Abstract
Background
We evaluated whether combined heart liver transplant can protect the heart graft from the development of Cardiac allograft vasculopathy (CAV) using coronary 3D volumetric IVUS.
Methods
From 2004 to 2009, we identified 24 isolated heart transplant (HTx) and 10 combined heart liver transplant (H+Liver Tx) recipients in whom two coronary 3D IVUS studies were performed one year apart. Baseline 3D IVUS was performed at 0.22 [0.17, 1.16] years after transplant with follow up 3D IVUS exams performed 0.96 [0.83, 1.08] after baseline exam.
Results
Rate of plaque volume and plaque index (plaque volume/vessel volume) progression was attenuated in the H+Liver Tx group (0.3±1.1 mm3/mm vs. 1.5±2.9 mm3/mm; P=0.08, and 0.01±0.03 vs. 0.1±0.1; P=0.004 respectively). Rejection burden was much lower in the H+Liver Tx patients. Outcome analysis in 66 consecutive patients (56 HTx and 10 H+Liver Tx) was performed irrespective of performance of second coronary IVUS. Combined heart and liver transplant was associated with reduced rate of cardiac events (p=0.04), which remained significant when adjusted for the difference in the primary etiology for heart disease (p=0.05).
Conclusions
Our preliminary serial 3D coronary IVUS data show that combined heart and liver transplant attenuates CAV by decreasing the rate of plaque volume and plaque index progression and improves coronary related outcomes. Because of the small numbers and the differences in etiology of heart disease our data should be interpreted cautiously, and larger clinical trials would be required to recommend combined heart liver transplant for improved coronary remodeling.
Keywords: Heart transplant, Liver Transplant, Cardiac allograft vasculopathy, 3D intravascular ultrasound
Cardiac allograft vasculopathy (CAV) remains the leading cause morbidity and mortality in heart transplant recipients. (1, 2) Attempts at combined liver and renal transplantation against a positive cross-match (3–6) suggest that the liver graft protect the subsequent renal transplant from damage due to circulating pre-formed antibodies.
Numerous reports suggest that any “multi-organ” transplant that includes more than the heart is associated with less cardiac rejection and CAV. (7–11) The experience with combined heart and liver transplant is small and limited mainly to patients with familial amyloidosis (FA), an autosomal-dominant disease.
We have reported our single-center experience of combined heart and liver transplantation (H+Liver Tx) (12) in which we found no significant CAV in any of the H+Liver Tx patients by using routine angiography while in the comparable isolated heart transplant (HTx) group, angiographic CAV was diagnosed in 38% of patients. Based on these initial observations, we used serial coronary 3D volumetric IVUS to precisely delineate the occurrence and rate of progression of the CAV in patients with combined heart and liver transplant.
Results
Patient Characteristics
Table 1 shows the baseline characteristics of patients, and angiographically estimated CAV (categorized using the ISHLT guidelines) with HTx and H+Liver Tx. Prevalence of ischemic cardiomyopathy was lower and prevalence of restrictive cardiomyopathy (8 familial amyloidosis and one idiopathic restrictive cardiomyopathy) higher in the H+Liver Tx group. Triglyceride levels were lower in the H+Liver Tx patients as well. There were no differences between the groups in all other parameters.
Table 1.
Baseline Demographic and Clinical Characteristics of the Patients stratified to combined heart liver transplant or heart transplant
| Variable | Combined heart liver (n=10) | Heart alone (n=25) | P value |
|---|---|---|---|
| Recipient age, years | 61.3±9.0 | 54.3±11.5 | 0.07 |
| Gender—male, n (%) | 8 (80) | 19 (76) | 0.8 |
| Time from transplant to 1st IVUS (years) | 1.5±3.3 | 2.2±3.9 | 0.6 |
| Time between IVUS exams | 0.9±0.2 | 1.0±0.3 | 0.3 |
| ICMP, n (%) | 1 (10) | 9 (36) | 0.1 |
| Recipient diagnosis | ICMP 1 (10); DCMP 0 (0) RCM 8 (80); OTH 1 (10) |
ICMP 9 (36); DCMP 10 (40) RCM 3 (12); OTH 3 (12) |
0.0006 |
| ISHLT CAV score baseline (%) | 0 [0,1] | 0 [0,1] | 0.8 |
| Donor age (years) | 32.0±13.8 | 38.1±12.9 | 0.2 |
| Ischemic time (min) | 182.6±30.3 | 172.1±34.2 | 0.4 |
| BMI (kg/m2) | 25.4±5.5 | 26.4±4.6 | 0.6 |
| Uric acid (mg/dL) | 7.3±1.9 | 7.1±2.2 | 0.7 |
| GFR mL/min/1.73 m2 | 56.5±17.4 | 53.5±15.8 | 0.7 |
| Triglycerides (mg/dL) | 99.5±33 | 155.8±121 | 0.04 |
| HDL cholesterol (mg/dL) | 51.6±16.8 | 53.7±16.4 | 0.7 |
| LDL cholesterol (mg/dL) | 92.7±29.3 | 99.3±27.4 | 0.5 |
| Diabetes, n (%) | 3 (30) | 7 (28) | 0.9 |
| Positive cytotoxicity cross match n (%) | 0/9 (0) | 0/20 (0) | 1.0 |
| Positive flow cytometric crossmatch n (%) | 0/5 (0) | 1/7 (14) | 0.3 |
| Positive virtual crossmatch n (%) | 0/5 (0) | 3/13 (23) | 0.5 |
| Statin n (%) | 10 (100) | 21 (84) | 0.3 |
| Aspirin, n (%) | 2 (20) | 11 (44) | 0.3 |
| MMF vs AZA, n (%) | MMF 7 (70); AZA 3 (30) | MMF 16 (64); AZA 9 (36) | 0.7 |
| Prednisone, n (%) | 7 (70) | 12 (48) | 0.3 |
ICMP: Ischemic cardiomyopathy; DCMP, dilated cardiomyopathy; RCM: Restrictive cardiomyopathy; OTH: other; BMI: body mass index; MMF: Mycophenolate mophetil; AZA: Azathioprine.
Volumetric Changes in the H+Liver Tx and HTx groups
Volumetric data by 3D coronary IVUS at baseline and 0.96 [0.83, 1.08] years are shown in Table 2. We found no significant differences in the baseline or follow up vessel volume between the groups. Plaque volume increased significantly between coronary IVUS exams in the HTx group (5.8±2.9 mm3/mm vs. 4.3±1.9 mm3/mm; p=0.02) but did not change in the H+Liver Tx. The combined impact of no change in vessel volume and accelerated plaque progression resulted in significant progression in the plaque index which is a dimensionless index of plaque burden (0.39±0.14 vs. 0.28±0.8; p=0.0006) in the HTx group but not in the H+Liver Tx group. The progression in plaque index in the H+Liver Tx group was significantly slower than in the HTx (0.01±0.03 vs. 0.1±0.1; p=0.004 in absolute change and 4.7±11.6% vs. 40.2±50.6%; p=0.002 in percent change).
Table 2.
Comparison of vascular geometry and progression of allograft vasculopathy in one year by 3D IVUS in patients with HTx and H+Liver Tx.
| Combined heart liver (n=10) | Heart alone (n=25) | P value | |
|---|---|---|---|
| VV/SL (mm3/mm) | |||
| Baseline | 13.1±3.5 | 14.9±4.7 | 0.2# |
| Follow up | 13.1±3.0 | 15.6±5.9 | 0.1* |
| Absolute difference | −0.01±3.2 | 0.64±4.5 | 0.6# |
| Change in % | −4.4±33.6 | −1.4±30.3 | 0.8# |
| P-value | 0.9¶ | 0.5¶ | |
| PV/SL (mm3/mm) | |||
| Baseline | 4.2±1.9 | 4.3±1.9 | 0.8# |
| Follow up | 4.5±2.0 | 5.8±2.9 | 0.1* |
| Absolute difference | 0.3±1.1 | 1.5±2.9 | 0.08# |
| Change in % | −1.6±44.0 | 17.8±37.5 | 0.2# |
| P-value | 0.4¶ | 0.02¶ | |
| PI (%) | |||
| Baseline | 0.31±0.1 | 0.28±0.08 | 0.5# |
| Follow up | 0.32±0.11 | 0.39±0.14 | 0.2* |
| Absolute difference | 0.01±0.03 | 0.1±0.1 | 0.004# |
| Change in % | 4.7±11.6 | 40.2±50.6 | 0.002# |
| P-value | 0.2¶ | 0.0006¶ | |
SL: segment length; VV: vessel volume; LV: lumen volume; PV: plaque volume; PI: plaque index; HTx: Heart transplant; H+Liver Tx: combined heart and liver transplant
ANCOVA test; baseline value is a covariate.
T-test
Paired t test
Sub group analysis
The two groups differed significantly in terms of etiology for heart failure. In 9/25 (36%) of the HTx group the etiology was ischemic which is considered a risk factor for accelerated plaque progression post transplant (13). To correct for this possible bias we also analyzed the 3D coronary IVUS data in the subgroup with no ischemic heart disease (9 patients in the combined group and 16 in the HTx). The progression of plaque index remained accelerated in the combined group even when patients with an ischemic etiology where excluded (Figure 1). Treatment with either azathioprine or mycophenolate mophetil as a secondary immunosuppressant did not have a significant effect on the rate of plaque progression in either group (H+Liver Tx; 0.008±0.03 vs. 0.01±0.04; p=0.70 for azathioprine vs. mycophenolate mophetyl) (HTx; 0.12±0.09 vs. 0.10±0.15; p=0.6). Patients with H+Liver Tx demonstrated attenuated plaque progression irrespective if the secondary immunosuppressant was azathioprine or mycophenolate mophetyl compared with patients with HTx (0.008±0.02 vs. 0.12±0.09; p=0.009 for the combined group vs. isolated heart on azathioprine; 0.01±0.04 vs. 0.09±0.14; p=0.07 for combined vs. isolated heart on mycophenolate mophetil).
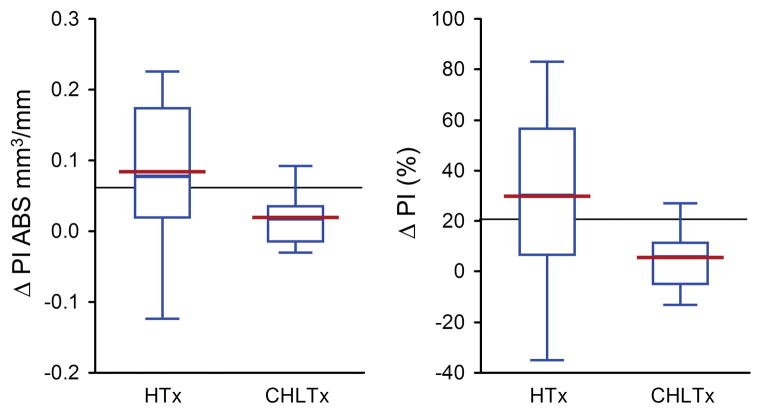
Figure 1. Effect of Combined Liver and Heart Transplant on Plaque Index Progression in Nonischemic Patients.
Relative and absolute changes in plaque index (PI) progression between the baseline and second IVUS examination in patients treated with combined heart and liver transplant (CHLTx) or isolated heart transplant (HTx) excluding patients with ischemic cardiomyopathy.
a) The graph presents absolute changes (in mm3/mm) in plaque index (plaque volume/vessel volume) between the baseline IVUS examination performed during the first year after transplant and the second IVUS exam performed a year afterwards in patients with no ischemic cardiomyopathy undergoing isolated heart transplant or combined heart and liver transplant. There was significant attenuation of plaque index progression in the combined heart and liver group even when excluding all patients with ischemic etiology (p=0.05) b) The graph presents relative changes (in percent) in plaque index (plaque volume/vessel volume) between the baseline IVUS examination performed during the first year after transplant and the second IVUS exam performed a year afterwards in patients with no ischemic cardiomyopathy undergoing isolated heart transplant or combined heart and liver transplant. Combined heart and liver transplant was significantly associated with attenuated plaque index progression even when analyzing patients without ischemic etiology for their heart failure (p=0.04).
Results are represented by box plots (middle hash of the box indicates the median; 25th to 75th percentiles are represented by end caps of the box; whiskers extend to the last observed value still within 1.5 times the interquartile range [difference between the 25th and 75th percentiles] above or below the 25th and 75th percentiles). The blue line connected the mean value for both groups.
CHLTx: Combined heart and liver transplant; HTx: Isolated heart transplant; PI: Plaque index; CAV: Cardiac allograft vasculopathy; ABS: Absolute.
Parameters associated with plaque index progression in consecutive 3D coronary IVUS
H+Liver Tx was strongly associated with less plaque index progression in univariate analysis (unadjusted risk ratio 0.4[0.23–0.73], p=0.003]. The performance of H+Liver Tx remained significantly associated with attenuated rate of plaque index progression when adjusted for the difference in ischemic cardiomyopathy prevalence, triglyceride levels at baseline, uric acid levels at the end of follow up, age and gender (adjusted risk ratio 0.46[0.25–0.83], p=0.01) but not when adjusted for total rejection score (TRS) (p=0.07), emphasizing the link between H+Liver Tx – reduced rejection burden, and attenuated plaque progression.
Vascular events and clinical outcome at follow up
Table 1 in the Supplemental Digital Content shows the end of follow up parameters in both groups. The only differences in laboratory parameters at the end of follow up were a higher uric acid level in the HTx. There was a marked difference in the rejection burden (assessed by TRS and ARS) between the groups with significantly lower rejection scores in the H+Liver Tx patients (P<0.0001 for both). Although angiographically assessed vasculopathy severity at baseline was similar between the groups the patients in the HTx group showed a trend for higher angiographically assessed vasculopathy score (using the ISHLT nomenclature) compared to H+Liver Tx group at the end of follow up.
Table 3 shows the baseline characteristics in the 76 patients included in our outcome analysis. The only differences between the groups were that patients in the HTx group had higher prevalence of ischemic cardiomyopathy and hypertension.
Table 3.
Baseline Demographic and Clinical Characteristics of the 76 consecutive patients transplanted until June 2009, performing at least one IVUS exam, analyzed for outcome.
| Variable | Isolated heart (n = 66) | Combined heart and liver (n = 10) | P-value |
|---|---|---|---|
| Recipient age, years | |||
| Gender—male, Number (%) | 49 (74) | 7 (70) | 0.4 |
| Ejection fraction (%) | 63 [60, 66] | 64 [62, 68] | 0.6 |
| ICMP, Number (%) | 37 (56) | 1 (10) | 0.004 |
| Donor age (years) | 28.3 [19.6, 42.8] | 28.5 [20.9, 36.5] | 0.7 |
| Ischemic time (min) | 175 [148, 200] | 156 [151, 190] | 0.5 |
| BMI (kg/m2) | 26.3 [23.6, 29.8] | 24.8 [22.1, 27.3] | 0.3 |
| Glucose (mg/dL) | 97 [92, 118] | 93 [85, 113] | 0.3 |
| Uric acid (mg/dL) | 6.3 [5.6, 7.8] | 6.6 [5.3, 7.3] | 0.6 |
| Creatinine (mg/dL) | 1.4 [1.2, 1.6] | 1.4 [1.1, 1.7] | 0.9 |
| GFR mL/min/1.73 m2 | 61 [49, 69] | 63 [45, 69] | 0.8 |
| Triglycerides (mg/dL) | 141 [96, 232] | 106 [67, 179] | 0.2 |
| HDL cholesterol (mg/dL) | 54 [41, 67] | 59 [47, 63] | 0.7 |
| LDL cholesterol (mg/dL) | 96 [75, 118] | 98 [70, 126] | 0.9 |
| Hypertension, Number (%) | 32 (48) | 2 (20) | 0.05 |
| Diabetes, Number (%) | 24 (36) | 1 (10) | 0.1 |
Thirteen of the 66 patients (19.6%) died during follow up, all of them in the HTx group (p=0.04) from which 4 deaths were related to severe CAV (2 with MI on pathologic examination, 1 from cardiogenic shock after a failed coronary intervention and one died suddenly and had known severe CAV). Five-year survival trended to be improved with H+Liver Tx (100.0±0.0 % vs. 81.8±5.2%; p=0.2).
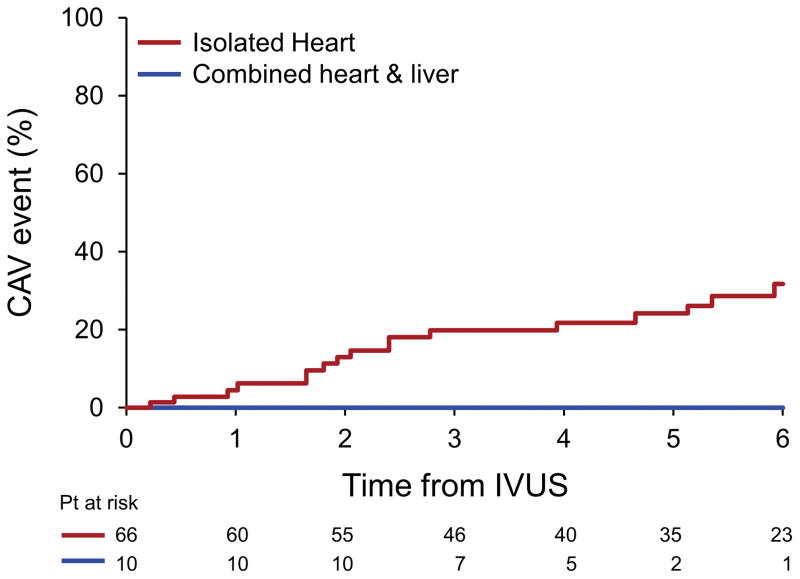
Eight patients required readmission because of graft failure related to severe CAV during follow up, (12.1%), 2 patients had a MI, and 5 patients underwent percutaneous interventions for CAV, all in the HTx group. Eighteen patients (27.2%) had at least one CAV related event during follow up, all in the isolated group. The performance of H+Liver Tx resulted in a lower incidence of the combined CAV outcome in 5 years (0.0±0.0 % vs. 24.3±5.7 %; p=0.13; figure 2).
Figure 2.
Cardiac allograft vasculopathy related outcome of patients after heart transplant comparing those undergoing combined heart and liver transplant and isolated heart transplant.
The graphs indicate cardiac events (coronary related death, vasculopathy related graft failure, need for per-cutaneous coronary intervention or non fatal myocardial infarction) after diagnosis.
Note that there are more cardiac events in patients treated with isolated heart transplant.
The performance of H+Liver Tx was associated with a trend for reduced all cause mortality (p=0.08) and a significant reduction in CAV related events in univariable analysis (p=0.04), which remained significant when adjusted for the difference in the primary etiology for heart disease (p=0.05).
Discussion
Our study is the first to analyze the impact of H+Liver Tx on the progression of CAV by repeated 3D IVUS exams. We show that patients with H+Liver Tx have slower progression of CAV. The attenuation in coronary plaque progression by the addition of the liver graft is significant even when adjusting for the lower prevalence of ischemic heart disease and the differences in traditional coronary risk factors. Furthermore, we show that the attenuated plaque progression is translated to better coronary related clinical outcomes in unadjusted and adjusted analysis.
Patient Characteristics
The patients in the H+Liver Tx group differed from the heart transplant group by a higher prevalence of FA and restrictive cardiomyopathy and a lower prevalence of ischemic cardiomyopathy as indication for heart replacement therapy. Because ischemic cardiomyopathy is a risk factor for accelerated CAV (13), the etiologic difference may have biased the results towards accelerated plaque progression in the isolated heart transplant patients. Furthermore, the H+Liver Tx group also had lower triglyceride levels and lower prevalence of hypertension before transplant, both possible risk factors for accelerated vasculopathy. We have therefore analyzed the subgroup of patients without ischemic cardiomyopathy and found that the protective effect of the liver graft on the coronary vessels was maintained (Figure 1). Adjustment for the differences in traditional risk factors and prevalence of ischemic cardiomyopathy by multivariate analysis shows that the performance of H+Liver Tx remained significantly associated with attenuated plaque index progression. We believe that these analyses suggest that the liver graft has protective effects on the coronary vasculature that are not related just to the difference in baseline characteristics between the groups although we acknowledge that due to the differences in etiology of heart disease our data should be interpreted cautiously, and larger clinical trials would be required to recommend combined heart liver transplant for improved coronary remodeling.
Volumetric Changes in the H+Liver Tx and isolated heart transplant groups
3D IVUS is presently considered the gold standard for the evaluation of CAV (14–16) and quantifies both intimal thickening and changes in external elastic membrane area (arterial remodeling). This is important because lumen loss in CAV is caused not only by intimal thickening but also by changes in external elastic membrane area. (16–18) We show that the addition of a liver graft attenuates plaque progression with no effect on vessel volume progression. This suggests that the mechanism of slower progression of CAV with H+Liver Tx involves a reduced rate of intimal hyperplasia and plaque progression with no effect on vessel remodeling, at least in the first two years after transplant.
We show that not only is plaque progression attenuated, as observed by 3D IVUS and routine coronary angiography, but that this attenuation is translated to improved coronary related clinical outcomes. We also show that the improved clinical outcomes with H+Liver Tx remain significant even after adjustment for the lower prevalence of ischemic cardiomyopathy in the HTx group suggesting that the grafted liver protects the heart transplant by other mechanisms.
Mechanism of attenuation of CAV
The diffuse nature of CAV suggests an immune etiology and immune activation and increased cellular rejection burden may lead to an inflammatory process in the vascular endothelium and potentiation of CAV. (19–23) Cell mediated heart rejection was less frequent in the H+Liver Tx patients, which in view of the association between rejection burden and CAV may have played a role in the attenuation of plaque progression. To explain the favorable low rejection rate, and attenuated progression of CAV induction of partial tolerance has been proposed (12). Previous reports have shown that donor myeloid cells migrate after liver transplantation into recipients T- dependent areas of lymphoid tissue (24) (25, 26), where they or their progeny appear to persist indefinitely and may induce donor leukocyte chimerism. (27, 28) The induction of mixed chimerism may eliminate cells in the thymus that are reactive to donor antigen (central deletion), and result in unresponsiveness to these antigens, delaying or attenuating CAV. (27) Another intriguing and possible explanation for the attenuated CAV seen in the combined heart and liver transplant patients may be switching from central deletion to peripheral mechanism that may include regulatory T cells. (28) It has been recently shown that orthotopic liver transplantation in mice results in expansion of Foxp3 expressing CD 4+ regulatory T cells (Treg) in the recipient spleen, and that adoptive transfer of those spleen cells significantly prolongs donor heart graft survival. (29) High levels of such FoxP3+ Tregs have been demonstrated to play a role in tolerance and long term survival of kidney allografts without immunosupression in patients in which chimerism was induced by non-myeloablative combined bone marrow and kidney transplant. (28) Another possible mechanism may be that the liver may permit acceptance of other simultaneously transplanted organs by means of shedding soluble human leukocyte antigen (HLA) antigens (30). It has been hypothesized that maintaining a proper concentration of soluble HLA in circulation would lead to tolerance to the allotype of the soluble HLA. This concept may help explain the protection of a simultaneous kidney and heart transplant by a successful human liver transplant. (3, 4, 31–33)
Heart recipients transplanted in the presence of donor-specific anti- HLA antibodies have a lower graft survival (34, 35) suggesting a possible role for the humoral arm of the immune system in the pathogenesis of CAV. The mechanism may be related to the fact that endothelial cells express both HLA I and II molecules in the context of organ transplantation endowing them with the capacity to present antigen to the recipient T cells but also to be a target for allo-immune responses from donor specific antibodies towards both HLA I and HLA II antigens (36, 37). These alloantibodies are capable to activate endothelial exocytosis of granules that contain pro-thrombotic mediators, resulting in exaggerated mitosis and inflammation of the vessel (38) which may result in a “smoldering” inflammatory response, and accelerated plaque progression. In that respect, it has been reported that a positive cross-match can become negative after liver transplantation, and that donor anti HLA antibodies may be absorbed or neutralized by the liver graft (39–41) which may have played a significant role in the attenuation of the rate of CAV.
Clinical implications
We demonstrate that it is possible to achieve excellent outcome and survival with H+Liver Tx and that the liver allograft may play an important role in delaying the development of CAV. Recent reports have described the use of a partial auxiliary liver in the context of kidney transplant. (40, 42) showing that the auxiliary liver may offer a protective effect when transplanted together with a kidney from the same donor, despite a positive cross-match between the donor and the recipient. The results of the antibody analyses in these reports have supported the hypothesis that liver grafts reduce the levels of specific anti-donor reactivity. Whether such partial auxiliary liver transplants may be performed in the context of heart transplant to protect from accelerated CAV is speculative. However, this study provides preliminary data, which suggests that such an approach should perhaps be considered especially in the context of multiple pre-formed antibodies, in whom finding a donor can be especially challenging, or younger recipients whose longer term outcome may be greatly limited by CAV.
Study Limitations
The lack of randomization and especially the significant differences in etiology of heart disease between the groups may have been a source of bias. Importantly, we lacked the power to make definitive conclusions about clinical outcomes due to the small number of patients, the short period of follow up and the marked difference in etiology of heart disease between the groups. We also acknowledge the fact that the data may be skewed by referral bias as it comes from a center very accustomed to treating patients with familial amyloidosis and need for combined heart and liver transplant. We believe that our data should be considered preliminary and be expanded in further studies.
We routinely perform H+Liver Tx in every patient with severe cardiomyopathy related to FA, and therefore lack patients with amyloidosis and isolated heart transplant, which would have consisted the optimal control group.
Methods
Study Design
The study was a nonrandomized, single-center study approved by the Mayo Clinic institutional review board. From January 2004 to April 2009, we identified a total of 10 H+Liver Tx recipients in which at least two coronary 3D IVUS were performed in the first two years after transplant. All the H+Liver Tx patients were treated with calcineurin inhibitors as primary immunosuppressant during the time period of IVUS exams (cyclosporine [n=5] or tacrolimus [n=5]). During the same time period, a total of 122 HTx were also performed in our program. Eighty-two of these patients did not have 3D coronary IVUS exams performed in the first and second year after transplant and were excluded from our IVUS analysis. The remaining 40 patients were divided into those on calcineurin inhibitors (n=24) and on sirolimus (n=16) as primary immunosuppressant. To avoid the potential bias incurred by the use of sirolimus, which has been shown to produce significant attenuation of CAV when used as primary immunosuppressant (15, 43) we excluded in our primary analysis all the patients on sirolimus remaining with 24 patients in the HTx group for the IVUS analysis.
Baseline characteristics were collected immediately before the first IVUS exam. Exclusion of patients based on the presence or absence of IVUS exams may have resulted in bias in outcome analysis. To correct for this possible bias, survival, and time to CAV related adverse events were calculated in all 76 patients (66 HTx and 10 H+Liver Tx) transplanted between January 2004 and April 2009, maintained on Calcineurin inhibitors, irrespective of performance of IVUS examinations. Immunosupression as well as routine endomyocardial biopsies were performed and managed as previously described. (15) Total rejection score (TRS) and any rejection score (ARS) were calculated as previously described. (8)
Follow-Up and Clinical Outcomes
Clinical follow-up was obtained by review of medical records, surveys, and telephone interviews. The cause of death was determined by review of medical records and death certificates. Death related to CAV was defined as death due to myocardial infarction (MI), or abrupt death occurring in the setting of progressive CAV. Heart failure related to CAV was defined as readmission because of graft failure in a patient with known CAV and no other demonstrable etiology for the clinical. The combined CAV related outcome was defined as CAV related mortality, or MI, or heart failure related to CAV or need for percutaneous intervention for CAV.
Coronary IVUS Examination and Analysis
The methods for conducting coronary IVUS have been described elsewhere. (15, 44) Briefly, coronary IVUS was performed from the mid to distal left anterior descending coronary artery to the left main coronary artery with a dedicated imaging catheter and IVUS scanner (Volcano Therapeutics Inc, Rancho Cordova, Calif). Offline volumetric analysis of IVUS data was performed (echo Plaque 2, version 2.5, INDECSystems Inc, Santa Clara, Calif) by operators who were unaware of treatment assignment. The Simpson rule for volumetric measurement was used. Starting with the first complete vascular ring distal to the bifurcation with the left circumflex artery lumen, plaque and vessel volume were analyzed. Each measured volume was normalized to the examined segment length (mm3/mm) to compensate for differences in examined vessel segment length. A plaque index was calculated as follows: (plaque volume/vessel volume). Changes in plaque volume, lumen volume, and vessel volume or plaque index were defined as follow-up minus baseline volume measures value and as percent change. The semi automated contour detection of both the lumen and the media-adventitia interface was performed at intervals of either 16 or 32 frames, depending on the heterogeneity of the image. All other measurements were carried out automatically. Border detection was corrected manually in all frames after automatic border detection.
Statistical Analysis
Continuous parameters were presented as means ± SD and compared using the Student’s t-test. Ordinal data were presented by median, 1st and 3rd quartiles and compared using the exact nonparametric Wilcoxon rank sum test. Differences from baseline to the follow-up IVUS exams were compared by use of a paired t-test. IVUS values at end of follow up were compared between groups by ANCOVA, with the baseline value of the term included in the analysis as a covariate. Categorical data were compared between groups using the χ2 or the Fisher’s exact test. The association between plaque index progression and the performance of H+Liver Tx was analyzed by univariate analysis. To analyze the independent association between the H+Liver Tx and the rate of plaque index progression multivariate analysis (with the plaque index progression in percent as dependent variable and the performance of H+Liver Tx as independent variable) was performed. Adjustment for the prevalence of ischemic cardiomyopathy was performed first, and then other traditional risk factors for CAV were added.
Cox proportional hazard was used to analyze the association of the performance of H+Liver Tx with the time to vascular cardiac events with calculation of hazard ratios (HR) and confidence intervals. Survival distributions were calculated from the time of first IVUS according to the Kaplan–Meier method and compared by means of the log-rank test. All P values were two-sided, and values of less than 0.05 were considered to indicate statistical significance. All data were analyzed with the JMP System software version 8.0 (SAS Institute, Inc, Cary, NC). All authors participated in designing the study, collecting and analyzing data, and drafting and revising the manuscript.
Supplementary Material
Footnotes
Authorship Contributions: Yan Topilsky, MD (Y.T): 1,2,3,5
Eugenia Raichlin (E.R): 1,2,3,5
Barry A. Boilson (B.A.B):1,2,3,5
John A. Schirger (J.A.S): 1,2,3,5
Naveen L. Pereira, MD (N.L.P): 1,5
Brooks S. Edwards, MD (B.S.E): 1,2
Alfredo L. Clavell (A.L.C): 1,2
Richard J. Rodheffer (R.J.R): 1,2
Manish J. Gandhi, MD (M.J.G): 1,2,3,4
Participated in research design
Participated in the writing of the paper
Participated in the performance of the research
Contributed new reagents or analytic tools
Participated in data analysis
Disclosures
The authors of this manuscript have no conflict of interest to disclose.
References
- 1.Taylor DO, Edwards LB, Boucek MM, Trulock EP, Aurora P, Christie J, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult heart transplant report--2007. J Heart Lung Transplant. 2007 Aug;26(8):769–81. doi: 10.1016/j.healun.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Miller LW, Schlant RC, Kobashigawa J, Kubo S, Renlund DG. 24th Bethesda conference: Cardiac transplantation. Task Force 5: Complications. J Am Coll Cardiol. 1993 Jul;22(1):41–54. doi: 10.1016/0735-1097(93)90814-h. [DOI] [PubMed] [Google Scholar]
- 3.Neumann UP, Lang M, Moldenhauer A, Langrehr JM, Glanemann M, Kahl A, et al. Significance of a T-lymphocytotoxic crossmatch in liver and combined liver-kidney transplantation. Transplantation. 2001 Apr 27;71(8):1163–8. doi: 10.1097/00007890-200104270-00025. [DOI] [PubMed] [Google Scholar]
- 4.Flye MW, Duffy BF, Phelan DL, Ratner LE, Mohanakumar T. Protective effects of liver transplantation on a simultaneously transplanted kidney in a highly sensitized patient. Transplantation. 1990 Dec;50(6):1051–4. [PubMed] [Google Scholar]
- 5.Rana A, Robles S, Russo MJ, Halazun KJ, Woodland DC, Witkowski P, et al. The combined organ effect: protection against rejection? Ann Surg. 2008 Nov;248(5):871–9. doi: 10.1097/SLA.0b013e31817fc2b8. [DOI] [PubMed] [Google Scholar]
- 6.Opelz G, Margreiter R, Dohler B. Prolongation of long-term kidney graft survival by a simultaneous liver transplant: the liver does it, and the heart does it too. Transplantation. 2002 Nov 27;74(10):1390–4. doi: 10.1097/00007890-200211270-00008. discussion 70-1. [DOI] [PubMed] [Google Scholar]
- 7.Pinderski LJ, Kirklin JK, McGiffin D, Brown R, Naftel DC, Young KR, Jr, et al. Multi-organ transplantation: is there a protective effect against acute and chronic rejection? J Heart Lung Transplant. 2005 Nov;24(11):1828–33. doi: 10.1016/j.healun.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Raichlin E, Bae JH, Kushwaha SS, Lennon RJ, Prasad A, Rihal CS, et al. Inflammatory burden of cardiac allograft coronary atherosclerotic plaque is associated with early recurrent cellular rejection and predicts a higher risk of vasculopathy progression. J Am Coll Cardiol. 2009 Apr 14;53(15):1279–86. doi: 10.1016/j.jacc.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 9.Raichlin E, Kushwaha SS, Daly RC, Kremers WK, Frantz RP, Clavell AL, et al. Combined heart and kidney transplantation provides an excellent survival and decreases risk of cardiac cellular rejection and coronary allograft vasculopathy. Transplant Proc. 2011 Jun;43(5):1871–6. doi: 10.1016/j.transproceed.2011.01.190. [DOI] [PubMed] [Google Scholar]
- 10.Vermes E, Grimbert P, Sebbag L, Barrou B, Pouteil-Noble C, Pavie A, et al. Long-term results of combined heart and kidney transplantation: a French multicenter study. J Heart Lung Transplant. 2009 May;28(5):440–5. doi: 10.1016/j.healun.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Hermsen JL, Nath DS, del Rio AM, Eickstaedt JB, Wigfield C, Lindsey JD, et al. Combined heart-kidney transplantation: the University of Wisconsin experience. J Heart Lung Transplant. 2007 Nov;26(11):1119–26. doi: 10.1016/j.healun.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Raichlin E, Daly RC, Rosen CB, McGregor CG, Charlton MR, Frantz RP, et al. Combined heart and liver transplantation: a single-center experience. Transplantation. 2009 Jul 27;88(2):219–25. doi: 10.1097/TP.0b013e3181ac60db. [DOI] [PubMed] [Google Scholar]
- 13.Taylor DO, Stehlik J, Edwards LB, Aurora P, Christie JD, Dobbels F, et al. Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Heart Transplant Report-2009. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2009 Oct;28(10):1007–22. doi: 10.1016/j.healun.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987 May 28;316(22):1371–5. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 15.Raichlin E, Bae JH, Khalpey Z, Edwards BS, Kremers WK, Clavell AL, et al. Conversion to sirolimus as primary immunosuppression attenuates the progression of allograft vasculopathy after cardiac transplantation. Circulation. 2007 Dec 4;116(23):2726–33. doi: 10.1161/CIRCULATIONAHA.107.692996. [DOI] [PubMed] [Google Scholar]
- 16.Tsutsui H, Ziada KM, Schoenhagen P, Iyisoy A, Magyar WA, Crowe TD, et al. Lumen loss in transplant coronary artery disease is a biphasic process involving early intimal thickening and late constrictive remodeling: results from a 5-year serial intravascular ultrasound study. Circulation. 2001 Aug 7;104(6):653–7. doi: 10.1161/hc3101.093867. [DOI] [PubMed] [Google Scholar]
- 17.Lim TT, Liang DH, Botas J, Schroeder JS, Oesterle SN, Yeung AC. Role of compensatory enlargement and shrinkage in transplant coronary artery disease. Serial intravascular ultrasound study. Circulation. 1997 Feb 18;95(4):855–9. doi: 10.1161/01.cir.95.4.855. [DOI] [PubMed] [Google Scholar]
- 18.Pethig K, Heublein B, Meliss RR, Haverich A. Volumetric remodeling of the proximal left coronary artery: early versus late after heart transplantation. J Am Coll Cardiol. 1999 Jul;34(1):197–203. doi: 10.1016/s0735-1097(99)00159-x. [DOI] [PubMed] [Google Scholar]
- 19.Jimenez J, Kapadia SR, Yamani MH, Platt L, Hobbs RE, Rincon G, et al. Cellular rejection and rate of progression of transplant vasculopathy: a 3-year serial intravascular ultrasound study. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2001 Apr;20(4):393–8. doi: 10.1016/s1053-2498(00)00249-7. [DOI] [PubMed] [Google Scholar]
- 20.Brunner-La Rocca HP, Schneider J, Kunzli A, Turina M, Kiowski W. Cardiac allograft rejection late after transplantation is a risk factor for graft coronary artery disease. Transplantation. 1998 Feb 27;65(4):538–43. doi: 10.1097/00007890-199802270-00015. [DOI] [PubMed] [Google Scholar]
- 21.Raichlin E, Edwards BS, Kremers WK, Clavell AL, Rodeheffer RJ, Frantz RP, et al. Acute cellular rejection and the subsequent development of allograft vasculopathy after cardiac transplantation. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2009 Apr;28(4):320–7. doi: 10.1016/j.healun.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Narrod J, Kormos R, Armitage J, Hardesty R, Ladowski J, Griffith B. Acute rejection and coronary artery disease in long-term survivors of heart transplantation. The Journal of heart transplantation. 1989 Sep-Oct;8(5):418–20. discussion 20-1. [PubMed] [Google Scholar]
- 23.Vassalli G, Gallino A, Weis M, von Scheidt W, Kappenberger L, von Segesser LK, et al. Alloimmunity and nonimmunologic risk factors in cardiac allograft vasculopathy. European heart journal [Research Support, Non-US Gov’t Review] 2003 Jul;24(13):1180–8. doi: 10.1016/s0195-668x(03)00237-9. [DOI] [PubMed] [Google Scholar]
- 24.Bosma BM, Metselaar HJ, Gerrits JH, van Besouw NM, Mancham S, Groothuismink ZM, et al. Migration of allosensitizing donor myeloid dendritic cells into recipients after liver transplantation. Liver Transpl. 2010 Jan;16(1):12–22. doi: 10.1002/lt.21961. [DOI] [PubMed] [Google Scholar]
- 25.Lu L, Woo J, Rao AS, Li Y, Watkins SC, Qian S, et al. Propagation of dendritic cell progenitors from normal mouse liver using granulocyte/macrophage colony-stimulating factor and their maturational development in the presence of type-1 collagen. J Exp Med. 1994 Jun 1;179(6):1823–34. doi: 10.1084/jem.179.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomson AW, Lu L, Wan Y, Qian S, Larsen CP, Starzl TE. Identification of donor-derived dendritic cell progenitors in bone marrow of spontaneously tolerant liver allograft recipients. Transplantation. 1995 Dec 27;60(12):1555–9. doi: 10.1097/00007890-199560120-00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell PS, Chase CM, Sykes M, Ito H, Shaffer J, Colvin RB. Tolerance, mixed chimerism, and chronic transplant arteriopathy. J Immunol. 2001 Nov 15;167(10):5731–40. doi: 10.4049/jimmunol.167.10.5731. [DOI] [PubMed] [Google Scholar]
- 28.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008 Jan 24;358(4):353–61. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W, Carper K, Zheng XX, Kuhr CS, Reyes JD, Liang Y, et al. The role of Foxp3+ regulatory T cells in liver transplant tolerance. Transplant Proc. 2006 Dec;38(10):3205–6. doi: 10.1016/j.transproceed.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 30.Davies HS, Pollard SG, Calne RY. Soluble HLA antigens in the circulation of liver graft recipients. Transplantation [Research Support, Non-US Gov’t] 1989 Mar;47(3):524–7. doi: 10.1097/00007890-198903000-00025. [DOI] [PubMed] [Google Scholar]
- 31.Saidman SL, Duquesnoy RJ, Demetris AJ, McCauley J, Ramos H, Mazariegos G, et al. Combined liver-kidney transplantation and the effect of preformed lymphocytotoxic antibodies. Transpl Immunol. 1994;2(1):61–7. doi: 10.1016/0966-3274(94)90080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mjornstedt L, Friman S, Backman L, Rydberg L, Olausson M. Combined liver and kidney transplantation against a positive cross match in a patient with multispecific HLA-antibodies. Transplant Proc. 1997 Nov;29(7):3164–5. doi: 10.1016/s0041-1345(97)00827-0. [DOI] [PubMed] [Google Scholar]
- 33.Morrissey PE, Gordon F, Shaffer D, Madras PN, Silva P, Sahyoun AI, et al. Combined liver-kidney transplantation in patients with cirrhosis and renal failure: effect of a positive cross-match and benefits of combined transplantation. Liver Transpl Surg. 1998 Sep;4(5):363–9. doi: 10.1002/lt.500040512. [DOI] [PubMed] [Google Scholar]
- 34.Tambur AR, Bray RA, Takemoto SK, Mancini M, Costanzo MR, Kobashigawa JA, et al. Flow cytometric detection of HLA-specific antibodies as a predictor of heart allograft rejection. Transplantation. 2000 Oct 15;70(7):1055–9. doi: 10.1097/00007890-200010150-00011. [DOI] [PubMed] [Google Scholar]
- 35.Przybylowski P, Balogna M, Radovancevic B, Frazier OH, Susskind B, Van Buren C, et al. The role of flow cytometry-detected IgG and IgM anti-donor antibodies in cardiac allograft recipients. Transplantation. 1999 Jan 27;67(2):258–62. doi: 10.1097/00007890-199901270-00012. [DOI] [PubMed] [Google Scholar]
- 36.Taflin C, Charron D, Glotz D, Mooney N. Immunological function of the endothelial cell within the setting of organ transplantation. Immunol Lett. May 27; doi: 10.1016/j.imlet.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 37.Al-Lamki RS, Bradley JR, Pober JS. Endothelial cells in allograft rejection. Transplantation. 2008 Nov 27;86(10):1340–8. doi: 10.1097/TP.0b013e3181891d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamakuchi M, Kirkiles-Smith NC, Ferlito M, Cameron SJ, Bao C, Fox-Talbot K, et al. Antibody to human leukocyte antigen triggers endothelial exocytosis. Proc Natl Acad Sci U S A. 2007 Jan 23;104(4):1301–6. doi: 10.1073/pnas.0602035104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manez R, Kelly RH, Kobayashi M, Takaya S, Bronsther O, Kramer D, et al. Immunoglobulin G lymphocytotoxic antibodies in clinical liver transplantation: studies toward further defining their significance. Hepatology. 1995 May;21(5):1345–52. doi: 10.1002/hep.1840210519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olausson M, Mjornstedt L, Norden G, Rydberg L, Molne J, Backman L, et al. Successful combined partial auxiliary liver and kidney transplantation in highly sensitized cross-match positive recipients. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons [Clinical Trial Research Support, Non-US Gov’t] 2007 Jan;7(1):130–6. doi: 10.1111/j.1600-6143.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- 41.Gugenheim J, Amorosa L, Gigou M, Fabiani B, Rouger P, Gane P, et al. Specific absorption of lymphocytotoxic alloantibodies by the liver in inbred rats. Transplantation. 1990 Aug;50(2):309–13. doi: 10.1097/00007890-199008000-00027. [DOI] [PubMed] [Google Scholar]
- 42.Olausson M, Mjornstedt L, Norden G, Rydberg L, Lindner P, Backman L, et al. Auxiliary liver and combined kidney transplantation prevents hyperacute kidney rejection in highly sensitized patients. Transplant Proc [Research Support, Non-US Gov’t] 2002 Dec;34(8):3106–7. doi: 10.1016/s0041-1345(02)03577-7. [DOI] [PubMed] [Google Scholar]
- 43.Kushwaha SS, Khalpey Z, Frantz RP, Rodeheffer RJ, Clavell AL, Daly RC, et al. Sirolimus in cardiac transplantation: use as a primary immunosuppressant in calcineurin inhibitor-induced nephrotoxicity. J Heart Lung Transplant. 2005 Dec;24(12):2129–36. doi: 10.1016/j.healun.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 44.Bae JH, Rihal CS, Edwards BS, Kushwaha SS, Mathew V, Prasad A, et al. Association of angiotensin-converting enzyme inhibitors and serum lipids with plaque regression in cardiac allograft vasculopathy. Transplantation. 2006 Oct 27;82(8):1108–11. doi: 10.1097/01.tp.0000230378.61437.a5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.