Abstract
Heme oxygenase 1 expression is increased in pediatric patients with malaria. The carboxyhemoglobin level (a measure of heme oxygenase 1 activity) has not been assessed in adult patients with malaria. Results of pulse co-oximetry revealed that the mean carboxyhemoglobin level was elevated in 29 Indonesian adults with severe falciparum malaria (10%; 95% confidence interval [CI], 8%–13%) and in 20 with severe sepsis (8%; 95% CI, 5%–12%), compared with the mean levels in 32 patients with moderately severe malaria (7%; 95% CI, 5%–8%) and 36 controls (3.6%; 95% CI, 3%–5%; P < .001). An increased carboxyhemoglobin level was associated with an increased odds of death among patients with severe malaria (odds ratio, 1.2 per percentage point increase; 95% CI, 1.02–1.5). While also associated with severity and fatality, methemoglobin was only modestly increased in patients with severe malaria. Increased carboxyhemoglobin levels during severe malaria and sepsis may exacerbate organ dysfunction by reducing oxygen carriage and cautions against the use of adjunctive CO therapy, which was proposed on the basis of mouse models.
Keywords: Severe malaria, Plasmodium falciparum, carboxyhemoglobin, methemoglobin
Case-fatality rates in severe malaria remain at 10%–30%, even with use of highly effective antiparasitic drugs [1]. Development of adjunctive agents to improve outcomes requires better understanding of the pathogenesis of severe disease.
The diatomic molecules nitric oxide (NO) [2] and carbon monoxide (CO) [3] are postulated to have key roles in ameliorating the pathogenesis of severe malaria. Erythrocyte rupture in malaria and other acute inflammatory hemolytic diseases decrease NO bioavailability because of quenching by cell-free hemoglobin [4], which is metabolized to globin chains and free heme [3]. Heme is proposed to have pathogenic roles in severe malaria, owing to its proinflammatory and pro-oxidative properties [3]. Heme oxygenase 1 (HO-1) is an inducible enzyme responsible for the breakdown of heme into CO, biliverdin IXα, and iron, with up to 85% of endogenous CO produced by this pathway [5]. The CO generated binds to hemoglobin, forming carboxyhemoglobin and prevents further release of heme [3]. Increasing HO-1 activity or administering CO before development of severe disease protected against experimental murine cerebral malaria by attenuating the effects of heme, with each being proposed as adjunctive therapies [3]. However, few clinical studies have measured HO-1 activity or carboxyhemoglobin levels or have assessed the association with disease severity in malaria. Kenyan children did not have increased carboxyhemoglobin levels in cerebral malaria, compared with levels in children with moderately severe malaria, with inadequate HO-1 induction proposed as a contributory factor [6]. In contrast, increased HO-1 expression was associated with severe malaria in Gambian children [7] and with cerebral malaria in Malawian autopsy studies [8]. Studies in Angolan children and Myanmar adults have also linked an increased risk of cerebral malaria with HO-1 gene promoter polymorphisms associated with increased expression [9, 10].
In malaria, hemoglobin tetramers released during erythrocyte-rupture disaggregate into dimers, which oxidize to non–oxygen-carrying methemoglobin [11]. Elevated methemoglobin levels have been reported in African children in proportion to malarial severity and may contribute to pathogenesis by reducing oxygen-carrying capacity [11]. The carboxyhemoglobin level (a measure of HO-1 activity) and the methemoglobin level have not been reported in Asian adults with malaria, and their relationship with disease severity is unknown. We used pulse co-oximetry to compare levels of carboxyhemoglobin and methemoglobin among adults with severe malaria, those with moderately severe malaria, those with severe sepsis, and healthy controls.
METHODS
The study was conducted at Mitra Masyarakat Hospital, Timika, Papua, Indonesia, an area with unstable malaria transmission. Written informed consent was obtained from patients or relatives. The study was approved by the ethics committees of the National Institute of Health Research and Development, Indonesia, and the Menzies School of Health Research, Australia.
Patients ≥18 years of age with severe or moderately severe Plasmodium falciparum malaria, or severe sepsis and healthy controls were enrolled as previously described [12]. Severe malaria was defined as the presence of at least 1 of the modified World Health Organization (WHO) criteria [12]; individuals with moderately severe malaria were inpatients hospitalized with fever within the preceding 48 hours, parasite counts of >1000 parasites/µL, and no WHO-specified warning signs or severe criteria. Patients with hemoglobin levels of <60 g/L or those receiving antimalarials for >18 hours were excluded. Severe sepsis was defined as clinical evidence of infection, the presence of ≥3 systemic inflammatory response syndrome criteria, and dysfunction of ≥1 organ, with or without septic shock, according to American College of Chest Physicians criteria [12], with no parasites by microscopy or rapid diagnostic testing. Healthy controls were nonrelated hospital visitors with no history of fever and no parasitemia on microscopy. Standardized history and physical examination findings were documented. Parasite counts were determined by thick and thin film microscopy. Hemoglobin, creatinine, and lactate levels were measured with a bedside analyzer (i-STAT), and plasma histidine-rich protein 2 (HRP2) levels were determined by an enzyme-linked immunosorbent assay as described elsewhere [12]. Patients were managed by non–study hospital physicians, using standard hospital protocols as described elsewhere [12].
Carboxyhemoglobin and Methemoglobin Measurements
Oxygen saturation, carboxyhemoglobin, and methemoglobin levels were measured using a Food and Drug Administration–approved pulse co-oximeter (Masimo Rad-7). The co-oximeter compares differential absorption, using a multiple-wavelength sensor with 8 visible and infrared light-emitting diodes attached to the index or middle finger. Studies comparing carboxyhemoglobin levels measured by pulse co-oximetry with those measured by venous blood analysis have reported mean differences of 1.1%–1.4% [13]. Three measurements over a 20-minute period were taken, and the mean value was recorded if there was a difference of <2% between readings. Same-patient measurements with wider fluctuations within the 20-minute period were not recorded or analyzed. Measurements were repeated daily until discharge or death.
Statistical Methods
Statistical analysis was performed using Stata, version 11. Intergroup differences were compared by analysis of variance or the Kruskal-Wallis test, where appropriate. Pearson or Spearman correlation coefficients were determined depending on the normality of distributions. Longitudinal associations were assessed by mixed-effects modeling using generalized estimating equations. Logistic regression was used to determine the association between binary outcomes. A 2-sided P value of <.05 was considered statistically significant.
RESULTS
Patients
Patient profiles have previously been published [12]. Pulse co-oximeter readings were obtained for all healthy controls; for 32 of 33 patients with moderately severe malaria; for 29 of 36 with severe malaria, including 7 of the 9 who died; and for 20 of 24 with severe sepsis, including all 4 with fatal cases. Baseline patient characteristics and laboratory results are shown in Table 1. Among patients with severe malaria, 9 (31%) had 1 severity criterion, 7 (24%) had 2, and 13 (45%) had >2. Patients with severe malaria received intravenous artesunate, and patients with moderately severe malaria received intravenous artesunate initially followed by oral artemisinin combination therapy, once they could tolerate oral therapy. Patients with severe sepsis received antibiotics as previously described [12].
Table 1.
Baseline Demographic Characteristics and Clinical and Laboratory Values
| Variable | Healthy Controls (n=36) | Patients With Moderately Severe Malaria (n=32) | Patients With Severe Malaria (n=29) | Patients With Severe Sepsis (n=20) |
|---|---|---|---|---|
| Age, y, mean (range) | 29 (19–54) | 31 (18–50) | 30 (18–55) | 30 (20–50) |
| Male sex | 27 (75) | 17 (53) | 23 (79) | 14 (70) |
| Current smoker | 14 (39) | 10 (31) | 11 (38) | 8 (40) |
| Fever duration before presentation, d, mean (range) | NA | 4 (1–14) | 5 (1–14) | 5 (1–12) |
| Systolic blood pressure, mm Hg, mean (range)a | 118 (101–140) | 106 (95–136) | 110 (78–153) | 105 (61–152) |
| Diastolic blood pressure, mm Hg, mean (range)a | 70 (57–89) | 63 (47–90) | 63 (40–90) | 64 (31–85) |
| Pulse, beats/min, mean (range)a | 67 (52–108) | 82 (55–115) | 93 (70–120) | 109 (69–175) |
| Oxygen saturation, %, mean (range) | 99 (96–100) | 99 (94–100) | 97 (75–100) | 97 (86–100) |
| Temperature, °C, mean (range) a | 36.3 (35–37) | 37.1 (35.3–40.4) | 37.3 (35.1–39.6) | 37.5 (35.4–39.4) |
| White blood cell count, ×103 cells/µL, mean (range) a | 6.3 (2.8–9.2) | 4.9 (2–11.6) | 9.5 (2.7–18.5) | 14.3 (1.7–39) |
| Hemoglobin level, g/dL, mean (range)* | 12.9 (8.8–16.5) | 11.0 (6.8–15) | 10.6 (6.1–17) | 11.3 (6.8–14.6) |
| Platelet count, ×109 platelets/L, mean (range)a | 158 (112–247) | 54 (12–143) | 50 (11–188) | 112 (22–229) |
| Creatinine level, mmol/L, mean (range)a | NA | 94 (22–180) | 317 (66–1190) | 184 (57–626) |
| Lactate level, mmol/L, mean (range)a | NA | 1.3 (0.45–3.2) | 4.3 (0.54–15.5) | 2.9 (1.0–8.4) |
| Parasite density, parasites/µL, geometric mean (95% CI)a | NA | 9953 (6892–13 564) | 44 070 (11 240–180 797) | NA |
| Carboxyhemoglobin level, %, mean (95% CI)a | ||||
| Overall | 3.6 (3–5) | 7 (5–8) | 10 (8–13) | 8 (5–12) |
| Smokers | 5 (3–7) | 8 (2–13) | 11 (8–14) | 4 (2–7) |
| Nonsmokers | 3 (2–5) | 6 (4–8) | 9 (4–14) | 11 (6–16) |
| Methemoglobin level, %, median (IQR)a | 0.6 (0.4–0.9) | 0.8 (0.7–1.6) | 1.2 (0.9–1.4) | 1 (0.7–1.6) |
| O2-carrying capacity, mL O2/dL, mean (range)a,b | 15.9 (9.4–21.2) | 12.9 (6.3–17.6) | 12.8 (4.5–20.1) | 13.3 (7.5–17.1) |
Data are no. (%) of subjects, unless otherwise indicated.
Abbreviations: CI, confidence interval; IQR, interquartile range.
a P < .05 (by analysis of variance, the Kruskal-Wallis test, or the χ2 test for comparison o patients with severe malaria, patients with moderately severe malaria, and healthy controls).
b Calculated as [1.34 × hemoglobin level] × [oxygenated hemoglobin level], where hemoglobin level is given in g/dL and the oxygenated hemoglobin level is defined as the O2 saturation level – carboxyhemoglobin level – methemoglobin level.
Carboxyhemoglobin Level, Disease Severity, and Mortality
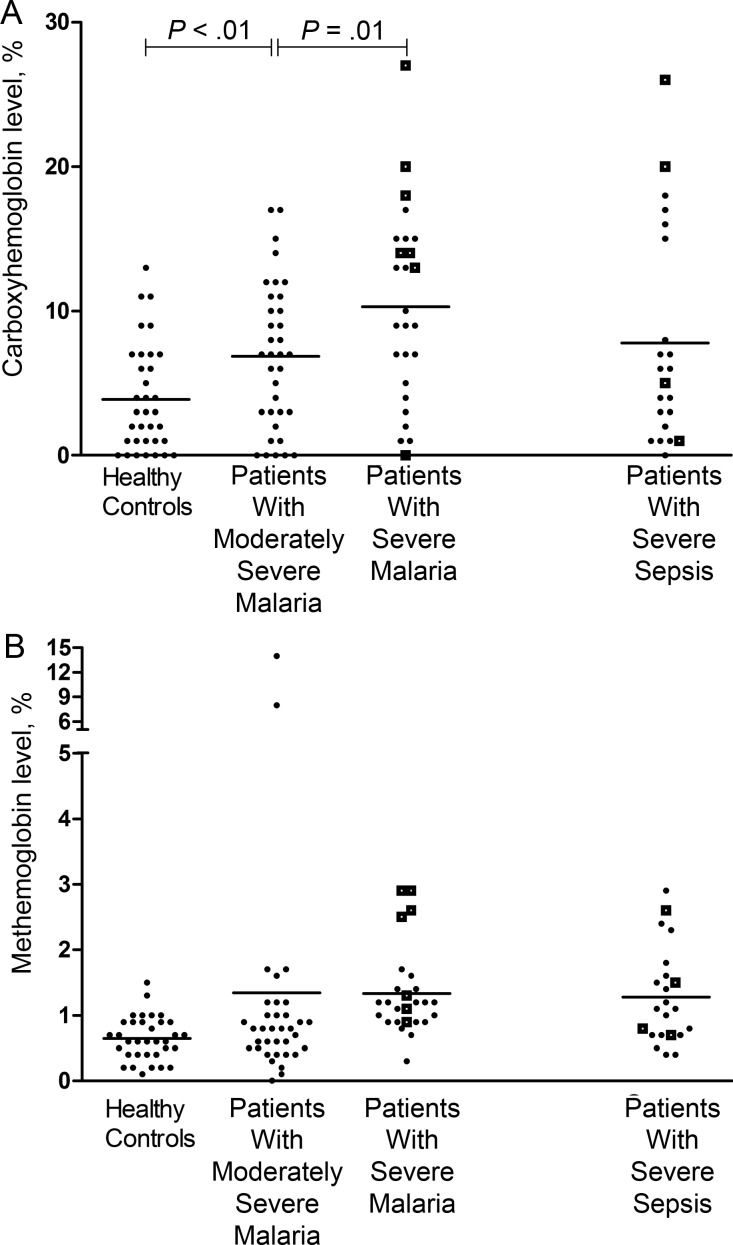
Carboxyhemoglobin levels were increased in the severe malaria and severe sepsis groups, compared with the moderately severe malaria and healthy control groups (P < .001; Table 1 and Figure 1A). Among patients with severe malaria, there was an association between carboxyhemoglobin level and disease severity (P = .02) and blood lactate level (r = 0.37; P = .04), with levels in patients who died significantly higher than those in patients who survived (P = .02; Figure 1A). Univariate logistic regression revealed that the odds of death increased by 1.2 (95% confidence interval [CI], 1.02–1.5) with each percentage point increase in carboxyhemoglobin level (P = .04); however, this was not significant after adjustment for hemoglobin level (P = .07). The prognostic value of the carboxyhemoglobin level for death in patients with severe malaria, as measured by the area under the receiver operating curve, was 0.73 (95% CI, .52–.97), compared with a value of 0.69 (95% CI, .46–.96; P = .4) for the venous lactate level. There was no significant change with carboxyhemoglobin levels with clinical recovery and time.
Figure 1.
A, Carboxyhemoglobin levels in healthy controls, patients with moderately severe malaria, patients with severe malaria (P < .001, by analysis of variance), and patients with severe sepsis. Open squares represent fatal cases, and horizontal lines represent mean values for each group. Horizontal bars represent pairwise comparisons between disease groups. B, Methemoglobin levels in healthy controls, patients with moderately severe malaria, patients with severe malaria, and patients with severe sepsis (P = .01, comparing all groups by the Kruskal-Wallis test). Open squares represent fatal cases, and horizontal lines represent the mean values for each group.
Carboxyhemoglobin Level and Hematological and Parasitological Measures
There was an inverse association between hemoglobin levels at enrollment and carboxyhemoglobin levels in patients with malaria (r = −0.36; P = .002) and those with severe malaria (r = −0.61; P = .001) but not in those with moderately severe malaria (r = −0.27; P = .1) or severe sepsis (r = −0.28; P = .1). There was also a significant association between plasma HRP2 concentrations (parasite biomass) and carboxyhemoglobin levels in all 41 patients with malaria (r = 0.38; P = .02) but not in 24 patients with moderately severe malaria (r = 0.28; P = .1) or 17 with severe malaria (r = 0.34; P = .1).
Methemoglobin Levels and Disease Severity
Methemoglobin levels were also increased in proportion to malaria disease severity (P = .01; Table 1 and Figure 1B), but levels were modest compared to carboxyhemoglobin levels. In patients with severe malaria, methemoglobin levels were higher in those with a fatal outcome (mean, 2.0%; 95% CI, 1.3%–2.9%), compared with levels among survivors (mean, 1.1%; 95% CI, 1.0%–1.2%; P = .002), and were comparable to levels among patients with severe sepsis (Figure 1B). There was no association between methemoglobin levels and hemoglobin levels, lactate levels, and parasite biomass. There was no change in methemoglobin levels with clinical recovery or time in all patients with malaria, in patients with severe malaria, or in patients with moderately severe malaria.
DISCUSSION
Carboxyhemoglobin levels were increased in Indonesian adults with falciparum malaria in proportion to disease severity. In patients with severe malaria, carboxyhemoglobin levels predicted mortality, with a prognostic value comparable to that of lactate levels. The increase in methemoglobin levels in patients with severe malaria was more modest.
The increased carboxyhemoglobin levels in patients with severe malaria are consistent with findings of studies involving African children and Myanmar adults, which showed that increased HO-1 expression or polymorphisms associated with increased HO-1 expression were associated with disease severity [7, 9, 10]. These results suggest that, as in children, there is increased HO-1 activity in response to increased hemolysis [7], with a marked elevation in the carboxyhemoglobin level. Results suggest that heme released during hemolysis may induce HO-1 and increase endogenous CO production, as suggested by the inverse relationship between hemoglobin and carboxyhemoglobin levels, but that an increased carboxyhemoglobin level may be insufficient to prevent further release of free heme and heme-related toxicity. The more modest increase in the methemoglobin level suggests that either plasma hemoglobin rapidly dissociates to free heme, the major inducer of HO-1, or that increased CO binding to hemoglobin incompletely prevents further breakdown of cell-free hemoglobin and oxidation to methemoglobin. HO-1 activity was not associated with mortality in pediatric patients with severe malaria but is predictive of death in adults. While moderate increases in the carboxyhemoglobin level during moderately severe malaria could protect against severe disease, the association between increasing levels and mortality suggests further increases were either insufficiently protective or harmful.
A previous study involving African children reported greater elevation in methemoglobin levels in patients with severe disease than in adult patients with severe malaria, with most of these having cerebral malaria and/or severe anemia [11]. Mean hemoglobin concentrations in these children were approximately half of those in our study adults with severe disease, perhaps contributing to a higher percentage of methemoglobin. Our findings suggest that impaired oxygen-carrying capacity in adult patients with severe malaria is related much more to an increased carboxyhemoglobin level than to an increased methemoglobin level. Similar to methemoglobin, carboxyhemoglobin is incapable of participating in oxygen transport and may exacerbate tissue hypoxia in patients with severe malaria.
Murine models demonstrated that prophylactic inhalation of CO at 250 parts per million, given after infection but before development of severe disease, significantly reduced mortality from experimental cerebral malaria by increasing carboxyhemoglobin levels to 23% [3, 14]. In contrast, in human experimental endotoxemia, CO had no antiinflammatory effects when administered at 500 parts per million, which increased carboxyhemoglobin levels to a mean of 7% [14]. These results and the high levels of carboxyhemoglobin seen in patients with severe malaria caution against the use of therapies to increase CO bioavailability, including inhaled CO, as adjunctive agents in Asian adults with severe malaria. Increasing carboxyhemoglobin levels from the mean of 10% seen in patients with severe malaria to 23% achieved with CO prophylaxis in the mouse models [3] would result in a further estimated decrease of 19.4% in the oxygen-carrying capacity.
Our study had several limitations. Carboxyhemoglobin and methemoglobin levels in blood were not directly measured because of a lack of available equipment and reagents. Co-oximetry–determined carboxyhemoglobin levels in control patients appeared to be higher than those previously reported with other methods, but this would not explain the relative increases in carboxyhemoglobin levels seen in patients with malaria or sepsis. In addition, the carboxyhemoglobin levels in patients with severe sepsis were comparable to those reported in Japanese adults with sepsis, whose levels were measured with a gas chromatography analyzer [15]. High rates of cigarette smoking could also explain increased carboxyhemoglobin levels, but the proportion of smokers was similar across groups. We were unable to exclude an artifactual component of measured carboxyhemoglobin and methemoglobin levels due to hemozoin in patients with severe malaria; however, it is notable that both were comparably high in severe bacterial sepsis.
In conclusion, the carboxyhemoglobin level and, to a lesser extent the methemoglobin level are elevated in proportion to disease severity in adults with falciparum malaria and are predictive of death. Results suggest that HO-1 activity and CO production are increased in adult patients with severe malaria but may be insufficient to prevent the breakdown of cell-free hemoglobin to heme and its deleterious consequences. The deleterious reduction in oxygen-carrying capacity due to increased levels of endogenous carboxyhemoglobin in patients with severe malaria cautions against adjunctive therapy with CO.
Notes
Acknowledgments. We thank Retno Gitawati, Indri Rooslamiati, Sri Muliati, and Erens Meokbum, for their support; Yohanes Kalvein Mira Mangngi, for nursing assistance; Ferryanto Chalfein, Prayoga, and Kim Piera, for technical and logistical assistance; Mitra Masyarakat Hospital staff, for clinical support; and Mauritz Okeseray, Paulus Sugiarto, Jeanne Rini Poespoprodjo, and Lembaga Pengembangan Masyarakat Amungme Kamoro, for support and assistance.
Financial support. This work was funded by the Australian National Health and Medical Research Council (ICRG ID 283321; grants 605807 and 496600; and fellowships 490307 [to N. M. A.] and 605831 [to T. W. Y.]) and the Wellcome Trust (ICRG GR071614MA and senior fellowship to R. N. P.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.SEAQUAMAT Group. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366:717–25. doi: 10.1016/S0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 2.Yeo TW, Lampah DA, Gitawati R, et al. Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med. 2007;204:2693–704. doi: 10.1084/jem.20070819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pamplona A, Ferreira A, Balla J, et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat Med. 2007;13:703–10. doi: 10.1038/nm1586. [DOI] [PubMed] [Google Scholar]
- 4.Yeo TW, Lampah DA, Tjitra E, et al. Relationship of cell-free hemoglobin to impaired endothelial nitric oxide bioavailability and perfusion in severe falciparum malaria. J Infect Dis. 2009;200:1522–9. doi: 10.1086/644641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryter SW, Otterbein LE. Carbon monoxide in biology and medicine. Bioessays. 2004;26:270–80. doi: 10.1002/bies.20005. [DOI] [PubMed] [Google Scholar]
- 6.Cunnington AJ, Kendrick SF, Wamola B, Lowe B, Newton CR. Carboxyhemoglobin levels in Kenyan children with Plasmodium falciparum malaria. Am J Trop Med Hyg. 2004;71:43–7. [PubMed] [Google Scholar]
- 7.Walther M, De Caul A, Aka P, et al. HMOX1 Gene Promoter Alleles and High HO-1 Levels Are Associated with Severe Malaria in Gambian Children. PLoS Pathog. 2012;8:e1002579. doi: 10.1371/journal.ppat.1002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark IA, Awburn MM, Harper CG, Liomba NG, Molyneux ME. Induction of HO-1 in tissue macrophages and monocytes in fatal falciparum malaria and sepsis. Malar J. 2003;2:41. doi: 10.1186/1475-2875-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeda M, Kikuchi M, Ubalee R, et al. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to cerebral malaria in Myanmar. Jpn J Infect Dis. 2005;58:268–71. [PubMed] [Google Scholar]
- 10.Sambo MR, Trovoada MJ, Benchimol C, et al. Transforming growth factor beta 2 and heme oxygenase 1 genes are risk factors for the cerebral malaria syndrome in Angolan children. PLoS One. 2010;5:e11141. doi: 10.1371/journal.pone.0011141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anstey NM, Hassanali MY, Mlalasi J, Manyenga D, Mwaikambo ED. Elevated levels of methaemoglobin in Tanzanian children with severe and uncomplicated malaria. Trans R Soc Trop Med Hyg. 1996;90:147–51. doi: 10.1016/s0035-9203(96)90118-2. [DOI] [PubMed] [Google Scholar]
- 12.Yeo TW, Lampah DA, Kenangalem E, Tjitra E, Price RN, Anstey NM. Impaired skeletal muscle microvascular function and increased skeletal muscle oxygen consumption in severe falciparum malaria. J Infect Dis. 2013;207:528–36. doi: 10.1093/infdis/jis692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shamir MY, Avramovich A, Smaka T. The current status of continuous noninvasive measurement of total, carboxy, and methemoglobin concentration. Anesth Analg. 2012;114:972–8. doi: 10.1213/ANE.0b013e318233041a. [DOI] [PubMed] [Google Scholar]
- 14.Mayr FB, Spiel A, Leitner J, et al. Effects of carbon monoxide inhalation during experimental endotoxemia in humans. Am J Respir Crit Care Med. 2005;171:354–60. doi: 10.1164/rccm.200404-446OC. [DOI] [PubMed] [Google Scholar]
- 15.Takaki S, Takeyama N, Kajita Y, et al. Beneficial effects of the heme oxygenase-1/carbon monoxide system in patients with severe sepsis/septic shock. Intensive Care Med. 2010;36:42–8. doi: 10.1007/s00134-009-1575-4. [DOI] [PubMed] [Google Scholar]