Abstract
Goal-directed reaching is important for activities of daily living. Populations of neurons in the primary motor cortex (M1) that project to spinal motor circuits are known to represent kinematics of reaching movements. We investigated whether repetitive practice of goal-directed reaching movements induces use-dependent plasticity of those kinematic characteristics, in a manner similar to finger movements, as had been shown previously. Transcranial magnetic stimulation (TMS) was used over the scalp to evoke upper extremity movements while the forearm was resting in a robotic cradle. Plasticity was measured by the change in kinematics of these evoked movements following goal-directed reaching practice. Baseline direction of TMS-evoked arm movements was determined for each subject. Subjects then practiced 3 blocks of 160 goal-directed reaching movements in a direction opposite to the baseline direction (14 cm reach 180° from baseline direction) against a 75 N·m spring field. Changes in TMS-evoked whole arm movements were assessed after each practice block and after 5 minutes following the end of practice. Direction and the position of the point of peak velocity of TMS-evoked movements were significantly altered following training and at a 5-minute interval following training, while amplitude did not show significant changes. This was accompanied by changes in the motor evoked potentials (MEPs) of the shoulder and elbow agonist muscles that partly explained the change in direction, mainly by increase in agonist MEP, without significant changes in antagonists. These findings demonstrate that the arm representation accessible by motor cortical stimulation demonstrates rapid plasticity induced by goal-directed robotic reach training in healthy subjects.
Goal-directed reaching is a movement that is fundamental for many human endeavors, and for performance of many activities of daily living. The kinematic parameters of reaching movements, such as direction, are determined through activity in the primary motor cortex (M1) (Graziano et al., 2002; Paninski et al., 2004; Hatsopoulos et al., 2007; Matsuzaka et al., 2007; Rickert et al., 2009). Previous research in our laboratory employed transcranial magnetic stimulation (TMS) applied over M1 to map TMS-evoked movement representation, using a robotic device to measure movement kinematics. These M1 movement maps varied between subjects and stimulus locations, but within a given location, the direction and extent of the evoked movements were remarkably consistent over time (Jones-Lush et al., 2010). The stability of TMS-evoked movements in the controlled environment of the rehabilitation robot therefore allows practice-related changes in motor representation to be characterized.
Kinematic and kinetic parameters of goal-directed movements such as direction and force can be deduced from M1 activity. Multiple studies have investigated how kinematic parameters of movements represented in corticospinal projections are modified during adaptation of reaching movements in a setting of a systematic force or visual perturbation (Gandolfo et al., 2000; Arce et al., 2010; Orban de Xivry et al., 2011). However, little is known about how kinematic parameters of movements represented in M1, with its projections to distal motor networks, are altered with repetitive practice of goal-directed reaching movements in the absence of a perturbation. Such typical goal-directed reaching movements are practiced in rehabilitation settings and are distinct form of practice than adaptation to force fields or visuomotor transformations (Huang et al., 2011; Krakauer and Mazzoni, 2011).
Repetitive practice of motor tasks induces changes in movement characteristics of those tasks and associated neurophysiology that likely form the neural basis for recovery of motor deficits after CNS injury (Butefisch et al., 2000; Muellbacher et al., 2001). One paradigm that demonstrates this physiological plasticity is TMS-evoked thumb movements (Classen et al., 1998). Stereotyped thumb movements are evoked in a consistent direction by TMS. Subsequent practice of movements in the direction opposite to the evoked movements results in a reversal in the direction of post-practice TMS-evoked finger movement for several minutes (Classen et al., 1998). While highly repetitive practice of simple single-joint finger movements demonstrates clear neural plasticity, such practice is very different from the multi-joint goal-directed reaching movements that characterize clinical rehabilitation, and evidence for plasticity related to proximal upper extremity movements is scarce. Further, it is not known which kinematic parameters of movements represented in corticospinal system (by which we mean M1 and the subcortical and spinal networks to which it projects) are amenable to change with repetitive practice of goal-directed unperturbed reaching movements.
Here, we investigate the temporal evolution of practice-induced changes in the kinematic characteristics of the reaching movements evoked by TMS applied over M1. First, baseline stability of the kinematic features of TMS-evoked arm movements was assessed over time. Then, changes in those features were assessed as participants practiced goal-directed reach movements in a direction opposite to the one evoked at baseline. We hypothesized that repetitive goal-directed reaching practice would trigger use-dependent plasticity that would enhance representation of the practiced reaching movements accessible by transcranial stimulation of the motor cortex. We further explored the relationship between kinematic characteristics of TMS-evoked reach-like movements and motor evoked potentials (MEPs) to begin to relate the changes in movement representation to changes in muscle activity.
Experimental procedures
2.1 Participants
Twenty-two healthy volunteers (7 females, mean age ±SD: 27±3.15 years, one left-handed) with no history of neurological disease participated in the study. All participants met TMS safety criteria (Wassermann, 1998). All provided informed consent and were evaluated per a protocol approved by the University of Maryland Institutional Review Board and the local Veterans Administration Research Committee.
2.2 Data collection
Participants were seated comfortably in front of a 2 degree-of-freedom planar robot (Interactive Motion Technologies, Cambridge, MA) with their dominant arm in the robotic arm cradle. The forearm was secured to the robot’s molded arm cradle with two straps (Jones-Lush et al., 2010) that maintained the participant’s elbow just below the horizontal plane as compared to the hand and shoulder (Figure 1A). Subjects were instructed to remain relaxed with their hand resting around the handle at the end of the cradle. A center-acting spring-like force (75 N·m) was applied by the robot to prevent the arm from drifting and to return it to the initial configuration after movements were made, without any subject effort. The robot encoders recorded the position and velocity of all movements in the horizontal X,Y plane. Data was digitized at 200 Hz and stored for offline analysis. Surface electromyography (EMG) from the right arm muscles of four principal shoulder/elbow contributors (anterior deltoid-AD, posterior deltoid-PD, biceps brachii-BB, and triceps brachii-TB) was visually and auditorily monitored to ensure that the subjects were at rest prior to TMS stimulation. In 11 of 22 participants, EMG was recorded for offline analysis. In the other 11 participants, these proximal muscle recordings had a long-lasting TMS-induced artifact that contaminated many motor evoked potentials (MEP), limiting ability to analyze MEP plasticity in these participants (despite using standard Ag/AgCl disk electrodes and a TMS-specific amplifier from James Long, Caroga Lake, NY). In the remaining 11 participants, we used microamplifiers with integrated dry metal electrodes (B & L Engineering, Santa Ana, CA) that effectively eliminated the TMS artifact. EMG was digitized at 2 kHz using a Dell computer equipped with an A/D board (National Instruments, Austin, TX), and were time-synchronized with the position data. A 100-msec period after stimulus was examined.
Figure 1.
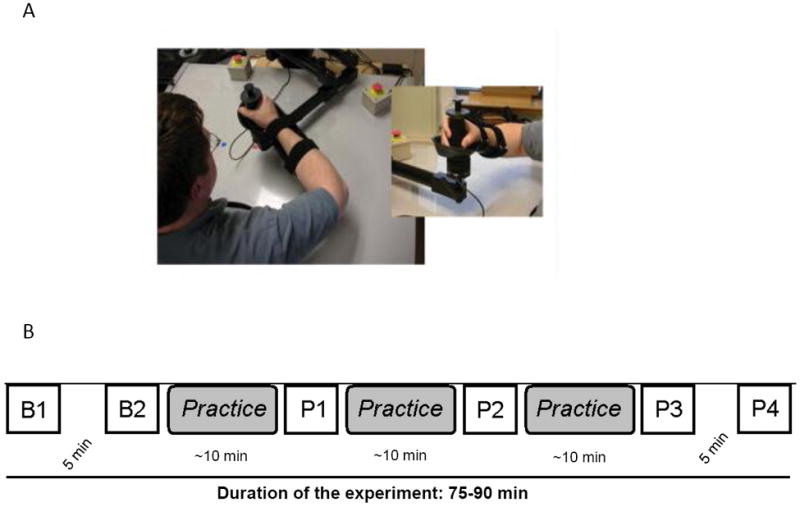
(A) Experimental setup: Participants sat in a chair with their arm secured in the robotic cradle as shown. Participants assumed a comfortable position and were relaxed during TMS stimulation. Note the level of the elbow was slightly below that of the hand and shoulder.
(B) Experimental Design: Participants were tested for TMS-evoked movements at two baselines B1 and B2 using single pulses at 120% of the movement threshold. B1 and B2 were separated by a 5-minute interval during which participants rested with their arm in the robotic cradle. Then participants practiced goal-directed reaching to in a direction opposite to the TMS-evoked movements at B1 and B2. Practice was administered in three practice blocks of 160 trials each. After each practice block, TMS-evoked movements were assessed again (P1, P2 and P3). After P3, participants rested with their arm in the robotic cradle before P4 when TMS-evoked movements were assessed.
2.3 TMS
Single pulse stimulation of dominant (contralateral to the practice arm) motor cortex was performed using a MagStim 200 (MagStim Ltd., Wales, UK) with a 90 mm loop-diameter figure-of-8 coil. Coil and head position were recorded by a frameless sterotaxic system (BrainSight, Rogue Research, Montréal, QC), and coregistered with an anatomical MRI of standard brain template to allow for precise localization of stimulation location throughout the course of the experiment. During stimulation the coil was held tangential to the scalp with the handle pointing backward and laterally at a 45° angle to the sagittal plane.
2.4 Baseline measurements
Arm movements were elicited by methodically stimulating the area over the dominant primary motor cortex (M1), guided by a 5 × 5 cm grid centered over the anatomical landmark of the hand knob and aligned with the anterior-posterior and medial-lateral axes of the head (Jones-Lush et al., 2010). Movement hotspots were located for each subject, defined as the location that, when stimulated, produced the largest movement recorded by the planar robot. Movement thresholds were then determined at the hotspot for each subject, defined as the lowest stimulation level that elicited movement of 1 mm or more in at least 5/10 stimulations. Baseline movements were elicited by applying 10 pulses of TMS (5 sec between stimulations) over the hotspot at 120% of the movement threshold (mean ± standard deviation = 71 ±16 Maximum Stimulator Output, MSO). Applying TMS over M1 as the arm secured in the robotic arm cradle elicited upper extremity movements within the robotic environment. To assess the stability of the baseline TMS-elicited movements over time, a second baseline assessment was performed in 13 of 22 participants (Figure 1B).
2.5 Training
Participants then practiced three blocks of 160 active, goal-directed reaching movements (Figure 1B). A visual target (1 cm diameter) was displayed on the monitor in front of the participant. The goal of each movement was to move the cursor to the target, accomplished by a 14 cm handle movement in the direction opposite to the baseline TMS-evoked movements. For example, if the dominant movement direction at the hotspot during baselines was forward and to the left (North-West – NW), then the training direction was back towards the body and to the right (South-East – SE).
Every trial began once the participant held the robotic arm in the starting position (1 cm circle). Once in the starting position, the participant received a ready cue indicated by the target color turning red. After two seconds, the participant received the “go” cue that was signaled by the target turning green. Participants were instructed to reach to the target as fast as possible once the target turned green. While reaching to the target, the participants were required to move against a spring-like 75 N·m force that passively returned the hand to the center starting position after the active reach to the target. Participants received visual feedback about their hand position represented as a cursor throughout the movement. When they reached the target position, the target turned yellow, indicating a successful reach.
2.6 Post-training measurements
The effect of goal-directed reaching practice on kinematic parameters of TMS-evoked movements was assessed. TMS-evoked-movements (10 stimuli, 5 sec apart, 120% threshold at hotspot) were collected for all subjects at rest after each practice block (Figure 1B: P1, P2, P3) and after a 5 min. rest period following the completion of all training (P4) to study the progression of plasticity and persistence of changes.
2.7 Data analysis
Movement vectors were calculated offline from the robot-recorded X,Y data for each TMS-elicited upper extremity movement. Vectors were defined from the starting position of the robot handle (pre-stimulation) to the X,Y point occurring at first peak velocity of the TMS-evoked movement (post-stimulation, Figure 2A). We operationally defined this point of first peak velocity of the TMS-evoked movement as the end-point of movement, as this part of the movement was dominated by the evoked forces, before any voluntary correction or the spring force began to return the handle to the starting position. The direction of the movement was determined as the angle made by the vector from origin to end-point with the zero degree vector (Figure 2B). Mean direction was obtained from the average of ten movement vectors at each assessment point. Our primary dependent measure was change in the direction of the mean end-point vector from the baseline 1 at baseline 2 (B2), after each practice block (P1-P3) and 5 minutes following end of practice (P4). Because directional change may not capture all practice-induced changes in evoked movement characteristics, we quantified the change in the end-point position (from B1) at B2, and P1-P4. Position change of the end-point was calculated for each movement vector as the Euclidean distance between the end-points at each time (Figure 2C). Another dependent measure was the change in amplitude of the TMS-evoked movement vectors (Figure 2B).
Figure 2.
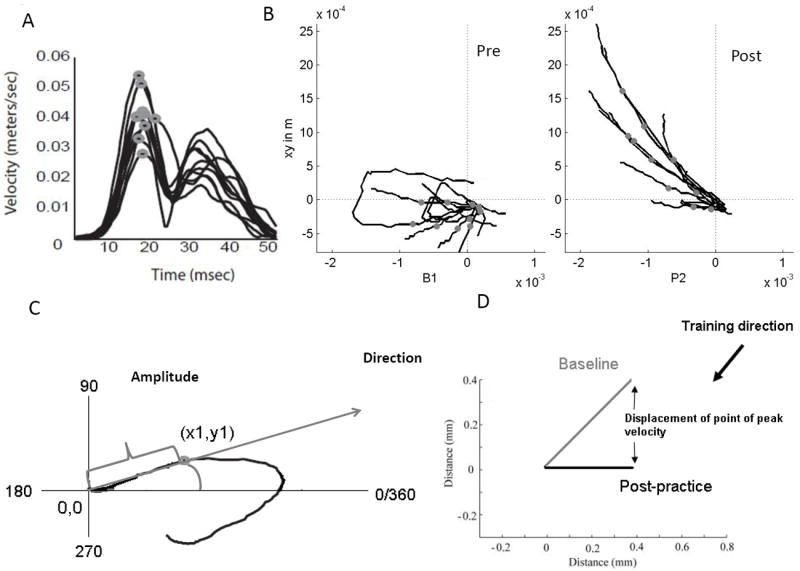
Dependent measures
(A) The X-Y position of the TMS-evoked arm movements was analyzed offline. Velocity profile was derived for each trajectory. Point of first peak velocity was determined on the position trajectory.
(B) Movement vectors were defined using the trajectories of TMS evoked movements from the starting position of the robot handle to the X, Y point of first peak velocity of the TMS-evoked movements before practice (Pre) and after practice (Post).
(C) Direction of the vector was determined as the angle made by the movement vector between the zero degree vector and the movement vector. Movement amplitude was defined as the distance between the starting position of the robot handle and the point of peak velocity.
(D) Shift in the point of first peak velocity over time was determined as the Euclidian distance between the baseline and post-practice measurement.
To assess the stability of TMS evoked movements, comparisons were first made between the two baselines. For the direction data, each direction was treated as a unit length vector with an angle given by the direction value. A paired-t test was conducted to assess the difference in direction and amplitude between the two baselines. For end-point position change over the baseline, a one-sided t test was conducted to assess the endpoint position change between the two baselines.
To evaluate the effect of practice, deviation from baseline 1 was calculated for post-training (P1, P2, P3), and retention (P4) for direction, end-point position, and amplitude. For direction, amplitude and end-point position, mixed linear models for repeated data were used to compare the change at baseline 2 and probes 1-4, with time being the fixed effect and subject a random effect. Statistically significant results were followed by multiple comparisons of changes in post-training with change between baselines, using Dunnett’s method to compute p values, adjusting for 4 multiple comparisons. All non-missing values were included in the analysis.
Movements during training and MEPs evoked during TMS measurements were recorded for 11 participants and stored for off-line analysis. Movement time (MT) was quantified as time taken by the participant to reach the target after the go cue (target turning green). Average MT was calculated for 20 trials. A repeated measures ANOVA was used to analyze the effect of practice on the movement time. Peak-to-peak amplitude for each MEP was measured offline using a MATLAB script. Mean MEP amplitude of 10 trials was calculated for each testing point (two baselines: B1 and B2; and P1-P4). Based on the training movement direction, we classified the shoulder and elbow muscles as agonists and antagonists. Thus we classified the MEP data into four muscle groups based on individual subject training direction: shoulder agonists, shoulder antagonists, elbow agonists and elbow antagonists. For example, if the training direction for a particular subject (e.g. Figure 4: TR45) was 100°, then AD and TB were classified as shoulder and elbow agonists respectively. Subsequently, PD and BB were classified as shoulder and elbow antagonists for that subject. Stability of the MEP amplitude over the two baselines was computed using a TOST equivalence test. The absolute changes in the MEP measures at each time point (P1, P2 P3 and P4) from the average of the 2 baselines were computed for each muscle group. A mixed linear model for repeated data were used to assess whether the changes were significantly different from baseline, with time being the fixed effect and subject being the random effect (and p values were adjusted for 4 multiple comparisons).
Figure 4.
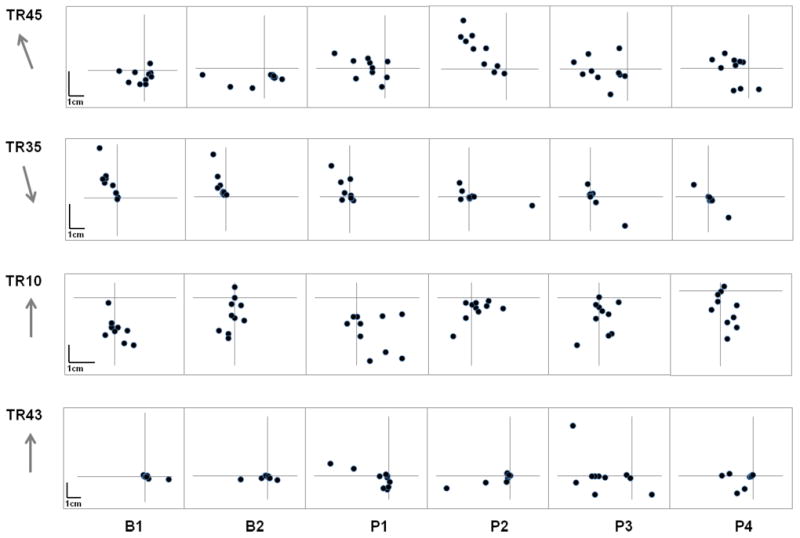
Individual participant data demonstrating the change in the position of peak velocity of TMS evoked movements at different time points over the course of the experiment. The grey arrows indicate the direction of training. Interindividual differences were evident for change in TMS-evoked movements in response to practice.
Results
3.1 Stability of Baseline movements
TMS evoked ballistic movements of arm, braked by the spring-field with passive return to home position, as previously reported (Jones-Lush, et al., 2010). An individual’s movements tended to cluster within a quadrant of the plane, but mean movement vector varied by individual subject. In 13 out of 22 participants, we conducted two baselines to assess the change in the TMS-evoked movement kinematics (X-Y position, amplitude and direction) over time. A paired t test revealed no significant difference between the directions of movement vectors across the two baselines, indicating that the direction of the TMS-evoked movements was stable (p = 0.339). Amplitudes at B1 and B2 were not significantly different (Paired t test, p = 0.881). However, a one-sided t test revealed a significant change in the Euclidean distance between the two baseline end-points (Figure 3), indicating some instability in this combined measure of direction and amplitude.
Figure 3.
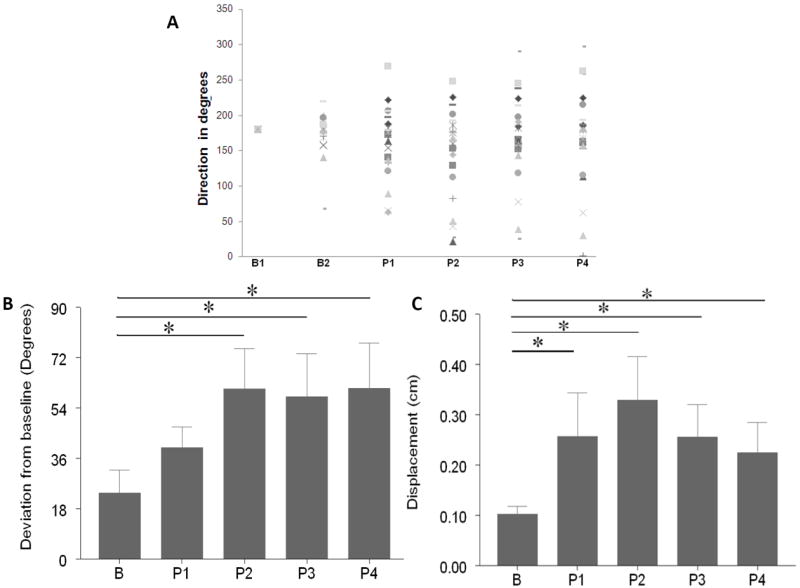
(A) Individual participant data showing the time course of direction over baseline (B1, B2), practice (P1-P3) and at retention (P4). The direction for baseline 1 (B1) was transformed to 180° for each participant. Subsequently, directions at each test (B2, P1-4) for each subject were transformed by adding (or subtracting) the difference between B1 raw direction and 180 degrees.
(B) Time course of the change in the direction from baseline 1. “B” refers to the change (mean+SE) in direction between the two baselines (data for 13 participants). Bars P1-P4 illustrate the change in direction from the baseline over the course of practice and after 5 minutes post practice.
(C) Change in the position from the baseline 1. “B” refers to the change (mean+SE) in the position of peak velocity between the two baselines (data for 13 participants). Bars P1-P4 illustrate the change in the position of the peak velocity from the baseline over the course of practice and after 5 minutes post practice (for all participants).
3.2 Training-induced plasticity
All participants reduced their movement time over practice (p < 0.001). Participants were accurate in their practice direction (mean deviation from the target direction = 2.5° ± 0.66°). Repetitive practice of goal-directed reaching changed the characteristics of TMS-evoked upper extremity movements. Direction effects: The mixed linear model for repeated measures revealed a significant effect of time in direction (RM-ANOVA, p=0.01, Figure 3 A and B). Post-hoc comparisons indicated that direction at P1 was not significantly different from baseline 2 (p = 0.4594). However, at P2 and P3 the direction was significantly different than the baseline 1 (P2: p = 0.0046, and P3: p = 0.0495; Figure 3B). There was a significant difference from the baseline at each probe (Figure 3C; mixed linear models for repeated data; P1: p = 0.003, P2: p = 0.0002, and P3: p = 0.002). This indicated that repetitive goal-directed reaching practice significantly changed the end-point position of TMS-evoked movements compared to the baseline. Amplitude: There was no significant change in the amplitude of TMS-evoked movements over time (p = 0.37, 0.40, 0.59 for P1, P2 and P3 respectively).
Analysis of individual participant data demonstrated different patterns that led to a change in end-point position. While some participants (n=6) demonstrated an almost complete reversal in the direction of TMS-evoked movements following practice (Figure 4; TR45) a few participants showed reduction (or change) in amplitude, but no change in direction (Figure 4; TR35). For some participants, the post-practice TMS-evoked movement vectors fell between the original and the practiced movements (approximately a 90° change, but on either side of the line between trained and untrained movements, n=11; Figure 4;TR43). A few others did not show any consistent change in end-point position with practice (n=5; Figure 4; TR10).
3.3 Duration of plasticity
To assess short-term retention of practice-related movement plasticity, TMS-elicited movements were additionally probed at 5-minute intervals after training ended (P4). Participants retained the practice-induced change in direction of TMS-evoked movements over the 5-minute interval. There was a significant change in direction at P4 compared to baseline 2 (p = 0.0316; P4 in Figure 3B). Similarly, there was a significant change in the end-point location at P4 compared to baseline 2 (P4 in Figure 3C). There was no significant difference in amplitude of TMS-evoked responses at P4 compared to baseline 2 (p = 0.49).
3.4 Motor evoked potential (MEP) changes with practice
Because changes in end-effector movements must result from changes in muscle activation, we investigated the changes in MEP produced by practice. Figure 5 shows representative subject data mean peak-to-peak MEP amplitude for the four muscles over baseline, practice (P1-P3) and at a 5-min interval after practice (P4). Change in the direction of evoked movements in this participant was accompanied by an increase in the MEP amplitude of shoulder and elbow agonists. Analysis of the group data revealed that MEP amplitude was stable over the two baselines except for shoulder antagonists (p = 0.08). Over practice and after 5-min interval, there was a significant change in the MEP amplitudes of the agonist muscles compared to the average of two baselines (Figure 6). Both shoulder and elbow agonists demonstrated a significant increase in MEP amplitude over practice (RM-ANOVA: p = 0.039 for shoulder agonist and p = 0.02 for elbow agonist) and after 5 min. post-practice, indicating an effect of training on muscle representations in the motor cortex.
Figure 5.
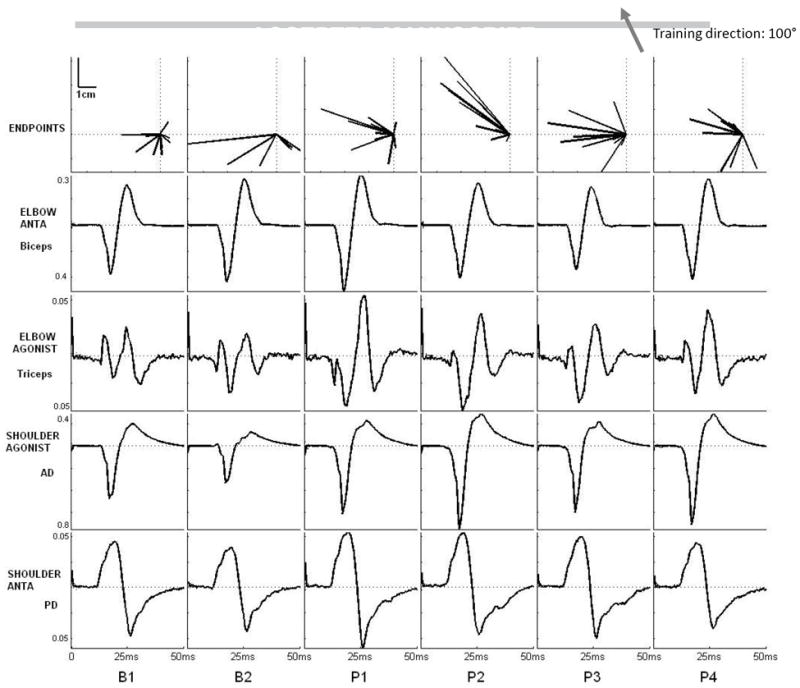
Representative subject data showing change in the movement vectors over practice and concomitant changes in MEP amplitudes evoked by TMS in shoulder and elbow muscles. MEP shown is the ensemble average of 10 MEPs evoked by TMS at each testing point with the arm constrained in the robotic cradle. This participant practiced goal-directed reaching in the 100° direction (depicted by the grey arrow on upper left corner). The shoulder agonist (anterior deltoid, AD), elbow agonist (triceps) and shoulder antagonist (posterior deltoid, PD) showed an increase in average MEP amplitude over practice compared to baseline. In contrast, the elbow antagonist (biceps) showed a progressive decrease over practice (P1, 2, 3) that reverted back to baseline after rest (P4).
Figure 6.
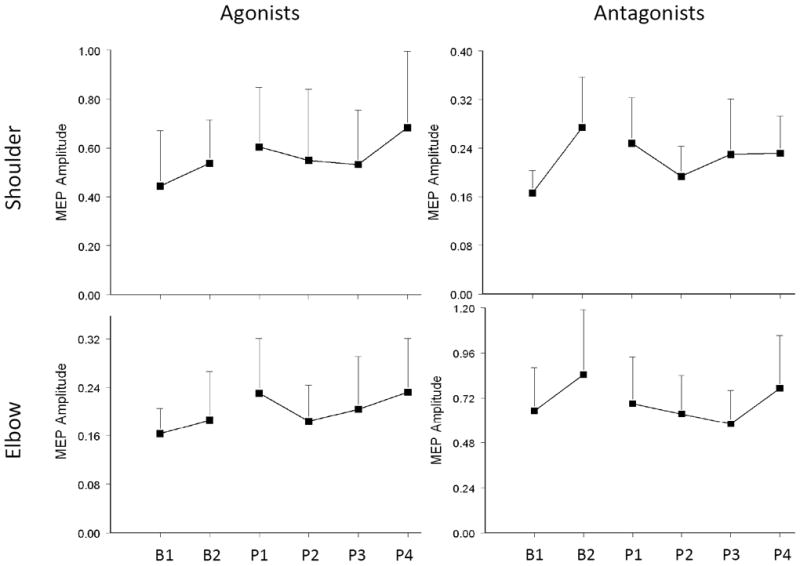
Change in the MEP amplitude (mean±SD) of shoulder and elbow muscles evoked by TMS at each testing point. After a general increase in the MEP size during the baseline period, the agonists of both shoulder and elbow for the trained direction showed an increase in MEP amplitude. In contrast, the antagonists showed a decrease over training.
Discussion
In this study, we investigated changes in the kinematic characteristics of TMS-evoked proximal upper extremity movements and MEPs in healthy participants practicing goal-directed reaching within a robotic environment. Repetitive reaching practice significantly affected the kinematic characteristics of TMS-evoked movements. After practice without any force-field or visuomotor perturbation, there was a change in the direction and position of point of peak velocity of TMS-evoked movements. These kinematic changes were accompanied by an increase in MEP amplitude of shoulder and elbow agonists for the training direction. The effects were relatively rapid in onset, and lasted at least 5 minutes beyond the termination of practice.
4.1 Stability of TMS-evoked movements
In this experiment, we assessed the stability of the TMS-evoked upper extremity movements for 13 participants over a 5-minute period during which the participant sat with their hand secured to the robotic arm cradle. Similar to our previously reported results (Jones-Lush 2010), we observed little change in the direction and amplitude, but some drift in the combined end-point measure, of the TMS-evoked movements over time. TMS evoked motor responses have been demonstrated to have considerable variability over time that may represent inherent variability of the motor system. Further, short-term immobilization has been shown to affect motor cortical excitability (Liepert et al., 1995; Zanette et al., 2004; Ngomo et al., 2012).
4.2 Practice-induced change in TMS-evoked arm movements
We demonstrated that the direction of TMS-evoked arm movements could change with as few as 320 trials of repetitive robotic reach training. Following 160 trials (at P1), the deviation in direction was not significantly different than the deviation at baseline 2, indicating that directional change was beyond the inherent variability of the dependent measure. However, with additional practice, the deviation was significantly larger (at P2 and P3) than that could be accounted for by the variability at rest. This suggests that there may be a critical dose of practice for change in kinematic parameters of TMS-evoked movements. Previous studies have demonstrated similar motor cortex plasticity following repetitive ballistic thumb movements and adaptation to force or visual perturbations (Karni et al., 1998; Gandolfo et al., 2000; Hlustik et al., 2004; Jensen et al., 2005; Arce et al., 2010; Orban de Xivry et al., 2011). Our study supports this notion for goal-directed reaching practice typically practiced with robotic and other forms of rehabilitation. Secondly, we provide evidence that the healthy motor cortex is capable of rapid corticospinal plasticity for goal-directed multi-joint reaching movements as has been previously found for single-joint movements (Classen et al., 1998). This provides a starting point from which to gauge robotic therapy-induced plasticity in the motor cortex of stroke subjects in the future.
The change in direction of TMS-evoked arm movements in our study was not as robust as those demonstrated for the thumb movements in the Classen et al (1998). The neuroplastic changes related to the proximal arm movements may have important differences with those related to distal hand movements, particularly as there are likely more subcortical motor centers involved. In addition to subcortical elements, reaching movement engage fronto-parietal networks, so changes in corticospinal system that are accessible with TMS may represent just one of the many aspects of practice-related plasticity. We designed the practice reaching movements to be ballistic movements in a defined direction, much like the training movements used by Classen et al. (1998). However there are significant differences in the type of movements practiced in the current study. First, reach training involved multijoint goal-directed movements. These movements are considerably different than the ballistic thumb movements. Second, amplitude and speed or practiced movements were different compared to TMS-evoked movements and to ballistic thumb movements. However, movements practiced in our study more resemble functional reaching activities that engage multiple proximal muscle groups. Second, while it is hard to compare the amount of training for the multijoint movements used here with thumb movements (Classen et al., 1998), the number of movements made and time spent making movements (480 movements, 15 minutes) were considerably lower than those used in the thumb study (1 Hz for 30 minutes = ~1800 movements). This may partly explain why we saw a more inter-individual variability in the neuroplastic response to practice than reported in the thumb study. That we observed changes in movement direction with only 15 minutes of training suggests that longer periods of training, increased frequency of training, or increased number of training sessions should be investigated and may yield longer lasting changes in TMS induced movements.
Lastly and as touched on above, multi-joint, goal-directed reaching movements that require visuomotor processing are considerably more complex than repetitive finger movements. Thus plasticity associated with such movements is likely more complex, involving multiple areas of the brain. It is therefore likely that all elements involved in practice related plasticity are not accessible to TMS stimulation of M1; hence the TMS-evoked movements represent a biased sample of all the motor elements. This may partly account for the observation that in some subjects, TMS-evoked movements did not change completely in the direction of practice. (Often, movements appeared to rotate around or move through the origin, as if they were on their way towards the practiced direction.)
Concomitant increase in MEP amplitudes of the shoulder and elbow agonists with practice represents yet another dimension of practice-related plasticity for reaching movements. The longitudinal MEP data suggests a selective alteration in output evoked by central stimulation and partly explains the short-term plasticity in the movement data. Interpretation of the relationship of MEP changes to overall TMS-evoked movement patterns is complex, but the changes were in the direction one would predict by positive plasticity in the representation of activated muscles. For instance, triceps MEP amplitude was increased significantly with repetitive elbow extension. However, antagonists did not significantly decrease, as might have been expected if negative plasticity affected their representation.
4.3 Interindividual variability and practice-related factors
While some participants showed changes in direction and position of movement endpoint post-training, some subjects showed little or no change. The basis for this lack of response in some participants is not known, but has also been noted for other exercise protocols and non-invasive brain stimulation protocols aimed to induce motor plasticity (Stefan et al, 2006, Kriváneková et al, 2011). Further, some participants with stroke undergoing robotic therapy consisting of reaching movements such as those employed here do not show substantial functional improvement even after 12 weeks of practice (1 hour, 3 times a week). While studies of repetitive practice-based stroke rehabilitation paradigms do show modest functional gains (Wolf et al., 2002; Richards et al., 2006), they often also reveal non-responders. It is conceivable that longer periods of practice or increased practice frequency may convert some of the non-responders to responders in this experimental paradigm. Further, increasing the resistance to reaching movements may increase task difficulty and promote plasticity in the motor cortex and corticospinal system. Further research is needed to characterize the structure of practice that may facilitate practice-induced plasticity. Vigorous, repeated movements of the upper extremity could induce fatigue at multiple levels in the neuromuscular system. However, all participants were able to complete the training movements, implying that any fatigue was not severe enough to prevent voluntary muscle activation. Further, fatigue effects would have resulted in opposite changes in TMS-evoked movements by reducing competing muscle effects, and this was never seen.
4.4 Representation of movements in primary motor cortex
The representational role of upper motor neurons in the primary motor cortex remains an area of lively debate (Shemmell et al., 2007; Churchland et al., 2010). Muscle activity patterns, movement endpoint information, intrinsic or extrinsic movement components, movement parameters such as distance, direction, speed, curvature, some combination of these or any other complex biomechanical property of an upcoming movement may be represented or decoded from M1 (Hayashi et al., 2006; Hotermans et al., 2008; Romei et al., 2009). The motor cortex may be better thought of as a dynamic system in which the changing neuronal activity will result in intended movement, an idea recently supported by Churchland, Sussillo, Abbott and others (Churchland et al., 2010). Results from the current study contribute to this ongoing discussion by demonstrating that arm movements evoked by TMS pulses delivered to the same cortical location could be altered by as little as 15 minutes of practice in healthy subjects. We do not suggest that this study answers any questions about representation of movement in the corticospinal system, and its outputs. But it does provide practical data related to potential use of TMS to enhance goal-directed reaching practice, with the demonstration of a significant training effect on kinematics of TMS-evoked movements.
These findings further demonstrate that a specific area of the corticospinal system activated with the same input from the same starting conditions can result in different movement patterns depending on the previous history of activity. This suggests that even brief periods of training and even immobilization can change the baseline state of the motor cortex and/or the corticospinal targets.
4.5 Limitations
By using a single point on the distal limb (the center of the robot handle), to monitor the endpoint of reaching movements we were able to show statistically significant changes in TMS-evoked upper extremity movements with practice. We could not determine if the changes seen after practice were due to changes in evoked torque in a particular joint, or more distributed changes. Using a planar system to transduce and record TMS-elicited arm movements further inhibits the measurement of contributing torque components, as movements in the vertical plane are obscured. TMS-evoked forces that would drive the hand up or down would not result in measurable movement because the hand was constrained in the robotic cradle in a way that prevented any vertical movement of the hand. The complexities of a 3D support system that would have isotropic resistance to movement are considerable, and the present study using a 2D system was considered to be a necessary research stage. Finally, the sites of plasticity in the neuraxis (e.g. cortical, brainstem or spinal) cannot be ascertained. Future studies can be designed to address each of these limitations, and they could include: 1. measurement of spinal excitability through reflex studies, 2. startle responses to measure brainstem state, 3. paired-pulse inhibition, and even 4. transcranial electrical stimulation. Finally, a number of practice controls are possible, including absence of training and training in the direction of TMS evoked-movements. Nevertheless, the fact that the post-practice direction change was, on average, greater than the baseline variability provides confidence in the practice effects.
4.6 Conclusion
The TMS-accessible corticospinal representation of arm movements demonstrates rapid plasticity induced by goal-directed robotic reach training in healthy subjects. This plasticity was also reflected by changes in the motor evoked potentials (MEPs) of the shoulder and elbow agonist muscles indicating an increase in the evoked responses for practiced muscles. But the relationship between reach practice and TMS-evoked motor output was less robust and more complex than for ballistic thumb movements.
Highlights.
TMS-evoked movements were used to assess plasticity of primary motor cortex (M1).
Goal-directed reaching practice in robotic environment induced M1 plasticity.
M1 plasticity encoded kinematic features of practiced movements.
M1 excitability of agonist muscles for reaching was enhanced with practice.
Acknowledgments
We thank Jamie Lush for assistance with data collection and analyses.
GRANTS
This work was funded by the National Institute of Health Grant R01 HD061462 to G.F. Wittenberg and a Senior Fellowship from KU Leuven.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Shailesh S. Kantak, Email: skantak@som.umaryland.edu.
Lauren M. Jones-Lush, Email: ljones-lush@som.umaryland.edu.
Priya Narayanan, Email: PNarayanan@som.umaryland.edu.
Timothy N. Judkins, Email: tjudkins@i-a-i.com.
George F. Wittenberg, Email: gwittenb@grecc.umaryland.edu.
References
- Arce F, Novick I, Mandelblat-Cerf Y, Vaadia E. Neuronal correlates of memory formation in motor cortex after adaptation to force field. J Neurosci. 2010;30:9189–9198. doi: 10.1523/JNEUROSCI.1603-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci U S A. 2000;97:3661–3665. doi: 10.1073/pnas.050350297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Cunningham JP, Kaufman MT, Ryu SI, Shenoy KV. Cortical preparatory activity: representation of movement or first cog in a dynamical machine? Neuron. 2010;68:387–400. doi: 10.1016/j.neuron.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 1998;79:1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- Gandolfo F, Li C, Benda BJ, Schioppa CP, Bizzi E. Cortical correlates of learning in monkeys adapting to a new dynamical environment. Proc Natl Acad Sci U S A. 2000;97:2259–2263. doi: 10.1073/pnas.040567097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano MS, Taylor CS, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002;34:841–851. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- Hatsopoulos NG, Xu Q, Amit Y. Encoding of movement fragments in the motor cortex. J Neurosci. 2007;27:5105–5114. doi: 10.1523/JNEUROSCI.3570-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, Shimura K, Kasai T. Modulations of use-dependent excitability changes of human motor cortex (M1) by practice condition. Percept Mot Skills. 2006;103:697–702. doi: 10.2466/pms.103.3.697-702. [DOI] [PubMed] [Google Scholar]
- Hlustik P, Solodkin A, Noll DC, Small SL. Cortical plasticity during three-week motor skill learning. J Clin Neurophysiol. 2004;21:180–191. doi: 10.1097/00004691-200405000-00006. [DOI] [PubMed] [Google Scholar]
- Hotermans C, Peigneux P, de Noordhout AM, Moonen G, Maquet P. Repetitive transcranial magnetic stimulation over the primary motor cortex disrupts early boost but not delayed gains in performance in motor sequence learning. Eur J Neurosci. 2008;28:1216–1221. doi: 10.1111/j.1460-9568.2008.06421.x. [DOI] [PubMed] [Google Scholar]
- Huang VS, Haith A, Mazzoni P, Krakauer JW. Rethinking motor learning and savings in adaptation paradigms: model-free memory for successful actions combines with internal models. Neuron. 2011;70:787–801. doi: 10.1016/j.neuron.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JL, Marstrand PC, Nielsen JB. Motor skill training and strength training are associated with different plastic changes in the central nervous system. J Appl Physiol. 2005;99:1558–1568. doi: 10.1152/japplphysiol.01408.2004. [DOI] [PubMed] [Google Scholar]
- Jones-Lush LM, Judkins TN, Wittenberg GF. Arm movement maps evoked by cortical magnetic stimulation in a robotic environment. Neuroscience. 2010;165:774–781. doi: 10.1016/j.neuroscience.2009.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Mazzoni P. Human sensorimotor learning: adaptation, skill, and beyond. Curr Opin Neurobiol. 2011 doi: 10.1016/j.conb.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Liepert J, Tegenthoff M, Malin JP. Changes of cortical motor area size during immobilization. Electroencephalogr Clin Neurophysiol. 1995;97:382–386. doi: 10.1016/0924-980x(95)00194-p. [DOI] [PubMed] [Google Scholar]
- Matsuzaka Y, Picard N, Strick PL. Skill representation in the primary motor cortex after long-term practice. J Neurophysiol. 2007;97:1819–1832. doi: 10.1152/jn.00784.2006. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Cohen L, Hallett M. Role of the human motor cortex in rapid motor learning. Exp Brain Res. 2001;136:431–438. doi: 10.1007/s002210000614. [DOI] [PubMed] [Google Scholar]
- Ngomo S, Leonard G, Mercier C. Influence of the amount of use on hand motor cortex representation: Effects of immobilization and motor training. Neuroscience. 2012;220:208–214. doi: 10.1016/j.neuroscience.2012.06.018. [DOI] [PubMed] [Google Scholar]
- Orban de Xivry JJ, Marko MK, Pekny SE, Pastor D, Izawa J, Celnik P, Shadmehr R. Stimulation of the human motor cortex alters generalization patterns of motor learning. Journal of Neuroscience. 2011;31:7102–7110. doi: 10.1523/JNEUROSCI.0273-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paninski L, Fellows MR, Hatsopoulos NG, Donoghue JP. Spatiotemporal tuning of motor cortical neurons for hand position and velocity. J Neurophysiol. 2004;91:515–532. doi: 10.1152/jn.00587.2002. [DOI] [PubMed] [Google Scholar]
- Richards L, Gonzalez Rothi LJ, Davis S, Wu SS, Nadeau SE. Limited dose response to constraint-induced movement therapy in patients with chronic stroke. Clin Rehabil. 2006;20:1066–1074. doi: 10.1177/0269215506071263. [DOI] [PubMed] [Google Scholar]
- Rickert J, Riehle A, Aertsen A, Rotter S, Nawrot MP. Dynamic encoding of movement direction in motor cortical neurons. J Neurosci. 2009;29:13870–13882. doi: 10.1523/JNEUROSCI.5441-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei V, Thut G, Ramos-Estebanez C, Pascual-Leone A. M1 contributes to the intrinsic but not the extrinsic components of motor-skills. Cortex. 2009;45:1058–1064. doi: 10.1016/j.cortex.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Shemmell J, Riek S, Tresilian JR, Carson RG. The role of the primary motor cortex during skill acquisition on a two-degrees-of-freedom movement task. J Mot Behav. 2007;39:29–39. doi: 10.3200/JMBR.39.1.29-39. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Blanton S, Baer H, Breshears J, Butler AJ. Repetitive task practice: a critical review of constraint-induced movement therapy in stroke. Neurologist. 2002;8:325–338. doi: 10.1097/01.nrl.0000031014.85777.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanette G, Manganotti P, Fiaschi A, Tamburin S. Modulation of motor cortex excitability after upper limb immobilization. Clin Neurophysiol. 2004;115:1264–1275. doi: 10.1016/j.clinph.2003.12.033. [DOI] [PubMed] [Google Scholar]


