Abstract
Background. The association between initiation of antiretroviral therapy (ART) for human immunodeficiency virus (HIV) infection and possible herpes simplex virus type 2 (HSV-2) shedding and genital ulcer disease (GUD) has not been evaluated.
Methods. GUD and vaginal HSV-2 shedding were evaluated among women coinfected with HIV and HSV-2 (n = 440 for GUD and n = 96 for HSV-2 shedding) who began ART while enrolled in a placebo-controlled trial of HSV-2 suppression with acyclovir in Rakai, Uganda. Monthly vaginal swabs were tested for HSV-2 shedding, using a real-time quantitative polymerase chain reaction assay. Prevalence risk ratios (PRRs) of GUD were estimated using log binomial regression. Random effects logistic regression was used to estimate odds ratios (ORs) of HSV-2 shedding.
Results. Compared with pre-ART values, GUD prevalence increased significantly within the first 3 months after ART initiation (adjusted PRR, 1.94; 95% confidence interval [CI], 1.04–3.62) and returned to baseline after 6 months of ART (adjusted PRR, 0.80; 95% CI, .35–1.80). Detection of HSV-2 shedding was highest in the first 3 months after ART initiation (adjusted OR, 2.58; 95% CI, 1.48–4.49). HSV-2 shedding was significantly less common among women receiving acyclovir (adjusted OR, 0.13; 95% CI, .04–.41).
Conclusions. The prevalence of HSV-2 shedding and GUD increased significantly after ART initiation, possibly because of immune reconstitution inflammatory syndrome. Acyclovir significantly reduced both GUD and HSV-2 shedding and should be considered to mitigate these effects following ART initiation.
Keywords: herpes simplex virus type 2 (HSV-2), human immunodeficiency virus (HIV), immune reconstitution inflammatory syndrome (IRIS), acyclovir, reactivation, Uganda
Herpes simplex virus type 2 (HSV-2) is a common sexually transmitted pathogen and is a major cause of genital ulceration worldwide [1–5]. Observational studies have found that HSV-2 seropositivity is also associated with a 3-fold increased risk for acquiring human immunodeficiency virus type 1 (HIV-1) [1, 2, 6, 7], possibly because HSV-2 causes genital ulcers and recruitment of dendritic cells and CD4+ T cells expressing CCR5 into areas of HSV-2 replication [1, 8, 9]. While randomized trials of HSV-2 suppression failed to show reductions in HIV acquisition or transmission [10–12], HSV-2 suppression slows HIV disease progression [13, 14].
HIV infection also causes HSV-2 reactivation [15], and genital ulcer disease (GUD) is more frequent and persistent during HIV infection [16], possibly increasing the risk of HIV transmission. There are reported cases of increased genital lesions immediately after antiretroviral therapy (ART) initiation [17–19]. All previous studies of HSV-2 have been limited to individuals receiving long-term ART, compared with those not receiving ART. These studies showed that long-term ART reduces the number of HSV-2 lesions but has no impact on HSV-2 shedding [20–22]. However, no longitudinal studies of HSV-2 reactivation among individuals initiating ART have been reported.
Here, we report the prevalence of genital ulcers and HSV-2 shedding among HIV-positive, HSV-2–coinfected individuals who were followed prospectively before and after initiation of ART in a randomized trial of HSV-2 suppression in Rakai, Uganda.
METHODS
Study Population
Individuals aged ≥18 years who were coinfected with HIV and HSV-2 and had a CD4+ T-cell count of 300–400 cells/µL were enrolled in a double-blind, individually randomized placebo-controlled trial of HSV-2 suppression to assess HIV disease progression in Rakai, Uganda, as previously reported [14]. Individuals who had AIDS-defining illnesses or were receiving ART were excluded. The primary outcome was defined as HIV-1 disease progression to a CD4+ T-cell count of <250 cells/μL or World Health Organization (WHO) stage IV disease, at which time ART was initiated. Participants provided written informed consent. Individuals were randomly assigned to receive placebo or 400 mg of acyclovir twice daily for 24 months. Serologic testing (for HIV and HSV-2), CD4+ T-cell count testing, physical examinations (including clinical evaluation for GUD), self-collection of a vaginal swab specimen, and interviews to ascertain sociodemographic characteristics were conducted at baseline. Serum was stored at a temperature of −80°C.
Participants were seen monthly for drug refill and adverse event review. Enrollment was conducted between May 2007 and November 2008. All trial participants were followed monthly until trial closure, in October 2010. Thus, the initial enrollees were followed for an additional 22 months, and this protracted follow-up allowed assessment of effects after initiation of ART for participants who reached a study end point. The median number of monthly visits was 32 (interquartile range [IQR], 26–38), with 22 individuals being followed for the maximum of 42 monthly visits. Participants only received acyclovir or placebo during the first 24 months of follow-up.
All 440 individuals in the trial were evaluated monthly for genital lesions by clinical examination. All trial participants were included in the analysis of GUD, irrespective of whether they initiated ART or were assigned to receive acyclovir. At each study visit, female participants provided a self-collected vaginal swab specimen. They were instructed to squat, insert a 20-cm Dacron or cotton-tipped swab high into the vagina vault, and rotate the swab. After the swab specimen was collected, a field worker placed the swab in specimen transport medium (Digene, Gaithersburg, MD). The specimens were maintained at a temperature of 4°C–10°C for <6 hours, until they were frozen at –80°C. There were 96 women who provided monthly vaginal swabs for at least 6 months before and 6 months after ART initiation, and their specimens were evaluated for HSV-2 shedding. No male genital swab specimens were collected. These 96 women were included in the analysis of HSV-2 shedding.
All individuals (in the intervention and control arms) with documented GUD were treated with acyclovir. At enrollment and every 6 months thereafter, a general physical examination and repeat CD4+ T-cell count and HIV load testing were performed. Even individuals who reached the primary outcome of ART eligibility (defined as a CD4+ T-cell count of <250 cells/µL or WHO stage IV disease) continued to receive placebo or acyclovir for the first 24 months after enrollment.
The trial was approved by the Uganda National Council for Science and Technology (Kampala, Uganda), the Uganda Virus Research Institute Science and Ethics Committee, and the National Institute of Allergy and Infectious Diseases Intramural Institutional Review Board. The trial was registered with Clinicaltrials.gov (NCT00405821).
HSV-2, HIV, and CD4+ T-Cell Count Testing
HIV-1 serostatus was determined using 2 different enzyme immunoassays (Vironostika HIV-1, Organon Teknika [Charlotte, NC], and Cambridge Biotech [Worcester, MA]), with Western blot confirmation of all discordant enzyme immunoassays (HIV-1 WB Bio-Merieux-Vitek, St. Louis, MO). HSV-2 serostatus was determined using the Focus HerpeSelect-2 enzyme immunoassay (Focus Technologies, Cypress, CA), with a cutoff of 3.4 to improve specificity [23]. CD4+ T-cell count testing was performed using a FACSCalibur (Becton Dickenson, Franklin Lakes, NJ), and HIV load testing was done using the Roche Monitor v1.5 assay (Roche Diagnostics, Indianapolis, IN)
A real-time quantitative polymerase chain reaction assay with primers to glycoprotein B was used to detect HSV DNA in vaginal swabs [24]. HSV-2 shedding/reactivation was defined as a positive result of >150 HSV DNA copies /mL [25].
Statistical Analysis
Among 440 men and women in the trial, clinician-diagnosed GUD was assessed by study arm (acyclovir vs placebo) and time in months with respect to ART initiation (no ART exposure and 1–3, 4–6, and >6 months after ART initiation). Individuals were only evaluated for GUD during the 24 months when they received either acyclovir or placebo. Prevalence risk ratios (PRRs) and 95% confidence intervals (CIs) of current GUD at the time of a study visit were estimated using log binomial regression with GEE robust variance estimates to account for multiple observations within the same individual.
Women were evaluated for possible HSV-2 shedding during the entire trial period, including the time after acyclovir was discontinued. Study characteristics among the 96 women in whom HSV-2 shedding was assessed were tabulated by study arm. Differences were assessed by χ2 and Wilcoxon–Mann-Whitney tests for categorical and continuous variables, respectively. All P values were 2-sided. The primary outcome was detection of HSV-2 shedding before and after ART initiation. The intensity of HSV-2 shedding (measured in copies per milliliter) was also analyzed as a secondary outcome. HSV-2 shedding was assessed longitudinally such that each of the 96 female participants could contribute multiple outcomes before and after ART initiation. Pre-ART CD4+ T-cell count and HIV load measurements were defined using the last measurement prior to ART initiation, whereas post-ART CD4+ T-cell count and HIV-1 load measurements were defined as the first measurement 2–9 months following ART initiation. We further defined immune reconstitution as a CD4+ T-cell count increase of >50 cells/µL between these pre- and post-ART measurements [26]. HIV-1 virologic suppression was defined as an undetectable HIV-1 viral load (ie, <400 copies/mL) in the post-ART period. A descriptive analysis to examine the probability of HSV shedding with respect to the times before and after ART initiation and the immunologic outcomes related to ART initiation was conducted.
Random effects logistic regression with an exchangeable correlation structure was used to estimate odds ratios (ORs) of univariate and multivariate associations of any HSV-2 shedding, adjusted for baseline participant characteristics, such as age, and for time-varying covariates, including acyclovir treatment, ART status, and month before and after ART initiation. Age, months from ART initiation, HIV load, and CD4+ T-cell count covariates were analyzed as categorical and continuous variables.
Covariates that were statistically associated with the study outcome (ie, those with a P value of <.15) in univariate analysis and potential confounders of the ART and GUD relationship were entered into an adjusted model. Analyses were conducted in Stata, version 11.0, and R, version 2.14.
RESULTS
Prevalence of Symptomatic GUD
Among the 440 participants (220 acyclovir recipients and 220 placebo recipients) enrolled in the trial, 132 (30.0%) initiated ART during the 24-month follow-up period. There were 148 cases of symptomatic GUD (1.5% of 10 136 study visits). Current GUD increased significantly within the first 3 months after HIV-positive men and women initiated ART (adjusted PRR, 1.94; 95% CI, 1.04–3.62; Table 1). The prevalence of GUD returned to baseline levels after 6 months of ART. While current GUD was less common among individuals who received acyclovir than placebo (adjusted PRR, 0.42; 95% CI, .23–.74), an increased prevalence of GUD after ART initiation was seen in both trial arms (Table 1). There was no difference in GUD frequency between men and women (P = .85).
Table 1.
Current Genital Ulcer Disease Prevalence Over 24 Months Among Men and Women, Stratified by Study Arm and Duration of Antiretroviral Therapy (ART)
| Visits, Proportion (%)a |
Prevalence Rate Ratio (95% CI) |
||||
|---|---|---|---|---|---|
| ART Duration | Acyclovir Arm | Placebo Arm | Total | Unadjusted | Adjustedb |
| None received | 29/4529 (0.6) | 92/4367 (2.1) | 121/8896 (1.4) | 1.00 (reference) | 1.00 (reference) |
| 0–3 mo post | 4/212 (1.9) | 9/252 (3.6) | 13/464 (2.8) | 1.98 (1.05–3.75) | 1.94 (1.04–3.62) |
| 4–6 mo post | 5/107 (4.7) | 3/151 (2.0) | 8/258 (3.1) | 2.08 (0.99–4.38) | 1.99 (.94–4.22) |
| >6 mo post | 2/238 (0.8) | 4/280 (1.4) | 6/518 (1.2) | 0.81 (0.35–1.87) | 0.80 (.35–1.80) |
Abbreviations: CI, confidence interval; HSV-2, herpes simplex virus 2.
a Data are no. of visits during which HSV-2 shedding was detected/no. of visits HSV-2 shedding was evaluated (%), unless otherwise indicated.
b Analyses were adjusted for sex and trial arm (acyclovir or placebo).
Characteristics of Women Assessed for HSV-2 Shedding
The 96 women evaluated for monthly HSV-2 shedding were highly compliant with study visits and contributed swabs during 1080 of 1152 monthly study visits (93.8%). Of these 96 women, 43 (44.8%) were assigned to receive acyclovir, and 53 (55.2%) were assigned to receive placebo (Table 2). The median age was 34.5 years (IQR, 30.5–44.0 years) and did not differ significantly between trial arms (P = .36). All women received ART, and the majority (84.4%) received zidovudine/lamivudine/nevirapine. Before ART initiation, the majority (58.3%; 56/96) had a CD4+ T-cell count of >200 cells/µL (median, 217 cells/µL). Between 2 and 9 months after ART initiation, the median CD4+ T-cell count increase was 106.5 cells/µL, and the majority of women (73.3%) achieved immune reconstitution. There was no difference in pre-ART viral load, CD4+ T-cell count, or the percentage who achieved immune reconstitution by trial arm.
Table 2.
Characteristics of Women Assessed for Herpes Simplex Virus 2 Shedding
| Characteristic | Acyclovir Arm (n = 43) | Placebo Arm (n = 53) | P |
|---|---|---|---|
| Age at enrollment, y | .36 | ||
| 20–29 | 7 (16.3) | 16 (30.2) | |
| 30–39 | 20 (46.5) | 21 (39.6) | |
| 40–49 | 11 (25.6) | 13 (24.5) | |
| ≥50 | 5 (11.6) | 3 (5.7) | |
| HIV ART regimen | .58 | ||
| AZT/3TC/NVP | 39 (90.7) | 42 (79.2) | |
| CBV/EFV | 1 (2.3) | 4 (7.5) | |
| d4T/3TC/NVP | 2 (4.7) | 4 (7.5) | |
| TDF/3TC/NVP | 1 (2.3) | 2 (3.8) | |
| TVD/EFV | 0 | 1 (1.9) | |
| Pre-ART viral load, copies/mL | .81 | ||
| <10 000 | 5 (11.6) | 10 (18.9) | |
| 10 000–49 999 | 10 (23.3) | 11 (20.8) | |
| 50 000–99 999 | 6 (13.9) | 7 (13.2) | |
| >100 000 | 22 (51.2) | 25 (47.2) | |
| CD4+ T-cell count, cells/µL | |||
| Before ART | .07 | ||
| <100 | 4 (9.3) | 0 | |
| 100–200 | 14 (32.6) | 22 (41.5) | |
| >200 | 25 (58.1) | 31 (58.5) | |
| Change after ART initiation, median (IQR)a | 97 (40–158) | 118 (46–162) | .64 |
| Immune reconstitutiona,b | 30 (73.2) | 36 (73.5) | .98 |
| Undetectable HIV load after ARTc | 40 (93.0) | 45 (84.9) | .21 |
| Follow-up duration, mo | |||
| Before ART | .12 | ||
| Median (IQR) | 6 (6–6) | 6 (5–6) | |
| Range | 4–6 | 2–6 | |
| After ART | |||
| Median (IQR) | 6 (6–6) | 6 (6–6) | .32 |
| Range | 3–6 | 1–6 |
Data are no. (%) of subjects, unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; AZT, zidovudine; CBV, zidovudine-lamivudine; d4T, stavudine; EFV, efavirenz; HIV, human immunodeficiency virus; IQR, interquartile range; NVP, nevirapine; TDF, tenofovir disoproxil fumarate; TDV, tenofovir disoproxil fumarate–emtricitabine; 3TC, lamivudine.
a Data exclude 6 individuals (2 in the acyclovir arm and 4 in the placebo arm) who did not have follow-up CD4+ T-cell counts.
b Defined as a CD4+ T-cell count increase of >50 cells/µL.
c Defined as <400 copies/mL.
HSV-2 Shedding
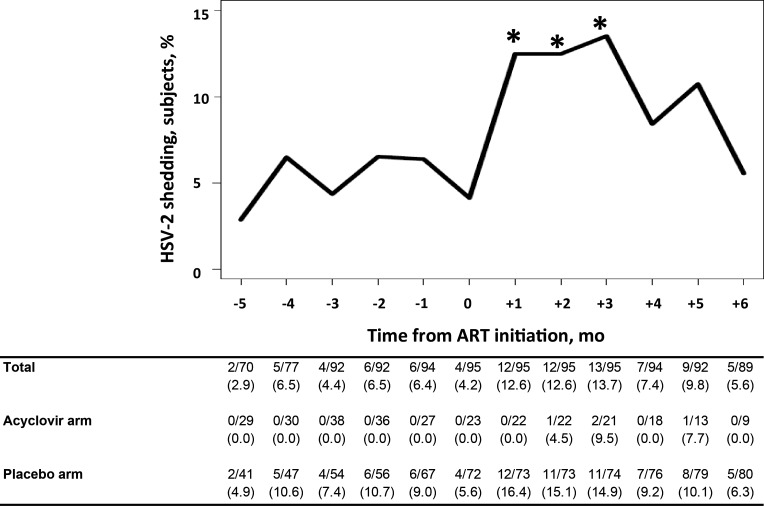
HSV-2 shedding was detected in 48 women (50.0%) and at 85 monthly visits (7.9%). Before ART initiation, HSV-2 shedding was detected in 5.2% (27/520) of the monthly visits among women receiving placebo and none of the monthly visits among women receiving acyclovir (Figure 1 and Table 3). The frequency of HSV-2 shedding increased immediately after ART initiation and remained elevated for 3 months (Figure 1 and Table 3). Compared to the 6 months before ART initiation, HSV-2 shedding after ART initiation was significantly higher at month 1 (OR, 2.83; 95% CI, 1.33–6.04), month 2 (OR, 2.84; 95% CI, 1.33–6.05), and month 3 (OR, 3.15; 95% CI, 1.50–6.60) but not at month 4 (OR, 1.50; 95% CI, .61–3.68), month 5 (OR, 2.07; 95% CI, .91–4.74), or month 6 (OR, 1.09; 95% CI, .39–3.00). Overall, HSV-2 shedding was highest in the first 3 months after ART initiation, compared with the pre-ART period (adjusted OR, 2.58; 95% CI, 1.48–4.49).
Figure 1.
Prevalence of herpes simplex virus 2 (HSV-2) shedding among women before and after initiation of antiretroviral therapy (ART). As indicated by the asterisk, the prevalence of HSV-2 shedding was significantly higher 1, 2, and 3 months after ART initiation, compared with 6 months before ART initiation. Data in the table are no. of visits during which HSV-2 shedding was detected/no. of visits HSV-2 shedding was evaluated (%).
Table 3.
Characteristics Associated With the Detection of Vaginal Herpes Simplex Virus 2 (HSV-2) Shedding
| Characteristic | HSV-2 Positivity, Visits, Proportion (%)a | Odds Ratio (95% CI) |
|
|---|---|---|---|
| Unadjusted | Adjusted | ||
| Age, y | |||
| 20–29 | 34/260 (13.1) | 1.00 (reference) | 1.00 (reference) |
| 30–39 | 29/468 (6.2) | 0.41 (.20–.84) | 0.43 (.22–.83) |
| 40–49 | 20/263 (7.6) | 0.46 (.20–1.04) | 0.62 (.29–1.34) |
| >50 | 2/89 (2.2) | 0.14 (.03–.72) | 0.21 (.04–1.04) |
| Pre-ART viral load, copies/mL | |||
| <10 000 | 6/167 (3.6) | 1.00 (reference) | 1.00 (reference) |
| 10 000–49 999 | 8/239 (3.4) | 0.95 (.28–3.23) | 1.05 (.31–3.57) |
| 50 000–99 999 | 7/146 (4.8) | 1.41 (.39–5.11) | 1.19 (.33–4.30) |
| >100 000 | 64/528 (12.1) | 4.00 (1.46–10.92) | 4.01 (1.47–10.93) |
| Pre-ART CD4+ T-cell count, cells/µL | |||
| <100 | 4/46 (8.7) | 1.00 (reference) | … |
| 100–200 | 32/402 (8.0) | 0.89 (.19–4.24) | … |
| >200 | 49/632 (7.8) | 0.84 (.18–3.90) | … |
| Immune reconstitutionb,c | |||
| No | 16/269 (5.9) | 1.00 (reference) | … |
| Yes | 65/751 (8.7) | 1.47 (.69–3.13) | … |
| ART duration | |||
| None received | 27/520 (5.2) | 1.00 (reference) | 1.00 (reference) |
| 0–3 mo | 37/285 (13.0) | 2.94 (1.70–5.06) | 2.58 (1.48–4.49) |
| 4–6 mo | 21/275 (7.6) | 1.54 (.84–2.85) | 1.23 (.66–2.30) |
| Acyclovir | |||
| No | 81/792 (10.2) | 1.00 (reference) | 1.00 (reference) |
| Yes | 4/288 (1.4) | 0.10 (.03–.31) | 0.13 (.04–.41) |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval.
a Data are no. of visits during which HSV-2 shedding was detected/no. of visits HSV-2 shedding was evaluated (%)
b Data exclude 6 individuals (2 in the acyclovir arm and 4 in the placebo arm) who did not have follow-up CD4+ T-cell counts.
c Defined as a CD4+ T-cell count increase of >50 cells/µL.
While the elevated frequency of HSV-2 shedding after ART initiation occurred in both study arms, shedding was significantly less frequent among women receiving acyclovir (adjusted OR, 0.13; 95% CI, .04–.41; Table 3). However, among women with detectable shedding of HSV-2, the median HSV-2 load was not different between those who were receiving acyclovir (3.52 log10 DNA copies/mL; IQR, 2.85–4.30 log10 DNA copies/mL), compared with placebo (3.57log10 DNA copies/mL; IQR, 2.94–4.48 log10 DNA copies/mL; P = .82).
Only 2 women in the acyclovir arm had detectable HSV-2 shedding in the post-ART period; these 2 women contributed 4 episodes (Table 3). One woman was highly compliant with acyclovir therapy (pill count–based compliance, 99.7% during the 6 months after ART) but had 3 episodes of HSV-2 shedding. The other woman was >98% compliant with acyclovir therapy during 5 of the first 6 months after ART initiation. However, she had an episode of HSV-2 shedding during the month of poor compliance, when she only completed 57.1% of her acyclovir dose.
Table 3 shows the univariate and multivariate associations with any HSV-2 shedding. In addition to the associations with ART described above, the frequency of HSV-2 shedding and reactivation was highest in the youngest women (age, 20–29 years; 13.1%) and decreased with age (>50 years, 2.2%; OR, 0.14; 95% CI, .03–.72). The frequency of HSV-2 shedding did not vary by pre-ART CD4+ T-cell count. While not statistically significant, HSV-2 shedding was also elevated among women with immune reconstitution, compared with those without immune reconstitution (OR, 1.47; 95% CI, .69–3.13). HSV-2 shedding was most common among women with an HIV load of >100 000 copies/mL, compared with women with an HIV load of <10 000 copies/mL (adjusted OR, 4.01; 95% CI, 1.47–10.93).
There was no significant difference in the median HSV-2 load among women with HSV-2 shedding before ART initiation (3.7 log10 DNA copies/mL; IQR, 3.2–4.5 log10 DNA copies/mL), compared with after ART initiation (3.4log10 DNA copies/mL; IQR, 2.8–4.5 log10 DNA copies/mL; P = .14).
DISCUSSION
Among women initiating ART, the frequency of HSV-2 shedding significantly increased during the first 3 months after initiation of placebo or acyclovir therapy. HSV-2 shedding was most common among the youngest women (age, 20–29 years) and those with the highest HIV loads (>100 000 copies/mL). In addition, both men and women had significantly higher numbers of symptomatic GUD episodes during the 3-month period after ART initiation. This is the first study to demonstrate that ART initiation is associated with HSV-2 reactivation and symptomatic GUD.
Of the women in this study, 50% experienced HSV-2 shedding. These rates are higher than reported in either HIV-negative or HIV-positive women in the United States [21, 22, 27, 28]. However, the rates are similar to those previously reported among women in Africa [20]. HSV-2 shedding in this study was highest among women <30 years old, which is consistent with previous findings that HSV-2 shedding is more frequent among younger individuals, who are more likely to have recently acquired HSV-2 [27, 29].
The increased frequency of HSV-2 shedding and GUD after ART initiation is possibly due to immune reconstitution inflammatory syndrome (IRIS). HIV-associated ART restores the host immune response, as indicated by an increased CD4+ T-cell count. Individuals with advanced immunodeficiency may experience IRIS, which leads to unmasking of subclinical infections or paradoxical worsening of treated opportunistic infections [30]. IRIS is typically defined by symptoms of an inflammatory process after initiation of ART [30] and has been documented with several viruses of the herpes family, such as varicella zoster virus, Kaposi sarcoma–associated herpes virus (ie, human herpesvirus 8), and cytomegalovirus [17, 30, 31]. In general, viral shedding is a manifestation of the nearly continuous reactivation of HSV-2 in the sacral ganglion, with migration of virus to the sensory nerve endings in the genital epithelium and release [32]. The finding in our longitudinal study that the frequencies of GUD and HSV-2 shedding were highest within the first 3 months after ART initiation, as is typical of IRIS [31], certainly suggests that the altered immune response seen after institution of ART may enhance viral shedding. Reports have shown IRIS to be more common among individuals with higher viral loads [33], which is compatible with our finding that HSV-2 shedding was most common among women with the highest HIV load prior to ART initiation. Thus, the increased HSV-2 shedding may be due to IRIS.
Acyclovir suppresses HSV-2 by acting as a DNA chain terminator. In this study, there was an 86% reduction in HSV-2 shedding among women in the acyclovir arm, supporting previous findings that anti–HSV-2 therapy reduces HSV-2 recurrence [34] and the finding in our study of a 69% reduction in the frequency of symptomatic GUD among men and women in the acyclovir arm [14]. Despite HSV-2 suppression, short episodes of subclinical HSV-2 shedding may still occur [28]. In addition, acyclovir treatment in this study did not affect the HSV-2 load among those with detectable shedding. The lack of complete suppression of HSV-2 shedding despite acyclovir treatment may help to explain the lack of efficacy of HSV-2 suppression to reduce HIV acquisition or transmission [10–12]. However, acyclovir appears to be effective in reducing the frequency of HSV-2 shedding and GUD symptoms immediately after the initiation of ART.
This study has limitations. GUD was clinically diagnosed, and the rates may be an underestimate because of omission of lesions that resolved in the interval between monthly visits. Although GUD etiology has not been determined, preliminary data indicate that 95.8% of all genital ulcers in this trial with a defined etiology were due to HSV-2. While GUD rates were increased with ART among both men and women in the study, HSV-2 shedding was only assessed among the women. Thus, further investigation among men is needed. The HSV-2 shedding rate in this study may be underestimated because of the use of monthly sampling and the lack of daily sampling. While women obtained vaginal swab specimens monthly for HSV-2 detection, measurement of the CD4+ T-cell count was limited to every 6 months, and we cannot ascertain the exact timing of pre-ART CD4+ T-cell count and immune reconstitution after ART. Measurements of CD4+ T-cell counts ranged from 2 to 9 months after ART initiation. In a sensitivity analysis of 49 women with 578 observations who had CD4+ T-cell count within 4 months of ART initiation, there was a stronger effect for HSV-2 shedding among women with immune reconstituted, compared with those without immune reconstitution (OR, 1.89; 95% CI, .59–6.06), but it remained non–statistically significant. While the increased HSV-2 shedding and GUD frequencies are likely due to IRIS, one cannot exclude the possibility that these events were due to changes in sexual behavior or were concurrent with the emergence of opportunistic infections. Individuals were closely monitored in this study; the median CD4+ T-cell count at ART initiation was 217 cells/µL, and only 4 women (4.2%) developed a CD4+ T-cell count of <100 cells/µL. Since IRIS is most common among individuals with a CD4+ T-cell count of <50 cells/µL [30], this study likely underestimates the number of episodes of HSV-2 shedding and GUD that were potentially due to IRIS.
Individuals starting ART should be counseled on the possible increased risk of GUD and HSV-2 shedding, and new treatment guidelines should be considered after evaluating the best method to reduce the occurrence of HSV-2 shedding and GUD among individuals initiating ART. The increased number of genital lesions due to HSV-2 after initiation of ART could increase the risk HIV transmission. Acyclovir treatment among individuals initiating ART appears to reduce frequencies of both GUD and HSV-2 shedding. Thus, addition of acyclovir or other HSV-2 suppression therapy should be considered during this period.
Notes
Acknowledgments. We thank the study participants and the Rakai Community Advisory Board, whose commitment and cooperation made this study possible.
Disclaimer. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Financial support. This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH; and in part by program project grant PO1 AI-30731-19); the National Cancer Institute, NIH (contract HHSN261200800001E); the NIH (grant 1K23AI093152-01A1 to A. A. R. T.); and the Doris Duke Charitable Foundation (Clinician Scientist Development Award 22006.02 to A. A. R. T.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007;370:2127–37. doi: 10.1016/S0140-6736(07)61908-4. [DOI] [PubMed] [Google Scholar]
- 2.Tobian AA, Quinn TC. Herpes simplex virus type 2 and syphilis infections with HIV: an evolving synergy in transmission and prevention. Curr Opin HIV AIDS. 2009;4:294–9. doi: 10.1097/COH.0b013e32832c1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tobian AA, Ssempijja V, Kigozi G, et al. Incident HIV and herpes simplex virus type 2 infection among men in Rakai, Uganda. AIDS. 2009;23:1589–94. doi: 10.1097/QAD.0b013e32832d4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suntoke TR, Hardick A, Tobian AA, et al. Evaluation of multiplex real-time PCR for detection of H. ducreyi, T. pallidum, HSV-1, and HSV-2 in the diagnosis of genital ulcer disease in Rakai District, Uganda. Sex Transm Infect. 2009;85:97–101. doi: 10.1136/sti.2008.034207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tobian AAR, Charvat B, Ssempijja V, et al. Factors associated with the prevalence and incidence of herpes simplex virus type 2 infections among men in Rakai, Uganda. J Infect Dis. 2009;199:945–9. doi: 10.1086/597074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. Aids. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 7.Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004;35:435–45. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 8.Rebbapragada A, Wachihi C, Pettengell C, et al. Negative mucosal synergy between Herpes simplex type 2 and HIV in the female genital tract. Aids. 2007;21:589–98. doi: 10.1097/QAD.0b013e328012b896. [DOI] [PubMed] [Google Scholar]
- 9.Holmberg SD, Stewart JA, Gerber AR, et al. Prior herpes simplex virus type 2 infection as a risk factor for HIV infection. Jama. 1988;259:1048–50. [PubMed] [Google Scholar]
- 10.Watson-Jones D, Weiss HA, Rusizoka M, et al. Effect of Herpes Simplex Suppression on Incidence of HIV among Women in Tanzania. N Engl J Med. 2008;358:1560–71. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Celum C, Wald A, Hughes J, et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:2109–19. doi: 10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362:427–39. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lingappa JR, Baeten JM, Wald A, et al. Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet. 2010;375:824–33. doi: 10.1016/S0140-6736(09)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds SJ, Makumbi F, Newell K, et al. Daily acyclovir to prevent disease progression among HIV-1/HSV-2 co-infected individuals: a randomized, double-blinded placebo controlled trial in Rakai, Uganda. Lancet Infect Dis. 2012;12:441–8. doi: 10.1016/S1473-3099(12)70037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McClelland RS, Wang CC, Overbaugh J, et al. Association between cervical shedding of herpes simplex virus and HIV-1. Aids. 2002;16:2425–30. doi: 10.1097/00002030-200212060-00007. [DOI] [PubMed] [Google Scholar]
- 16.Serwadda D, Gray RH, Sewankambo NK, et al. Human immunodeficiency virus acquisition associated with genital ulcer disease and herpes simplex virus type 2 infection: a nested case-control study in Rakai, Uganda. J Infect Dis. 2003;188:1492–7. doi: 10.1086/379333. [DOI] [PubMed] [Google Scholar]
- 17.French MA, Lenzo N, John M, et al. Immune restoration disease after the treatment of immunodeficient HIV-infected patients with highly active antiretroviral therapy. HIV Med. 2000;1:107–15. doi: 10.1046/j.1468-1293.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 18.Couppie P, Sarazin F, Clyti E, et al. Increased incidence of genital herpes after HAART initiation: a frequent presentation of immune reconstitution inflammatory syndrome (IRIS) in HIV-infected patients. AIDS Patient Care STDS. 2006;20:143–5. doi: 10.1089/apc.2006.20.143. [DOI] [PubMed] [Google Scholar]
- 19.Graham SM, Masese L, Gitau R, et al. Increased risk of genital ulcer disease in women during the first month after initiating antiretroviral therapy. J Acquir Immune Defic Syndr. 2009;52:600–3. doi: 10.1097/QAI.0b013e3181b065cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayaud P, Nagot N, Konate I, et al. Effect of HIV-1 and antiretroviral therapy on herpes simplex virus type 2: a prospective study in African women. Sex Transm Infect. 2008;84:332–7. doi: 10.1136/sti.2008.030692. [DOI] [PubMed] [Google Scholar]
- 21.Posavad CM, Wald A, Kuntz S, et al. Frequent reactivation of herpes simplex virus among HIV-1-infected patients treated with highly active antiretroviral therapy. J Infect Dis. 2004;190:693–6. doi: 10.1086/422755. [DOI] [PubMed] [Google Scholar]
- 22.Ameli N, Bacchetti P, Morrow RA, et al. Herpes simplex virus infection in women in the WIHS: epidemiology and effect of antiretroviral therapy on clinical manifestations. AIDS. 2006;20:1051–8. doi: 10.1097/01.aids.0000222078.75867.77. [DOI] [PubMed] [Google Scholar]
- 23.Laeyendecker O, Henson C, Gray RH, et al. Performance of a commercial, type-specific enzyme-linked immunosorbent assay for detection of herpes simplex virus type 2-specific antibodies in Ugandans. J Clin Microbiol. 2004;42:1794–6. doi: 10.1128/JCM.42.4.1794-1796.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jerome KR, Huang ML, Wald A, Selke S, Corey L. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. J Clin Microbiol. 2002;40:2609–11. doi: 10.1128/JCM.40.7.2609-2611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magaret AS, Wald A, Huang ML, Selke S, Corey L. Optimizing PCR positivity criterion for detection of herpes simplex virus DNA on skin and mucosa. J Clin Microbiol. 2007;45:1618–20. doi: 10.1128/JCM.01405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuboi SH, Pacheco AG, Harrison LH, et al. Mortality associated with discordant responses to antiretroviral therapy in resource-constrained settings. J Acquir Immune Defic Syndr. 2010;53:70–7. doi: 10.1097/QAI.0b013e3181c22d19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tronstein E, Johnston C, Huang ML, et al. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA. 2011;305:1441–9. doi: 10.1001/jama.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston C, Saracino M, Kuntz S, et al. Standard-dose and high-dose daily antiviral therapy for short episodes of genital HSV-2 reactivation: three randomised, open-label, cross-over trials. Lancet. 2012;379:641–7. doi: 10.1016/S0140-6736(11)61750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wald A, Zeh J, Selke S, Ashley RL, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med. 1995;333:770–5. doi: 10.1056/NEJM199509213331205. [DOI] [PubMed] [Google Scholar]
- 30.Muller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:251–61. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis. 2009;48:101–7. doi: 10.1086/595006. [DOI] [PubMed] [Google Scholar]
- 32.Koelle DM, Corey L. Herpes simplex: insights on pathogenesis and possible vaccines. Annu Rev Med. 2008;59:381–95. doi: 10.1146/annurev.med.59.061606.095540. [DOI] [PubMed] [Google Scholar]
- 33.Novak RM, Richardson JT, Buchacz K, et al. Immune reconstitution inflammatory syndrome: incidence and implications for mortality. AIDS. 2012;26:721–30. doi: 10.1097/QAD.0b013e3283511e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lebrun-Vignes B, Bouzamondo A, Dupuy A, Guillaume JC, Lechat P, Chosidow O. A meta-analysis to assess the efficacy of oral antiviral treatment to prevent genital herpes outbreaks. J Am Acad Dermatol. 2007;57:238–46. doi: 10.1016/j.jaad.2007.02.008. [DOI] [PubMed] [Google Scholar]