Abstract
Caveolae, plasma membrane invaginations of 60–80 nm in diameter, are a subset of lipid rafts enriched in cholesterol and sphingolipids. Caveolae are expressed in various tissues and cell types, such as endothelial cells, macrophages, neutrophils and adipocytes. The functions of caveolae are diverse and include endocytosis, transcytosis, potocytosis, calcium signaling, and regulation of various signaling events. Although growing evidence has increased our understanding of caveolae function, the role of caveolae in sepsis is still a controversial issue. In this review, we present a number of studies addressing caveolae and sepsis and describe the signaling pathways involved, including the LPS-eNOS-TLR4-NFκB, MKK3/p38 MAPK, cPLA2/p38 MAPK, STAT3/NFκB and IL-1β-IL-1R1 pathways. Different studies using endotoxemia and bacteremia animal models have provided distinct conclusions about the function of caveolae, and we discuss these inconsistencies. Taken together, the current data suggest that the function of caveolae in sepsis, which involves a number of signaling pathways, is complex and warrants further studies.
Caveolae, plasma membrane invaginations of 60–80 nm in diameter, were first identified in the 1950s by electron microscopy (Palade, 1953). Caveolae are a subset of lipid rafts that are enriched in cholesterol and sphingolipids. Caveolae are expressed in various tissues and cell types, such as smooth muscle, fibroblasts, endothelial cells, macrophages and adipocytes. The functions of caveolae are diverse and include endocytosis, transcytosis, potocytosis, calcium signaling, and regulation of various signaling events (Parton and Simons, 2007).
The major constituent of caveolae is the protein caveolin (Rothberg et al., 1992). There are three isoforms of caveolin, caveolin-1, 2, and 3. Caveolin-1 and 2 are expressed ubiquitously, whereas caveolin-3 is muscle-specific. Caveolin-1 is the most studied of these and the first member of the caveolin family to be identified. It is a 22-kDa tyrosine-phosphorylated protein and is known to be a structural component of caveolae and of transport vesicles derived from the trans-Golgi network (Igbavboa et al., 2009; Lin et al., 2009). Caveolin-1 is ubiquitously expressed, albeit at different levels in specific tissues. Disruption of the caveolin-1 gene leads to loss of caveolae (Drab et al., 2001), indicating an essential role of caveolin-1 in caveolae formation. As caveolin-1 is the defining and functional protein of caveolae, most studies have focused on it, especially those addressing the roles of caveolae in sepsis (Zemans and Downey, 2008).
1. Function of caveolae and caveolin
Mice genetically deficient in caveolin-1 have been employed for studying the functions of caveolae and caveolin-1 (Drab et al., 2001). Caveolin-1-deficient mice generally appear healthy, but they are physically weak, and their lungs display severe abnormalities, with increased cell numbers and disorganized architecture. Subsequent studies have uncovered the functions of caveolin-1 in multiple biological processes, regulating cholesterol trafficking, signal transduction and tumorigenesis.
1.1 Cholesterol
Cholesterol is required for the formation and maintenance of caveolae. Caveolin-1 can bind free cholesterol and has been implicated in the intracellular transport and regulation of cholesterol. A series of studies have demonstrated that caveolin-1 directly binds and traffics cholesterol through the cytoplasm to the plasma membrane in a lipoprotein chaperone complex (Igbavboa et al., 2009; Lin et al., 2009).The chaperone complex consists of caveolin-1, cyclophilin A, cyclophilin 40 and HSP56. A caveolin-1 lipoprotein chaperone complex was also shown to facilitate the uptake of caveolae cholesterol (Lin et al., 2009). Caveolin-1 facilitates this uptake by forming a lipoprotein complex consisting of caveolin-1, cyclophilin A, cyclophilin 40 and annexin II. Thus, caveolin-1 appears to shuttle between the plasma membrane and the Golgi by a multi-step process. Evidence also suggests that caveolin-1 is involved in the transport of newly synthesized cholesterol from the ER to the plasma membrane. Fu et al. (Fu et al., 2010) reported that cholesterol increases the adhesion of monocytes to the endothelium by moving adhesion molecules out of caveolae, suggesting that caveolin-1 may also affect inflammatory processes through its interaction with cholesterol.
1.2 Signal transduction
Previous reports have presented caveolae as lipid-based signaling platforms that both compartmentalize and concentrate signaling molecules. However, recent studies emphasize the importance of caveolins, including caveolin-1, as negative regulators of diverse cellular signaling pathways (Dessy et al., 2010). Specific motifs within the caveolin proteins serve to recruit lipids and proteins to caveolae, thus facilitating intracellular trafficking of the cellular machinery and regulation of signaling pathways. Sequestered within caveolae, through interaction with caveolin-1, are many G protein receptors, Gα subunits, tyrosine kinases and receptor tyrosine kinases, GTPases, components of the MAPK pathway, and others (Cao et al., 2002; Vargas et al., 2002; Vihanto et al., 2006). In many of these interactions, caveolin-1 appears to dampen signaling pathways by inhibiting the associated proteins, including c-Src, H-Ras, mitogen-activated protein (MAP) kinases (Engelman et al., 1998) and eNOS (Brouet et al., 2001; Garrean et al., 2006; Ju et al., 1997; Mirza et al., 2010; Sessa, 2005; Shin et al., 1996; Wang and Abdel-Rahman, 2005). Some of these proteins are important participants in sepsis, implying a role for caveolae in this process.
2. The role of caveolae and caveolin in sepsis
2.1 Distribution of caveolae and caveolin in immune cells
Although caveolae and caveolin have been implicated in immune response processes, their presence in immune cells is still a contentious issue (Harris et al., 2002a). Most research suggests that they are commonly found in myeloid cells. Caveolae or caveolin have been identified in murine macrophages and mast cells, in human dendritic cells and in bovine monocytes, macrophages and dendritic cells (Harris et al., 2002b; Shin et al., 2000; Wang et al., 2006). Caveolin has also been reported in human neutrophils (Hu et al., 2008), the professional phagocytic immune cells, suggesting that caveolae and caveolin play an important role in specific immune cell functions. Most recent evidence suggests that caveolae and caveolin are present in all types of immune cells, including lymphocytes, although different results have been presented (Fra et al., 1995; Medina et al., 2006b; Vallejo and Hardin, 2005). The presence of caveolae or caveolin in human and murine lymphocytes might depend on the cell type studied, and their expression or distribution might also be dependent on the activation and/or maturation state of the cell. The universal distribution of caveolae and caveolin-1 in immune cells suggests they might be involved in sepsis.
2.2 The role of caveolae in pathogen internalization
Caveolae have long been suggested to play a role in innate immunity, and pathogen–caveolae interactions have been reported. In many cases, particularly for bacterial pathogens and their exotoxins, such interactions might have evolved to facilitate the entry of the pathogen into host cells, avoiding routes that would lead to pathogen destruction.
Under serum-free conditions, E. coli that binds to macrophages through FimH (a mannose-binding fimbrial lectin that promotes bacterial adherence and colonization of mucosal surfaces) can survive inside the macrophage (Baorto et al., 1997). The receptor for FimH is GPI-linked CD48, which is concentrated in the caveolae of macrophages and mast cells. Internalization of FimH-expressing E. coli is inhibited by the caveolae-disrupting agents filipin, nystatin and methyl-β-cyclodextrin (Baorto et al., 1997; Shin et al., 2000). Under nonopsonic conditions, Chlamydia trachomatis has been shown to enter HEp-2 and HeLa 299 endothelial cells, as well as J-774A.1 mouse macrophage/monocyte cells, through caveolin-positive membrane domains enriched in glycosphingolipids and cholesterol; this entry can be inhibited with nystatin or filipin (Norkin et al., 2001). Caveolae have also been implicated in the cellular transport of viruses, including simian virus 40, respiratory syncytial virus and human immunodeficiency virus. The presence of caveolae thus facilitates the survival of pathogens inside the host cells, implicating caveolae as important regulators of infectious diseases.
2.3 The role of caveolae signaling in the response to LPS-induced endotoxemia
Using different cell lines, Lei et al. (Lei and Morrison, 2000) observed different changes in caveolin-1 expression in response to various LPS concentrations. Similarly, Tiruppathi et al. (Tiruppathi et al., 2008) reported that LPS challenge of human lung microvascular endothelial cells induced concentration- and time-dependent increases in the expression of caveolin-1 mRNA and protein, which were associated with increased endothelial permeability. These data suggest that caveolin-1 expression is associated with LPS signaling/internalization, leading us to the idea of a caveolin-inflammation relationship (Kamoun et al., 2006).
2.3.1 eNOS-TLR4-NFκB
The interaction of caveolin-1 with endothelial NO synthase (eNOS) is the only direct protein-protein interaction that can be demonstrated for caveolin-1 at this time. When it is not activated, eNOS is primarily located in caveolae, where its activation can be modulated through either direct steric inhibition of calmodulin binding of caveolin-1 or regulation of upstream and downstream signaling (Gratton et al., 2000; Mirza et al., 2010; Zhao et al., 2009). NO is a crucial in vivo determinant of lung inflammation. NO not only exerts direct bactericidal and immunomodulating effects but also plays a multifaceted role in the regulation of NFκB and de novo synthesis of proinflammatory proteins, such as ICAM-1 and inducible NO synthase (iNOS) (Shin et al., 1996; Spiecker et al., 1997; Tsao et al., 1997). Caveolin-1 acts as an inhibitory regulator of eNOS via their direct interaction (Feron et al., 1996; Garcia-Cardena et al., 1996; Ju et al., 1997). The caveolin-1-eNOS interaction has been implicated in the pathogenesis of atherosclerosis (Brouet et al., 2001) and hepatic cirrhosis (Wang and Abdel-Rahman, 2005; Yokomori et al., 2005). Garrean et al. (Garrean et al., 2006) further investigated the role of caveolin-1 in modulation of NO signaling and the lung inflammatory response to LPS-induced endotoxemia. In the model of LPS challenge, caveolin-1, by regulating eNOS-mediated nitric oxide production, is considered to be a crucial regulator of NFκB activation, and the resulting pulmonary inflammation, in response to LPS.
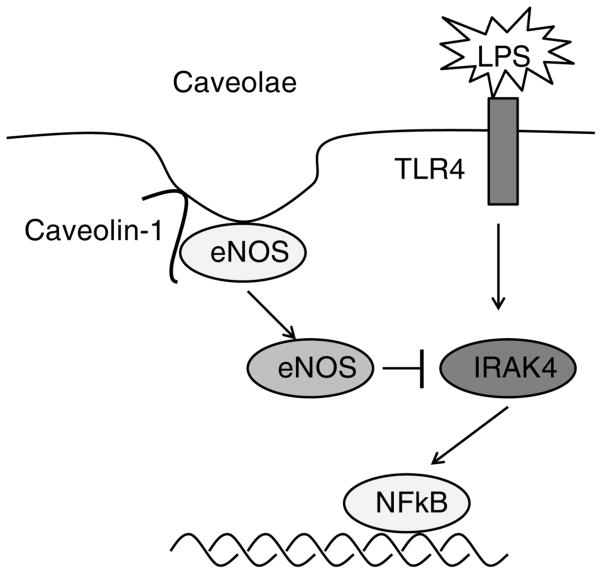
TLR4 binding by LPS causes activation of NFκB, a regulator of immunity and inflammation, leading to the production of an array of pro-inflammatory cytokines. The serine-threonine kinase interleukin-1R-associated kinase 4 (IRAK4), which is a signaling component required for NFκB activation and innate immunity, plays a critical role in the LPS-TLR4 pathway. IRAK4 is prominently nitrated in caveolin-1-deficient endothelial cells. Using double-mutant mice with genetic deletions of caveolin-1 and eNOS, Mirza et al. (Mirza et al., 2010) observed an eNOS-dependent decrease in the plasma concentration of pro-inflammatory cytokines and marked improvement of survival in caveolin-1-deficient mice following LPS challenge (Fig 1). Chronic eNOS activation due to loss of caveolin-1 serves a crucial immunomodulatory function through tyrosine nitration-mediated impairment of IRAK4; this results in decreased activation of nuclear factor-κB in response to LPS challenge, thereby protecting the animals from LPS-induced lung injury. Wang et al. (Wang et al., 2009) have shown that the regulation of the caveolin-1/TLR4 interaction by heme oxygenase-1/carbon monoxide activity reduces the production of tumor necrosis factor-α and interleukin-6 in response to LPS-induced in ammation in macrophages. Taken together, the data above suggest direct or indirect interactions among caveolin-1, eNOS, TLR4 and NFκB.
Figure 1.
A model describing the regulation of eNOS-TLR4-NFκB signaling by caveolin-1. Caveolin-1 is a critical negative regulator of eNOS via direct interaction. Caveolin-1 deficiency leads to eNOS translocation and activation, resulting in IRAK4 nitration. IRAK4 nitration inhibits TLR4 signaling in response to LPS challenge. Impaired TLR4 signaling results in dampening of the inflammatory response through decreased NFκB activation.
2.3.2 The MKK3/p38 MAPK pathway
Using LPS challenge of murine alveolar and peritoneal macrophages and RAW264.7 cells, Wang et al. (Wang et al., 2006) observed that caveolin-1 decreases LPS-induced TNF-α and IL-6 production and augments IL-10 production. p38 mitogen-activated protein kinase (MAPK) phosphorylation is increased by overexpressing caveolin-1 in RAW264.7 cells, whereas phosphorylation of c-Jun N-terminal kinase, the extracellular signal-regulated kinase MAPK, and Akt is inhibited. These data suggest that caveolin-1 acts as a potent immunomodulatory effecter molecule in immune cells and that its regulation of LPS-induced cytokine production involves the MKK3/p38 MAPK pathway. These findings seems to conflict with the high death rate induced by LPS in caveolin-1 expressing mice, underscoring the complicated regulatory role of caveolin-1 in sepsis.
2.3.3 The cPLA2/p38 MAPK pathway
Lv et al. (Lv et al., 2010) studied the effect of caveolin-1 on cytosolic phospholipase A2 (cPLA2), p38 mitogen-activated protein kinase (p38 MAPK) and NFκB during the inflammatory response of mouse lung alveolar type-1 cells (AT-1 cells) induced by LPS. They found that overexpression of caveolin-1 aggravates the AT-1 cell injury induced by LPS, and this involves regulation of cPLA2 through the cPLA2/p38 MAPK pathway.
2.4 The role of caveolae signaling in bacterial infection-related sepsis
Our previous review has suggested that mice lacking caveolin-1 are more resistant to LPS-induced inflammatory injury to the lung (Garrean et al., 2006). However, a contrasting phenomenon was recently reported, in which caveolin-1 was associated with bacterial infections of certain species. Medina et al. (Medina et al., 2006a) sought to determine the role of caveolin-1 expression in Salmonella enterica serovar Typhimurium pathogenesis. Caveolin-1-deficient mice displayed a significant decrease in survival when challenged with Salmonella. They also displayed increased production of inflammatory cytokines, chemokines, and nitric oxide, yet were unable to control the systemic infection of Salmonella. The increased chemokine production in caveolin-1-deficient mice resulted in greater infiltration of neutrophils into granulomas. Taken together, these data suggest that caveolin-1-deficient mice have defects in the innate immunity and in inflammatory immune responses during Salmonella infection (Medina et al., 2006a). Gadjeva et al. (Gadjeva et al., 2010) examined the sensitivity of caveolin-1-deficient mice to a P. aeruginosa challenge. They found that caveolin-1-deficient mice have increased sensitivity to P. aeruginosa infection, as represented by elevation in mortality rate, in bacterial burdens recovered from lungs and spleens, and in inflammatory responses. Using a respiratory infection model with Pseudomonas aeruginosa, Yuan et al. observed an enhanced inflammatory response in caveolin-1-deficient mice, characterized by elevated inflammatory cytokines, decreased phagocytic ability of macrophages, and increased superoxide release (Yuan et al., 2011). They further revealed that the caveolin-1/STAT3/NFκB axis is responsible for the dysregulated cytokine response. Inconsistent with LPS-induced endotoxemia, these findings indicate a protective role for caveolin-1 in bacterial infection-related sepsis and suggest that the additional events associated with bacterial infection could be more important than LPS signaling during sepsis.
Systemic infection in clinical patients usually consists of not only endotoxemia but also bacteremia, which is called sepsis. Although the LPS-endotoxemia animal model is widely used for the study of sepsis, and LPS plays an essential role in sepsis, the endotoxemia animal model does not fully mimic the changes observed in sepsis (Eskandari et al., 1992).Therefore, results from endotoxemia and sepsis studies may seem to conflict. For example, TLR4 mutant mice are resistant to LPS-induced endotoxic animal death but are highly susceptible to bacteria-induced septic death (Echtenacher et al., 2001; Poltorak et al., 1998; Vazquez-Torres et al., 2004). The reason for this phenomenon is still unclear. In response to LPS, wild-type macrophages can respond with exaggerated generation of inflammatory cytokines, which are critical for killing the invading bacteria but can also damage normal tissue. Inflammatory cytokines thus constitute a two-edged sword during sepsis. The cytokines that mainly mediate bacteria killing during sepsis may predominantly induce tissue damage during LPS challenge. Given the distinct differences between endotoxemia and sepsis, Feng et al. (Feng et al., 2010) have assessed the role of caveolin in sepsis using a well established and more clinically relevant septic animal model, cecal ligation and puncture (CLP). They demonstrated that mice deficient in caveolin-1 are more susceptible to polymicrobial septic death than wild-type littermates. Further analysis revealed that caveolin-1 protects against septic death, likely through its roles in modulating inflammatory responses, in alleviating bacterial burdens and in suppressing thymocyte apoptosis. This study provides direct evidence for the role of caveolin-1 in sepsis.
2.5 Other signaling pathways
There is evidence indicating that caveolae participate in additional inflammatory signaling pathways.
2.5.1 IL-1β-IL-1R1
Recent evidence suggests that signaling by the proinflammatory cytokine interleukin-1β (IL-1β) is dependent on reactive oxygen species derived from NADPH oxidase. Redox signaling in response to IL-1β is known to require endocytosis of its cognate receptor (IL-1R1) following ligand binding and the formation of redox-active signaling endosomes that contain Nox2 (also called redoxosomes). The consequent generation of reactive oxygen species by redoxosomes is responsible for the downstream recruitment of IL-1R1 effectors (IRAK, TRAF6, and I-κB kinase kinases) and ultimately for activation of the transcription factor NFκB. Oakley et al. (Oakley et al., 2009) demonstrated that caveolin-1-dependent endocytosis of Nox2 and IL-1R1 into the redoxosome is responsible for IL-1β-dependent activation of NFκB. They also showed that Vav1, a Rac1 guanine exchange factor and activator of Nox2, is recruited to lipid rafts following IL-1β stimulation and that it is required for NFκB activation.
2.5.2 Cyclooxygenase-2 (COX-2)
COX-2 is an inducible enzyme responsible for the formation of inflammatory prostanoids, such as prostaglandins and thromboxane. Its role in the pathophysiology of inflammatory states such as sepsis is increasingly recognized (Bitto et al., 2012; El-Achkar et al., 2007; Modzelewski and Janiak, 2004). COX-2 is upregulated in pathological states such as sepsis. The upregulation of COX-2 acts as a pro-inflammatory factor contributing to the multi-organ failure observed in sepsis. Clinical trials have been performed using COX-2 inhibitors to disrupt the sepsis process. Liou et al. (Liou et al., 2001) demonstrated the colocalization and interaction of COX-2 with caveolin-1 in human fibroblasts. In addition, Chen et al. (Chen et al., 2010) found that COX-2 protein levels in lung and colon tissues are higher in caveolin-1-null mice than in wild-type mice. Their findings suggest that caveolin-1 binds COX-2 in the endoplasmic reticulum (ER) and promotes its degradation there. The C-terminal region of COX-2 is required for caveolin-1 binding and degradation. These studies suggest a novel function for caveolin-1 in controlling COX-2 expression and thus, possibly, in regulating inflammatory responses.
2.5.3 Rac1 and Rac2
Hu et al. (Hu et al., 2008) studied the functional responses of bone marrow neutrophils from caveolin-1-null mice and revealed that caveolin-1-null neutrophils are defective in agonist-induced oxidant production, adhesion, and transendothelial migration compared with wild-type neutrophils. The study provides mechanistic data linking caveolin-1 to activation of Rac1 and Rac2, small GTPases known to act as molecular switches in agonist-induced pathways leading to antimicrobial responses of neutrophils (such as activation of the NADPH oxidase, chemoattractant-induced actin reorganization, and migration). Furthermore, overexpression of recombinant caveolin-1 in “engineered phagocytes” (Cos-phox cells) resulted in enhanced chemoattractant-induced superoxide production, providing strong evidence for a signal-enhancing role of caveolin-1 in this response.
2.6 Caveolin-2/3 and sepsis
In addition to caveolin-1, other caveolin isoforms have been associated with inflammatory responses. Caveolin-2-deficient mice exhibit increased sensitivity to endotoxemia, which is linked to the increased phosphorylation of STAT-1 at tyrosine 701 in intestinal cells (de Almeida et al., 2011). LPS-induced inhibition of connexin43 gap junction communication in astrocytes is mediated by downregulation of caveolin-3 (Liao et al., 2010).
3. Summary and future Studies
Different reports have presented distinct findings, especially in various septic animal models, suggesting complex roles for caveolae in regulating signaling in sepsis. In LPS animal models, the pro- versus anti-survival roles of caveolin-1 in endotoxemia are likely to depend on animal genetic background and severity of endotoxemia. In septic animal models infected with pathogenic bacteria or challenged with CLP, consistent results were reported, revealing protective roles of caveolin-1. Caveolin-1 thus regulates cytokine production, enhances phagocytosis of macrophage and plays as-yet undefined roles in the adaptive immunity. These mechanisms could be more important than LPS challenge-associated signaling in microbial sepsis (Fig 2). Given the multiple roles of caveolae, one would expect mice deficient in caveolin to be nonviable. However, caveolin-1, caveolin-2, and caveolin- 3 knockout mice are all viable and fertile, suggesting that some functions of caveolae are performed by lipid rafts in the caveolin-1 knockout mice. Obviously, a great deal of additional research is required for understanding the interactions between caveolae and lipid rafts. An exciting development has been the generation of loxP-caveolin-1 mice by Dr. Cao et al. (Cao et al., 2003). While Dr. Cao used this technique to generate whole-body caveolin-1 knockout mice, my laboratory bred the loxP-caveolin-1 mice with LysMcre transgenic mice and confirmed that the expression of caveolin-1 is abolished specifically in macrophages (unpublished data). We expect that this unique animal model will provide further insight into the roles of caveolin-1 in specific tissues.
Figure 2.
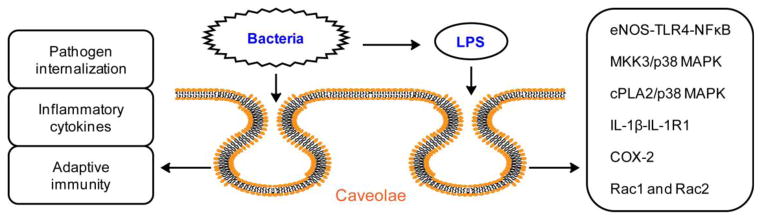
Caveolae/caveolin-1 show complexity in regulating cell signaling during sepsis. In endotoxemia challenged with LPS, contrasting survival outcomes are observed, which are likely to depend on animal background and LPS dosage. The associated signaling pathways regulated by caveolae/caveolin-1 include eNOS-TLR4-NFκB, MKK3/p38 MAPK, cPLA2/p38 MAPK, IL-1β-IL-1R1, COX-2, Rac1 and Rac2. However, in sepsis induced by bacterial infection or CLP, a protective role of caveolae/caveolin-1 is observed in most studies. Additional mechanisms besides LPS signaling include regulation of pathogen internalization, inflammatory cytokine production, and undefined roles in adaptive immunity.
Acknowledgments
Funding Sources: This publication was made possible by grants R01GM085231, R01GM085231-2S1 and R01GM085231-5S1 to X-A Li from NIGMS/NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIGMS or NIH. This work was also supported by a grant from the Children’s Miracle Network.
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baorto DM, Gao Z, Malaviya R, Dustin ML, van der Merwe A, Lublin DM, Abraham SN. Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature. 1997;389:636–639. doi: 10.1038/39376. [DOI] [PubMed] [Google Scholar]
- Bitto A, Minutoli L, David A, Irrera N, Rinaldi M, Venuti FS, Squadrito F, Altavilla D. Flavocoxid, a dual inhibitor of COX-2 and 5-LOX of natural origin, attenuates the inflammatory response and protects mice from sepsis. Crit Care. 2012;16:R32. doi: 10.1186/1364-8535-16-R32. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Brouet A, Sonveaux P, Dessy C, Moniotte S, Balligand JL, Feron O. Hsp90 and caveolin are key targets for the proangiogenic nitric oxide-mediated effects of statins. Circ Res. 2001;89:866–873. doi: 10.1161/hh2201.100319. [DOI] [PubMed] [Google Scholar]
- Cao G, Yang G, Timme TL, Saika T, Truong LD, Satoh T, Goltsov A, Park SH, Men T, Kusaka N, et al. Disruption of the caveolin-1 gene impairs renal calcium reabsorption and leads to hypercalciuria and urolithiasis. Am J Pathol. 2003;162:1241–1248. doi: 10.1016/S0002-9440(10)63920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Courchesne WE, Mastick CC. A phosphotyrosine-dependent protein interaction screen reveals a role for phosphorylation of caveolin-1 on tyrosine 14: recruitment of C-terminal Src kinase. J Biol Chem. 2002;277:8771–8774. doi: 10.1074/jbc.C100661200. [DOI] [PubMed] [Google Scholar]
- Chen SF, Liou JY, Huang TY, Lin YS, Yeh AL, Tam K, Tsai TH, Wu KK, Shyue SK. Caveolin-1 facilitates cyclooxygenase-2 protein degradation. J Cell Biochem. 2010;109:356–362. doi: 10.1002/jcb.22407. [DOI] [PubMed] [Google Scholar]
- de Almeida CJ, Witkiewicz AK, Jasmin JF, Tanowitz HB, Sotgia F, Frank PG, Lisanti MP. Caveolin-2-deficient mice show increased sensitivity to endotoxemia. Cell Cycle. 2011;10:2151–2161. doi: 10.4161/cc.10.13.16234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessy C, Feron O, Balligand JL. The regulation of endothelial nitric oxide synthase by caveolin: a paradigm validated in vivo and shared by the ‘endothelium-derived hyperpolarizing factor’. Pflugers Arch. 2010;459:817–827. doi: 10.1007/s00424-010-0815-3. [DOI] [PubMed] [Google Scholar]
- Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- Echtenacher B, Freudenberg MA, Jack RS, Mannel DN. Differences in innate defense mechanisms in endotoxemia and polymicrobial septic peritonitis. Infect Immun. 2001;69:7271–7276. doi: 10.1128/IAI.69.12.7271-7276.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Achkar TM, Plotkin Z, Marcic B, Dagher PC. Sepsis induces an increase in thick ascending limb Cox-2 that is TLR4 dependent. Am J Physiol Renal Physiol. 2007;293:F1187–1196. doi: 10.1152/ajprenal.00217.2007. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Chu C, Lin A, Jo H, Ikezu T, Okamoto T, Kohtz DS, Lisanti MP. Caveolin-mediated regulation of signaling along the p42/44 MAP kinase cascade in vivo. A role for the caveolin-scaffolding domain. FEBS Lett. 1998;428:205–211. doi: 10.1016/s0014-5793(98)00470-0. [DOI] [PubMed] [Google Scholar]
- Eskandari MK, Bolgos G, Miller C, Nguyen DT, DeForge LE, Remick DG. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J Immunol. 1992;148:2724–2730. [PubMed] [Google Scholar]
- Feng H, Guo L, Song Z, Gao H, Wang D, Fu W, Han J, Li Z, Huang B, Li XA. Caveolin-1 protects against sepsis by modulating inflammatory response, alleviating bacterial burden, and suppressing thymocyte apoptosis. J Biol Chem. 2010;285:25154–25160. doi: 10.1074/jbc.M110.116897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J Biol Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- Fra AM, Williamson E, Simons K, Parton RG. De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc Natl Acad Sci U S A. 1995;92:8655–8659. doi: 10.1073/pnas.92.19.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C, He J, Li C, Shyy JY, Zhu Y. Cholesterol increases adhesion of monocytes to endothelium by moving adhesion molecules out of caveolae. Biochim Biophys Acta. 2010;1801:702–710. doi: 10.1016/j.bbalip.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Gadjeva M, Paradis-Bleau C, Priebe GP, Fichorova R, Pier GB. Caveolin-1 modifies the immunity to Pseudomonas aeruginosa. J Immunol. 2010;184:296–302. doi: 10.4049/jimmunol.0900604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cardena G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc Natl Acad Sci U S A. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrean S, Gao XP, Brovkovych V, Shimizu J, Zhao YY, Vogel SM, Malik AB. Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J Immunol. 2006;177:4853–4860. doi: 10.4049/jimmunol.177.7.4853. [DOI] [PubMed] [Google Scholar]
- Gratton JP, Fontana J, O’Connor DS, Garcia-Cardena G, McCabe TJ, Sessa WC. Reconstitution of an endothelial nitric-oxide synthase (eNOS), hsp90, and caveolin-1 complex in vitro. Evidence that hsp90 facilitates calmodulin stimulated displacement of eNOS from caveolin-1. J Biol Chem. 2000;275:22268–22272. doi: 10.1074/jbc.M001644200. [DOI] [PubMed] [Google Scholar]
- Harris J, Werling D, Hope JC, Taylor G, Howard CJ. Caveolae and caveolin in immune cells: distribution and functions. Trends Immunol. 2002a;23:158–164. doi: 10.1016/s1471-4906(01)02161-5. [DOI] [PubMed] [Google Scholar]
- Harris J, Werling D, Koss M, Monaghan P, Taylor G, Howard CJ. Expression of caveolin by bovine lymphocytes and antigen-presenting cells. Immunology. 2002b;105:190–195. doi: 10.1046/j.1365-2567.2002.01362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Ye RD, Dinauer MC, Malik AB, Minshall RD. Neutrophil caveolin-1 expression contributes to mechanism of lung inflammation and injury. Am J Physiol Lung Cell Mol Physiol. 2008;294:L178–186. doi: 10.1152/ajplung.00263.2007. [DOI] [PubMed] [Google Scholar]
- Igbavboa U, Sun GY, Weisman GA, He Y, Wood WG. Amyloid beta-protein stimulates trafficking of cholesterol and caveolin-1 from the plasma membrane to the Golgi complex in mouse primary astrocytes. Neuroscience. 2009;162:328–338. doi: 10.1016/j.neuroscience.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem. 1997;272:18522–18525. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- Kamoun WS, Karaa A, Kresge N, Merkel SM, Korneszczuk K, Clemens MG. LPS inhibits endothelin-1-induced endothelial NOS activation in hepatic sinusoidal cells through a negative feedback involving caveolin-1. Hepatology. 2006;43:182–190. doi: 10.1002/hep.20940. [DOI] [PubMed] [Google Scholar]
- Lei MG, Morrison DC. Differential expression of caveolin-1 in lipopolysaccharide-activated murine macrophages. Infect Immun. 2000;68:5084–5089. doi: 10.1128/iai.68.9.5084-5089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CK, Wang SM, Chen YL, Wang HS, Wu JC. Lipopolysaccharide-induced inhibition of connexin43 gap junction communication in astrocytes is mediated by downregulation of caveolin-3. Int J Biochem Cell Biol. 2010;42:762–770. doi: 10.1016/j.biocel.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Lin YC, Lin CH, Kuo CY, Yang VC. ABCA1 modulates the oligomerization and Golgi exit of caveolin-1 during HDL-mediated cholesterol efflux in aortic endothelial cells. Biochem Biophys Res Commun. 2009;382:189–195. doi: 10.1016/j.bbrc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Liou JY, Deng WG, Gilroy DW, Shyue SK, Wu KK. Colocalization and interaction of cyclooxygenase-2 with caveolin-1 in human fibroblasts. J Biol Chem. 2001;276:34975–34982. doi: 10.1074/jbc.M105946200. [DOI] [PubMed] [Google Scholar]
- Lv XJ, Li YY, Zhang YJ, Mao M, Qian GS. Over-expression of caveolin-1 aggravate LPS-induced inflammatory response in AT-1 cells via up-regulation of cPLA2/p38 MAPK. Inflamm Res. 2010;59:531–541. doi: 10.1007/s00011-010-0157-9. [DOI] [PubMed] [Google Scholar]
- Medina FA, de Almeida CJ, Dew E, Li J, Bonuccelli G, Williams TM, Cohen AW, Pestell RG, Frank PG, Tanowitz HB, et al. Caveolin-1-deficient mice show defects in innate immunity and inflammatory immune response during Salmonella enterica serovar Typhimurium infection. Infect Immun. 2006a;74:6665–6674. doi: 10.1128/IAI.00949-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina FA, Williams TM, Sotgia F, Tanowitz HB, Lisanti MP. A novel role for caveolin-1 in B lymphocyte function and the development of thymus-independent immune responses. Cell Cycle. 2006b;5:1865–1871. doi: 10.4161/cc.5.16.3132. [DOI] [PubMed] [Google Scholar]
- Mirza MK, Yuan J, Gao XP, Garrean S, Brovkovych V, Malik AB, Tiruppathi C, Zhao YY. Caveolin-1 deficiency dampens Toll-like receptor 4 signaling through eNOS activation. Am J Pathol. 2010;176:2344–2351. doi: 10.2353/ajpath.2010.091088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modzelewski B, Janiak A. Pentoxyphilline as a cyclooxygenase (cox-2) inhibitor in experimental sepsis. Medical science monitor : international medical journal of experimental and clinical research. 2004;10:BR233–237. [PubMed] [Google Scholar]
- Norkin LC, Wolfrom SA, Stuart ES. Association of caveolin with Chlamydia trachomatis inclusions at early and late stages of infection. Exp Cell Res. 2001;266:229–238. doi: 10.1006/excr.2001.5202. [DOI] [PubMed] [Google Scholar]
- Oakley FD, Smith RL, Engelhardt JF. Lipid rafts and caveolin-1 coordinate interleukin-1beta (IL-1beta)-dependent activation of NFkappaB by controlling endocytosis of Nox2 and IL-1beta receptor 1 from the plasma membrane. J Biol Chem. 2009;284:33255–33264. doi: 10.1074/jbc.M109.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade GE. An electron microscope study of the mitochondrial structure. J Histochem Cytochem. 1953;1:188–211. doi: 10.1177/1.4.188. [DOI] [PubMed] [Google Scholar]
- Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Sessa WC. Regulation of endothelial derived nitric oxide in health and disease. Mem Inst Oswaldo Cruz. 2005;100(Suppl 1):15–18. doi: 10.1590/s0074-02762005000900004. [DOI] [PubMed] [Google Scholar]
- Shin JS, Gao Z, Abraham SN. Involvement of cellular caveolae in bacterial entry into mast cells. Science. 2000;289:785–788. doi: 10.1126/science.289.5480.785. [DOI] [PubMed] [Google Scholar]
- Shin WS, Hong YH, Peng HB, De Caterina R, Libby P, Liao JK. Nitric oxide attenuates vascular smooth muscle cell activation by interferon-gamma. The role of constitutive NF-kappa B activity. J Biol Chem. 1996;271:11317–11324. doi: 10.1074/jbc.271.19.11317. [DOI] [PubMed] [Google Scholar]
- Spiecker M, Peng HB, Liao JK. Inhibition of endothelial vascular cell adhesion molecule-1 expression by nitric oxide involves the induction and nuclear translocation of IkappaBalpha. J Biol Chem. 1997;272:30969–30974. doi: 10.1074/jbc.272.49.30969. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Shimizu J, Miyawaki-Shimizu K, Vogel SM, Bair AM, Minshall RD, Predescu D, Malik AB. Role of NF-kappaB-dependent caveolin-1 expression in the mechanism of increased endothelial permeability induced by lipopolysaccharide. J Biol Chem. 2008;283:4210–4218. doi: 10.1074/jbc.M703153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao PS, Wang B, Buitrago R, Shyy JY, Cooke JP. Nitric oxide regulates monocyte chemotactic protein-1. Circulation. 1997;96:934–940. doi: 10.1161/01.cir.96.3.934. [DOI] [PubMed] [Google Scholar]
- Vallejo J, Hardin CD. Expression of caveolin-1 in lymphocytes induces caveolae formation and recruitment of phosphofructokinase to the plasma membrane. Faseb J. 2005;19:586–587. doi: 10.1096/fj.04-2380fje. [DOI] [PubMed] [Google Scholar]
- Vargas L, Nore BF, Berglof A, Heinonen JE, Mattsson PT, Smith CI, Mohamed AJ. Functional interaction of caveolin-1 with Bruton’s tyrosine kinase and Bmx. J Biol Chem. 2002;277:9351–9357. doi: 10.1074/jbc.M108537200. [DOI] [PubMed] [Google Scholar]
- Vazquez-Torres A, Vallance BA, Bergman MA, Finlay BB, Cookson BT, Jones-Carson J, Fang FC. Toll-like receptor 4 dependence of innate and adaptive immunity to Salmonella: importance of the Kupffer cell network. J Immunol. 2004;172:6202–6208. doi: 10.4049/jimmunol.172.10.6202. [DOI] [PubMed] [Google Scholar]
- Vihanto MM, Vindis C, Djonov V, Cerretti DP, Huynh-Do U. Caveolin-1 is required for signaling and membrane targeting of EphB1 receptor tyrosine kinase. J Cell Sci. 2006;119:2299–2309. doi: 10.1242/jcs.02946. [DOI] [PubMed] [Google Scholar]
- Wang X, Abdel-Rahman AA. Effect of chronic ethanol administration on hepatic eNOS activity and its association with caveolin-1 and calmodulin in female rats. Am J Physiol Gastrointest Liver Physiol. 2005;289:G579–585. doi: 10.1152/ajpgi.00282.2004. [DOI] [PubMed] [Google Scholar]
- Wang XM, Kim HP, Nakahira K, Ryter SW, Choi AM. The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J Immunol. 2009;182:3809–3818. doi: 10.4049/jimmunol.0712437. [DOI] [PubMed] [Google Scholar]
- Wang XM, Kim HP, Song R, Choi AM. Caveolin-1 confers antiinflammatory effects in murine macrophages via the MKK3/p38 MAPK pathway. Am J Respir Cell Mol Biol. 2006;34:434–442. doi: 10.1165/rcmb.2005-0376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomori H, Oda M, Wakabayashi G, Kitajima M, Yoshimura K, Nomura M, Hibi T. High expressions of caveolins on the proliferating bile ductules in primary biliary cirrhosis. World J Gastroenterol. 2005;11:3710–3713. doi: 10.3748/wjg.v11.i24.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K, Huang C, Fox J, Gaid M, Weaver A, Li G, Singh BB, Gao H, Wu M. Elevated inflammatory response in caveolin-1-deficient mice with Pseudomonas aeruginosa infection is mediated by STAT3 protein and nuclear factor kappaB (NF-kappaB) J Biol Chem. 2011;286:21814–21825. doi: 10.1074/jbc.M111.237628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemans R, Downey GP. Role of caveolin-1 in regulation of inflammation: different strokes for different folks. Am J Physiol Lung Cell Mol Physiol. 2008;294:L175–177. doi: 10.1152/ajplung.00488.2007. [DOI] [PubMed] [Google Scholar]
- Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HH, Vogel SM, Brovkovych V, Yuan JX, Wharton J, Malik AB. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest. 2009;119:2009–2018. doi: 10.1172/JCI33338. [DOI] [PMC free article] [PubMed] [Google Scholar]