Abstract
Cholesterol transport is an essential process in all multicellular organisms. In this study we applied two recently developed approaches to investigate the distribution and molecular mechanisms of cholesterol transport in Caenorhabditis elegans. The distribution of cholesterol in living worms was studied by imaging its fluorescent analog, dehydroergosterol, which we applied to the animals by feeding. Dehydroergosterol accumulates primarily in the pharynx, nerve ring, excretory gland cell, and gut of L1–L3 larvae. Later, the bulk of dehydroergosterol accumulates in oocytes and spermatozoa. Males display exceptionally strong labeling of spermatids, which suggests a possible role for cholesterol in sperm development. In a complementary approach, we used a photoactivatable cholesterol analog to identify cholesterol-binding proteins in C. elegans. Three major and several minor proteins were found specifically cross-linked to photocholesterol after UV irradiation. The major proteins were identified as vitellogenins. rme-2 mutants, which lack the vitellogenin receptor, fail to accumulate dehydroergosterol in oocytes and embryos and instead accumulate dehydroergosterol in the body cavity along with vitellogenin. Thus, uptake of cholesterol by C. elegans oocytes occurs via an endocytotic pathway involving yolk proteins. The pathway is a likely evolutionary ancestor of mammalian cholesterol transport.
INTRODUCTION
Sterols are important constituents of the eukaryotic cell plasma membrane. Cholesterol, which is found predominantly in animal cells, influences permeability barrier properties and fluidity of the lipid bilayer. Data accumulated in recent years demonstrate that cholesterol is not only a structural component of the membrane that renders the membrane less fluid, but it is also actively involved in the modulation of cell signaling. This modulation is thought to occur via cholesterol- and glycosphingolipid-enriched microdomains within the plasma membrane, often referred to as glycolipid-enriched membranes (GEMs) or “rafts” (Simons and Ikonen, 1997). These specialized domains are envisaged as a lateral association of specific lipids and proteins in which cholesterol plays an organizing role. However, most of the information about specialized lipid domains comes from studies in model membrane systems (Brown and London, 2000) and in tissue culture cells (Mukherjee and Maxfield, 2000). In this study, we investigated the roles played by cholesterol in a living organism, the nematode C. elegans. The power of genetics and the availability of its entire genome sequence make this organism an excellent model for studying many biological processes. As a first step toward understanding sterol traffic and its biological roles in Caenorhabditis elegans, we investigated the distribution and transport of sterol in living worms.
One of the main advantages to with the use of C. elegans as a model system is that nematodes are auxotrophic for sterols because they do not possess the enzymes necessary for de novo sterol biosynthesis (Hieb and Rothstein, 1968; Chitwood and Lusby, 1991). The main sources of sterols for the soil worm C. elegans are animal feces or yeast/plant remnants. C. elegans is routinely propagated in the laboratory on agar plates containing cholesterol. Thus, the organism's sterols can be replaced with fluorescent or photochemically reactive analogs to investigate the distribution and function of sterols in a living organism.
Cholesterol in C. elegans is probably involved in the structural and functional organization of the plasma membrane, which is similar to other animal cells. Worms grown on plates depleted of cholesterol display defects in molting (Yochem et al., 1999). If cholesterol is substituted by its nonfunctional analog 25-azacoprostane, growth and reproduction of animals is strongly inhibited (Chitwood et al., 1984; Bottjer et al., 1985). Recently, while investigating the function of a C. elegans homologue of caveolin, we found that the cholesterol level in gonads influences signal transduction (Scheel et al., 1999). In particular, cholesterol depletion led to the acceleration of meiotic cell cycle progression via exit from the pachytene arrest. This process is known to be regulated by the Ras/MAP-kinase pathway (Church et al., 1995).
Despite the vital importance of sterols for the worm, cholesterol distribution and the molecular mechanisms of its transport are poorly understood. It is not even clear whether sterols are taken up by C. elegans via the digestive tract or through the cuticle. Very little is known about the molecules involved in the transport of cholesterol into different organs of the adult worm or embryos. One previously identified candidate for cholesterol uptake is a homologue of gp330/megalin, a member of the low-density lipoprotein (LDL)-receptor superfamily (Yochem et al., 1999). However, its exclusive localization to the apical surface of the hypodermis called into question the involvement of this receptor in cholesterol uptake by the gut. Interestingly CHE-14 is another recently described potential cholesterol-binding protein, which was localized to the apical side of ectodermal cells (Michaux et al., 2000).
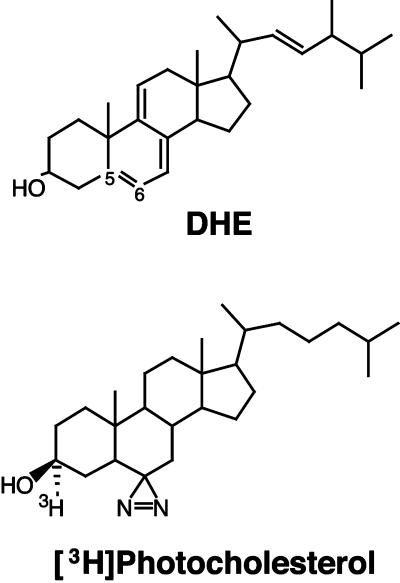
One reason for the paucity of information on cholesterol distribution and transport has been the lack of methods for imaging cholesterol or for identification of cholesterol-binding proteins. Until recently only indirect methods were available (Liscum and Dahl, 1992; Liscum and Munn, 1999). The optical transparency of C. elegans provides a unique opportunity to apply approaches of fluorescence imaging and photoaffinity labeling in a whole organism. However, imaging of biological sterols in living cells was not possible until recently because of the lack of a suitable fluorescent probe that mimics cholesterol's biophysical and biological properties. Dehydroergosterol (DHE, Figure 1A) is a naturally occurring fluorescent analog of cholesterol that mimics many of its properties (Schroeder et al., 1991, 1995), but it was not generally useful for microscopic imaging because it absorbs in the far UV region and emits in the near UV region of the spectrum. Only recently, with the use of a camera that had high efficiency in the UV range as well as high UV throughput of various optical components of the microscope, was it possible to image DHE and thus detect the distribution of sterol in living cultured cells (Mukherjee et al., 1999). In contrast, labeling of cells with a fluorescent derivative of cholesterol, 25-NBD-cholesterol, differed dramatically from the labeling with DHE, including labeling of organelles such as the nuclear envelope that are known to have low levels of sterol. A fluorescent cholesterol-binding polyene antibiotic, filipin, gave similar although quantitatively different labeling of cells compared with DHE (Mukherjee et al., 1999). In our preliminary experiments we found that application of filipin for studies in living C. elegans is complicated because of poor ability of this reagent to penetrate the inner organs of the worm. Moreover, autofluorescence of the worm in the region of the filipin emission spectrum is very high, making its detection in dissected animals almost impossible.
Figure 1.
Structures of DHE and [3H]photocholesterol.
Another advance in studying cholesterol transport comes from the recent synthesis of a biologically active, photoactivatable cholesterol analog, [3H]photocholesterol (Figure 1A). This analog has made it possible to identify proteins that bind cholesterol in vivo (Thiele et al., 2000).
In this study we used DHE and [3H]photocholesterol to investigate the distribution of sterol in the worm and to identify molecules involved in its transport. We show that in contrast to filipin or 25-NBD-cholesterol, DHE accumulates specifically in certain tissues of the worm. Two major sites of accumulation are the oocytes and developing sperm. With the use of [3H]photocholesterol, we identified vitellogenins as interaction partners of cholesterol, and we found that rme-2 worms, which lack the vitellogenin receptor (Grant and Hirsh, 1999), fail to accumulate DHE in oocytes and embryos. Because vitellogenins have homology to mammalian apolipoprotein B-100 (Baker, 1988; Spieth et al., 1991), and the vitellogenin receptor, RME-2, is a member of the LDL receptor superfamily (Grant and Hirsh, 1999), vitellogenins and their receptor may be regarded as evolutionary predecessors of LDL and its receptor in higher organisms. The photocross-linking approach has also led to identification of a 37-kDa protein that binds cholesterol in hermaphrodites as well as in males.
MATERIALS AND METHODS
Materials
All reagents for propagation of C. elegans, DHE (ergosta-5,7,9(11),22-tetraen-3β-ol), filipin, and M-βCD (methyl-β–cyclodextrin) were from Sigma-Aldrich Chemicals (Deisenhoff, Germany); 25-NBD-cholesterol was purchased from Molecular Probes (Eugene, OR); M9 medium was prepared as previously described (Sulston and Hodgkin, 1988); chambered coverglasses were a product of Nunc GmbH (Wiesbaden, Germany). [3H]cholesterol with specific activity of 47 Ci/mmol was purchased from Amersham-Pharmacia (Amersham, England). The synthesis of [3H]photocholesterol, [3α-3H]-azi-5α-cholestan-3β-ol, with a specific activity of 5.0 Ci/mmol, was described previously (Thiele et al., 2000).
C. elegans Strains
Maintenance of the strains used was according to (Brenner, 1974). N2, variety Bristol, was a wild-type strain; fer-1(lc1) was kind gift of Dr. Adam Antebi (MPI for Molecular Genetics, Berlin). This strain was propagated at 16°C, and for labeling experiments L1 larvae were transferred to 25°C; him-8(e1489) was provided by the Caenorhabditis Genetics Center (University of Minnesota). Strains bIs1[vit-2::gfp], rme-2(b1005), and rme-2(b1008) were described previously (Grant and Hirsh, 1999).
Preparation of DHE containing Bacteria and Labeling of Worms
An overnight culture of Escherichia coli (0.5 ml; strain OP50) was mixed with an equal volume of 5 mM DHE in ethanol and dialyzed against water (two times; 100 ml). After dialysis, the mixture was mixed with live OP50 bacteria in the ratio 1:5, and 40 μl was spread on a 1.7% agarose plate not containing cholesterol. For some experiments a ratio of 1:25, 1:125, or 1:625 of DHE mixture to living bacteria was used. Plates were ready for labeling experiments after growth of the bacterial lawn overnight at 16°C. In our experience the mixture of DHE with bacteria is stable for at least 1 month when kept at −20°C, whereas plates should be used within 1 week; otherwise, fluorescence of DHE abruptly diminishes.
For staging experiments all strains used were bleached with hypochlorite according to (Sulston and Hodgkin, 1988). Hatched L1 larvae were kept overnight on a plate without food and then transferred to DHE-containing plates. Different larval and adult stages were distinguished by the degree of gonad development and were picked and transferred to a chambered coverglass containing 0.5 ml of 10 mM sodium azide in M9 buffer. Worms were analyzed by fluorescence microscopy after 5–10 min, by which time they stopped pharyngeal pumping and lay without motion. In some experiments 1 mM tetramizole in M9 buffer was used instead of azide.
Some hermaphrodites or males were dissected in a chambered coverglass with the use of two 24-gauge needles. In this case sperm medium SM (Nelson et al., 1982) was used instead of M9. Hermaphrodites were cut near to the pharynx, whereas males were cut at the distal third.
Staining with 25-NBD-cholesterol was performed as described above for DHE. For staining with filipin, worms were soaked in 10 μM solution prepared in M9 buffer for 2–5 h at room temperature
Fluorescence Microscopy
Twelve-bit fluorescent or DIC images were obtained with the use of an inverted microscope (Axiovert-100; Zeiss, Göttingen, Germany) equipped with a SPOT RT monochrome CCD camera (Diagnostic Instruments, Inc., Sterling Heights, MI). For detection of DHE a filter set described previously (Mukherjee et al., 1999) and a Plan-NEOFLUAR ×40 (N.A = 1.3; Zeiss) oil-immersion objective were used. For imaging of GFP a standard filter set for FITC was used.
Images were acquired with the use of Metaview (Universal Imaging, West Chester, PA) and processed by Adobe Photoshop 5.0.2 (Adobe Systems Inc., Edinburgh, UK) programs.
Preparation of [3H]Photocholesterol-containing Bacteria and Labeling of Worms
The preparation of [3H]cholesterol- or [3H]photocholesterol-containing bacteria was similar to that of DHE. [3H]photocholesterol (10 μl; 2 mCi/ml) was mixed with equal volume of bacteria and after dialysis were spread onto an agarose plate devoid of cholesterol. L1 larvae prepared by bleaching of mixed populations were transferred to plates and grown until L4 or mixed populations had developed. For irradiation experiments worms were washed from the plate with M9 buffer and further washed with M9 buffer several times.
To compare labeling of hermaphrodites and males, him-8 animals were fed with [3H]photocholesterol, and 100 adult animals of each sex were irradiated with UV. Both samples were processed for SDS-PAGE.
Irradiation with UV
Irradiation of worms was performed in 12-well plastic cell-culture plates cooled on ice. Worms were irradiated for 5 min with the use of a high-pressure 50 W mercury lamp. The beam was reflected and thus filtered (λ > 310 nm) with the use of a 0.3-cm-thick commercially available glass mirror.
Analysis of Cross-linked Proteins by SDS-PAGE
After irradiation worms were transferred to a fresh centrifuge tube, washed twice with M9, and resuspended in 50 μl of SDS-PAGE sample buffer. The suspension was frozen in liquid nitrogen and subsequently thawed. After repeating this procedure three times, worms were homogenized with a manual Teflon homogenizer. The homogenate was boiled for 5 min, and after short centrifugation the supernatant was applied to SDS-PAGE. Preparation of 12 and 5% gels was as previously described (Scheel et al., 1999). Gels were stained with Coomassie Brilliant Blue and after destaining were incubated in Amplify solution (Amersham Pharmacia Inc.) for 30 min. Dried gels were exposed to Hyperfilm (Amersham Pharmacia Inc).
Extraction of Labeled Worms with Triton X-114 and Subsequent Phase Separation
Worms were fed with [3H]photocholesterol and irradiated with UV as described above. After two washes with M9, they were homogenized in 100 μl of buffer containing 25 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.5 mM CaCl2, and 1% Triton X-114. The homogenate was adjusted to 500 μl with the same buffer, and the phases were separated by incubation for 15 min at 37°C. After centrifugation at room temperature (10 min, 13,000 rpm), the aqueous phase was precipitated with 10% TCA. The detergent phase was again adjusted to 500 μl, and the phase separation procedure was repeated twice. The detergent phase together with the resulting washes were precipitated with TCA. Pellets dissolved in SDS-PAGE sample buffer were subjected to electrophoresis.
Immunoprecipitation Techniques
For immunoprecipitation of vitellogenins, worms were UV cross-linked and then homogenized in a buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, a mixture of protease inhibitors, and 1% Triton X-100. One-half milliliter of each homogenate was incubated overnight at 4°C with primary antibodies with the use of dilutions of 1:100. Immunoprecipitates were collected by incubation with a mixture of Protein-A- and Protein-G-Sepharose (1:1) for 2 h at 4°C. After rigorous washing with buffer, precipitates were desorbed from the resin by boiling in SDS-PAGE sample buffer and subsequently separated on a 5% acrylamide gel. The following antibodies were used: polyclonal immunoglobulin fraction from rats immunized with electrophoretically purified yolk 170-kDa polypeptides (antiyp170) and monoclonal mouse antibodies PIIA3 and OIC1. All these antibodies are described in Sharrock et al. (1990).
Separation of Vitellogenin Complexes on a Sucrose Gradient
Vitellogenin complexes from mixed populations of rme-2 mutants or wild-type worms were isolated with the use of a procedure similar to that described in Sharrock et al. (1990). However, instead of embryos, adult animals were taken. [3H]photocholesterol–labeled worms were homogenized in 100 μl of detergent-free buffer (50 mM Tris-HCl, pH 7.8, 200 mM NaCl, 5 mM MgCl2, 0.5 mM CaCl2). After clarification at 13.000 rpm for 5 min, the supernatant was sonicated for four 20-s periods with the SH219 tip of a Bandolin electronics instrument, and the preparation was allowed to cool on ice between sonication periods. The sonicated material was adjusted to 200 μl and layered onto a 5–20% (two times, 2 ml) sucrose gradient prepared with the same buffer. The centrifugation (46,000 rpm for 15.5 h) was performed in SW-60 rotor with the use of a Beckman Avanti centrifuge (Beckman Coulter, Unterschessheim-Lohhof, Germany). Gradients were fractionated, aliquots were collected from each fraction, and either total radioactivity or radioactivity after TCA precipitation was measured. TCA-precipitates were analyzed on a 12% SDS-PAGE. Catalase (11.3 S) was used as a marker for sedimentation.
Determination of Sterols in Vitellogenin Complexes
Vitellogenin complexes from rme-2 worms fed with [3H]photocholesterol or [3H]cholesterol were obtained as described above. However, instead of being centrifuged, they were extracted with two volumes of chloroform/methanol (1:1). After rigorous shaking at 37°C for 15 min, the lower organic phase was dried with the use of a Speedvac centrifuge. The dried material was dissolved in 10 μl of methanol and applied to a glass TLC plate covered with 0.25 mm silica gel 60 (Merck, Darmstadt, Germany). The chromatogram was developed with hexane:ethyl ester (2:1). After the chromatography plates were dried, sprayed with scintillant (Ultima Gold, Canberra-Packard, Dreilich, Germany), and exposed to Hyperfilm. Cholesterol and its several esters (e.g., palmitoyl, oleyl) were used as markers. Nonradioactive sterols were visualized by spraying of plates with 20% sulfuric acid and heating to 135°C for 20 min. Under theses conditions, cholesterol and its esters are stained dark red. Fluorograms were scanned on a Hitachi scanner and quantified with the use of MacBas II software (Fuji Photo Film Europe, Düsseldorf, Germany).
RESULTS
Labeling of Hermaphrodites and Males with DHE
To follow the localization of cholesterol in living C. elegans, we used its fluorescent analog DHE and a microscope equipped with a CCD camera that is highly sensitive to UV light (Mukherjee et al., 1999). Worms were labeled by growing them on agarose plates containing pads of bacteria mixed with DHE (Figure 1). Animals grown on DHE for several generations did not show any visible difference compared with those propagated on plates containing equivalent concentrations of cholesterol. Because C. elegans cannot be propagated in the absence of dietary cholesterol, these results indicate that DHE can functionally replace cholesterol for growth and development in the nematode.
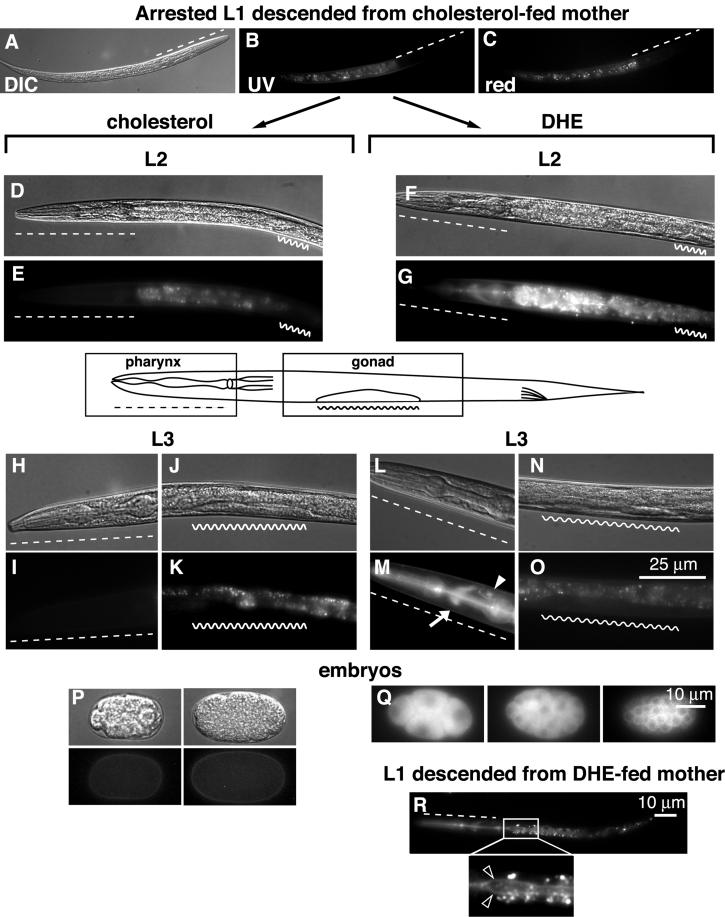
We began the labeling procedure with wild-type L1 larvae descended from a hermaphrodite that was grown on cholesterol. Gravid worms were bleached with alkaline hypochlorite, and derived eggs were hatched on plates lacking food (Sulston and Hodgkin, 1988). Such animals arrest development at the L1 stage. Without application of DHE, fluorescence microscopy revealed only the autofluorescence of intestinal lysosomes called gut granules (Figure 2A), visible also in green and red fluorescence channels (Figure 2, B and C). Gut granules are thought to contain autofluorescent lipofuscins, causing strong fluorescence over a wide range of the spectrum (Clokey and Jacobson, 1986; White, 1988).
Figure 2.
Labeling of L1, L2, and L3 larval stages with DHE. (A–C) L1 larva descending from cholesterol-fed mother. L2 larva produced by the transfer of L1 onto cholesterol- (D and E) or DHE-containing (F and G) plate. Pharingeal (H and I) and gonadal (J and K) regions of L3 larva grown on control plate and correspondingly pharingeal (L and M) and gonadal (N and O) regions of DHE-fed worm. Different developmental stages of C. elegans embryos grown in control conditions (P) and on DHE plate (Q). DHE fluorescence distribution in L1 larvae descended from DHE-fed mother (R). Inset in R shows magnified region of gut. Bright spots around the apical surfaces of the gut are autofluorescent gut granules. Dashed line, pharyngeal region; wavy line, gonad; arrow, nerve ring; closed arrowhead, excretory gland cell; open arrowheads, apical surfaces of the gut.
Unlabeled L1 larvae were transferred to DHE-containing plates, and we viewed DHE fluorescence in living animals at various life stages. Labeled organs and cells were identified by a combination of fluorescence and Nomarski (DIC-differential interference contrast) microscopy.
Figure 2 shows labeling of L2 and L3 hermaphrodite larvae (Figure 2, D–O). Already after 5 h of growth on DHE, fluorescent labeling was detected in the L2 pharyngeal region (Figure 2G, dashed line). This labeling, more intense in L3 (Figure 2M) remains strong during the life span of the worm. The main structures labeled in the pharyngeal region were the pharynx itself, the nerve ring (white arrow), and the excretory gland cell (arrowhead). Not surprisingly, the intestine was also strongly labeled. The immature germ cells of L2–L3 animals (wavy lanes) were devoid of DHE.
Figure 2, P and Q, shows several embryos dissected from a DHE-labeled hermaphrodite. Although membrane staining was evident in all embryonic stages, early embryos displayed mostly cytoplasmic labeling (Figure 2Q). When DHE-labeled mothers were bleached and the isolated eggs were hatched on plates lacking food, L1 larvae (Figure 2R) showed strong labeling of the pharynx, nerve ring, and intestine. The apical membranes of the pharynx and intestine appeared most intensely labeled (Figure 2R, inset; open arrowheads). This shows that the mother provides a significant amount of sterol to her progeny, which is incorporated into newly hatched larvae. This result also shows that localization of DHE to apical membranes of the pharynx and intestine is independent of feeding.
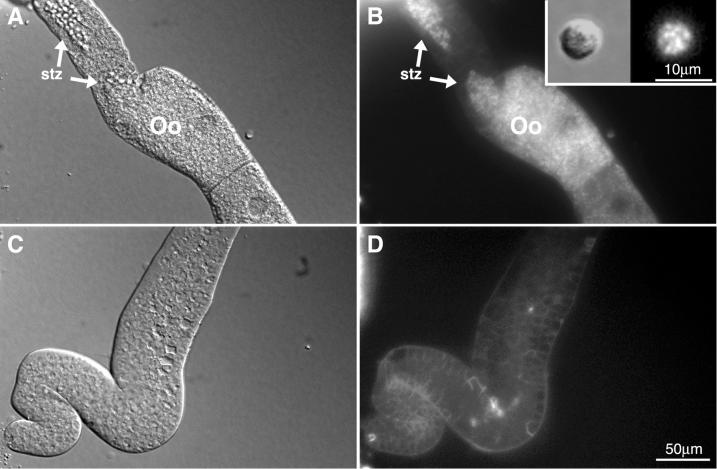
Spermatogenesis begins at the late L4 larval stage (Ward and Carrel, 1979). At this point strong DHE labeling of spermatids started to become visible (Figure 3D, white arrow). This labeling continued to increase with age, peaking in the young adult before production of the first mature oocytes (Figure 3H). At this time spermatids still reside in the oviduct. In addition, we detected intense labeling of the spermathecal valve (white arrowhead). After maturation, the first ovulated oocyte pushes the spermatids into the spermatheca where they are activated to become spermatozoa. The strongly labeled spermatozoa in the spermatheca are seen in Figure 3L. It can be seen that at this time that two to three nearly full-grown oocytes also became DHE-labeled.
Figure 3.
Labeling of late L4 larvae (A–D), young adult hermaphrodite (E–H), adult hermaphrodite (I–L), and adult virgin male (M–P) with DHE. (A, B, E, F, I, J, M, and N) Gonadal regions of worms grown on cholesterol and (C, D, G, H, K, L, O, P) on DHE plates. std, spermatids; stz, spermatozoa; arrowhead, proximal spermathecal valve.
The labeling of males with DHE was similar to that of hermaphrodites. Besides the pharynx, nerve ring, and excretory gland cell (not shown), DHE accumulated in the testis, with a very intense labeling of the seminal vesicle (Figure 3P).
The labeling seen in the spermatheca or seminal vesicle corresponds to individual spermatozoa. This is evident in Figure 4, A and B, where a preparation of a dissected hermaphrodite is shown. Oocytes contain punctate DHE fluorescence in the cytoplasm. These puncta may be yolk granules (see below). In nonactivated male spermatids (Figure 4B, inset) the DHE was localized predominantly in dispersed cytoplasmic structures.
Figure 4.
Hermaphrodite and male sperm from dissected animals. (A and B) Spermatheca and proximal gonad of a dissected hermaphrodite; (C and D) distal gonad of the same animal. Inset in B displays a spermatid dissected from him-8 male grown on DHE plate. Oo, oocyte; stz, spermatozoa.
The distal part of the hermaphrodite gonad (Figure 4, C and D) also displayed membrane labeling. However, the fluorescence intensity was about five times less than that of spermatozoa or oocytes (compare Figure 4, B and D). A very different picture was obtained when 25-NBD-cholesterol or filipin were used as tracers of cholesterol instead of DHE. In either case only the pharyngeal region and intestine but not germ cells were labeled by these compounds (not shown). Dietary filipin was toxic to worms, so we bathed the animals in a solution of this compound for labeling.
Depletion of Cholesterol during Spermatogenesis Does Not Interfere with Development of the Embryo
The intense labeling of sperm with DHE raises a fundamental question about its biological role in the fertilization process. Is cholesterol in sperm transferred to the zygote upon gamete fusion, or is its removal from the sperm required for effective fertilization?
We determined whether any of the DHE in embryos is derived from sperm by examining cross-progeny embryos derived from unlabeled hermaphrodites crossed with labeled males. For this experiment fer-1 mutant animals were used, because at 25°C fer-1 hermaphrodites do not produce functional sperm. On mating with wild-type males fer-1 hermaphrodites produce normal progeny. When males were fed DHE and then mated with unlabeled hermaphrodites, no label in embryos was detected (not shown). In contrast, fer-1 hermaphrodites grown on DHE produced strongly labeled unfertilized oocytes. These data demonstrate that the bulk of the cholesterol in embryos is provided by the oocyte and not the sperm.
To assess directly the importance of cholesterol for fertilization, we compared the progeny of 3 fer-1 mutant L4 larvae, grown at 25°C, crossed with 6 him-8 males grown either on cholesterol-containing or cholesterol-free plates. After 36 h the number of offspring was counted. In five independent experiments there was no significant difference between the number of offspring per plate from hermaphrodites mated with cholesterol-depleted and control males, 453 ± 142 and 457 ± 165 (mean ± SD), respectively. Hermaphrodites without crossing with males produced an average of 9 offspring each (n = 15). We conclude that the high level of cholesterol in sperm is not required for efficient fertilization.
Identification of Vitellogenins as Cholesterol-binding Proteins
To identify proteins that bind cholesterol and are possibly involved in its transport or function in the cell, we used a photoaffinity labeling approach. A recently described photoactivatable analog of cholesterol, [3H]photocholesterol (Figure 1), was synthesized, and worms were fed with this compound as described for DHE. Similar to DHE, worms grown on nonradioactive photocholesterol for eight generations did not show any difference compared with those propagated on plates containing cholesterol. On irradiation with UV, photocholesterol produces a carbene radical that can insert into neighboring chemical groups. Thus, radioactive [3H]photocholesterol can be covalently cross-linked to cholesterol-binding proteins, allowing their characterization and eventual identification.
Feeding of mixed population of worms with [3H]photocholesterol and subsequent irradiation with UV resulted in labeling of three high-molecular-mass proteins, ranging from 90 to 190 kDa (Figure 5A). In contrast, similar exposure times of gels containing protein lysates from late L4 or virgin young adults did not display any significant labeling with [3H]photocholesterol. The labeled bands coincided very well with some Coomassie-stained bands of the mixed population extract (Figure 5B). In mixed populations several weakly labeled bands were also visible (indicated with dots). One of these bands with a 37-kDa molecular mass was also visible in L4/young adults. To determine more exactly the molecular masses of the major labeled proteins, we performed separation on 5% gels. Under these conditions we estimated the mass of the three bands as 170, 115, and 90 kDa (Figure 5B). After irradiation we extracted lysates with Triton X-114 and allowed the phases to separate at 37°C. All three major bands were found in the aqueous phase (Figure 5C, AP). Thus, all three proteins are soluble. By comparison, a weakly labeled 31-kDa band segregated almost entirely into the detergent phase (DP), indicating that it is an integral membrane protein.
Figure 5.
Labeling of mixed population (MP) and L4/young adults with [3H]photocholesterol (A). Major bands of high-molecular mass are indicated with arrowheads and minor bands with dots. The separation was performed on a 12% acrylamide gel. Lysates from UV-irradiated (+) and from nonirradiated (−) worms. (B) Separation of labeled bands from mixed population on a 5% gel. (C) Extraction of labeled proteins with Triton X-114 and subsequent phase separation. AP, aqueous phase; W1 and W2, washes 1 and 2, respectively; DP, detergent phase. (D) Comparison of the labeling in adult hermaphrodites and males. (E) Immunoprecipitation of cross-linked bands with nonimmune mouse IgG, polyclonal anti-YP170 and monoclonal antibodies PIIA3 and OIC1. (F) Labeling of YP170::gfp strain with [3H]photocholesterol. wt, lysate from wild-type worms.
The absence of these proteins from L4 and young adult animals, their solubility in Triton X-114, their coincidence with major Coomassie-stained bands in crude lysates, and their estimated molecular masses led us to suspect that these [3H]photocholesterol–labeled proteins are vitellogenins. Yolk proteins (vitellogenins) of molecular masses 170 kDa (Figure 5, A and B) and 115 and 88 kDa are known to be expressed exclusively in adult hermaphrodites' intestines, secreted into the body cavity, and taken up into storage vesicles in oocytes (Kimble and Sharrock, 1983). To further test this hypothesis, we compared the labeling of hermaphrodites and males, because males are devoid of vitellogenins. When 100 adult hermaphrodites or 100 males were labeled with [3H]photocholesterol, only hermaphrodites gave strong labeling of high-molecular-mass bands (Figure 5D). A band of ∼37 kDa was labeled in individuals of both sexes.
The final proof that the labeled bands are indeed vitellogenins came from immunoprecipitation experiments. As seen in Figure 5E, the 170-kDa band could be immunoprecipitated either with rat polyclonal antibodies against vitellogenin 170B or mAb PIIA3. A mAb, recognizing both 170-kDa proteins (OIC1), immunoprecipitated all three bands. This fits well with previously obtained immunoprecipitation patterns with the use of these antibodies, which showed the existence of two vitellogenin complexes (Sharrock et al., 1990; see also below). In contrast, nonimmune IgG did not immunoprecipitate the labeled bands. Moreover, when a transgenic strain expressing a vitellogenin 170B::green fluorescent protein (YP170::GFP) fusion protein was cross-linked with [3H]photocholesterol, an additional band of ∼200 kDa (YP170B+GFP) was observed (Figure 5F). These data allow us to conclude that the [3H]photocholesterol binds to vitellogenins in C. elegans. Our results indicate that the main binding component is YP170B.
Uptake of DHE into Oocytes and Embryos Is Compromised in rme-2 Mutants
Recently, several mutants of C. elegans deficient in receptor-mediated endocytosis were isolated (rme-1–12; Grant and Hirsh, 1999). RME-2, a new member of the LDL receptor superfamily, was identified as the yolk receptor in C. elegans. If yolk proteins are the primary transporters of cholesterol into oocytes, then animals lacking the yolk receptor, RME-2, should fail to accumulate cholesterol in the oocytes and instead accumulate it in the body cavity.
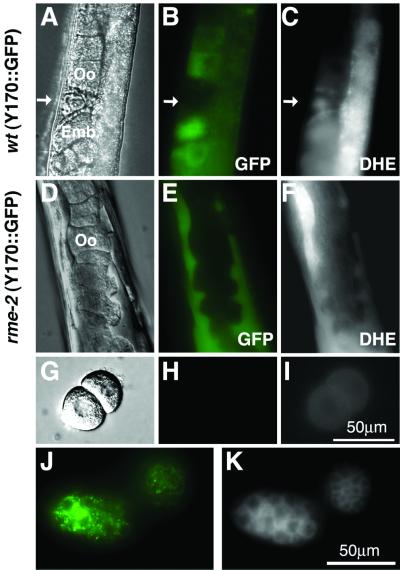
Indeed, we found that very little DHE accumulated in the oocytes of rme-2 mutants, but instead it accumulated to high levels in large pools within the body cavity. We also compared the localization of a GFP-tagged yolk protein (YP170::GFP) with DHE in wild-type and rme-2 mutants. In wild-type worms, YP170::GFP labeled the same nearly full-grown oocytes that were also labeled by DHE (Figure 6, A–C). In rme-2 mutants most DHE colocalized with YP170::GFP in pools located within the body cavity (compare Figure 6, E and F). We conclude that the bulk of the cholesterol entering oocytes is transported via vitellogenins and their receptor, RME-2.
Figure 6.
Uptake of DHE in rme-2 strain. Adult hermaphrodite of vit-2::gfp strain, grown on DHE plate, observed with the use of DIC optics (A) or in the GFP (B) and DHE (C) fluorescence channels. rme-2 mutant expressing YP170::GFP was fed with DHE and observed in DIC (D), GFP (E), and DHE (F) channels. Oocytes from rme-2 mutant expressing YP170::GFP grown on DHE imaged with the use of DIC (G), GFP (H) and DHE (I) channels. Embryos from YP170:: GFP strain grown on DHE, observed in GFP (J) and DHE (K) channels. Green, GFP fluorescence; arrow, spermatozoa; Oo, oocyte; Emb, embryo.
We did observe some residual accumulation of DHE in full-grown oocytes of rme-2 mutants. This is most evident in isolated oocytes laid by DHE-fed rme-2 mutant hermaphrodites (compare Figure 6, H and I). We conclude that the bulk of cholesterol is transported into oocytes via vitellogenins/RME-2, but an alternative minor pathway probably exists for cholesterol uptake into oocytes. Such a pathway might mediate cholesterol uptake in all cells.
Previous investigations demonstrated that vitellogenins in embryos form two types of complexes (Sharrock et al., 1990). One of them, an 8S complex, contains two 170B molecules. Another one, a 12S complex, consists of 170A, 115- and 88-kDa vitellogenins. However, lipid analysis showed that both complexes contain very little cholesterol. Indeed, the DHE/YP170::GFP double-labeled embryos show very weak overlap of GFP and DHE fluorescence at the subcellular level (Figure 6, J and K). Also the level of cross-linking of [3H]photocholesterol and vitellogenins in embryos was very low compared with that of whole adult worms (our unpublished results). These data indicate that after transport into the oocyte, cholesterol is largely separated from vitellogenins. This is similar to the separation of cholesterol from LDL particles after uptake into the late endosomes of mammalian cells.
Because rme-2 mutants accumulate unendocytosed vitellogenin complexes in the body cavity, they provide an excellent opportunity to determine the cholesterol content of vitellogenin complexes before their uptake into oocytes. rme-2 mutants and wild-type animals were fed with [3H]cholesterol or [3H]photocholesterol, and after homogenization in a buffer without detergent, the complexes were separated on a sucrose gradient by centrifugation. As seen in Figure 7 for [3H]photocholesterol fed rme-2 animals, almost all of the radioactivity was found in 8S and 12S complexes. In contrast, in wild-type animals significant amount of the radioactivity is found in the upper part of the gradient, probably as a complex with low-molecular-weight soluble proteins. Additionally, quite high quantity of the radioactivity is found in these animals in the membrane fraction, probably representing cholesterol in complexes with membrane proteins. Interestingly, the 12S complex contains more of the total radioactivity than the 8S complex (squares). In contrast, after irradiation the 8S complex shows an equal amount of covalently attached radioactivity. The interaction of YP170B, found in the 8S complex, with cholesterol could be stronger than YP170A, found in the 12S complex. Alternatively the cross-linking of cholesterol to vitellogenins may be sterically disadvantageous in the 12S complex. We have analyzed the contents of vitellogenin complexes by TLC. Most of the [3H]photocholesterol detected was free. Only ∼25% was esterified. This fits well with previously determined quantities of esterified sterol in C. elegans (Chitwood et al., 1984). Altogether, these results show that cholesterol is bound to vitellogenins in 8S and 12S complexes within the body cavity before uptake by the oocytes. These complexes, which contain a mixture of free and esterified sterols, are endocytosed via the RME-2 receptor. After transport into oocytes, cholesterol–vitellogenin complexes disassemble, and cholesterol is distributed to new locations within the oocyte.
Figure 7.
Separation of vitellogenin complexes from rme-2 mutants by centrifugation on a sucrose gradient. The graphs with closed squares and circles depict respectively total radioactivity and radioactivity after TCA-precipitation in the fractions from rme-2 extract. □, total radioactivity; ○, radioactivity in TCA-precipitated fractions of the extract from wild-type worms. Inset: labeled bands from TCA-precipitated fractions 10 and 13 of rme-2 extract, separated on a 12% SDS-PAGE.
DISCUSSION
DHE and [3H]Photocholesterol as Tools for Studying the Distribution and Transport of Cholesterol In Vivo
Investigation of cholesterol uptake and distribution in living cells or organisms is of enormous importance, and C. elegans is well suited for such studies. It is small and optically transparent, which makes possible applications of fluorescence imaging or photoaffinity labeling in whole organisms. In addition, mutant strains of C. elegans can be used to delineate the molecular mechanisms of sterol transport and regulation. Because of their noninvasive nature, DHE provides the ability to follow the uptake and distribution of cholesterol in living organisms, whereas [3H]photocholesterol is an excellent reagent for the identification of cholesterol-binding proteins. Both mimic cholesterol reliably in vivo in mammalian systems (Mukherjee et al., 1999; Thiele et al., 2000), and as shown herein, they can also support the growth and development of C. elegans lacking other sources of sterols.
Enrichment of Cholesterol in Germ Line Cells
Our in vivo DHE labeling experiments show that cholesterol accumulation is most prominent in the pharynx, nerve ring, excretory gland cell, gut, and germ-line cells. DHE labeling of the apical surface of the pharynx and intestine cannot be explained entirely by direct contact with DHE-containing food because L1 larvae derived from DHE-fed mothers, but hatched in the absence of DHE, display strong apical DHE fluorescence in the intestine. Rather, the DHE pattern indicates a true enrichment of cholesterol in these membranes. Enrichment of cholesterol in the apical surface of the pharynx and intestine may be needed to make it rigid or resistant to harsh environmental conditions in the lumen. Furthermore, recent studies in mammalian systems indicate the importance of cholesterol-rich domains in the microvilli of epithelial cells (Röper et al., 2000). The importance of cholesterol accumulation in the nerve ring, a structure composed entirely of axonal processes is unknown, although cholesterol-rich domains are thought to be important for axonal vs. dendritic protein sorting in other systems (Ledesma et al., 1998). These neurons are known to be involved in several sensory processes including chemotaxis, thermotaxis, or a decision to form dauer larvae (Bargmann and Mori, 1988).
Enrichment of the oocyte with cholesterol is not surprising because these cells must store high levels of membrane components in order to facilitate the rapid growth and development of the embryo. After the formation of the eggshell, no additional uptake from the surrounding environment is possible.
In contrast, the reason for high-level accumulation of cholesterol in spermatids and spermatozoa is not as obvious. The amount of cholesterol in sperm compared with that of the much larger oocyte is insignificant, making it unlikely that it serves an important role in supplying the developing embryo. Our experiments, and published studies, show that cholesterol accumulation in sperm per se is not necessary for fertilization. For instance, it is known that cholesterol depletion by growth on cholesterol-free medium or growth on 25-azacoprostane, a nonfunctional analog of cholesterol, has no visible effect on the first generation of treated animals (Chitwood et al., 1984; Bottjer et al., 1985; Yochem et al., 1999). Only the second generation shows defects in molting and growth.
Moreover, the overall depletion of cholesterol in the gonad results in increased egg-laying activity, accelerating the meiotic cell cycle and exit from pachytene arrest (Scheel et al., 1999). In mammals newly ejaculated sperm is inactive and needs to be capacitated by several factors (Chang, 1951; Austin, 1952). It is well established that cholesterol efflux plays an important role in the capacitation of mammalian sperm (Langlais et al., 1988). This efflux triggers a signaling cascade that includes phosphorylation of several sperm proteins and elevation of Ca2+ concentration (Visconti et al., 1999). It is tempting to speculate that this capacitation process is evolutionarily conserved, and nematodes have similar mechanisms for keeping sperm inactive until the appropriate moment. Cholesterol in spermatozoa could act as a negative regulator, controlling this process, and its efflux could be necessary for successful fertilization.
Yolk Proteins as Carriers of Cholesterol
One of the major findings of the present study is that vitellogenins bind cholesterol and are directly involved in its transport into oocytes. The sequence homology of C. elegans yolk proteins to apolipoproteins was reported >10 years ago (Baker, 1988; Spieth et al., 1991). The recent identification of RME-2 as a receptor for vitellogenins in C. elegans strengthened the apparent similarity between endocytosis of LDL and yolk proteins because RME-2 is a new member of the LDL receptor superfamily (Grant and Hirsh, 1999; Willnow et al., 1999). Also, in other organisms the homology between the LDL receptor and yolk protein receptors was noticed (Bujo et al., 1994; Schonbaum et al., 1995). Despite these indications, no data on direct interactions between vitellogenins and cholesterol have been presented. Moreover, because vitellogenin complexes isolated from oocytes contained little cholesterol (Sharrock et al., 1990), the involvement of vitellogenins in cholesterol transport was neglected. Several types of data presented here demonstrate a crucial role for vitellogenins and their receptors in cholesterol transport. In rme-2 mutants that lack the vitellogenin receptor, the uptake of sterol into oocytes is very strongly diminished. Moreover, analysis in an rme-2 mutant showed that the bulk of cholesterol before its transport into oocytes resides in the 8S and 12S complexes of vitellogenins. Interestingly, although the 12S complex contained more [3H]photocholesterol, the YP170B vitellogenin that forms an 8S complex was cross-linked with [3H]photocholesterol to a greater extent. Either YP170B binds cholesterol most strongly or the [3H]photocholesterol the 12S complex is sterically hindered from binding YP170A covalently.
We found that there is little colocalization of DHE and vitellogenins within oocytes. We postulate that soon after uptake into the endosomal system of the oocyte, cholesterol is released from vitellogenin complexes and is rapidly redistributed to cellular membranes. This could explain why previous studies found very little cholesterol complexed with vitellogenin isolated from oocytes (Sharrock et al., 1990).
It is clear that vitellogenins cannot be the only transporters of cholesterol in C. elegans. Uptake of cholesterol by hermaphrodite larvae starts much earlier than the expression of yolk proteins. In addition, males do not express vitellogenins but still accumulate significant levels of cholesterol. We identified a 37-kDa cholesterol-binding protein, common to males and hermaphrodites throughout the life cycle. This protein is a candidate to be a general cholesterol transporter in C. elegans and may account for the majority of cholesterol transport that is independent of vitellogenins.
The results presented in this article show that the use of chemical analogs of cholesterol that are either photoreactive or naturally fluorescent provide a powerful set of tools to investigate cholesterol transport and distribution in a living organism. The initial results of this approach, which are reported here, demonstrate a remarkable similarity in two aspects of sterol traffic between C. elegans and mammals. First, we observed a striking accumulation of sterol in developing sperm, which is not necessary for fertilization. This is reminiscent of the high levels of cholesterol in mammalian sperm, which becomes reduced as a key part of the capacitation process. Finally, we found that the vitellogenin:vitellogenin receptor system is used by oocytes for import of sterols, suggesting that this is an evolutionary ancestor of the LDL:LDL receptor system. The methods described in this article should be useful for studying many other aspects of sterol transport and regulation.
ACKNOWLEDGMENTS
The authors are indebted to members of Kurzchalia laboratory for fruitful discussions. This work was partially supported by a Human Frontier Scientific Program grant (to F.R.M. and T.V.K.) and by NIH grant DK-27083 ( to F.R.M.). V.M. was supported by EG-project HPRN-CT-2000-00077 to T.V.K. We thank Dr. Adam Antebi (MPI for Genetics, Berlin) and C.G.C. (University of Minnesota, Minneapolis) for providing some C. elegans mutant strains.
Abbreviations used:
- DHE
dehydroergosterol
- LDL
low-density lipoprotein
- M-βCD
methyl-β-cyclodextrin
REFERENCES
- Austin CR. The 'capacitation' of the mammalian sperm. Nature. 1952;170:326. doi: 10.1038/170326a0. [DOI] [PubMed] [Google Scholar]
- Baker ME. Is vitellogenin an ancestor of apolipoprotein B-100 of human low-density lipoprotein and human lipoprotein lipase? Biochem J. 1988;255:1057–1060. doi: 10.1042/bj2551057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI, Mori I . a.C.o.C.e. Researchers, editors. Chemotaxis and thermotaxis. In: Wood WB, editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. pp. 717–737. [Google Scholar]
- Bottjer KP, Weinstein PP, Thompson MJ. Effects of an azasteroid on growth, development and reproduction of the free-living nematodes Caenorhabditis briggsae and Panagrellus redivivus. Comp Biochem Physiol. 1985;82B:99–106. doi: 10.1016/0305-0491(85)90135-x. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- Bujo H, Hermann M, Kaderli MO, Jacobsen L, Sugawara S, Nimpf J, Yamamoto T, Schneider WJ. Chicken oocyte growth is mediated by an eight ligand binding repeat member of the LDL receptor family. EMBO J. 1994;13:5165–5175. doi: 10.1002/j.1460-2075.1994.tb06847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MC. Fertilization capacity of spermatozoa deposited into the Fallopian tubes. Nature. 1951;168:697–698. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- Chitwood DJ, Lusby WR. Metabolism of plant sterols by nematodes. Lipids. 1991;26:619–627. doi: 10.1007/BF02536426. [DOI] [PubMed] [Google Scholar]
- Chitwood DJ, Lusby WR, Lozano R, Thompson MJ, Svoboda JA. Sterol metabolism in the nematode Caenorhabditis elegans. Lipids. 1984;19:500–505. doi: 10.1007/BF02534482. [DOI] [PubMed] [Google Scholar]
- Church DL, Guan KL, Lambie EJ. Three genes of the MAP kinase cascade, mek-2, mpk-1/sur-1 and let-60 ras, are required for meiotic cell cycle progression in Caenorhabditis elegans. Development. 1995;121:2525–2535. doi: 10.1242/dev.121.8.2525. [DOI] [PubMed] [Google Scholar]
- Clokey GV, Jacobson LA. The autofluorescent “lipofuscin granules” in the intestinal cells of Caenorhabditis elegans are secondary lysosomes. Mech Ageing Dev. 1986;35:79–94. doi: 10.1016/0047-6374(86)90068-0. [DOI] [PubMed] [Google Scholar]
- Grant B, Hirsh D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol Biol Cell. 1999;10:4311–4326. doi: 10.1091/mbc.10.12.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieb WF, Rothstein M. Sterol requirement for reproduction of a free-living nematode. Science. 1968;160:778–780. doi: 10.1126/science.160.3829.778. [DOI] [PubMed] [Google Scholar]
- Kimble J, Sharrock WJ. Tissue-specific synthesis of yolk proteins in Caenorhabditis elegans. Dev Biol. 1983;96:189–196. doi: 10.1016/0012-1606(83)90322-6. [DOI] [PubMed] [Google Scholar]
- Langlais J, Kan FW, Granger L, Raymond L, Bleau G, Roberts KD. Identification of sterol acceptors that stimulate cholesterol efflux from human spermatozoa during in vitro capacitation. Gamete Res. 1988;20:185–201. doi: 10.1002/mrd.1120200209. [DOI] [PubMed] [Google Scholar]
- Ledesma MD, Simons K, Dotti CG. Neuronal polarity: essential role of protein-lipid complexes in axonal sorting. Proc Natl Acad Sci USA. 1998;95:3966–3971. doi: 10.1073/pnas.95.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum L, Dahl NK. Intracellular cholesterol transport. J Lipid Res. 1992;33:1239–1254. [PubMed] [Google Scholar]
- Liscum L, Munn NJ. Intracellular cholesterol transport. Biochim Biophys Acta. 1999;1438:19–37. doi: 10.1016/s1388-1981(99)00043-8. [DOI] [PubMed] [Google Scholar]
- Michaux G, Gansmuller A, Hindelang C, Labouesse M. CHE-14, a protein with a sterol-sensing domain, is required for apical sorting in C. elegans ectodermal epithelial cells. Curr Biol. 2000;10:1098–1107. doi: 10.1016/s0960-9822(00)00695-3. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Maxfield FR. Role of membrane organization and membrane domains in endocytic lipid trafficking. Traffic. 2000;1:203–211. doi: 10.1034/j.1600-0854.2000.010302.x. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Zha X, Tabas I, Maxfield FR. Cholesterol distribution in living cells: fluorescence imaging using dehydroergosterol as a fluorescent cholesterol analog. Biophys J. 1999;75:1915–1925. doi: 10.1016/S0006-3495(98)77632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson GA, Roberts TM, Ward S. Caenorhabditis elegans spermatozoan locomotion: amoeboid movement with almost no actin. J Cell Biol. 1982;92:121–131. doi: 10.1083/jcb.92.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röper K, Corbeil D, Huttner W. Retention of prominin in microvilli reveals distinct cholesterol-based lipid microdomains in the apical plasma membrane. Nat Cell Biol. 2000;2:582–592. doi: 10.1038/35023524. [DOI] [PubMed] [Google Scholar]
- Scheel J, Srinivasan J, Honnert U, Henske A, Kurzchalia TV. Involvement of caveolin-1 in meiotic cell-cycle progression in Caenorhabditis elegans. Nat Cell Biol. 1999;1:127–129. doi: 10.1038/10100. [DOI] [PubMed] [Google Scholar]
- Schonbaum CP, Lee S, Mahowald AP. The Drosophila yolkless gene encodes a vitellogenin receptor belonging to the low density lipoprotein receptor superfamily. Proc Natl Acad Sci USA. 1995;92:1485–1489. doi: 10.1073/pnas.92.5.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder F, Jefferson JR, Kier AB, Knittel J, Scallen TJ, Wood WG, Hapala I. Membrane cholesterol dynamics: cholesterol domains and kinetic pools. Proc Soc Exp Biol Med. 1991;196:235–252. doi: 10.3181/00379727-196-43185. [DOI] [PubMed] [Google Scholar]
- Schroeder F, Woodford JK, Kavecansky J, Wood WG, Joiner C. Cholesterol domains in biological membranes. Mol Membr Biol. 1995;12:113–119. doi: 10.3109/09687689509038505. [DOI] [PubMed] [Google Scholar]
- Sharrock WJ, Sutherlin ME, Leske K, Cheng TK, Kim TY. Two distinct yolk lipoprotein complexes from Caenorhabditis elegans. J Biol Chem. 1990;265:14422–14431. [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Spieth J, Nettleton M, Zucker-Aprison E, Lea K, Blumenthal T. Vitellogenin motifs conserved in nematodes and vertebrates. J Mol Evol. 1991;32:429–438. doi: 10.1007/BF02101283. [DOI] [PubMed] [Google Scholar]
- Sulston J, Hodgkin J . a.C.o.C.e. Researchers, editors. Methods. In: Wood WB, editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. pp. 587–606. [Google Scholar]
- Thiele C, Hannah MJ, Fahrenholz F, Huttner WB. Cholesterol binds to synaptophysin and is required for biogenesis of synaptic vesicles. Nat Cell Biol. 2000;2:42–49. doi: 10.1038/71366. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Galantino-Homer H, Ning X, Moore GD, Valenzuela JP, Jorgez CJ, Alvarez JG, Kopf GS. Cholesterol efflux-mediated signal transduction in mammalian sperm. beta-cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation. J Biol Chem. 1999;274:3235–3242. doi: 10.1074/jbc.274.5.3235. [DOI] [PubMed] [Google Scholar]
- Ward S, Carrel JS. Fertilization and sperm competition in the nematode Caenorhabditis elegans. Dev Biol. 1979;73:304–321. doi: 10.1016/0012-1606(79)90069-1. [DOI] [PubMed] [Google Scholar]
- White J. The Nematode Caenorhabditis elegans, W.B. Wood and a.C.o.C.e. Researchers. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. The Anatomy; pp. 81–122. [Google Scholar]
- Willnow TE, Nykjaer A, Herz J. Lipoprotein receptors: new roles for ancient proteins. Nat Cell Biol. 1999;1:E157–E162. doi: 10.1038/14109. [DOI] [PubMed] [Google Scholar]
- Yochem J, Tuck S, Greenwald I, Han M. A gp330/megalin-related protein is required in the major epidermis of Caenorhabditis elegans for completion of molting. Development. 1999;126:597–606. doi: 10.1242/dev.126.3.597. [DOI] [PubMed] [Google Scholar]