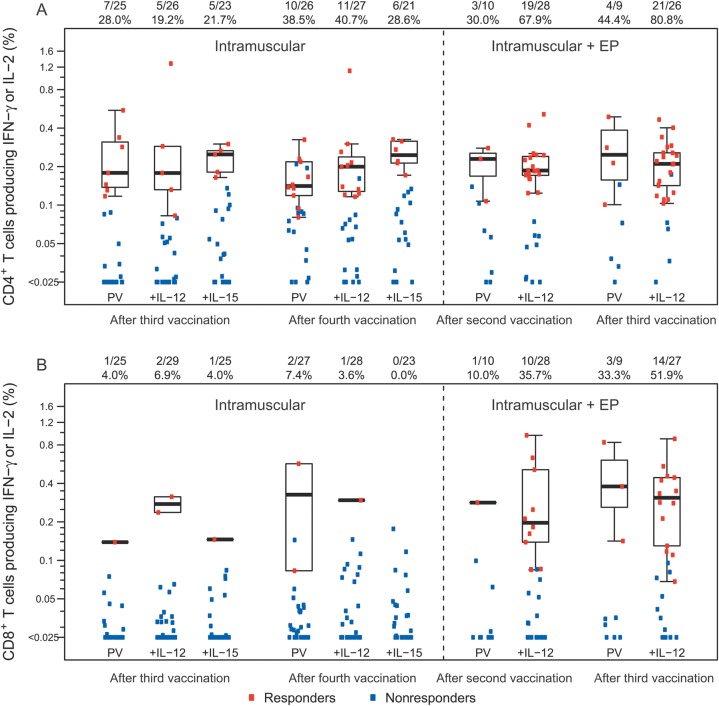
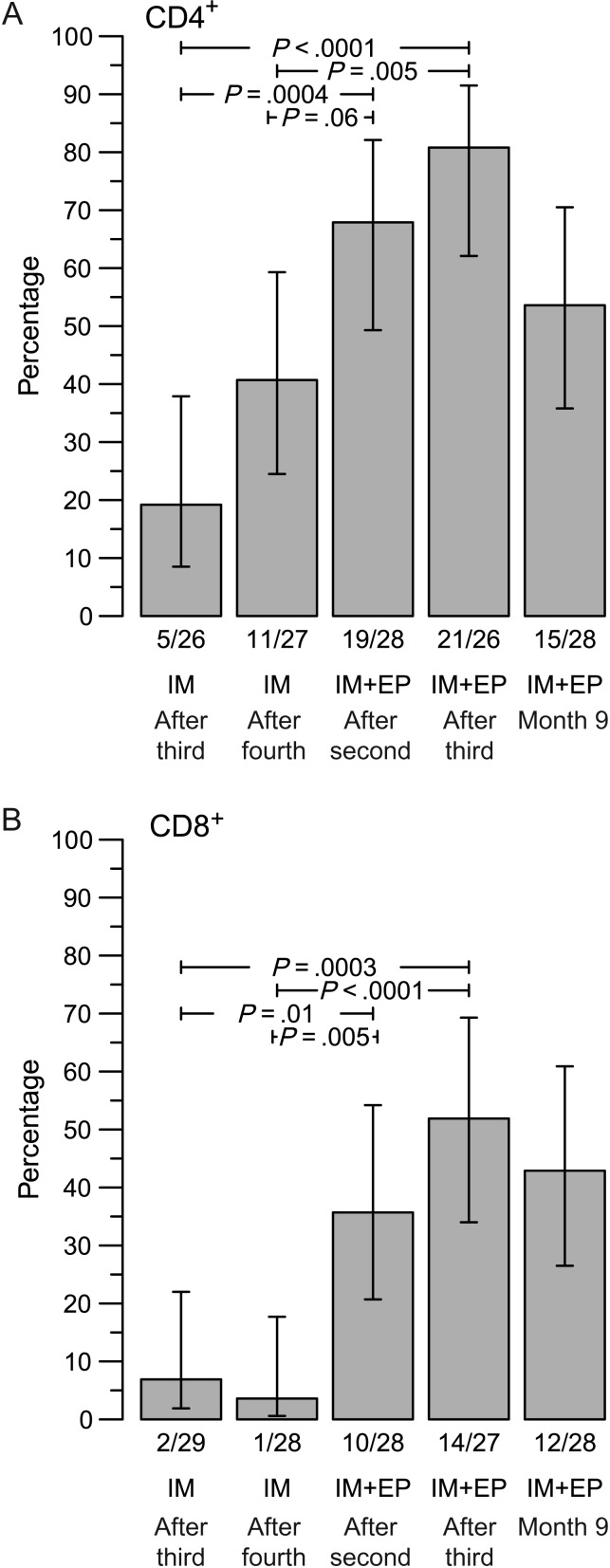
Subjects receiving vaccine developed both HIV-specific CD4+ and CD8+ T-cell responses (Figure 3). In the case of intramuscular injection, 38.5% of subjects (10/26) receiving PV alone developed an HIV-specific CD4+ T-cell response after the fourth vaccination. The addition of IL-12 plasmid (40.7%; 11 of 27 subjects) or IL-15 plasmid (28.6%; 6 of 21 subjects) did not increase the number of responders nor the magnitude of responses. Electroporation increased these response rates considerably, even with lower doses of vaccine. After 2 vaccinations involving intramuscular delivery plus electroporation, 30.0% of PV recipients (3/10) and 67.9% of PV plus IL-12 recipients (19/28) developed HIV-specific CD4+ T-cell responses, and after 3 vaccinations, 44.4% of PV recipients (4/9) and 80.8% of recipients of PV plus IL-12 (21/26) responded. This latter response rate was higher than after 4 vaccinations with PV plus IL-12 without electroporation (P = .005). The response rate for intramuscularly delivered PV plus IL-12 with electroporation was still relatively high (53.6%; 15/28) 6 months after the third vaccination (month 9 in Figure 4).
Figure 3.
Magnitude of the human immunodeficiency virus (HIV)–specific CD4+ and CD8+ T-cell response magnitude. The percentage of CD4+ T-cells (A) and CD8+ T-cells (B) producing interferon γ (IFN- γ) and/or interleukin-2 (IL-2) in response to Env, Gag, or Pol global potential T-cell epitope (PTE) peptide pools 2 weeks after the indicated immunization, as measured by intracellular staining assay. Responders are shown in red colored circles, and nonresponders are shown in blue circles. Box plots show the distribution of the magnitude of response in positive responders only. The box indicates the median and interquartile range (IQR); whiskers extend to the furthest point within 1.5 times the IQR from the upper or lower quartile. Numbers at the top of each panel show the number of responders/number with an assay result and the percentage with positive response. Data from the groups that received PENNVAX-B DNA vaccine (PV) plus 0.8 mg of interleukin 15 (IL-15) intramuscularly are not shown. Abbreviations: EP, electroporation; IFN-γ, interferon γ; IL-12, interleukin 12.
Figure 4.
T-cell response rates for PENNVAX-B DNA vaccine plus interleukin 12 arms, comparing intramuscular (IM) delivery and IM delivery with electroporation (EP). Vertical bars denote 95% confidence intervals calculated by the score method. P values are from Fisher exact tests.
Figure 5.
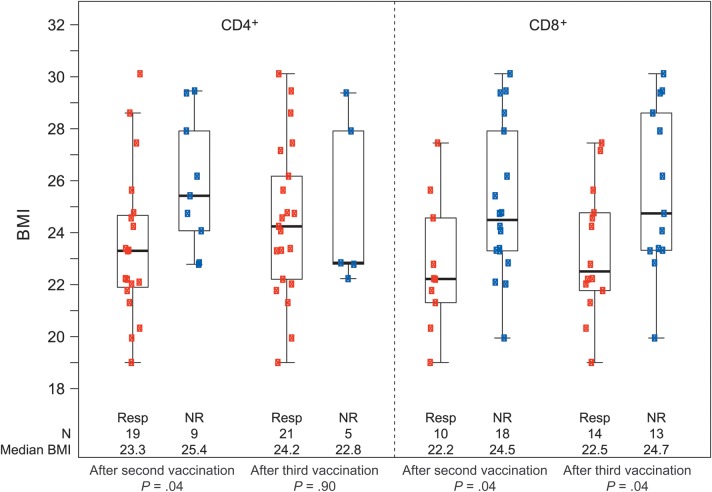
Body mass index (BMI; calculated as the weight in kilograms divided by the height in meters squared) by human immunodeficiency virus (HIV)–specific CD4+ and CD8+ T-cell responses for individuals who received PENNVAX-B DNA vaccine plus interleukin 12 intramuscularly with electroporation. P values are from Wilcoxon rank sum tests comparing the distribution of BMI between responders (Resp) and nonresponders (NR).
Adding electroporation increased the frequency of CD8+ T-cell responses even more dramatically. After 4 vaccinations by intramuscular injection, only 7.4% of individuals (2/27) had a detectable CD8+ T-cell response, and this result was not improved by the addition of plasmid IL-12 (3.6%; 1 of 28 individuals) or IL-15 (0%; 0 of 23 individuals; Figure 3). In contrast, after 3 intramuscularly delivered vaccinations with electroporation, 33.3% of PV recipients and 51.9% of PV plus IL-12 recipients (14/27; P < .0001, compared with the group that received PV plus IL-12 without electroporation) were responders, and 6 months after the third vaccination 42.9% (12/28) still responded (Figure 3). Overall, 71.4% of individuals vaccinated with PV plus IL-12 plasmid intramuscularly with electroporation developed a CD4+ or CD8+ T-cell response after the second vaccination, and 88.9% developed a CD4+ or CD8+ T-cell response after the third vaccination (Figure 4).
In addition to rates of response, we also evaluated the magnitude of responses among responders. No significant differences were observed between arms for either protocol or between like-arms with or without electroporation. For individuals who received PV plus IL-12 intramuscularly with electroporation and responded after the second and third vaccinations, there was no increase in the magnitude of responses between these vaccinations. Only 1 individual with a CD4+ T-cell response after the second vaccination did not respond following the third vaccination, and 2 others did not have an assay result following the third vaccination.
Immune Responses by HIV Protein
For PV plus IL-12 delivered intramuscularly with electroporation, overall immune responses were most often detected against HIV Pol (CD4+ T-cell response, 79.2% [18/26]; CD8+ T-cell response, 58.1% [13/27]), compared with HIV Gag (CD4+ T-cell response, 65.4% [17/26]; CD8+ T-cell response, 7.4% [2/27]) and HIV Env (CD4+ T-cell response, 0% [0/26]; CD8+ T-cell response, 14.8% [4/27]). In contrast to other DNA vaccination studies [2, 3] and a macaque study of the same plasmid [35], very few individuals responded to HIV Env peptides. There was some concern that the PTE peptide set was not well matched to the epitopes encoded by the plasmid DNA. However, additional assays that used a set of peptides matched for the vaccine Env plasmid identified 1 more CD4+ T-cell responder and 3 additional CD8+ T-cell responders (which did not alter response rates significantly).
Immune Responses in Relation to Demographic Characteristics
We excluded subjects from the electroporation trial if their BMI was >30, out of concern that the needle depth (18 mm) might not reach the deltoid muscle. After adjustment for BMI and the other demographic factors in a logistic regression model, the peak CD4+ T-cell response rate difference between the group that received PV plus IL-12 intramuscularly and the group that received PV plus IL-12 intramuscularly with electroporation was statistically significant (odds ratio, 5.5; P = .02). With only 1 CD8+ T-cell responder in the intramuscularly delivered PV plus IL-12 group, modeling was not possible. However, limiting the intramuscular delivery group to those with a BMI of ≤30 and an age of ≤41 years resulted in a response rate of 6.7% (1/15), compared with a response rate of 51.9% observed with intramuscular delivery of PV plus IL-12 with electroporation (P = .003).
As show in Figure 5, individuals who responded to intramuscular delivery of PV plus IL-12 with electroporation after the second vaccination had a significantly lower BMI than nonresponders (median BMI, 23.3 vs 25.4; P = .04). However, after the third vaccination, the majority of subjects had a CD4+ T-cell response, and this difference was not significant. In contrast, for CD8+ T-cell responses, the BMI of responders was lower than that of nonresponders after 2 or 3 vaccinations (P = .04 at both time points). We found no relationship between sex and either CD4+ or CD8+ T-cell response among subjects who received intramuscularly delivery with electroporation.
DISCUSSION
DNA vaccines primarily elicit CD4+ T-cell responses in humans [3]. Previous studies have not yielded appreciable CD8+ T-cell responses without ex vivo expansion of PBMCs for as long as 7 days [36]. However, DNA vaccination can increase CD8+ T-cell responses as part of a prime-boost regimen [37]. While CD4+ T-cell responses dominated in our trials as well, to our knowledge this is the first demonstration that a DNA vaccine can elicit CD8+ T-cell responses of significant magnitude and in >50% of the vaccine recipients. We did not detect significant humoral immune responses over the course of this study. This is not surprising, as these plasmids were not designed to present conformational epitopes for immune recognition. Here, we demonstrate that a combined optimization approach with electroporation significantly enhances the immunogenicity of DNA vaccination and elicits magnitudes of cellular immune responses comparable to those in recently reported HVTN trials of DNA prime-vector–based boost vaccine regimens [38, 39].
In human trials, repeated boosting with DNA alone has not shown appreciable increases in the magnitudes of responses [3, 39]. While we saw increases in the response rates between the second and third vaccination delivered intramuscularly with electroporation, we found no obvious increase in the magnitude of responses in individuals recognizing the same peptide pools after the third vaccination, in contrast to macaque studies [26, 35, 40–43]. The magnitude of these immune responses did not decrease appreciably 6 months after final vaccination with electroporation in the majority of responders.
PV delivered intramuscularly with electroporation demonstrated not only superior immunogenicity, but also a significant dose-sparing effect on the amount of DNA required for a response. We found only modest CD4+ T-cell responses to 6 mg of PV given intramuscularly, and there was no significant increase in the response rate with the addition of IL-12 or IL-15. This dosing required injections at 2 sites. In contrast, higher levels of immune responses were achieved with a single injection of 3 mg of PV DNA and 1 mg of IL-12 DNA intramuscularly with electroporation. In the small group that received PV intramuscularly with electroporation without IL-12, 4 of 9 evaluable individuals developed CD4+ T-cell immune responses, and 3 of 9 developed CD8+ T-cell responses. While these response rates appear lower than with PV plus IL-12 delivered intramuscularly with electroporation, these differences were not statistically significant. Future larger trials may help answer whether IL-12 has a significant effect on the immunogenicity of DNA delivered via electroporation.
Vaccinations in each trial were well tolerated. Electroporation is associated with initial discomfort, but pain diminished rapidly within 25 minutes of vaccination, and the majority of participants indicated a willingness to accept vaccination intramuscularly with electroporation for an important infectious disease.
Despite restricting enrollment in the electroporation protocol to individuals with a BMI of ≤30, we observed a relationship between CD8+ T-cell responses and BMI. Even after 3 vaccinations delivered intramuscularly with electroporation, the response rate tended to be lower in individuals with higher BMIs. The injection needle and the electroporation array had a maximal penetration depth of 18 mm, and it is possible that in some individuals a portion of the vaccine dose did not reach muscle or that some of the dose was outside of the electroporation field. We did not perform deltoid skinfold measurements to evaluate whether this strictly correlated with BMI. New delivery systems for electroporation include devices that deliver DNA intradermally, which may reduce the effects of BMI.
In contrast to other trials, we predominantly found Pol- and Gag-specific immune responses. The reason for the lack of Env-specific immune responses in this study is not clear. Vasan et al published a small trial of ADVAX DNA (clade C/B Env, Gag, Pol, and Nef-Tat) [27] delivered with and without electroporation. After 3 vaccinations, the responses to DNA delivered via electroporation, although modest, were higher than to DNA vaccination alone, reaching a median value of 400 spot-forming cells per million cells, and Env-specific responses were dominant [44]. We used a synthetic consensus clade B Env designed specifically to generate T-cell responses, which elicited balanced responses to Env and Gag in a macaque study [35]. Additional formulations may answer this question, as we only tested a single ratio of plasmid antigens at 1 dose and schedule per trial. Studies in progress are now examining Env designs that focus more on B-cell as well as T-cell responses. It will be interesting to evaluate the effects of electroporation and IL-12 adjuvant with these new designs.
Notes
Acknowledgments. We thank the participants in this trial and the dedicated staff at each of the HVTN clinical sites and laboratories who made the study possible.
D. W. developed the concept; B. M., S. K., S. P., S. E., M. E., D. K., and N. S. designed the study; C. B., M. P., J. B., and J. Y. oversaw preclinical safety or immunogenicity evaluations and/or product testing; N. S., D. B. W., A. K., and J. E. developed and/or supplied the vaccine, adjuvant, or electroporation device; S. K., K. R., S. E., S. P., J. F., B. K., I. F., P. J., M. K., and L. B. oversaw study conduct and managed participants at study sites; S. P., P. J., S. E., S. K., M. A., M. C., A. S., and M. E. provided medical monitoring and study oversight; J. H., S. D., D. C., J. B., and J. K. oversaw performance of immunogenicity assays and interpretation of results; S. K., M. E., and B. M. analyzed the data; and S. K., M. E., B. M., J. H., and D. W. wrote the manuscript.
Disclaimer. This article was written by authors in their capacity as National Institutes of Health (NIH)employees, but the views expressed here do not necessarily represent those of the NIH.
Representatives from the University of Pennsylvania, Profectus BioSciences, which supplied IL-12 plasmid, and Inovio Pharmaceuticals, which supplied DNA vaccine and the electroporation device, had no role in evaluation of the immunogenicity assays or analysis of the data.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grants UM1 AI068614 [to the HVTN Core FHCRC], UM1 AI068635 [to SCHARP], UM1 AI068618 [to the HVTN Laboratory program FHCRC], UM1 AI069452 [to UAB], UM1AI069418 [to Emory], UM1AI069511 [to Rochester], UM1AI069421 [to GHESKIO], UM1 AI069412 [to BWH], UM1 AI069470 [to the New York Blood Center–Bronx, NY Blood Center–Union Square, and Columbia University], UM1 AI069439 [to Vanderbilt], UM1 AI069534 [to UPENN], UM1 AI069496 [to SFDPH], P01-AI071739 [to Penn], HVDDT contract HHSN272200800063C [to Inovio], and CTSA award no. UL1TR000445 to Vanderbilt.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hokey DA, Weiner DB. DNA vaccines for HIV: challenges and opportunities. Springer Semin Immunopathol. 2006;28:267–79. doi: 10.1007/s00281-006-0046-z. [DOI] [PubMed] [Google Scholar]
- 2.Catanzaro AT, Roederer M, Koup RA, et al. Phase I clinical evaluation of a six-plasmid multiclade HIV-1 DNA candidate vaccine. Vaccine. 2007;25:4085–92. doi: 10.1016/j.vaccine.2007.02.050. [DOI] [PubMed] [Google Scholar]
- 3.Graham BS, Koup RA, Roederer M, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. J Infect Dis. 2006;194:1650–60. doi: 10.1086/509259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. 2008;9:776–88. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacGregor RR, Boyer JD, Ugen KE, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J Infect Dis. 1998;178:92–100. doi: 10.1086/515613. [DOI] [PubMed] [Google Scholar]
- 6.MacGregor RR, Ginsberg R, Ugen KE, et al. T-cell responses induced in normal volunteers immunized with a DNA-based vaccine containing HIV-1 env and rev. AIDS. 2002;16:2137–43. doi: 10.1097/00002030-200211080-00005. [DOI] [PubMed] [Google Scholar]
- 7.Muthumani K, Zhang D, Dayes NS, et al. Novel engineered HIV-1 East African Clade-A gp160 plasmid construct induces strong humoral and cell-mediated immune responses in vivo. Virology. 2003;314:134–46. doi: 10.1016/s0042-6822(03)00459-8. [DOI] [PubMed] [Google Scholar]
- 8.Chapman BS, Thayer RM, Vincent KA, Haigwood NL. Effect of intron A from human cytomegalovirus (Towne) immediate-early gene on heterologous expression in mammalian cells. Nucleic Acids Res. 1991;19:3979–86. doi: 10.1093/nar/19.14.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haigwood NL, Pierce CC, Robertson MN, et al. Protection from pathogenic SIV challenge using multigenic DNA vaccines. Immunol Lett. 1999;66:183–8. doi: 10.1016/s0165-2478(98)00156-4. [DOI] [PubMed] [Google Scholar]
- 10.Baden LR, Blattner WA, Morgan C, et al. Timing of plasmid cytokine (IL-2/Ig) administration affects HIV-1 vaccine immunogenicity in HIV-seronegative subjects. J Infect Dis. 2011;204:1541–9. doi: 10.1093/infdis/jir615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barouch DH, Santra S, Schmitz JE, et al. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–92. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 12.Morrow MP, Pankhong P, Laddy DJ, et al. Comparative ability of IL-12 and IL-28B to regulate Treg populations and enhance adaptive cellular immunity. Blood. 2009;113:5868–77. doi: 10.1182/blood-2008-11-190520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraynyak KA, Kutzler MA, Cisper NJ, et al. Plasmid-encoded interleukin-15 receptor alpha enhances specific immune responses induced by a DNA vaccine in vivo. Hum Gene Ther. 2009;20:1143–56. doi: 10.1089/hum.2009.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 15.Wolf SF, Temple PA, Kobayashi M, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991;146:3074–81. [PubMed] [Google Scholar]
- 16.Giri JG, Anderson DM, Kumaki S, Park LS, Grabstein KH, Cosman D. IL-15, a novel T cell growth factor that shares activities and receptor components with IL-2. J Leukoc Biol. 1995;57:763–6. doi: 10.1002/jlb.57.5.763. [DOI] [PubMed] [Google Scholar]
- 17.Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–8. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 18.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–90. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker TC, Wherry EJ, Boone D, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–8. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wherry EJ, Becker TC, Boone D, Kaja MK, Ma A, Ahmed R. Homeostatic proliferation but not the generation of virus specific memory CD8 T cells is impaired in the absence of IL-15 or IL-15Ralpha. Adv Exp Med Biol. 2002;512:165–75. doi: 10.1007/978-1-4615-0757-4_22. [DOI] [PubMed] [Google Scholar]
- 21.Wherry EJ, Teichgraber V, Becker TC, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–34. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 22.Cemazar M, Golzio M, Sersa G, Rols MP, Teissie J. Electrically-assisted nucleic acids delivery to tissues in vivo: where do we stand? Curr Pharm Des. 2006;12:3817–25. doi: 10.2174/138161206778559740. [DOI] [PubMed] [Google Scholar]
- 23.Favard C, Dean DS, Rols MP. Electrotransfer as a non viral method of gene delivery. Curr Gene Ther. 2007;7:67–77. doi: 10.2174/156652307779940207. [DOI] [PubMed] [Google Scholar]
- 24.Gothelf A, Mir LM, Gehl J. Electrochemotherapy: results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat Rev. 2003;29:371–87. doi: 10.1016/s0305-7372(03)00073-2. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Kjeken R, Mathiesen I, Barouch DH. Recruitment of antigen-presenting cells to the site of inoculation and augmentation of human immunodeficiency virus type 1 DNA vaccine immunogenicity by in vivo electroporation. J Virol. 2008;82:5643–9. doi: 10.1128/JVI.02564-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luckay A, Sidhu MK, Kjeken R, et al. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J Virol. 2007;81:5257–69. doi: 10.1128/JVI.00055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Y, Chen Z, Zhang W, et al. Design, construction, and characterization of a dual-promoter multigenic DNA vaccine directed against an HIV-1 subtype C/B’ recombinant. J Acquir Immune Defic Syndr. 2008;47:403–11. doi: 10.1097/QAI.0b013e3181651b9d. [DOI] [PubMed] [Google Scholar]
- 28.Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol. 2011;23:421–9. doi: 10.1016/j.coi.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalams SA, Parker S, Jin X, et al. Safety and immunogenicity of an HIV-1 gag DNA vaccine with or without IL-12 and/or IL-15 plasmid cytokine adjuvant in healthy, HIV-1 uninfected adults. PLoS One. 2012;7:e29231. doi: 10.1371/journal.pone.0029231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McElrath MJ, De Rosa SC, Moodie Z, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao HX, Sutherland LL, Xia SM, et al. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C HIV-1 primary viruses. Virology. 2006;353:268–82. doi: 10.1016/j.virol.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goepfert PA, Tomaras GD, Horton H, et al. Durable HIV-1 antibody and T-cell responses elicited by an adjuvanted multi-protein recombinant vaccine in uninfected human volunteers. Vaccine. 2007;25:510–8. doi: 10.1016/j.vaccine.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 33.Tomaras GD, Yates NL, Liu P, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol. 2008;82:12449–63. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montefiori DC. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol. 2009;485:395–405. doi: 10.1007/978-1-59745-170-3_26. [DOI] [PubMed] [Google Scholar]
- 35.Hirao LA, Wu L, Khan AS, et al. Combined effects of IL-12 and electroporation enhances the potency of DNA vaccination in macaques. Vaccine. 2008;26:3112–20. doi: 10.1016/j.vaccine.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang R, Doolan DL, Le TP, et al. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science. 1998;282:476–80. doi: 10.1126/science.282.5388.476. [DOI] [PubMed] [Google Scholar]
- 37.De Rosa SC, Thomas EP, Bui J, et al. HIV-DNA priming alters T cell responses to HIV-adenovirus vaccine even when responses to DNA are undetectable. J Immunol. 2011;187:3391–401. doi: 10.4049/jimmunol.1101421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Churchyard GJ, Morgan C, Adams E, et al. A phase IIA randomized clinical trial of a multiclade HIV-1 DNA prime followed by a multiclade rAd5 HIV-1 vaccine boost in healthy adults (HVTN204) PLoS One. 2011;6:e21225. doi: 10.1371/journal.pone.0021225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goepfert PA, Elizaga ML, Sato A, et al. Phase 1 safety and immunogenicity testing of DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis. 2011;203:610–9. doi: 10.1093/infdis/jiq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosati M, Bergamaschi C, Valentin A, et al. DNA vaccination in rhesus macaques induces potent immune responses and decreases acute and chronic viremia after SIVmac251 challenge. Proc Natl Acad Sci U S A. 2009;106:15831–6. doi: 10.1073/pnas.0902628106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winstone N, Wilson AJ, Morrow G, et al. Enhanced control of pathogenic Simian immunodeficiency virus SIVmac239 replication in macaques immunized with an interleukin-12 plasmid and a DNA prime-viral vector boost vaccine regimen. J Virol. 2011;85:9578–87. doi: 10.1128/JVI.05060-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schadeck EB, Sidhu M, Egan MA, et al. A dose sparing effect by plasmid encoded IL-12 adjuvant on a SIVgag-plasmid DNA vaccine in rhesus macaques. Vaccine. 2006;24:4677–87. doi: 10.1016/j.vaccine.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 43.Hirao LA, Wu L, Satishchandran A, et al. Comparative analysis of immune responses induced by vaccination with SIV antigens by recombinant Ad5 vector or plasmid DNA in rhesus macaques. Mol Ther. 2010;18:1568–76. doi: 10.1038/mt.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vasan S, Hurley A, Schlesinger SJ, et al. In vivo electroporation enhances the immunogenicity of an HIV-1 DNA vaccine candidate in healthy volunteers. PLoS One. 2011;6:e19252. doi: 10.1371/journal.pone.0019252. [DOI] [PMC free article] [PubMed] [Google Scholar]