Abstract
Purpose
Standard treatment for glioblastoma (GBM) is surgery followed by radiation (RT) and temozolomide (TMZ). While there is variability in survival based on several established prognostic factors, the prognostic utility of other factors such as tumor size and location are not well established.
Experimental Design
The charts of ninety two patients with GBM treated with RT at the National Cancer Institute (NCI) between 1998 and 2012 were retrospectively reviewed. Most patients received RT with concurrent and adjuvant TMZ. Topographic locations were classified using preoperative imaging. Gross tumor volumes were contoured using treatment planning systems utilizing both pre-operative and post-operative MR imaging.
Results
At a median follow-up of 18.7 months, the median overall survival (OS) and progression-free survival (PFS) for all patients was 17.9 and 7.6 months. Patients with the smallest tumors had a median OS of 52.3 months compared to 16.3 months among patients with the largest tumors, P = 0.006. The patients who received bevacizumab after recurrence had a median OS of 23.3 months, compared to 16.3 months in patients who did not receive it, P = 0.0284. The median PFS and OS in patients with periventricular tumors was 5.7 and 17.5 months, versus 8.9 and 23.3 months in patients with non-periventricular tumors, P = 0.005.
Conclusions
Survival in our cohort was comparable to the outcome of the defining EORTC-NCIC trial establishing the use of RT+TMZ. This study also identifies several potential prognostic factors that may be useful in stratifying patients.
Introduction
Glioblastoma multiforme (GBM) is the most common primary central nervous system malignancy in adults, with approximately 14,000 newly diagnosed patients in the United States each year [1]. Despite multi-modality treatment, it remains one of the most aggressive tumors, with a median overall survival of only 14 months [2]. Nitrosoureas were the mainstay of adjuvant chemotherapy despite limited evidence of benefit [3] until 2005, when the European Organization for Research and Treatment of Cancer (EORTC) and the National Cancer Institute of Canada published a randomized prospective trial comparing surgery followed by either radiation therapy alone, or radiation therapy plus the addition of concurrent daily temozolomide and 6 months of adjuvant temozolomide (RT+TMZ) [2]. There was an improvement in median overall survival in the RT+TMZ group of 14.6 months compared to 12.1 months, and a 2-year overall survival of 27% compared to 11%. This regimen has been widely adapted as the standard of care since then.
Although the median survival is 14.6 months, there is a range of survival times when patients are placed in subsets using the recursive partitioning analysis (RPA) developed in 1993 (4). The RPA identified four risk groups based on several prognostic factors, with age (greater or less than 50 years) being the most significant determinant of survival, followed by performance status, mental status, neurological function, extent of resection, and radiotherapy dose. The prognostic significance of the RPA was validated in patients receiving RT+TMZ in 2006 [4] and simplified to include three distinct prognostic groups defined by age, performance status, extent of resection, and neurological function. The majority of recurrences occur locally with the predominance of failures occurring within the high dose radiation fields [5]. Consistent with this, in a recent dose escalation study of intensity-modulated RT (IMRT) using doses of 66 to 81 Gy, 18 out of 28 recurrent tumors had at least 80% of the recurrent volume within the 95% prescription isodose line [6].
Presented here are the experience and outcomes of patients treated at the National Cancer Institute in Bethesda, Maryland using the radiation and temozolomide protocol. Included are the radiographic recurrence patterns. Also reported are possible new prognostic factors, such as tumor location (periventricular versus non-periventricular) and primary tumor size, that may be useful in stratifying patients in future research protocols.
Methods
Patients
A total of 100 consecutive adult patients with histologically-confirmed (World Health Organization) grade IV GBM were treated with radiation therapy at the NCI in Bethesda, Maryland between 1998 and 2012 on a National Cancer Institute institutional review board approved protocol after given written informed consent. Eight patients were excluded from analysis: two patients who died during treatment, and six patients who did not complete radiation treatment because of clinical deterioration. Ninety two patients were included in the final analysis. All patients underwent surgery [gross total resection (GTR), sub-total resection (STR) or biopsy (Bx)], followed by external beam, involved field RT. Demographic factors including age, performance status, working status, and extent of resection prior to treatment were collected, and an RPA score was derived for all patients [4]. Other clinical parameters were collected for each patient, including gender, tumor location, chemotherapy regimens, time of last follow-up, and patient status at the last follow-up (alive or deceased).
Tumor Location
The available pre-operative magnetic resonance imaging (MRI) sequences were reviewed. Patients were classified as “periventricular” if the contrast-enhancing lesion seen in T1-weighted MRI was in contact with the lateral ventricle, and all other patients were classified as “non-periventricular”.
Treatment Plan
Patients were simulated and treated using a custom thermoplastic face mask for immobilization. A computerized tomography (CT) scan of the head and upper neck was obtained during simulation using a Philips Brilliant Big Bore CT scanner, and images were transferred to a Varian Eclipse treatment planning system. The MR images, including post-contrast T1 images, T2 images or fluid attenuated inversion recovery (FLAIR) images, were fused (co-registered) to the CT images, as previously described [7]. The majority of patients (91% of patients) received RT at a dose of 2 Gy given once daily, 5 days per week, for a total dose of 60 Gy over the course of 6 weeks. Gross tumor volumes (GTVs) were contoured using T2 or FLAIR MRI, and T1 MRI. The initial gross tumor volume, GTV1, was defined as the enhancing lesion and surrounding edema seen on T2 or FLAIR. The boost volume, GTV2, was defined as the contrast-enhancing lesion only, as seen on T1 MRI. The planning target volumes (PTVs) were volumetric expansions within the skull of the GTVs. PTV1 consisted of a 2 cm expansion of GTV1 and was treated to a total dose of 46 Gy in 23 fractions. The cone down volume, PTV2, consisted of a 2.5 cm expansion of GTV2, and was treated with seven additional 2 Gy fractions to a total dose of 60 Gy. The maximum dose limits to normal tissues and organs at risk were: 7 Gy to the lenses, 50 Gy to the retinae, 55 Gy to the optic nerves, 56 Gy to the optic chiasm, and 60 Gy to the brainstem. Target volumes were obtained using preexisting contoured 3D tumor volumes, and recorded in cubic centimeters (cc) using the “calculate volume” function in the treatment planning system.
Chemotherapy
The majority of patients received RT with concurrent daily TMZ (90% of patients), followed by adjuvant monthly temozolomide (75%). Concurrent TMZ was prescribed at a dosage of 75 mg/m2/day, and adjuvant TMZ was prescribed at a dosage of 150 to 200 mg/m2/day for 5 days every 28 days for 6–12 cycles, unless the patient experienced disease progression, or treatment-related toxicity. Patients were treated with various therapies, including bevacizumab, after tumor progression, at the discretion of their treating physician. Anti-seizure medications and steroids were given as needed and doses were recorded at each treatment visit.
Pattern of Failure
Conventional MRI was obtained at 1 month post-radiation and every 2–3 months thereafter. Response was defined using MacDonald criteria and more recently the RANO criteria [8]. For patients with MRI documented failures, the T1 MRI showing tumor recurrence was fused to the original CT used for treatment planning, and the contrast-enhancing lesion was delineated as the recurrent gross tumor volume (rGTV). The dosimetric location of the recurrence was determined by overlaying the dose distribution on the planning CT, and observing where the rGTV was located in relation to the 90% isodose line. Recurrence was defined as “central” when the entire tumor recurrence resided within the 90% isodose surface, as “marginal” if the tumor recurrence crossed the 90% isodose surface, and as “distant” was the tumor recurrence resided entirely outside of the 90% isodose surface [9].
Statistical Analysis
The date of tumor progression was determined based on clinical symptoms and MRI-documented progression of disease. The date of death was determined based on clinic notes or using the internet-based Social Security Death Index. Progression-free survival and overall survival were measured from the date of diagnosis to the date of progression, death, or last follow-up. Time-to-event distributions were estimated using the Kaplan-Meier method and compared with the log-rank test. Cox regression was used for multivariate analysis.
Results
Patient Characteristics, Recurrence Pattern and Survival
The final patient cohort included 92 patients with GBM who were seen at the National Cancer Institute between July 1998 and January 2012. Patient characteristics for the entire cohort are listed in Table 1. The median patient age at diagnosis was 57 years (range: 31–79 years). Temozolomide was given concurrently to 83 patients, and in an adjuvant setting to 69 patients. Eighty patients completed the prescribed concurrent temozolomide. Of those patients who received adjuvant monthly temozolomide, 55 received temozolomide for ≥6 months. At a median follow-up of 18.7 months (range: 2.3–116.0 months), 70 patients had evidence of tumor progression, and 61 patients had died. The median OS for all patients was 17.9 months (95% CI: 16.3–23.9, Fig. 1) and the median PFS for all patients was 7.6 months (95% CI: 6.8–9.1, Fig. 1). Of the patients who had progressed, 56 had complete datasets including the radiation treatment plans and MRIs showing recurrence. Forty eight (86%) of the patients had a central recurrence; four (7%) had a marginal recurrence; and three (5%) had a distant recurrence. Twenty nine of the patients that recurred received bevacizumab, either as a mono-therapy or as part of combination therapy.
Table 1. Patient and Tumor Characteristics.
| Subcategory | n (%) | |
| Age (years) | ||
| <50 | 20 (22%) | |
| ≥50 | 72 (78%) | |
| median (range) | 57 (31–79) | |
| Sex | ||
| female | 31 (34%) | |
| male | 61 (66%) | |
| KPS | ||
| 90–100 | 76 (83%) | |
| 70–80 | 13 (14%) | |
| <70 | 2 (2%) | |
| Working/Not Working | ||
| W | 77 (84%) | |
| NW | 15 (16%) | |
| RPA | ||
| 3 | 19 (21%) | |
| 4 | 49 (53%) | |
| 5 | 24 (26%) | |
| Extent of Surgery | ||
| Gross total resection | 32 (35%) | |
| Subtotal resection | 44 (48%) | |
| Biopsy only | 16 (17%) | |
| Bevacizumab at 1st recurrence | ||
| Yes | 29 (41%) | |
| No | 33 (47%) | |
| Concurrent temozolomide | ||
| Yes | 83 (90%) | |
| No | 9 (10%) | |
| Adjuvant temozolomide | ||
| Yes | 69 (75%) | |
| No | 18 (20%) | |
| Corticosteroids during RT | ||
| Yes | 67 (73%) | |
| No | 25 (27%) | |
| Levetiracetam during RT | ||
| Yes | 56 (61%) | |
| No | 36 (39%) | |
| Hemisphere | ||
| right | 38 (41%) | |
| left | 53 (58%) | |
| both | 1 (1%) | |
| Location | ||
| Temporal | 23 (25%) | |
| Parietal | 18 (20%) | |
| Frontal | 22 (24%) | |
| Occipital | 2 (2%) | |
| Temporal-Parietal | 5 (5%) | |
| Occipital-Parietal | 6 (7%) | |
| Frontal-Temporal | 6 (7%) | |
| Frontal-Parietal | 4 (4%) | |
| Other | 5 (5%) | |
| Failures | ||
| central | 49 (88%) | |
| marginal | 4 (7%) | |
| distant | 3 (5%) | |
| Tumor Location | ||
| Periventricular | 27 (29%) | |
| Nonperiventricular | 59 (64%) | |
| Unsure | 6 (7%) |
Abbreviations: KPS, Karnofsky Performance Status; W, Working; NW, Not working; RPA, Recursive Partitioning Analysis; RT, Radiation Therapy. Patient demographics, treatment details, and characteristics of primary tumor before surgery/chemoirradiation.
Figure 1. Kaplan-Meier analysis of progression-free survival (PFS) and overall survival (OS) in all patients.
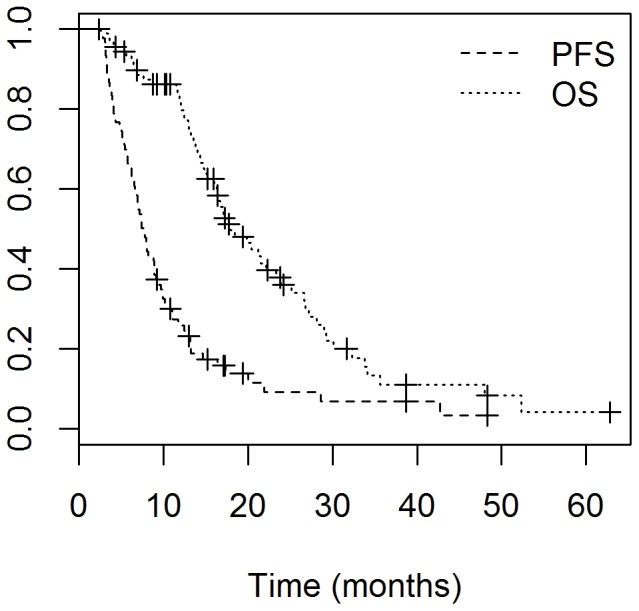
The median OS for all patients was 17.9 months (95% CI: 16.3–23.9) and the median PFS for all patients was 7.6 months (95% CI: 6.8–9.1).
Prognostic Factors
Tumor size
While previous analyses have shown that age and resection status are the primary prognostic factors for survival, tumor size and location are often prognostic in other tumor histologies. To explore the relationship of tumor size defined as the smallest quartile versus the largest quartile [10] and prognosis in GBM, calculated treatment planning volumes were compared to patient outcomes. Clinical variables for the small and large volumes are shown in Table 2. As shown in figure 2, using log rank analysis, patients with the smallest GTV1 tumors (GTV1 in the lowest 25th percentile) had a median OS of 52.3 months compared to 16.3 months among patients with the largest tumors (GTV1 in the highest 25th percentile), P = 0.006. Likewise, the median PFS was 12.5 months for patients with the smallest GTV1 tumors and 6.2 months for patients with the largest, P = 0.008. On multivariate analysis using tumor volume, age, resection status and location, only tumor volume was statistically significant, P = 0.02, Table 2. Similarly, the OS among patients with the smallest PTV1 tumors (lowest 25th percentile) had not reached the median at a follow-up of 17.9 months, compared to 14.8 months in patients with the largest PTV1 tumors (highest 25th percentile), P = 0.0125. Patients with the smaller PTV1 tumors had a median PFS of 9.7 months and patients with the larger PTV1 tumors had a median PFS 6.2 months, P = 0.0186.
Table 2. Tumor Volume.
| Subcategory | Median | Range | Small | Large | HR | SE | p-value | |
| Number | 21 | 21 | ||||||
| Age | ||||||||
| <50 | 6(29%) | 2(10%) | ||||||
| >50 | 15(71%) | 19(90%) | 0.009 | 0.51 | NS | |||
| Median | 54 | 61 | ||||||
| Resection Status | ||||||||
| GTR | 11(52%) | 3(15%) | 0.66 | 0.88 | NS | |||
| STR | 6(29%) | 13(62%) | 0.55 | 0.82 | NS | |||
| Biopsy | 4(20%) | 5(24%) | ||||||
| Location | ||||||||
| Non-periventricular | 16(84%) | 9(45%) | ||||||
| Periventricular | 3(16%) | 11(55%) | 0.23 | 0.60 | NS | |||
| Treatment Planning Volumes | ||||||||
| GTV1 | 90.4 cc | 2.7–385 cc | 1.44 | 0.63 | 0.022 | |||
| GTV2 | 28.4 cc | 1.6–166 cc | ||||||
| PTV1 | 424.7 cc | 76–1124 cc | ||||||
| PTV2 | 340 cc | 79–859 cc |
Abbreviations: GTR, gross total resection; STR, sub-total resection; GTV, gross tumor volume; PTV, planning tumor volume.
Figure 2. Kaplain-Meier analysis comparing overall survival of patients with the smallest tumors in the bottom quartile (Q1) and patients with the largest tumors in the top quartile (Q4), based on GTV1 volumes.
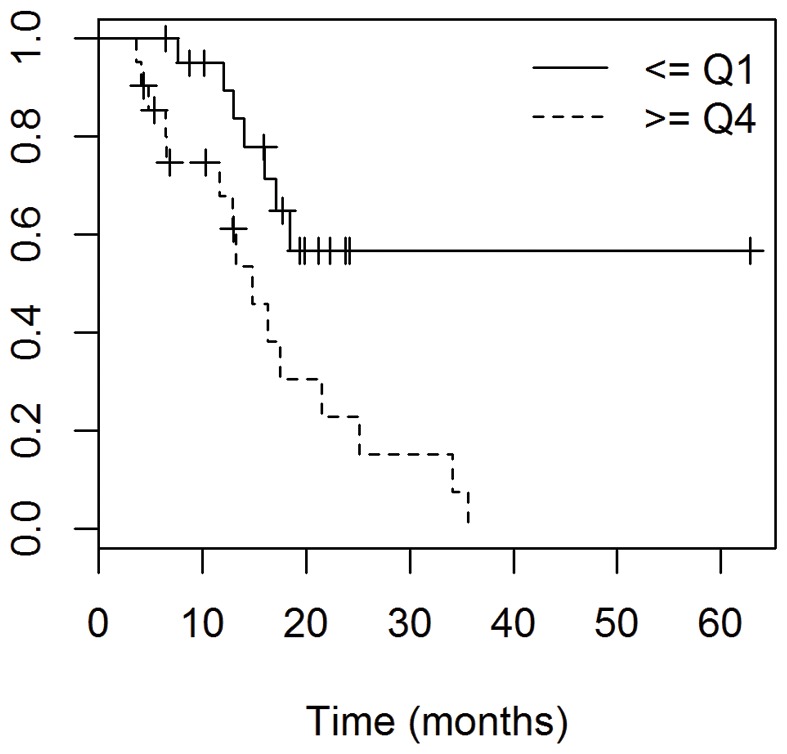
Median OS for patients with the smallest tumors (Q1) was 52.3 months compared to 16.3 months among patients with the largest tumors, P = 0.006.
Tumor location
Among the 86 patients, there were 27 (31%) patients with primary tumors categorized as periventricular, and 59 (69%) patients with non-periventricular tumors. Using a log rank analysis, there was a decreased time to progression between the periventricular group and the non-periventricular group, however, there was no significant difference in OS between the groups. The median PFS in patients with periventricular tumors was 5.7 months (95% CI: 5.0–7.9) versus 8.9 months (95% CI: 7.4–11.0) in patients with non-periventricular tumors, P = 0.005. The OS was 17.5 months (95% CI: 12.4–26.8) in patients with periventricular tumors, and 23.3 months (95% CI: 16.5–29.0) in patients with non-periventricular tumors, P = 0.176. On multivariate analysis using tumor location, age, resection status and tumor volume, PFS for tumor location was no longer statistically significant, Table 3.
Table 3. Tumor Location.
| Subcategory | Non-PV | PV | HR | SE | p-value | |
| Number | 59 | 27 | ||||
| Age | ||||||
| <50 | 14(24%) | 4(15%) | ||||
| >50 | 45(76%) | 23(85%) | 0.34 | 0.31 | NS | |
| Median | 56 | 59 | ||||
| Resection Status | ||||||
| GTR | 28(47%) | 4(15%) | 0.45 | 0.56 | NS | |
| STR | 25(42%) | 15(56%) | 0.54 | 0.55 | NS | |
| Biopsy | 6(10%) | 8(30%) | ||||
| Tumor Volume | ||||||
| GTV1 | 84.7 | 104.9 | 0.009 | 0.003 | 0.0007 |
Abbreviations: PV, periventricular: GTR, gross total resection; STR, sub-total resection.
Use of Bevacizumab at Recurrence
Of the 70 patients who progressed, 62 continued to be followed at the NIH. Of those patients, 28 received bevacizumab after their first progression, either as mono-therapy or as part of combination therapy, and 34 did not receive bevacizumab after their first progression. Using log rank analysis, the patients who received bevacizumab had a significant improvement in overall survival. Patients receiving bevacizumab had a median OS of 23.3 months (95% CI: 17.1–35.6), compared to 16.3 months (95% CI: 13.8–25.1) in patients who did not receive it, P = 0.0284, Fig. 3. On multivariate analysis using bevacizumab usage, age, resection status and tumor location, bevacizumab usage was statistically significant, P = 0.04, Table 4.
Figure 3. Overall survival of patients who received bevacizumab after progression compared to those who didn’t.
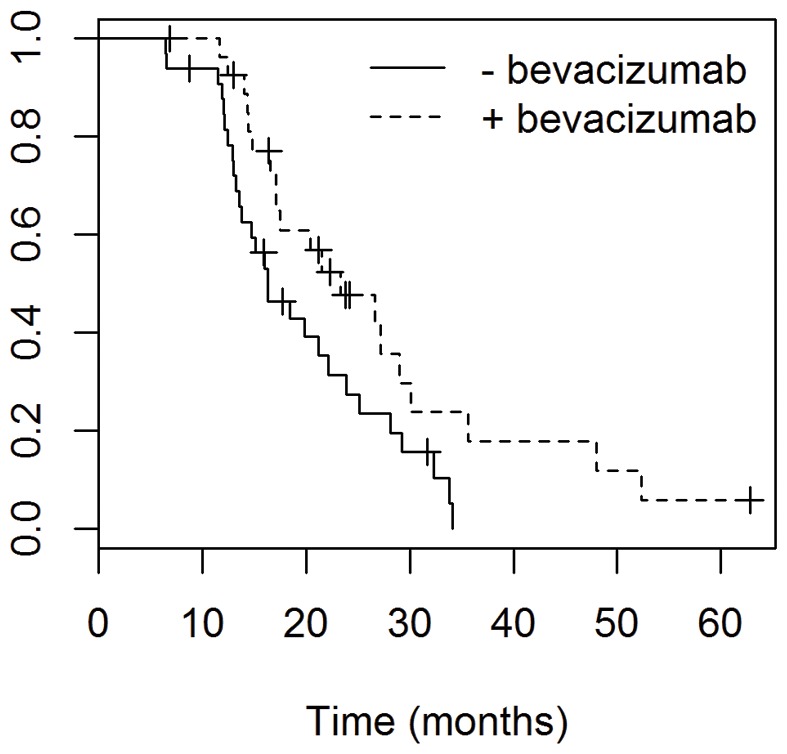
Patients receiving bevacizumab had a median OS of 23.3 months (95% CI: 17.1–35.6), compared to 16.3 months (95% CI: 13.8–25.1) in patients who did not receive it, P = 0.0284.
Table 4. Bevacizumab Usage at Recurrence.
| Subcategory | Yes | No | HR | SE | p-value | |
| Number | 28 | 34 | 0.90 | 0.44 | 0.04 | |
| Age | ||||||
| <50 | 4(14%) | 7(21%) | ||||
| >50 | 24(86%) | 27(79%) | 0.66 | 0.44 | NS | |
| Median | 59 | 57 | ||||
| Resection Status | ||||||
| GTR | 9(32%) | 16(47%) | 0.85 | 0.68 | NS | |
| STR | 13(46%) | 16(47%) | 0.57 | 0.67 | NS | |
| Biopsy | 6(21%) | 2(6%) | ||||
| Location | ||||||
| Non-periventricular | 18(67%) | 24(73%) | ||||
| Periventricular | 9(33%) | 9(27%) | 0.011 | 0.004 | 0.001 |
Abbreviations: GTR, gross total resection; STR, sub-total resection.
Bevacizumab/Tumor size
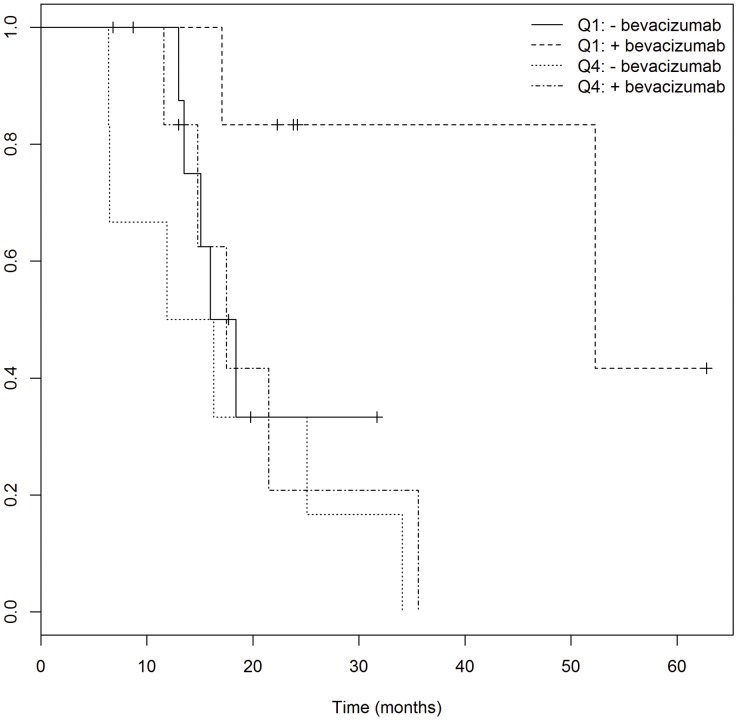
Moreover, we analyzed the survival outcomes of the patients with the smallest tumors versus those with the largest tumors, and stratified both groups by whether they had received bevacizumab at first recurrence or not (Fig. 4). The survival benefit of bevacizumab was only evident in the group of patients with the smallest tumors (lowest 25th percentile of GTV1 and PTV1). The median OS for the group of patients with the smallest GTV1 tumors who also received bevacizumab was 52.3 months, compared to 17.2 months for patients with the smallest tumors without bevacizumab, 17.5 months for patients with the largest tumors receiving bevacizumab, and 14.1 months for patients with the largest tumors not receiving bevacizumab. This survival benefit remained evident when examining PTV1 volumes: the median OS of patients with the smallest PTV1s who received bevacizumab had not yet reached the median at a follow-up of 26.3 months, whereas the OS for patients with small tumors not receiving bevacizumab, those with large tumors receiving bevacizumab, and those with large tumors not receiving bevacizumab was 18.4, 17.5, and 13.2, respectively.
Figure 4. The median OS for the group of patients with the smallest GTV1 tumors who also received bevacizumab (Q1:+bevacizumab) was 52.3 months, compared to 17.2 months for patients with the smallest tumors without bevacizumab (Q1: −bevacizumab), 17.5 months for patients with the largest tumors receiving bevacizumab (Q4: +bevacizumab), and 14.1 months for patients with the largest tumors not receiving bevacizumab (Q4: −bevacizumab).
Discussion
Herein, we report on a single institution's experience treating 92 consecutive patients with GBM, the majority treated with concurrent radiotherapy and temozolomide, followed by adjuvant temozolomide. Our median PFS and OS of 7.6 and 17.9 months, respectively, are comparable to the EORTC trial results showing a median PFS and OS of 6.9 and 14.6 months, respectively, among patients treated with concurrent and adjuvant TMZ(2). These results validate the efficacy of the EORTC regimen when implemented in routine clinical practice. Two international single center experiences have reported OS of 16.4 and 18.3 months, which are also consistent with our findings [11], [12].
There has been a trend toward improvement in OS among more contemporary GBM studies compared to those treated in the EORTC trial (13–14). Patients from the recent New Approaches to Brain Tumor Therapy (NABTT) single-agent phase II trials, in which all patients received standard RT+TMZ therapy, showed a median OS of 19.6 months [13]. Similarly, another recent phase II trial used its own historical institutional control cohort, which had a median OS of 21.1 months [14]. However, the majority (89%) of those patients received bevacizumab at tumor recurrence, which may have an effect on overall survival. These improvements in survival may be attributed to selection bias in recruitment for phase II trials, or may reflect an improvement in the care of patients with GBM perhaps mirroring the increasing experience using TMZ and a more meticulous monitoring of tumor progression. It is important to consider these improvements in survival when comparing outcomes of phase II studies to historical controls.
It remains obvious that not all patients with GBM have the same prognosis, and that there is a heterogeneous population with varying outcomes. The RTOG-RPA, which was published before the incorporation of TMZ into treatment, reported that certain prognostic factors (age, performance status, tumor histopathology) were stronger prognostic factors than modifications in therapy [15]. The recent validation of these prognostic factors in patient populations treated with TMZ and advanced therapies supports their continued importance relative to changes in therapy [4], [16], [17].
In our study, we report the correlation between volumetric tumor size and progression-free survival and overall survival. The volumetric parameters we examined were the gross tumor volumes using T2 weighted MRI and the initial planning target volume. Our results show that a larger pre-operative, pre-treatment gross tumor volume was associated with a reduction in both PFS and OS, when compared to smaller tumor volume. Importantly, there were an equal percentage of patients that had only a biopsy in each group. Several recent studies have reported an association between GBM tumor size and survival [10], [18]–[20]. One recent study showed a negative impact of pre-operative enhancing tumor, pre-operative necrosis volume, and residual non-enhancing volume prior to radiochemotherapy on outcome [10]. However, they found no association with the preoperative T2 abnormality. A second study concluded that there was no association between pre-operative absolute anatomic lesion volumes and survival, but noted that patients with a large percentage of the T2 lesion containing enhancement and necrosis had a decreased survival [20]. However, when analyzing post-surgery, pre-radiochemotherapy tumor volumes, the same group reported an effect on survival of increasing volumes of all anatomic lesions including T2 and contrast-enhancing lesions [19]. Other studies have reported varying results on whether tumor volume is associated with survival, but were limited by either small sample size or lack of up to date TMZ chemotherapy [21]–[27]. Thus, our data is consistent with previously published data reporting volumetric tumor size as a prognostic marker. In contrast to other studies showing no relationship between T2 abnormality and survival [17], [18], [20], we report that this volume may have prognostic significance, underscoring the importance of an accurate assessment of tumor burden including the most distal tumor cells and edema evident from T2 images. Our data suggest that in the concurrent TMZ era, preoperative tumor volumes on T2 MRI are prognostic of PFS and OS.
A second prognostic factor in our study was the location of the tumor (periventricular vs. non-periventricular). Recent data suggest a role of the cells of the subventricular zone (SVZ) in the GBM stem cell theory. Our study suggests that tumors in contact with the SVZ (periventricular tumors) have a shorter PFS than those patients with non-periventricular tumors, although there was no significant difference in OS. Importantly, there were more patients in the periventricular group that had only a biopsy as their surgical procedure. Two other retrospective studies support an association with tumors involving the SVZ and a decreased survival [28], [29]. However, in the first study, only 58% of patients were evaluated after undergoing a primary resection, and only 27% of patients received TMZ therapy [29]. The second study only evaluated 39 total patients, none of whom received a GTR [28]. Additional studies concluded that patients with tumors adjacent to the SVZ were more likely to be multifocal at diagnosis and to have noncontiguous tumor recurrences [30]. Moreover, those with subependymal spread had decreased survival [31]. Although it remains unknown what underlying biology distinguishes periventricular tumors, our study and others lend support that periventricular tumors may be associated with a decrease in survival even when controlling for extent of resection and use of temozolomide.
Our study also examined the relationship between bevacizumab therapy at tumor recurrence and initial tumor size, showing that among patients receiving bevacizumab, only those with the smaller GTV1 tumors had an increased overall survival of 52.3 months, compared to 17.5 months in those with larger GTV1 tumors (which was comparable to the OS of patients who did not receive bevacizumab at first recurrence). This suggests that bevacizumab might be more effective in smaller tumors, and perhaps the anti-angiogenic effects are less successful once the tumor has reached a certain size. In contrast to our results, one study examined 16 patients with recurrent GBM and noted that hyperperfusion volume was correlated with time to progression, but found no effect of tumor volume at recurrence [32]. Other studies have reported the use of advanced, non-conventional MRI techniques to report response to bevacizumab in recurrent GBM [33]–[35]. One study examining patients with recurrent GBM found that contrast-enhancing volume seen on MRIs taken before bevacizumab initiation were associated with improved PFS but not OS [36]. They reported that a pretreatment ratio of FLAIR to contrast-enhancing volume was associated with PFS and OS [36]. Our study suggests that the tumor volume seen on T2 may be associated with survival. Further studies are needed to clarify whether tumor size can be used as an indicator of potential response to bevacizumab, and whether this can be used to guide treatment decisions.
In conclusion, our data supports previous evidence that preoperative tumor size and tumor location may have prognostic value. Furthermore in our cohort of patients smaller preoperative tumor size is predictive for improved OS when treated with bevacizumab. These findings support current translational research exploring the heterogeneous biology of GBMs and its impact on treatment outcomes.
Funding Statement
This work was supported by the intramural research program of the National Cancer Institute, NIH. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.American Cancer Society (2009) Cancer Facts & Figures. Atlanta: American Cancer Society.
- 2. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, et al. (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10): 987–996. [DOI] [PubMed] [Google Scholar]
- 3. Stewart LA (2002) Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet 359(9311): 1011–1018. [DOI] [PubMed] [Google Scholar]
- 4. Mirimanoff RO, Gorlia T, Mason W, Van den Bent MJ, Kortmann RD, et al. (2006) Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol 24(16): 2563–2569. [DOI] [PubMed] [Google Scholar]
- 5. Milano MT, Okunieff P, Donatello RS, Mohile NA, Sul J, et al. (2010) Patterns and timing of recurrence after temozolomide-based chemoradiation for glioblastoma. Int J Radiat Oncol Biol Phys 78(4): 1147–1155. [DOI] [PubMed] [Google Scholar]
- 6. Tsien CI, Brown D, Normolle D, Schipper M, Piert M, et al. (2012) Concurrent temozolomide and dose-escalated intensity-modulated radiation therapy in newly diagnosed glioblastoma. Clin Cancer Res 18(1): 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stall B, Zach L, Ning H, Ondos J, Arora B, et al. (2010) Comparison of T2 and FLAIR imaging for target delineation in high grade gliomas. Radiat Oncol 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8(7): 1277–1280. [DOI] [PubMed] [Google Scholar]
- 9. Oppitz U, Maessen D, Zunterer H, Richter S, Flentje M (1999) 3D-recurrence-patterns of glioblastomas after CT-planned postoperative irradiation. Radiother Oncol 53(1): 53–57. [DOI] [PubMed] [Google Scholar]
- 10. Iliadis G, Kotoula V, Chatzisotiriou A, Televantou D, Eleftheraki AG, et al. (2012) Volumetric and MGMT parameters in glioblastoma patients: survival analysis. BMC Cancer 12: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jalali R, Basu A, Gupta T, Munshi A, Menon H, et al. (2007) Encouraging experience of concomitant Temozolomide with radiotherapy followed by adjuvant Temozolomide in newly diagnosed glioblastoma multiforme: single institution experience. Br J Neurosurg 21(6): 583–587. [DOI] [PubMed] [Google Scholar]
- 12. Jeon HJ, Kong DS, Park KB, Lee JI, Park K, et al. (2009) Clinical outcome of concomitant chemoradiotherapy followed by adjuvant temozolomide therapy for glioblastaomas: single-center experience. Clin Neurol Neurosurg 111(8): 679–682. [DOI] [PubMed] [Google Scholar]
- 13. Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, et al. (2010) Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res 16(8): 2443–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lai A, Tran A, Nghiemphu PL, Pope WB, Solis OE, et al. (2011) Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol 29(2): 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Curran WJ Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, et al. (1993) Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst 85(9): 704–710. [DOI] [PubMed] [Google Scholar]
- 16. Paravati AJ, Heron DE, Landsittel D, Flickinger JC, Mintz A, et al. (2011) Radiotherapy and temozolomide for newly diagnosed glioblastoma and anaplastic astrocytoma: validation of Radiation Therapy Oncology Group-Recursive Partitioning Analysis in the IMRT and temozolomide era. J Neurooncol 104(1): 339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li J, Wang M, Won M, Shaw EG, Coughlin C, et al. (2011) Validation and simplification of the Radiation Therapy Oncology Group recursive partitioning analysis classification for glioblastoma. International journal of radiation oncology, biology, physics 81(3): 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramakrishna R, Barber J, Kennedy G, Rizvi A, Goodkin R, et al.. (2010) Imaging features of invasion and preoperative and postoperative tumor burden in previously untreated glioblastoma: Correlation with survival. Surg Neurol Int 1. [DOI] [PMC free article] [PubMed]
- 19. Saraswathy S, Crawford FW, Lamborn KR, Pirzkall A, Chang S, et al. (2009) Evaluation of MR markers that predict survival in patients with newly diagnosed GBM prior to adjuvant therapy. J Neurooncol 91(1): 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crawford FW, Khayal IS, McGue C, Saraswathy S, Pirzkall A, et al. (2009) Relationship of pre-surgery metabolic and physiological MR imaging parameters to survival for patients with untreated GBM. J Neuroonco 91(3): 337–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tralins KS, Douglas JG, Stelzer KJ, Mankoff DA, Silbergeld DL, et al. (2002) Volumetric analysis of 18F-FDG PET in glioblastoma multiforme: prognostic information and possible role in definition of target volumes in radiation dose escalation. J Nucl Med 43(12): 1667–1673. [PubMed] [Google Scholar]
- 22. Keles GE, Lamborn KR, Chang SM, Prados MD, Berger MS (2004) Volume of residual disease as a predictor of outcome in adult patients with recurrent supratentorial glioblastomas multiforme who are undergoing chemotherapy. J Neurosurg 100(1): 41–46. [DOI] [PubMed] [Google Scholar]
- 23. Cao Y, Nagesh V, Hamstra D, Tsien CI, Ross BD, et al. (2006) The extent and severity of vascular leakage as evidence of tumor aggressiveness in high-grade gliomas. Cancer Res 66(17): 8912–8917. [DOI] [PubMed] [Google Scholar]
- 24. Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, et al. (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95(2): 190–198. [DOI] [PubMed] [Google Scholar]
- 25. Wang YY, Yang GK, Li SY, Baol XF, Wu CY (2004) Prognostic factors for deep situated malignant gliomas treated with linac radiosurgery. Chin Med Sci J 19(2): 105–110. [PubMed] [Google Scholar]
- 26.Kowalczuk A, Macdonald RL, Amidei C, Dohrmann G, 3rd, Erickson RK, et al. (1997) Quantitative imaging study of extent of surgical resection and prognosis of malignant astrocytomas. Neurosurgery 41(5): 1028–1036; discussion 1036–1028. [DOI] [PubMed]
- 27. Hammoud MA, Sawaya R, Shi W, Thall PF, Leeds NE (1996) Prognostic significance of preoperative MRI scans in glioblastoma multiforme. J Neurooncol 27(1): 65–73. [DOI] [PubMed] [Google Scholar]
- 28. Young GS, Macklin EA, Setayesh K, Lawson JD, Wen PY, et al. (2011) Longitudinal MRI evidence for decreased survival among periventricular glioblastoma. J Neurooncol 104(1): 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chaichana KL, McGirt MJ, Frazier J, Attenello F, Guerrero-Cazares H, et al. (2008) Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J Neurooncol 89(2): 219–224. [DOI] [PubMed] [Google Scholar]
- 30. Lim DA, Cha S, Mayo MC, Chen MH, Keles E, et al. (2007) Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol 9(4): 424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parsa AT, Wachhorst S, Lamborn KR, Prados MD, McDermott MW, et al. (2005) Prognostic significance of intracranial dissemination of glioblastoma multiforme in adults. J Neurosurg 102(4): 622–628. [DOI] [PubMed] [Google Scholar]
- 32. Sawlani RN, Raizer J, Horowitz SW, Shin W, Grimm SA, et al. (2010) Glioblastoma: a method for predicting response to antiangiogenic chemotherapy by using MR perfusion imaging–pilot study. Radiology 255(2): 622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pope WB, Kim HJ, Huo J, Alger J, Brown MS, et al. (2009) Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology 252(1): 182–189. [DOI] [PubMed] [Google Scholar]
- 34. Ellingson BM, Malkin MG, Rand SD, LaViolette PS, Connelly JM, et al. (2011) Volumetric analysis of functional diffusion maps is a predictive imaging biomarker for cytotoxic and anti-angiogenic treatments in malignant gliomas. J Neurooncol 102(1): 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen W, Delaloye S, Silverman DH, Geist C, Czernin J, et al. (2007) Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a pilot study. J Clin Oncol 25(30): 4714–4721. [DOI] [PubMed] [Google Scholar]
- 36. Ellingson BM, Cloughesy TF, Lai A, Nghiemphu PL, Mischel PS, et al. (2011) Quantitative volumetric analysis of conventional MRI response in recurrent glioblastoma treated with bevacizumab. Neuro Oncol 13(4): 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]