Abstract
Background The human papillomavirus (HPV) vaccine offers an opportunity to reduce health inequalities associated with cervical cancer provided the vaccine is delivered equitably at population level.
Method We reviewed evidence of inequalities in HPV vaccine uptake in young women after undertaking a comprehensive search of databases from inception to March 2012. Studies that compared HPV vaccination initiation and/or completion by at least one ethnicity or socioeconomic-related variable in adolescent young women were included. There were no language restrictions. Data were extracted by two reviewers and pooled in a meta-analysis using a random-effects model; sub-analyses and meta-regression were undertaken to investigate sources of heterogeneity.
Results In all, 29 publications related to 27 studies were included in the review. Black young women were less likely to initiate HPV vaccination compared with White young women (combined OR: 0.89, 95% CI: 0.82–0.97). In the USA, young women without healthcare insurance were less likely to initiate (combined OR: 0.56, 95% CI: 0.40–0.78). There was no strong evidence that lower family income (combined OR: 1.16, 95% CI: 1.00–1.34) or lower parental education (combined OR 1.06, 95% CI: 0.92–1.22) influenced HPV vaccination initiation.
Conclusions We found strong evidence for differences in HPV vaccination initiation by ethnicity and healthcare coverage, but did not find a strong association with parental education or family income variables. The majority of studies originated from the USA. Population-based studies reporting both initiation and completion of the HPV vaccination programme are required to establish patterns of uptake in different healthcare contexts.
Keywords: HPV vaccine; socioeconomic factors; ethnic disparity; immunization, adolescents; public health
Introduction
Since 2006, bivalent and quadrivalent HPV vaccines have been licensed globally for females aged between 9 and 26 years.1 If administered before sexual debut, both vaccines offer protection against HPV 16 and 18 which are responsible for approximately 70% of cases of cervical cancer.2 The World Health Organization recommends a three-dose immunization schedule for females aged between 9 and 13 years.3
Socioeconomic disparities of cervical cancer persist throughout countries and populations worldwide; the most disadvantaged groups of women experience an incidence approximately twice that of the least disadvantaged, regardless of the existence of national screening programmes.4 In the USA, age-adjusted cervical cancer incidence is 2 and 1.5 times as frequent for Hispanic and African American women, respectively, in comparison with non-Hispanic White women.5
In recent years, many countries have introduced the HPV vaccine into their national immunization programmes. There is the potential to increase health inequalities if uptake is lower amongst already disadvantaged communities.4,5 Therefore, the aim of this systematic review and meta-analysis is to summarize evidence on the uptake of HPV vaccination programmes in adolescent young womenby ethnicityand socioeconomic status.
Method
Data sources
We followed the PRISMA guidelines throughout the design, conduct and reporting of this systematic review.6 A comprehensive search strategy was developed using a combination of text words and the following indexing terms (MeSH): ‘papillomavirus’, ‘wart virus’, ‘vaccination’, ‘immunization’, ‘immunization programs’, ‘wart virus vaccines’, ‘socioeconomics’, ‘healthcare disparity’, ‘minority health’, ‘minority groups’ and ‘ethnic groups’ (available from authors on request). The following databases were searched from inception to 9 March 2012: Cumulative Index to Nursing and Allied Health Literature (CINAHL), Embase, Medline, PsycINFO and ISI Web of Science and ISI Proceedings. Abstracts were saved using Endnote X3.
Study selection
Studies were eligible if vaccine uptake in young women aged ≤18 years was reported by at least one of the following: (i) primary caregiver/household highest educational attainment; (ii) area-level measures of deprivation; (iii) primary caregiver/household annual income; (iv) healthcare insurance coverage status (US-based studies only); (v) ethnicity; (vi) religion, and; (vii) frequency of religious attendance. Studies reporting country of birth as a proxy for ethnicity, or intention to receive the HPV vaccine, were excluded.
Conference abstracts were included if they reported sufficient information related to the variables of interest. Reviews, editorials, dissertations, letters and books were included if they presented original data. Where cohorts were reported in multiple publications, the priority for inclusion was the publication reporting the greatest number of variables of interest. Publications reporting initiation and completion separately were reported together.
Titles and abstracts of all identified studies and relevant full texts were assessed by two independent reviewers (H.F. and K.M-W.). Authors were contacted to provide additional information if data were not reported in a suitable format for data synthesis. If not provided, the study was excluded. No language restrictions were applied.
Quality assessment of the included studies was undertaken to illustrate potential sources of bias using an appraisal tool adapted for use in observational studies7 by one reviewer (H.F.). Studies were not excluded on this basis in view of the predominantly observational nature of the primary studies.
Data extraction
Information relevant to the study characteristics including delivery site of vaccine, study methodology and study results were extracted by one reviewer (H.F.) and double-checked by another (S.A.).
For ethnicity, data were grouped by the following categories: ‘White’, ‘Black’, ‘Latina’ and ‘Asian’. Adjusted and unadjusted ORs and the corresponding 95% CIs pertaining to each category were extracted, taking the White ethnic group as the reference group.
To facilitate comparisons, data relating to the highest and lowest category of primary caregiver/household education, primary caregiver/household income and area-level deprivation were extracted, taking the highest level as the reference category. Household/primary caregiver income and area-level deprivation data were treated separately throughout analyses. For USA-based studies only, healthcare insurance status indicators were grouped as insured and not insured and relevant data extracted. Insured was treated as the reference category. Additional data were extracted by sub-categories to investigate heterogeneity.
Statistical methods
Total heterogeneity between studies independent of number of studies was evaluated using the Q-statistic and the I2-statistics.8 Evidence of heterogeneity was classified as weak, moderate and strong for corresponding I2 of 25%, 50% and 75%, respectively. Pooled results from a random-effects model were reported if heterogeneity was weak or moderate. If heterogeneity was strong, studies were presented narratively. Final analyses comprized adjusted ORs (aORs) where available, with unadjusted ORs used if not reported.
To identify potential study-level factors contributing to heterogeneity, meta-regression modelling was undertaken. Dummy variables were created for study design, verification of HPV vaccination status, high uptake and adjustment for socioeconomic and other variables of interest. Study year was added to the model as a categorical variable. The natural logarithm OR of each socioeconomic and ethnicity variable was used as the dependent variable and study-level factors as the independent variables.
Results
Of 1093 records initially identified through the database searches, 699 abstracts were reviewed and 123 full-text articles were assessed for eligibility. Full-text studies were excluded for not reporting uptake of HPV vaccination by ethnicity or socioeconomic variable of interest (n = 48), not reporting original data on uptake (n = 28), duplication of study (n = 13), and initiation not reported by the age group of interest (n = 2). A total of 29 publications reporting uptake in 27 studies met the inclusion criteria (Figure 1).
Figure 1.
Flow diagram of study selection procedure
Overall, 359 260 of 905 536 (39.7%, range 9.4–70.6%) young women aged between 8 and 18 years initiated HPV vaccination. In studies reporting completion, 78 327 of 157 017 (49.9%, range 26.9–85.3%) young women who had initiated HPV vaccination completed the series. The proportion of young women initiating and completing the HPV vaccine varied substantially both by ethnicity and socioeconomic indicators (Supplementary Table 1, available as Supplementary data at IJE online).
The majority of studies were from the USA (n = 22, 81.5%) with additional studies from Canada (2) and Europe (one each from Belgium, The Netherlands and the UK). Most of the studies were cross-sectional questionnaires (13, 48.1%) or retrospective chart reviews (12, 44.4%). Two were prospective cohort studies. Study participants were sampled from the general population (15, 55.6%), from a healthcare setting (9, 33.3%) or schools (3, 11.1%). The majority of studies were in relation to healthcare based vaccination programmes (24, 88.8%). A wide range of demographic (daughters’ age, parental age, primary caregiver education, parental marital status, race/ethnicity, region), socioeconomic (income and healthcare insurance coverage related), behavioural (sexually active), healthcare-related (healthcare visit type, usual source of care for daughter) and HPV-specific variables were adjusted for in the analyses (Table 1).
Table 1.
Descriptive characteristics of studies eligible for the review
| Year | Authors | Country | Study time period | Study design | Study location (geographical) | Study population | Vaccine delivery mechanism | Data extracted | Variables adjusted | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|
| 2011 | Bastani R et al.26 | USA | January 2009 to November 2009 | Cross-sectional survey | Los Angeles County | 490 mothers, or primary caregivers, of girls aged 9 to 18 years | Healthcare setting | OR, aOR | Daughter age, parental age, parental education, race/ethnicity, usual source of care for daughter and HPV specific questions | Moderate |
| 2009 | Caskey R et al.19 | USA | November 2010 | Cross-sectional survey | Nationally representative research panel of 60 000 US households | 412 adolescents aged 13 to 17 years | Healthcare setting | Raw | Moderate | |
| 2009 | Chao C et al.34 | USA | October 2006 to September 2007 October 2006 to March 2008 | Retrospective chart review | Kaiser Permanente, Southern California, Los Angeles County | 179 580 female members aged 9 to 17 years | Healthcare setting | Raw | Low | |
| 2010 | Chao C et al.9 | |||||||||
| 2010 | Cook R, et al.10 | USA | July 2006 to June 2008 | Retrospective chart review | Participants of Medicaid Florida | 167 082 females aged 11 to 18 years | Healthcare setting | Raw, OR, aOR | Age, geographical region, Medicaid plan type, month of vaccination, number of enrolled months, outpatient visit (yes/no), race and sexually active | Low |
| 2010 | Dempsey A et al.15 | USA | January 2007 to March 2008 | Retrospective chart review | University of Michigan Health System, Michigan | 10 082 females aged 9 to 18 years | Healthcare setting | Raw, OR, aOR | Age, insurance coverage, race/ethnicity, medical specialty and visit type | Low |
| 2011 | Dorell C et al.16 | USA | January 2008 to February 2010 | Cross-sectional survey | 50 states | 18 228 girls aged 13 to 17 years | Healthcare setting | Raw, OR, aOR | Age, health insurance, maternal education, maternal marital status, poverty level, race/ethnicity, year, well-child visit at age 11-12 years | Moderate |
| Niccolai L et al.36 | ||||||||||
| 2009 | Gerend M et al.27 | USA | January 2008 to June 2008 | Cross-sectional survey | 4 paediatric clinics in southeastern USA | 32 parents of girls aged 9 to 17 | Healthcare setting | Raw | High | |
| 2011 | Gold R et al.35 | USA | 2007 to 2008 | Retrospective chart review | School-based health centres, Oregon | 450 adolescents aged 9 to 17 years | Healthcare setting | Raw | Low | |
| 2009 | Gottlieb S et al.20 | USA | July to October 2007 | Cross-sectional survey | 5 counties in southeastern North Carolina | 889 parents of girls aged 10 to 18 years | Healthcare setting | OR | Moderate | |
| 2011 | Guerry S et al.31 | USA | October 2007 to June 2008 | Cross-sectional survey | Schools in Los Angeles County | 509 study caregivers of girls aged 11 to 18 years | Healthcare setting | OR | High | |
| 2012 | Keenan K et al.12 | USA | 2008 | Prospective cohort study | Population-based sample, Pittsburgh | 2098 adolescents aged 12 to 15 years | Healthcare setting | OR, aOR | Age, poverty, pubertal status race, sexual behaviour | Moderate |
| 2011 | Lefevere E et al.28 | Belgium | January 2007 and June 2009 | Retrospective chart review | Members of the National Alliance of Christian Mutualities, Flanders, Belgium | 117 151 mother-daughter pairs of girls aged 12 to 18 years | Healthcare setting | Raw | Low | |
| 2010 | Mathur M et al.25 | USA | August 2007 to February 2008 | Cross-sectional survey | Two schools, San Francisco Bay Area | 156 high-school girls | Healthcare setting | Raw | High | |
| 2010 | Rondy M et al.29 | Netherlands | Retrospective chart review | Netherlands | 384 869 girls aged 11 to 16 years | Healthcare setting | Raw | Low | ||
| 2010 | Ogilvie G et al.17 | Canada | September 2008 to June 2009 | Cross-sectional survey | Province of British Columbia | 2025 parents of girls in grade 6 (aged 12 to 13 years) | School setting | Raw, OR | Moderate | |
| 2012 | Palli S et al.18 | USA | 2007 to 2008 | Cross-sectional survey | USA | 91 642 interviews of parents of adolescent girls aged 12 to 17 years | Healthcare setting | aOR | Age, ethnicity, healthcare coverage, medical home type, Metropolitan Statistical Area status, perceived health of child, preventive medical care visits, parental age, parental education, parental health and region | Moderate |
| 2010 | Pruitt S et al.21 | USA | 2008 | Cross-sectional survey | 270 counties, and 6 US states | 1709 girls aged 13 to 17 years | Healthcare setting | OR, aOR | Age, area level deprivation, annual household income, parental education, parental healthcare insurance, and race/ethnicity | Moderate |
| 2010 | Reiter P et al.14 | USA | July to October 2007 | Cross-sectional survey | State of North Carolina | 617 female adolescents aged 10 to 17 years and their parents | Healthcare setting | Raw, OR | Moderate | |
| 2008 | Roberts S et al.11 | UK | February 2007 to July 2008 | Prospective cohort study | Schools in 2 primary care trusts in Manchester | 2817 girls aged 12 to 13 years | School setting | OR, aOR | Area level deprivation and ethnicity | Low |
| 2008 | Rosenthal S et al.32 | USA | April 2007 to January 2008 | Cross-sectional survey | University-based primary care clinic | 153 female care providers of adolescent girls aged 11 to 17 years | Healthcare setting | OR, aOR | Health belief statements, parental education, parenting and sexually transmitted infection history | High |
| 2011 | Schluterman N et al.46 | USA | August 2006 to January 2010 | Retrospective chart review | University of Maryland Medical Center, Maryland | 8069 patients aged 9 to 26 years | Healthcare setting | OR, aOR | Age at first visit, insurance type and race | Low |
| 2011 | Shelton R et al.24 | USA | September 2007 to January 2008 | Cross-sectional survey | Nationally representative research panel of 60 000 US households | 836 primary caregivers of adolescent girls aged 9 to 17 years | Healthcare setting | OR, aOR | Age, education and race | Moderate |
| 2011 | Smith L et al.30 | Canada | September 2007 to April 2010 | Retrospective chart review | Kingston, Frontenac, Lennox and Addington, Ontario | 2519 girls aged 12 to 13 years | School setting | OR | Low | |
| 2011 | Tan W et al.33 | USA | June 2008 to October 2009 | Retrospective chart review | North Carolina Immunization Registry | 105 922 girls aged 9 to 18 years | Healthcare setting | Raw | Moderate | |
| 2011 | Taylor L et al.13 | USA | 2007 to 2008 | Cross-sectional survey | USA | 620 girls aged 11 to 18 years | Healthcare setting | Raw | Moderate | |
| 2011 | Tiro J et al.22 | USA | March 2007 to December 2009 | Retrospective chart review | 4 safety net clinics in Dallas, Texas | 700 girls aged 11 to 18 years | Healthcare setting | OR | Moderate | |
| 2010 | Yeganeh N et al.23 | USA | May 2008 to June 2008 | Cross-sectional survey | Free community clinic in Los Angeles, California | 95 primary caregivers of girls aged 11 to 17 years | Healthcare setting | Raw, OR | High |
HPV vaccination initiation by ethnicity
Overall, 14 studies9–22 reported data facilitating comparison of HPV vaccination initiation by ethnicity. There was strong evidence of heterogeneity for analyses comparing Latina and Asian young women with White young women and these estimates were not pooled (P < 0.001, I2 = 93.5% and P < 0.01, I2 = 78.4%, respectively). Pooled estimates indicate that on average Black young women were less likely to initiate HPV vaccination than White young women (combined OR: 0.89, 95% CI: 0.82–0.97, P < 0.01, I2 = 63.5%) (Figure 2).
Figure 2.
Odds ratios of HPV vaccination initiation of Black young women in comparison with White young women
Of the eight studies comparing HPV vaccination initiation between White and Latina young women, two studies indicated that young Latina women had a higher odds of initiation10,16, two indicated lower odds of initiation13,19 and three were equivocal.19,22 In the remaining study the proportion of Latina women was too small to interpret the results with confidence.20
Of the four studies permitting comparison of HPV vaccination initiation between White and Asian young women, one study showed strong evidence that Asian young women were less likely to initiate HPV vaccination,9 whereas the others showed no evidence of a difference.11,16,17
HPV vaccination initiation by religion and frequency of religious attendance
There was no strong evidence that religion and frequency of attendance at a place of worship were related to HPV vaccination initiation. Two studies showed no evidence of differences for HPV vaccination initiation by religious faith.17,23 Another reported weak evidence that Catholic religious beliefs were associated with increased initiation.24 One study reported that more frequent religious service attendance was associated with initiation,24 whereas another suggested the opposite.25
HPV vaccination initiation by income or area-level indicators
There were nine studies13,14,16,18–21,26,27 that facilitated comparison of HPV vaccination initiation by income indicators for primary caregiver or household, and five studies11,21,28–30 by area-level deprivation. There was strong evidence of heterogeneity in the analysis comparing area-level deprivation and estimates were not pooled (P < 0.001, I2 = 97.9). Four non USA-based studies indicated that young women living in the most deprived areas were less likely to initiate HPV vaccination than those living in the least.11,28–30) One USA-based study reported findings counter to this.21 The combined OR showed no strong association between low income and initiation of HPV vaccination (combined OR: 1.16, 95% CI: 1.00–1.34) (Figure 3).
Figure 3.
Odds ratios of HPV vaccination initiation in young women belonging to lowest family or household annual income category in comparison with highest
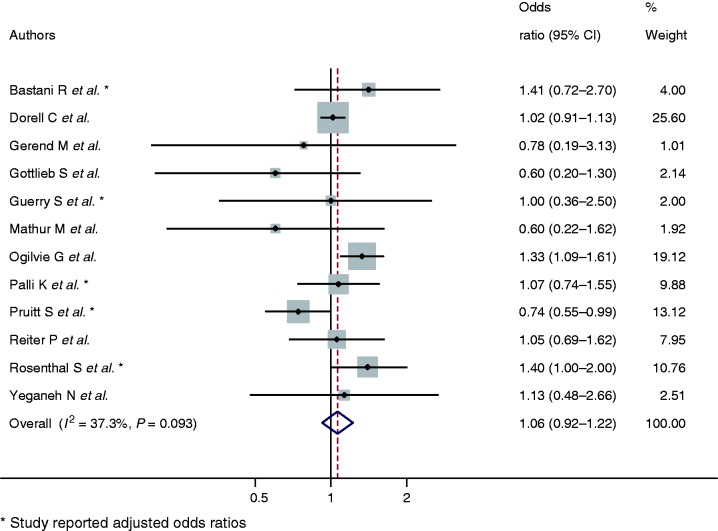
HPV vaccination initiation by primary caregiver educational attainment
There were 12 studies14,16–18,20,21,23,25–27,31,32 that reported data facilitating comparison by primary caregiver educational attainment. The pooled estimates indicate no evidence for difference in HPV vaccination initiation in young women by primary caregiver educational attainment category (combined OR 1.06, 95% CI: 0.92–1.22, P = 0.09, I2 = 37.3) (Figure 4).
Figure 4.
Odds ratios of HPV vaccination initiation by lowest primary caregiver education category in comparison with highest
HPV vaccination initiation by healthcare insurance coverage
Eight USA-based studies13–16,18,20,21,26 reported initiation by healthcare insurance coverage. The pooled results indicated lower odds of initiating HPV vaccination for young women who had no healthcare insurance coverage in comparison with those who had healthcare insurance coverage (combined OR: 0.56, 95% CI: 0.40–0.78) (Figure 5).
Figure 5.
Odds ratios of HPV vaccination initiation by young women without healthcare insurance coverage in comparison with young women with healthcare insurance coverage
Other sub-analyses
Further ad hoc sub-analyses excluding studies considered to be at high risk of bias resulted only in minor changes to combined effect sizes and heterogeneity by ethnic and socioeconomic variables of interest. There were insufficient studies to permit sub-analysis either by vaccination programme delivery setting or countries with publicly funded HPV vaccination programmes.
Investigating sources of heterogeneity
To investigate sources of heterogeneity, meta-regression analysis was undertaken. Adjustment for the study-level variables ‘self-report measurement of HPV status’ and ‘adjustment for primary caregiver education status’ reduced heterogeneity for the analysis comparing initiation by White and Black ethnic group (I2 = 45.0%). Reductions in heterogeneity were not observed across other analyses.
HPV vaccination completion by ethnicity
Completion of the HPV vaccine course by ethnicity was reported by six studies.10,15,21,33–35 Four studies10,33–35 provided evidence that young Black women were less likely to complete the HPV vaccine course, whereas two were equivocal.15,21 Two studies provided evidence that Latina young women were less likely to complete HPV vaccination series than White young women, whereas results from four other studies showed no difference.21,34–36 Two studies reported that Asian young women were more likely to complete HPV vaccination series than White young women.33,34
HPV vaccination completion by income or area-level indicators
Few studies reported completion by income; one study presented strong evidence to support that young women belonging to lower family income categories are less likely to complete HPV vaccination.36 Similarly for area-level deprivation indicators: one study offered evidence to suggest that young women living in the most deprived neighbourhood quintile were less likely to complete the vaccine series.30
HPV vaccination completion by primary caregiver educational attainment
Few studies reported completion by primary caregiver education; one study indicated young women belonging to families with lower education attainment are less likely to complete.36
HPV vaccination completion by healthcare insurance coverage
In one study, young women with private insurance were more likely to have completed the vaccination series that those with public insurance.33
Discussion
Key findings
This systematic review indicates that Black young women are less likely to initiate HPV vaccination in comparison with White young women (combined OR: 0.89, 95% CI: 0.82–0.97). In the USA, young women without healthcare insurance coverage were less likely to initiate HPV vaccination (combined OR: 0.56, 95% CI: 0.40–0.78). However, there was no strong evidence for differences of initiation by family income or primary caregiver educational achievement.
Strengths and limitations
We followed a systematic and comprehensive process including: a search strategy applied to multiple databases to uncover all relevant studies; no restrictions on the basis of publication date or language; titles, abstracts and full-text studies independently reviewed by two of the authors; and study authors contacted to provide additional information to minimize study selection bias. We were also able to incorporate some meta-analyses and investigate sources of heterogeneity through sub-analyses and meta-regression techniques.
Nonetheless there are several potential limitations. There was considerable heterogeneity between studies including differences in study design, reporting of socioeconomic and ethnicity variables, type and number of confounders, and definition of reference groups. This limited the application of meta-analysis. Examining possible causes of heterogeneity by meta-regression yielded little additional insight into sources of heterogeneity. Potential explanations for residual heterogeneity include: variation in the populations from which study samples were drawn; modality of healthcare insurance coverage scheme, and; vaccination delivery setting.
The measures of ethnicity as used in this study are crude and do not reflect different cultural beliefs and norms within ethnic sub-populations. Few studies reported other ethnicity-related variables, such as religion, which could conceivably influence HPV vaccination uptake.
Recall and response bias may be present in studies using self-report measures of HPV vaccination status.13,14,17–19,21,24–26,31,32 Both, unadjusted and aORs have been presented because of inconsistency of reporting. Examining HPV vaccination uptake by ethnicity, without accounting for socioeconomic circumstances, does not accurately portray the impact of cultural differences. Adjustment for these confounders in the studies included in this review generally resulted in ORs deviating towards the null and results may be overestimated. Studies reporting both socioeconomic position and ethnicity allowing adjustment for confounding are required.
The majority of studies included in the review are from the USA where insurance is the predominant model of healthcare and the HPV vaccine is usually delivered in the primary care setting. The results may not be generalizable to different healthcare systems offering universal health care, population types or different vaccination delivery methods, such as school-based programmes delivered free at the point of care, frequently implemented in other countries. All of the studies meeting the inclusion criteria were from higher resource countries. No studies were retrieved from the African and Asian continents, where the majority of cases of cervical cancer occur,37 despite the availability of the HPV vaccine in a number of countries.
Finally, completion (rather than initiation) of the HPV vaccination series was reported too infrequently to permit meta-analyses.
Findings in relation to other studies
A systematic review reporting hypothetical acceptance suggested there would be no difference in acceptability of HPV vaccination by ethnicity, but lower education and higher income was associated with higher acceptability.38 Our meta-analyses indicate that high acceptability has not been translated into HPV vaccination uptake in Black young women, which is of concern given the higher incidence of cervical cancer in this population group.5
The factors affecting HPV vaccination uptake in Black young women are not yet fully understood. They may relate to lower socioeconomic status experienced by many ethnic groups, or cultural differences in relation to attitudes to preventive health care. Qualitative studies to elicit cultural or religious beliefs related to HPV vaccination initiation may help explain some differences. In addition, targeted media publicity campaigns and physician recommendation may be helpful to promote vaccination uptake.
We found that young women without healthcare insurance coverage were less likely to initiate HPV vaccination. This is an important finding as lower uptake could exasperate existing inequalities in cervical cancer incidence4 and screening attendance39 in disadvantaged populations. Cost and access to healthcare are perceived as barriers to vaccination by young women without healthcare insurance coverage, despite free availability through the Vaccines for Children programme in the USA.40 Informing uninsured young women and their primary caregivers who are eligible about available resources may help alleviate some of the perceived barriers to vaccination.
A previous systematic review reported that higher HPV vaccine initiation was associated with having numerous factors, including health insurance, older age, receipt of childhood vaccines and positive vaccine attitudes.41 The authors also suggested that African American young women are less likely to initiate, although the results from individual studies were not pooled in a meta-analysis. The current study supports and strengthens this argument by focusing on ethnic and socioeconomic differences in uptake, reporting the results from approximately double the number of studies, and including some meta-analysis.
The highest uptake rates reported in this study were achieved through school-based vaccination programmes11,17,30 which have been shown to be acceptable and convenient to parents/carers.42,43 The observed lower uptake of the HPV vaccine in the general practice setting may be as a result of a reliance on opportunistic strategies for vaccine delivery. For a population who typically utilize healthcare infrequently, this approach may not be appropriate if high coverage is to be achieved. This study demonstrated inequalities in uptake in general practice-based setting, even in a setting with universal healthcare.29 School-based programmes could be advantageous in overcoming practical barriers to healthcare access in the primary care setting, such as transport issues or appointment restrictions,44,45 and promoting more equitable coverage of the HPV vaccine. More detailed understanding of the contextual factors contributing to differences of uptake by vaccination delivery setting would be beneficial to inform future HPV vaccination programmes or other health initiatives.
Conclusions
We found strong evidence for differences in HPV vaccination initiation by ethnicity and healthcare coverage, but not for parental education or family income. Future population-based studies reporting initiation and completion of the HPV vaccination series are required to establish whether the patterns of initiation reported here are replicated in healthcare settings outside the USA. Further research should identify barriers and develop interventions to improve uptake in specific populations identified with lower uptake.
Supplementary Data
Supplementary data are available at IJE online.
Funding
The work was supported by the Centre for the Development and Evaluation of Complex Interventions for Public Health Improvement (DECIPHer) which receives funding from: the British Heart Foundation; Cancer Research UK; Economic and Social Research Council (RES-590-28-0005); Medical Research Council; the Welsh Assembly Government; and the Wellcome Trust (WT087640MA), under the auspices of the UK Clinical Research Collaboration.
Supplementary Material
Acknowledgements
The authors would like to thank Alison Weightman (University of Cardiff) and Cath Borwick (University of Bristol) for their valuable input into the design of the search strategy. The following contributions to the manuscript were made by the authors: concept and design of the study (H.F., C.T., S.A., M.H.); reviewing titles and abstracts (H.F., K.M-W.); data extraction (H.F.); data analyses (H.F.); interpretation of data (H.F., S.A., C.T., M.H.); preparing the initial draft of manuscript (H.F.); and reviewing drafts and approving the final manuscript as submitted (H.F., C.T., S.A., K.M-W., M.H.). The lead author will act as a guarantor for the paper.
Conflict of interest: None declared.
KEY MESSAGES.
Widespread HPV vaccination has the potential to reduce existing cervical cancer inequalities.
Published studies, predominantly from the USA, show initiation of HPV vaccination is lower in Black young women and young women without healthcare cover insurance, but there is no evidence for a difference of HPV vaccination initiation by parental education or income.
Population-based studies reporting both initiation and completion of the HPV vaccination programme are required to establish patterns of uptake in different countries and healthcare contexts.
Further research is required to identify barriers to initiation and completion of HPV vaccination and develop interventions to improve uptake in populations identified with lower uptake.
References
- 1.Koulova A, Tsui J, Irwin K, Van Damme P, Biellik R, Aguado MT. Country recommendations on the inclusion of HPV vaccines in national immunization programmes among high-income countries, June 2006–January 2008. Vaccine. 2008;26:6529–41. doi: 10.1016/j.vaccine.2008.08.067. [DOI] [PubMed] [Google Scholar]
- 2.Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121:621–32. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Human Papillomavirus Vaccines. WHO position paper; Wkly Epidemiol Rec 2009;84:118–31. [Google Scholar]
- 4.Parikh S, Brennan P, Boffetta P. Meta-analysis of social inequality and the risk of cervical cancer. Int J Cancer. 2003;105:687–91. doi: 10.1002/ijc.11141. [DOI] [PubMed] [Google Scholar]
- 5.Watson M, Saraiya M, Benard V, et al. Burden of cervical cancer in the United States, 1998–2003. Cancer. 2008;113:2855–64. doi: 10.1002/cncr.23756. [DOI] [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CASP NHS Public Health Resource Unit. Critical Appraisal Skills Programme (CASP) appraisal tools. 2003 Available from http://www.casp-uk.net/wp-content/uploads/2011/11/CASP_RCT_Appraisal_Checklist_14oct10.pdf (12 March 2013, date last accessed) [Google Scholar]
- 8.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 9.Chao C, Velicer C, Slezak JM, Jacobsen SJ. Correlates for human papillomavirus vaccination of adolescent girls and young women in a managed care organization. Am J Epidemiol. 2010;171:357–67. doi: 10.1093/aje/kwp365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook RL, Zhang JY, Mullins J, et al. Factors associated with initiation and completion of human papillomavirus vaccine series among young women enrolled in Medicaid. J Adolescent Health. 2010;47:596–99. doi: 10.1016/j.jadohealth.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Roberts SA, Brabin L, Stretch R, et al. Human papillomavirus vaccination and social inequality: results from a prospective cohort study. Epidemiol Infect. 2011;139:400–05. doi: 10.1017/S095026881000066X. [DOI] [PubMed] [Google Scholar]
- 12.Keenan K, Hipwell A, Stepp S. Race and sexual behavior predict uptake of the human papillomavirus vaccine. Health Psychol. 2012;31:31–34. doi: 10.1037/a0026812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor LD, Hariri S, Sternberg M, Dunne EF, Markowitz LE. Human papillomavirus vaccine coverage in the United States, National Health and Nutrition Examination Survey, 2007-2008. Prev Med. 2011;52:398–400. doi: 10.1016/j.ypmed.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Reiter PL, Cates JR, McRee A-L, et al. Statewide HPV vaccine initiation among adolescent females in North Carolina. Sex Transm Dis. 2010;37:549–56. doi: 10.1097/OLQ.0b013e3181d73bf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dempsey A, Cohn L, Dalton V, Ruffin M. Patient and clinic factors associated with adolescent human papillomavirus vaccine utilization within a university-based health system. Vaccine. 2010;28:989–95. doi: 10.1016/j.vaccine.2009.10.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics. 2011;128:830–39. doi: 10.1542/peds.2011-0950. [DOI] [PubMed] [Google Scholar]
- 17.Ogilvie G, Anderson M, Marra F, et al. A population-based evaluation of a publicly funded, school-based HPV vaccine program in British Columbia, Canada: parental factors associated with HPV vaccine receipt. PLoS Med. 2010;7:e1000270. doi: 10.1371/journal.pmed.1000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palli SR MS, Aparasu RR. Prevalence and predictors of human papillomavirus vaccination in adolescent girls. J Am Pharm Assoc. 2012;52:52–58. doi: 10.1331/JAPhA.2012.10195. [DOI] [PubMed] [Google Scholar]
- 19.Caskey R, Lindau ST, Alexander GC. Knowledge and early adoption of the HPV vaccine among girls and young women: results of a national survey. J Adolescent Health. 2009;45:453–62. doi: 10.1016/j.jadohealth.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb SL, Brewer NT, Sternberg MR, et al. Human papillomavirus vaccine initiation in an area with elevated rates of cervical cancer. J Adolesc Health. 2009;45:430–37. doi: 10.1016/j.jadohealth.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 21.Pruitt SL, Schootman M. Geographic disparity, area poverty, and human papillomavirus vaccination. AJPM. 2010;38:525–33. doi: 10.1016/j.amepre.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiro JA, Pruitt SL, Bruce CM, et al. Multilevel correlates for human papillomavirus vaccination of adolescent girls attending safety net clinics. Vaccine. 2012;30:2368–75. doi: 10.1016/j.vaccine.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 23.Yeganeh N, Curtis D, Kuo A. Factors influencing HPV vaccination status in a Latino population; and parental attitudes towards vaccine mandates. Vaccine. 2010;28:4186–91. doi: 10.1016/j.vaccine.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Shelton R, Snavely A, De Jesus M, Othus M, Allen J. HPV vaccine decision-making and acceptance: does religion play a role? J Relig Health. 2011;11:1–11. doi: 10.1007/s10943-011-9553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathur MB, Mathur VS, Reichling DB. Participation in the decision to become vaccinated against human papillomavirus by California high school girls and the predictors of vaccine status. J Pediatr Health Care. 2010;24:14–24. doi: 10.1016/j.pedhc.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Bastani R, Glenn BA, Tsui J, et al. Understanding suboptimal human papillomavirus vaccine uptake among ethnic minority girls. Cancer Epidemiol Biomarkers Prev. 2011;20:1463–72. doi: 10.1158/1055-9965.EPI-11-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerend MA, Lee SC, Shepherd JE. Predictors of human papillomavirus vaccination acceptability among underserved women. Sex Transm Dis. 2007;34:468–71. doi: 10.1097/01.olq.0000245915.38315.bd. [DOI] [PubMed] [Google Scholar]
- 28.Lefevere E, Hens N, De Smet F, Van Damme P. Dynamics of HPV vaccination initiation in Flanders (Belgium) 2007-2009: a Cox regression model. BMC Public Health. 2011;11:470. doi: 10.1186/1471-2458-11-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rondy M, van Lier A, van de Kassteele J, Rust L, de Melker H. Determinants for HPV vaccine uptake in the Netherlands: A multilevel study. Vaccine. 2010;28:2070–75. doi: 10.1016/j.vaccine.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 30.Smith LM, Brassard P, Kwong JC, Deeks SL, Ellis AK, Levesque LE. Factors associated with initiation and completion of the quadrivalent human papillomavirus vaccine series in an Ontario cohort of grade 8 girls. BMC Public Health. 2011;11:645. doi: 10.1186/1471-2458-11-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guerry SL, De Rosa CJ, Markowitz LE, et al. Human papillomavirus vaccine initiation among adolescent girls in high-risk communities. Vaccine. 2011;29:2235–41. doi: 10.1016/j.vaccine.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 32.Rosenthal SL, Rupp R, Zimet GD, et al. Uptake of HPV vaccine: demographics, sexual history and values, parenting style, and vaccine attitudes. J Adolesc Health. 2008;43:239–45. doi: 10.1016/j.jadohealth.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Tan W, Viera AJ, Rowe-West B, Grimshaw A, Quinn B, Walter EB. The HPV vaccine: Are dosing recommendations being followed? Vaccine. 2011;29:2548–54. doi: 10.1016/j.vaccine.2011.01.066. [DOI] [PubMed] [Google Scholar]
- 34.Chao C, Velicer C, Slezak JM, Jacobsen SJ. Correlates for completion of 3-dose regimen of HPV vaccine in female members of a managed care organization. Mayo Clin Proc. 2009;84:864–70. doi: 10.4065/84.10.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gold R, Naleway AL, Jenkins LL, et al. Completion and timing of the three-dose human papillomavirus vaccine series among adolescents attending school-based health centers in Oregon. Prev Med. 2011;52:45658. doi: 10.1016/j.ypmed.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Niccolai LM, Mehta NR, Hadler JL. Racial/ethnic and poverty disparities in human papillomavirus vaccination completion. AJPM. 2011;41:428–33. doi: 10.1016/j.amepre.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 37.Arbyn M, Castellsagué X, de Sanjosé S, et al. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22:2675–86. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 38.Brewer NT, Fazekas KI. Predictors of HPV vaccine acceptability: A theory-informed, systematic review. Prev Med. 2007;45:107–14. doi: 10.1016/j.ypmed.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 39.McKinnon B, Harper S, Moore S. Decomposing income-related inequality in cervical screening in 67 countries. Int J Public Health. 2011;56:139–52. doi: 10.1007/s00038-010-0224-6. [DOI] [PubMed] [Google Scholar]
- 40.Teitelman AM, Stringer M, Nguyen GT, Hanlon AL, Averbuch T, Stimpfel AW. Social cognitive and clinical factors associated with HPV vaccine initiation among urban, economically disadvantaged women. J Obstet Gynecol Neonatal Nurs. 2011;40:691–701. doi: 10.1111/j.1552-6909.2011.01297.x. [DOI] [PubMed] [Google Scholar]
- 41.Kessels SJM, Marshall HS, Watson M, Braunack-Mayer AJ, Reuzel R, Tooher RL. Factors associated with HPV vaccine uptake in teenage girls: A systematic review. Vaccine. 2012;30:3546–56. doi: 10.1016/j.vaccine.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 42.Cooper Robbins SC, Bernard D, McCaffery K, Brotherton JM, Skinner S. “I just signed”: Factors influencing decision-making for school-based HPV vaccination of adolescent girls. Health Psychol. 2010;29:618–25. doi: 10.1037/a0021449. [DOI] [PubMed] [Google Scholar]
- 43.Gordon D, Waller J, Marlow LAV. Attitudes to HPV vaccination among mothers in the British Jewish community: Reasons for accepting or declining the vaccine. Vaccine. 2011;29:7350–56. doi: 10.1016/j.vaccine.2011.07.083. [DOI] [PubMed] [Google Scholar]
- 44.Brown KF, Kroll JS, Hudson MJ, et al. Factors underlying parental decisions about combination childhood vaccinations including MMR: A systematic review. Vaccine. 2010;28:4235–48. doi: 10.1016/j.vaccine.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 45.Roberts KA, Dixon-Woods M, Fitzpatrick R, Abrams KR, Jones DR. Factors affecting uptake of childhood immunisation: a Bayesian synthesis of qualitative and quantitative evidence. Lancet. 2002;360:1596–99. doi: 10.1016/S0140-6736(02)11560-1. [DOI] [PubMed] [Google Scholar]
- 46.Schluterman NH, Terplan M, Lydecker AD, Tracy JK. Human papillomavirus (HPV) vaccine uptake and completion at an urban hospital. Vaccine. 2011;29:3767–72. doi: 10.1016/j.vaccine.2011.03.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.