Abstract
Over-expression of ABCG2 is linked to multidrug resistance in cancer chemotherapy. We have previously shown that functionalized aurones effectively reduced the efflux of pheophorbide A (an ABCG2 substrate) from ABCG2 over-expressing MDA-MB-231/R (“R”) cells. In the present report, we investigated the functional relevance of this observation and the mechanisms by which it occurs. Aurones and related analogs were investigated for re-sensitization of R cells to mitoxantrone (MX, a chemotherapeutic substrate of ABCG2) in cell-based assays, accumulation of intracellular MX by cell cytometry, interaction with ABCG2 by biochemical assays and in vivo efficacy in MX resistant nude mice xenografts. We found that methoxylated aurones interacted directly with ABCG2 to inhibit efflux activity, possibly by competing for occupancy of one of the substrate binding sites on ABCG2. The present evidence suggests that they are not transported by ABCG2 although they stimulate ABCG2-ATPase activity. Alteration of ABCG2 protein expression was also discounted. One member was found to re-sensitize R cells to MX in both in vitro and in vivo settings. Our study identified methoxylated aurones as promising compounds associated with low toxicities and potent modulatory effects on the ABCG2 efflux protein. Thus, they warrant further scrutiny as lead templates for development as reversal agents of multidrug resistance.
Keywords: ABCG2, ABC transporter, multidrug resistance, functionalized aurones, reversing agents
1. Introduction
Multidrug resistance (MDR) is a phenomenon associated with the resistance of tumor cells to the cytostatic or cytotoxic actions of structurally dissimilar and functionally divergent drugs commonly used in cancer chemotherapy [1, 2]. The most common mechanism by which cancer cells acquire resistance is through the increased expression and activity of ATP-binding cassette (ABC) transport proteins. These transporters utilize the energy of cellular ATP to actively efflux anti-cancer drugs out of cells, hence limiting the intracellular accumulation of these agents to levels that are inadequate for therapeutic effect [3]. ABC transporters that play key roles in the development of MDR are ABCB1 (P-glycoprotein, P-gp), ABCC1 (multidrug resistant protein 1, MRP1) and ABCG2 (breast cancer resistant protein BCRP) [3, 4, 5]. Of these MDR transporters, ABCG2 is the most recent to be described [6, 7, 8]. MDR-linked ABC transporters display broad substrate specificity, consistent with their behavior as multidrug efflux pumps. Thus, they confer resistance to clinically relevant anticancer drugs such as anthracyclins, vinca alkaloids, methotrexate and mitoxantrone. The efflux activities of ABCB1 and ABCG2 have been recognized by the International Transporter Consortium to contribute significantly towards clinically relevant drug-drug interactions by affecting absorption and disposition of drugs [9].
Various strategies have been proposed to combat ABC drug transporter-mediated MDR in cancer cells [10]. The most common approach is to employ specific and high affinity inhibitors to block the efflux activity of transporters at the cell surface. Other less explored alternatives are to inhibit transcription factors involved in modulating transporter gene expression / amplification or to inhibit signaling pathways that control the over-expression of these transporters. Given the complexity and multi-factorial nature of MDR, modulators that simultaneously inhibit transporter function at the cell surface and at the signal transduction / gene expression level, may stand a better chance of success [10]. However, until these approaches have been adequately explored and validated, the reliance on small molecule transport modulators/inhibitors to address the problem of MDR will remain. In this regard, natural products and their synthetic derivatives have been a fruitful source of novel target molecules. For example, the mycotoxin, fumitremorgin C (FTC) was the first ABCG2 inhibitor to be reported [11], and its synthetic analogue Ko 143 has been shown to be more potent and less toxic [12]. Curcumin derived from tumeric powder and various curcuminoids like tetrahydrocurcumin have been characterized as inhibitors of ABCB1, ABCC1 and ABCG2 [13, 14, 15]. Flavonoids have likewise been found to be multi-targeting transport inhibitors [16, 17, 18, 19].
In an earlier report, we showed that a group of lesser known flavonoids called aurones (2-benzylidenebenzofuran-3(2H)-ones), re-sensitized ABCG2-overexpressing cancer cells to mitoxantrone (an anticancer agent and ABCG2 substrate) at sub-micromolar concentrations [20]. A more detailed structure-activity relationship study further confirmed the potential of the aurone template as a lead for developing potent non-toxic modulators of ABCG2 and ABCB1 [21]. Notably, we identified several methoxylated aurones and related analogs that were equipotent to FTC, an established ABCG2 inhibitor, in reducing the efflux of pheophorbide A (PhA, an ABCG2 substrate) in ABCG2 over-expressing human breast cancer cells (MDA-MB-231/R). However, there are several gaps in our understanding of how these compounds moderate ABCG2 efflux activity. Foremost is the question relating to the functional relevance of the in vitro results and whether the diminished efflux of PhA from MDA-MB-231/R cells is due to the modulation of ABCG2 efflux activity or competition with PhA for occupancy of substrate binding sites on ABCG2.
We have attempted to answer some of these questions in the present report. Selected aurones and related analogs were investigated for their ability to re-sensitize MDA-MB-231/R cells to mitoxantrone and when observed, to determine if there was a concurrent increase in the intracellular accumulation of mitoxantrone. The direct interaction of the test compound with ABCG2 was investigated on two biochemical assays, namely the ABCG2-ATPase assay and the photo-affinity labeling of ABCG2 with a transport substrate, [125I]-Iodoarylazidoprazosin. The question as to whether these compounds interacted with ABCG2 as substrates or non-substrates was addressed by comparing their differential growth inhibitory activities on ABCG2 over-expressing and parental (wild-type) MDA-MB-231 cells. To determine if the diminished efflux activity of ABCG2 involved down-regulation of protein expression, Western blot analysis of ABCG2 levels in cells incubated with one of the more potent compounds uncovered in this investigation A-2 (Figure 1) was carried out. A-2 was also administered together with mitoxantrone to mice bearing an MDA-MB-231/R-induced xenograft to determine if its in vitro ABCG2 modulatory activity could be translated to an in vivo setting. Taken together, results from the present study revealed that modulation of ABCG2 by functionalized aurones and its related structural analogs involves direct interaction with ABCG2 and aurone A-2 is identified as a potent compound for further development as a clinically useful MDR-reversal agent.
Figure 1. Chemical structures of test compounds studied in this work.
The EC50 value of each compound for Pheophorbide A accumulation in ABCG2 over-expressing MDA-MB-231/R cells is given at the bottom of the structure.
2. Methods
2.1. Cell lines and materials for biological assay
Mitoxantrone (MX), fumitremorgin C (FTC), dimethyl sulfoxide (DMSO, pharmaceutical grade), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich, St Louis, Mo (USA). Pheophorbide A (PhA) was purchased from Frontier Scientific, Logan, UT (USA). Ketamine was obtained from Parnell Laboratories Pte Ltd (Australia). Medetomidine and atipamezole were purchased from Pfizer New Zealand Ltd (Auckland, NZ). Mouse monoclonal antibody BXP-21 (against ABCG2) was acquired from Signet Laboratories, Inc. (Dedham, MA, USA) and anti-mouse secondary antibody was from Amersham Biosciences Inc. (Piscataway, NJ, USA). The breast cancer cell line MDA-MB-231, stably transfected with expression vectors for wild type 482R ABCG2 (R cells) and pcDNA3.1 (parental V cells) were kindly provided by Dr. Douglas D. Ross (Greenebaum Cancer Center, University of Maryland, Baltimore, USA). Both MDA-MB-231/V and MDA-MB-231/R cells were cultured in 75-cm2 flasks with RPMI 1640 (Invitrogen Corporation, CA, USA) culture media supplemented with 10% fetal bovine serum (Hyclone, UT, USA) at 37°C in a 5% CO2 humidified atmosphere. The culture media contained 0.1 mg/ml streptomycin sulfate and 0.1 mg/ml penicillin G (Sigma Chemical Co., St. Louis, MO, USA) and 1.0 mg/ml geneticin (Invitrogen Corporation, CA, USA). MDA-MB-231/R and V cells for in vivo testing were assessed to be pathogen-free by Laboratory Animal Centre of the National University of Singapore. Cells were sub-cultured when they reached 80-90% confluency and used within 10 passages for assays. The syntheses and purification (to at least 95% purity) of the compounds investigated in this study (Table 1) have been reported by the authors [21]. Their systematic nomenclatures are given in Supplementary Information. All other chemicals were purchased from Sigma-Aldrich, St Louis, Mo (USA).
Table 1. Effect of selected test compounds on cytotoxicity of mitoxantrone in ABCG2-expressing MDA-MD-231/R cells.
| Compound | Concentration (μM) | IC50 of mitoxantrone (μM)a | Resistance indexb |
|---|---|---|---|
| Mitoxantrone (MX) | 0.92 (0.06) | 0.92/0.07 = 13.1 | |
| MX + | 0.05 | ||
| A-2 | 0.37 (0.14)* | 5.3 | |
| A-3 | 0.52 (0.09) | 7.4 | |
| HA-1 | 1.37 (0.42) | 19.6 | |
| I-2 | 0.21 (0.07)** | 3.0 | |
| AZ-1 | 0.56 (0.19) | 8.0 | |
| C-2 | 0.21 (0.04)** | 3.0 | |
| F2 | 0.10 (0.02)*** | 1.4 | |
| FTC | 0.52 (0.05) | 7.4 | |
| MX + | 0.5 | ||
| A-2 | 0.06 (0.02)*** | 0.9 | |
| A-3 | 0.09 (0.03)*** | 1.3 | |
| HA-1 | 0.36 (0.1)*** | 5.1 | |
| I-2 | 0.02 (0.01)*** | 0.3 | |
| AZ-1 | 0.16 (0.07)*** | 2.3 | |
| C-2 | 0.07 (0.01)*** | 1.0 | |
| F-2 | 0.07 (0.03)*** | 1.0 | |
| FTC | 0.38 (0.25)*** | 5.4 | |
| MX + | 1 | ||
| A-2 | 0.05 (0.03)*** | 0.7 | |
| I-2 | 0.03 (0.02)*** | 0.4 |
Mean of three or more independent determinations. SD is given in parentheses. Significant difference (*p < 0.05, **p < 0.01, ***p < 0.001) between IC50 of MX in MDA-MB-231/R cells in the absence of test compound versus that in the presence of test compound (One-way ANOVA, Bonferroni post-hoc).
Resistance index = IC50 of MX R cells + test compound / IC50 of MX V cells. IC50 value of MX in MDA-MB-231/V cells = 0.07 (0.01) μM. A compound that totally re-sensitized R cells to MX would have a resistance index of ≤ 1.
2.2. Re-sensitization of MDA-MB-231/R cells to MX
The growth inhibitory IC50 of MX on MDA-MB-231/R cells was determined in the presence of test compound (A-2, A-3, HA-1, I-2, AZ-1, C-2, F-2) using the MTT assay [20]. Briefly, cells (104) were seeded in 96-well plates and incubated for 24 h, after which the medium (RPMI) was replaced by fresh medium containing MX and the test compound (0.05 μM, 0.5 μM or 1μM). After 72 h, the drug-containing medium was removed, cells were washed with PBS and MTT was added to each well (3h, 37°C). The MTT solution was then removed, DMSO was added to dissolve the formazan crystals and readings were made at 590 nm on a Tecan Infinite M200 microplate reader. The IC50 values of MX in both MDA-MB-231/R and MDA-MB-231/V cells were concurrently determined as controls in each experiment.
2.3. Mitoxantrone (MX) accumulation studies
The accumulation of MX was performed by flow cytometry on MDA-MB-231/R and MDA-MB-231/V cells by a previously described method [20]. The experiments were carried out on compounds A-2, A-3, DA-1, HA-1, AZ-1, I-2, F-2 and C-2 at a concentration of 5μM, with FTC (10 μM) as positive control. Briefly, cells (106cells/ml) were incubated with test compound in serum-free RPMI medium for 15 min, 37°C, followed by addition of MX (3 μM) for 30 min. Ice-cold PBS was added, cells were removed by centrifugation and the pellet resuspended in PBS for the determination of MX on the flow cytometer. Cells were excited at 488 nm and emission at 680 nm was recorded for the detection of MX fluorescence. MX levels in the treated cells were normalized to the vehicle control (0.1% DMSO) which was taken as 100%.
2.4. ATPase assay of ABCG2
The ATPase assay was carried out as described previously [22]. Briefly, crude membrane protein (10 μg crude membrane protein per 100μl) from High Five insect cells expressing ABCG2 were incubated with test compound at various concentrations in the presence and absence of beryllium fluoride (BeFx) (0.2 mmol/L beryllium sulfate and 2.5mmol/L sodium fluoride) in ATPase assay buffer (50 mM MES-Tris (pH 6.8), 50 mM KCl, 5 mM NaN3, 1 mM EGTA, 1 mM ouabain, 2 mM DTT, 10 mM MgCl2) for 3-5 min at 37°C. The reaction was initiated by the addition of 5 mM ATP. After 20 min at 37°C, 2.5% SDS solution was added to terminate the reaction. The release of inorganic phosphate was quantified by a colorimetric reaction. ABCG2-specific ATPase activity was recorded as BeFx-sensitive ATPase activity [23, 24].
2.5. Photoaffinity labeling of ABCG2 with [125I]-Iodoarylazidoprazosin ([125 I]-IAAP)
The photoaffinity labeling of ABCG2 with [125 I]-IAAP was carried out as described previously [22, 23]. Briefly, crude membranes (50μg/ml) from MCF-7 FLV1000 cells were incubated with 5μM of test compound or FTC for 10 minutes at room temperature in 50 mM Tris-HCl (pH 7.5), after which was added 3-6 nM [125I] IAAP (2,200 Ci/mmol, Perkin Elmer Life Sciences, Wellesley, MA). The samples were incubated for an additional 5 min under subdued light, then exposed to ultravolet (365nm) light for 10 min at room temperature. The labeled ABCG2 was immunoprecipitated using the BXP-21 antibody and processed as described previously [23]. The radioactivity in the ABCG2 band was quantified using the STORM 860 PhosphorImager system (Molecular Dynamics, Sunnyvale, CA) and ImageQuaNT software (Molecular Dynamics).
2.6. Western blot analysis
Compound A-2 was incubated at 0.5μM or 1μM with MDA-MB-231/R cells for 72 hours following the method described in Section 2.2. MDA-MB-231/R cells were also separately exposed to MX (0.1 μM) alone and a combination of A-2 (0.5 μM or 1 μM) and MX (0.1μM). MDA-MB-231/V cells were similarly exposed to A-2 (0.5 μM or 1 μM), MX (0.01μM) and a combination of A-2 (0.5 μM or 1 μM) and MX (0.01 μM). 0.1 μM and 0.01 μM of MX were chosen as test concentrations on R cells and V cells respectively so as not to affect the viability of the cells. These concentrations were 9-fold and 7-fold lower than the IC50 of MX on R cells (0.92 ± 0.06 μM) and V cells (0.07 ± 0.01 μM) respectively. After 72 hours, the cells were trypsinized and lysates were subjected to electrophoresis on 7.5% SDS-PAGE and transferred to nitrocellulose membranes for overnight blocking. The blots were probed with anti-ABCG2 (BXP-21), followed by horseradish peroxidase-conjugated anti-mouse secondary antibody as described previously [20].
2.7. Cell Cytotoxicity by MTT assay
The cytotoxicity profiles of the test compounds (Figure 1) were determined by the MTT assay as described in Section 2.2. Stock solutions of test compounds were prepared in DMSO and diluted with media to give concentrations of 5 μM and 10 μM for investigations on MDA-MB-231/V and R cells. The final concentration of DMSO in the well was kept at 1% v/v. Cell survival was determined as a % of control cells (cells in media containing 1% DMSO) and at least two determinations were made for each concentration of test compound.
2.8. In vivo studies
Experimental protocols were approved by the National University of Singapore Institutional Animal Care and Use Committee [IACUC 106/09(A) 09] and were in accordance to the National Advisory Committee for Laboratory Animal Research guidelines for the care and use of animals for scientific purposes. Balb/c female athymic nude mice (18 to 20g body weight, 8 weeks old) were obtained from the Biological Resource Centre (Singapore). Animals were kept under controlled environmental conditions (19-26°C, relative humidity < 70%, 12 h dark-light cycle) with free access to water and standard feed.
To establish the tumor xenograft, the mice were sedated by an intraperitoneal (IP) injection of an anaesthetic cocktail (0.1 ml/10g) comprising ketamine (0.75 ml of 100 mg/ml stock solution) and medetomidine (1 ml of 1 mg/ml stock solution) diluted to 10 ml with 0.9% saline. Once sedated, MDA-MB-231/V or MDA-MB-231/R cells (15 million cells per ml of RPMI-1640 supplemented with 10% fetal bovine serum, 0.1mg/ml streptomycin sulfate and penicillin G, and 1.0mg/ml geneticin) at a density of 15 million cells/ml were injected orthotopically into one of the mammary fat pads of the animal. To reverse the sedation, the animal was injected IP with the reversal agent atipamezole (0.1 ml of 5 mg/ml stock solution in 9.9ml of 0.9% saline) in a volume equivalent to the amount of anaesthetic used.
Treatment was started 13 to 18 days post injection of tumor cells when the animals developed palpable mammary tumors of 5 to 9 mm diameter at the injection site. The mice bearing R or V xenografts were randomized into their treatment groups, with at least 4 mice in each treatment group. For animals in the R group, the treatment groups were animals receiving (i) vehicle control (1% v/v DMSO in saline); (ii) test compound A-2 (0.2 mg/kg, equivalent to 10 μM A-2); (iii) MX (4mg/kg); and (iv) a combination of A-2 (0.2 mg/kg) and MX (4 mg/kg). Similar treatment groups were extended to animals bearing the V xenografts. All drug solutions were freshly prepared prior to injection and sterilized by filtration using a 0.20 micron size DMSO-safe Acrodisc syringe filter. The final concentration of DMSO in these solutions was ≤1% (v/v). The solutions were administered intratumorally in a volume of 0.1ml. Only one administration was made throughout the entire study period.
Mice were closely monitored, weighed and tumor size was measured at least 3 times weekly. For tumor size measurement, length (L= the longer diameter) and width (W= the shorter diameter) of the tumor were measured with electronic vernier calipers and reported up to 2 decimal places. Tumor volume (V) was calculated as V = L × (W2/2). Survival (in days) of mice in the different treatment groups were monitored throughout the period of study. Mice were euthanized according the criteria of the IACUC-approved treatment protocol, namely when they were observed to have (i) tumor size > 1.5 cm in diameter; (ii) ulcerated, infected or inflamed tumor; (iii) ruffled fur, hunched back appearance or inappetent state; (iv) 10% or more loss of body weight over 24 hours, or 20% or more loss of body weight compared to control group or (v) be in a moribund or pre-moribund state. Mice that did not meet the criteria for euthanasia were sacrificed 60 days post tumor inoculation.
The Kaplan-Meier survival analysis required the identification of censored events. These were events in which the animal (i) remained alive after 60 days post tumor inoculation, (ii) died due to unrelated causes such as sudden death after tumor inoculation, (iii) put down due to other conditions cited for euthanasia unrelated to tumor size > 1.5 cm in diameter and (iv) developed tumors that deviated from an ellipsoidal shape such as secondary growth next to the original tumor.
2.9. Statistical analysis
Data were analyzed for statistical significance using parametric one-way ANOVA followed by a Bonferroni or Dunnett's post-hoc test (2-sided). Non-parametric Spearman correlation analysis was employed for some data sets. In vivo data were analyzed by Kaplan-Meier survival analysis with log rank test to assess significance [25]. All analyses were carried out on SPSS version 15.0 for Windows (Chicago, IL) and p values < 0.05 were considered significant.
3. Results
3.1. Test compounds investigated in this report
Figure 1 lists the structures of compounds investigated in this report. They comprise methoxylated aurones (A-1, A-2, A-3), aurone analogs (unsubstituted aurone UA-1, hydroxylated aurone HA-1, dehydroaurone DA-1, isoaurone IA-1, azaaurone AZ-1), indanones (I-1, I-2, I-3), flavones (F-1, F-2, F-3) and chalcones (C-1, C-2, C-3). Included in Figure 1 are their previously reported EC50 values for increasing PhA content in ABCG2 over-expressing MDA-MB-231/R cells [21]. It can be seen that several compounds (A-2, A-3, I-2, I-3, F-2, F-3) were as potent as FTC (EC50 PhA: 1.3 μM) in this respect. The remaining compounds were less potent and of these, a handful (A-1, I-1, F-1, C-2, C-3) had determinable EC50 values while the rest had negligible activity even when tested at high concentrations (> 30 μM).
3.2. A-2 and other potent inhibitors of PhA efflux re-sensitized ABCG2-overexpressing MDA-MB-231 /R cells to MX
Having shown that several test compounds were as potent as FTC in reducing the efflux of PhA from ABCG2 over expressing MDA-MB-231/R cells (“R cells”), we proceeded to assess the functional relevance of these findings by determining the ability of these compounds to re-sensitize R cells to the cytotoxic effects of the ABCG2 substrate and anticancer agent, mitoxantrone (MX).
The growth inhibitory IC50 of MX on parental MDA-MB-231/V cells (“V cells”) which have constitutive levels of ABCG2 was 0.07 μM. On the ABCG2 over-expressing R cells, IC50 of MX was increased 13-fold to 0.92 μM (Table 1). This reduction in susceptibility (described here as “resistance index”) is ascribed to the higher levels of ABCG2 in the R cells, leading to efflux of MX from the cells and hence the need for a higher concentration to affect cell viability.
When R cells were treated with FTC, they became more susceptible to the growth inhibitory effects of MX. Thus, the IC50 of MX on R cells was reduced to 0.52 μM in the presence of 0.05 μM FTC, and was further decreased to 0.38 μM when 0.5 μM FTC was present.
Here, we tested seven compounds for re-sensitization of R cells to MX. They are A-2, A-3, I-2, F-2 (equipotent to FTC in terms of EC50 PhA) and HA-1, AZ-1, C-2 (less potent than FTC). At 0.05 μM, four out of seven compounds significantly reduced the IC50 of MX. When tested at a higher concentration of 0.5 μM, all of the seven test compounds were shown to significantly reduce the IC50 of MX in R cells. Notably, at this concentration, A-2, A-3, I-2, C-2 and F-2 (but not the less potent analogs HA-1 and AZ-1) fully restored MX sensitivity of R cells to the same level as that for V cells (resistance indices ≤ 1.0). In comparison, FTC at 0.5 μM could not fully restore sensitivity of R cells to MX.
It was noted that the methoxylated indanone I-2 at 0.5 μM significantly enhanced MX sensitivity of R cells, to the extent that these cells were now 3 times more sensitive to MX than the parental V cells (resistance index 0.3). To determine if I-2 could further lower the IC50 of MX on R cells, it was tested at a higher concentration of 1 μM. As seen from Table 1, no further increase in sensitivity was observed, and this was also true for another potent re-sensitizer A-2. Thus, there appears to be a limit to which I-2 and A-2 could re-sensitize R cells to the growth inhibitory effects of MX.
3.3. A-2 and other potent inhibitors of PhA efflux increase MX accumulation in ABCG2-over-expressing MDA-MB-231 /R cells
To determine if the re-sensitization of R cells to MX in the presence of the test compound (Section 3.2) is accompanied by an increase in the concentration of MX in the treated cells, we measured the accumulation of fluorescent MX in R cells exposed to test compound (5 μM) and MX. The results are presented in Figure 2. It is seen that all the compounds that re-sensitized R cells to MX (Section 3.2) significantly increased the content of MX in the R cells compared to the untreated control. The most potent compounds (I-2, A-2, A-3, AZ-1) increased MX content by more than 3-fold. Only the dehydroaurone DA-1, which did not reduce PhA efflux from R cells (EC50 PhA > 30 μM) [21], failed to significantly increase MX concentration in the treated R cells. We did not find significant differences in the levels of accumulated MX in cells treated with FTC or test compound (with the exception of DA-1) but it should be noted that FTC was tested at a 2-fold higher concentration (10 μM) than the other compounds. It is likely that if FTC is tested at a lower than 5 μM concentration, it may not inhibit the activity completely. We also investigated the effects of the test compounds (5 μM) on MX accumulation in parental V cells under similar experimental conditions. Here, we found no significant difference in the levels of MX accumulated in treated and untreated V cells (Supplementary Information), thus corroborating a role for the inhibition of ABCG2-mediated efflux of MX from R cells by the test compounds.
Figure 2. Accumulation of mitoxantrone in MDA-MB-231/R cells in the absence or presence of 5 μM test compounds.
MDA-MB-231/R cells (106cells/ml) were incubated in the absence (control) or presence of 5μM test compound in serum-free RPMI medium for 15 min, 37°C, followed by addition of MX (3 μM) for 30 min. The cells were then washed with ice-cold PBS and resuspended in PBS for the determination of MX on the flow cytometer as described in Methods. 10μM FTC was used a positive control. The MX accumulation in MDA-MB-231/R cells was calculated as a percentage of control (0.1% DMSO) as described previously (Sim et al., 2008). Data points are expressed as mean and error bars represent SD for n = 3 - 4 independent determinations. Statistical difference (*p < 0.05) between MX accumulation in control and treated groups were analyzed using one-way ANOVA analysis followed by Dunnett post-hoc test.
3.4. A-2 and other potent inhibitors of PhA efflux stimulate ABCG2-ATPase activity
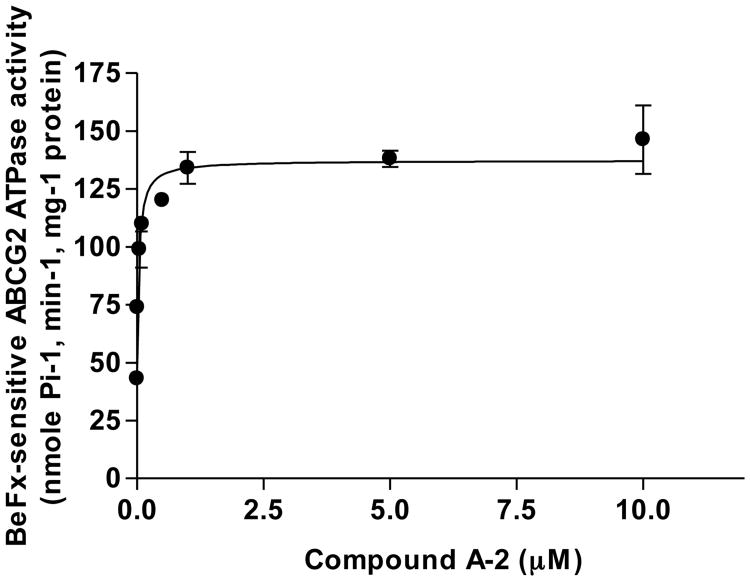
Next, the compounds listed in Table 1 were examined for their effects on ABCG2-mediated ATP hydrolysis in membranes isolated from High Five insect cells that over-express ABCG2. All the compounds stimulated the beryllium fluoride-sensitive basal ABCG2-ATPase activity in a concentration dependent manner (Supplementary Information). Figure 3 is a representative plot obtained with A-2. A-2 increased ABCG2-ATPase activity by approximately 3-fold and at 37 nM, increased ATPase activity to 50% of the maximum level (Table 2). Other potent stimulators of ATPase activity were A-3, I-3 and F-2, which like A-2 stimulated basal ATPase activity by ca 3-fold and required nanomolar concentrations for half-maximal stimulation of ATPase activity. The least potent compounds were the dehydroaurone DA-1 and flavone F-3, both of which increased basal ATPase activity by less than 2-fold and brought about half-maximum stimulation of ATPase activity at micromolar concentrations. The poor activity of F-3 was traced to its poor solubility. The remaining compounds had moderate affinities, stimulating ATPase activities to 50% of maximum levels at < 1 μM.
Figure 3. The effect of compound A-2 on the ATPase activity of ABCG2.
The ABCG2-expressing High-Five cells crude membrane proteins (10 μg membrane protein/100 μl) was incubated with increasing concentrations of A-2 in the absence or presence of beryllium fluoride in ATPase assay buffer for 3-5 min at 37°C. The reaction was initiated by the addition of 5 mM ATP. After 20 min at 37°C, 2.5% SDS solution was added to terminate the reaction. ABCG2-specific ATPase activity was recorded as BeFx-sensitive ATPase activity as described previously (Shukla et al., 2006; Shukla et al., 2009). The concentration required for 50% stimulation with A-2 was 0.037 ± 0.013 μM (SD, n = 3 independent determinations).
Table 2.
Comparison of concentrations of test compounds required for half-maximal stimulation of ABCG2 ATPase activity in High-Five insect cell crude membranes and EC50 PhA values for pheophorbide A accumulation in MDA-MB-231/R cells
| Compound | Concentration required for half-maximal stimulation of ATPase activity (μM)a | ATPase activity (fold stimulation)b | EC50 PhA (μM)c |
|---|---|---|---|
| A-1 | 0.14(0.055) | 3.4 | 6.72 (0.53) |
| A-2 | 0.037 (0.013) | 3.4 | 0.91 (0.06) |
| A-3 | 0.048 (0.021) | 3.1 | 1.07 (0.07) |
| DA-1 | 1.1 (0.19) | 2.5 | >50 |
| UA-1 | 0.19 (0.076) | 2.8 | >30 |
| IA-1 | 0.20 (0.062) | 3.0 | >30 |
| HA-1 | 0.37 (0.084) | 3.0 | >30 |
| AZ-1 | 0.37 (0.083) | 2.8 | >30 |
| I-1 | 0.31 (0.13) | 3.0 | 9.10 (0.55) |
| I-2 | 0.22 (0.11) | 2.9 | 2.06 (0.10) |
| I-3 | 0.043 (0.014) | 3.2 | 1.17 (0.07) |
| F-1 | 0.16 (0.025) | 2.8 | 13.45 (0.95) |
| F-2 | 0.065 (0.025) | 3.1 | 2.32 (0.38) |
| F-3 | 4.5 (1.5)d | 1.9 | 2.45 (0.23) |
| C-1 | 0.66 (0.11) | 2.8 | >50 |
| C-2 | 0.21 (0.071) | 2.9 | 2.89 (0.19) |
| C-3 | 0.19 (0.054) | 2.6 | 6.13 (0.58) |
Data are expressed as the mean of three independent determinations. SD is given in parentheses.
Fold stimulation of ABCG2 ATPase activity was determined by taking basal activity as 1 or 100%.
EC50 PhA values (concentration required to achieve 50% of maximal PhA accumulation in MDA-MB-231/R cells) were previously reported (Sim et al., 2011).
Solubility problems were encountered with F-3.
Interestingly, we found that the concentrations for half-maximal stimulation of ATPase activity were significantly correlated to the EC50 PhA values of the compounds listed in Table 2 (Spearman's rho value of 0.623, p < 0.008) (data not shown). Thus compounds that interacted with greater affinity with ABCG2 were also those that reduced PhA efflux from R cells to a greater extent.
It should be noted that the ATPase assay does not provide insight on the functional status of the test compound. While there are ABCG2 inhibitors that inhibit ABCG2-associated ATPase activity (for example FTC) [26], there are inhibitors like curcumin that stimulate ATPase activity [14]. Furthermore, the ABCG2 substrate PhA has a biphasic effect on ABCG2 ATPase activity, stimulating ATP hydrolysis at low concentrations and inhibiting it at higher concentrations [27]. A recent report by Kannan et al [28] showed that tariquidar inhibits ABCG2 efflux activity at high concentration but stimulates its ATPase activity at lower concentrations. Here, we find that the potent analogs A-2, A-3, I-2, F-2 which have profiles aligned to that of ABCG2 inhibitors (Section 3.2, 3.3), strongly stimulated ATPase activity.
3.5. A-2 and other potent inhibitors of PhA efflux inhibit the photoaffinity labeling of ABCG2 with [125I] Iodoarylazidoprazosin ([125I]-IAAP)
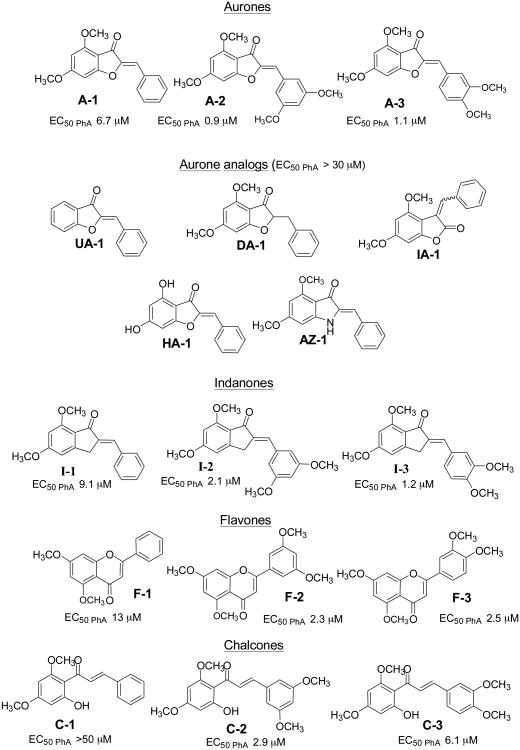
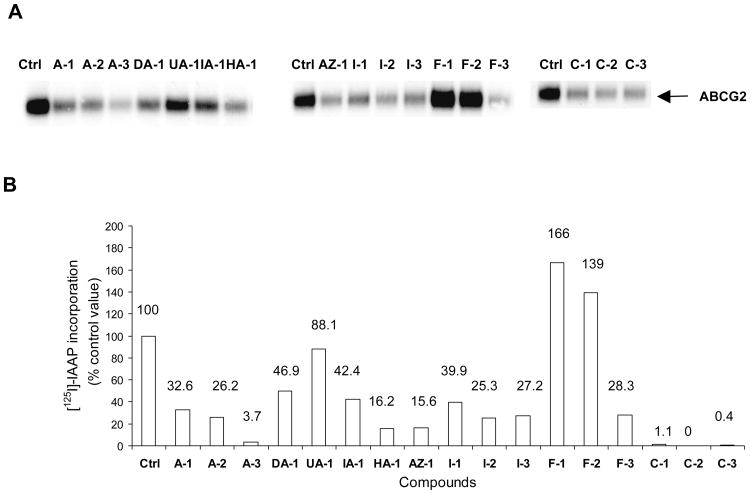
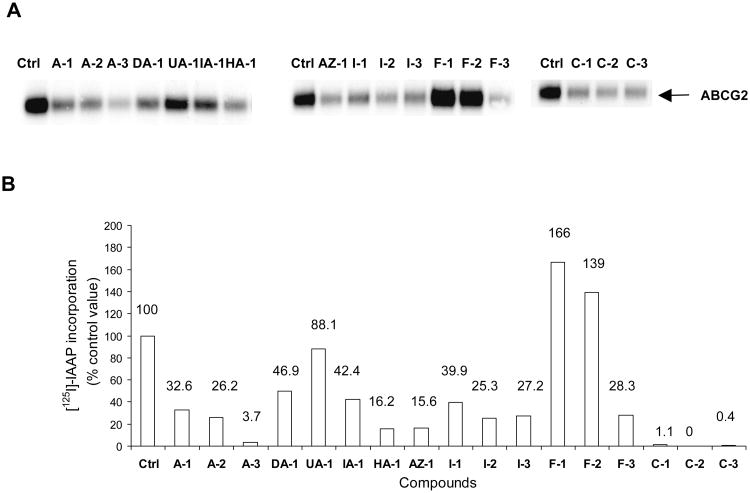
The same compounds tested in Section 3.4 were investigated for their effects on the photo-labeling of ABCG2 by [125I]-IAAP in membranes isolated from ABCG2 over-expressing human breast cancer cells (MCF-7 FLV 1000). Briefly, the membranes were incubated with [125I]-IAAP and then irradiated to promote covalent linkage of the labeled compound to the protein. The radioactively labeled ABCG2 protein was immunoprecipitated with BXP-21, separated by gel electrophoresis and protein labeling was visualized and quantified by autoradiography (Figure 4). If a compound has a greater affinity for the binding site than IAAP, then photo-affinity labeling of the site is diminished and the intensity of the photo-labeled ABCG2 band is correspondingly reduced. Compounds were tested at 5 μM.
Figure 4. Effect of aurones and their analogs on the photoaffinity labelling of ABCG2 with [125I]-IAAP.
Panel A: Autoradiogram showing the photoaffinity labeling of ABCG2 with [125I]-IAAP in the absence or presence of 5μM test compounds. ABCG2-containing crude membranes (50μg protein/ml) were incubated with 5 μM of test compound or FTC for 10 minutes at room temperature in 50 mM Tris-HCl (pH 7.5), after which 3-6 nM [125I] IAAP was added. The samples were incubated for an additional 5 min under subdued light, then exposed to ultravolet (365nm) light for 10 min at room temperature. The labeled ABCG2 was immunoprecipitated using the BXP-21 antibody and processed as described previously (Shukla et al., 2006). The radioactivity incorporated into ABCG2 was determined by exposing the gel to an X-ray film at −70°C and quantified using a phosphoimager and ImageQuaNT software. Panel B: %[125I]-IAAP incorporation = [125I]-IAAP signals obtained in the presence of test compound (5 μM) – [125I]-IAAP labelling obtained in the presence of FTC (5 μM). Values were average from two independent experiments.
As seen in Figure 4, the methoxyaurone A-3 and chalcones C-1, C-2 and C-3 completely inhibited the photolabelling of ABCG2 by [125I]-IAAP, indicating strong affinity for the IAAP binding site by these compounds. In contrast, the flavones F-1, F-2 and the unsubstituted aurone UA-1 caused minimal changes to the intensities of the photo-labeled ABCG2 bands, implying poor affinities for the IAAP site. The remaining compounds reduced [125I]-IAAP incorporation by 50% or more, and is probably associated with moderate affinities.
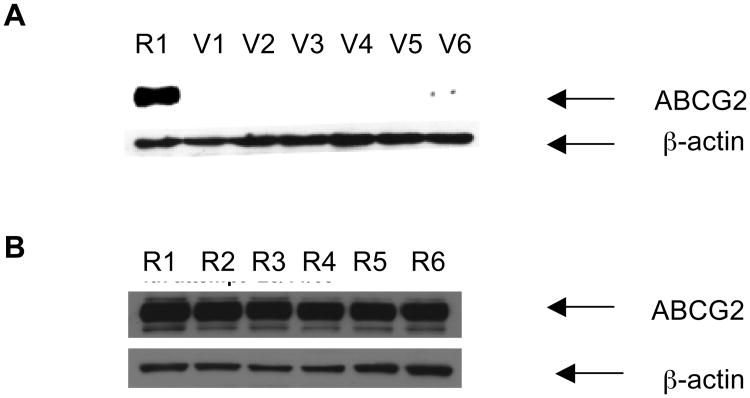
3.6. Western blot analysis shows that A-2 does not reduce ABCG2 protein levels of R cells
Next, we asked the question if the compounds interfered with transcription and translation processes involved in the expression of ABCG2. Inhibition of these processes would reduce ABCG2 expression and result in diminished efflux activity. These experiments were carried out with A-2 which is one of the more potent compounds affecting re-sensitization of R cells to MX (Section 3.2). Briefly, A-2 was incubated with R cells at 1 μM or 0.5 μM which are the concentrations at which it successfully re-sensitized R cells to MX. The R cell lysates were then analyzed by Western blotting to determine if ABCG2 levels are reduced compared to untreated R cell lysates. The experiments were repeated with parental V cells which have constitutional levels of ABCG2.
As seen from Figure 5A, control R cell lysates (Lane R1) showed a distinct ABCG2 band that was not observed in control V cell lysates (Lane V1). V cell lysates treated with A-2 at 0.5 μM (Lane V2) and 1 μM (Lane V3) were also not different from those obtained with untreated V cells. R cell lysates incubated with A-2 at similar concentrations of 0.5 μM (Lane R2) and 1 μM (Lane R3) gave ABCG2 bands that were as intense as those observed from control lysates (Figure 5B). Thus, A-2 did not affect the expression of ABCG2 protein levels in either R or V cells.
Figures 5. Western blot analyses showing the effect of compound A-2 (alone or in combination with mitoxantrone) on ABCG2 expression in MDA-MB-231/V (A) and R cells (B).
Cells were treated under different conditions for 72 hours after which they were trypsinized and 15 μg protein of R and V cell lysates were subjected to electrophoresis on 7.5% SDS-PAGE and transferred to nitrocellulose membranes for overnight blocking at room temperature. The blots were probed with anti-ABCG2 (BXP-21), followed by horseradish peroxidase-conjugated anti-mouse secondary antibody as described previously (Sim et al., 2008). β-Actin was used as a positive control in the Western blot analyses. Blots presented are representative of those from three independent determinations. Lanes marked R or V refer to lysates derived from R or V cells respectively. In panel A, lane R1 and lane V1, control R and V cells in 1% DMSO; lane V2 and V3, V cells exposed to A-2 at 0.5 μM and 1 μM; lane V4, V cells exposed to MX at 0.01 μM; and lane V5 and V6, V cells exposed to A-2 (0.5μM) + MX (0.01 μM) and A-2 (1 μM) + MX (0.01 μM). In panel B, lane R2 and R3, R cells exposed to A-2 at 0.5μM and 1 μM; lane R4, R cells exposed to MX at 0.1 μM; and lane R5 and R6, R cells exposed to A-2 (0.5 μM) + MX (0.1 μM) and A-2 (1 μM) + MX (0.1 μM).
In the re-sensitization experiments (Section 3.2), we showed that A-2 at 0.5 μM and 1 μM effectively increased the sensitivity of R cells to MX. The Western blot analysis confirmed that A-2 at these concentrations did not increase ABCG2 protein levels. To ensure that ABCG2 levels in R cells were also not affected by the combined presence of both MX and A-2 in the re-sensitization experiments, we determined the ABCG2 content in R cell lysates that were exposed to both A-2 and MX, as well as to MX alone. The concentration of MX used in these experiments (0.1 μM) was lower than its IC50 on R cells (0.92 μM), in order not to adversely affect the viability of the cells. As seen in Figure 5B, the ABCG2 band intensities in Lane R5 (A-2 0.5 μM + MX 0.1 μM) and Lane R6 (A-2 1 μM + MX 0.1 μM) are not diminished compared to the control lane R1. MX at 0.1 μM (Lane R4) also had no effect on the ABCG2 band intensity. In the same way, ABCG2 levels in V cells treated with MX or MX and A-2 were negligible (Figure 5A). Hence, it is evident that the re-sensitization of R cells to MX by A-2 is due to effects on the functional activity of ABCG2 and not due to reduced ABCG2 protein levels.
3.7. A-2 and other test compounds do not demonstrate ABCG2 substrate-like behavior based on their effects on the viability of ABCG2 over-expressing R cells and parental V cells
The preceding sections (3.4, 3.5) have shown that the test compounds (including potent analogs A-2, A-3, I-2 and I-3) interacted with ABCG2 to stimulate ATPase activity and competed with IAAP for binding to its site on the protein. The question is then asked if these compounds are themselves transported out of cells by ABCG2. If they are indeed substrates, they would be able to compete with other substrates (MX, PhA, IAAP) for efflux by ABCG2, especially if their structural features permit occupancy of these binding sites. It would also be in keeping with their ability to stimulate ATPase activity (Section 3.4), a property normally associated with substrates. To address this question, we determine the growth inhibitory effects of the test compound (at fixed concentrations of 5 μM and 10 μM) on R and V cells. The rationale is that if the test compound is a substrate, it would be transported out of the R cells by ABCG2 and hence affects the viability of R cells to a lesser degree than V cells. On the other hand, if it is not a substrate of ABCG2, viability of R and V cells would be affected to the same extent. The results are presented in Table 3.
Table 3. Effect of selected compounds on viability of MDA-MB-231/R and MDA-MB-231/V cellsa.
| Compound | % Viability | |||
|---|---|---|---|---|
| MDA-MB-231/R | MDA-MB-231/V | |||
| 10 μM | 5 μM | 10 μM | 5 μM | |
| A-1 | 122 (9) | NDb | 101 | ND |
| A-2 | 32 (4)c | 35 (7) | 37 (3)c | 65 (19) |
| A-3 | 65 (4)c | 82 (7) | 86 (4)c | 90 (2) |
| DA-1 | 56 (7)c | 59 (6)d | 108 (10)c | 108 (8)d |
| UA-1 | 60 (8)c | 68 (10)d | 103 (7)c | 98 (9)d |
| IA-1 | 68 (6)c | 91 (10)d | 97 (8)c | 102 (6)d |
| HA-1 | 77 (21)c | 88 (24) | 108 (4)c | 112 (7) |
| AZ-1 | 93 (9) | 101 (15) | 110 (3) | 111 (7) |
| I-1 | 94 (10) | 92 (10) | 108 (5) | 109 (14) |
| I-2 | 61 (10)c | 65 (14) | 93 (4)c | 100 (9) |
| I-3 | 73 (13) | 83 (10) | 101 (5) | 109 (13) |
| F-1 | 70 (3)c | 94 (16) | 97 (3)c | 105 (6) |
| F-2 | 68 (4)c | 88 (6) | 88 (1)c | 101 (3) |
| F-3 | 63 (16) | 76 (13) | 91 (4) | 103 (6) |
| C-1 | 75 (4)c | 112 (23) | 92 (3)c | 103 (4) |
| C-2 | 75 (14) | 71 (2)d | 84 (3) | 90 (2)d |
| C-3 | 71 (12) | 84 (4) | 89 (1) | 92 (6) |
Percentage viability = (Average absorbance of wells with test compounds / Average absorbance of control wells with medium and 1% DMSO) × 100%. Values are mean (SD) of three independent determinations.
ND = Not detected.
Significant difference (p< 0.05) between % viability of R cells and V cells at 10μM by paired t test (2-sided).
Significant difference (p< 0.05) between % viability of R cells and V cells at 5μM by paired t test (2-sided).
It can be seen from Table 3 that none of the compounds increased the viability of R cells to a greater extent than V cells. In fact, the compounds either affected viabilities of R and V cells to the same degree (no significant difference in % viability at one or both concentrations) or reduced viability of R cells to a greater degree than V cells (at one or both concentrations). Not withstanding the preliminary nature of this assay, it would seem that none of the compounds showed a substrate-like profile (viability of R cells > V cells) but several were non-substrates (viability of R cells = V cells) or had a profile that defied classification (viability of R cells < V cells). Of the potent re-sensitizers of R cell susceptibility to MX (A-2, A-3, I-2, F-2), all were associated with a non-substrate profile at 5 μM. Quantifying cell viabilities in terms of IC50 would provide a more accurate assessment of the differential activities of the test compounds on the R and V cells.
3.8. Nude mice bearing xenografts induced by R cells survive longer when treated with A-2 and MX, compared to MX alone
Xenografts were induced in the mammary fat pad of nude mice by injection of R cells. Reports by others [29, 30] have identified the mammary fat pad to be the preferred site for orthotopic induction of tumor xenografts due to its higher “take up” rate. The mice were then treated with either MX, A-2 or combined MX and A-2 at the start of the experiment. A-2 was tested at 0.2 mg/kg (equivalent to 10 μM) while MX was administered at a relatively low dose of 4 mg/kg in an effort to minimize toxicity to the animal [31]. Administration was done intra-tumorally as the pharmacokinetic profile of A-2 is not known. A-2 was also used at a higher concentration (0.2 mg/kg, equivalent to 10 μM) as compared to that employed (1 μM) in the re-sensitization experiments (Section 3.2). It is hoped that this would compensate for possible losses incurred during distribution and metabolic processes. Animals in the various treatment arms were monitored for changes in weight, tumor volume and life span throughout the investigation. Mice bearing xenografts induced by V cells were subjected to the same treatment arms.
The weights of the animals on the different treatment arms remained relatively constant throughout the experiment (Supplementary Data), which indicates that no serious toxicity issues were encountered with the drugs at the doses employed. Tumor volume was not found to be a suitable basis for analysis of the in vivo efficacy of A-2 because variations in tumor volume within each treatment group were found to be too narrow for comparison. Hence, the survival times of the treated animals were monitored and the Kaplan Meier survival analysis was used to compare the various treatment arms in mice with R and V xenografts (Table 4).
Table 4. Kaplan-Meier analysisa of treatment schemes in mice with MDA-MB-231/V induced xenografts and MDA-MB-231/R induced xenografts.
| Human cell line xenograft | Treatment groups | |||
|---|---|---|---|---|
| Control (1% DMSO) | A-2 | Mitoxantrone | A-2 + Mitoxantrone | |
| MDA-MB-231/V | ||||
| Number treated | 5 | 5 | 5 | 4 |
| Number of censored eventsb | 0 | 2 | 1 | 2 |
| Median survival time (days) | 12 | 15 | 29 | 25 |
| ILS (%)c | - | 25 | 142 | 108 |
| Log-rank test (p)d | 0.356 | 0.013* | 0.008** | |
| MDA-MB-231/R | ||||
| Number treated | 4 | 4 | 5 | 5 |
| Number of censored eventsb | 0 | 1 | 1 | 1 |
| Median survival time (days) | 12 | 15 | 17 | 25 |
| ILS (%)c | - | 25 | 42 | 108 |
| Log-rank test (p)d | 0.169 | 0.055 | 0.007** | |
Kaplan-Meier analysis was carried out on SPSS Ver 15.0 (Chicago, IL)
Death attributed to causes unrelated to tumor size > 1.5 cm (censured events). These are described in Section 2.8.
ILS= % increase in median life span = [(median survival time for treated group-median survival time for control)/median survival time for control] × 100%
Statistical significance between treatment group and control group
p<0.05 ;
p<0.01.
The median survival time for mice bearing V xenografts (control) was 12 days and this was increased to 15 days in mice treated with A-2 (Table 4). This is equivalent to a 25% increase in median life span (ILS) compared to the control /untreated animals but the log-rank test did not identify the increase in life span to be a significant event (p > 0.05). While A-2 did not increase the life span of the animal, it also did not hasten its demise, implying that it is essentially non-toxic at the dose used. In animals treated with MX, the median survival time increased to 29 days or 142% of the control survival time, a significant lengthening of life span (p < 0.05). In the case of mice treated with A-2 and MX, mean survival time was 25 days or 108% of the control. In spite of the relatively modest increase (compared to the 142% ILS observed for MX), it was deemed to be a significant increase in survival (p < 0.01). These results are largely anticipated since the xenograft was established with parental V cells which had constitutive ABCG2 levels. Thus, MX would retain its effectiveness against the tumor and prolonged survival of the animals. The significant increase in survival of animals treated with A-2 and MX probably reflected a dominant contribution by MX, since A-2 by itself did not prolong survival.
In the case of animals bearing the R cell induced xenograft, mean survival time for the control (untreated) arm was similar to that obtained for animals bearing the V xenograft (12 days, Table 4). Treatment with A-2 did not significantly prolong survival time which is indicative of its low toxicity as well as the absence of a direct cytotoxic /anti-proliferative effect by A-2 on the tumor cells of the xenograft.
As expected, treatment with MX caused only a modest increase in median life span (42% increase, p > 0.05) in line with the fact that MX is a substrate of ABCG2 and would accumulate to a lesser degree in the xenograft induced by R cells. On the other hand, the treatment arm comprising A-2 and MX increased survival time to 108%, a significant increase (p < 0.01) from the control arm. Ironically, animals on this treatment arm did live significantly longer (median survival 25 days) than those treated with MX alone (median survival 17 days). Thus, there is only indirect evidence that A-2 enhanced efficacy of MX on R cell-induced xenografts. There are several possible reasons why survival times of animals on the MX and combined MX + A-2 treatment arms were not significantly different. The dosages of MX and A-2 have not been optimized in this study and a higher dose of A-2 (currently 20-fold less than that of MX) could have given a different outcome. Using a larger number of animals for each treatment arm may also influence the results.
4. Discussion
In an earlier report, we identified several methoxylated aurones A-2, A-3, indanones I-2, I-3 and flavones F-2, F-3 that strongly reduced the efflux of PhA from ABCG2 over-expressing human MDA-MB-231/R cells [21]. Notably, these compounds were comparable to FTC, a well-established and potent ABCG2 inhibitor. The present study was undertaken to establish the functional relevance of these findings and to provide a better understanding of how these novel templates interact with the ABCG2 protein. To this end, we employed several experimental approaches to provide insight to these questions.
First, we showed that A-2, A-3, I-2 and F-2 effectively restored the sensitivity of human ABCG2 over-expressing R cells to MX at a low concentration of 0.5 μM. In fact, R cells exposed to this concentration of test compound were found to be as responsive to the cytotoxic effects of MX as the parental V cells. Since none of the compounds at 0.5 μM significantly affected the viability of R cells (Section 3.7), their contribution to the diminished viability of R cells in the re-sensitization experiments is negligible. Hence, the nanomolar EC50 of MX in R cells concurrently treated with these compounds is solely attributed to the enhanced potency of MX. The investigations on MX accumulation in R cells provide further support for this notion. It is seen that A-2, A-3 and I-2 increased the content of MX in R cells by almost 3-fold but did not change MX levels in parental V cells subjected to the same treatment. Clearly, the re-sensitization of R cells to MX in the presence of these compounds is linked to a reduction in the efflux activity of ABCG2, leading to an increase in the intracellular concentration of MX.
A majority of inhibitors of ABC transport proteins do so by competing with substrate binding or impeding ATP binding /hydrolysis. Thus far, several compounds (A-2, A-3, I-2, I-3, F-2) have demonstrated potent modulatory effects on the efflux activity of ABCG2. To confirm that these effects arise from a direct interaction with the protein, we investigated their effects on the beryllium fluoride-sensitive basal ABCG2-ATPase activity and the photo-affinity labeling of ABCG2 with a labeled substrate [125 I]-IAAP.
The transport of substrates and the hydrolysis of ATP are closely linked events in ABC transporters [32]. Theoretically, the effect of a compound on the ATPase activity of the transporter should give valuable clues on the nature of its interaction with the protein. Activation of ATPase activity leading to an increase in ATP turnover suggests the presence of a substrate while a reduction in ATPase activity (if examined in a fully activated transporter) indicates an inhibitor or a substrate with a low transport rate [33]. However as pointed out earlier, there are many exceptions to this relationship. There are inhibitors like curcumin that stimulate ATPase activity [14] and substrates like PhA that have a biphasic effect on ATPase activity [27]. Here, we found that all the test compounds stimulated ATPase activity, albeit with varying levels of potency as reflected in the concentrations required for 50% maximal stimulation.
[125 I]-IAAP is a photoaffinity analog of prazosin that has been widely used to characterize the substrate binding sites of ABCG2 [23]. A compound that reduces the photo-labeling of ABCG2 is deduced to interact with the IAAP binding site on the protein although the reduction in IAAP binding could be due to conformational changes in the protein induced by the test compound. Here, we found that most of the compounds that stimulated ATPase activity also reduced the photo-affinity labeling of ABCG2. Exceptions are the flavones F-1, F-2 and the unsubstituted aurone UA-1. Thus, the present findings support an interaction with the IAAP binding site by the test compounds, including the potent methoxylated aurones (A-2, A-3) and indanones (I-2, I-3).
The photoaffinity labeling assay also serves the purpose of highlighting the structural diversity of substrates whose binding to ABCG2 has been disrupted by the test compounds. This is relevant because ABCG2 is known to have multiple substrate binding sites [34, 35] and a compound that is able to modulate the efflux of different substrates is potentially more useful than one whose activity is limited to a single substrate. MX is a synthetic anthracenedione, PhA is a naturally occurring chlorophyll metabolite and prazosin is a methoxylated quinozalineamine. The ability of the test compounds to interfere with the transport /binding of these chemically diverse substrates is a good indication of their potential usefulness as modulators of ABCG2 activity.
Although there is evidence to support a direct interaction between the test compounds and ABCG2 (ATPase assay, photo-labeling with labeled IAAP), there is still a possibility that these compounds reduce ABCG2 content by interfering with the upstream processes of transcription and translation involved in its synthesis. This likelihood was excluded, at least for A-2 which is one of the more potent compounds identified in this investigation. Western blot analysis on A-2 showed that it did not reduce ABCG2 content in R cells at 0.5 μM and 1 μM, which were the same concentrations used to demonstrate the re-sensitization of R cells to MX by A-2.
While it is evident that most of the test compounds competed with other substrates for occupancy of their binding sites, it is not known if they are themselves substrates of ABCG2. Here we carried out a preliminary and indirect assessment based on the differential growth inhibitory effects of the compounds on R and V cells. It is found that none of the compounds, in particular the more potent analogs (A-2, A-3, I-2, I-3, F-2, F-3), have a substrate-like profile (viability of R cells > V cells). Further confirmation of their status would require monitoring their bi-directional transport on cell lines that over-expresses ABCG2 [9].
Having identified A-2 as a potent re-sensitizer of R cells to MX in an in vitro setting, we proceeded to demonstrate its efficacy in mice bearing MX unresponsive xenografts induced by R cells. Analysis of the life spans of mice bearing R or V cell-induced xenografts on the three different treatment arms (A-2, MX, combined A-2 and MX) provided the following insights. First, treatment of mice bearing V or R xenografts with A-2 did not significantly increase their life spans. Clearly, A-2 had no effect on the progression of the tumor and was essentially non-toxic at the dose used. Second, treatment with MX resulted in significantly longer life spans for mice with xenografts induced by V cells but not R cells, which is in keeping with the MX-resistant character of the R cell induced xenograft. Lastly, life spans of mice with V and R xenografts were increased when treated with both MX and A-2. The significantly longer life span of mice bearing R xenografts on this treatment arm (compared to control) stood in stark contrast to the same mice receiving MX alone where life spans were not increased. Clearly, the ABCG2 modulatory property of A-2 plays an important role in increasing the sensitivity of the tumor to the cytotoxic effects of MX. On the other hand, the efficacy of the combined A-2 and MX treatment in mice bearing V xenografts is attributed to the presence of MX in the combination, since MX alone could significantly lengthened the survival of the treated animals.
Taken together, the present investigations have shown that the methoxylated aurones A-2 and A-3 interacted directly with ABCG2 to inhibit efflux activity. These compounds competed for occupancy of one or more substrate binding sites on ABCG2, thus inhibiting the efflux of the bona fide substrate by ABCG2. In the absence of a substrate, the present evidence suggests that they are not transported by ABCG2, implying that these compounds do not bind with sufficient affinity to their putative sites to stimulate the hydrolysis of ATP necessary for transport. The ability of A-2 to re-sensitize R cells to MX was observed in both in vitro and in vivo settings. Notably, concurrent administration of A-2 and MX significantly prolonged the survival of mice bearing ABCG2 over-expressing tumor xenografts.
In conclusion, methoxylated aurones are promising compounds associated with low toxicities and potent modulatory effects on the ABCG2 efflux protein. Thus, aurones warrant further scrutiny as lead templates for potential MDR reversal agents. Inevitably, there are still areas that require further attention, such as a better understanding of aurones pharmacokinetic profiles, their interaction with the ATP binding site and their effects on the localization of ABCG2. The latter is relevant as the modulatory effects of the aurones may be mediated by induced shifts in the localization of ABCG2 from the cell membrane to the cytosol, and consequent effects on cellular signalling pathways.
Supplementary Material
Acknowledgments
We wish to thank Dr Douglas D. Ross (Greenebaum Cancer Centre, University of Maryland, Baltimore, USA) for the generous gift of parental and transfected MDA-MD-231 cells. This work was made possible by funding to MLG from the National University of Singapore (RP 148000084112), NUS research scholarship to HMS. CP Wu and SV Ambudkar were supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
References
- 1.Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–98. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Tomas R. Multidrug resistance: retrospect and prospects in anti-cancer drug treatment. Curr Med Chem. 2006;13:1859–76. doi: 10.2174/092986706777585077. [DOI] [PubMed] [Google Scholar]
- 3.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 4.Boumendjel A, Florin A, Boutonnat J. Reversal agents of multidrug resistance mediated by multidrug resistance-associated proteins (MRPs) In: Boumendjel A, Boutonnat J, Robert J, editors. ABC Transporters and Multidrug Resistance. Hoboken, New Jersey: John Wiley & Sons, Inc; 2009. pp. 261–80. [Google Scholar]
- 5.Lee C. Reversing agents for ATP-binding cassette drug transporters. Methods Mol Biol. 2010;596:325–40. doi: 10.1007/978-1-60761-416-6_14. [DOI] [PubMed] [Google Scholar]
- 6.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95:15665–70. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- 8.Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58:5337–9. [PubMed] [Google Scholar]
- 9.Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–36. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shukla S, Wu CP, Ambudkar SV. Development of inhibitors of ATP-binding cassette drug transporters: present status and challenges. Expert Opin Drug Metab Toxicol. 2008;4:205–23. doi: 10.1517/17425255.4.2.205. [DOI] [PubMed] [Google Scholar]
- 11.Rabindran SK, Ross DD, Doyle LA, Yang W, Greenberger LM. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000;60:47–50. [PubMed] [Google Scholar]
- 12.Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, et al. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1:417–25. [PubMed] [Google Scholar]
- 13.Chearwae W, Anuchapreeda S, Nandigama K, Ambudkar SV, Limtrakul P. Biochemical mechanism of modulation of human P-glycoprotein (ABCB1) by curcumin I, II, and III purified from Turmeric powder. Biochem Pharmacol. 2004;68:2043–52. doi: 10.1016/j.bcp.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Chearwae W, Shukla S, Limtrakul P, Ambudkar SV. Modulation of the function of the multidrug resistance-linked ATP-binding cassette transporter ABCG2 by the cancer chemopreventive agent curcumin. Mol Cancer Ther. 2006;5:1995–2006. doi: 10.1158/1535-7163.MCT-06-0087. [DOI] [PubMed] [Google Scholar]
- 15.Limtrakul P, Chearwae W, Shukla S, Phisalphong C, Ambudkar SV. Modulation of function of three ABC drug transporters, P-glycoprotein (ABCB1), mitoxantrone resistance protein (ABCG2) and multidrug resistance protein 1 (ABCC1) by tetrahydrocurcumin, a major metabolite of curcumin. Mol Cell Biochem. 2007;296:85–95. doi: 10.1007/s11010-006-9302-8. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed-Belkacem A, Pozza A, Macalou S, Perez-Victoria JM, Boumendjel A, Di Pietro A. Inhibitors of cancer cell multidrug resistance mediated by breast cancer resistance protein (BCRP/ABCG2) Anti-Cancer Drugs. 2006;17:239–43. doi: 10.1097/00001813-200603000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez AI, Real R, Pérez M, Mendoza G, Prieto JG, Merino G. Modulation of the activity of ABC transporters (P-glycoprotein MRP2 BCRP) by flavonoids drug response. J Pharm Sci. 2010;99:598–617. doi: 10.1002/jps.21851. [DOI] [PubMed] [Google Scholar]
- 18.Boumendjel A, Di Pietro A, Dumontet C, Barron D. Recent advances in the discovery of flavonoids and analogs with high-affinity binding to P-glycoprotein responsible for cancer cell multidrug resistance. Med Res Rev. 2002;22:512–29. doi: 10.1002/med.10015. [DOI] [PubMed] [Google Scholar]
- 19.Boumendjel A, Baubichon-Cortay H, Trompier D, Perrotton T, Di Pietro A. Anticancer multidrug resistance mediated by MRP1: recent advances in the discovery of reversal agents. Med Res Rev. 2005;25:453–72. doi: 10.1002/med.20032. [DOI] [PubMed] [Google Scholar]
- 20.Sim HM, Lee CY, Ee PL, Go ML. Dimethoxyaurones: Potent inhibitors of ABCG2 (breast cancer resistance protein) Eur J Pharm Sci. 2008;35:293–306. doi: 10.1016/j.ejps.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Sim HM, Loh KY, Yeo WK, Lee CY, Go ML. Aurones as Modulators of ABCG2 and ABCB1: Synthesis and Structure-Activity Relationships. ChemMedChem. 2011;6:713–24. doi: 10.1002/cmdc.201000520. [DOI] [PubMed] [Google Scholar]
- 22.Wu CP, Shukla S, Calcagno AM, Hall MD, Gottesman MM, Ambudkar SV. Evidence for dual mode of action of a thiosemicarbazone, NSC73306: a potent substrate of the multidrug resistance linked ABCG2 transporter. Mol Cancer Ther. 2007;6:3287–96. doi: 10.1158/1535-7163.MCT-07-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shukla S, Robey RW, Bates SE, Ambudkar SV. The calcium channel blockers, 1,4-dihydropyridines, are substrates of the multidrug resistance-linked ABC drug transporter, ABCG2. Biochemistry. 2006;45:8940–51. doi: 10.1021/bi060552f. [DOI] [PubMed] [Google Scholar]
- 24.Shukla S, Robey RW, Bates SE, Ambudkar SV. Sunitinib (Sutent, SU11248), a small-molecule receptor tyrosine kinase inhibitor, blocks function of the ATP-binding cassette (ABC) transporters P-glycoprotein (ABCB1) and ABCG2. Drug Metab Dispos. 2009;37:359–65. doi: 10.1124/dmd.108.024612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan Y. Biostatistics 203. Survival analysis. Singapore Med J. 2004;45:249–56. [PubMed] [Google Scholar]
- 26.Henrich CJ, Robey RW, Takada K, Bokesch HR, Bates SE, Shukla S, et al. Botryllamides: natural product inhibitors of ABCG2. ACS Chem Biol. 2009;4:637–47. doi: 10.1021/cb900134c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henrich CJ, Robey RW, Bokesch HR, Bates SE, Shukla S, Ambudkar SV, et al. New inhibitors of ABCG2 identified by high-throughput screening. Mol Cancer Ther. 2007;6:3271–8. doi: 10.1158/1535-7163.MCT-07-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kannan P, Telu S, Shukla S, Ambudkar SV, Pike VW, Halldin C, et al. The “Specific” P-Glycoprotein Inhibitor Tariquidar Is Also a Substrate and an Inhibitor for Breast Cancer Resistance Protein (BCRP/ABCG2) ACS Chem Neurosci. 2011;2:82–9. doi: 10.1021/cn100078a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price JE, Polyzos A, Zhang RD, Daniels LM. Tumorigenicity and metastasis of human breast carcinoma cell lines in nude mice. Cancer Res. 1990;50:717–21. [PubMed] [Google Scholar]
- 30.Clarke R. Issues in experimental design and endpoint analysis in the study of experimental cytotoxic agents in vivo in breast cancer and other models. Breast Cancer Res Treat. 1997;46:255–78. doi: 10.1023/a:1005938428456. [DOI] [PubMed] [Google Scholar]
- 31.Almond B, Hadba A, Freeman S, Cuevas B, York A, Detrisac C, et al. Efficacy of mitoxantrone-loaded albumin microspheres for intratumoral chemotherapy of breast cancer. J Controlled Release. 2003;91:147–55. doi: 10.1016/s0168-3659(03)00214-1. [DOI] [PubMed] [Google Scholar]
- 32.Ambudkar SV, Cardarelli CO, Pashinsky I, Stein WD. Relation between the turnover number for vinblastine transport and for vinblastine stimulated ATP hydrolysis by human P-glycoprotein. J Biol Chem. 1997;272:21160–6. doi: 10.1074/jbc.272.34.21160. [DOI] [PubMed] [Google Scholar]
- 33.Hegedus C, Szakacs G, Homolya L, Orban TI, Telbisz A, Jani M, et al. Ins and outs of the ABCG2 multidrug transporter: an update on in vitro functional assays. Adv Drug Deliv Rev. 2009;61:47–56. doi: 10.1016/j.addr.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Ejendal K, Hrycyna C. Differential sensitivities of the human ATP-binding cassette transporters ABCG2 and P-glycoprotein to cyclosporin A. Mol Pharmacol. 2005;67:902–11. doi: 10.1124/mol.104.001701. [DOI] [PubMed] [Google Scholar]
- 35.Giri N, Agarwal S, Shaik N, Pan G, Chen Y, Elmquist WF. Substrate-dependent breast cancer resistance protein (Bcrp1/Abcg2)-mediated interactions: consideration of multiple binding sites in in vitro assay design. Drug Metab Dispos. 2009;37:560–70. doi: 10.1124/dmd.108.022046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.