Abstract
The objective of this study was to evaluate some of the mechanisms involved in the activation of the immune system in patients with advanced-stage cancer (n = 7) who received an autologous dendritic cell vaccine. We examined the immune response mediated by macrophages (CD14+), natural killer cells (CD56+), and B lymphocytes (CD19+) by flow cytometry and assessed the expression of Th1 (IFN-γ, TNF-α, IL-2, and IL-12), Th2 (IL-4), and Treg (TGF-β) cytokines by flow cytometry and an enzyme-linked immunosorbent assay. The CD14+ TNF-α+ population was significantly increased (P < 0.04) when patients received the vaccine; IL-2 expression in both NK cells and in B lymphocytes was increased after a transient initial increase showed a nearly significant decrease (P < 0.07 and P < 0.06 respectively), whereas the CD19+ and CD56+ populations did not show significant changes. Dendritic cell-based immunotherapy led to increased secretion of IFN-γ and IL-12 and reduced secretion of TGF-β. In conclusion, it is likely that the autologous dendritic cell vaccine stimulated the immune cells from the peripheral blood of patients with cancer and generally increased the production of Th1 cytokines, which are related to immunomodulatory responses against cancer.
Keywords: immune response, cancer, dendritic cells, immunotherapy
Introduction
Tumor cells engage in complex interactions with the immune system of the host, involving various cell lineages and mediators. In some hosts, the immune response can inhibit tumor growth, leading to spontaneous remission; in others, it may promote chronic inflammation, induced by tumor progression and angiogenesis.1 Population studies show that patients who maintain a chronic inflammatory state are more predisposed to develop cancer.2 In contrast, other authors have found extensive infiltration of natural killer (NK) cells in gastric and colorectal cancers and have associated this finding with an improved prognosis.3,4
When the immune system is stimulated, inflammatory cells are recruited and activated. These cells include innate immune cells and macrophages, dendritic cells (DCs), NK cells, mastocytes, and neutrophils, which participate in the first line of defense against pathogens and depend on the synthesis and liberation of specific cytokines and chemokines.5 The antitumor immune response is mediated by these innate immune cells that, in turn, form complex activation chains of adaptive immunity that involve participation of T and B lymphocytes and liberation of further immunoregulatory components. In addition, the IL-12 produced by macrophages and other antigen- presenting cells stimulates NK cells to exercise cytotoxicity and produce more IFN-γ, increasing the tumoricidal potential of the macrophages.6–10
Macrophage infiltrates are frequently found in neoplastic tissues, known as tumor-associated macrophages, which can be polarized into different functional phenotypes according to the stimulus and the microenvironment. Studies distinguish two populations of macrophages. The M1 population is found in active states and basically produces cytokines and immunoregulatory agents of the proinflammatory profile, such as interleukin (IL)-12, IL-23, tumor necrosis factor (TNF)-α, inducible nitric oxide synthase (iNOS), major histocompatibility complex I (MHCI), and MHCII. The M2 population is frequently found in tumors; it expresses cytokines and anti-inflammatory agents, such as IL-10 and transforming growth factor (TGF)-β, and provides a favorable atmosphere for tumor development.10
Other important cells of the mononuclear phagocytic system include the DCs. Through their ability to present antigens, DCs act between innate and acquired immune responses. Studies have been conceived to develop a vaccine for patients with neoplasias based on tumor antigen-pulsed and in vitro-matured DCs. In this new form of therapeutic intervention, the immune system is stimulated to generate a specific response and to discriminate its targets with great precision, making the attack mechanism highly directed towards the neoplastic tissue. The vaccine against cancer causes few side effects because it does not lead to immunosuppression, unlike chemotherapy or radiation therapy.11–14
In a previous study, we evaluated the action of immunotherapy with a DC vaccine on the acquired immune response in patients with advanced cancer.15
Another important element to highlight is the interaction between innate and acquired immunity when confronted with DC therapy. The innate immune system is potentially able to recognize mutated cells and to induce an antitumor response, orchestrated in transition with acquired immune cells, particularly DCs. The objective of this study was to evaluate the activation of key cells that defend against tumors, including macrophages, NK cells, and B lymphocytes, as well as the expression of cytokines with Th1 (IL-2, IL-12, TNF-α and IFN-γ), Th2 (IL-4), and Treg (TGF-β) profiles in patients with advanced cancer receiving an autologous DC vaccine.
Methods
We evaluated the innate immune response, represented by NK cells (CD56+) and macrophages (CD14+), the humoral immune response, represented by B lymphocytes (CD19+), and serum cytokine expression levels (IFN-γ, IL-4, IL-12, and TGF-β).
Patients and DC vaccine
The volunteers and procedures for DC vaccine used in this study were described previously by our group.15 Inclusion criteria were advanced or recurrence of solid tumor in an adult and patients who refused conventional treatment. Exclusion criteria were immunosuppressive diseases, such as AIDS, autoimmune diseases, patients who had not received any treatment (chemotherapy, radiotherapy or hormone therapy) for at least two months before the DC vaccine.
The patients received doses of vaccine DCs every two weeks and were evaluated by clinical examination and laboratory tests, such as blood count, cervico-vaginal cytology, colposcopy, ultrasound, and computed tomography for analysis of stability or progression of the disease. Table 1 shows a summary of the general characteristics for each patient participating in the study. The patients receiving treatment did not have any major side effects, except for one patient with vaginal melanoma who showed an exacerbation of a previous disease (vitiligo).
Table 1.
Patient characteristics based on age, tumor type, stage, and previous treatments.
| Patient | Age (y) | Tumor type | Stage (TNM)* | Previous treatments | Treatment period (number of applications) |
|---|---|---|---|---|---|
| 1 | 76 | Vaginal cancer | IIIB (T3N1M0) | – | 2 years and 6 months (60) |
| 2 | 77 | Vaginal melanoma | IIC (T4NxMx) | – | 1 year and 3 months (30) |
| 3 | 48 | Vaginal recurrence of cervical cancer | I (T1N0M0) | Radiotherapy, surgery, IFN | 2 years (48) |
| 4 | 66 | Breast cancer | IV (T4dN2M1) | Chemotherapy, radiation therapy, surgery | 4 months (8) |
| 5 | 39 | Cervical cancer | IVB (T2bN0M1) | – | 3 months (6) |
| 6 | 80 | Breast cancer | IIIC (T4dN3Mx) | Chemotherapy, radiation therapy, surgery | 5 months (10) |
| 7 | 27 | Breast cancer | IV (T2N1M1) | Chemotherapy, radiation therapy, surgery | 5 months (10) |
Note:
TNM = classification of cancer staging, where T refers to the tumor size, N refers to any involved lymph nodes, and M refers to the presence of metastasis.
Flow cytometry
Peripheral blood samples were drawn from the patients and cells were evaluated by flow cytometry (BD FACS Calibur cytometer and cell sorter, BD Biosciences, Franklin Lakes, NJ, USA). Cytometry protocols were deployed in accordance with those suggested by the manufacturer. The peripheral blood cells were collected to examine the following markers in all patients: B lymphocytes (CD19+), macrophages (CD14+), and NK cells (CD56+). The procedure was performed prior to initiating therapy with DCs (pretreatment analysis). Additional analysis was performed every 15 days.
Leukocytes were isolated from peripheral blood samples via centrifugation at 4 °C by using a standard cell lysing protocol (FACSTM Lysing Solution, BD Biosciences) in accordance with the manufacturer’s instructions. Cells were initially resuspended in phosphate-buffered saline (PBS) for extracellular tagging with α-CD19 PE, α-CD14 PE, α-CD56 PE, and α-CD25 fluorescein isothiocyanate (FITC). After extracellular tagging, the cells were incubated at 4 °C for 30 min, rinsed twice by centrifugation with PBS, and incubated with a fixation and permeabilization solution (BD Cytofix/CytopermTM) for 20 min at 4°C. The cells were rinsed twice with Perm/Wash buffer (BD Biosciences) prior to the second tagging.
For intracellular tagging, α-IL-2 FITC antibodies were utilized for B cells. Macrophages were also tagged with α-TNF-α FITC and α-IFN-γ FITC antibodies. An α-IL-2 FITC antibody was used to tag NK cells. After intracellular tagging, cells were incubated at 4 °C for 30 min and resuspended in 500 μL of PBS for cytometric analysis with a BD FACSCalibur™. To determine which cells corresponded to lymphocytes and macrophages, we identified the region to be analyzed by constructing a gate according to a chart control for relative size (forward scatter; FSC) and granularity and complexity (side scatter; SSC) in each experiment and for each patient.
Cytokine levels
Serum cytokine levels (IL-4, IL-12, IFN-γ, and TGF-β) were measured using an enzyme-linked immunosorbent assay (ELISA) with pairs of monoclonal antibodies from BD OptEIA™ (BD Biosciences). The procedure was performed in accordance with the manufacturer’s protocol. Plates (384-well) were sensitized with 25 μL of specific monoclonal antibodies for uptake of the desired cytokine diluted in a coating buffer. For the standards, 25 μL of recombinant cytokine were added to wells in the first row of each plate according to a 1:2 dilution series based on initial concentrations. To the other rows, 25 μL of serum containing the cytokine to be dosed were added to each well. The plates were incubated at room temperature for 2 hours and washed five times with a solution containing 0.05% PBS-Tween. Next, detector antibody (25 μL/well) for the cytokine to be dosed was added. The plates were incubated for 1 hour at room temperature and washed again five times in PBS-Tween.
After this step, 25 μL/well of TMB Substrate Reagent Set (BD OptEIATM) was added. After 30 min, 25 μL/well of Stop Solution (2 N phosphoric acid) was added. The ELISA plate was read with a Spectramax 384 Plus automatic reader. The results reflected the difference between the absorbance at 450 and 570 nm. The serum concentration of each cytokine was expressed in pg/mL by comparison with a standard curve, which was obtained simultaneously. The sensitivities of the assay for the cytokines were as follows: 4.7–300 pg/mL for IFN-γ, 7.8–500 pg/mL for IL-4, 31.3–2000 pg/mL for IL-12, and 125–8000 pg/mL for TGF-β.
Statistical analysis
The tables show the results of statistical analysis, including the average and standard error of the mean for serum cytokine dosages. We evaluated the pretreatment steps and 10 post treatment analyses for the DC vaccine.
Data are shown as the mean and standard error of the mean (SEM). The results were analyzed using the Kruskal-Wallis nonparametric test. Statistical analysis and graphing were performed with GraphPad Prism version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Results were considered statistically significant when P ≤ 0.05.
Results
An evaluation of the macrophage population revealed oscillations in the percentage of fluorescence after the start of treatment. High values were obtained between the second and fourth doses of the vaccine, with a slight decrease between the fifth and sixth doses and a return to a peak after the eighth application (Table 2). IFN-γ expression by macrophages showed a marked stimulation after the start of DC immunotherapy, although the fluorescence percentage for the CD14/IFN-γ double marker generally decreased after the eighth analysis. In analyzing the TNF-α expression by the macrophages, we observed significant changes (P = 0.04) at the start of therapy, with a representative peak at the sixth dose of the vaccine.
Table 2.
Distribution in percentage of the expression of CD14, CD19, CD25, and CD56 with IL-2, IFN-γ, and TNF-α in patients treated with the DC vaccine.
| Marking | Pretreatment (%) | Posttreatment (%) | P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||
| CD14+ | 1.33 ± 1.21 | 0.31 ± 0.18 | 1.94 ± 0.88 | 0.64 ± 0.38 | 1.74 ± 1.73 | 0.7 ± 0.46 | 0.14 ± 0.13 | 0.02 ± 0.01 | 0.18 ± 0.09 | 0.64 ± 0.49 | 1.38 ± 0.92 | 0.53 |
| CD14+/IFN-γ | 13.24 ± 5.64 | 6.07 ± 4.26 | 7.50 ± 2.24 | 15.35 ± 13.7 | 6.39 ± 4.62 | 7.14 ± 3.4 | 32.30 ± 23.5 | 27.02 ± 26.5 | 28.26 ± 27.5 | 0.57 ± 0.25 | 4.14 ± 2.55 | 0.60 |
| CD14+/TNF-α | 2.2 ± 1.85 | 0.84 ± 0.81 | 1.50 ± 0.77 | 3.62 ± 2.61 | 2.99 ± 2.95 | 0.46 ± 0.44 | 22.2 ± 22.1 | 0.52 ± 0.51 | 0.87 ± 0.84 | 0.05 ± 0.02 | 0.02 ± 0.01 | 0.04 |
| CD19+ | 1.09 ± 0.2 | 0.54 ± 0.15 | 0.83 ± 0.22 | 0.67 ± 0.17 | 1.91 ± 0.64 | 0.63 ± 0.27 | 0.48 ± 0.24 | 0.91 ± 0.6 | 2.07 ± 1.79 | 2.04 ± 1.33 | 0.29 ± 0.03 | 0.20 |
| CD19+/IL-2 | 0.86 ± 0.63 | 0.18 ± 0.04 | 1.37 ± 0.65 | 0.35 ± 0.15 | 0.4 ± 0.09 | 0.13 ± 0.06 | 0.34 ± 0.26 | 0.18 ± 0.1 | 0.66 ± 0.57 | 0.52 ± 0.2 | 0.62 ± 0.5 | 0.06 |
| CD56+ | 0.92 ± 0.88 | 0.03 ± 0.01 | 0.51 ± 0.26 | 0.8 ± 0.75 | 0.05 ± 0.04 | 0.06 ± 0.03 | ND | 0.01 ± 0.01 | ND | 0.13 ± 0.13 | 0.01 ± 0.01 | 0.73 |
| CD56+/CD25+ | 0.62 ± 0.56 | 0.02 ± 0.01 | 0.82 ± 0.58 | 0.55 ± 0.38 | 0.07 ± 0.06 | ND | ND | 0.01 ± 0.01 | ND | 0.03 ± 0.01 | 0.01 ± 0.01 | 0.24 |
| CD56+/IL-2 | 0.87 ± 0.68 | 0.2 ± 0.06 | 0.86 ± 0.27 | 0.43 ± 0.21 | 0.47 ± 0.21 | 0.14 ± 0.09 | 0.03 ± 0.03 | 0.11 ± 0.02 | 0.24 ± 0.2 | 0.38 ± 0.27 | 0.08 ± 0.02 | 0.07 |
Notes: Results represent the mean ± SEM for the percentage of fluorescence (%). P-values by Kruskal-Wallis test.
Abbreviation: ND, not detected.
The NK cells (CD56+) showed peak in fluorescence around the third analysis, with a decrease occurring over the course of therapy. The expression of the alpha chain of the IL-2 receptor (CD25+) by the NK cells, which provides evidence of cell activation, showed a representative increase after the start of DC-based immunotherapy. However, the CD25+ expression generally decreased from the third analysis onward. IL-2 expression by NK cells varied from the start to approximately the fourth analysis (P = 0.07).
The induction of the humoral immune response can be evaluated using the lymphocyte B (CD19+) marker. This marker showed an oscillation in fluorescence after the start of immunotherapy, with higher positivity peaks between the fourth, eighth, and ninth analyses, after which there was a period of reduction. The IL-2 cytokine expression by B lymphocytes (CD19+/IL-2) showed a sharp decline after immunotherapy, with a reversion starting from the third analysis and generally increasing up to the tenth analysis (P = 0.06); however, there was no evidence of statistically significant differences between these analyses.
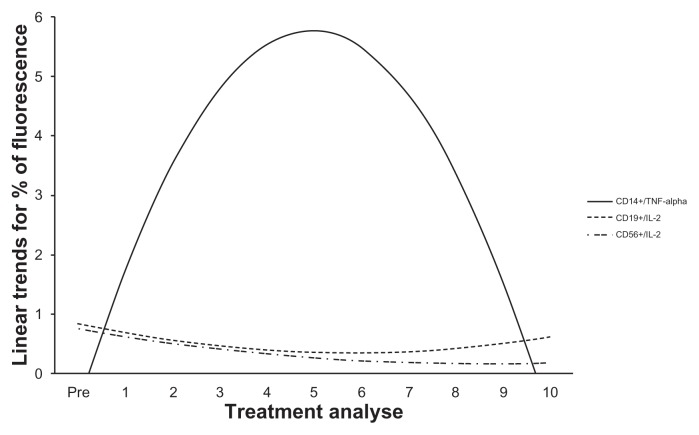
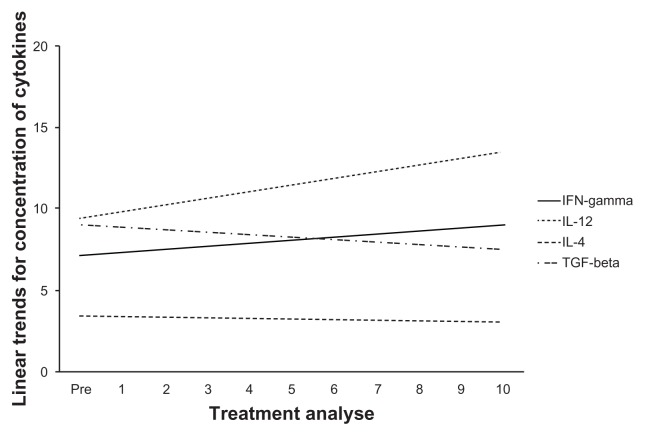
Figure 1 shows the tendencies of the markers to show significant or near-significant changes when we analyzed the influence of the DC vaccine in treating patients with advanced cancer. Before and during DC-based immunotherapy, we collected blood samples to measure serum cytokine levels. Th1 cytokines were capacitated by DC-based immunotherapy (Table 2). Over the course of therapy, the IFN-γ cytokine showed an arguable increase in synthesis (Fig. 2). IL-12 showed a substantial, but not significant, increase in production after therapy with DCs and over the course of treatment (Fig. 2). The serum expressions of IL-4 and TGF-β generally decreased after the start of treatment (Table 3 and Figure 2).
Figure 1.
Linear trends for the percentage of fluorescence for samples showing significant expression of CD14, CD19, and CD56 with IL-2 and TNF-α in patients receiving treatment with the DC vaccine.
Figure 2.
Linear trends for the concentration of samples that expressed Th1 cytokines versus Th2 or Treg cytokines in patients with advanced-stage cancer receiving treatment with the DC vaccine.
Table 3.
Concentrations of Th1, Th2, and Treg cytokines in patients with advanced-stage cancer receiving treatment with the DC vaccine.
| Marker | Pretreatment (pg/mL) | Posttreatment (pg/mL) | P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||
| IFN-γ | 1.42 ± 0.61 | 2.04 ± 0.98 | 5.64 ± 3.49 | 4.61 ± 1.9 | 32.7 ± 21.2 | 3.29 ± 1.91 | 3.28 ± 1.91 | 25.3 ± 23.9 | 1.13 ± 1.13 | 9.5 ± 5.26 | 0.02 ± 0.02 | 0.35 |
| IL-12 | 9.11 ± 3.23 | 9.49 ± 4.77 | 9.13 ± 3.65 | 12.6 ± 5.58 | 15.5 ± 7.58 | 11 ± 5.39 | 2.44 ± 1.5 | 6.94 ± 4.51 | 27.1 ± 14.3 | 11.5 ± 7.13 | 10.8 ± 5.7 | 0.90 |
| IL-4 | 4.41 ± 2.15 | 3.53 ± 1.09 | 3.07 ± 1.73 | 2.15 ± 1.2 | 0.81 ± 0.42 | 1.69 ± 0.82 | 8.43 ± 6.52 | 3.25 ± 2.35 | 2.0 ± 0.81 | 5.17 ± 2.86 | 1.03 ± 0.45 | 0.80 |
| TGF-β | 851 ± 331 | 1358 ± 537 | 712 ± 385 | 707 ± 436 | 619 ± 337 | 762 ± 466 | 642 ± 449 | 1037 ± 720 | 913 ± 635 | 745 ± 745 | 740 ± 740 | 0.99 |
Note:P-values by Kruskal-Wallis test. Results are expressed as mean ± SEM.
Discussion
DCs capture and process tumor antigens and present parts of those antigens through the major histocompatibility complex (MHC) for T-effector cells.16 In this study, we performed immunotherapy with autologous DCs differentiated in vitro and sensitized with antigens obtained through biopsies of the primary tumor site (breast or vagina) or the metastatic site (lungs or liver) of the patient. The patients receiving treatment did not have any major side effects, such as coetaneous rash, hypotension, itching, hyperthermia, myalgia, edema, or infection, which has been observed in previous studies.17,18 Only one patient with vaginal melanoma showed an exacerbation of a previous disease, vitiligo. This exacerbation may have occurred through activation of an immune response against melanocytes during treatment, a common finding in patients with this type of tumor.19 Similar studies involving the treatment of melanoma have demonstrated the potential induction of systemic antitumor immune response and tumor regression with low toxicity.20
With respect to the intercellular expression of TNF-α by the macrophages, our results showed a significant increase (P ≤ 0.05) during the first cell analyses after DC immunotherapy. Our results indicate that these cells are stimulated and can participate through innate immunity in the process of inducing and maintaining antitumor responses. Studies examining adoptive cell immunotherapy with monocytes/macrophages have demonstrated biological responses related to an increase in TNF-α in the peritoneal fluid, although significant tumor regression has not been achieved.21
We evaluated the expression of IL-2 and the α chain of its receptor, CD25, in NK cells. However, nearly significant decreases in IL-2 expression in NK occurred successively (P < 0.07). We observed an increase in these markers after the first DC vaccines. Our findings suggest that DC immunotherapy can induce effector immune responses in NK cells, stimulating a Th1 profile response after the start of immunotherapy.
We observed that the IFN-γ level in the blood was higher during therapy than in the pretreatment period. Soleimani et al (2009)22 observed similar results, demonstrating that patients with metastatic renal carcinoma and treated with an autologous pulsed DC vaccine with allogeneic tumor lysate showed an increase in IFN-γ levels, mainly after the 13th application of the vaccine and most relevant in patients with disease stabilization. There was also an increase in the levels of IL-12 after the fourth and sixth applications of the vaccine. We also observed generally increasing IL-12 levels.
Tumor-derived TGF-β can increase expression of chemokine receptors in immature DCs and suppress expression of CCR7, thereby impeding the migration of DCs through the lymph nodes and maintaining the DCs immature at the tumor site.23 In our patients, serum TGF-β levels oscillated considerably. The levels of this cytokine generally decreased between pretreatment and the end of therapy, as well as after various applications of the vaccine.
The immunotherapy developed in this study did not cause damage to the patients, but did increase the quality of life and stabilized the disease in nearly all cases. This fact has already been verified in a phase II study in patients with small-cell lung cancer.24 Therefore, the transient increase in these cell populations indicates that DC immunotherapy positively influenced the target cells for innate and acquired immunity in patients with advanced cancer. However, after a period of therapy, there was a decline in all cell populations, most obviously between the eighth and tenth analyses. These findings indicate that even with an initial stimulation of immune response, after a specific period the tumors can develop new tumor escape mechanisms.
There are many possible mechanisms involved in the ineffectiveness of the DC vaccine for treating cancer, such as heterogeneity of the DC populations, inadequate protocols for vaccine preparation, an incomplete stage of DC maturity and inability of DC trafficking, mutations in the tumor antigen, tumor immunosuppression mediated by self-tolerance, use of tumor antigens from biopsy tissues with an abundance of proteins, carbohydrates, nucleic acids, or other components that are also present in healthy cells, low avidity in the immune response cells for antigens associated with the tumor, and suppression by T regulatory cells.25–28 The DC vaccine is a new therapeutic approach for treating cancer. The state of the disease directly influences the success of immunotherapy. Because it is still an investigatory therapeutic measure, patients are normally directed to this approach at an advanced or terminal stage of disease when they have severe immunosuppression, compromising the effectiveness of immunotherapeutic treatment.
The association of clinical and immunological parameters indicates a direct correlation between the improvement of clinical outcomes and the favorable outcomes to DC vaccine-activated immune response, as demonstrated by the increase in NK cells and macrophages activities, as well as increased synthesis of Th1 cytokines such as IL-12, associated with a reduction of TGF-β, likely a regulatory T profile.
These results show an activation of the systemic immune response in patients receiving autologous DC immunotherapy. This activation generally increased cytokines of the Th1 profile (IFN-γ and IL-12), which act on immune cell response, and reduced cytokines of the Th2 profile (IL-4 and TGF-β), which stimulate the humoral immune response.
Footnotes
Author Contributions
Conceived and designed the experiments: MAM, EFCM. Analyzed the data: MAM, EFCM, DRA, LM, BFM, TMO, CMR. Wrote the first draft of the manuscript: MAM, EFCM, DRA, LM. Contributed to the writing of the manuscript: MAM, EFCM, DRA, LM, BFM, TMO, CMR. Agree with manuscript results and conclusions: MAM, EFCM, DRA, LM, BFM, TMO, CMR. Jointly developed the structure and arguments for the paper: MAM, EFCM, DRA, LM, BFM, TMO, CMR. Made critical revisions and approved final version: MAM, EFCM, DRA, LM, BFM, TMO, CMR. All authors reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
The authors would like to thank the Studies and Projects Funding Body (Financiadora de Estudos e Projetos, FINEP), the Foundation for Research Assistance of the State of Minas Gerais (Fundação de Amparo à Pesquisa do Estado de Minas Gerais, FAPEMIG), the National Council for Scientific and Technical Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq), and the Uberaba Foundation for Teaching and Research (Fundação de Ensino e Pesquisa de Uberaba, FUNEPU) for financial assistance.
References
- 1.Dougan M, Dranoff G. Immune therapy for cancer. Ann Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 2.Karin M, Greten FR. NF-κB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5(10):749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 3.Coca S, Perez-Piqueras J, Martinez D, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79(12):2320–8. doi: 10.1002/(sici)1097-0142(19970615)79:12<2320::aid-cncr5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 4.Ishigami S, Natsugoe S, Tokuda K, et al. Clinical impact of intratumoral natural killer cell and dendritic cell infiltration in gastric cancer. Cancer Lett. 2000;159(1):103–8. doi: 10.1016/s0304-3835(00)00542-5. [DOI] [PubMed] [Google Scholar]
- 5.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425(6957):516–21. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 6.Enzler T, Gillessen S, Manis JP, et al. Deficiencies of GM-CSF and interferon-gamma link inflammation and cancer. J Exp Med. 2003;197(9):1213–9. doi: 10.1084/jem.20021258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 8.Dirkx AE, Oude Egbrink MG, Wagstaff J, Griffioen AW. Monocyte/macrophage infiltration in tumours: modulators of angiogenesis. J Leukoc Biol. 2006;80(6):1183–96. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- 9.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 10.Hao NB, Lü MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumour microenvironments and the progression of tumours. Clin Dev Immunol. 2012;2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nestle FO. Dendritic cell vaccination for cancer therapy. Oncogene. 2000;19(56):6673–9. doi: 10.1038/sj.onc.1204095. [DOI] [PubMed] [Google Scholar]
- 12.Vari F, Hart DN. Loading DCs with Ag. Cytotherapy. 2004;6(2):111–21. doi: 10.1080/14653240410005230. [DOI] [PubMed] [Google Scholar]
- 13.Hung CF, Monie A, Alvarez RD, Wu TC. DNA vaccines for cervical cancer: from bench to bedside. Exp Mol Med. 2007;39(6):679–89. doi: 10.1038/emm.2007.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baxevanis CN, Perez SA, Papamichail M. Combinatorial treatments including vaccines, chemotherapy and monoclonal antibodies for cancer therapy. Cancer Immunol Immunother. 2009;58(3):317–24. doi: 10.1007/s00262-008-0576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigues CM, Matias BF, Murta EFC, Michelin MA. The role of T lymphocytes in cancer patients undergoing immunotherapy with autologous dendritic cells. Clin Med Insights Oncol. 2011;5:107–15. doi: 10.4137/CMO.S6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soliman H. Developing an effective breast cancer vaccine. Cancer Control. 2010;17(3):183–90. doi: 10.1177/107327481001700307. [DOI] [PubMed] [Google Scholar]
- 17.Ernstoff MS, Nair S, Bahnson RR, et al. A phase IA trial of sequential administration recombinant DNA-produced interferons: combination recombinant interferon γ and recombinant interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 1990;8:1637–49. doi: 10.1200/JCO.1990.8.10.1637. [DOI] [PubMed] [Google Scholar]
- 18.Shwaab T, Schwarzer A, Wolf B, et al. Clinical and immunologic effects of intranodal autologous tumor lysate-dendritic cell vaccine with aldesleukin (interleukin 2) and IFN-α2a therapy in metastatic renal cell carcinoma patients. Clin Cancer Res. 2009;15(15):4986–92. doi: 10.1158/1078-0432.CCR-08-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg SA, White DE. Vitiligo in patients with melanoma: normal tissue antigens can be targets for cancer immunotherapy. J Immunother Emphasis Tumor Immunol. 1996;19(1):81–4. [PubMed] [Google Scholar]
- 20.Sun Y, Paschen A, Schadendorf D. Cell-based vaccination against melanoma—background, preliminary results, and perspective. J Mol Med (Berl) 1999;77(8):593–608. doi: 10.1007/s001099900039. [DOI] [PubMed] [Google Scholar]
- 21.Andreesen R, Scheibenbogen C, Brugger W, et al. Adoptive transfer of tumor cytotoxic macrophages generated in vitro from circulating blood monocytes: a new approach to cancer immunotherapy. Cancer Res. 1990;50(23):7450–6. [PubMed] [Google Scholar]
- 22.Soleimani A, Berntsen A, Svane IM, Pedersen AE. Immune responses in patients with metastatic renal cell carcinoma treated with dendritic cells pulsed with tumor lysate. Scand J Immunol. 2009;70(5):481–9. doi: 10.1111/j.1365-3083.2009.02322.x. [DOI] [PubMed] [Google Scholar]
- 23.Bennaceur K, Chapman J, Brikci-Nigassa L, Sanhadji K, Touraine JL, Portoukalian J. Dendritic cells dysfunction in tumour environment. Cancer Lett. 2008;272(2):186–96. doi: 10.1016/j.canlet.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Antonia SJ, Mirza N, Fricke I, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12(3 Pt 1):878–87. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 25.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5(4):296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 26.Mathis D, Benoist C. Back to central tolerance. Immunity. 2004;20:509–16. doi: 10.1016/s1074-7613(04)00111-6. [DOI] [PubMed] [Google Scholar]
- 27.Evel-Kabler K, Chen SY. Dendritic cell-based tumor vaccines and antigen presentation attenuators. Mol Ther. 2006;13(5):850–8. doi: 10.1016/j.ymthe.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Lokhov PG, Balashova EE. Cellular cancer vaccines: an update on the development of vaccines generated from cell surface antigens. J Cancer. 2010;1:230–41. doi: 10.7150/jca.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]