Abstract
Krüppel-like factor 5 (KLF5) is a multifunctional transcription factor involved in cell proliferation, differentiation and carcinogenesis. In addition to frequent inactivation in different types of human cancers including breast cancer, KLF5 has been identified as an essential co-factor for the TGF-β tumor suppressor. In our previous study demonstrating a negative regulation of ER (estrogen receptor alpha) function by KLF5 in breast cancer cells, we noticed that estrogen reduced the protein level of KLF5. In this study, we tested whether and how estrogen-ER signaling regulates KLF5 protein. We found that estrogen caused the degradation of KLF5 protein, and the degradation was sensitive to proteasome inhibitors but not other inhibitors. The estrogen-inducible E3 ligase EFP was identified as a key player in estrogen-mediated degradation of KLF5, as knockdown and over-expression of EFP increased and decreased KLF5 protein levels respectively, and the decrease continued even when protein synthesis was blocked. EFP-mediated degradation impaired the function of KLF5 in gene transcription. While only unubiquitinated EFP interacted with KLF5, overexpression of EFP appeared to prevent the ubiquitination of KLF5 while resulting in heavy ubiquitination of the E3 itself. Furthermore, ubiquitination of EFP interrupted its interaction with KLF5. Although the mechanism for how EFP degrades KLF5 remains to be determined, these results suggest that estrogen causes the degradation of KLF5 protein by inducing the expression of EFP in ER-positive breast cancer cells.
Keywords: KLF5, EFP, estrogen, ER, breast cancer
INTRODUCTION
Krüppel is a segmentation gene in Drosophila melanogaster which encodes a protein composed of several zinc finger motifs [1]. Krüppel-like factors (KLFs) are mammalian proteins homologous to the zinc finger part of Krüppel. KLF5 (also named IKLF or BTEB2) is a transcription factor with a proline-rich N-terminal region and a C-terminus that contains three consecutive zinc finger motifs [2]. Preceding the zinc finger motifs is a short basic region that may contribute to the ability of KLF5 to bind to a GC-rich promoter. By regulating target genes such as PDGF-α, PPARδ, NF-κB, cyclinD1, p15 and Myc [3-9], KLF5 modulates a variety of cellular processes including proliferation, differentiation, and apoptosis [4, 6, 8, 10-13]. Consequently, KLF5 has been implicated in various physiological and pathological processes including carcinogenesis [13].
In epithelial proliferation, KLF5 has dual functions, being stimulatory in basal-like cells but inhibitory in epithelial cells treated with TGF-β [7-9, 13-21]. One mechanism for the reversal of KLF5 function is that TGF-β recruits p300 to acetylate KLF5, which alters the KLF5 transcriptional complex and reverses KLF5 function in gene regulation [8, 9, 14].
KLF5 appears to play a role in breast cancer, but its precise function remains to be determined. On one hand, the KLF5 locus at chromosome 13 is frequently deleted in human breast cancer and its protein is degraded by the WWP1 oncogenic ubiquitin E3 ligase [19, 22, 23], which suggests a tumor suppressor function of KLF5. On the other hand, increased expression of KLF5 is associated with the expression of the HER2 oncoprotein and shorter survival in patients [24], which suggests an oncogenic function of KLF5 in breast cancer. In our effort to understand the role of KLF5 in breast cancer, we examined whether and how KLF5 affects the function of estrogen receptor alpha (ER hereafter) in breast cancer cells, and found that KLF5 inhibits estrogen-promoted cell proliferation and gene regulation in ER-positive breast cancer cells, which involves the interaction of KLF5 with ER [25]. We noticed during that study that treatment of ER-positive cells with estrogen (E2) decreased protein levels of KLF5.
EFP (also named ZNF147 and TRIM25) is an estrogen-responsive RING finger protein that has an E3 ligase activity in both ubiquitination and ISGylation of proteins [26, 27]. While present in the cytoplasm of normal mammary epithelial cells, EFP expression in breast cancer is positively associated with lymph node metastasis and worse patient survival [28], and functional studies have demonstrated that Efp plays an oncogenic role in breast cancer at least by targeting proteolysis of 14-3-3 sigma, a tumor suppressor that suppresses cell cycle progression [29]. In antivirial response of human cells, interferon upregulates EFP and induces its conjugation with the ubiquitin-like protein ISG15 [30], and EFP is indeed an ISG15 E3 ligase for 14-3-3 sigma and its ISGylation enzyme activity is RING domain-dependent [27]. Interestingly, EFP is also autoISGylated, and the autoISGylation negatively regulates its ISG15 E3 ligase activity [31].
In this study, we characterized whether and how ER signaling regulates KLF5. We found that ER signaling regulated KLF5 at both the RNA and protein levels, mediating a dual regulation. However, the regulation of RNA transcript was observed to be a late event while KLF5 protein regulation was observed to be a prominent and early event. We found that ER signaling down-regulated KLF5 protein through the proteasome machinery in ER-positive breast cancer cells, and the estrogen-induced EFP E3 ubiquitin ligase played a crucial role in ER-caused proteolysis of KLF5. EFP interacted with KLF5, but did not cause obvious ubiquitination of KLF5. Rather, EFP itself was ubiquitinated, and its ubiquitination appeared to prevent the ubiquitination of KLF5. These results suggest the dual regulation of KLF5 at both the RNA and protein levels by ER signaling and that the protein regulation of KLF5 precedes the RNA level regulation of KLF5. KLF5 protein is down-regulated by ER signaling through the EFP ubiquitin E3 ligase.
EXPERIMENTAL
Plasmid constructions and transient transfection
Expression plasmids pcDNA3-KLF5, pcDNA3-FLAG-KLF5 (FLAG tag added to the N-terminus), pcDNA3-KLF5-FLAG (FLAG tag added to the C-terminus), HA-Ub (HA-tagged ubiquitin) and Myc-WWP1 (Myc tagged WWP1) were described in our previous study [32]. The pcDNA3-FLAG-EFP plasmid (FLAG-EFP) was constructed using a PCR-based approach with the following primers (restriction enzyme sites are underlined): 5’-GGGGGTACCAT GGCAGAGCTGTGCCCCCTG-3’ (forward) and 5’-CCCGAATTCCTACTTGGGGGAGC AGATGGAGAGTG-3’ (reverse). For the construction of KLF5 plasmid pcDNA3-HA-KLF5-His, in which HA-tag was added to the N-terminus and His-tag was to C-terminus of KLF5, we first made the pcDNA3-HA-His vector by inserting a fragment of DNA containing HA tag, multiple cloning sites EcoRI, XhoI, HindIII, BamHI, NotI, PstI and XbaI, and His tag into the pcDNA3.1 vector (Invitrogen, Carlsbad, CA) between restriction enzyme sites NcoI and ApaI. The KLF5 cDNA was then cloned into this pcDNA3-HA-His vector between HA and His tags at EcoRI and NotI sites using a PCR-based approach with the following primers (restriction enzyme sites are underlined): 5’-TTTGAATTCATGGCTACAAGGGTGC-3’ (forward); 5’-TTTGCGGCCGCGTTCTGGTGCCTCTTCATA-3’ (reverse). All the plasmid transfections were carried out using the Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions.
Construction of EFP mutants
Deletion mutants of EFP were created by PCR-based approaches with FLAG-EFP as templates and FLAG-pcDNA3 as vectors. Primer sequences for creating deletion mutants FLAG-EFP-M1 and FLAG-EFP-M2 have been summarized in Table S1 (Supplementary Data). To construct FLAG-EFP-cS and FLAG-EFP-kR substitution mutation, PCR-directed mutagenesis method was used using FLAG-EFP as the template. PCR primers used for these mutants have been summarized in Table S2.
Cell culture and treatment
ER positive MCF-7 breast cancer cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA) and maintained in Eagle's minimum essential medium supplemented with 10% fetal bovine serum (FBS), 0.01 M HEPES, 1 mM sodium pyruvate, 0.15% sodium bicarbonate, 0.45% glucose, and penicillin and 1% streptomycin. ER positive T-47D cells were also purchased from ATCC and maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 0.01 M HEPES, 1 mM sodium pyruvate, 0.15% sodium bicarbonate, 0.45% glucose, and penicillin and 1% streptomycin. The COS-1 African green monkey kidney cell line, which is ER-negative, was also purchased from ATCC and propagated following ATCC's procedures. Estrogen (17 β -estradiol, E2), tamoxifen (Tam), proteasome inhibitors MG132, MG115 and PS341 and lysosome inhibitor ammonium chloride were purchased from Sigma (St Louis, MO). These reagents were dissolved and used following the manufacturer's recommendations and our previously published papers [22, 33].
To investigate the direct role of estrogen responsiveness of EFP and KLF5 degradation, MCF-7 and T-47D cells were maintained in phenol red-free medium supplemented with 10% charcoal-dextran-stripped fetal bovine serum for at least 3 days. Subsequently, the cells were treated with 1 μM of 17β-estradiol (E2) and were harvested at indicated times to isolate total RNA and protein for analysis. In a similar way, MCF-7 cells cultured in hormone free medium were treated with 1 μM of tamoxifen for 4 hours and subsequently total RNA and protein were isolated for analysis.
Western blot analysis and immunoprecipitation (IP)
A total of 5 × 105 cells were seeded onto each well of a 6-well plate, to which plasmids were transfected using the Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) Forty-eight hours (h) after transfection, cells were treated with 20 μM MG132 for 4 h, and collected with RIPA buffer (50 mM Tris–HCl, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% DOC, 0.1% SDS and 1% Sigma cocktail proteinase inhibitors I) for western blot analysis. Anti-FLAG, anti-β-actin and anti-HA rabbit polyclonal antibodies were purchased from Sigma (St Louis, MO). Anti-EFP mouse monoclonal antibody (mAb) was purchased from BD Bioscience (Franklin Lakes, NJ). Anti-Ub mAb and all secondary antibodies were purchased from Cell Signaling Technology (Danvers, MA). For immunoprecipitation, cell lysates were prepared from 60-mm dishes with 0.5 ml RIPA buffer, and incubated with 30 μl of FLAG-M2 beads with rotation at 4°C for overnight. Beads were then washed three times with washing buffer (20 mM Tris–HCl, pH 8.0, 100 mM KCl, 5 mM MgCl2, 0.2 mM EDTA, 10% glycerol, and 0.1% Tween-20, with 10 mM 2-mercaptoethanol and 1% proteinase inhibitors cocktail added just prior to use). Protein precipitates were boiled with 2 × SDS loading buffer (Bio-Rad, Hercules, CA) and subjected to western blot analysis.
SiRNA transfection
A small interfering RNA (siRNA) with the sequence of 5’-GCACUGGAUGAUGUGAGAA-3’ was used to target EFP. A siRNA targeting the luciferase gene with the sequence is 5’-CUUACGCUGAGUACUUCGAUU-3’was used as a negative control. Both the siRNAs were chemically synthesized (Dharmacon, Chicago, IL). MCF-7 cells were transfected with 100 nM of each siRNA and transfection was carried out by using the siPORT Amine Reagent (Ambion, Austin, TX) in 6-well plates. Forty-eight hours after transfection, total protein was collected for analysis using western blot.
Cycloheximide (CHX) chase assay
COS-1 cells were seeded onto 6-well plates at a density of 5 × 105 cells per well. After incubating at 37°C overnight, cells were transfected with untagged KLF5 construct in the presence or absence of EFP plasmid with Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Twenty-four hours after transfection, cells were treated with 50 μg/ml CHX for different time periods. Total proteins were collected and analyzed by western blot for KLF5, EFP and β-actin. The intensity of protein bands on a film was measured using the Image J program (http://rsb.info.nih.gov/ij), and the degradation curves were plotted as detailed in the legend for figure 2.
Figure 2. Estrogen-induced expression of EFP degrades KLF5 protein.
(A, B) Transfection of siRNA against EFP (siEFP) into MCF-7 cells cultured in regular medium (A) or in hormone-free medium but treated with 1 μM estrogen (E2) for 18 hours (B) increases protein levels of KLF5, as determined by western blotting. (C) MCF-7 cells grown in regular medium were treated with proteasome inhibitors MG132, MG115 and PS341 or lysosome inhibitor ammonium chloride (NH4Cl), and protein expression of KLF5, EFP and β-actin was evaluated by western blotting. (D) Different amounts of EFP plasmid (from 0 to 0.8 μg) were co-transfected with 0.8 μg of KLF5 plasmid into COS-1 cells, and western blotting was performed to detect protein levels of KLF5 and EFP in the presence (+) or absence (−) of MG132. (E) MCF-7 cells in regular medium (panel at left) and COS-1 cells (panel at right) were transfected with expression plasmids for FLAG-tagged EFP and untagged KLF5, KLF5 with a FLAG tag at N-terminus (F-KLF5), or KLF5 with a FLAG tag at C-terminus (KLF5-F), and cell lysates were analyzed by western blotting for protein expression of KLF5 and EFP. Presence or absence of EFP is indicated by “+” or “−”. (F, G) Cycloheximide (CHX) chase assay of COS-1 cells transfected with KLF5 and vector control or EFP. Western blotting was performed with cells treated with CHX for the indicated times to detect protein levels of KLF5 and EFP (F). Signal intensities for bands of KLF5 and β-actin were quantitated using the Image J program, and relative levels of KLF5 were plotted against times of CHX treatment (G).
RNA extraction and real-time quantitative RT–PCR
An RNeasy kit (Qiagen, Valencia, CA) was used to isolate total RNA, and the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) was used to perform reverse transcription. SYBR-based real-time PCR was done to examine the expression of KLF5, EFP and β-actin. The primers used for the study have been summarized in Table S3. The ABI PRISM 7000 sequence detection system (AB Applied Biosystems, Foster City, CA) was used to perform SYBR real-time PCR in triplicate. The ΔCt method, in which β-actin was co-amplified to normalize the amount of RNA added to a reaction, was applied to determine threshold values according to published procedures [34].
Nickel-nitrilotriacetic acid (Ni-NTA)-agarose purification
COS-1 cells were co-transfected with HA- and His-tagged KLF5 (pcDNA3-HA-KLF5-His), HA-tagged ubiquitin, and FLAG-tagged EFP or Myc-tagged WWP1 for 48 hours. After the treatment of MG132 at 20 μM for 4 hours, cells were washed with phosphate-buffered saline (PBS) and lysed in RIPA buffer containing 10 mM imidazole. Twenty μl of Ni-NTA-agarose beads (Qiagen, Valencia, CA) were added to cell lysate and rotated at 4°C for 6 hours. Precipitates were washed three times with washing buffer containing 20 mM imidazole, boiled with 2 × SDS loading buffer, and subjected to western blot analysis.
Promoter-luciferase reporter assay
The FGF-BP proximal promoter (−128 to +61) cloned in the pGL3-BASIC plasmid was provided by Dr. Ceshi Chen (Albany, NY) [35]. MCF-7 or T-47D cells were seeded in a 12 well plate at a density of 1 × 105 cells per well. On the next day these cells were transfected with the FGF-BP promoter reporter construct (0.5 μg per well) and siEFP (75 nM final concentration) using the Lipofectamine 2000 reagent following manufacturer's protocol. The siRNA for KLF5, siKLF5, was described previously [9], and the siRNA for luciferase (siLuc) was used as controls. Forty-eight hours after transfection, cells were lysed and luciferase assay was carried out using Promega's luciferase assay kit as described earlier [36]. Triplicate of each set of experiment were used for the analysis.
RESULTS
Estrogen immediately down-regulates the protein but not RNA level of KLF5 in ER-positive MCF-7 breast cancer cells
In our previous study describing the role of KLF5 in regulating ER function, we noticed that estrogen treatment decreased the protein level of transfected KLF5 in the MCF-7 ER-positive breast cancer cell line, while depletion of hormone in culture medium slightly increased the protein level of KLF5 [25]. This result led us to hypothesize that ER signaling might cause the degradation of KLF5 protein. To test this possibility, we cultured MCF-7 cells in hormone-free medium, and then added estrogen (E2) into the medium for different time periods. As shown in Figure 1A, estrogen (E2) treatment mediated a time-dependent decrease in KLF5 protein level. Estrogen (E2) decreased the protein amount of KLF5 as early as two hours after treatment, which corresponded to the induction of EFP protein by estrogen (E2) (Fig. 1A). Similarly, in another ER positive cell line T-47D, estrogen (E2) treatment also mediated the time dependent decrease in KLF5 protein with a corresponding increase in EFP protein (Fig. 1B). KLF5 protein was observed to decrease as early as 6 hours after treatment with estrogen (E2) with increase in EFP level. In another ER-positive cell line, ZR-75-1, the same treatment also decreased KLF5 protein, but the effect was not detectable until 24 hours after treatment (Fig. S1A). Consistently, induction of EFP protein was not noticeable until 24 hours after estrogen (E2) treatment in ZR-75-1 cells (Fig. S1A). Induction of EFP correlated with decrease of KLF5 at different time points, suggesting a role of EFP in the downregulation of KLF5 protein.
Figure 1. Estrogen treatment causes the degradation of KLF5 in ER-positive breast cancer cells, and the ER-induced EFP E3 ligase is involved.
(A, B) MCF-7 (A) or T-47D (B) cells grown in hormone-free medium and treated with 1 μM estrogen (E2) for the indicated times were subjected to western blotting to detect protein expression of KLF5, EFP and β-actin. (C, D) MCF-7 (C) or T-47D (D) cells in panels A and B were subjected to real-time PCR for RNA expression of EFP and KLF5. (E, F) MCF-7 cells grown in hormone free medium and treated with 1 μM of the ER inhibitor tamoxifen (Tam) and/or estrogen (E2) for 4 hours were subjected to western blotting for protein expression of KLF5, EFP and β-actin (E) and real-time PCR for RNA expression of EFP and KLF5 (F).
We also examined the effect of estrogen treatment on the RNA levels of KLF5 and EFP at different time points. In MCF-7 cells, estrogen-induced EFP transcription was detectable as early as 2 hours after treatment (Fig. 1C), which is consistent with the increase in its protein levels; in T-47D cells, estrogen (E2) induced EFP expression as early as 4 hours (Fig. 1D) with the increase in its protein level at 6 hours (Fig. 1B); but in ZR-75-1 cells the induction was not detectable until 8 hours after treatment (Fig. S1B). In all the cell lines, the transcription of KLF5 showed no changes within 24 hours but decreased at 48 or 72 hours after estrogen (E2) treatment (Fig. 1C, 1D, S1B), indicating that estrogen also downregulates KLF5 transcription, but the decrease in RNA level is not as early as the decrease in protein level.
We also treated MCF-7 cells maintained in hormone free medium with the non-steroidal anti-estrogen tamoxifen (Tam) and estrogen (E2) individually and in combination, and measured KLF5 protein levels. Treatment of cells with estrogen for 4 hours resulted in a decreased level of KLF5 protein (Fig. 1E). The decrease in KLF5 protein level was not seen when the cells were treated with either tamoxifen alone or a combination of tamoxifen with estrogen (Fig. 1E). RNA expression of KLF5 was not affected by either Tam or estrogen (E2) alone or in combination treatment (Fig. 1F). We also treated MCF-7 cells maintained in regular medium with the non-steroidal anti-estrogen tamoxifen, and measured KLF5 protein level. Although weak, treatment of cells with Tam for 4 hours or longer resulted in a detectable level of KLF5 protein (Fig. S1C) and the RNA expression of KLF5 were not affected by Tam treatment (Fig. S1D). These results suggests that inhibition of the estrogen-ER signaling leads to an increased protein level of KLF5, while the RNA level is not affected
The estrogen-responsive protein EFP, an ubiquitin E3 ligase, plays a role in estrogen-mediated downregulation of KLF5 protein
Previous studies indicate that KLF5 is regulated by the ubiquitin proteasome pathway (UPP). WWP1 [22, 33] and Fbw7 [37, 38] have been identified as an E3 ubiquitin ligases mediating the ubiquitination and degradation of KLF5. However, neither WWP1 nor Fbw7 is estrogen- or ER-responsive (Fig. S1E). It is thus possible that one or more other E3 ligases mediate estrogen-caused degradation of KLF5. There are at least seven ubiquitin E3 ligases that are ER-responsive, including SKP2, Cul-4A, E6-AP, EFP, BCA2, MDM2, and RNF11. In our search for ER-responsive E3 ligases that are inducible by estrogen and ER in ER-negative cells after transfection with ER and treatment with estrogen (E2), we found that EFP was the only ER-responsive E3 ligase among these seven in ER-negative cells (Dong XY et al., manuscript in preparation). We therefore tested whether EFP could be the E3 ligase involved in the degradation of KLF5. As expected, the expression of EFP was induced by estrogen (E2) treatment at both the RNA and protein levels in MCF-7, T-47D, and ZR-75-1 cells, although the induction in ZR-75-1 cells was slower than that in MCF-7 and T-47D cells (Fig. 1A-D, S1A-B). The increase in EFP protein correlated with the decrease in KLF5 protein at different time points in these cell lines (Fig. 1A, 1B, S1A), suggesting a role of EFP in the degradation of KLF5. Consistently, treatment for 4 hours with tamoxifen, an anti-estrogen agent, interfered with the induction of EFP by estrogen (E2) at both the RNA and protein levels and the decrease of KLF5 protein level (Fig. 1E, 1F). Again, the RNA level of KLF5 was not affected by tamoxifen with 4 hours of treatment (Fig. 1F).
To further test whether EFP functions in estrogen-mediated degradation of KLF5, we knocked down the expression of EFP by RNAi in MCF-7 cells grown in either regular medium (Fig. 2A) or hormone-free medium supplemented with estrogen (E2) (Fig. 2B), and analyzed the expression of KLF5 protein. Although it is more difficult to detect KLF5 protein due to degradation in cells in normal medium, we could still detect an increase in the protein level of KLF5 upon the knockdown of EFP (Fig. 2A). In MCF-7 cells cultured in hormone-free medium and treated with estrogen (E2), knockdown of EFP also increased the protein level of KLF5 in cells treated with or without estrogen (E2) (Fig. 2B). Similar experiments were done in MCF-7 cells using different siRNAs against EFP and it was observed that the knockdown of EFP did increase the KLF5 protein level (Fig S2A-E). These results indicate that the ER-responsive EFP E3 ubiquitin ligase plays a role in estrogen-mediated downregulation of KLF5 protein.
Protein degradation is responsible for EFP-mediated downregulation of KLF5
To determine whether the proteasome pathway is responsible for estrogen-mediated decrease in KLF5 protein, we treated MCF-7 cells grown in regular medium with proteasome inhibitors MG132, MG115 and PS-341. The lysosome inhibitor ammonium chloride was also used. While KLF5 protein was hardly detectable in MCF-7 cells grown in regular medium, each of the three proteasome inhibitors enriched KLF5 protein to a detectable level (Fig. 2C). Cells treated with ammonium chloride still had no detectable KLF5 (Fig. 2C).
To further test whether protein degradation is responsible for EFP-mediated down-regulation of KLF5, we transfected KLF5 plasmid and different amounts of EFP plasmid into COS-1 cells in the presence or absence of the MG132 proteasome inhibitor. Ectopic expression of EFP caused a dose-dependent decrease in KLF5 protein level, and treatment with MG132 enriched protein levels of KLF5, but did not completely inhibit the effect of EFP (Fig 2D). We also transfected expression plasmids for EFP and different forms of KLF5 (wildtype, N-terminally tagged, and C-terminally tagged) into MCF-7 cells in regular medium and found that ectopic expression of EFP dramatically decreased protein expression for each form of KLF5 (Fig. 2E, panel at left). In a similar way, we transfected the same sets of plasmids into the COS-1 monkey kidney cells, and again found a dramatic decrease in KLF5 protein level in EFP-expressing cells (Fig. 2E, panel at right). Because KLF5 expression was driven by the CMV promoter in these experiments, and CMV promoter is a viral promoter not usually affected by the regulatory mechanisms in mammalian cells, these results suggest that protein degradation rather than transcriptional regulation is responsible for EFP-mediated reduction in the KLF5 protein level. To examine whether KLF5 degradation caused by EFP is a specific event, we transfected expression plasmids for GFP and KLF5 into COS-1 cells along with EFP, and found that ectopic expression of EFP dramatically decreased the protein level of KLF5 but had no effect on GFP protein (Fig. S3A). We also transfected a fusion expression plasmid in which GFP is cloned at the N-terminus of KLF5, and observed that ectopic expression of EFP could no longer degrade the GFP-KLF5 fusion protein (Fig. S3B).
To more definitely determine whether EFP decreases KLF5 protein level by protein degradation, we performed a cycloheximide (CHX) chase assay by transfecting EFP and KLF5 into COS-1 cells, treating cells with the protein synthesis inhibitor CHX, and measuring KLF5 protein expression by western blotting (Fig. 2F) and signal quantification (Fig. 2G). Consistent with previous findings that showed protein degradation of KLF5 in different cells [22, 32, 33], KLF5 protein expression decrease in EFP-expressing cells treated with CHX for 2, 4 and 6 hours (Fig. 2F, 2G), while KLF5 protein level did not change in cells without EFP expression (Fig. 2F, 2G). The EFP-caused decrease in KLF5 protein levels was 20%, 38%, and 84% of control level for CHX treatments of 2, 4 and 6 hours respectively (Fig. 2G). We also performed CHX chase assay in MCF-7 cells where we depleted hormone for 3 days and then treated cells with estrogen and CHX for the indicated times and performed western blotting to examine protein expression. We found that hormone depletion increased the level of KLF5 protein, and treatment with CHX did not decrease the level of KLF5 in the absence of estrogen (Fig. S4). Similarly, CHX treatment did not alter EFP level in the absence of estrogen (Fig. S4). In the presence of estrogen, while KLF5 level decreased considerably and EFP level increased as expected, CHX treatment unexpectedly increased KLF5 level and decreased EFP level (Fig. S4), and the increase in KLF5 levels with different CHX concentrations was significantly correlated with the decrease in EFP levels (P < 0.005, linear regression and correlation test). These results suggest that EFP itself could undergo protein degradation, and EFP degradation could then lead to increased KLF5 protein level. These results further suggest that EFP down-regulates KLF5 through protein degradation.
EFP interacts with KLF5 at the protein level
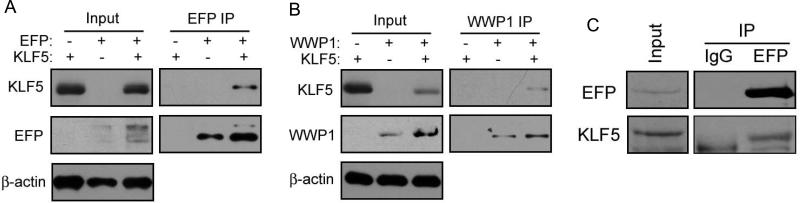
It is well established that in the proteasome pathway, E3 ligases interact with substrates to transfer ubiquitin and cause their degradation by the proteasome. To test whether EFP interacts with KLF5, we transfected plasmids for KLF5 and FLAG-tagged EFP into COS-1 cells, and performed IP with anti-FLAG antibody. Expression of transfected genes were confirmed by western blotting (Fig. 3A, Input). In the protein complex pulled down by antibody for EFP, KLF5 protein was detected (Fig. 3A). We used WWP1 as a positive control, which was previously identified as an E3 ligase that interacts with KLF5 to ubiquitinate and degrade KLF5 [22]. As expected, KLF5 was detected in the protein complex precipitated by antibody for WWP1 (Fig. 3B).
Figure 3. Detection of protein interaction between KLF5 and EFP by immunoprecipitation (IP) combined with western blotting in COS-1 and MCF-7 cells.
(A, B) COS-1 cells were co-transfected with KLF5 and FLAG-tagged EFP (FLAG-EFP) (A) or myc-tagged WWP1 (B) for 48 hours and treated with 20 μM MG132 for 4 hours. Cell lysates were subjected to western blotting (Input) or to IP with anti-FLAG antibody (for EFP) or anti-myc antibody (for WWP1) with subsequent western blotting to detect proteins of KLF5 and EFP (EFP IP in panel A) or KLF5 and WWP1 (WWP1 IP in panel B). The WWP1 panel serves as a positive control. (C) MCF-7 cells grown in hormone-free medium for 2 days were lysed and subjected to IP with EFP antibody (EFP) or mouse immunoglobulin G (IgG). Western blotting was performed to detect endogenous EFP and KLF5 in the precipitates (IP) or in cell lysates not subjected to IP (Input).
To determine whether the KLF5-EFP interaction occurs between endogenous KLF5 and EFP, we performed IP with EFP antibody in MCF-7 cells, which express both EFP and KLF5 (Fig. 1). Because MCF-7 cells in regular medium have almost no detectable KLF5 protein while hormone depletion could significantly enrich KLF5 protein (Fig. 1A), we grew MCF-7 cells in hormone-free medium (phenol red-free medium supplemented with 10% charcoal-dextran stripped FBS) for 2 days before applying IP to cells. As a negative control for monoclonal mouse anti-EFP antibody, mouse IgG was used in the IP. As shown in Figure 3C, KLF5 was detected in the protein complex pulled down by EFP antibody, indicating that endogenous EFP and KLF5 proteins interact with each other.
While EFP itself is ubiquitinated, it did not appear to ubiquitinate KLF5
To determine whether EFP ubiquitinates KLF5 as an E3 ligase, we further characterized its interaction with KLF5 in the presence of ectopically expressed ubiquitin and the MG132 proteasome inhibitor. We transfected COS-1 cells with KLF5, FLAG-tagged EFP and HA-tagged ubiquitin, treated cells with MG132, and performed IP and western blotting. Expression of KLF5, EFP and ubiquitin was confirmed by western blotting (Fig. 4, Input). We first precipitated ubiquitinated proteins using the anti-HA antibody for ubiquitin. As expected, a smear of proteins was detected with the anti-HA antibody in each group regardless of EFP and KLF5, and the smear was much darker in precipitated samples than in input samples (Fig. 4A, Ub panel). For KLF5, its expression was strong, EFP did not make an obvious difference, and no ladder of ubiquitinated KLF5 was visible in the input samples (Fig. 4A, KLF5 panel, lanes 1, 3). In the precipitated ubiquitin complexes, on the other hand, a ladder of ubiquitinated KLF5 was strong in cells without EFP, while no KLF5 band was visible in unubiquitinated form (Fig. 4A, KLF5 panel, lane 4). Unexpectedly, expression of EFP dramatically decreased the level of ubiquitinated KLF5, leaving only a weak band of KLF5 that appeared to have one unit of ubiquitin (Fig. 4A, KLF5 panel, lane 6). For EFP, strong smears were detected in cells transfected with EFP (Fig. 4A, EFP panel, lanes 2, 3), and the smears were darker in ubiquitin precipitates (Fig. 4A, EFP panel, lanes 5, 6), indicating that EFP is heavily ubiquitinated with the cotransfection of ubiquitin.
Figure 4. Characterization of KLF5 ubiquitination, EFP ubiquitination, and protein interaction between EFP and KLF5 in COS-1 cells co-transfected with ubiquitin and treated with MG132.
COS-1 cells were transfected with different combinations of plasmids, as indicated above the images, for 48 hours. MG132 treatment of 20 μM was applied to cells during the last 15 hours of transfection. Cell lysates were subjected to western blotting (Input, all panels), IP combined with western blotting (IP, panels A-C), or Ni-NTA purification combined with western blotting (KLF5 pull down, panel D) to detect different proteins indicated at the left of each panel. HA-tagged ubiquitin plasmid and anti-HA antibody were used for ubiquitin (Ub), Myc-tagged WWP1 plasmid and anti-Myc antibody for WWP1, and FLAG-tagged EFP plasmid and anti-FLAG antibody for EFP. For KLF5, untagged pcDNA3-KLF5 plasmid was used in panels A-C, while pcDNA3-HA-KLF5-His plasmid was used in panel D. Anti-KLF5 serum was used to detect KLF5 protein in all panels. Arrows indicate the two EFP bands predominantly detected in cells transfected with EFP but not ubiquitin.
As a positive control for the ubiquitination of KLF5, we also transfected WWP1 into COS-1 cells and performed IP with anti-HA antibody and western blotting with antibodies for WWP1, KLF5 and ubiquitin. Again, a smear of proteins was detected with the anti-HA antibody for ubiquitin in each group regardless of WWP1 and KLF5, and the smear was much darker in precipitated samples (Fig. 4B, Ub panel, both Input and IP). For KLF5, it was again strongly expressed in the unubiquitinated form in cells not subjected to IP, and there were no obvious bands of ubiquitinated KLF5 (Fig. 4B, KLF5 panel, lanes 1, 3). A band of WWP1 was detected in WWP1-transfected cells not subjected to IP (Fig. 4B, WWP1 panel, lane 3), but no WWP1 was detected in ubiquitin precipitate (Fig. 4B, WWP1 panel, lane 6). In ubiquitin complexes precipitated with anti-HA antibody for ubiquitin, a ladder of ubiquitinated KLF5 was detected in cells with and without WWP1 transfection (Fig. 4B, KLF5 panel, lanes 4, 6), and expression of WWP1 did not obviously decrease larger bands of ubiquitinated KLF5 while dramatically decreasing the smallest band of ubiquitinated KLF5 (close to the size of KLF5 with one unit of ubiquitin) (Fig. 4B, KLF5 panel, lane 6).
We then precipitated EFP complexes with anti-FLAG antibody in cells transfected with ubiquitin and different combinations of KLF5 and EFP, and performed western blotting to detect each of the molecules. Consistent with the results in Figure 4A, a smear of EFP was detected in cells transfected with EFP, regardless of IP or KLF5 expression (Fig. 4C, EFP panel, both Input and IP lanes), and similar signals were also detected by the anti-HA antibody for ubiquitin in the precipitate (Fig. 4C, Ub panel). For KLF5, while strong KLF5 expression was detected in cells not subjected to IP (Fig. 4C, KLF5 panel, lanes 1, 3), which is consistent with results in Figure 4A and 4B, there was no detectable KLF5 signal in the EFP precipitates (Fig. 4C, KLF5 panel, lanes 4, 6). This result suggests that ubiquitinated EFP no longer interacts with KLF5.
We also examined the presence of EFP and ubiquitin in KLF5 protein complexes in cells transfected with His-tagged KLF5, HA-tagged ubiquitin, and FLAG-tagged EFP or myc-tagged WWP1. Cell lysates were incubated with NTA-agarose beads to pull down KLF5 protein complexes, which were then subjected to western blotting. WWP1 was used as a positive control that interacts with KLF5. While expression of either WWP1 or EFP decreased the protein level of KLF5 in cells not subjected to pull-down, abundant KLF5 was detected in each of the pull-down groups (Fig. 4D, KLF5 panel). In the KLF5 protein complex, both WWP1 and EFP were clearly detected (Fig. 4D, pull down). Worth noting is that EFP bands detected in the KLF5 protein complex were primarily in unubiquitinated form (Fig. 4D, EFP panel, lane 6), which is different from those detected in EFP or ubiquitin precipitates.
Role of the RING finger domain of EFP in the degradation of KLF5
EFP comprises of three essential domains: RING-finger, a B box-coiled-coil domain, and a C-terminal SPRY domain [39]. The essential E3 ligase activity of EFP is mostly conserved to its RING-finger and the cysteine residues in the RING finger. We examined the role of the RING-finger domain in degrading KLF5. We generated some deletion mutants of EFP (Fig. 5A), and analyzed their effect on the degradation of KLF5 by western blotting. We also analyzed the interaction between KLF5 and EFP by immunoprecipitation. As shown in Figure 5B, the EFP-M1 mutant, comprising of only the RING-finger, could not interact with and degrade KLF5, suggesting that the RING finger alone is not sufficient for the EFP-KLF5 interaction and the degradation of KLF5. The EFP-M2 mutant, in which the RING-finger was deleted, could not degrade KLF5 either, although it still interacted with KLF5 (Fig. 5B). When the two conserved cysteine residues in the RING finger of EFP were replaced with serines, EFP could no longer interact with KLF5; nor could it degrade KLF5 (Fig. 5C). The EFP-kR mutant, in which the lysine at 117 was changed to arginine thus the ISGylation of EFP was interrupted, as demonstrated in a previous study [31], had no obvious effect on the EFP-KLF5 interaction and the degradation of KLF5 (Fig. 5C). Therefore the degradation of KLF5 by EFP is dependent on both its interaction with KLF5 and its RING finger domain including the conserved cysteine residues.
Figure 5. Role of different domains of EFP in the degradation of KLF5.
(A) Schematic diagram depicting different mutants of FLAG-EFP in which different domains or residues were deleted to mutated. (B,C) Effect of different mutations on the interaction of EFP with KLF5 and the degradation of KLF5. FLAG-EFP or its mutants (M1 and M2 in panel B or cS and kR in panel B) were co-transfected with KLF5 into COS-1 cells, and cell lysates were subjected to IP with FLAG antibody and immunoblotting (IB) with indicated antibodies (panels at the right). Input panels (left) were IB results from cell lysates not subjected to IP. Arrows indicate wildtype and mutant EFP proteins. In C, the panels in the middle and right were from the same cell lysates, while the panel at left was from another batch of cell lysates.
EFP negatively regulates the function of KLF5
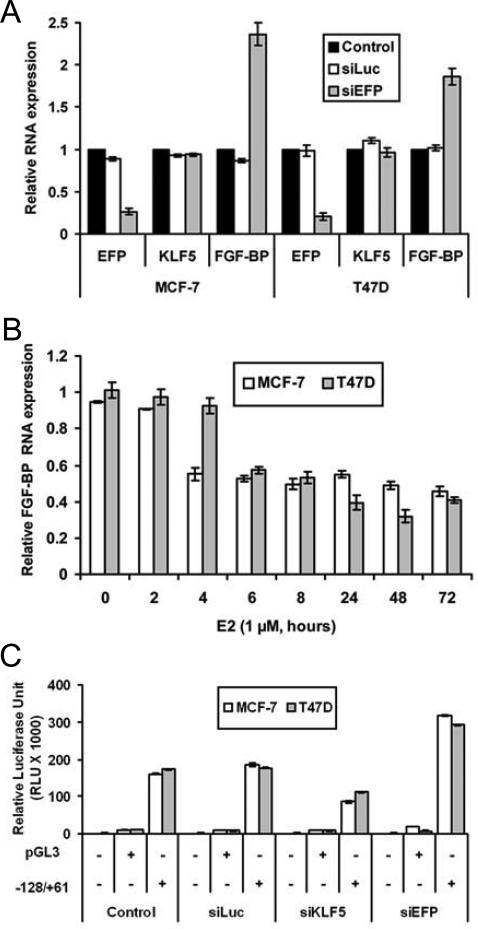
Among the KLF5 regulated genes, fibroblast growth factor binding protein 1 (FGF-BP) has been shown as a direct target of KLF5 that involves the binding of KLF5 to the GC-rich/Sp1 site of the FGF-BP promoter [35]. Furthermore, upregulation of FGF-BP partially mediates the pro-proliferation function of KLF5 in breast cells [35]. Therefore, we examined the effect of EFP on the function of KLF5 in the regulation of FGFBP1. Knock down of EFP in MCF-7 and T-47D cells grown in regular medium, which did not change the RNA level of KLF5 but increased the protein level of KLF5, significantly increased the RNA level of FGFBP1 (Fig. 6A). Consistently, estrogen treatment in MCF-7 and T-47D cells, which significant decreased KLF5 protein levels (Fig. 1C, 1D), also decreased the RNA levels of FGF-BP in these cells (Fig. 6B). With the FGFBP1 promoter-luciferase activity as a readout, knockdown of EFP increased the promoter activity of FGFBP1 in both MCF-7 and T-47D cells (Fig. 6C). These results suggest that EFP can negatively regulate the transcriptional function of KLF5.
Figure 6. Negative regulation of KLF5 function by EFP in ER positive breast cancer cells.
(A) Knockdown of EFP by RNAi increased the expression of FGFBP1 in MCF-7 or T-47D cells cultured in regular medium, as determined by real-time PCR. (B) Expression of FGFBP1 in the same MCF-7 or T-47D cells from Figure 1, which were treated with estrogen for different times, as measured by real-time PCR analysis. (C) Knockdown of EFP by RNAi increased the promoter activity of FGFBP1 in MCF-7 and T-47D cells cultured in regular medium. FGF-BP promoter reporter plasmid was cotransfected with siRNAs for 48 hours, and the luciferase assay was performed. Control, no siRNA transfection; siLuc, siRNA against the luciferase gene (negative control); SiKLF5, siRNA against KLF5 (positive control); siEFP, siRNA against EFP.
DISCUSSION
Estrogen causes KLF5 degradation primarily through the estrogen-inducible E3 ubiquitin ligase EFP
In eukaryotic cells, the proteasome is the major intracellular proteolytic machinery for protein destruction [40, 41]. Previous studies have established that the KLF5 transcription factor is degraded by the proteasome, and the degradation could be ubiquitin-dependent or independent [32, 33]. The WWP1 E3 ubiquitin ligase has been demonstrated to interact with KLF5, causing its ubiquitination and degradation [22]. In this study, we found that in the ER-positive MCF-7, T-47D and ZR-75-1 breast cancer cell lines, the activation of estrogen-ER signaling downregulated KLF5 protein while inducing the EFP ubiquitin E3 ligase (Fig. 1A,1B, S1A), and inhibitors for proteasome but not for lysosome could interrupt ER-mediated downregulation of KLF5 (Fig. 2C). In MCF-7 cells grown in both regular medium and hormone-depleted medium supplemented with estrogen (E2), knockdown of the estrogen-inducible E3 ubiquitin ligase EFP increased endogenous KLF5 protein (Fig. 2A, 2B, S2A-D), while ectopic expression of EFP decreased ectopically expressed KLF5 protein (Fig. 2E). Furthermore, transfection of EFP into ER-negative COS-1 cells also decreased the protein level of transfected KLF5 (Fig. 2E), and the decrease was sensitive to the inhibition of protein synthesis (Fig. 2F, 2G). Functionally, the estrogen–induced EFP impaired the function of KLF5 in the regulation of its target genes such as FGFBP1, while knockdown of EFP enhanced the function of KLF5 in gene regulation (Fig. 6). These results not only indicate that the estrogen-ER signaling causes the degradation of KLF5, they also establish EFP as another E3 ligase that mediates the degradation and modulates the function of KLF5.
While EFP interacts with KLF5, it does not appear to ubiquitinate KLF5, and the mechanism for EFP-mediated KLF5 degradation remains to be determined
Proteasomal degradation often involves ubiquitination, in which the substrate is attached with a chain of polyubiquitin through its interaction with an E3 ligase. Ubiquitinated substrates are then recognized by the 19S regulatory complex of the 26S proteasome for degradation. For example, the WWP1 HECT-domain-containing E3 ligase mediates the ubiquitination and degradation of KLF5 through its interaction with KLF5 [22, 42]. Similarly, the RING-finger-containing EFP E3 ligase has been shown to ubiquitinate 14-3-3σ to mediate its degradation [29]. In the degradation of KLF5 by EFP, EFP interacted with KLF5 at the protein level (Fig. 3, 4D), and such an interaction appeared to be necessary for KLF5 degradation, as deletion of the B-box-coiled coil domain of EFP interrupted its interaction with and the degradation of KLF5 (Fig. 5A, 5B). As expected for a typical E3 ligase in protein degradation, the RING domain and its conserved cysteine residues of EFP are essential for KLF5 degradation (Fig. 5). Unexpectedly, EFP did not cause detectable ubiquitination of KLF5 (Fig. 4A, KLF5 panel, lane 6). In fact, expression of EFP appeared to abolish the ubiquitination of KLF5 (Fig. 4A, KLF5 panel, lane 6), which is different from the WWP1 positive control (Fig. 4B, KLF5 panel, lane 6). It is likely that in the presence of ubiquitin, EFP has more preference of being ubiquitinated over KLF5.
We further noticed that EFP itself was ubiquitinated (Fig. 4) and co-expression of exogenous ubiquitin enhanced the ubiquitination of EFP (Fig. 4A, 4C), which is consistent with a previous report in which auto-ubiquitination of the EFP E3 ligase was described [43]. Unexpectedly, ubiquitination of EFP prevented the interaction of EFP with KLF5, as no KLF5 was detected in the protein complexes pulled down by EFP antibody (Fig. 4C, KLF5 panel, lane 6) or by ubiquitin antibody (Fig. 4A, KLF5 panel, lane 6), while strong smears were detected for both ubiquitin and EFP. It is unlikely that the lack of KLF5 ubiquitination is due to excessive degradation caused by EFP, because the MG132 proteasome inhibitor was applied to cells and abundant unubiquitinated KLF5 was detected in the input (Fig. 4A-C, Input for KLF5). Furthermore, in precipitates pulled down by KLF5 antibody (Fig. 4D), the predominant form of EFP was unubiquitinated. Therefore, in the complex between EFP and KLF5, it appears that neither EFP nor KLF5 is ubiquitinated.
In addition to its ubiquitin E3 ligase activity, EFP is also an ISG15 E3 ligase that mediates the ISGylation of 14-3-3 sigma in viral response [27]. Interestingly, EFP is autoISGylated, and its autoISGylation impairs its ISG15 E3 ligase activity [31]. Whereas the ISGylation activity of EFP appears to be dispensable for its function in KLF5 degradation in our study where no interferon treatment or viral infection was applied (the kR mutant in Fig. 5), we speculate that ubiquitination of EFP could impair its function as an E3 ligase in the degradation of KLF5 similar to the effect of its autoISGylation, and when too much EFP is available, more EFP becomes ubiquitinated to decrease its activity in KLF5 degradation. It is even possible that ubiquitination of EFP leads to its protein degradation, because in the CHX chase assay, EFP protein level decreased in CHX-treated MCF-7 cells in the presence of estrogen (Fig. S4). Whether ubiquitination of EFP causes its degradation and alters its function in the degradation of KLF5 remains to be determined, as does how EFP mediates the degradation of KLF5.
Mutation of the two cysteines in EFP's RING finger, which dramatically impairs the catalytic activity of EFP in its autoISGylation [27], almost abolished the interaction between KLF5 and EFP (Fig. 5C), although deletion of the entire RING finger did not have an obvious effect on the interaction (Fig. 5B). One possibility is that a third protein – protein X – could be ubiquitinated by EFP and interact with both EFP and KLF5, and ubiquitinated protein X could be responsible for the transfer of KLF5 to the proteasome for degradation. EFP with the cysteine mutations could still fold and bind to protein X, but could not efficiently ubiquitinate protein X. Consequently, mutated EFP could prevent other E3 ligases from binding and ubiquitinating protein X, which could otherwise occur as compensation if the RING domain is deleted.
A negative regulatory relationship between KLF5 and estrogen-ER signaling
In our previous study, we found that KLF5 inhibits estrogen-induced gene transcription and cell proliferation [25]. In this study we found that estrogen negatively regulates KLF5 in two ways. Firstly, estrogen promptly induces one of the E3 ligases, EFP, which causes protein degradation of KLF5, although the mechanism of the degradation is unclear. Secondly, estrogen gradually down-regulates the transcription of KLF5, which takes a longer time to occur. Taken together, from our previous and current studies it appears that KLF5 and estrogen signaling regulate each other through a feedback mechanism (Fig. 7). As the presence of more KLF5 helps inhibit the function of estrogen by interacting with ER, control in the level of KLF5 is achieved by the up-regulation of EFP, which immediately downregulates the protein level of KLF5 by protein degradation. The estrogen-ER signaling also gradually downregulates the RNA level of KLF5 for a more extensive control of KLF5 (Fig. 7).
Figure 7. Feedback regulation of KLF5 and estrogen signaling in ER positive breast cancer cells.
Based on results from this study and a previous study [25], we propose that wherein KLF5 inhibits estrogen-induced cell proliferation and gene regulation through protein interaction with ER and thus disrupts the functional effect of estrogen [25], estrogen-ER signaling inhibits KLF5 function by immediate protein degradation through the induction of the EFP E3 ligase and gradual downregulation of KLF5 transcription to accomplish tight control in the overall expression of KLF5.
EFP is predominantly expressed in estrogen responsive tissues including mammary glands, uteri, and osteoblasts [44-46]. As an E3 ligase, EFP has been shown to ubiquitinate and degrade a number of proteins including DDX58/RIGI [47], ERα [43], 14-3-3 sigma [29], thus playing a critical role in the regulation of various pathways. Functional and expression studies suggest an oncogenic role of EFP in human cancer [26, 29]. KLF5, on the other hand, has been suggested as a tumor suppressor because it has frequent genomic deletion in breast cancer, its re-expression inhibits the proliferation of breast cancer cells [19, 48], and it participates in the inhibitory function of the TGF-β tumor suppressor in cell proliferation [8, 9, 14]. It also appears to interact with estrogen receptor beta to suppress tumor growth [49]. While the KLF5-EFP interaction could play a role in the structure and function of normal mammary tissue, interruption of this interaction could contribute to mammary tumorigenesis.
In summary, we found that the estrogen-ER signal regulates KLF5 at both the RNA and protein level. This dual regulation is accomplished at an early stage by increasing the EFP E3 ligase level, which mediates the degradation of KLF5 protein, and at a late stage by decreasing the RNA transcript level. We found that the estrogen-ER signal caused the degradation of KLF5 in ER-positive MCF-7 breast cancer cells through the proteasome machinery. These results suggest that the estrogen-ER signal negatively regulates the KLF5 transcription factor, and EFP-mediated proteasome degradation is one of the mechanisms for the negative regulation of KLF5 in breast epithelial cells.
Supplementary Material
Acknowledgments
FUNDING
This work was supported by the National Institute of Health/National Cancer Institute through grant R01 CA087921 and grant from Shanghai Municipal Education Commission (11ZZ106).
Abbreviations
- EFP
estrogen-responsive finger protein
- KLF5
Krüppel-like factor 5
- CHX
cycloheximide
- siRNA
short interfering RNA
- PDGF-α
platelet-derived growth factor-α
- TGF-β
transforming growth factor β
- UPP
ubiquitin proteasome pathway
Footnotes
AUTHOR CONTRIBUTION
Ke-Wen Zhao, Deepa Sikriwal, Xueyuan Dong, Peng Guo, and Xiaodong Sun performed the experiments, analyzed and interpreted the data. Ke-Wen Zhao, Deepa Sikriwal and Jin-Tang Dong designed the research project, analyzed data, and drafted and finalized the manuscript and figures.
REFERENCES
- 1.Wieschaus E, Nusslein-Volhard C, Kluding H. Kruppel, a gene whose activity is required early in the zygotic genome for normal embryonic segmentation. Dev. Biol. 1984;104:172–186. doi: 10.1016/0012-1606(84)90046-0. [DOI] [PubMed] [Google Scholar]
- 2.Sogawa K, Imataka H, Yamasaki Y, Kusume H, Abe H, Fujii-Kuriyama Y. cDNA cloning and transcriptional properties of a novel GC box-binding protein. BTEB2. Nucleic Acids Res. 1993;21:1527–1532. doi: 10.1093/nar/21.7.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aizawa K, Suzuki T, Kada N, Ishihara A, Kawai-Kowase K, Matsumura T, Sasaki K, Munemasa Y, Manabe I, Kurabayashi M, Collins T, Nagai R. Regulation of platelet-derived growth factor-A chain by Kruppel-like factor 5: new pathway of cooperative activation with nuclear factor-kappaB. J. Biol. Chem. 2004;279:70–76. doi: 10.1074/jbc.M306621200. [DOI] [PubMed] [Google Scholar]
- 4.Oishi Y, Manabe I, Tobe K, Tsushima K, Shindo T, Fujiu K. Krüppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. 2005;1:27–39. doi: 10.1016/j.cmet.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Chanchevalap S, Nandan MO, McConnell BB, Charrier L, Merlin D, Katz JP, Yang VW. Kruppel-like factor 5 is an important mediator for lipopolysaccharide-induced proinflammatory response in intestinal epithelial cells. Nucleic Acids Res. 2006;34:1216–1223. doi: 10.1093/nar/gkl014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C, Benjamin MS, Sun X, Otto KB, Guo P, Dong XY, Bao Y, Zhou Z, Cheng X, Simons JW, Dong JT. KLF5 promotes cell proliferation and tumorigenesis through gene regulation in the TSU-Pr1 human bladder cancer cell line. Int. J. Cancer. 2006;118:1346–1355. doi: 10.1002/ijc.21533. [DOI] [PubMed] [Google Scholar]
- 7.Bateman NW, Tan D, Pestell RG, Black JD, Black AR. Intestinal tumor progression is associated with altered function of KLF5. J. Biol. Chem. 2004;279:12093–12101. doi: 10.1074/jbc.M311532200. [DOI] [PubMed] [Google Scholar]
- 8.Guo P, Zhao KW, Dong XY, Sun X, Dong JT. Acetylation of KLF5 alters the assembly of P15 transcription factors in TGFbeta-mediated induction in epithelial cells. J. Biol. Chem. 2009;284:18184–18193. doi: 10.1074/jbc.M109.007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo P, Dong XY, Zhao KW, Sun X, Li Q, Dong JT. Opposing effects of KLF5 on the transcription of Myc in epithelial proliferation in the context of TGFbeta. J. Biol. Chem. 2009;284:28243–28252. doi: 10.1074/jbc.M109.036160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun R, Chen X, Yang VW. Intestinal-enriched kruppel-like factor (kruppel-like factor 5) is a positive regulator of cellular proliferation. J. Biol. Chem. 2001;276:6897–6900. doi: 10.1074/jbc.C000870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adam PJ, Regan CP, Hautmann MB, Owens GK. Positive- and negative-acting Kruppel-like transcription factors bind a transforming growth factor beta control element required for expression of the smooth muscle cell differentiation marker SM22alpha in vivo. J. Biol. Chem. 2000;275:37798–37806. doi: 10.1074/jbc.M006323200. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Goldstein BG, Chao HH, Katz JP. KLF4 and KLF5 Regulate Proliferation, Apoptosis and Invasion in Esophageal Cancer Cells. Cancer Biol. Ther. 2005;4:1216–1221. doi: 10.4161/cbt.4.11.2090. [DOI] [PubMed] [Google Scholar]
- 13.Dong JT, Chen C. Essential role of KLF5 transcription factor in cell proliferation and differentiation and its implications for human diseases. Cell. Mol. Life Sci. 2009;66:2691–2706. doi: 10.1007/s00018-009-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo P, Dong XY, Zhang X, Zhao KW, Sun X, Li Q, Dong JT. Pro-proliferative factor KLF5 becomes anti-proliferative in epithelial homeostasis upon signaling-mediated modification. J. Biol. Chem. 2009;284:6071–6078. doi: 10.1074/jbc.M806270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sur I, Rozell B, Jaks V, Bergstrom A, Toftgard R. Epidermal and craniofacial defects in mice overexpressing Klf5 in the basal layer of the epidermis. J. Cell Sci. 2006;119:3593–3601. doi: 10.1242/jcs.03070. [DOI] [PubMed] [Google Scholar]
- 16.Nandan MO, McConnell BB, Ghaleb AM, Bialkowska AB, Sheng H, Shao J, Babbin BA, Robine S, Yang VW. Kruppel-like factor 5 mediates cellular transformation during oncogenic KRAS-induced intestinal tumorigenesis. Gastroenterology. 2008;134:120–130. doi: 10.1053/j.gastro.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McConnell BB, Klapproth JM, Sasaki M, Nandan MO, Yang VW. Kruppel-like factor 5 mediates transmissible murine colonic hyperplasia caused by Citrobacter rodentium infection. Gastroenterology. 2008;134:1007–1016. doi: 10.1053/j.gastro.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein BG, Chao HH, Yang Y, Yermolina YA, Tobias JW, Katz JP. Overexpression of Kruppel-like factor 5 in esophageal epithelia in vivo leads to increased proliferation in basal but not suprabasal cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G1784–1792. doi: 10.1152/ajpgi.00541.2006. [DOI] [PubMed] [Google Scholar]
- 19.Chen C, Bhalala HV, Qiao H, Dong JT. A possible tumor suppressor role of the KLF5 transcription factor in human breast cancer. Oncogene. 2002;21:6567–6572. doi: 10.1038/sj.onc.1205817. [DOI] [PubMed] [Google Scholar]
- 20.Chen C, Bhalala HV, Vessella RL, Dong JT. KLF5 is frequently deleted and down-regulated but rarely mutated in prostate cancer. Prostate. 2003;55:81–88. doi: 10.1002/pros.10205. [DOI] [PubMed] [Google Scholar]
- 21.Chanchevalap S, Nandan MO, Merlin D, Yang VW. All-trans retinoic acid inhibits proliferation of intestinal epithelial cells by inhibiting expression of the gene encoding Kruppel-like factor 5. FEBS Lett. 2004;578:99–105. doi: 10.1016/j.febslet.2004.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen C, Sun X, Guo P, Dong XY, Sethi P, Cheng X, Zhou J, Ling J, Simons JW, Lingrel JB, Dong JT. Human Kruppel-like factor 5 is a target of the E3 ubiquitin ligase WWP1 for proteolysis in epithelial cells. J. Biol. Chem. 2005;280:41553–41561. doi: 10.1074/jbc.M506183200. [DOI] [PubMed] [Google Scholar]
- 23.Chen C, Sun X, Guo P, Dong XY, Sethi P, Zhou W, Zhou Z, Petros J, Frierson HF, Vessella RL, Atfi A, Dong JT. Ubiquitin E3 ligase WWP1 as an oncogenic factor in human prostate cancer. Oncogene. 2007;26:2386–2394. doi: 10.1038/sj.onc.1210021. [DOI] [PubMed] [Google Scholar]
- 24.Tong D, Czerwenka K, Heinze G, Ryffel M, Schuster E, Witt A, Leodolter S, Zeillinger R. Expression of KLF5 is a prognostic factor for disease-free survival and overall survival in patients with breast cancer. Clin. Cancer Res. 2006;12:2442–2448. doi: 10.1158/1078-0432.CCR-05-0964. [DOI] [PubMed] [Google Scholar]
- 25.Guo P, Dong XY, Zhao KW, Sun X, Li Q, Dong JT. Estrogen-induced interaction between KLF5 and estrogen receptor (ER) suppresses the function of ER in ER-positive breast cancer cells. Int. J. Cancer. 2010;126:81–89. doi: 10.1002/ijc.24696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeda K, Orimo A, Higashi Y, Muramatsu M, Inoue S. Efp as a primary estrogen-responsive gene in human breast cancer. FEBS Lett. 2000;472:9–13. doi: 10.1016/s0014-5793(00)01421-6. [DOI] [PubMed] [Google Scholar]
- 27.Zou W, Zhang DE. The interferon-inducible ubiquitin-protein isopeptide ligase (E3) EFP also functions as an ISG15 E3 ligase. J. Biol. Chem. 2006;281:3989–3994. doi: 10.1074/jbc.M510787200. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki T, Urano T, Tsukui T, Horie-Inoue K, Moriya T, Ishida T, Muramatsu M, Ouchi Y, Sasano H, Inoue S. Estrogen-responsive finger protein as a new potential biomarker for breast cancer. Clin. Cancer Res. 2005;11:6148–6154. doi: 10.1158/1078-0432.CCR-05-0040. [DOI] [PubMed] [Google Scholar]
- 29.Urano T, Saito T, Tsukui T, Fujita M, Hosoi T, Muramatsu M, Ouchi Y, Inoue S. Efp targets 14-3-3 sigma for proteolysis and promotes breast tumour growth. Nature. 2002;417:871–875. doi: 10.1038/nature00826. [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi T, Inoue S, Yokosawa H. Identification and Herc5-mediated ISGylation of novel target proteins. Biochem. Biophys. Res. Commun. 2006;348:473–477. doi: 10.1016/j.bbrc.2006.07.076. [DOI] [PubMed] [Google Scholar]
- 31.Zou W, Wang J, Zhang DE. Negative regulation of ISG15 E3 ligase EFP through its autoISGylation. Biochem. Biophys. Res. Commun. 2007;354:321–327. doi: 10.1016/j.bbrc.2006.12.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen C, Zhou Z, Guo P, Dong JT. Proteasomal degradation of the KLF5 transcription factor through a ubiquitin-independent pathway. FEBS Lett. 2007;581:1124–1130. doi: 10.1016/j.febslet.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 33.Chen C, Sun X, Ran Q, Wilkinson KD, Murphy TJ, Simons JW, Dong JT. Ubiquitin-proteasome degradation of KLF5 transcription factor in cancer and untransformed epithelial cells. Oncogene. 2005;24:3319–3327. doi: 10.1038/sj.onc.1208497. [DOI] [PubMed] [Google Scholar]
- 34.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng HQ, Zhou Z, Huang J, Chaudhury L, Dong JT, Chen C. Kruppel-like factor 5 promotes breast cell proliferation partially through upregulating the transcription of fibroblast growth factor binding protein 1. Oncogene. 2009;28:3702–3713. doi: 10.1038/onc.2009.235. [DOI] [PubMed] [Google Scholar]
- 36.Chen C, Zhou Y, Zhou Z, Sun X, Otto KB, Uht RM, Dong JT. Regulation of KLF5 involves the Sp1 transcription factor in human epithelial cells. Gene. 2004;330:133–142. doi: 10.1016/j.gene.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 37.Zhao D, Zheng HQ, Zhou Z, Chen C. The Fbw7 tumor suppressor targets KLF5 for ubiquitin-mediated degradation and suppresses breast cell proliferation. Cancer Res. 2010;70:4728–4738. doi: 10.1158/0008-5472.CAN-10-0040. [DOI] [PubMed] [Google Scholar]
- 38.Liu N, Li H, Li S, Shen M, Xiao N, Chen Y, Wang Y, Wang W, Wang R, Wang Q, Sun J, Wang P. The Fbw7/human CDC4 tumor suppressor targets proproliferative factor KLF5 for ubiquitination and degradation through multiple phosphodegron motifs. J. Biol. Chem. 2010;285:18858–18867. doi: 10.1074/jbc.M109.099440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inoue S, Orimo A, Hosoi T, Kondo S, Toyoshima H, Kondo T, Ikegami A, Ouchi Y, Orimo H, Muramatsu M. Genomic binding-site cloning reveals an estrogen-responsive gene that encodes a RING finger protein. Proc. Natl. Acad. Sci. U. S. A. 1993;90:11117–11121. doi: 10.1073/pnas.90.23.11117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annu. Rev. Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 41.Rechsteiner M, Hill CP. Mobilizing the proteolytic machine: cell biological roles of proteasome activators and inhibitors. Trends Cell Biol. 2005;15:27–33. doi: 10.1016/j.tcb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 42.Jariel-Encontre I, Bossis G, Piechaczyk M. Ubiquitin-independent degradation of proteins by the proteasome. Biochim. Biophys. Acta. 2008;1786:153–177. doi: 10.1016/j.bbcan.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Nakajima A, Maruyama S, Bohgaki M, Miyajima N, Tsukiyama T, Sakuragi N, Hatakeyama S. Ligand-dependent transcription of estrogen receptor alpha is mediated by the ubiquitin ligase EFP. Biochem. Biophys. Res. Commun. 2007;357:245–251. doi: 10.1016/j.bbrc.2007.03.134. [DOI] [PubMed] [Google Scholar]
- 44.Orimo A, Inoue S, Ikeda K, Noji S, Muramatsu M. Molecular cloning, structure, and expression of mouse estrogen-responsive finger protein Efp. Co-localization with estrogen receptor mRNA in target organs. J. Biol. Chem. 1995;270:24406–24413. doi: 10.1074/jbc.270.41.24406. [DOI] [PubMed] [Google Scholar]
- 45.Inoue S, Urano T, Ogawa S, Saito T, Orimo A, Hosoi T, Ouchi Y, Muramatsu M. Molecular cloning of rat efp: expression and regulation in primary osteoblasts. Biochem. Biophys. Res. Commun. 1999;261:412–418. doi: 10.1006/bbrc.1999.0874. [DOI] [PubMed] [Google Scholar]
- 46.Das N, Wang J, Dey SK. Uterine preparation for implantation in the mouse is associated with coordinate expression of estrogen-responsive finger protein and estrogen receptor. Mol. Reprod. Dev. 1997;46:499–506. doi: 10.1002/(SICI)1098-2795(199704)46:4<499::AID-MRD8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 47.Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L, Takeuchi O, Akira S, Chen Z, Inoue S, Jung JU. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- 48.Knuutila S, Aalto Y, Autio K, Bjorkqvist AM, El-Rifai W, Hemmer S, Huhta T, Kettunen E, Kiuru-Kuhlefelt S, Larramendy ML, Lushnikova T, Monni O, Pere H, Tapper J, Tarkkanen M, Varis A, Wasenius VM, Wolf M, Zhu Y. DNA copy number losses in human neoplasms. Am. J. Pathol. 1999;155:683–694. doi: 10.1016/S0002-9440(10)65166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakajima Y, Akaogi K, Suzuki T, Osakabe A, Yamaguchi C, Sunahara N, Ishida J, Kako K, Ogawa S, Fujimura T, Homma Y, Fukamizu A, Murayama A, Kimura K, Inoue S, Yanagisawa J. Estrogen Regulates Tumor Growth Through a Nonclassical Pathway that Includes the Transcription Factors ER{beta} and KLF5. Sci Signal. 2011;4:ra22. doi: 10.1126/scisignal.2001551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.