Abstract
Background. Type 2 diabetes mellitus (DM) is a major risk factor for the development of active pulmonary tuberculosis, although the immunological mechanisms underlying this interaction remain unexplored. The influence of poorly controlled diabetes on pathogen-specific T-helper 1 (Th1) and T-helper 17 (Th17) responses have not been examined.
Methods. To identify the role of Th1 and Th17 cells in tuberculosis with coincident DM, we examined mycobacteria-specific immune responses in the whole blood of individuals who had tuberculosis with DM and compared them to those in individuals who had tuberculosis without DM.
Results. Tuberculosis coincident with DM is characterized by elevated frequencies of monofunctional and dual-functional CD4+ Th1 cells following Mycobacterium tuberculosis antigen stimulation and elevated frequencies of Th17 subsets at both baseline and following antigen stimulation. This was associated with increased systemic (plasma) levels of both Th1 and Th17 cytokines and decreased baseline frequencies of natural regulatory T cells but not interleukin 10 or transforming growth factor β.
Conclusions. Therefore, our data reveal that tuberculosis in persons with DM is characterized by elevated frequencies of Th1 and Th17 cells, indicating that DM is associated with an alteration in the immune response to tuberculosis, leading to a biased induction of Th1- and Th17-mediated cellular responses and likely contributing to increased immune pathology in M. tuberculosis infection.
Keywords: T cells, bacterial, cytokines, tuberculosis, diabetes mellitus
The association between diabetes mellitus (DM) and tuberculosis and their synergistic role in causing human disease has been appreciated for a long time but has only lately become a major topic of clinical and fundamental research [1]. While tuberculosis continues to be a major disease burden in developing countries, the rise in prevalence of type 2 DM has been rapid and relentless, and the dual burden of diabetes and tuberculosis clearly represents a serious global public health concern [2]. There is growing evidence that DM is an important risk factor for developing active pulmonary tuberculosis [3]. Based on murine models and human studies, immunity to Mycobacterium tuberculosis requires T-helper 1 (Th1) responses and (to a lesser extent) T-helper 17 (Th17) responses [4, 5]. Thus, interleukin 12, interferon γ (IFN-γ), and tumor necrosis factor α (TNF–α; along with interleukin 17 [IL–17] and interleukin 23) all play important roles in the induction and maintenance of protective immune responses against tuberculous [6–11]. Furthermore, multifunctional T cells, defined by their ability to coexpress ≥2 cytokines, have also been associated with resistance to infection in animal models [12] and in some human studies [13, 14].
To study influence of DM on CD4+ T-cell responses in active pulmonary tuberculosis, we examined baseline, antigen–specific, and polyclonal induction of single-, double-, and triple-cytokine–producing cells of the Th1 and Th17 subsets in individuals with active tuberculosis and coincident DM and compared them to individuals with active tuberculosis without diabetes. We show that those with tuberculosis coincident with DM have elevated frequencies of single- and double-cytokine–producing CD4+ Th1 cells, as well as increased frequencies of Th17 subsets following mycobacterial antigen stimulation in comparison to individuals with tuberculosis and without DM. We also show that this expansion of Th1 and Th17 cells is associated with increased systemic (plasma) levels of Th1- and Th17-associated cytokines. Thus, our data demonstrate that diabetes is associated with a profound alteration in the CD4+ T-cell response to M. tuberculosis, which possibly contributes to increased severity and/or immune-mediated pathology in tuberculosis.
MATERIALS AND METHODS
Study Population
We studied a group of 44 individuals in south India with active pulmonary tuberculosis; 22 had DM, and 22 did not have DM. Pulmonary tuberculosis was diagnosed on the basis of both sputum smear and culture positivity. DM was diagnosed on the basis of glycated hemoglobin (HbA1c) levels and random measurement of blood glucose level, according to the American Diabetes Association criteria (all DM individuals had HbA1c levels of >6.5% and randomly measured blood glucose levels of >200 mg/dL). All individuals were human immunodeficiency virus negative. The 2 groups did not differ significantly in terms of sputum smear grades or radiological extent of disease. All individuals were naive to antituberculous treatment. Anthropometric measurements, including height, weight, and waist circumference, and biochemical parameters, including plasma glucose level, lipid profile, and HbA1c level, were obtained using standardized techniques as detailed elsewhere [15]. Hematologic analysis was performed on all individuals, using the Act-5 Diff hematology analyzer (Beckman Coulter). All individuals were examined as part of a clinical protocol approved by the Institutional Review Board of the National Institute of Research in Tuberculosis (clinical trials registration NCT01154959), and informed, written consent was obtained from all participants.
Ex vivo Analysis
All antibodies used in the study were from BD Biosciences, BD Pharmingen, eBioscience, or R&D Systems. Absolute numbers of CD4+ T cells were enumerated in whole blood, using the BD Multiset 6-Color TBNK cocktail (BD Biosciences). Naive and memory T-cell phenotyping was performed using CD45RA and CCR7 staining on CD4+ T cells and natural regulatory T-cell (nTreg) phenotyping was performed using CD25, Foxp3, and CD127. Ex vivo intracellular staining for Ki-67 expression on CD4+ T cells was performed.
Antigens
M. tuberculosis antigens used were purified protein derivative (PPD; Serum Statens Institute), early secreted antigen-6 (ESAT-6; Fitzgerald Industries), and culture filtrate protein-10 (CFP-10; Fitzgerald Industries). Final concentrations were 10 µg/mL for PPD, ESAT-6, and CFP-10 and 5 µg/mL for anti–CD3.
In Vitro Culture
Whole-blood-cell cultures were performed to determine the intracellular levels of cytokines. Briefly, whole blood was diluted 1:1 with Roswell Park Memorial Institute 1640 medium supplemented with penicillin/streptomycin (100 U/100 mg/mL), l-glutamine (2 mM), and HEPES (10 mM; all from Invitrogen) and distributed in 12-well tissue culture plates (Costar). The cultures were then stimulated with ESAT-6, CFP-10, or anti-CD3 or with medium alone in the presence of the costimulatory molecules CD49d/CD28 at 37°C for 6 hours. Brefeldin A (10 µg/mL) was added after 2 hours. After 6 hours, centrifugation, washing, and red blood cell lysis was performed. The cells were fixed using cytofix/cytoperm buffer (BD Biosciences) and cryopreserved at −80°C.
Intracellular Cytokine Staining
The cells were thawed, washed, and stained with surface antibodies for 30–60 minutes. Surface antibodies used were CD3, CD4, and CD8. The cells were washed and permeabilized with BD Perm/Wash buffer (BD Biosciences) and stained with intracellular cytokines for an additional 30 minutes before washing and acquisition. Cytokine antibodies used were IFN-γ, TNF-α, interleukin 2 (IL-2), interleukin 10 (IL-10), IL-17A and interleukin 22 (IL-22). Eight-color flow cytometry was performed on a FACSCanto II flow cytometer with FACSDiva software, version 6 (Becton Dickinson). The lymphocyte gating was set by forward and side scatter, and 100 000 lymphocytes events were acquired. Data were collected and analyzed using Flow Jo software (TreeStar). All data are depicted as the frequency of CD4+ T cells expressing cytokine(s). Baseline values following stimulation with medium are depicted as baseline frequency, while frequencies following stimulation with antigens are depicted as net frequencies (with baseline values subtracted).
Enzyme-Linked Immunosorbent Assay (ELISA)
IL–2, IFN–γ, TNF–α, IL–10, and IL-17A levels were measured using Bioplex multiplex cytokine assay system (Biorad), and IL-22 and transforming growth factor β (TGFβ) levels were measured by ELISA, using kits from R&D Systems.
Statistical Analysis
Data analyses were performed using GraphPad PRISM (GraphPad Software). Geometric means (GMs) were used for measurements of central tendency. Statistically significant differences between 2 groups were analyzed using the nonparametric Mann–Whitney U test. Multiple comparisons were corrected using the Holm correction. To observe whether the effects of DM persist after controlling for factors that tend to be related to DM, we fit linear models on the log-transformed values to the DM status, including a binary term for sex and linear terms for age, body mass index (BMI; calculated as the weight in kilograms divided by the height in meters squared), cholesterol level, and triglyceride levels. We then tested for significance in this model by the likelihood ratio test. The linear models were fit in R (version 2.15.2).
RESULTS
Study Population Characteristics
Baseline characteristics, including demographic characteristics and clinical and biochemical features, of the study population are shown in Table 1. As can be seen, compared with subjects without diabetes, those with diabetes had higher fasting blood glucose, glycated hemoglobin, serum cholesterol, low-density lipoprotein cholesterol, and triglyceride levels but lower high-density lipoprotein cholesterol levels. The groups did not differ in their measured hematological parameters (Table 2), nor were there differences in the 2 groups in the absolute numbers of CD4+ and CD8+ T cells, B cells, and natural killer cells (data not shown).
Table 1.
Demographic Profile of Patients With Pulmonary Tuberculosis, by Presence or Absence of Diabetes Mellitus (DM)
| Characteristic | DM Present (n = 22) | DM Not Present (n = 22) | P |
|---|---|---|---|
| Age, y | 49.1 (40–58) | 45.4 (40–55) | NS |
| Sex, M/F | 16/6 | 18/5 | NS |
| BMIa | 23.5 (18.43–28.55) | 21.4 (15.62–31.22) | NS |
| Diabetes duration, y | 4.5 (1–28) | ||
| Systolic blood pressure, mm Hg | 116.4 (91–169) | 118.08 (90–150) | NS |
| Diastolic blood pressure, mm Hg | 73.6 (58–103) | 74.7 (40–112) | NS |
| Random glucose level, mg/dL | 280.4 (147–587) | 95.5 (76–177) | <.0001 |
| Glycated hemoglobin level, % | 11.20 (7.54–14.78) | 5.33 (4.46–6.12) | <.0001 |
| Total cholesterol level, mg/dL | 209.09 (163–259) | 177.51 (134–235) | .0021 |
| Serum triglyceride level, mg/dL | 199.14 (84–679) | 96.83 (57–433) | <.0001 |
| High-density lipoprotein cholesterol level, mg/dL | 39.15 (29–57) | 49.93 (25–86) | .0066 |
| Low-density lipoprotein cholesterol level, mg/dL | 119.84 (47–185) | 102.16 (62–136) | .0460 |
Data are no. of subjects or median (range)
Abbreviation: NS, not significant.
a Body mass index (BMI) is calculated as the weight in kilograms divided by the square of the height in meters.
Table 2.
Hematologic Profile of Patients With Pulmonary Tuberculosis, by Presence or Absence of Diabetes Mellitus (DM)
| Characteristic | DM Present | DM Not Present | P |
|---|---|---|---|
| Red blood cell count, ×106 cells/μL | 4.9 (3.7–7.6) | 4.2 (3.3–5.7) | NS |
| White blood cell count, ×103 cells/μL | 14 911.5 (7900–19700) | 10 101.5 (5400–21 200) | NS |
| Lymphocyte count, cells/mL | 1725 (1012–2240) | 1687 (850–3315) | NS |
| Neutrophil count, cells/mL | 8455.3 (4929.6–16 075.2) | 6891.1 (3364.2–16735.9) | NS |
| Monocyte count, cells/mL | 875.6 (539.5–1033.6) | 780.3 (286.2–1632.8) | NS |
| Eosinophil count, cells/mL | 197.1 (91.3–394.4) | 224.8 (64.8–258.7) | NS |
| Basophil count, cells/mL | 78.4 (49.8–108.8) | 84.4 (23.1–99.5) | NS |
| Platelet count, ×103 platelets/μL | 346.7 (166–501) | 320.4 (113–551) | NS |
Abbreviation: NS, not significant.
Tuberculosis Coincident With DM Is Associated With Increased Frequencies of Antigen–Specific Mono- and Dual-Functional CD4+ Th1 cells
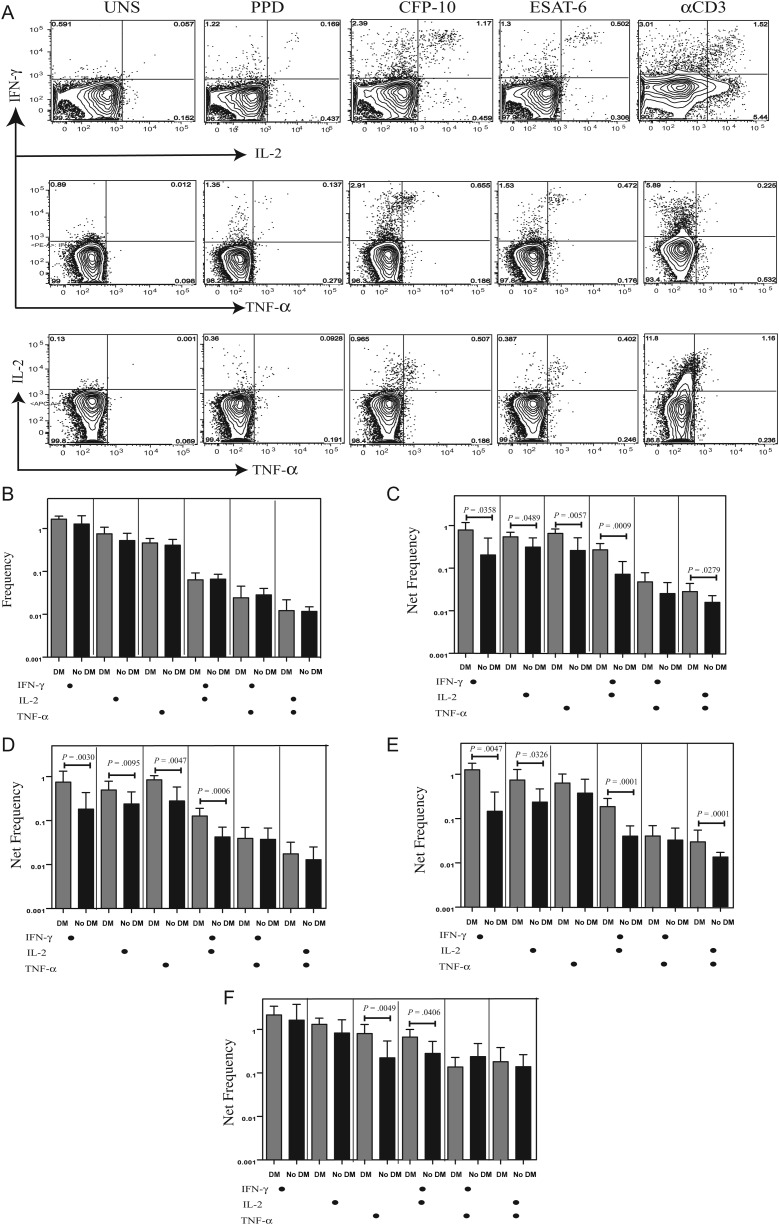
CD4+ T cells play a key role in immune control of M. tuberculosis infection, and the frequency of multifunctional, cytokine-producing, antigen-specific Th1 cells has been associated with control of infection [12, 16]. To determine the influence of DM on Th1 cells in active tuberculosis, we used multiparameter flow cytometry to define the frequencies CD4+ T cells expressing IFN-γ, IL-2, and/or TNF-α at baseline and following stimulation with either mycobacterial antigens or anti-CD3 (Figure 1A). As shown in Figure 1B, there were no differences in the frequencies of CD4+ T cells expressing either only 1, 2, or 3 cytokines at baseline in patients with DM as compared to those without DM. In contrast, in response to PPD (Figure 1C), CFP-10 (Figure 1D), and ESAT-6 (Figure 1E), we observed significantly elevated frequencies of CD4+ T cells expressing single cytokines (IFN-γ, IL-2, or TNF-α) or different combinations of 2 cytokines (IFN-γ and IL-2, IFN-γ and TNF-α, or IL-2 and TNF-α) in patients with DM, compared with those without DM. The frequency of multifunctional CD4+ T cells expressing all 3 cytokines was below the threshold of detection. Finally, stimulation with anti-CD3 did not induce significant differences in the net frequencies of Th1 cells between the 2 groups, with the exception of CD4+ T cells expressing TNF-α alone or both IFN-γ and IL-2 (Figure 1E), indicating that the increased frequency of Th1 cells induced in patients with DM was predominantly pathogen specific.
Figure 1.
Elevated antigen-specific frequencies of mono- and dual-functional T-helper 1 (Th1) cells in patients with tuberculosis coincident with diabetes mellitus (DM). A, Flow data from a representative whole-blood intracellular cytokine assay for a patient with tuberculosis coincident with DM showing expression of interferon γ (IFN-γ), interleukin 2 (IL-2), and tumor necrosis factor α (TNF-α). The plots shown are gated on CD3+CD4+ T cells. B, The baseline frequencies of CD4+ T cells expressing 1, 2, or 3 cytokines (IFN-γ, IL-2, and/or TNF-α) are shown as bar graphs, with the bars representing the geometric mean of the frequency of CD4+ T cells expressing the respective cytokine(s) and the error bars representing the 95% confidence interval in patients with tuberculosis coincident with DM (n = 22) and those with tuberculosis only (n = 22). C–E, The net frequency of CD4+ T cells expressing 1, 2, or 3 cytokines in response to purified protein derivative (PPD; C), culture filtrate protein-10 (CFP-10; E), and early secreted antigen-6 (ESAT-6; E) is shown in patients with tuberculosis coincident with DM and those with tuberculosis only. F, The net frequency of CD4+ T cells expressing the different cytokines in response to anti-CD3 stimulation is shown in patients with tuberculosis coincident with DM and those with tuberculosis only. Net frequencies were calculated by subtracting baseline frequencies from antigen- or anti-CD3–stimulated frequencies. P values were calculated using the Mann–Whitney U test.
Tuberculosis Coincident With DM Is Associated With Increased Frequencies of Baseline and Antigen–Specific CD4+ Th17 Subsets
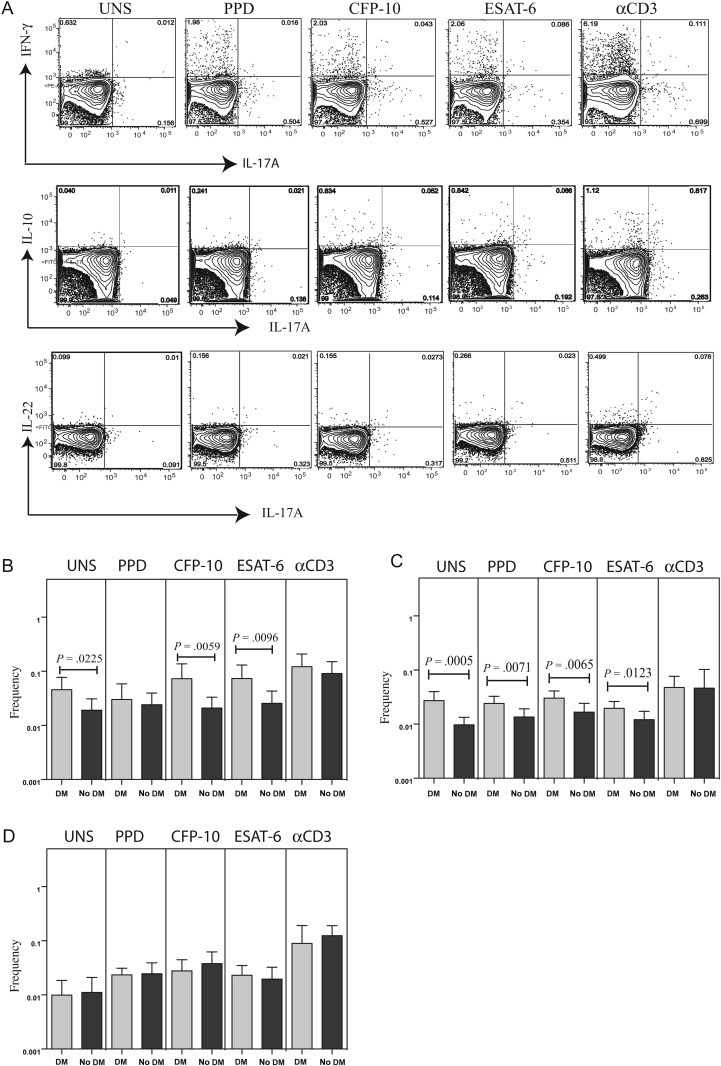
CD4+ Th17 cells are thought to play an important role in memory responses to M. tuberculosis infection, but IL-17 is also thought to contribute to pathology [17]. To determine the influence of DM on Th17 subsets in active tuberculosis, we measured the frequencies of several CD4+ Th17 subsets—CD4+ T cells expressing IL-17A, IFN-γ or IL-17A, IL-22 or IL-17A, IL-10 (Figure 2A)—in the absence of stimulation (baseline) and following stimulation with mycobacterial antigens or anti-CD3. As shown in Figure 2B and 2C, there were significantly elevated frequencies of CD4+ T cells expressing IL-17A, IFN-γ or IL-17A, IL-10 at baseline and following M. tuberculosis antigen stimulation but not after anti-CD3 stimulation in patients with DM, compared with individuals without DM. On the other hand, no significant differences in the frequency of CD4+ T cells expressing IL-17A, IL-22 were observed between patients with DM and those without DM (Figure 2D). Thus, DM is associated with enhanced frequencies of CD4+ Th17 subsets in individuals with active tuberculosis.
Figure 2.
Elevated baseline and antigen-specific frequencies of T-helper 17 (Th17) subsets in patients with tuberculosis coincident with diabetes mellitus (DM). A, Flow data from a representative whole-blood intracellular cytokine assay from an individual with tuberculosis coincident with DM, showing expression of interleukin 17A (IL-17A), interferon γ (IFN-γ), interleukin 22 (IL-22), and interleukin 10 (IL-10). The plots shown are gated on CD3+CD4+ T cells. B–D, The baseline or net frequencies of CD4+ T cells coexpressing IL-17A and IFN-γ (B), IL-17A and IL-10 (C), or IL-17A and IL-22 (D) in response to purified protein derivative (PPD), culture filtrate protein-10 (CFP-10), early secreted antigen-6 (ESAT-6), and anti-CD3 are shown as bar graphs, with the bar representing the geometric mean of the frequency of CD4+ T cells expressing the respective cytokine(s), and the error bar representing the 95% confidence interval in patients with tuberculosis coincident with DM (n = 22) and those with tuberculosis only (n = 22). Net frequencies were calculated by subtracting baseline frequencies from antigen- or anti-CD3–stimulated frequencies. P values were calculated using the Mann–Whitney U test.
Tuberculosis Coincident With DM Is Associated With Increased Systemic Levels of Th1 and Th17 Cytokines
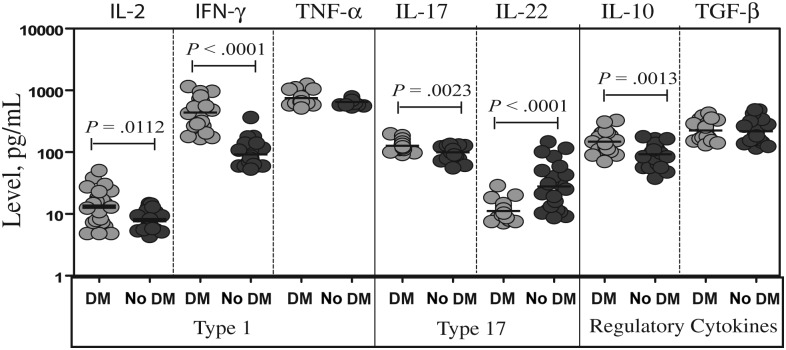
To study the association between the frequencies of Th1 and Th17 cells and systemic immune responses in patients with tuberculosis coincident with DM, we measured the plasma levels of Th1 (IFN-γ, TNF-α, and IL-2)–associated and Th17 (IL-17A and IL-22)–associated cytokines, as well as the known regulatory cytokines IL-10 and TGF-β. As shown in Figure 3, plasma levels of IL-2 (GM, 13.1 pg/mL in patients with DM and 7.9 pg/mL in those without DM; P = .0112), IFN-γ (GM, 439.2 and 91.6 pg/mL, respectively; P < .0001), and IL-17A (GM, 126.5 and 99.1 pg/mL, respectively; P = .0023) were significantly higher in patients with DM as compared to individuals without DM. In contrast, plasma levels of IL-22 were significantly lower in patients with DM, compared with patients with DM (GM, 10.0 vs 31.3 pg/mL; P < .0001). Finally, the circulating levels of IL-10 (GM, 148.5 vs 98.9 pg/mL; P = .0013) but not TGF-β were higher in patients with DM than in those without DM. Thus, while systemic levels of proinflammatory cytokines are higher in patients with tuberculosis coincident with DM, this is not reflective of a corresponding decrease in immunoregulatory cytokines.
Figure 3.
Tuberculosis coincident with diabetes mellitus (DM) is associated with alterations in the levels of T-helper 1 (Th1) and T-helper 17 (Th17) cytokines. The plasma levels of Th1 (interleukin 2 [IL-2], interferon γ [IFN-γ], and tumor necrosis factor α [TNF-α]), Th17 (IL-17A and interleukin 22 [IL-22]), and immunoregulatory (interleukin 10 [IL-10] and transforming growth factor β [TGF-β]) cytokines were measured by enzyme-linked immunosorbent assay in patients with tuberculosis coincident with DM and tuberculosis-only individuals. The results are shown as scatter plots with each circle representing a single individual. P values were calculated using the Mann–Whitney U test.
Multivariate Analysis Confirms the Association of Tuberculosis Coincident With DM With Increased Antigen-Driven Th1 Cells and Proinflammatory Cytokines
To confirm the association of tuberculosis coincident with DM with elevated frequencies of Th1 cells and proinflammatory cytokines, we tested for the effect of DM on each variable after controlling for the effects of age, sex, BMI, cholesterol level, and triglyceride levels. We performed a likelihood ratio test for the DM effect, controlling for age, sex, BMI, cholesterol level, and triglyceride levels, by comparing 2 linear models: (1) a model with the flow readout as the response (log transformed) and independent variables for age (continuous), sex, BMI (continuous), cholesterol level (continuous), and triglyceride levels (continuous), and (2) a model as in (1), but also with an independent variable for DM (yes or no). DM was associated with significant increases in the frequency of most PPD-, ESAT-6–, and CFP-10–induced mono- and dual-functional Th1 cells. The magnitude of the significant associations was as follows: for PPD: IFN-γ (adjusted relative risk [RR], 3.6; adjusted P = .0131), TNF-α (RR, 3.5; P = .0101), and IL-2 and TNF-α (RR, 2.1; P = .0416); for CFP-10: IL-2 (RR, 2.8, P = .0422), TNF-α (RR, 2.7; P = .0243), and IFN-γ and IL-2 (RR, 3.5; P = .0051); for ESAT-6: IFN-γ (RR, 5.8; P = .0119), IL-2 (RR, 3.1; P = .0175), IFN-γ and IL-2 (RR, 4.6; P < .0001), and IL-2 and TNF-α (RR, 2.3; P = .0156). Similarly, DM was also independently associated with the increased circulating levels of proinflammatory cytokines [IFN-γ (RR, 4.8; P < .0001), IL-2 (RR, 1.6; P = .0294), and IL-17 (RR, 1.3; P = .0303)], as well as IL-10 (RR, 1.5; P = .0081), in individuals with active tuberculosis. Thus, DM appears to profoundly influence the immune responses to tuberculosis, independent of other common covariables.
Tuberculosis Coincident With DM Is Associated With Decreased Baseline Frequencies of nTregs but Not Central or Effector Memory T Cells
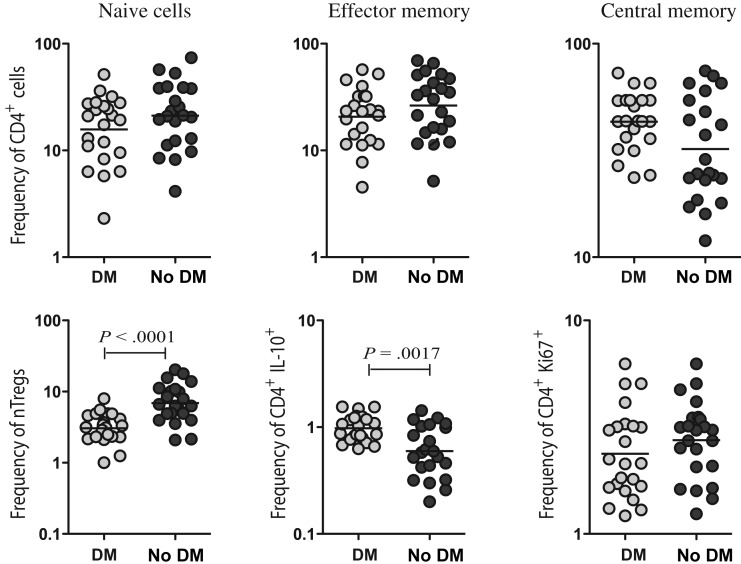
We next examined the frequencies of CD4+ T cell memory subsets, frequencies of nTregs, or CD4+ T cells expressing IL-10 and the recent activation/proliferation status of CD4+ T cells at baseline, because alterations in any of the above-mentioned parameters could influence the frequency of Th1 and Th17 cells in patients with tuberculosis coincident with DM. Our data reveal that DM is not associated with significant alterations in the frequencies of naive, central memory, and effector memory CD4+ T cells, compared with no DM (Figure 4A). Since nTregs have been shown to be associated with downregulation of Th1 responses in active M. tuberculosis infection [18, 19], we also examined the baseline frequency of nTregs in patients with and patients without DM. Interestingly, the nTreg population was present at a significantly lower frequency in patients with DM as compared to those without DM (Figure 4B). In addition, as shown in Figure 4B, DM was associated with increased rather than decreased frequencies of CD4+ T cells expressing IL-10. Finally, we measured Ki-67 expression to determine baseline activation/proliferation of CD4+ T cells and found no significant difference between the 2 groups. Thus, our data suggest that decreased frequencies of nTregs are associated enhanced CD4+ Th1 and Th17 cytokine responses in patients with tuberculosis coincident with DM.
Figure 4.
Tuberculosis coincident with diabetes mellitus (DM) is associated with decreased frequencies of natural regulatory T-cells, but not central or effector memory T cells. A, Percentages of naive (defined as CD45RA+CCR7+), central memory (defined as CD45RA−CCR7+), and effector memory (defined as CD45RA−CCR7−) CD4+ T cells in patients with tuberculosis coincident with DM and tuberculosis-only individuals. B, Percentages of natural regulatory T cells (defined as CD4+, CD25+, Foxp3+, and CD127dim), CD4+ T cells expressing interleukin 10 (IL-10), and CD4+ T cells expressing Ki-67 in patients with tuberculosis coincident with DM and those with tuberculosis only. The results are shown as scatter plots, with each circle representing a single individual. P values were calculated using the Mann–Whitney U test.
DISCUSSION
Multiple epidemiologic studies have shown that DM is a major risk factor for active pulmonary tuberculosis, as well as a predictor of poor treatment outcomes and reduced survival among those with tuberculosis [1]. A recent meta-analysis of 13 observational studies on the risk for tuberculosis in diabetic individuals determined that diabetic patients were 3.1 times more likely to have tuberculosis than nondiabetic individuals [3]. Several studies have also suggested a dose effect, with tuberculosis risk increasing with DM severity [3, 20]. In recent decades, in developing countries such as India, the incidence of tuberculosis remains high, while the prevalence of DM is rising alarmingly. In fact, a recent study in Chennai demonstrated that the prevalence of type 2 diabetes in patients with tuberculosis attending outpatient clinics was approximately 25% and that another 25% of these patients were prediabetic [21]. The immunological basis for this susceptibility to tuberculosis among individuals with DM is not well understood. One possible mechanism is that an impaired immune response in diabetic patients facilitates either primary infection with M. tuberculosis or reactivation of latent tuberculosis [22]. Studies examining the innate and adaptive immune response to microbial antigens in diabetic patients suggest that these responses are compromised, particularly in patients with chronic hyperglycemia [23–25]. Whether this applies to M. tuberculosis infection remains unclear. Indeed, mice with chronic hyperglycemia exhibit deficient priming of the adaptive immune response, resulting in a higher bacterial burden in the lung that is associated with an exuberant (not impaired) Th1 response, leading to immune-mediated pathology [26]. Interestingly, these data mirror the finding that patients with tuberculosis coincident with DM overexpress cytokines that are normally protective during tuberculosis [27].
Our findings reveal 2 interesting features. First, while there were no differences in the spontaneously expressed frequency of CD4+ Th1 cells of the mono- and dual-functional variety, M. tuberculosis antigen stimulation resulted in an exaggerated expansion of these cells in diabetic individuals. Second, the expansion of mono- and dual-functional Th1 cells in patients with tuberculosis coincident with DM was relatively pathogen specific, since the differences in the Th1 frequency profiles between the 2 groups of patients with tuberculosis were almost completely abolished when stimulation with a polyclonal stimulus (anti-CD3) was compared. Our study, therefore, confirms an important role for Th1 cells in the pathogenesis of tuberculosis in DM and suggests that elevated frequencies of Th1 cells might actually reflect an enhanced severity of disease or may reflect a higher antigen load. Since unrestrained expansion of Th1 cells is also known to contribute to pathology in several infectious and autoimmune diseases [28], our findings also suggest that this expanded population could possibly contribute to lung pathology in diabetic individuals.
Although it is well recognized that CD4+ Th1 cells are critical in cellular responses to tuberculosis, it is also clear that these responses alone are not sufficient to eliminate infection [4]. However, because of the potential for IL-17 to mediate immune pathology, as seen in autoimmune diseases and infection models [29], it is also postulated that IL-17 may have detrimental effects in chronic bacterial infectious diseases, such as tuberculosis. Therefore, the balance between Th17-mediated protection and pathology is key in defining the outcome of M. tuberculosis infections [30]. Our data on the examination of Th17 subsets reveal that 2 different Th17 subsets (expressing IL-17A, IFN-γ or IL-17A, IL-10 but not IL-17A, IL-22) are present at an increased frequency in patients with tuberculosis coincident with DM. Th17 cells coexpressing IL-17A and IFN-γ have been found to be important for responses to the fungal pathogen Candida albicans, whereas Th17 cells coexpressing IL-17A and IL-10 are important for responses to the bacterial pathogen Staphylococcus aureus [31]. Although the initial Th17 response might therefore be beneficial in establishing resistance to pathogens, exaggerated Th17 responses have been shown to represent unchecked tissue damaging inflammation in the setting of ineffective antimicrobial mechanisms [32]. Interestingly, the third subset that we examined, IL-17A– and IL-22–expressing CD4+ T cells, is not elevated and therefore warrants further investigation. Therefore, our data suggest that an exaggerated Th17 response, similar to the Th1 response, occurs in individuals with active tuberculosis coincident with diabetes and possibly contributes to immune-mediated pathology, as well. It is also possible that the increased Th1/Th17 responses do not contribute directly to pathology but are actually more indicative of increased bacterial burdens in diabetic individuals or an inability of these individuals to mount an effective antibacterial response.
Interestingly, the enhanced CD4+ T-cell repertoire involving Th1 and Th17 subsets is associated with significantly higher systemic levels of Th1- and Th17-associated cytokines. Thus, IL-2, IFN-γ, and IL-17A are all present at significantly higher levels in the circulation of diabetic individuals, compared with nondiabetic individuals, with pulmonary tuberculosis. T-cell profiles in patients with insulin resistance are known to be altered, which could indicate that T-cell factors are linked to disturbed insulin sensitivity [33]. In patients with type 2 DM, circulating T cells produce increased levels of IL-17 and IFN-γ and promote inflammation, and several cytokines produced by Th1 or Th17 cells have been linked to insulin resistance [33, 34]. Thus, underlying poorly controlled diabetes by itself could contribute to the increase in proinflammatory cytokines observed in patients with tuberculosis coincident with DM. However, coupled with the altered frequency of M. tuberculosis antigen–specific CD4+ T cells in the periphery, our data strongly support an important role for diabetes in contributing to an acutely proinflammatory environment during M. tuberculosis infections.
One potential mechanism for the increase in baseline levels of proinflammatory cytokines and Th17 subsets in patients with tuberculosis coincident with DM could be a concomitant decrease in the levels of systemic immunoregulatory cytokines or the frequency of CD4+ T cells expressing IL-10. However, as revealed by the increased systemic levels of IL-10 and the unaltered levels of TGF-β, it is not only cytokines with inflammatory potential that are elevated in patients with tuberculosis coincident with DM, but also cytokines with major immunoregulatory potential. Moreover, the frequency of CD4+ T cells expressing IL-10 is actually increased in patients with tuberculosis coincident with DM, suggesting that these cells have little or no role to play in curbing the expansion of Th1/Th17 cells or that they are expanded with delayed kinetics, resulting an inability to suppress the increased Th1/Th17 responses. Thus, the exuberant CD4+ Th1 and Th17 response in individuals with diabetes is not associated with decreased systemic IL-10 or TGF-β levels. Our data also suggest that it is unlikely that an alteration in T-cell numbers or subset distribution or recent T-cell activation was the primary cause for the enhanced expansion of cytokine-producing T cells. Another important mechanism that could account for the exaggerated Th1 and Th17 response is the impairment in the induction of nTregs. Our data reveal that, at baseline, patients with tuberculosis coincident with DM exhibit a significantly lower frequency of nTregs, which could potentially account for the exaggerated proinflammatory response engendered by M. tuberculosis infection in diabetic individuals. Since diabetes and other metabolic disorders are known to have profound effects on the development of nTregs [34], our data imply that diabetes-induced impairment in Tregs could play an important role in tuberculosis pathogenesis.
Our study provided important insights into the influence of poorly controlled type 2 DM on the pathogenesis of tuberculosis. Our data supporting the effects of an excessive but otherwise intact adaptive immune response to M. tuberculosis during diabetes provided a rational basis for testing combined antimicrobial and antiinflammatory therapies in diabetic patients with tuberculosis. Our study also provides an impetus to perform longitudinal studies examining the role of immunological biomarkers in the development of tuberculosis in diabetic patients. Finally, our data support the prediction that diabetes exacerbates tuberculosis severity to a significant degree and therefore provides a rationale for treating latent tuberculosis in the diabetic population in India. Future studies examining immune responses in diabetic patients with latent tuberculosis should provide additional insights into the pathogenesis of tuberculosis by dissecting the complex immunological interaction between diabetes and tuberculosis.
Notes
Acknowledgments. We thank the staff of Department of Clinical Research and the Department of Social Work, NIRT, especially Ms Kalaiselvi, and Government Stanley Hospital, Chennai, for valuable assistance in recruiting the patients for this study; and R. Anuradha, V. Gopinath, and Jovvian George of the NIH–ICER, for technical assistance.
Financial support. This work was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9:737–46. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harries AD, Lin Y, Satyanarayana S, et al. The looming epidemic of diabetes-associated tuberculosis: learning lessons from HIV-associated tuberculosis. Int J Tuberc Lung Dis. 2011;15:1436–44. doi: 10.5588/ijtld.11.0503. i. [DOI] [PubMed] [Google Scholar]
- 3.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev. 2008;226:191–204. doi: 10.1111/j.1600-065X.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 10.Khader SA, Bell GK, Pearl JE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 11.Khader SA, Guglani L, Rangel-Moreno J, et al. IL-23 is required for long-term control of Mycobacterium tuberculosis and B cell follicle formation in the infected lung. J Immunol. 2011;187:5402–7. doi: 10.4049/jimmunol.1101377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forbes EK, Sander C, Ronan EO, et al. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol. 2008;181:4955–64. doi: 10.4049/jimmunol.181.7.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Day CL, Abrahams DA, Lerumo L, et al. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J Immunol. 2011;187:2222–32. doi: 10.4049/jimmunol.1101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harari A, Rozot V, Enders FB, et al. Dominant TNF-alpha+ Mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nat Med. 2011;17:372–6. doi: 10.1038/nm.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deepa M, Pradeepa R, Rema M, et al. The Chennai Urban Rural Epidemiology Study (CURES)–study design and methodology (urban component) (CURES-I) J Assoc Physicians India. 2003;51:863–70. [PubMed] [Google Scholar]
- 16.Caruso AM, Serbina N, Klein E, Triebold K, Bloom BR, Flynn JL. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J Immunol. 1999;162:5407–16. [PubMed] [Google Scholar]
- 17.Torrado E, Cooper AM. IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev. 2010;21:455–62. doi: 10.1016/j.cytogfr.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hougardy JM, Place S, Hildebrand M, et al. Regulatory T cells depress immune responses to protective antigens in active tuberculosis. Am J Respir Crit Care Med. 2007;176:409–16. doi: 10.1164/rccm.200701-084OC. [DOI] [PubMed] [Google Scholar]
- 19.Sharma PK, Saha PK, Singh A, Sharma SK, Ghosh B, Mitra DK. FoxP3+ regulatory T cells suppress effector T-cell function at pathologic site in miliary tuberculosis. Am J Respir Crit Care Med. 2009;179:1061–70. doi: 10.1164/rccm.200804-529OC. [DOI] [PubMed] [Google Scholar]
- 20.Leung CC, Lam TH, Chan WM, et al. Diabetic control and risk of tuberculosis: a cohort study. Am J Epidemiol. 2008;167:1486–94. doi: 10.1093/aje/kwn075. [DOI] [PubMed] [Google Scholar]
- 21.Viswanathan V, Kumpatla S, Aravindalochanan V, et al. Prevalence of diabetes and pre-diabetes and associated risk factors among tuberculosis patients in India. PLoS One. 2012;7:e41367. doi: 10.1371/journal.pone.0041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponce-De-Leon A, Garcia-Garcia Md Mde L, Garcia-Sancho MC, et al. Tuberculosis and diabetes in southern Mexico. Diabetes Care. 2004;27:1584–90. doi: 10.2337/diacare.27.7.1584. [DOI] [PubMed] [Google Scholar]
- 23.Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999;26:259–65. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 24.Nikolajczyk BS, Jagannathan-Bogdan M, Shin H, Gyurko R. State of the union between metabolism and the immune system in type 2 diabetes. Genes Immun. 2011;12:239–50. doi: 10.1038/gene.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–8. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- 26.Vallerskog T, Martens GW, Kornfeld H. Diabetic mice display a delayed adaptive immune response to Mycobacterium tuberculosis. J Immunol. 2010;184:6275–82. doi: 10.4049/jimmunol.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Restrepo BI, Fisher-Hoch SP, Pino PA, et al. Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells. Clin Infect Dis. 2008;47:634–41. doi: 10.1086/590565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazarevic V, Glimcher LH. T-bet in disease. Nat Immunol. 2011;12:597–606. doi: 10.1038/ni.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters A, Lee Y, Kuchroo VK. The many faces of Th17 cells. Curr Opin Immunol. 2011;23:702–6. doi: 10.1016/j.coi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torrado E, Robinson RT, Cooper AM. Cellular response to mycobacteria: balancing protection and pathology. Trends Immunol. 2011;32:66–72. doi: 10.1016/j.it.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zielinski CE, Mele F, Aschenbrenner D, et al. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484:514–8. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 32.Sallusto F, Zielinski CE, Lanzavecchia A. Human Th17 subsets. Eur J Immunol. 2012;42:2215–20. doi: 10.1002/eji.201242741. [DOI] [PubMed] [Google Scholar]
- 33.Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012;8:709–16. doi: 10.1038/nrendo.2012.114. [DOI] [PubMed] [Google Scholar]
- 34.Matarese G, Procaccini C, De Rosa V. At the crossroad of T cells, adipose tissue, and diabetes. Immunol Rev. 2012;249:116–34. doi: 10.1111/j.1600-065X.2012.01154.x. [DOI] [PubMed] [Google Scholar]