Abstract
NK subsets have activating and inhibitory receptors that bind MHC-I. Ly49A is a mouse inhibitory receptor that binds with high affinity to H2d in both a cis- and trans-manner. Ly49A cis-associations limit trans-interactions with H2d-expressing targets as well as monoclonal antibody (mAb)-binding. We demonstrate that cis-interactions affect mAb effector functions. In vivo administration of anti-Ly49A depleted NK cells in H2b but not H2d mice. Despite lack of depletion, in vivo treatment with anti-Ly49A reduced NK killing capabilities and inhibited activation, partially due to its agonistic effect. These data explain the previously described in vivo effects on bone marrow allograft rejection observed with anti-Ly49A treatment in H2d-haplotype mice. However, prior treatment of mice with poly(I:C) or MCMV infection resulted in increased Ly49A expression and Ly49A+ NK cell depletion in H2d mice. These data indicate that although Ly49 mAbs can exert similar in vivo effects in mice with different MHC-haplotypes, these effects are mediated via different mechanisms of action correlating with Ly49A expression levels and can be altered within the same strain contingent on stimuli. This illustrates the marked diversity of mAb effector functions due to the regulation of the level of expression of target antigens and responses by stimulatory incidents such as infection.
INTRODUCTION
Natural killer (NK) cells represent an important immune cell that contributes to anti-viral and anti-tumor responses (1). NK activation is regulated by the balance of activating and inhibitory receptors (2, 3). NK function was identified by the spontaneous lysis of tumor cells and transplantation studies where parental bone marrow (BM) grafts were rejected by irradiated F1-hybrid mice (4, 5). The phenomenon of hybrid-resistance was later explained by the “missing-self” theory, which proposed that the lack of proper MHC class-I (MHC-I) expression resulted in NK cell-mediated rejection (6, 7). The identification of MHC-I-specific inhibitory receptors that suppress NK activation revealed another pathway regulating NK function dependent on the MHC-I level on target cells (7–9). MHC-I expression is also required for the acquisition of NK function and tolerance (10–14). NK cells isolated from b2-microglobulin (β2m) deficient mice are less responsive than NK cells isolated from wild-type mice (12). Furthermore, the transfer of NK cells from B2m-deficient mice into MHC-I-sufficient recipients restores NK function (15). The enigmatic presence of NK cells with inhibitory receptors for MHC-I molecules that lack self-reactivity was explained by NK “licensing” (13, 14, 16). This hypothesis states that NK cells lacking inhibitory receptors that bind self-MHC-I are unlicensed NK cells and remain hyporesponsive in comparison to licensed NK cells, which are capable of binding to self-MHC-I in vitro (13) and in vivo (17).
In mice, MHC-I-binding inhibitory receptors belong to the C-type lectin-like Ly49 family; whereas in humans these receptors are classified in the Killer cell Immunoglobulin-like Receptor family (KIR) which is homologous to Ly49s in function but structurally unrelated (3). Inhibitory Ly49s and KIRs contain immunoreceptor tyrosine-based inhibitory motifs (ITIMs). Upon MHC engagement, the tyrosine residues of the ITIM are phosphorylated and allow for the recruitment of tyrosine phosphatases through the SCR-homology 2-domain (SHP-1, SHP-2 or SHIP) and results in decreased phosphorylation of multiple intracellular signaling proteins involved in NK activation such as ZAP70, SLP76 or Vav-1 (3, 18).
Ly49A is an inhibitory receptor that strongly binds to H2Dd and H2Dk (19, 20) through residues of the α2 and α3 domains and b2-microglobulin(21–24). Accordingly, Ly49A+ NK cells from H2d and H2k strain mice produced higher amounts of IFNγ upon stimulation compared to H2b, H2q and H2s strains, indicating licensing of the Ly49A NK subset in H2d and H2k strain mice (13, 25). It was observed that H2d and H2k mice had lower expression of Ly49A in comparison to animals with a different H2-haplotype (26–28). Ly49A is able to bind to H2d expressed on the same cell resulting in cis-binding (12, 28). This interaction occurs laterally at the same site (site-2) where trans-interaction with MHC-I ligand on target cells occurs, preventing simultaneous cis- and trans-interactions (22, 23, 28–30). The binding site for MHC-I seems to be critical not only for NK inhibition by trans-binding but also for NK licensing via Ly49A and H2d interactions as transgenic mice with altered site-2 in H2d (R6A-H2d KODO mice) resulted in lower Ly49A-dependent licensing compared to wild-type H2d KODO mice (31). Cis-binding has also been associated with NK licensing (32). It has been postulated that cis-binding restricts the ability of Ly49A to interact in trans by reducing the proportion of free Ly49A (28, 33) and limiting the formation of immunological synapses (34). Antibodies againstLy49A appear to bind less to the receptor in H2d compared to H2b NK cells (26, 28). The function of cis-interactions seems to be to reduce the threshold necessary for NK cell activation (30). Although cis-associations have not been described for KIRs, they have been shown in the human leukocyte immunoglobulin-like receptor (LILR)-B2 and its mouse orthologue paired Ig-like receptor (PIR) (35).
The function of NK cell subsets has been explored predominantly by in vivo depletion with mAbs. Although cis-interaction has been shown to limit mAb binding (26–28), its effect on mAb functions has not yet been described. Here we have addressed the influence of Ly49A and MHC-I cis-interactions on the ability of anti-Ly49A to deplete NK cells in vivo.
MATERIAL AND METHODS
Mice
The University of California, Davis Institutional Animal Care and Use Committees approved studies and protocols. Female B10 (H2b), B10.D2 (H2d) and B10.BR (H2k) mice were purchased from Jackson Laboratory (Bar Harbor, ME). Female C57BL/6 (H2b), DBA (H2d), B6D2F1 (H2bxd) mice were obtained from the Animal Production Area, National Cancer Institute (Frederick, MD). All mice were used at 8–12 weeks of age and housed under specific pathogen-free conditions.
Antibodies Treatment and Flow Cytometry
For in vivo or in vitro treatments, 300ug anti-Ly49G2 (clone 4D11), anti-Ly49C/I (5E6), anti-NK1.1 (PK136) and anti-Ly49A (YE1/32) and/or rat-IgG (rIgG, Jackson Immunoresearch) were used. Dr. Lewis Lanier (UCSF) generously provided anti-Ly49A (YE1/48). Mice were intraperitoneally (ip) treated in vivo with 300ug of anti-Ly49A (YE1/32 or YE1/48) and/or anti-Ly49G2 (4D11) or rIgG two days prior to tissue collection, BMT or MCMV infection. In some studies, 200ug of poly(I:C) were injected into mice to promote NK cell activation two days prior to tissue collection. Splenocyte single-cell suspensions were prepared and antibody staining and analysis was performed as previously described (36) two days after in vivo treatment, 24h after BMT or 7-days post-MCMV infection.
Allogeneic Bone Marrow Transplantation and CFU-c Assay
B10.D2 recipient mice were treated with indicated mAbs two days prior to irradiation (950cGy) and 15 million B10 BM cells infusion. BMT and CFU-c assay were performed as previously described (17). In some experiments, spleens were collected 24h after BMT to determine NK cell subset depletion by mAbs.
NK Stimulation Assay
Two days after in vivo mAb treatment, 10 million splenocytes were in vitro incubated for 12h in 10ug NK1.1 (PK136) plate-bound 24-wells with one μl/ml Golgiplug and Golgistop (DB pharmingen). Intracellular staining for granzyme-B was performed using the BD Cytofix/Cytoperm Fixation/Permeabilization kit following the manufacturer instructions.
In some experiments, splenocytes from resting B10 or B10.D2 mice were incubated for 7 days with 1000 IU/ml recombinant-human IL-2 (NCI repository) and 20μg/ml anti-Ly49A (YE1/32), anti-Ly49C/I (5E6) or isotype-matched control IgG. Adherent lymphokine-activated killer cells (ALAKs) were collected and cultured for 4 additional hours in the presence of Golgiplug and Golgistop (DB Bioscience). Intracellular staining of phosphorylated ERK1/2 was determined by using BD™ Phosphoflow mouse anti-ERK1/2 (pT202/pY204) or isotype control following manufacturer’s instructions.
Cytotoxic Assays
Cytolytic activity of ALAKs was determined by a 4-hour Cr51-release assay against several tumors according to standard procedures (37, 38). The NK-sensitive tumor cell line YAC-1 was obtained from ATCC (Manassas, VA). Dr. Stephen K. Anderson (NCI) generously provided the rat-myeloma cell line YB transfected with H2Dd or H2Db. Tumor cells were grown as previously described (38).
In vitro assessment of Ly49A expression
Cells from B10.D2 and C57BL/6 mice were acid stripped as previously described to interrupt cis-interactions Cells were stained for CD45, CD3, NK1.1 and Ly49A (YE1/48) for analysis by flow cytometry. To determine the presence of anti-Ly49A (clone YE1/32), in vivo treated splenocytes were collected and incubated at 4C with 20ug/mL of rIgG or anti-Ly49A (YE1/32). After 1hr cells were washed, and stained with the secondary Ab goat anti-ratIgG (BD, anti-rIgG) and anti-CD3 (Hamster mAb) to determine surface expression of Ly49A and/or anti-Ly49A in vivo binding. For intracellular detection, the BD Cytofix/Cytoperm Fixation/Permeabilization kit was used following manufacturer’s instructions prior Ly49A staining.
Competitive Assay
To prevent the mAb binding, splenocytes of B6 and B10.D2 mice were incubated with the indicated doses of APC-labeled H2Ld MCMV pp89-derived peptide YPHFPTNL or H2Db MCMV M45985-993 tetramers (NIH Tetramer Core Facility at Emory University) for 2 hr at 37C. Then cells were washed and stained as previously described.
MCMV infection
B10.D2 mice were treated with 300μg of mAbs two days prior infection with 5×104 plaque forming units (PFU) of MCMV ip (Smith strain obtained from American Type Culture Collection, Manassas, VA). Seven days post-infection, spleens and livers were collected for NK cell analysis by flow cytometry and determination of viral loads, respectively. The virus was maintained by repeated salivary gland passage in BALB/c mice and salivary gland homogenates were prepared in RPMI-1640 medium (Gibco Laboratories, Grand Island, NY) as described elsewhere (39).
Quantification of MCMV
On day 7 post-infection, livers were collected and DNA was extracted using DNeasy Tissue Kit (Qiagen, Valencia, CA). Real-time PCR was used as previously described for the quantification of MCMV(39). The data are represented as IE1 gene copies/100mg of tissue.
Statistical Analysis
Each experiment was performed with 3–4 mice per group and repeated at least two times. Statistical significance was determined by using Student’s two-tailed t-test, one-way Anova (Tukey post-test analysis) or two-way ANOVA (Bonferroni post-test analysis) as appropriate. P-values were considered statistically significant when p<0.05.
RESULTS
Biological effect of anti-Ly49A (clone YE1/32) on the role of NK cell subsets in rejection of allogeneic BMC in H2d strain mice
We have previously shown the preferential role of host licensed NK subsets in the in vivo rejection of allogeneic BMCs using in vivo depletion of NK cell subsets (17). To confirm the effect of licensed NK cells in allogeneic BM rejection, B10.D2 (H2d) mice were treated with rIgG or anti-Ly49G2 and/or anti-Ly49A (Ly49G2 and Ly49A bind H2d) two days prior toallogeneic BMT. Hematopoietic recovery and engraftment was measured by the CFU assay 7-days after BMT as previously described (17). We observed that while anti-Ly49A treatment did not affect the rejection of allogeneic BMCs, the depletion of the Ly49G2 NK cell subset resulted in higher engraftment compared to control (Figure 1A) in accordance with our previous results (17). Furthermore, combined treatment with anti-Ly49A and anti-Ly49G2 mAbs significantly improved the BMC engraftment compared to anti-Ly49G2 treatment alone (s-CFU: 3067.22±340.97 vs. 39.84±26.8 p<0.001) (Figure 1A and (17, 40)). These data demonstrate and confirm the previous report that in vivo treatment of H2d strain mice with anti-Ly49A mAb results in increased engraftment of H2b BMCs (17, 40).
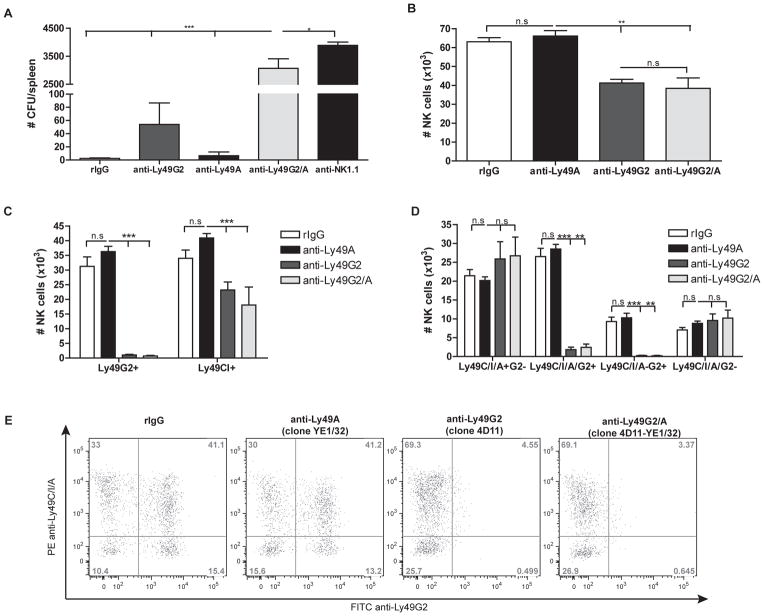
Figure 1. Treatment with anti-Ly49A and anti-Ly49G2 improves allogeneic BMC engraftment synergistically in B10.D2 (H2d) hosts.
B10.D2 recipient mice were treated with rIgG, anti-NK1.1, anti-Ly49G2 and/or anti-Ly49A two days prior lethal radiation and intravenous infusion of 15×106 B10 BMCs. (A) The hematopoietic progenitor content of spleens (Total CFU-c/spleen) was assessed seven days post-BMT. (B–E) Twenty-four hr post-BMT splenocytes were stained for NK cells (CD45, CD3, NK1.1, Ly49G2, Ly49C/I or Ly49A). (B) Total number of NK cells (CD45+CD3−NK1.1+) and (C) total number of Ly49G2+ or Ly49C/I+ NK cells is shown. (D) Total number or (E) representative dot plots of the frequency of Ly49G2, Ly49C/I and Ly49A NK subsets previously gated on CD45+CD3−NK1.1+ cells is shown. Data are representative of two experiments with three mice per group (mean ± SEM). One-Way Anova was used to assess significance (*p<0.05, **p<0.01, ***p<0.001, n.s: not significant).
The effect of mAb treatment observed in allogeneic BMC engraftment was initially thought to be due to the depletion of the host Ly49A+ and Ly49G2+ NK cells. To confirm NK depletion by mAbs, we measured the host NK cell subset distribution after mAb treatment. Spleens were collected 24h post-allogeneic BMT and NK numbers were calculated by flow cytometry. The treatment of host B10.D2 mice with anti-Ly49G2 (4D11) prior to allogeneic BMT resulted in a significant reduction of NK cells (63.08×104±2.14 vs. 41.29×104±1.94 p<0.001) 24h post-BMT (Figure 1B). Moreover, anti-Ly49G2 treatment resulted in an efficient depletion of Ly49G2+ NK cells (Figure 1C–E) and an approximately 50% reduction of Ly49C/I+ NK cells (Figure 1C–E) as expected (Supplemental Figure 1). In contrast, anti-Ly49A (YE1/32) treatment did not have an effect on the total number of NK cells (Figure 1B) despite Ly49A+ NK cells representing approximately 20% of total NK cells. Similarly, the distribution of Ly49G2 and Ly49C/I was not affected (Figures 1C–E) compared to rIgG control treated mice, but a 20% reduction in total numbers of each NK cell subset occurred as expected (Supplemental Figure 1). No differences were found in NK cell numbers between the use of anti-Ly49G2 alone and anti-Ly49G2 combined with anti-Ly49A (Figure 1B–E) indicating that as opposed to anti-Ly49G2 administration with the depletion of Ly49G2+ NK cells, anti-Ly49A administration did not result in comparable depletion of Ly49A+ NK cells in these mice.
Anti-Ly49A (clone YE1/32) depletes Ly49A+ NK cells in H2b strains but not in H2d strains
We analyzed the impact of MHC-I haplotype in the ability of anti-Ly49A (YE1/32) to eliminate Ly49A+ NK cells. We treated resting B10 (H2b) and B10.D2 (H2d) mice with control rIgG, anti-Ly49A and/or anti-Ly49G2 and the effect on Ly49A depletion was determined indirectly by analyzing the number of NK cells and the distribution of Ly49G2 and Ly49C/I subsets We choose this strategy because of limitations in the detection of Ly49A by flow cytometry (Supplemental Figure 1). B10.D2 Ly49A can only be shown using the clone YE1/48, but not C57BL/6 A1 clone. However, when YE1/48 was used to directly determine the effect of in vivo treatment with anti-Ly49A (clone YE1/32) a population stained positive in all the strains analyzed whereas the use of A1 clone in B10 mice did show Ly49A depletion after YE1/32 treatment for this strain suggesting an unspecific binding for YE1/48. Thus, as expected, we observed a reduction in the percentage and numbers of total NK cells as well as the total numbers of Ly49G2+ or Ly49C/I+ NK cells after anti-Ly49G2 administration in both strains (Figure 2A–E(17)). However, anti-Ly49A treatment was only effective in B10 mice resulting in significant reduction of total NK cells and Ly49G2+ or Ly49C/I+ NK cell subsets (Figure 2A–E). The effect on NK cell depletion by anti-Ly49A in B10.D2 mice was not dependent on antibody amounts as administration of higher doses did not reduce the total number of NK cells (Supplemental Figure 2).
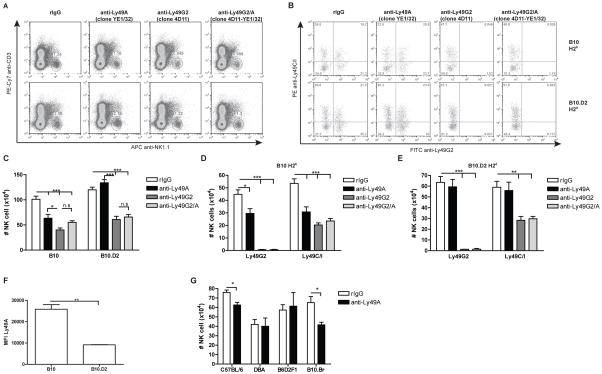
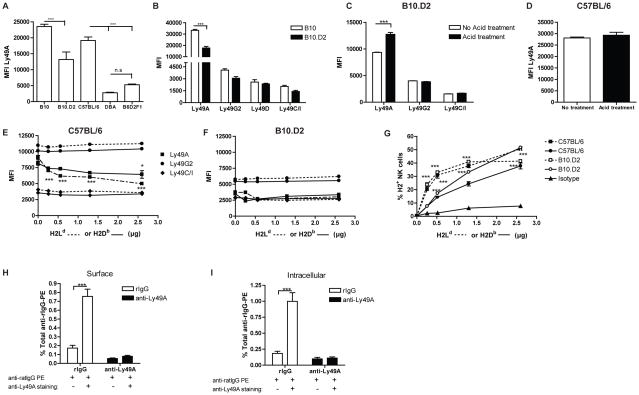
Figure 2. H2d expression on the cell surface limits the ability of anti-Ly49A (clone YE1/32) to deplete NK cells.
B10 (H2b), B10.D2 (H2d), C57BL/6 (H2b), DBA (H2d), B6D2F1 (H2bxd) and B10.BR (H2k) mice were treated with control rIgG, 4D11 and/or YE1/32 mAbs two days prior to harvest. Splenocytes were stained for NK cells (CD45, CD3, NK1.1, Ly49G2 and Ly49C/I). (A–E) Representative dot plots (A–B) and total number of NK cells (CD45+CD3−NK1.1+) or Ly49G2+ or Ly49C/I+ NK cells (C–E) for B10 (A–D) and B10.D2 (A–C, E) are shown. (F) The median fluorescence intensity (MFI) is shown for Ly49A+ NK cells. (G) Total number of NK (CD45+CD3−NK1.1+) for C57BL/6, DBA and B6D2F1 and B10.BR is shown. Data are representative of two or three experiments with three mice per group (mean ± SEM). One-Way Anova was used to assess significance (*p<0.05, **p<0.01, ***p<0.001, n.s: not significant).
Previously it has been shown that the Ly49A receptor can bind to H2d displayed on the NK cell in a cis-manner, thereby reducing Ab-binding (28). When we analyzed the Ly49A expression of NK cells from B10 and B10.D2 mice we observed a reduction of MFI for Ly49A in B10.D2 compared with B10 mice consistent with possible cis-binding in H2d strains (Figure 2F), which might limit Ly49A detection by mAbs.
To exclude the possibility that the differential mAb function of anti-Ly49A was strain-specific, we treated C57BL/6 (H2b), DBA (H2d), B6D2F1 (H2bxd) and B10.BR (H2k) mice with anti-Ly49A (YE1/32) and assessed the NK distribution. As expected, the effect of anti-Ly49A treatment in C57BL/6 (H2b) mice on NK distribution was analogous to B10 (H2b) mice (Figure 2G and Supplemental Figure 3). In contrast and similar to effects in B10.D2 mice, administration of anti-Ly49A to DBA (H2d) mice did not deplete the NK cell population. Moreover, the expression of H2d seems to be sufficient to prevent NK depletion as anti-Ly49A treatment in B6D2F1 (C57BL/6 x DBA [H2bxH2d]) resulted in a comparable outcome to B10.D2 and its parental strain DBA with regard to lack of depletion (Figure 2G and Supplemental Figure 3). Surprisingly, when anti-Ly49A was injected into B10.BR (H2k) mice, the results were similar to those observed in B10 (H2b) mice showing a significant reduction in total numbers of NK cells and NK cell subsets. These data suggest that the absence of NK cell depletion by anti-Ly49A (YE1/32) is dependent on MHC-I haplotype, suggesting a cis-binding effect in H2d strains.
Lack of Ly49A+ NK cell depletion in H2d strains is not specific to the mAb clone
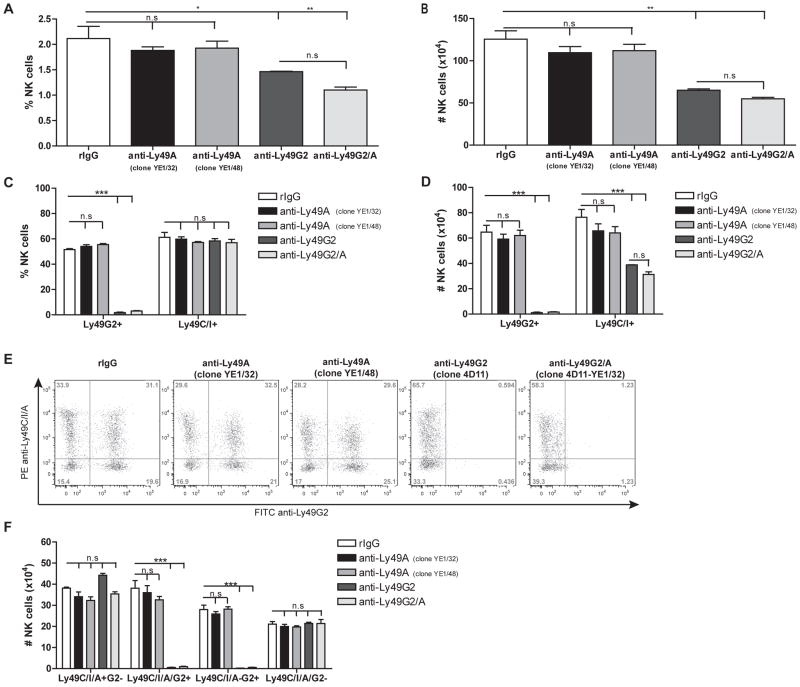
We next wanted to explore if the lack of NK depletion observed in H2d strains could be restricted to the specific clone of anti-Ly49A used. Therefore, we treated B10.D2 mice with anti-Ly49A clone YE1/48 in parallel with clone YE1/32. Similar to the results obtained previously with the YE1/32 clone, the use of YE1/48 did not reduce the percentage and the total number of NK cells (Figure 4A–B). Furthermore, the distribution of Ly49 NK cell subsets was not affected by administration of anti-Ly49A clone YE1/48 (Figure 4E–F). Anti-Ly49G2 treatment, however, did significantly reduce the number of total Ly49G2+ and Ly49C/I+ NK cells in comparison to controls (98.3%±0.8 and 40.23%±8.9 reduction) respectively (p<0.001), corroborating the depleting functions of this mAb in H2d strain mice (Figure 3). This demonstrates that Ly49A appears unique in its ability to resist depletion in mice expressing H2d.
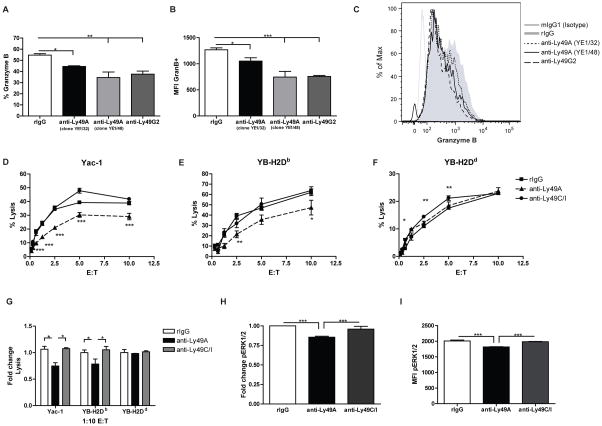
Figure 4. Impaired NK function after in vivo and in vitro treatment with anti-Ly49A.
(A–C) After in vivo mAb treatment, ten million B10.D2 splenocytes were stimulated for 12h with anti-NK1.1. Granzyme B expression were measured by intracellular staining. (A) The percentage and (B) MFI of Granzyme B and (C) Representative histograms of Granzyme B expression of cells previously gated on NK cells (CD45+CD3−NK1.1+) are represented. (D–I) Resting B10.D2 splenocytes were co-cultured with 103 IU/mL of IL-2 and 20ug/mL of control rIgG, anti-Ly49A (clone YE1/32) or anti-Ly49C/I (clone 5E6) for 7 days. Adherent lymphokine activated killer cells (ALAKs) were collected and a 4-hr Cr51-release assay was performed against Yac-1 (D), YB-H2Db (E) and YB-H2Dd (F) targeted cells. (G) Fold change on percentage of lysis for different tumors is shown at 1:10 effector:target ratio in comparison with rIgG-treated cells. (I) The level of ERK1/2 phosphorylation (pERK1/2) was also determined on NK cells by flow cytometry. Data are representative of at least two experiments with 2–3 mice per group (mean ± SEM). One-way or Two-way Anova were used to assess significance (*p<0.05, **p<0.01, ***p<0.001).
Figure 3. The lack of NK cell depletion by anti-Ly49A in H2d is not clone-specific.
B10.D2 mice were treated with control rIgG, anti-Ly49A (clones YE1/32 or YE1/48) or anti-Ly49G2 as previously described in figure 1. (A–B) The frequency and total number of NK (CD45+CD3−NK1.1+) cells are represented. (C–D) The percentage and total number of total Ly49G2+ or Ly49C/I+ NK cells are shown. (E–F) Representative dot plots and total number of Ly49G2, Ly49C/I and Ly49A NK subsets are shown. Data is representative of two experiments with three mice per group (mean ± SEM). One-way Anova was used to assess significance (*p<0.05, **p<0.01, ***p<0.001; n.s: not significant).
In vivo and in vitro treatment with anti-Ly49A results in impaired NK function in H2d strain mice
We have demonstrated that administration of anti-Ly49A depletes NK cells in H2b strains but not in H2d strains (Figure 1–3). However, the combination of anti-Ly49A and anti-Ly49G2 improves allogeneic BM engraftment in a synergistic manner in H2d mice (Figure 1A). These data suggest that although anti-Ly49A does not deplete NK cells, it may result in inhibition of Ly49A+ NK cells. Therefore, we next sought to evaluate NK function after in vivo anti-Ly49A treatment. B10.D2 mice were treated with anti-Ly49A (clones YE1/32 and YE1/48) in vivo as previously described. Cells were then stimulated in vitro with immobilized anti-NK1.1 and the production of granzyme B was assessed. Anti-Ly49A treatment decreased the percentages of granzyme B in comparison to rIgG (Figure 4A). The level of expression of granzyme B (MFI) was also reduced (Figure 4B–C). The decline of granzyme B production was comparable to that observed after anti-Ly49G2 treatment, which depletes the licensed Ly49G2 NK subset.
We also assessed the ability of anti-Ly49A (YE1/32) to inhibit NK function in vitro. We cultured splenocytes from B10.D2 mice in the presence of IL-2 (103 IU/mL) and 20ug/mL of anti-Ly49A, anti-Ly49C/I (unlicensed subset for B10.D2, binds H2b) or control rIgG. After seven days, ALAKs were collected and NK cell-mediated killing capability was measured by a chromium-release assay against Yac-1 and rat lymphoblast cell lines transfected with H2Dd or H2Db (YB-H2Dd and YB-H2Db). Anti-Ly49C/I treatment did not affect the ability of NK cells to lyse the tumor cell lines (Figure 4D–G) compared to rIgG. However, anti-Ly49A treatment reduced the ability of NK cells to eliminate Yac-1 and YB-H2Db tumor cells, in comparison to anti-Ly49C/I and rIgG (Figure 4D–G). The presence of H2Dd on the tumor cells resulted in inhibition of lysis through the interaction of H2Dd on target cells with Ly49G2 and Ly49A on the NK cells (38), which is demonstrated by the lower percentage of lysis for this tumor cell line compared to Yac-1 or YB-H2Db tumor cell lines (Figure 4D–G). Because there was no difference between groups in the ability to eliminate YB-H2Dd tumor cells, anti-Ly49A binding appears to cause NK inhibition to the same extent as H2d-Ly49A trans-binding.
The inhibition of NK cell cytotoxicity mediated by a mAb against Ly49A contrasts with a previous publication that showed that incubation with Fab’ anti-Ly49A (clone A1) resulted in enhanced NK cell function against H2d targets (41). In this study, Ly49A+ IL-2 activated NK cells were incubated with anti-Ly49A prior assessment of cytotoxicity. When we incubated IL-2 activated NK cells with anti-Ly49A (clone YE1/32) or anti-Ly49G2 (clone 4D11) 1-hour prior a Cr-release assay, improvement of NK cell lysis against H2d cells was detected (Supplemental Figure 4A–B). These data agree with previous studies that state that the incubation with mAbs against Ly49 inhibitory receptors block the interaction with the corresponding inhibitory receptor and MHC-I molecules expressed on tumor cells making them more susceptible to lysis (41–43). However, long-term culture with anti-Ly49A still inhibited NK cell function on B10.D2 NK cells (Figure 4 and Supplemental Figure 4). We did also analyze the effect of NK cell inhibition on IL-2 activated NK cell from B10 splenocytes. To our surprise, anti-Ly49C/I but not anti-Ly49A long-term incubation resulted in inhibition of NK cell function (Supplemental Figure 4C–D). Although Anti-Ly49C/I (clone 5E6) is able to efficiently deplete in vivo Ly49C/I+ NK cells in both H2d and H2b (17, 38), NK cell depletion is not expected to occur in vitro. Therefore under these conditions, we were able to reveal an inhibitory function of anti-Ly49C/I similar to the one observed with anti-Ly49A. Because NK cell inhibition was only observed in the H2d strain with anti-Ly49A, and in the H2b strain with anti-Ly49C/I, cis-interaction between MHC-I and the inhibitory receptor might be required to exert mAb-dependent NK cell inhibition.
We hypothesize that long-term incubation with mAbs mimics the SHP-1 dependent inhibitory effect observed upon recognition of self-MHC-I by the inhibitory receptor. Early studies in which Ly49A cDNA was transfected into the B-cell line A20 showed that the inhibitory function mediated by Ly49A was in part dependent on the recruitment of SH2 containing tyrosine phosphatase-1 (SHP-1) (44). Activation of SHP-1 is involved in the desphosphorylation of substrates critical for cell activation pathways such as those involved in the MAPK pathway. Because of the lower NK cell-mediated cytotoxic function after mAb treatment, we hypothesized that anti-Ly49A binds to the inhibitory receptor in a way that induces NK cell inhibition similar to MHC-I engagement. Therefore, we analyzed the level of phosphorylation of the extracellular signal-regulated kinase 1/2 (ERK1/2), a kinase located downstream of the MAPK pathway that is implicated in NK activation and cytokine degranulation (45). The level of pERK1/2 was significantly lower in NK cells that were cultured in the presence of IL-2 and anti-Ly49A compared with rIgG and anti-Ly49C/I (Figure 4H–I and Supplemental Figure 4F). Similarly, the phosphorylation of ERK1/2 was also reduced after anti-Ly49C/I long-term incubation of NK cells derived from H2b mice (Supplemental Figure 4). Taken together these data suggest that NK cell function is impaired after in vivo and in vitro anti-Ly49A binding due to cis-interaction with H2d
Decreased Ly49A expression in H2d strains correlates with impairment in NK cell depletion in vivo with anti-Ly49A
Previous studies have demonstrated that cis-interaction between H2d and Ly49A limits the number of free Ly49A (2, 25, 31, 33, 34). We hypothesized that the same cis-interaction could limit the ability of anti-Ly49A to bind to Ly49A+ NK cells, which could affect antibody-mediated cell depletion by antibody-dependent cellular cytotoxicity (ADCC) or complement pathways. When we analyzed the expression of Ly49A in the different strains, we observed that Ly49A MFI was significantly higher in B10 mice than in the congeneic strain B10.D2 mice (Figure 2 and Figure 5). Similarly, the Ly49A levels of DBA and B6D2F1 were significantly lower than C57BL/6 mice (Figure 5A). These data confirm previous work that demonstrated lower Ly49A MFI for H2d strains (25, 28, 31, 34).
Figure 5. Impact of H2d on anti-Ly49A binding capabilities.
Splenocytes from resting B10, B10.D2, C57BL/6, DBA and B6D2F1 were stained to identify Ly49A+ NK cells (CD45+CD3−NK1.1+Ly49A+). (A) MFI is shown for Ly49A+ NK cells for each strain. (B) B10.D2 and B10 resting spleen cells were also stained for Ly49G2, Ly49D and Ly49C/I and the MFI for each NK cell subsets and mouse strain is represented. (C–D) Ly49A MFI expression of B10.D2 (C) and C57BL/6 (D) NK cells after acid treatment. (E–F) Effect of H2Ld or H2Db tetramer binding in anti-Ly49A binding (MFI) is shown for C57BL/6 (E) and B10.D2 (F). (G) Percentage of H2Ld (dashed line) and H2Db (continues line) tetramer binding in C57BL/6 (black symbols) and B10.D2 (white symbols) NK cells (CD45+CD3−NK1.1+) is shown. (G–H) Surface or Intracellular expression of Ly49A NK cells of in vivo treated NK cells detected by secondary staining with anti-ratIgG. Data are representative of two (A–F) or one (G–H) experiments with three mice per group or by triplicate (mean ± SEM). Two-way Anova or two-tailed Student’s t-test was used to assess significance (*p<0.05, **p<0.01, ***p<0.001; n.s: not significant).
Early studies showed that Ly49A, Ly49G2 and Ly49D bind to H2d, resulting in NK inhibition or activation respectively (19). Therefore, one would expect that cis-interaction could also occur in Ly49G2 and Ly49D-positive NK cells and affect antibody binding. When we analyzed the MFI of these receptors, no significant differences between B10 and B10.D2 NK cells were observed (Figure 5B). Similarly, the MFI of Ly49C/I was not different between B10 and B10.D2 NK cells suggesting the anti-Ly49C/I (5E6) mAb binding site is not affected by cis-binding in H2b strains as well (Figure 5B), which could explain the in vivo depleting functions of these mAb (17, 38). These data also indicate the unique nature of Ly49A regarding cis-binding.
To further confirm cis-interaction as a mechanism that limits Ly49A surface expression in H2d strains, we exposed B10.D2 or C57BL/6 resting splenocytes to an acidic buffer (pH 3.3), which disrupts the MHC-I complex and allows Ab-binding (2, 33, 34). Acid treatment significantly enhanced Ly49A expression in cells from B10.D2 mice (Figure 5C), but did not have any effect on C57BL/6 mice (Figure 5D). In contrast, the expression of Ly49G2 was not affected by the distortion of MHC-I (Figure 5C), validating previous results that suggest the interaction of Ly49G2 with its mAb (4D11) is not affected by cis-binding and providing an explanation for the depleting functions of this mAb in H2d strain mice.
Several studies have demonstrated that the ability to recognize and bind to H2d is blocked by the addition of anti-Ly49A (22, 33). Additionally, the expression of H2d in H2d-transgenic C57BL/6 mice considerably reduces the MFI of NK cells stained with anti-Ly49A (2, 28, 32, 34). To mimic this effect in vitro and corroborate that H2d-Ly49A interaction interferes with anti-Ly49A binding, we incubated H2d and H2b splenocytes with H2d or H2b tetramers prior Ly49A staining. As expected, the binding of anti-Ly49A (YE1/48) was reduced by the presence of H2d in H2b-expressing cells in a dose dependent-manner (Figure 5E); whereas addition of H2d tetramer did not affect Ly49A recognition in H2d+ cells as H2d-Ly49A cis-binding is already formed (Figure 5F). The MFI of Ly49G2 and Ly49C/I was not reduced by the addition of H2d tetramer in neither H2d nor H2b stains (Figure 5E–F). Due to the ability of Ly49A to bind H2b with lower affinity, pre-incubation with H2b tetramers reduced anti-Ly49A binding to a lower extent compared to H2b tetramers (Figure 5E). Similar to Mehta et al. and in correlation with cis-binding, at high H2d tetramer doses the binding capabilities of the H2d tetramer was lower in H2d cells compared to H2b cells (46); whereas the binding capabilities were reversed with the H2b tetramer (Figure 5G). These data confirm the presence of H2d on Ly49A+ NK cells alters proper anti-Ly49A binding which prevents in vivo depletion when this mAb is used.
NKG2D downregulation upon NKG2D ligand engagement has been suggested to be due to internalization of the activating receptor that results in impaired NK cell function (47). To determine if Ly49A-anti-Ly49A interaction also induced internalization of the inhibitory receptor, intracellular staining of Ly49A+ NK cells was determined on in vivo anti-Ly49A treated cells by secondary staining with anti-rat IgG (anti-rIgG) that binds to rat anti-Ly49A YE1/32 mAb. However, no positive staining was detected in the surface of the treated cells or intracellularly (Figure 5H–I). It is possible that during internalization of the receptor, anti-Ly49A was released. To explore this possibility we incubated the cells with anti-Ly49A (clone YE1/32) after membrane permeabilization and prior secondary staining with anti-rIgG and again no positive staining was observed. As a positive control, in vivo rIgG treated splenocytes were stained with anti-Ly49A (clone YE1/32) or rIgG prior secondary staining with anti-rIgG. As expected, the incubation with purified anti-Ly49A resulted in the positive staining of approximately 0.8% of total splenocytes with anti-rIgG, which correlates with the total Ly49A+ NK cells present in the spleen and validates the use of anti-rIgG to detect the YE1/32 clone (Figure 5H–I). In rIgG-treated mice, Ly49A is primarily found on the NK cell surface because no differences were observed in the percentage (Figure 5H–I) or MFI (data not shown) of Ly49A+ NK cells between surface or intracellular staining. In conclusion, these data do not exclude Ly49A internalization as a possible mechanism of action of anti-Ly49A but suggest a degradation of the Ly49A receptor as it was not found on the surface nor intracellularly of anti-Ly49A in vivo treated cells.
Increased Ly49A expression after poly(I:C) treatment or MCMV infection allows for in vivo Ly49A+ NK cell subset depletion with anti-Ly49A
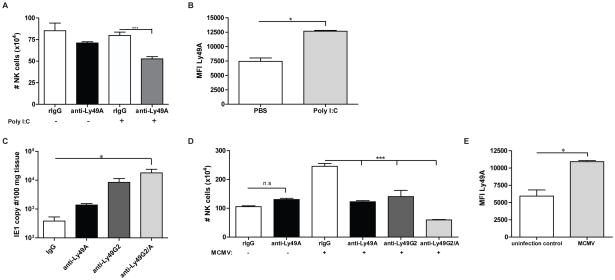
To investigate if increasing Ly49A expression could restore the ability of anti-Ly49A to deplete Ly49A+ NK cells in H2d mice similar to that observed in H2b or H2k strains, we treated B10.D2 mice with anti-Ly49A and poly(I:C) which has previously been demonstrated to activate NK cells and promote allogeneic BM rejection (17). The administration of poly(I:C) increased Ly49A expression as determined by MFI (Figure 6B) and as a consequence, anti-Ly49A mAb administration now resulted in depletion as demonstrated by reduced numbers of NK cells in vivo after concurrent poly(I:C) treatment (Figure 6A). Similar to the allogeneic BMT studies (Figure 1A), we have demonstrated the role of both licensed Ly49G2 and Ly49A NK cell subsets in the clearance of MCMV infection in B10.D2 mice by the use of mAbs (Figure 6C, Sungur et al., unpublished data), which corroborates the biological in vivo efficacy of both anti-Ly49A and anti-Ly49G2. Surprisingly, in contrast to the allogeneic BMT studies (Figure 1A), the total number of NK cells in the spleen of infected mice was significantly lower (P<0.001) after anti-Ly49A treatment in comparison to control rIgG-treated mice (Figure 6D). Interestingly, we observed that MCMV infection also resulted in an increase of Ly49A expression compared to that observed in uninfected mice (Figure 6E). These results indicate that the expression level of the Ly49A receptor plays a critical role in the depleting capabilities of the anti-Ly49A mAbs and that it is possible to modulate Ly49 receptor expression in H2d strain mice resulting in depletion.
Figure 6. Increased Ly49A expression in H2d strains allows for efficient NK cell depletion by anti-Ly49A.
B10.D2 mice were treated with control rIgG or anti-Ly49A (clone YE1/32) with poly(I:C) or PBS administration one day prior to harvest. (A) Total number of NK cells is shown. (B) Ly49A MFI was calculated on gated NK cells after PBS or poly(I:C) treatment. (C–E) B10.D2 mice were treated with control rIgG, anti-NK1.1, anti-Ly49A and/or anti-Ly49G2 two days prior infection with MCMV 5×104 PFU. (C) Seven days post-infection liver MCMV viral loads were measured by PCR. (D) Total numbers of NK cells were calculated from the spleen. (E) The MFI for Ly49A+ NK cells is shown for non-MCMV and MCMV infected mice. Data are representative of two experiments with 3–4 mice per group (mean ± SEM). Two-tailed Student’s t-test or One-way Anova was used to assess significance when appropriate (*p<0.05, **p<0.01, ***p<0.001; n.s: not significant).
DISCUSSION
We demonstrated that the differential effect of anti-Ly49A (YE1/32 and YE1/48) is dependent on MHC-I haplotype. In contrast to H2b strains, in H2d strains anti-Ly49A administration was unable to eliminate this NK subset likely due to cis-binding of MHC on the NK cell. We also demonstrate that although anti-Ly49A is not a depleting mAb in H2d strains, it can exert an agonistic inhibitory effect on NK function in resting conditions. However, the increase of Ly49A expression on the NK cell surface by poly(I:C) treatment or MCMV infection restored the ability of these mAbs to deplete Ly49A+ NK cells in vivo. Thus, these data illustrate the plasticity of function of mAbs that is modulated by the level of expression of the receptor to target.
To date, cis-binding has been demonstrated for those MHC-I molecules that have shown to display the highest affinity for the corresponding receptor, here H2Dd for Ly49A. Cis-interaction for Ly49A was previously suggested by the lower expression (MFI) of Ly49A in H2d-expressing cells (25, 31–34) and the disruption of the H2d complex (28, 33). Acid stripping (48) proved to increase the detection of Ly49A (YE1/48) in cells from H2d strains (28, 33) and provided evidence for the presence of a non-covalent union between H2d-Ly49A which impaired MHC-I trans-binding (28). It was also shown that prior administration of YE1/48 blocked H2d binding on H2b-expressing NK cells (33). Similarly, we have also shown that anti-Ly49A, but not anti-Ly49G2 binding, is blocked by the addition of H2d tetramer in C57BL/6 NK cells. In agreement with previous studies, the proportion of H2d tetramer able to bind to H2b+ NK cells is higher than H2b tetramer (46). Furthermore, we showed that both YE1/32 and YE1/48 were unable to deplete NK cells in H2d strains suggesting that both YE1/32 and YE1/48 behave similarly and presumably have a binding site for Ly49A that is in close proximity to the H2d-binding site. However, in contrast to YE1/48, the binding of anti-Ly49A JR9.318 clone was shown to not be diminished by cis-association (33).
Additionally, the MHC-I haplotype can also affect mAb binding capabilities as multiple MHC-I molecules are able to bind to inhibitory receptors with different affinities (19). Hanke et al. showed that Ly49A was also able to strongly bind to H2Dk, although with a lower affinity than to H2Dd (19). Therefore, Ly49A could potentially bind to H2k in a cis-manner. However, YE1/32 treatment depleted NK cells in B10.BR (H2k) mice. These results suggest that the Ab-binding site for YE1/32 is different in H2k-Ly49A interactions compared to H2d-Ly49A interactions. Furthermore, when Ly49A expression was determined in B10.BR mice with YE1/48 clone, lower MFI was detected compared with B10 mice implying that H2k-Ly49A affects the YE1/48 binding site but not the YE1/32 binding site. Andersson et al. showed that H2k multimeric binding was reduced, but not completely abrogated, in H2d-expressing Ly49A+ NK cells (33). Recent work has also demonstrated the binding of Ly49A to the non-classical MHC-I molecule H2-M3, which impacts Ly49A NK cell licensing in C57BL/6 H2b mice (49). Whether cis-binding between H2-M3 and Ly49A occurs in C57BL/6-derived NK cells is still unknown, but we can safely say that anti-Ly49A (clone YE1/32) is not affected by this cis-interaction if it does. Nevertheless, possible cis-interactions of inhibitory receptors with lower affinity MHC-I molecules or unknown non-classical MHC-I molecules might still occur and mAb binding could potentially be affected by it.
Because of the preferential binding of Ly49G2 to H2d, this receptor could also be associated to H2d in a cis-manner similar to Ly49A. Early studies showed that the binding of Ly49G2-transfected cells to H2d lymphoblast could be blocked by prior culture with mAb 4D11 (19). However, cis-binding has not been confirmed yet for Ly49G2. We and others have shown that anti-Ly49G2 (4D11) can efficiently deplete Ly49G2+ NK cells in multiple strains including H2d strains by flow cytometric analysis using another mAb against Ly49G2 (Cwy3) for detection or indirectly by observing a reduced number of NK cells (17, 38). Additionally, when Ly49G2 MFI was determined after acid treatment, no differences were detected. These results do not exclude a possible cis-association for Ly49G2, however, they do indicate that the interaction of the mAb (4D11) is not affected by this association and explains the depleting capabilities of this mAb in H2d strains. Similarly, in C57BL/6 (H2b) NK cells, Ly49I binding by clone YLI-90 or Ly49C-specific mAb 4L0 was not affected by H2b-Ly49C/I cis-interaction, whereas binding by Ly49C/I/F/H mAb 14B11 was affected (2). These differences depending on the mAb clone could also explain the in vivo efficacy of anti-Ly49C/I and anti-Ly49G2 mAbs independently of the haplotype (17, 38).
The Ab isotype could also influence the in vivo mAb functions. The IgG2a subclass binds to FcγRI with higher affinity (50), possibly resulting in improved ADCC mediated by monocytes and macrophages. 4D11 (anti-Ly49G2) and 5E6 (anti-Ly49C/I) are IgG2a mAbs whereas YE1/32 (anti-Ly49A) is an IgG2b mAb. However, YE1/48 (anti-Ly49A) is also an IgG2a mAb. Therefore the higher affinity for FcγRI by IgG2a isotypes does not explain the lack of NK depletion when anti-Ly49A mAbs are used in H2d strains.
It has been shown that cis-association reduces the number of accessible Ly49A sites to interact in trans with H2d molecules because they share the same site of interaction that reduces the threshold required for NK cell activation (22, 23, 28–30). Therefore, the inhibition of H2d-expressing target cells is less efficient in Ly49A+ NK cells from H2d strains (32, 41). Furthermore, the use of anti-Ly49A (YE1/48) in H2b strains blocks H2d-binding (33). Therefore, it is possible that the use of anti-Ly49A in H2d strains could block the remaining free receptors and further limit the interaction with H2d-expressing target cells resulting in stronger anti-tumor cytotoxicity against H2d target cells. In agreement with this idea, multiple studies showed that the blockade of inhibitory receptors with mAb caused increased NK cell lytic capabilities (41–43). In our study, the treatment with anti-Ly49A or anti-Ly49G2 did also improve NK cell lysis against H2d+ tumor cells if the incubation was done short-term prior to NK cell cytotoxicity assessment. However, long-term incubation with anti-Ly49A impaired NK cell function indicating an agonistic function of anti-Ly49A. Furthermore, mAb against the inhibitory receptors NKG2A and KIR3DL1 also showed to suppress NK cell function (51, 52).
YB-H2Dd tumor lysis was not affected by anti-Ly49A treatment as we expected because unlike previous studies that used sorted Ly49A NK cells, we used whole NK cell populations, which contained Ly49G2+ NK cells that could limit effects observed after anti-Ly49A treatment. Triggering Ly49G2+ NK cells could mediate a stronger NK inhibition despite the blockade and inhibition of Ly49A as Ly49G2+ NK cells represent a larger NK population than Ly49A and explains why no differences are found between rIgG and anti-Ly49A treated NK cells. The large presence of Ly49G2+ NK cells is also illustrated by the lower percentage of lysis observed in the YB-H2Dd tumor cell line compared to the other non-H2Dd tumor cell lines. However, NK cell cytotoxic function was significantly impaired for non-H2Dd-expressing cell lines. Furthermore, reduced ERK1/2 phosphorylation after in vitro IL-2 stimulation in anti-Ly49A-treated cells as well as granzyme B production of in vivo treated H2d NK cells suggest that the binding of anti-Ly49A mAb could be partially dependent on SHP-1 signaling in agreement with previous work (44). Inhibition of NK cell cytoxicity was also observed in H2b strains treated in vitro with anti-Ly49C/I but not with anti-Ly49A suggesting that cis-interaction might be necessary for the mAb-dependent NK cell inhibition.
It is also well known that NK cells can become activated due to the cross-linking of the Fc receptor on NK cells (CD16) by the Fc portion of IgG mAbs that results in the induction of ADCC (53). Because we used mAbs that contained the Fc portion for the in vitro studies, it is possible that NK cells could be activated during the culture with the mAbs through the Fc receptor and lead to the elimination of cells the antibody bound to. However we expect this effect to be minimal because we were able to observe differential killing capabilities when anti-Ly49A was used but not when anti-Ly49C/I or rIgG were used in H2d strains, and the opposite in H2b strains. Additionally, if Ly49C/I+ NK cells were depleted by anti-Ly49C/I, enhanced killing of YB-H2b tumor cells should have been observed but again no differences were found between rIgG and anti-Ly49C/I treatments. In agreement with our results, the ligation with F(ab)2 against Ly49A in a Ly49A-transfected A20 tumor B-cell line resulted in lower levels of phosphorylated ERK1/2 and p38 MAPK after IL-2 stimulation. Similarly, the reduction in ERK1/2 phosphorylation was mild suggesting that other inhibitory signaling pathways might be involved (44). Additionally, we also observed reduced granzyme B production after in vitro stimulation of NK cells that were in vivo treated with anti-Ly49A mAbs. Taken together, these results suggest that the use of anti-Ly49A (clones YE1/32 and YE1/48) can induce NK inhibition similar to that observed with H2d-Ly49A trans-binding in H2d strain mice acting as an agonistic mAbs in vivo and in vitro.
The use of mAbs has increased considerably in the last few decades not only to study multiple biological parameters but also as an immunotherapeutic weapon against cancer and autoimmune diseases. Furthermore, the study of NK cell biology has been facilitated by the use of mAbs. Depending on the mAb used, the effects can be to block, trigger, or eliminate particular cell populations. Here we provide evidence that supports differential Ab behavior depending on the Ab-clone used, the MHC-I haplotype, and the target expression. This differential effect seems to be based on the obstruction of the Ab-binding site with its ligand due to cis-interactions. Furthermore, alteration of the Ab-target cell surface expression can significantly modify the ability of mAbs to recognize and eliminate target cells, illustrating the plasticity of these mAbs modulated by cell surface expression of the particular target. In these studies we demonstrated that anti-Ly49A mAbs behave as agonistic mAbs when Ly49A surface levels are sequester by cis-binding with H2d, but as a depleting mAb when the expression of Ly49A is elevated. This increased level of expression of Ly49A observed after poly I:C treatment or MCMV infection also provides evidence for the differential expression of the inhibitory receptor dependent on stimulation. We and others have recently observed an increase of both percentage and/or level of expression of activating and inhibitory NK cell receptors during stimulatory environment such as Ly49G2 and NKG2A. Although the proportion of Ly49A is not altered after stimulation, the MFI is significantly increased similar to Ly49G2 (37, 38). This variability of NK cell receptor expression could definitely impact the usage of mAb against these receptors in NK-cell based therapies.
Our findings have also important clinical implications as the selection of licensed and unlicensed NK cells (as well as alloreactive NK cells) based on the presence of particular inhibitory and activating receptors could be used to improve cancer immunotherapy. The use of anti-KIRs to improve allogeneic and autologous NK alloreactivity is gaining importance in cancer treatment. The administration of anti-KIR mAb 1-7F9 improved NK cell-mediated cytotoxicity of HLA-matched AML blasts (54). Cis-interactions have not been described for KIRs yet, but it would be important to determine if the patient and donor HLA haplotype could impact the effectiveness of mAbs against KIRs or other inhibitory receptors such as NKG2A prior to their application in cancer therapy. Additionally, it will be important to determine the impact of human CMV or other inflammatory stimuli that could activate NK cells prior to anti-KIR therapy, which could markedly alter the biologic effects of the mAbs. Furthermore, the upregulation of the level of expression of inhibitory receptors upon stimulation could also be a mechanism to regulate NK cell responses. During infections, NK cell suppression and immune evasion could be mediated by upregulation of inhibitory receptors and therefore the use of mAb to eliminate or block those inhibitory receptors could be useful in the fight against infection or cancer. Therefore, careful analysis of these receptors prior and during mAb administration against inhibitory/activating receptors should be done, as the use of stimulatory cytokines such as IL-2 or IL-15 or opportunistic infections typically after hematopoietic stem cell transplantation could alter NK cell receptor expression during the course of cancer therapy and affect the efficacy of these mAbs. In conclusion, this study highlights the importance of understanding the factors that influence expression of desired NK cell targets and the consequent efficacy of NK cell-mediated therapies.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant R01-HL089905.
We thank Weihong Ma, Monja Metcalf, and Angela Baptise for their technical assistance; Dr. Lanier for helpful discussion and reagents; and the NIH Tetramer Core Facility for generously providing the tetramers.
References
- 1.Hallett WH, Murphy WJ. Natural killer cells: biology and clinical use in cancer therapy. Cell Mol Immunol. 2004;1:12–21. [PubMed] [Google Scholar]
- 2.Scarpellino L, Oeschger F, Guillaume P, Coudert JD, Levy F, Leclercq G, Held W. Interactions of Ly49 family receptors with MHC class I ligands in trans and cis. J Immunol. 2007;178:1277–1284. doi: 10.4049/jimmunol.178.3.1277. [DOI] [PubMed] [Google Scholar]
- 3.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 4.Murphy WJ, Kumar V, Bennett M. Acute rejection of murine bone marrow allografts by natural killer cells and T cells. Differences in kinetics and target antigens recognized. J Exp Med. 1987;166:1499–1509. doi: 10.1084/jem.166.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 6.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 7.Karre K. NK cells, MHC class I molecules and the missing self. Scandinavian journal of immunology. 2002;55:221–228. doi: 10.1046/j.1365-3083.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 8.Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- 9.Ljunggren HG, Karre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med. 1985;162:1745–1759. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bix M, Liao NS, Zijlstra M, Loring J, Jaenisch R, Raulet D. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature. 1991;349:329–331. doi: 10.1038/349329a0. [DOI] [PubMed] [Google Scholar]
- 11.Liao NS, Bix M, Zijlstra M, Jaenisch R, Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991;253:199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- 12.Hoglund P, Ohlen C, Carbone E, Franksson L, Ljunggren HG, Latour A, Koller B, Karre K. Recognition of beta 2-microglobulin-negative (beta 2m-) T-cell blasts by natural killer cells from normal but not from beta 2m- mice: nonresponsiveness controlled by beta 2m- bone marrow in chimeric mice. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:10332–10336. doi: 10.1073/pnas.88.22.10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliott JM, Wahle JA, Yokoyama WM. MHC class I-deficient natural killer cells acquire a licensed phenotype after transfer into an MHC class I-sufficient environment. J Exp Med. 2010;207:2073–2079. doi: 10.1084/jem.20100986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, Romagne F, Ugolini S, Vivier E. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Sun K, Alvarez M, Ames E, Barao I, Chen M, Longo DL, Redelman D, Murphy WJ. Mouse NK cell-mediated rejection of bone marrow allografts exhibits patterns consistent with Ly49 subset licensing. Blood. 2012;119:1590–1598. doi: 10.1182/blood-2011-08-374314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoglund P, Brodin P. Current perspectives of natural killer cell education by MHC class I molecules. Nat Rev Immunol. 2010;10:724–734. doi: 10.1038/nri2835. [DOI] [PubMed] [Google Scholar]
- 19.Hanke T, Takizawa H, McMahon CW, Busch DH, Pamer EG, Miller JD, Altman JD, Liu Y, Cado D, Lemonnier FA, Bjorkman PJ, Raulet DH. Direct assessment of MHC class I binding by seven Ly49 inhibitory NK cell receptors. Immunity. 1999;11:67–77. doi: 10.1016/s1074-7613(00)80082-5. [DOI] [PubMed] [Google Scholar]
- 20.Kane KP. Ly-49 mediates EL4 lymphoma adhesion to isolated class I major histocompatibility complex molecules. J Exp Med. 1994;179:1011–1015. doi: 10.1084/jem.179.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tormo J, Natarajan K, Margulies DH, Mariuzza RA. Crystal structure of a lectin-like natural killer cell receptor bound to its MHC class I ligand. Nature. 1999;402:623–631. doi: 10.1038/45170. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto N, Mitsuki M, Tajima K, Yokoyama WM, Yamamoto K. The functional binding site for the C-type lectin-like natural killer cell receptor Ly49A spans three domains of its major histocompatibility complex class I ligand. J Exp Med. 2001;193:147–158. doi: 10.1084/jem.193.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Whitman MC, Natarajan K, Tormo J, Mariuzza RA, Margulies DH. Binding of the natural killer cell inhibitory receptor Ly49A to its major histocompatibility complex class I ligand. Crucial contacts include both H-2Dd AND beta 2-microglobulin. The Journal of biological chemistry. 2002;277:1433–1442. doi: 10.1074/jbc.M110316200. [DOI] [PubMed] [Google Scholar]
- 24.Dam J, Guan R, Natarajan K, Dimasi N, Chlewicki LK, Kranz DM, Schuck P, Margulies DH, Mariuzza RA. Variable MHC class I engagement by Ly49 natural killer cell receptors demonstrated by the crystal structure of Ly49C bound to H-2K(b) Nat Immunol. 2003;4:1213–1222. doi: 10.1038/ni1006. [DOI] [PubMed] [Google Scholar]
- 25.Jonsson AH, Yang L, Kim S, Taffner SM, Yokoyama WM. Effects of MHC class I alleles on licensing of Ly49A+ NK cells. J Immunol. 2010;184:3424–3432. doi: 10.4049/jimmunol.0904057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Held W, Dorfman JR, Wu MF, Raulet DH. Major histocompatibility complex class I-dependent skewing of the natural killer cell Ly49 receptor repertoire. European journal of immunology. 1996;26:2286–2292. doi: 10.1002/eji.1830261003. [DOI] [PubMed] [Google Scholar]
- 27.Olsson MY, Karre K, Sentman CL. Altered phenotype and function of natural killer cells expressing the major histocompatibility complex receptor Ly-49 in mice transgenic for its ligand. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:1649–1653. doi: 10.1073/pnas.92.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doucey MA, Scarpellino L, Zimmer J, Guillaume P, Luescher IF, Bron C, Held W. Cis association of Ly49A with MHC class I restricts natural killer cell inhibition. Nat Immunol. 2004;5:328–336. doi: 10.1038/ni1043. [DOI] [PubMed] [Google Scholar]
- 29.Michaelsson J, Achour A, Rolle A, Karre K. MHC class I recognition by NK receptors in the Ly49 family is strongly influenced by the beta 2-microglobulin subunit. J Immunol. 2001;166:7327–7334. doi: 10.4049/jimmunol.166.12.7327. [DOI] [PubMed] [Google Scholar]
- 30.Held W, Mariuzza RA. Cis interactions of immunoreceptors with MHC and non-MHC ligands. Nat Rev Immunol. 2008;8:269–278. doi: 10.1038/nri2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi T, Ferris ST, Matsumoto N, Poursine-Laurent J, Yokoyama WM. Ly49-dependent NK cell licensing and effector inhibition involve the same interaction site on MHC ligands. J Immunol. 2011;186:3911–3917. doi: 10.4049/jimmunol.1004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chalifour A, Scarpellino L, Back J, Brodin P, Devevre E, Gros F, Levy F, Leclercq G, Hoglund P, Beermann F, Held W. A Role for cis Interaction between the Inhibitory Ly49A receptor and MHC class I for natural killer cell education. Immunity. 2009;30:337–347. doi: 10.1016/j.immuni.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 33.Andersson KE, Williams GS, Davis DM, Hoglund P. Quantifying the reduction in accessibility of the inhibitory NK cell receptor Ly49A caused by binding MHC class I proteins in cis. European journal of immunology. 2007;37:516–527. doi: 10.1002/eji.200636693. [DOI] [PubMed] [Google Scholar]
- 34.Back J, Chalifour A, Scarpellino L, Held W. Stable masking by H-2Dd cis ligand limits Ly49A relocalization to the site of NK cell/target cell contact. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3978–3983. doi: 10.1073/pnas.0607418104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masuda A, Nakamura A, Maeda T, Sakamoto Y, Takai T. Cis binding between inhibitory receptors and MHC class I can regulate mast cell activation. J Exp Med. 2007;204:907–920. doi: 10.1084/jem.20060631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raziuddin A, Longo DL, Bennett M, Winkler-Pickett R, Ortaldo JR, Murphy WJ. Increased bone marrow allograft rejection by depletion of NK cells expressing inhibitory Ly49 NK receptors for donor class I antigens. Blood. 2002;100:3026–3033. doi: 10.1182/blood.V100.8.3026. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen S, Dhedin N, Vernant JP, Kuentz M, Al Jijakli A, Rouas-Freiss N, Carosella ED, Boudifa A, Debre P, Vieillard V. NK-cell reconstitution after haploidentical hematopoietic stem-cell transplantations: immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood. 2005;105:4135–4142. doi: 10.1182/blood-2004-10-4113. [DOI] [PubMed] [Google Scholar]
- 38.Barao I, Alvarez M, Ames E, Orr MT, Stefanski HE, Blazar BR, Lanier LL, Anderson SK, Redelman D, Murphy WJ. Mouse Ly49G2+ NK cells dominate early responses during both immune reconstitution and activation independent of MHC. Blood. 2011 doi: 10.1182/blood-2010-11-316653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang-Feldman YJ, Wojtowicz A, Lochhead GR, Hale MA, Li Y, Pomeroy C. Use of quantitative real-time PCR (qRT-PCR) to measure cytokine transcription and viral load in murine cytomegalovirus infection. Journal of virological methods. 2006;131:122–129. doi: 10.1016/j.jviromet.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 40.Raziuddin A, Bennett M, Winkler-Pickett R, Ortaldo JR, Longo DL, Murphy WJ. Synergistic effects of in vivo depletion of Ly-49A and Ly-49G2 natural killer cell subsets in the rejection of H2(b) bone marrow cell allografts. Blood. 2000;95:3840–3844. [PubMed] [Google Scholar]
- 41.Olsson-Alheim MY, Salcedo M, Ljunggren HG, Karre K, Sentman CL. NK cell receptor calibration: effects of MHC class I induction on killing by Ly49Ahigh and Ly49Alow NK cells. J Immunol. 1997;159:3189–3194. [PubMed] [Google Scholar]
- 42.Koh CY, Blazar BR, George T, Welniak LA, Capitini CM, Raziuddin A, Murphy WJ, Bennett M. Augmentation of antitumor effects by NK cell inhibitory receptor blockade in vitro and in vivo. Blood. 2001;97:3132–3137. doi: 10.1182/blood.v97.10.3132. [DOI] [PubMed] [Google Scholar]
- 43.Vahlne G, Lindholm K, Meier A, Wickstrom S, Lakshmikanth T, Brennan F, Wilken M, Nielsen R, Romagne F, Wagtmann NR, Karre K, Johansson MH. In vivo tumor cell rejection induced by NK cell inhibitory receptor blockade: maintained tolerance to normal cells even in the presence of IL-2. European journal of immunology. 2010;40:813–823. doi: 10.1002/eji.200939755. [DOI] [PubMed] [Google Scholar]
- 44.Motoda K, Takata M, Kiura K, Nakamura I, Harada M. SHP-1/immunoreceptor tyrosine-based inhibition motif-independent inhibitory signalling through murine natural killer cell receptor Ly-49A in a transfected B-cell line. Immunology. 2000;100:370–377. doi: 10.1046/j.1365-2567.2000.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X, Allan DS, Krzewski K, Ge B, Kopcow H, Strominger JL. CD28-stimulated ERK2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10346–10351. doi: 10.1073/pnas.0604236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehta IK, Wang J, Roland J, Margulies DH, Yokoyama WM. Ly49A allelic variation and MHC class I specificity. Immunogenetics. 2001;53:572–583. doi: 10.1007/s002510100355. [DOI] [PubMed] [Google Scholar]
- 47.Doubrovina ES, Doubrovin MM, Vider E, Sisson RB, O’Reilly RJ, Dupont B, Vyas YM. Evasion from NK cell immunity by MHC class I chain-related molecules expressing colon adenocarcinoma. J Immunol. 2003;171:6891–6899. doi: 10.4049/jimmunol.171.12.6891. [DOI] [PubMed] [Google Scholar]
- 48.Storkus WJ, Zeh HJ, 3rd, Salter RD, Lotze MT. Identification of T-cell epitopes: rapid isolation of class I-presented peptides from viable cells by mild acid elution. Journal of immunotherapy with emphasis on tumor immunology : official journal of the Society for Biological Therapy. 1993;14:94–103. [PubMed] [Google Scholar]
- 49.Andrews DM, Sullivan LC, Baschuk N, Chan CJ, Berry R, Cotterell CL, Lin J, Halse H, Watt SV, Poursine-Laurent J, Wang CR, Scalzo AA, Yokoyama WM, Rossjohn J, Brooks AG, Smyth MJ. Recognition of the nonclassical MHC class I molecule H2-M3 by the receptor Ly49A regulates the licensing and activation of NK cells. Nat Immunol. 2012;13:1171–1177. doi: 10.1038/ni.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bevaart L, Jansen MJ, van Vugt MJ, Verbeek JS, van de Winkel JG, Leusen JH. The high-affinity IgG receptor, FcgammaRI, plays a central role in antibody therapy of experimental melanoma. Cancer research. 2006;66:1261–1264. doi: 10.1158/0008-5472.CAN-05-2856. [DOI] [PubMed] [Google Scholar]
- 51.Le Drean E, Vely F, Olcese L, Cambiaggi A, Guia S, Krystal G, Gervois N, Moretta A, Jotereau F, Vivier E. Inhibition of antigen-induced T cell response and antibody-induced NK cell cytotoxicity by NKG2A: association of NKG2A with SHP-1 and SHP-2 protein-tyrosine phosphatases. European journal of immunology. 1998;28:264–276. doi: 10.1002/(SICI)1521-4141(199801)28:01<264::AID-IMMU264>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 52.Yusa S, Catina TL, Campbell KS. SHP-1- and phosphotyrosine-independent inhibitory signaling by a killer cell Ig-like receptor cytoplasmic domain in human NK cells. J Immunol. 2002;168:5047–5057. doi: 10.4049/jimmunol.168.10.5047. [DOI] [PubMed] [Google Scholar]
- 53.Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202:1001–1012. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Romagne F, Andre P, Spee P, Zahn S, Anfossi N, Gauthier L, Capanni M, Ruggeri L, Benson DM, Jr, Blaser BW, Della Chiesa M, Moretta A, Vivier E, Caligiuri MA, Velardi A, Wagtmann N. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009;114:2667–2677. doi: 10.1182/blood-2009-02-206532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.