Abstract
Brain aging is a multifactorial process that is occurring across multiple cognitive domains. A significant complaint that occurs in the elderly is a decrement in learning and memory ability. Both rodents and zebrafish exhibit a similar problem with memory during aging. The neurobiological changes that underlie this cognitive decline are complex and undoubtedly influenced by many factors. Alterations in the birth of new neurons and neuron turnover may contribute to age-related cognitive problems. Caloric restriction is the only non-genetic intervention that reliably increases life span and healthspan across multiple organisms although the molecular mechanisms are not well-understood. Recently the zebrafish has become a popular model organism for understanding the neurobiological consequences but to date very little work has been performed. Similarly, few studies have examined the effects of dietary restriction in zebrafish. Here we review the literature related to memory decline, neurogenesis, and caloric restriction across model organisms and suggest that zebrafish has the potential to be an important animal model for understanding the complex interactions between age, neurobiological changes in the brain, and dietary regimens or their mimetics as interventions.
Keywords: Dietary Restriction, Age, Zebrafish, Neuron Turnover, Synapses
It was previously thought that brain aging occurred as a consequence of neuronal death. However, converging lines of evidence in non-human primates, rats, and mice suggest that significant neuron loss does not contribute to age-related cognitive decline. For example, in rats, there is no change in the hippocampal neuron number throughout the aging process [1,2]. Besides cell death, cell regeneration also plays an important role in body homeostasis. We know that cell regeneration is significantly reduced in aging process [3]. Until recently, it was assumed that neurons were not regenerated. Now, while it is occurring at a much slower rate when compared to other tissues like the colon or skin; it is clear that neurons regenerate and we call this process neurogenesis [4,5]. With the pioneering work done at the end of the 1990s, it is evident that new neurons are formed in hippocampus [6] and they are related to learning and memory [7]. Thus, it is thought that age-related diseases like Alzheimer’s and mild cognitive impairment may be related to problems with the generation of new neurons, i.e. neurogenesis. In this review, we will discuss changes in neuron number and how they relate to brain aging, and effects of an intervention, caloric restriction. Moreover, we will introduce the zebrafish as a model organism for studying aging and dietary interventions.
Brain Aging
Aging is a multifactorial process and changes occur across multiple cognitive domains. Older individuals often report complaints including changes in sensory abilities and attention. However, a significant and common complaint among elders is a decline in learning and memory ability. These changes in learning and memory ability can really affect a person’s quality of life and range from forgetting episodes in one’s past to an ability to navigate spatial environment. Both of these changes reflect compromised hippocampal function and could potentially lead to restrictions in daily activities, which would interfere with an aged person’s ability to maintain an independent lifestyle [8,9].
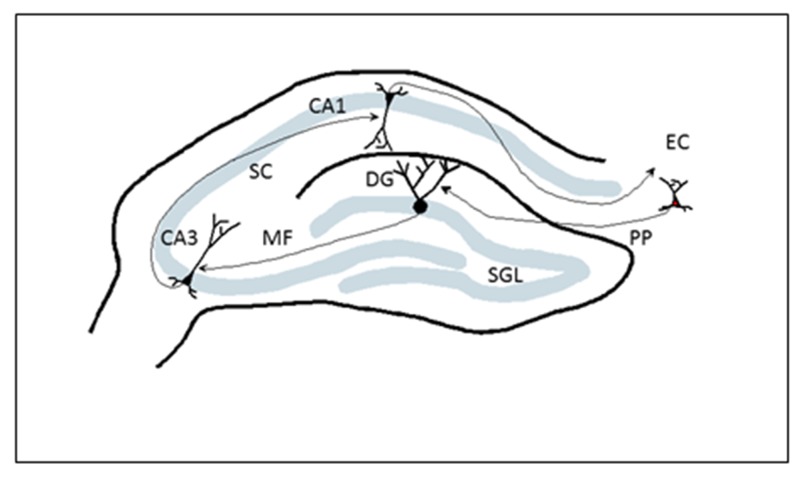
The hippocampus, also known as hippocampal formation, is a well-defined structure with three principal areas, including the dentate gyrus (DG), CA3, and CA1 regions (Figure 1). These areas are largely connected by unidirectional input that has been very well-described [10]. This input includes a projection from the entorhinal cortex (EC) to the DG via the perforant path, the mossy fiber (MF) input that comes from the DG to CA3 and the CA3 area projecting to the CA1 region via the schafer collateral (SC).
Figure 1:
Schematic diagram of the rodent hippocampus illustrating principal areas as well as main excitatory connectivity. CA1, CA3, dentate gyrus (DG), subgranular layer (SGL), perforant path (PP), entorhinal cortex (EC), mossy fiber (MF) and schafer collateral (SC) are shown.
Recent evidence from studies of neuronal number in the hippocampus of species from rodents to primates using unbiased stereological measurements has determined that significant cell loss does not occur in the aging hippocampus. Rapp and Gallagher demonstrated that in the CA1 and CA3 fields of hippocampus, the neuron numbers do not change between young and old age groups and neither is associated with cognitive status. Young (6 months old) and aged (27–28 months old) rats’ spatial learning and memory ability was assessed using a hippocampal-dependent, spatial version of the Morris water maze task [1]. This task measures the spatial bias for the position of a hidden escape platform based on the cues surrounding the test apparatus. Aged rats were segregated into two groups: those with and without learning impairments according to a spatial learning index [1]. Among the young and cognitively unimpaired and impaired aged animals, there were no differences in neuron numbers. In a similar study, neurons were counted in the hippocampus, parahippocampal region as a sum or in individual component areas of parahippocampal region such as perirhinal cortex, lateral entorhinal area, postrhinal cortex and medial entorhinal area [2]. Again, no differences were observed in the total neuron numbers among the groups.
Neurogenesis
Much of what we know about neurogenesis comes from rodent studies. There are two neurogenic areas in rodent brain, the subventricular zone (SVZ) and subgranular layer (SGL) of dentate gyrus. The cells that are born in SVZ migrate to the olfactory bulb through the rostral migratory system (RMS) and become olfactory neurons. Cells born in SGL remain in dentate gyrus and differentiate into granule cells (Figure 1) [5]. The fact that neurogenesis continues in the adult brain is an intriguing subject and draws much attention. We know that cells continue to proliferate but the rate of renewal and also whether these cells differentiate into functional neurons are the subjects of study, as well as the external factors that affect these processes. The most widely used method to observe neurogenesis is bromodeoxyuridine (BrdU) labeling. BrdU is a thymidine analog and incorporates into the DNA in the S-phase of cell division. It is possible to visualize these BrdU-incorporated cells with various tags and then to count them according to specific regions in the brain. Another method uses retroviral labeling which detects only the dividing cells and also enables researchers to visualize not only the soma but also the dendritic processes [11,12]. In a study using the technique of GFP labeling with a retroviral vector, the cells born in the dentate gyrus were labeled, and these newborn cells became glutaminergic neurons and formed functional synapses with hilar neurons, mossy cells and CA3 pyrimidal cells [12].
Many factors can both positively and negatively influence neurogenesis in the hippocampus and this has important implications for aging and caloric restriction. It is well-established that one of the environmental factors that has been found by a number of studies to accelerate neurogenesis is voluntary exercise (for a review please refer to [13]). In the pioneering study by van Praag et al., the newly formed cells were labeled with GFP specifically in the dentate gyrus. After 48 hours, there were GFP-positive cells in the runner and control group but after 4 weeks, a much higher percentage of these cells co-expressed neuronal markers in the runner group. Also, using immunocytochemical techniques to visualize synaptic terminals with synaptophysin and calbindin staining, it was shown that these newly formed GFP-positive cells received synaptic input [11]. These results supported an earlier study demonstrating that exercise enhances neurogenesis [14]. Although there are a vast number of studies showing a beneficial effect of voluntary running on neurogenesis, there is evidence that there are other underlying environmental factors that can influence the effects of exercise on neurogenesis. When three different strains of mice, one caught from wild, were tested in different rearing conditions, contrasting results were obtained. Running did not improve the cell numbers in dentate gyrus of wild-caught mice, as well as the laboratory mice that were raised in enriched environments [15]. This suggests that both exercise and environmental enrichment likely affect neurogenesis and the exact relationship among these variables is complex and intertwined.
Learning per se, especially hippocampal-dependent learning tasks, improves cell regeneration rates. In the rat dentate gyrus, the number of newly born neurons depends on associative learning tasks that require an intact hippocampus. It was shown that the newly formed cells had the morphology of granule neurons and expressed turned-on-after-division-64 kDA (TOAD-64), a marker for immature neurons. [7].
In the same way that exercise can accelerate the rates of neurogenesis, factors can have a negative effect on the birth of new neurons. Two of these are stress and radiation. One such study examined neuron number generation and spatial learning ability in adult mice that were exposed to prenatal stress. The results demonstrated that the number of granule cells was lower and also spatial learning was impaired in stressed mice as compared to the control group [16]. One of the more interesting findings from this work is that the effects of stress continue into the adulthood; the mice exhibit reduced neurogenesis even when they were 22-months old. Radiation is yet another factor that negatively affects neurogenesis. Twenty-one-day old mice exhibited reduced neurogenesis in the SGZ region after receiving irradiation. When tested after 3 months, they also exhibited learning impairments as tested with the Morris water maze task [17]. Also reductions in neurogenesis in dentate gyrus were correlated with impairments in hippocampal learning as evidenced by Barnes maze [18]. Thus, as an animal age, these effects on neurogenesis could have implications related to spatial learning and memory ability and then it would be important to examine different interventions, such as caloric restriction, which may alter the course of age-related changes.
The hippocampus plays a very important part in learning and memory. Since one of the two locations of neurogenesis is the dentate gyrus, whether the newly formed neurons are necessary for the formation of new memories or not is an important question. To answer this question, one group used a transgenic approach in young mice. With the conditional induction of transgenes, adult-born neurons in hippocampus were ablated and this reduction in new neuron formation in hippocampus affected spatial learning as tested by water maze experiments and contextual fear conditioning. [19]. This finding suggested that the acquisition of spatial relational memory is impaired by the inhibition of neurogenesis. In contrast to another study, after the knockdown of the presenilin 1 (PS1) gene in the dentate gyrus of mice, it was shown that the addition of new neurons was not required for memory formation [20]. Another group studied neurotrophin-3 (NT-3) and a link was found between neurogenesis and memory formation. Deletion of NT-3 had impacts on the differentiation of neurons in the dentate gyrus and since NT-3 mutants had impairments in spatial learning [21], it was suggested that neurogenesis, at least the differentiation process of new neurons, might be important in hippocampal memory formation.
Another approach to test neurogenesis and its relationship to cognitive functions that has been used previously is an antimitotic agent. Following treatment with methylazoxymethanol acetate (MAM), rats were tested in the Morris water maze to assess spatial learning and memory ability, as well as trace and conditional fear conditioning. These tasks all require an intact hippocampus and in MAM-treated animals, although the number of neurons with BrdU labeling was reduced extensively in dentate gyrus, only trace fear conditioning was affected. Contextual fear conditioning or spatial learning was not changed with the reduced cell numbers [22]. These data suggest that there is a relationship between neuron number and learning and memory ability although it is not conclusive.
Neurogenesis throughout Aging
The evidence is clear at this point that neurogenesis is not restricted to developmental stages and continues into adulthood. But the rate of cell renewal decreases and although it does not stop, it might play a role in cognitive aging as well as diseases such as dementia and Alzheimer’s. The degree of reduction in neurogenesis and the ways to prevent this reduction during aging is a hot topic among researchers.
One of the two sites of neurogenesis in the adult brain is SVZ and the architecture of the SVZ changes during aging. When tested in young, middle-aged and old mice, SVZ thinning is observed in aged animals [23]. Decreases in cell proliferation, the number of neuroblasts and transient amplifying progenitor cells were also observed. When tested throughout the gradual aging process, it was shown that the neuron proliferation decreases between 2 and 18 months of age and stays relatively stable between 18 and 24 months. Age-dependent decreases in proliferation were observed in the granular cell layer and hilus of hippocampus [24]. Moreover, in the aging brain, migration, survival, and neuronal fate choice remains stable but expression of neuronal markers and dendritic growth diminishes [25]. Therefore, there are age-related changes throughout the dentate gyrus, which is the site of neurogenesis in the hippocampus.
As mentioned previously there are ways to alter the number of neurons being born. Two of the ways of increasing the number of new neurons are experience and hormones. When adult and old mice were placed in an enriched environment, the survival of BrdU cells was increased and more cells in the dentate gyrus were differentiated into neurons in both groups [26]. Enriched environment in this situation meant more opportunities for social interaction, exploration, and physical activity for mice. Moreover, neurogenesis in the adult dentate gyrus decreases with age but is shown to be reversible with corticosteroid treatment in rats [27]. Similarly, when tested in young (5 months), middle-aged (18 months), and old (28 months) male Fisher 344XBrown Norway rats, neurogenesis decreased with increasing age but restoration of insulin-like growth factor-I (IGF-I) levels increased the neuronal production [28]. Neurogenesis and also the number of doublecortin (DCX), an immature neuronal marker, expressing neurons decreases in the DG, SGZ, and SVZ of 20 month-old mice as compared to 3 month-old animals. Fibroblast growth factor (FGF)-2 and heparin-binding epidermal growth factor-like growth factor (HB-EGF) restore the levels in both regions indicating that the brain can respond to external factors in making new neurons [29].
Neurogenesis and its relationship with hippocampal function have been studied in the context of aging. In young and aged rats, neurogenesis was compared in relationship to performance on the Morris water maze task. Interestingly, it was found that neurogenesis was not preserved in aged rats with intact cognitive abilities. These results suggested that neurogenesis might have different roles in young or old animals [30,31]. Thus, it may be that the process of neurogenesis is altered with age. p16INK4a is a cyclin-dependent kinase inhibitor and a well-known component of cancer related pathways, which is activated during cellular senescence [32]. It appears that p16INK4a also plays a role in the decline of neurogenesis throughout aging. It was shown that p16INK4a deficient mice exhibited reduced declines in neurogenesis in SVZ region but did not have any difference in the dentate gyrus [33].
The study of the process of neurogenesis in the context of aging is important and not limited to the rodent hippocampus. The pioneering work by Gould et al. in 1990s demonstrated that neurogenesis continues even at old age and in primates. Adult Old World monkeys were injected with BrdU and examined with neuronal and glial markers. The newly formed cells were located in dentate gyrus and expressed markers of mature neurons. After 2 hours the newly formed cells were neuron-specific enolase (NSE) negative whereas after 2 weeks the cells were immunoreactive for NSE and NeuN. Although low in number, old monkeys (age 23) exhibited BrdU positive cells indicating that neurogenesis continues in the old age [6].
Caloric Restriction
Caloric restriction (CR) extends life and health spans in diverse species. Currently, it is known as the only non-genetic intervention to delay age-related cognitive decline and diseases in mammals [34,35,36]. In 1935, Clive McCay et al. reported that reducing the amount of foods of rats resulted in extended maximum and average life span due to the retardation of growth [37]. However, CR was not widely studied as a scientific model that could delay the aging process until late 1900s [35]. In 1987, it was shown that learning and motor performance of calorically restricted aged mice increased [38]. In 1997, studies of CR in mice and rats proved to slow primary aging and protect against the deterioration in tissue structure and function, which would increase the longevity up to 50% by increasing survival rates [39,40]. Later in 20th century it was reported that CR might extend the life span of various other model organisms such as budding yeast [34,41,42], flies [43,44], spiders [45], worms [46,47], fish [48] and monkeys [34,49]. The findings are promising for humans who dream of a longer life span. Yet, the exact mechanism underlying CR remains elusive.
The aim of CR is to have a 30–40% reduction in the overall calorie uptake without causing any malnutrition. This reduction should be maintained over a certain time period and the method should not be confused with short-term or prolonged starvation. CR has always been associated with war-related extreme conditions or economic insufficiencies in poor countries. For instance, the number of centenarians per 100000 people in Okinawa, Japan is greater than any other parts of the world. Historically, poverty is believed to impose severe CR in older generations of Okinawa [50,51]. Similarly, a correlational study on Jewish women, who suffered from hunger under Nazi rule during the World War II, states that CR may increase the susceptibility for breast cancer [52]. Contrary to this study, 1944–1945 Dutch famine is thought to lower the risk of breast cancer since it might have imposed a transient CR [53]. Even though these ecologic cohort studies provide very interesting information regarding the effect of food on health and longevity, it should be noted that CR is not a form of starvation.
Since the regime and duration of CR differs according to the model organism of interest, there is a misconception in the public and the literature about how and why CR works. While some CR experiments use the alternate day feeding method, in which the food is provided every other day, some others chooses to reduce the daily portion of the meal. While CR is imposed on the budding yeast, Saccharomyces cerevisiae, by reducing the glucose concentration in rich media from 2% to 0.5% in some studies [34,41,54,55], in another study, substituting the ethanol-containing medium with water is suggested to provide an extreme CR condition [56]. CR is often referred as dietary restriction (DR), which is known to extend life and health span in a wide variety of organisms. However, DR does not necessarily result in the reduction of calorie uptake. It was reported that reduction of yeast extract or sugar could prevent primary aging and extend life span of Drosophila melanogaster; however, this reduction is not merely because of the reduction of the calorie intake. When the effects of yeast extract and sugar were tested separately, it was shown that the reduction in calorie intake itself does not extend the life span of fruit flies [57]. Additionally, the starting age and duration of CR should be well proposed to have significant and consistent results. If CR is applied to subjects for less than three weeks in the form of starvation, induction of acute stress is inevitable for the organism, which might overshadow the effects of CR. The logic underlying CR to extend the life span is likely to be the adaption of the organism evolutionarily to reallocate limited resources during the periods of food shortage [58,59]. On the other hand, it was previously reported that a short-term CR could regulate glucose ingestion in hypothalamus of patients with Type-2 diabetes [60], improve muscle stem cell availability and muscle repair in mice [61], increase growth hormone levels of short-obese children [62], and reduce the number of reactive oxygen species [63]. The power of the cohort studies cannot be underestimated. However, environmental changes and therefore the accumulation of mutations over time affect the life and health span negatively. Therefore, the most ideal experimental set up would include various birth cohorts and expose them to CR for a long time to assure the effects of CR at different developmental stages.
The applicability of CR to humans is controversial due to moral issues. It is anticipated that free-living people would not accept to undergo CR unless they are ensured that this diet is going to slow primary aging and protect against secondary aging as has been shown in rodent studies [37,38,39,40]. Therefore, it is much harder to have a controlled experiment on human subjects to see if CR works on humans. There are numerous external factors affecting the average and maximal life span of humans. To minimize the external factors, there have been several attempts to test CR in humans in the late 1900s. In 1991, four female and four male subjects aged between 28 and 67 were sealed inside an artificial ecosystem called Biosphere 2, where there was an emerging food scarcity for two years. Limited food availability caused an approximately 30% reduction in the daily calorie uptake through 6 months of closure; however, biospherians stated that the quality of their forced diet was excellent in terms of essential nutrient per calorie. According to the early results, this low-calorie low-fat nutrient-dense diet caused a 13% to 19% reduction in BMI of females and males, relatively [51,64,65]. Biospherians could observe a set of physiological changes including reduction in weight, blood pressure, total serum cholesterol, leukocyte count, fasting glucose, and triglyceride levels, which were previously shown in calorically restricted rodents [64]. The other attempt to test whether CR works in humans was initiated by the National Institute on Aging (NIA) in 2007 under a research program called CALERIE (Comprehensive Assessment of Long-Term Effects of Reducing Calorie Intake) [66]. To our knowledge, CALERIE is the first structured study to test the effects of prolonged CR in non-obese healthy human subjects.
CALERIE aims to unravel the similar or different effects of a 25% CR between humans and various model organisms when the weight changes are stabilized. CALERIE consists of two phases. In the first phase of study, the CALERIE research team found that 25% CR with or without exercise decreased cardiovascular disease risk [67], reduced DNA damage [68] and preserved the calcium intake confirming that this diet does not have any negative consequences on bone health [69]. The second phase of the study was initiated in April 2007. Stewart et al. describes the screening and recruitment methods of this study [70].
Another important aspect of CR is the examination of AL group. When an unlimited meal is provided to subjects, there is a possibility that the control group might consume more food and therefore consume more calories than they normally do. Several other health issues that emerge from this unhealthy diet may confound the results obtained in long-lasting costly CR experiments. To understand the effects of CR and to conclude a significant correlation with longevity, one should keep the food of the control group fixed. The main difference between CR and a regular diet is that people on ordinary diets aim to lose weight while all the proteins, carbohydrates, fat, minerals and vitamins are maintained at optimum level. However, a 30% reduction of calorie intake in humans requires much less food consumption, which would not only result in reductions in body temperature, blood pressure, and body/mass index, but also lead to depression.
It will likely be difficult for humans to comply to following a true CR diet. Thus, the development of CR-mimetics is of great interest to pharmaceutical companies. One molecular mechanism through which it is thought that CR exerts its effects is by blocking the mammalian target of rapamycin (mTOR) in the nutrient-signaling pathway. Thus, future studies need to be directed at altering the function of this molecule to determine whether CR directly mediates the effects.
Caloric Restriction and Synaptic Changes
In a series of consecutive studies, the effects of CR on synaptic levels were investigated. Rats were restricted throughout their life starting at 4 months of age. The restriction was a 40% reduction in the total daily caloric intake. Across lifes pan from young to middle and old age, a significant increase in the body weight of these rats was observed. Following the CR manipulation, the body weights were reduced significantly and stabilized across lifes pan [71]. In addition, key synaptic protein levels were stabilized across life span in CR animals as compared to their ad libitum-fed controls. One mechanism by which CR may be exerting its effects is by reducing IGF-1 levels. This polypeptide hormone plays a major role in the regulation of cellular processes in tissues throughout the body. In the nervous system, IGF-I is associated with neurotrophic effects and the amelioration of age-related cognitive impairment and it is a potent regulator of glutamate receptor levels. The plasma levels of IGF-I were tested in AL- and CR-fed animals. A modest decline was observed across life span and IGF-I levels have been reported to decline with age. Moreover, it was found that in the CR animals, IGF-I levels decreased as compared to their AL counterparts and the levels of this hormone was stabilized across lifes pan [72].
The AL-fed and CR animals, performance on the Morris water maze were tested in order to determine whether CR had a beneficial effect on hippocampal-dependent behavior. Both AL-fed and CR animals exhibited a decline in performance from young to middle age, however, AL continued to decline in old age whereas CR animals’ performance was stabilized. Thus, CR does not prevent an age-related decline but helps to stabilize a change that occurs at middle-age so as not to have a further decline in learning and memory performance [71].
Neurogenesis and Caloric Restriction
CR is one of the interventions that have been shown to extend life span and also to prevent brain aging. Although the exact mechanism of how a reduction in calories affects cognitive aging remains largely unknown, one possibility is that changes in neurogenesis might contribute to the altering the course of age-related cognitive decline. There are a limited number of studies investigating the relationship between neurogenesis and CR. Some of these are listed in Table 1.
Table 1:
A summary of the studies investigating the relationship between neurogenesis and caloric restriction
| Model organism | Age | Type of restriction | Reference | |
|---|---|---|---|---|
| Mice, male | 8 weeks | Alternate day feeding for 3 months | Increase in cell survival | [73] |
| Rats, male | 3 months old | Alternate day feeding | Increase in cell survival | [74] |
| Mice | 11 months old | Gradual decrease of calories* | Increase in cell numbers, glial? | [24] |
| Rats | 3–4 months old | Alternate day feeding for 3 months | Increase in neuronal phenotype | [75] |
| Rats, male | 21 days old or adult** | Protein restriction | Reduction in hippocampal progenitors | [76] |
CR was initiated at 10% at 14 weeks of age, lowered to 25% at 15 weeks of age and was set at 40% from 16 weeks onward
Fed with the protein restriction regime during lactation/weaning.
When 8 week-old mice were maintained on 3 months of dietary restriction (fed on alternate days), they were observed to have more cells survive in dentate gyrus when compared to ad libitum fed animals. Also in DR animals, brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) levels were shown to be increased [73]. In another study, caloric restriction and its effects on neurogenesis in mice were investigated. When BrdU-positive cells were counted, the hilus had more cells in the CR group. But when these cells were characterized, they were found to be glial cells since there was no co-labeling of BrdU with NeuN [24].
Two studies investigated CR in rats by applying an alternate day feeding regimen. In one study cell survival rates were found to be higher in the DR group when compared to AL group, suggesting that DR increases neurogenesis by increasing the survival of the newly generated cells in the dentate gyrus [74]. Likewise in the other study, DR enhanced the expression of the immature neuronal marker polysialic acid neural cell adhesion molecule (PSA-NCAM), BDNF and NT-3 [75].
In a study by de Godoy et al. [76], a different DR regimen was applied; the calorie content was kept constant in all groups, but in the test group the protein content was reduced to 8% instead of 20% of ad libitum fed animals. It was shown that in animals who were fed with a protein restricted diet during lactation and weaning, the progenitors are diminished in both the SGL and SVZ regions in adulthood. Interestingly, a decrease in SVZ was compensated by other progenitors whereas no such replacement was observed in SGL, emphasizing the importance of diet during early stages of life in terms of hippocampal development. Zebrafish as a Model Organism
The zebrafish (Danio rerio) is an ideal model organism to study human related diseases and especially aging. These fish live on average 3 years and age gradually like mammals [77]. Its genome is highly similar to human genome and there are orthologs for many human genes [78]. They have an integrated nervous system and exhibit advanced behavior properties like memory and social behavior [79]. Cognitive decline is observed during aging and this process is under the influence of external factors [80]. As the zebrafish age from 1 year to 3 years, it was documented that baseline motor activity declines and also learning is impaired as measured with conditioned place preference (CPP) and conditioned place avoidance (CPA) paradigms. Aging in zebrafish can be detected with senescence β-galactosidase (SA-β-Gal) activity. It was observed that SA-β-Gal staining increased from 18 to 36 months old. Also within the same age groups, SA-β-Gal staining was shown to increase after ionizing radiation [81]. A very interesting phenomena in zebrafish is that it has constitutive telomerase activity, unlike mammalian cells [82,77].
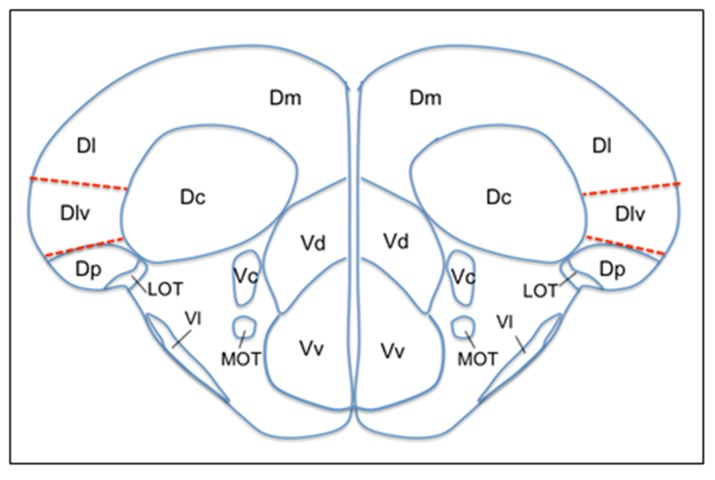
All the major regions in the zebrafish brain have been mapped and it is possible to locate the specific regions by taking cross-sectional slices and referring to the corresponding plate in the atlas by Wullimann et al. [83]. In their comprehensive review [84], Wullimann and Mueller contrast zebrafish as well as other teleostean forebrains to a mammalian’s brain. Among other well-defined regions of zebrafish brain, area dorsalis telencephali (pallium) and area ventralis (subpallium) is of particular interest. Medial pallium has been considered as the hippocampal equivalent and this region corresponds to the ventral division of the lateral zone of area dorsalis (Dlv) in zebrafish [84] (Figure 2).
Figure 2.
Schematic drawings of zebrafish brain anatomy showing the dorsal and ventral telencephalic regions. Drawing corresponds to the cross section 71 of the Wullimann atlas [83]. Medial pallium is shown with dashed lines which is the ventral division of the lateral zone of area dorsalis (Dlv) according to Wullimann and Mueller [84]. D, dorsal telencephalic area; Dc, central zone of D; Dl, lateral zone of D; Dm, medial zone of D; Dp, posterior zone of D; LOT, lateral olfactory tract; MOT, medial olfactory tract; V, ventral telencephalic area; Vc, central nucleus of V; Vd, dorsal nucleus of V; Vl, lateral nucleus of V; Vv, ventral nucleus of V.
Neurogenesis in Zebrafish
Neurogenesis is an ongoing and a widespread process in adult zebrafish. In their meticulous work [85], Zupanc et al. mapped the proliferation zones along with differentiation and migration patterns of newly formed cells in the zebrafish brain. The newly formed cells were shown to be present in a large number of areas in the zebrafish brain but with higher ratios in the regions of dorsal telencephalon, preoptic area of the diencephalon, torus longitudinalis, optic tectum of the mesencephalon and cerebellum [85]. Among these regions, dorsal telencephalon is especially notable. The posterior zone of the dorsal telencephalic area (Dp) and the ventral portion of the lateral zone of the dorsal telencephalic area (Dl) have been thought as the hippocampal equivalent in zebrafish [84]. And in these regions, Zupanc et al. have observed high proliferative activity and more importantly in the medial, lateral, and posterior zones of the dorsal telencephalic areas they showed that a large numbers of cells that were co-labeled with neuronal marker Hu protein and BrdU indicating that the newly formed cells are differentiated into neurons [85].
The zebrafish brain exhibits sexual dimorphism as demonstrated by the Dermon Lab. When adult fish were injected intraperitoneally with BrdU and tested for the presence of newly formed neurons after 24h (short survival) or 21 days (long survival), they showed that the long-term survival of BrdU-positive cells differed between sexes. The difference was shown to be mainly in the telencephalic Dm and the diencephalic PPv [86]. Also in the same study, the newly formed cells were found to start expressing neuronal markers from the day 21 and commit to be neurons [86]. In a previous study, regardless of the age, male and female zebrafish differed in short survival of cells in the molecular layer of corpus cerebelli (CCe) and the granular layer of the caudal lobe of the cerebellum (LCa) with an increased proliferation in males [87].
Caloric Restriction in Zebrafish
This gradual process of aging related decline in zebrafish allows for an extended timetable to determine the timing and duration for interventions such as moderate CR (MCR). The zebrafish model is an advantageous one for the evaluation of potential CR applications for interventions in humans in which lifelong MCR might not be feasible. To date there are very few studies that have examined CR in zebrafish and the regimen is not restriction but a fasting regime [88,89]. As was mentioned early, CR is thought to mediate its effects through the mTOR in the nutrient signaling pathway [90] and data on the neural consequences of CR and CR-mimetics at ages and durations relevant to clinical translation are sorely limited. Thus, the zebrafish is an excellent model organism for studying the molecular mechanisms of CR, as well as for performing drug screens to examine other possible CR-mimetics.
Concluding Remarks
The inconsistencies in cognitive performance may indicate that the timing and duration of CR could be important as to whether it has beneficial effects. The type of dietary regimen, as well as the sex of the animal will affect the neural responses to CR. Moreover, changes that are beneficial when CR is initiated in young animals may have different effects when initiated at older ages. As 1) the consequences of CR are heterogeneous, complex, and undoubtedly influenced by factors yet to be identified and 2) aging-related changes in the nervous system also are multifactorial and incompletely defined, introduction of CR to older animals is likely to result in a neural response that is altered significantly from that occurring when CR is begun in young animals. Thus, determining appropriate timing of CR is extremely important for possible translation to humans. Therefore, information about the neural consequences of CR in the zebrafish model is sorely lacking. More importantly, understanding the biological changes that are underlying age-related cognitive decline will provide targets for the development of possible mimetics that can be used in humans to prevent age-related cognitive decline, and these drugs could be easily tested and screened in a species such as the zebrafish.
References
- [1].Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci U S A. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rapp PR, Deroche PS, Mao Y, Burwell RD. Neuron number in the parahippocampal region is preserved in aged rats with spatial learning deficits. Cereb Cortex. 2002;12:1171–1179. doi: 10.1093/cercor/12.11.1171. [DOI] [PubMed] [Google Scholar]
- [3].Campisi J, d’ Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- [4].Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. Boca Raton (FL): CRC Press; 2007. [PubMed] [Google Scholar]
- [5].Lazarov O, Mattson MP, Peterson DA, Pimplikar SW, van Praag H. When neurogenesis encounters aging and disease. Trends Neurosci. 2010;33:569–579. doi: 10.1016/j.tins.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci USA. 1999;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nature Neuroscience. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- [8].Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, Sutherland RJ. The aging hippocampus: cognitive, biochemical and structural findings. Cereb Cortex. 2003;13:1344–1351. doi: 10.1093/cercor/bhg081. [DOI] [PubMed] [Google Scholar]
- [9].Moffat SD, Kennedy KM, Rodrigue KM, Raz N. Extrahippocampal contributions to age differences in human spatial navigation. Cereb Cortex. 2007;17:1274–1282. doi: 10.1093/cercor/bhl036. [DOI] [PubMed] [Google Scholar]
- [10].Andersen P, Morris R, Amaral D, Bliss T, O’Keefe J, editors. The Hippocampus book. Oxford: Oxford University Press; 2007. [Google Scholar]
- [11].van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vivar C, Potter MC, van Praag H. All About Running: Synaptic Plasticity, Growth Factors and Adult Hippocampal Neurogenesis. Curr Top Behav Neurosci. 2012;15:189–210. doi: 10.1007/7854_2012_220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schaefers ATU. Rearing conditions and domestication background determine regulation of hippocampal cell proliferation and survival in adulthood - Laboratory CD1 and C57Bl/6 mice versus wild house mice. Neuroscience. 2013;228:120–127. doi: 10.1016/j.neuroscience.2012.10.020. [DOI] [PubMed] [Google Scholar]
- [16].Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- [18].Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- [19].Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Feng R, Rampon C, Tang YP, Shrom D, Jin J, Kyin M, Sopher B, Miller MW, Ware CW, Martin GM, Kim SH, Langdon RB, Sisodia SS, Tsien JZ. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron. 32:911–926. doi: 10.1016/s0896-6273(01)00523-2. [DOI] [PubMed] [Google Scholar]
- [21].Shimazu K, Zhao M, Sakata K, Akbarian S, Bates B, Jaenisch R, Lu B. NT-3 facilitates hippocampal plasticity and learning and memory by regulating neurogenesis. Learn Mem. 2006;13:307–315. doi: 10.1101/lm.76006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC. The aging neurogenic subventricular zone. Aging Cell. 2006;5:139–152. doi: 10.1111/j.1474-9726.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- [24].Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol Aging. 2004;25:333–340. doi: 10.1016/S0197-4580(03)00083-6. [DOI] [PubMed] [Google Scholar]
- [25].Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci. 2005;21:464–476. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- [26].Kempermann G, Kuhn HG, Gage FH. Experience-Induced Neurogenesis in the Senescent Dentate Gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cameron, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- [28].Lichtenwalner R, Forbes M, Bennett S, Lynch C, Sonntag W, Riddle D. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- [29].Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, Logvinova A, Greenberg DA. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003;2:175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- [30].Bizon JL, Lee HJ, Gallagher M. Neurogenesis in a rat model of age-related cognitive decline. Aging Cell. 2004;3:227–234. doi: 10.1111/j.1474-9728.2004.00099.x. [DOI] [PubMed] [Google Scholar]
- [31].Bizon JL, Gallagher M. More is less: neurogenesis and age-related cognitive decline in Long-Evans rats. Sci Aging Knowledge Environ. 2005 Feb;7:re2. doi: 10.1126/sageke.2005.7.re2. [DOI] [PubMed] [Google Scholar]
- [32].Ozturk M, Arslan-Ergul A, Bagislar S, Senturk S, Yuzugullu H. Senescence and immortality in hepatocellular carcinoma. Cancer Letters. 2009;286:103–113. doi: 10.1016/j.canlet.2008.10.048. [DOI] [PubMed] [Google Scholar]
- [33].Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- [35].Roth GS, Ingram DK, Lane MA. Caloric restriction in primates and relevance to humans. Ann NY Acad Sci. 2001;928:305–315. doi: 10.1111/j.1749-6632.2001.tb05660.x. [DOI] [PubMed] [Google Scholar]
- [36].Weindruch R, Walford R. The Retardation of Aging and Disease by Dietary Restriction. Springfield, Illinois: Charles C Thomas; 1988. [Google Scholar]
- [37].McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. Nutrition. 1989;5:155–171. [PubMed] [Google Scholar]
- [38].Ingram DK, Weindruch R, Spangler EL, Freeman JR, Walford RL. Dietary restriction benefits learning and motor performance of aged mice. J Gerontol. 1987;42:78–81. doi: 10.1093/geronj/42.1.78. [DOI] [PubMed] [Google Scholar]
- [39].Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Flier JS, Underhill LH, Weindruch R, Sohal RS. Caloric Intake and Aging. New England Journal of Medicine. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jiang JC, Jaruga E, Repnevskaya MV, Jazwinski SM. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J. 2000;14:2135–2137. doi: 10.1096/fj.00-0242fje. [DOI] [PubMed] [Google Scholar]
- [43].Chapman T, Partridge L. Female fitness in Drosophila melanogaster: an interaction between the effect of nutrition and of encounter rate with males. Proc Biol Sci. 1996;263:755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- [44].Chippindale AK, Leroi AM, Kim SB, Rose MR. Phenotypic plasticity and selection in Drosophila life-history evolution. I Nutrition and the cost of reproduction. J Evol Biol. 1993;6:171–193. [Google Scholar]
- [45].Austad SN. Life extension by dietary restriction in the bowl and doily spider, Frontinella pyramitela. Exp Gerontol. 1989;24:83–92. doi: 10.1016/0531-5565(89)90037-5. [DOI] [PubMed] [Google Scholar]
- [46].Houthoofd K, Braeckman BP, Johnson TE, Vanfleteren JR. Life extension via dietary restriction is independent of the Ins/IGF-1 signalling pathway in Caenorhabditis elegans. Exp Gerontol. 2003;38:947–954. doi: 10.1016/s0531-5565(03)00161-x. [DOI] [PubMed] [Google Scholar]
- [47].Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev. 1977;6:413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- [48].Novak CM, Jiang X, Wang C, Teske JA, Kotz CM, Levine JA. Caloric restriction and physical activity in zebrafish (Danio rerio) Neurosci Lett. 2005;383:99–104. doi: 10.1016/j.neulet.2005.03.048. [DOI] [PubMed] [Google Scholar]
- [49].Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric Restriction Delays Disease Onset and Mortality in Rhesus Monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chan YC, Suzuki M, Yamamoto S. Nutritional status of centenarians assessed by activity and anthropometric, hematological and biochemical characteristics. J Nutr Sci Vitaminol. 1997;43:73–81. doi: 10.3177/jnsv.43.73. [DOI] [PubMed] [Google Scholar]
- [51].Holloszy JO, Fontana L. Caloric restriction in humans. Exp Gerontol. 2007;42:709–712. doi: 10.1016/j.exger.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Vin-Raviv N, Barchana M, Linn S, Keinan-Boker L. Severe caloric restriction in young women during World War II and subsequent breast cancer risk. Int J Clin Pract. 2012;66:948–958. doi: 10.1111/j.1742-1241.2012.02966.x. [DOI] [PubMed] [Google Scholar]
- [53].Elias SG, Peeters PHM, Grobbee DE, van Noord PAH. Breast cancer risk after caloric restriction during the 1944–1945 Dutch famine. J Natl Cancer Inst. 2004;96:539–546. doi: 10.1093/jnci/djh087. [DOI] [PubMed] [Google Scholar]
- [54].Li B, Skinner C, Castello PR, Kato M, Easlon E, Xie L, Li T, Lu SP, Wang C, Tsang F, Poyton RO, Lin SJ. Identification of potential calorie restriction-mimicking yeast mutants with increased mitochondrial respiratory chain and nitric oxide levels. J Aging Res. 2011;2011:673185. doi: 10.4061/2011/673185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- [56].Wei M, Fabrizio P, Hu J, Ge H, Cheng C, Li L, Longo VD. Life Span Extension by Calorie Restriction Depends on Rim15 and Transcription Factors Downstream of Ras/PKA, Tor, and Sch9. PLoS Genetics. 2008;4:e13. doi: 10.1371/journal.pgen.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mair W, Piper MDW, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Holliday R. Food, reproduction and longevity: is the extended lifespan of calorie-restricted animals an evolutionary adaptation? Bioessays. 1989;10:125–127. doi: 10.1002/bies.950100408. [DOI] [PubMed] [Google Scholar]
- [59].Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu Rev Biochem. 2008;77:727–754. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- [60].Teeuwisse WM, Widya RL, Paulides M, Lamb HJ, Smit JWA, de Roos A, van Buchem MA, Pijl H, van der Grond J. Short-term caloric restriction normalizes hypothalamic neuronal responsiveness to glucose ingestion in patients with type 2 diabetes. Diabetes. 2012;61:3255–3259. doi: 10.2337/db11-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cerletti M, Jang YC, Finley LWS, Haigis MC, Wagers AJ. Short-term calorie restriction enhances skeletal muscle stem cell function. Cell Stem Cell. 2012;10:515–519. doi: 10.1016/j.stem.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Rose SR, Burstein S, Burghen GA, Pitukcheewanont P, Shope S, Hodnicak V. Caloric restriction for 24 hours increases mean night growth hormone. J Pediatr Endocrinol Metab. 1999;12:175–183. doi: 10.1515/JPEM.1999.12.2.175. [DOI] [PubMed] [Google Scholar]
- [63].Wang C, Maddick M, Miwa S, Jurk D, Czapiewski R, Saretzki G, Langie SAS, Godschalk RWL, Cameron K, von Zglinicki T. Adult-onset, short-term dietary restriction reduces cell senescence in mice. Aging (Albany NY) 2010;2:555–566. doi: 10.18632/aging.100196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Walford RL, Harris SB, Gunion MW. The calorically restricted low-fat nutrient-dense diet in Biosphere 2 significantly lowers blood glucose, total leukocyte count, cholesterol, and blood pressure in humans. Proc Natl Acad Sci USA. 1992;89:11533–11537. doi: 10.1073/pnas.89.23.11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Redman LM, Ravussin E. Caloric restriction in humans: impact on physiological, psychological, and behavioral outcomes. Antioxid Redox Signal. 2011;14:275–287. doi: 10.1089/ars.2010.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Rickman AD, Williamson DA, Martin CK, Gilhooly CH, Stein RI, Bales CW, Roberts S, Das SK. The CALERIE Study: Design and methods of an innovative 25% caloric restriction intervention. Contemporary Clinical Trials. 2011;32:874–881. doi: 10.1016/j.cct.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lefevre M, Redman LM, Heilbronn LK, Smith JV, Martin CK, Rood JC, Greenway FL, Williamson DA, Smith SR, Ravussin E. Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis. 2009;203:206–213. doi: 10.1016/j.atherosclerosis.2008.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Redman LM, Rood J, Anton SD, Champagne C, Smith SR, Ravussin E. Calorie restriction and bone health in young, overweight individuals. Arch Intern Med. 2008;168:1859–1866. doi: 10.1001/archinte.168.17.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Stewart TM, Bhapkar M, Das S, Galan K, Martin CK, McAdams L, Pieper C, Redman L, Roberts S, Stein RI, Rochon J, Williamson DA. Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy Phase 2 (CALERIE Phase 2) screening and recruitment: methods and results. Contemp Clin Trials. 2013;34:10–20. doi: 10.1016/j.cct.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Adams MM, Shi L, Linville MC, Forbes ME, Long AB, Bennett C, Newton IG, Carter CS, Sonntag WE, Riddle DR, Brunso-Bechtold JK. Caloric Restriction and Age Affect Synaptic Proteins in Hippocampal CA3 and Spatial Learning Ability. Exp Neurol. 211:141–149. doi: 10.1016/j.expneurol.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Adams MM, Elizabeth Forbes M, Constance Linville M, Riddle DR, Sonntag WE, Brunso-Bechtold JK. Stability of local brain levels of insulin-like growth factor-I in two well-characterized models of decreased plasma IGF-I. Growth Factors. 2009;27:181–188. doi: 10.1080/08977190902863639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lee J, Seroogy KB, Mattson MP. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem. 2002;80:539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- [74].Lee J, Duan W, Long JM, Ingram DK, Mattson MP. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J Mol Neurosci. 2000;2:99–108. doi: 10.1385/JMN:15:2:99. [DOI] [PubMed] [Google Scholar]
- [75].Kumar S, Parkash J, Kataria H, Kaur G. Interactive effect of excitotoxic injury and dietary restriction on neurogenesis and neurotrophic factors in adult male rat brain. Neurosci Res. 2009;65:367–374. doi: 10.1016/j.neures.2009.08.015. [DOI] [PubMed] [Google Scholar]
- [76].de Godoy MA, de Souza AS, Alves Lobo M, Kress Sampaio OV, Moraes L, Baldanza Ribeiro M, Ribeiro Magri TP, Wernerck de Castro JPS, Tavares do Carmo MG, da Mota MS, Rocha S, Mendez-Otero R, Felippe Santiago M. Effects of protein restriction during gestation and lactation on cell proliferation in the hippocampus and subventricular zone: Functional implications. Protein restriction alters hippocampal/SVZ cell proliferation. Brain Res. 2012;1496:10–27. doi: 10.1016/j.brainres.2012.10.047. [DOI] [PubMed] [Google Scholar]
- [77].Kishi S, Uchiyama J, Baughman AM, Goto T, Lin MC, Tsai SB. The zebrafish as a vertebrate model of functional aging and very gradual senescence. Exp Geront. 2003;38:777–786. doi: 10.1016/s0531-5565(03)00108-6. [DOI] [PubMed] [Google Scholar]
- [78].Gerhard GS, Cheng KC. A call to fins! Zebrafish as a gerontological model. Aging Cell. 2002;1:104–111. doi: 10.1046/j.1474-9728.2002.00012.x. [DOI] [PubMed] [Google Scholar]
- [79].Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- [80].Yu L, Tucci V, Kishi S, Zhdanova IV. Cognitive Aging in Zebrafish. PLoS ONE. 2006;1:e14. doi: 10.1371/journal.pone.0000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Tsai SB, Tucci V, Uchiyama J, Fabian NJ, Lin MC, Bayliss PE, Neuberg DS, Zhdanova IV, Kishi S. Differential effects of genotoxic stress on both concurrent body growth and gradual senescence in the adult zebrafish. Aging Cell. 2007;6:209–224. doi: 10.1111/j.1474-9726.2007.00278.x. [DOI] [PubMed] [Google Scholar]
- [82].Kishi S. Functional Aging and Gradual Senescence in Zebrafish. Ann NY Acad Sci. 2004;1019:521–526. doi: 10.1196/annals.1297.097. [DOI] [PubMed] [Google Scholar]
- [83].Wullimann MF, Rupp B, Reichert H. Neuroanatomy of the zebrafish brain : a topological atlas. Basel; Boston: Birkhäuser Verlag; 1996. [Google Scholar]
- [84].Wullimann MF, Mueller T. Teleostean and mammalian forebrains contrasted: Evidence from genes to behavior. J Comp Neuro. 2004;475:143–162. doi: 10.1002/cne.20183. [DOI] [PubMed] [Google Scholar]
- [85].Zupanc GKH, Hinsch K, Gage FH. Proliferation, migration, neuronal differentiation, and long-term survival of new cells in the adult zebrafish brain. J Comp Neurol. 2005;488:290–319. doi: 10.1002/cne.20571. [DOI] [PubMed] [Google Scholar]
- [86].Ampatzis K, Makantasi P, Dermon CR. Cell proliferation pattern in adult zebrafish forebrain is sexually dimorphic. Neurosci. 2012;226:367–381. doi: 10.1016/j.neuroscience.2012.09.022. [DOI] [PubMed] [Google Scholar]
- [87].Ampatzis K, Dermon CR. Sex differences in adult cell proliferation within the zebrafish (Danio rerio) cerebellum. Eur J Neurosci. 2007;25:1030–1040. doi: 10.1111/j.1460-9568.2007.05366.x. [DOI] [PubMed] [Google Scholar]
- [88].Craig PM, Moon TW. Fasted zebrafish mimic genetic and physiological responses in mammals: a model for obesity and diabetes? Zebrafish. 2011;8:109–117. doi: 10.1089/zeb.2011.0702. [DOI] [PubMed] [Google Scholar]
- [89].Rodríguez F, López JC, Vargas JP, Gómez Y, Broglio C, Salas C. Conservation of spatial memory function in the pallial forebrain of reptiles and rayfinned fishes. J Neurosci. 2002;22:2894–2903. doi: 10.1523/JNEUROSCI.22-07-02894.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Chong ZZ, Shang YC, Zhang L, Wang S, Maiese K. Mammalian target of rapamycin: hitting the bull’s-eye for neurological disorders. Oxid Med Cell Longev. 2010;3:374–391. doi: 10.4161/oxim.3.6.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]