Abstract
Working memory (WM) declines with age. However it seems unclear, whether age related decline is more pronounced on verbal WM or on visuo-spatial WM. The present study compares the effect of aging on verbal and visuo-spatial modality of WM on native Hindi healthy speakers, in the age range of 40-to-above 80 years. It was found that normal aging affect both the verbal and visual working memory in similar way. Both modality declines with a similar rate up to 50–60 years and after 60 years relative saturation in span take place. Although verbal WM span is higher than visuo-spatial WM span, but no significant difference between verbal and visuo-spatial WM span were observed.
Keywords: Aging, verbal working memory, visuo-spatial working memory
Working memory (WM) is a capacity of an individual to maintain temporarily a limited amount of information in mind that support various abilities, including learning, reasoning, and preparation for action [1]. Although, WM active and relevant for a short period, but hold remembered stimulus “on-line” to guide behavior in the absence of external cues or prompts [2]. Various theoretical frameworks have been proposed to conceptualize the WM [1, 3–11]. Baddeley’s model has been regarded as gold standard in the area of WM research due to its ability to explain a majority of research data and its relative simplicity [12]. This model described WM as a multicomponent system consisted of phonological loop, visuo–spatial sketchpad and central executive. Phonological loop temporarily stores verbal information and responsible for rehearsing verbal information and recycling to refresh the memory trace [13]. Whereas, visuo–spatial sketchpad temporarily store and manipulate spatial and visual information, e.g. remembering shapes, colours and location [14]. Both are controlled and regulated by central executive.
Nearly all measures of working memory developed to date involved the measurement of span. Memory span is the maximum amount of sequential information an individual can remember accurately [15]. Amongst WM span tasks, counting span, operation span, and reading span tasks, is widely used to measure WM capacity. Moreover these tasks require participants to listen and recall serially the digits or letters. These verbal tasks are considered as reliable and valid measures of WM capacity [16]. Therefore, much more data are available from verbal working memory tasks as compared to other measure of WM capacity e.g., visuo-spatial WM span tasks [17, 18]. Amongst verbal working memory span tasks, the digit span task is most commonly used measures of immediate verbal recall, attentional capacity and in neuropsychological research and clinical evaluations [16]. However, visuo-spatial WM measure used jigsaw-puzzle task [19], corsi’s test [20].
Over the life span, WM increases at early development age [21, 22] and decreases with age in adulthood [23]. Aging associated decline in WM is not uniform. WM span has been found to increase upto the age of 20 years, followed by decline [24]. WM may begin to decline as early as the 30s, although initial decline may be slight [25].
Moreover conflicting results have been observed on comparing modality specific (verbal and visuo-spatial) WM tasks. Some authors have observed higher aging related decline for visuo-spatial WM than verbal WM [26, 27, 28]. In contrast, other studies reported more decline for verbal modality WM span task compared to visuo-spatial span tasks [19]. Apart from these, similar decline in both modalities were also reported [29, 30, 31].
At this point it is worth mentioning that effect of age on verbal and visuo-spatial WM appears to be less clear. It is still a debated issue whether age related decline is more pronounced on verbal WM or on visuo-spatial WM. The present study attempts to compare the effect of aging on verbal and visuo-spatial tasks modality of WM on native Hindi healthy speakers, in the age range of 40-to-above 80 years. WM hold the information and process it, which is relatively not dependent on modality of incoming information. Therefore, it was assumed that aging related decline in WM should be independent of task modality.
MATERIALS AND METHODS
Participants
The current sample comprised of 80 native Hindi speaking adults within the age range of 40 to above 80s years. Sample was divided into five age groups. Group I: aged 40–50 years (M = 45.12, SD = 2.7); Group II: aged 51–60 years (M = 55.87, SD = 3.5); Group III: aged 61–70 years (M = 65.81, SD = 2.5); Group IV: aged 71–80 years (M = 75.62, SD = 1.7); and Group V: aged above 80 years (M = 84.25, SD = 4.3). Each groups has equal number of participants (n = 16).
All the participants belonged to middle socioeconomic class, as measured on Kuppuswamy’s socioeconomic status scale [32]. All had education level higher than 9 years in Hindi medium. Mean schooling years for the sample was 11.08 (SD= 3.6). Participants had no present/past history of any neurological, psychological problems and or sensory deficits. It was ensured using Hindi Mental State Exam [33]. Participants scoring greater than or equal to 25 on the HMSE were taken up for the study. Individuals with any Axis I psychiatric diagnosis according to the DSM-IV-TR (APA, 2000) [34], presence of dementia, and severe untreated sight and hearing disorders were excluded from the study.
Materials and procedures
Participation in the study was voluntary. Informed consent form was obtained explaining about the objectives, justifications, and procedures of this investigation. Each participant was tested individually in a single session of approximately one hour. Prior to verbal and visuo-spatial WM tasks, demographic questionnaire and the HMSE were completed. Presentation order verbal and visuo-spatial WM task was counter balanced across participants.
Verbal working memory (VWM) task: Digit span test- This is a subtest of cognitive-linguistic assessment protocol for adults (CLAP) in Hindi [35]. The test comprised of digit forward span (DFS) and, digit backward span (DBS). Participants were asked to verbally repeat a set of digits in same sequence as the examiner in DFS task, and in reverse order for DBS task. Test starts from a set of 2 digits continuing to a maximum a set of 7 digits. Each set was trail thrice. Digits were presented at a rate of one digit per second. VWM span was calculated as a set of maximum digit, where two out of three trials were repeated correctly (Table 1). Digit span ranged from 2 to 7. If a minimum span of 2 was not achieved 0 was scored.
Table 1.
Example of verbal working memory span task.
| Set (trial) | Stimulus |
Responses
|
|||
|---|---|---|---|---|---|
| DFS | DBS | ||||
| 1 (i) | 2 3 | 2 3 | ✓ | 4 3 | ✓ |
| (ii) | 6 4 | 6 4 | ✓ | 7 4 | ✓ |
| (iii) | 9 5 | 9 5 | ✓ | 3 5 | ✓ |
|
| |||||
| 2 (i) | 5 7 2 | 5 7 2 | ✓ | 8 2 5 | X |
| (ii) | 6 8 2 | 6 8 6 | x | 5 6 6 | X |
| (iii) | 7 9 4 | 7 9 4 | ✓ | 3 4 9 | X |
|
| |||||
| 3 (i) | 1 7 5 8 | 1 7 5 8 | x | ||
| (ii) | 6 4 3 9 | 6 4 3 9 | ✓ | ||
| (iii) | 8 2 7 3 | 8 2 7 3 | x | ||
|
| |||||
| SPAN | 3 | 2 | |||
Sets of digit for digit forward span (DFS) and digit backward span (DBS) task are shown in stimulus column. Correct responses marked as ‘✓’ and wrong as ‘x’, shown in response column. Two out of three trials are correct up to a set of 3 digits and 2 digits in DFS and DBS task respectively. Therefore, DFS is 3 and DBS is 2.
Visuo-spatial working memory (VSWM) task: 5 × 5 matrices of blank squares were devised to assess VSWM span (Figure 1). Sequences of dots were presented in various squares, started with a set of two dots and continued up to a set of seven dots. Task was to recall the positions of those dots and indicate them in blank matrix. Each dot was displayed for one second and no dot was repeated in the same square in a set. Participants were asked to recall the dots position in same sequence as presented by examiner in visuo-spatial forward (VSF) task. However, visuo-spatial backward (VSB) task needed to recall the dots position in reverse order. Visuo-spatial working memory span was calculated as similar to verbal working memory span.
Figure 1.
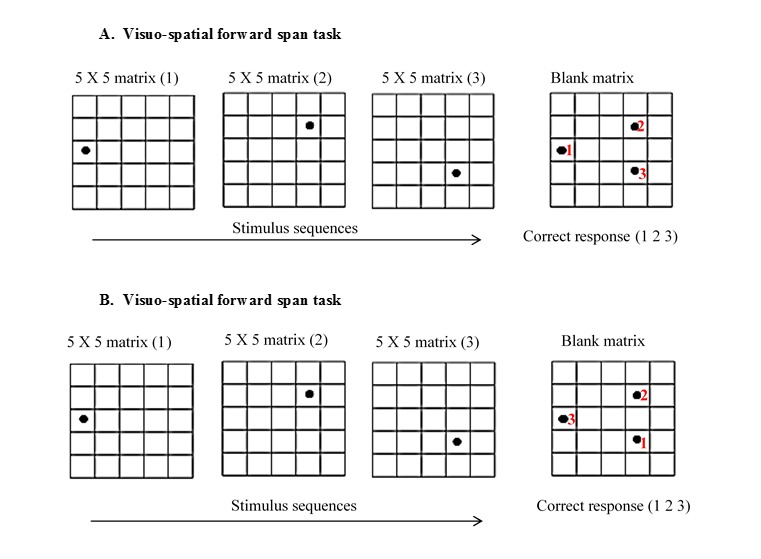
Example of material used in visuo-spatial forward span task (A), and visuo-spatial backward span task (B). Three dots are presented over three consecutive 5 x 5 matrices (1, 2, 3). Response was obtained in blank matrix. Numerical (1 2 3) in red colour indicates the sequence of response obtained.
Prior to experiment two practice trials were provided for familiarization with tasks. Experiments were carried out in a quiet, noise free environment at home or clinical setting. All the data was audio-video recorded with digital camera (Sony 1080). Finally the 10 percent of the audio-video recorded data were retested by three speech-language pathologists for inter-judge reliability.
Statistical analysis
The raw score of each individual was tabulated and statistical analysis was computed using Statistical Package for the Social Science (SPSS) version 17.0. A descriptive statistics (mean and standard deviation) were computed. Repeated measure ANOVA was used to determine the degree of difference across visual and verbal working memory tasks. Furthermore multivariate analysis of variances (MANOVA) was performed to determine the significance of differences across age group and gender in both verbal and visuo-spatial working memory tasks. Two-tailed tests and a significance level of 0.05 were used throughout the study.
RESULTS
Verbal working memory (VWM) and Visuo-spatial working memory (VSWM)
Mean and standard deviation (SD) of verbal WM span (DFS & DBS) are shown in Table 2. DFS was higher than DBS across all the age groups and a significant difference was found between them. ANOVA revealed a significant effect of age [F (4, 75) = 45.69, p < 0.01], and span (DFS and DBS) [F (1, 75) = 287, p < 0.01] on VWM span task. Furthermore a significant two-way interaction was found between verbal WM task and span [F (1, 75) = 64.28, p < 0.01].
Table 2.
Mean (M) and SD of verbal and visuo-spatial WM span tasks across gender and age groups.
| Age group | Gender | Verbal WM | Visuo-spatial WM | ||
|---|---|---|---|---|---|
|
| |||||
| DFS (Max.= 7) N = 80 | DBS (Max.= 7) N = 80 | VSFS (Max.= 7) N = 80 | VSBS (Max.= 7) N = 80 | ||
| M (SD) | M (SD) | M (SD) | M (SD) | ||
| 40–50 | Male | 6.50 (0.53) | 4.89 (0.35) | 4.0 (0.0) | 4.0 (0.0) |
| Female | 5.75 (0.46) | 4.39 (0.52) | 4.50 (0.53) | 4.50 (0.53) | |
| Total | 6.13 (0.61) | 4.63 (0.5) | 5.25 (0.68) | 4.25 (0.44) | |
|
| |||||
| 51–60 | Male | 6.0 (0.75) | 3.75 (0.70) | 4.13 (0.64) | 4.13 (0.64) |
| Female | 5.25 (0.46) | 3.87 (0.83) | 3.50 (0.53) | 3.50 (0.53) | |
| Total | 5.63 (0.71) | 3.81 (0.75) | 4.63 (0.88) | 3.81 (0.65) | |
|
| |||||
| 61–70 | Male | 4.50 (0.75) | 3.38 (0.52) | 3.0 (0.0) | 3.00 (0.0) |
| Female | 4.89 (0.64) | 3.13 (0.35) | 3.25 (0.46) | 3.25 (0.46) | |
| Total | 4.69 (0.7) | 3.25 (0.44) | 3.94 (0.57) | 3.13 (0.34) | |
|
| |||||
| 71–80 | Male | 4.88 (0.83) | 3.38 (0.52) | 3.75 (0.89) | 3.13 (0.35) |
| Female | 4.38 (0.74) | 3.13 (0.35) | 3.38 (0.51) | 3.0 (0.0) | |
| Total | 4.63 (0.8) | 3.25 (0.44) | 3.56 (0.72) | 3.06 (0.25) | |
|
| |||||
| Above 80s | Male | 5.0 (0.53) | 3.62 (0.74) | 3.38 (0.51) | 2.87 (0.35) |
| Female | 3.88 (0.64) | 3.00 (0.0) | 3.0 (0.0) | 2.75 (0.46) | |
| Total | 4.44 (0.81) | 3.31 (0.6) | 3.19 (0.4) | 2.81 (0.4) | |
Note: - DFS: Digit forward span; DBS: Digit backward span; VSFS: Visuo-spatial forward span; VSBS: Visuo-spatial backward span.
Mean and standard deviation (SD) of visuo-spatial WM span (VSFS & VSBS) are also shown in Table 2. Similar to VWM, Visuo-spatial forward span was significantly higher than backward span. Significant effect of age [p < 0.01], and span (VSFS and VSBS) [p < 0.01] on VSWM was found.
MANOVA revealed a significant effect of age group [F (16, 280) = 5.5, p < 0.01], and gender [F (4, 67) = 4.06, p < .01] on dependent measures of WM. F and p- value of age and gender effect and their interaction on WM span measures are shown in Table 3.
Table 3.
F and p-value for age group, gender and their interaction on verbal (DFS & DBS) and visuo-spatial (VSFS & VSBS) WM span tasks
| Parameters | F | P- value | |
|---|---|---|---|
| Age | DFS | 20.51 | 0.00** |
| DBS | 19.27 | 0.00** | |
| VSFS | 30.59 | 0.00** | |
| VSBS | 34.36 | 0.00** | |
|
| |||
| Gender | DFS | 14.35 | 0.00** |
| DBS | 6.14 | 0.01* | |
| VSFS | 4.21 | 0.04* | |
| VSBS | 0.07 | 0.78 | |
|
| |||
| Age * Gender | DFS | 3.01 | 0.02* |
| DBS | 1.13 | 0.34 | |
| VSFS | 5.17 | 0.00** | |
| VSBS | 4.40 | 0.00** | |
Note: DFS: Digit forward span; DBS: Digit backward span; VSFS: Visuo-spatial forward span; VSBS: Visuo-spatial backward span.
p < 0.05 = significant difference;
p<0.01 = highly significant difference.
Comparisons of VWM and VSWM across age groups are shown in Figure 2. VWM span was higher than VSWM, but no significant difference [p = 0.12] was found between them. Across VWM significant difference was found between age groups, except 61–70 years and 40–50 years; 61–70 years and above 80s; 71–80 years and above 80s. However across VSWM, significant differences were observed between the age groups except 61–70 years and 71–80 years; 71–80 years and above 80s.
Figure 2.
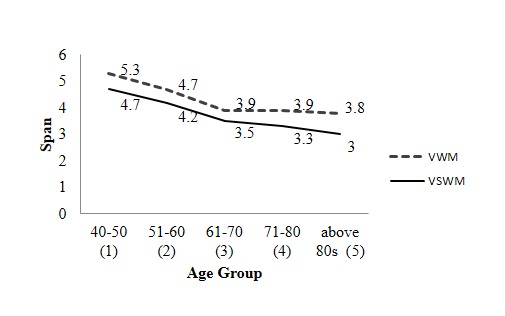
Verbal working memory (VWM) span and visuo-spatial working memory (VSWM) span declines with aging. Mean span on the Y-axis plotted against age groups in year on the X-axis.
Forward span and Backward span
Comparison of mean forward span and backward span in both verbal and visuo-spatial modalities across age groups are shown in Figure 3. No significant difference was found between verbal and visuo-spatial modality for both forward span (DFS & VSFS) [p = 0.09] and backward span (DBS &VSBS) [p = 0.07]. Significant differences between age groups on DFS, DBS, VSFS and VSBS over Bonferroni pair-wise comparison are shown in Table 4.
Figure 3.
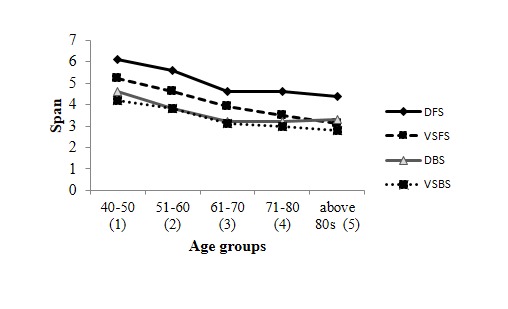
Digit forward span (DFS), visuo-spatial forward span (VSFS), digit backward span (DBS) and visuo-spatial backward span (VSBS) declines with aging. Mean span on the Y-axis plotted against age groups in year on the X-axis.
Table 4.
Example of verbal working memory span task
| Verbal WM | Visuo-spatial WM | |||
|---|---|---|---|---|
|
| ||||
| DFS | DBS | VSFS | VSBS | |
| Age group pairs | 40–50 & 71–80 yr 40–50 & above 80s 51–60 & 71–80 yr 51–60 & above 80s |
40–50 & 71–80 yr 40–50 & above 80s 51–60 & 71–80 yr 51–60 & above 80s |
40–50 & 71–80 yr 51–60 & 71–80 yr 51–60 & above 80s |
40–50 & above 80s, 51–60 & 71–80 yr, 51–60 & above 80s |
Note: - DFS: Digit forward span; DBS: Digit backward span; VSFS: Visuo-spatial forward span; VSBS: Visuo-spatial backward span.
Cronbach’s alpha coefficient for the VWM and VSWM tasks was 0.85 suggesting an adequate degree of internal reliability.
DISCUSSION
The present study investigated an effect of aging on WM, in particular whether there is a differential effect of aging on modality of information to be provided i.e., verbal or visuo-spatial. Results revealed that both verbal and visuo-spatial WM declines with aging and follow a similar pattern of decline although the rate of decline was not uniform. Pattern of decline might classify the adult life span into two groups. First group below 60 years of age, WM steep decline steeply. Second group above 60 years of age, relatively no change in WM span.
Reduced sensory acuity is pervasive with normal aging. Decline in sensory acuity might affect their cognitive tasks performance [36, 37, 38]. It was observed that participants above 61–70 years needed repetition of digits even presented louder. Even they listened the digits quite well to repeat them, had difficulty to memorizing and recalling digits. Similar difficulties were observed for visuo spatial tasks also. Pairwise comparison showed only a significant difference between after 60 years of age group and earlier age groups not amongst the age group after 60 years or below 60 years. Moreover, similar findings were found in previous studies that older adults are relatively more impaired than younger adults in verbal WM tasks [39, 40, 41, 42] and visuo-spatial WM tasks [43, 44].
Both verbal and visuo-spatial WM modalities were assessed for forward (DFS & VSFS) and backward span (DBS & VSBS) tasks. Forward spans were higher than backward spans in both modalities. Backward span task performances are regulated and control by central executive system [40]. Aging have a significant negative impact on central executive. Consequently, rate of decline for DBS should be more than DFS. But our result suggests a similar pattern of decline for both forward and backward span task. Moreover recent studies attributed a similar finding [40, 42]. These studies reported that a backward span task relies heavily on WM processing as compared to forward span tasks. Along with storing information as in the forward span, backward span needs concurrent processing that leads to lower span. In visuo-spatial the forward and backward span decline pattern was not as similar as verbal modalities. The VSFS decline at equal rate throughout 40-above 80 years. However, VSBS follow the DBS pattern. The asymmetrical pattern of decline might emphasize the differential role of central executive between both tasks. DFS was found within normal range of 5–8 digits [45]. However the DBS approximate towards the lowest value of normal range within 4–5 digits [46, 47]. Although, normative values for visuo-spatial modality were not reported in literature the VSFS and VSBS were found in the range of 3–4 and 2–4 respectively.
A parallel visuo-spatial version of the verbal WM task was developed in terms of the task demand characteristics, but verbal WM span was better than visuo-spatial WM span. Moreover, difference between verbal and visuo-spatial span was not statistically significant. Slightly better performance in verbal tasks might be due to difference between both modalities. Research attributed to WM found that the visuo-spatial WM may be much more limited in capacity than verbal WM [48]. If both modalities are differ significantly in their capacity then difference in span between these tasks should also be large and statistically significant. However, results of present finding violating the limited capacity view for verbal WM.
Verbal tasks are better compatible with speech than visuo-spatial tasks that help in retention of verbal information. Consequently, higher verbal WM span than visuo-spatial WM span across age groups. On the other hand, symbol used for verbal (digits) and visuo-spatial (dots) task modalities were different. Recalling positions or locations of dot sequences are not as common as digits. Consequently recalling dot involves more central executive control [49]. Future study should control the symbol used for assessing the WM ability in both modality.
Another fact explaining the asymmetry could be due to difference in rehearsal mechanism in both modalities. Verbal domain has well-practiced internal rehearsal mechanism using articulatory process of the phonological loop for the maintenance, which might be lacking in visuo-spatial domains [48]. In addition to that, visuo-spatial domain contributes to a continuous image generation process for overall image rather than for a single location [50].
Influence of symbols display in both modalities might also influence the WM span performance cannot be ignored [51]. Present study used the grid format for displaying the sequences location of dot that provides verbal re-coding of the locations of the dots. Contrary, our result revealed that verbal recoding did not influence the visuo-spatial WM task as compared to verbal tasks, as visuo-spatial span is lower than verbal span. Previous studies using a grid arrangement for visuo-spatial tasks have reported that task is sensitive to spatial movement rather than verbal dual tasks [52, 53]. Future study of visuo-spatial task should be carried out in absence of grid format for displaying the symbols to minimize phonological recoding.
CONCLUSION
The results of this study present some important facts. Normal aging affect both the verbal and visual working memory in relatively similar way. Both domains decline gradually up to 50-60 years, and after 60s relative saturation in span takes place. There is no significant difference between verbal and visuo-spatial WM span, but verbal WM span is relatively higher than visuo-spatial WM span. Apart from this, the study provides a data base for the working memory span of native healthy adult in both domains that might help in differentiating the normal versus pathological aging.
References
- [1].Baddeley AD, Hitch G. Working memory. New York: Academic Press; 1974. [Google Scholar]
- [2].Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:447–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- [3].Atkinson R, Shiffrin R. Human memory: A proposed system and its control processes. New York: Academic Press; 1968. [Google Scholar]
- [4].Baddeley A. Working memory. United Kingdom: Oxford University Press; 1986. [Google Scholar]
- [5].Baddeley A. The episodic buffer: A new component of working memory? Trends Cogn Sci. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- [6].Cowan N. Evolving conceptions of memory storage, selective attention, and their mutual constraints within the human information-processing system. Psychol Bull. 1988;104:163–191. doi: 10.1037/0033-2909.104.2.163. [DOI] [PubMed] [Google Scholar]
- [7].Cowan N. Attention and memory: An integrated framework. New York: Oxford University Press; 1995. [Google Scholar]
- [8].Cowan N. Working memory capacity. New York: Psychology Press; 2005. [Google Scholar]
- [9].Hasher L, Zacks RT, May CP. Inhibitory control, circadian arousal, and age. Cambridge: MIT Press; 1999. [Google Scholar]
- [10].Hasher L, Tonev ST, Lustig C, Zacks R. Inhibitory control, environmental support, and self-initiated processing in aging. Hove: Psychology Press; 2001. [Google Scholar]
- [11].Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and a new view. San Diego: Academic Press; 1988. [Google Scholar]
- [12].Baddeley AD, Logie RH. Working memory: The multiple component models. United Kingdom: Cambridge University Press; 1999. [Google Scholar]
- [13].Wright HH, Fergadiotis G. Conceptualizing and measuring working memory and its relationship to aphasia. Aphasiology. 2012;26:258–278. doi: 10.1080/02687038.2011.604304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Repovs G, Baddeley A. The multi-component model of working memory: Explorations in experimental cognitive psychology. Neuroscience. 2006;139:5–21. doi: 10.1016/j.neuroscience.2005.12.061. [DOI] [PubMed] [Google Scholar]
- [15].Gathercole SE, Pickering SJ, Ambridge B, Wearing H. The structure of working memory from 4 to 15 years of age. Dev Psychol. 2004;40:177–190. doi: 10.1037/0012-1649.40.2.177. [DOI] [PubMed] [Google Scholar]
- [16].Conway ARA, Bunting MF, Engle RW, Kane MJ, Hambrick DZ, Wilhelm O. Working memory span tasks: A methodological review and user’s guide. Psychon B Rev. 2005;12:769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- [17].Kane MJ, Hambrick DZ, Tuholski SW, Wilhelm O, Payne TW, Engle RW. The generality of working memory capacity: A latent-variable approach to verbal and visuo-spatial memory span and reasoning. J Exp Psychol Gen. 2004;133:189–217. doi: 10.1037/0096-3445.133.2.189. [DOI] [PubMed] [Google Scholar]
- [18].Shah P, Miyake A. The separability of working memory resources for spatial thinking and language processing: An individual differences approach. J Exp Psychol Gen. 1996;125:4–27. doi: 10.1037//0096-3445.125.1.4. [DOI] [PubMed] [Google Scholar]
- [19].Vecchi T, Richardson JTE, Cavallini E. Passive storage versus active processing in working memory: Evidence from age-related variations in performance. Eur J Cogn Psychol. 2005;17:521–539. [Google Scholar]
- [20].Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clin Neu. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- [21].Diamond A. The early development of executive functions. New York: Academic Press; 2006. [Google Scholar]
- [22].Hitch GJ. Working memory in children: A cognitive approach. Oxford: Oxford University Press; 2006. [Google Scholar]
- [23].Gick ML, Craik FIM, Morris RG. Task complexity and age differences in working memory. Mem Cognition. 1988;16:353–361. doi: 10.3758/bf03197046. [DOI] [PubMed] [Google Scholar]
- [24].Chiappe P, Hasher L, Siegel LS. Working memory, inhibitory control, and reading disability. Mem Cognition. 2000;28:8–17. doi: 10.3758/bf03211570. [DOI] [PubMed] [Google Scholar]
- [25].Park DC, Payer D. Lifespan cognition: Mechanisms of change. New York: Academic Press; 2006. [Google Scholar]
- [26].Bopp KL, Verhaeghen P. Age-related differences in control processes in verbal and visuospatial working memory: Storage, transformation, supervision, and coordination. Journal Gerontol Psychol Sci. 2007;62B:239–246. doi: 10.1093/geronb/62.5.p239. [DOI] [PubMed] [Google Scholar]
- [27].Jenkins L, Myerson J, Joerding JA, Hale S. Converging evidence that visuospatial cognition is more age-sensitive than verbal cognition. Psycholo Aging. 2000;15:157–175. doi: 10.1037//0882-7974.15.1.157. [DOI] [PubMed] [Google Scholar]
- [28].Myerson J, Emery L, White DA, Hale S. Effects of age, domain, and processing demands on memory span: Evidence for differential decline. Aging Neuropsychol C. 2003;10:20–27. [Google Scholar]
- [29].Borella E, Carretti B, DeBeni R. Working memory and inhibition across the adult life-span. Acta Psychol. 2008;128:33–44. doi: 10.1016/j.actpsy.2007.09.008. [DOI] [PubMed] [Google Scholar]
- [30].Borella E, Ghisletta P, De Ribaupierre A. Age differences in text processing: the role of working memory, inhibition and processing speed. Journal Gerontol Psychol Sci. 2011;66:311–320. doi: 10.1093/geronb/gbr002. [DOI] [PubMed] [Google Scholar]
- [31].Park D, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- [32].Kumar N, Gupta N, Kishore J. Kuppuswamy's socioeconomic scale: updating income range for the year 2012. Indian J Public Health. 2012;56:103–104. doi: 10.4103/0019-557X.96988. [DOI] [PubMed] [Google Scholar]
- [33].Ganguli M, Ratclife G, Chandra V, Sharma S, Gilbey J, Pandav R, Bellie S, Ryan C, Baker C, Seaberg E, Dekosky S. Hindi version of MMSE: The development of a cognitive screening instrument for a largely illiterate rural elderly population in India. Int J Geriatr Psychiatry. 1995;10:367–377. [Google Scholar]
- [34].American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4th ed, text rev. Washington, DC: Author; 2000. [Google Scholar]
- [35].Kumar N. Cognitive-Linguistic Assessment Protocol for adults in Hindi. University of Mysore; Mysore: 2012. Unpublished dissertation. [Google Scholar]
- [36].Baldwin CL. Cognitive implications of facilitating echoic persistence. Mem Cognition. 2007;35:774–780. doi: 10.3758/bf03193314. [DOI] [PubMed] [Google Scholar]
- [37].Lunner T, Rudner M, Ronnberg J. Background and basic processes: Cognition and hearing aids. Scand J Psychol. 2009;50:395–403. doi: 10.1111/j.1467-9450.2009.00742.x. [DOI] [PubMed] [Google Scholar]
- [38].Wingfield A, Tun PA, McCoy SL. Hearing loss in older adulthood. What it is and how it interacts with cognitive performance. Curr Dir Psychol Sci. 2005;14:144–148. [Google Scholar]
- [39].De Beni R, Palladino P. Decline in working memory updating through ageing: Intrusion error analyses. Memory. 2004;12:75–89. doi: 10.1080/09658210244000568. [DOI] [PubMed] [Google Scholar]
- [40].Hester RL, Kinsella GJ, Ong B. Effect of age on forward and backward span tasks. J Int Neuropsychol Soc. 2004;10:475–481. doi: 10.1017/S1355617704104037. [DOI] [PubMed] [Google Scholar]
- [41].Van der Linden M, Bredart S, Beerten A. Age related differences in updating working memory. Brit J Psychol. 1994;85:145–152. doi: 10.1111/j.2044-8295.1994.tb02514.x. [DOI] [PubMed] [Google Scholar]
- [42].Wilde NJ, Strauss E, Tulsky DS. Memory span on the Wechsler scales. J Clin Exp Neuropsyc. 2004;26:539–549. doi: 10.1080/13803390490496605. [DOI] [PubMed] [Google Scholar]
- [43].Bruyer R, Scailquin J. Assessment of visuospatial short-term memory and effect of aging. Eur Rev Appl Psychol. 1999;49:175–180. [Google Scholar]
- [44].Rowe G, Hasher L, Turcotte J. Age difference in visuospatial working memory. Psychol Aging. 2008;23:79–84. doi: 10.1037/0882-7974.23.1.79. [DOI] [PubMed] [Google Scholar]
- [45].Kaplan E, Fein D, Morris R, Delis D. WAIS-R as a neuropsychological instrument. San Antonio: Psychological Corporation; 1991. [Google Scholar]
- [46].Botwinick J, Storand M. Memory related functions and age. Springfield: Charles C. Thomas; 1974. [Google Scholar]
- [47].Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. 4th ed. New York: Oxford University Press; 2004. [Google Scholar]
- [48].Miyake A, Friedman NP, Rettinger DA, Shah P, Hegarty M. How are visuospatial working memories, executive functioning, and spatial abilities related? A latent-variable analysis. J Exp Psychol Gen. 2001;130:621–640. doi: 10.1037//0096-3445.130.4.621. [DOI] [PubMed] [Google Scholar]
- [49].Baddeley AD. Exploring the central executive. Q J Exp Psychol. 1996;49A:5–28. [Google Scholar]
- [50].Kosslyn SM. Mental imagery. Cambridge: MIT Press; 1990. [Google Scholar]
- [51].Fiore F, Borella E, Mammarella IC, De Beni R. Age differences in verbal and visuo-spatial working memory updating: Evidence from analysis of serial position curves. Memory. 2011. pp. 1–14. iFirst. [DOI] [PubMed]
- [52].Logie RH, Zucco G, Baddeley AD. Interference with visual short-term memory. Acta Psychol. 1990;75:55–74. doi: 10.1016/0001-6918(90)90066-o. [DOI] [PubMed] [Google Scholar]
- [53].Salway AFS, Logie RH. Visuospatial working memory, movement control and executive demands. Brit J Psychol. 1995;86:253–269. doi: 10.1111/j.2044-8295.1995.tb02560.x. [DOI] [PubMed] [Google Scholar]


