Abstract
Neurotransmitter release depends critically on Munc18-1, Munc13, the Ca2+ sensor synaptotagmin-1 and the SNAREs syntaxin-1, synaptobrevin and SNAP-25. In-vitro reconstitutions have shown that syntaxin-1-SNAP-25-liposomes fuse efficiently with synaptobrevin-liposomes in the presence of synaptotagmin-1-Ca2+, but neurotransmitter release also requires Munc18-1 and Munc13 in vivo. Here we found that Munc18-1 could displace SNAP-25 from syntaxin-1 and that fusion of syntaxin-1-Munc18-1-liposomes with synaptobrevin-liposomes required Munc13, in addition to SNAP-25 and synaptotagmin-1-Ca2+. Moreover, when starting with syntaxin-1-SNAP-25 liposomes, NSF-α-SNAP disassembled the syntaxin-1-SNAP-25 heterodimers and abrogated fusion, which then required Munc18-1 and Munc13. We propose that fusion does not proceed through syntaxin-1-SNAP-25 heterodimers, but starts with the syntaxin-1-Munc18-1 complex; Munc18-1 and Munc13 then orchestrate membrane fusion together with the SNAREs and synaptotagmin-1-Ca2+ in an NSF- and SNAP-resistant manner.
Neurotransmitter release by Ca2+-triggered synaptic vesicle fusion is crucial for neural function. Key components of the release machinery include (1, 2): i) the SNAP receptor (SNARE) proteins synaptobrevin, syntaxin-1 and SNAP-25, which form a tight four-helix bundle called SNARE complex (3) that brings the vesicle and plasma membranes together and is key for membrane fusion (4–6); ii) N-ethylmaleimide sensitive factor (NSF) and soluble NSF adaptor proteins (SNAPs), which disassemble the SNARE complex (3) to recycle the SNAREs for another round of fusion (7); iii) the Sec1-Munc18 (SM) protein Munc18-1, which binds to a self-inhibited ‘closed’ conformation of syntaxin-1 (8, 9) and to SNARE complexes (10, 11); iv) Munc13, which contains a large MUN domain that is critical for release (12) and catalyzes opening of syntaxin-1 (13); and v) the Ca2+ sensor synaptotagmin-1 (14). Despite important advances, a coherent model that integrates the functions of these eight central proteins has not emerged, and it is unclear why neurotransmitter release is totally abrogated in the absence of Munc18-1 and Munc13 (15–17). Munc18-1 is part of the conserved core fusion machinery (1, 2), and the Munc13 MUN domain is also likely to have a universal function in fusion (18, 19). However, reconstitutions of synaptic vesicle fusion have not explained the functional importance of Munc18-1 and Munc13. The ability of the neuronal SNAREs to induce lipid mixing between liposomes (20) can be enhanced by Munc18-1 (11), but without requiring Munc13. Similarly, Munc13-4 can enhance lipid mixing by the SNAREs (21) in the absence of Munc18-1. Moreover, these studies did not include synaptotagmin-1, which induces efficient fusion of liposomes containing syntaxin-1-SNAP-25 heterodimers with synaptobrevin-liposomes in the absence of Munc18-1 and Munc13 (22–26), despite the essential nature of Munc18-1 and Munc13 for release in vivo.
To resolve this gap between the reconstitution results and the physiological data, we performed reconstitution experiments that included the eight key components of the release machinery and were able to reproduce the functional requirement for Munc18-1 and Munc13.
Munc18-1 displaces SNAP-25 from syntaxin-1
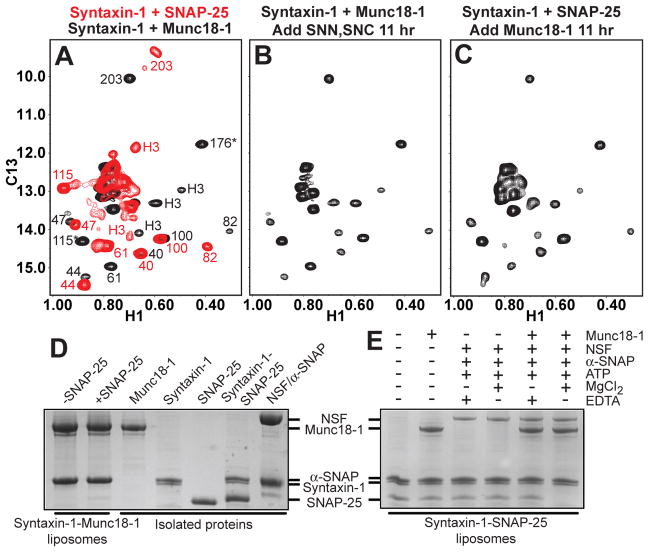
Most attempts to reconstitute synaptic fusion have used liposomes containing syntaxin-1-SNAP-25 heterodimers, assuming that these heterodimers constitute obligatory intermediates and serve as acceptors for synaptobrevin. The syntaxin-1-Munc18-1 complex is commonly assumed to form upstream. To test if syntaxin-1-SNAP-25 heterodimers can form when starting with the syntaxin-1-Munc18-1 complex, we used 1H-13C heteronuclear multiple quantum coherence (HMQC) NMR spectra of the syntaxin-1 cytoplasmic region specifically 1H,13C-labeled at Ile methyl groups (2H-Ile-13CH3-syntaxin-1) (Fig. 1A), which allow distinguishing different complexes of syntaxin-1 (13). The HMQC spectrum of the 2H-Ile-13CH3-syntaxin-1-Munc18-1 complex was not altered by addition of the SNARE motifs of SNAP-25 (Fig. 1B) even in the presence of the Munc13-1 MUN domain (Fig. S1A), which facilitates the transition from the syntaxin-1-Munc18-1 complex to the SNARE complex (13). Conversely, incubation of the 2H-Ile-13CH3-syntaxin-1-SNAP-25 complex with Munc18-1 led to the HMQC spectrum characteristic of the 2H-Ile-13CH3-syntaxin-1-Munc18-1 complex (Fig. 1C). Thus, SNAP-25 cannot open the conformation of syntaxin-1 bound to Munc18-1 without synaptobrevin, and Munc18-1 displaces SNAP-25 from syntaxin-1 in solution. The MUN domain accelerated this displacement (Fig. S1B), showing that Munc13-1 catalyzes not only the opening (13) but also the closing of syntaxin-1.
Figure 1.
Munc18-1 displaces SNAP-25 from syntaxin-1. (A-C) 1H-13C HMQC spectra of 2H-Ile-13CH3-syntaxin-1 bound to Munc18-1 (black) or to SNAP-25 (red) (A); initially bound to Munc18-1 and then incubated with the SNAP-25 SNARE motifs (SNN and SNC) for 11 hr (B); and initially bound to SNAP-25 and then incubated with Munc18-1 for 11 hr. In panel A, the available cross-peak assignments for the syntaxin-1-Munc18-1 complex are indicated in black and those for the syntaxin-1-SNAP-25 complex in red [for assignments, see ref. (13); H3 identifies the SNARE motif; * indicates tentative assignments]. Because of the 2:1 stoichiometry of the syntaxin-1-SNAP-25 complex, the cross-peak of I203 is double. (D) Proteoliposomes containing co-expressed syntaxin-1-Munc18-1 complex were incubated with SNAP-25 or buffer, co-floatation assays were performed, and the top fraction was analyzed by SDS-PAGE and Coomassie Blue staining (left two lanes). (E) Proteoliposomes containing syntaxin-1 were incubated with Munc18-1, SNAP-25, NSF, α-SNAP, ATP, Mg2+ and/or EDTA as indicated. Co-floatation assays were then performed and the results analyzed by SDS-PAGE and Coomassie Blue staining. The five lanes on the right of panel D show loading controls with soluble proteins.
To analyze the interplay between Munc18-1 and SNAP-25 using full-length syntaxin-1 in a membrane environment, we performed co-floatation assays with liposomes containing co-expressed syntaxin-1-Munc18-1 complex (27), which revealed that SNAP-25 does not displace Munc18-1 from membrane-anchored syntaxin-1 (Fig. 1D). In assays with liposomes containing only syntaxin-1, we observed co-floatation of SNAP-25, revealing efficient binding to syntaxin-1 (Figs. 1E and S2). Munc18-1 addition led to co-floatation with the proteoliposomes and only a small decrease in bound SNAP-25 (Fig. 1E), suggesting that most Munc18-1 bound to the syntaxin-1 N-terminal region of the syntaxin-1-SNAP-25 heterodimers (10, 11) without displacing SNAP-25. However, such displacement might have been hindered by the tendency of aggregation of syntaxin-1-SNAP-25 heterodimers, as suggested by gel filtration in detergent (Fig. S3). Because NSF-α-SNAP disassemble reconstituted syntaxin-1-SNAP-25 heterodimers (28), we tested their effect in the co-floatation assays. Addition of Munc18-1, NSF-α-SNAP and Mg2+-ATP completely released SNAP-25 from the proteoliposomes while Munc18-1 remained bound, but such release was not observed in the presence of ethylenediaminetetraacetic acid (EDTA) and/or absence of Munc18-1 (Fig. 1E). Thus, disassembly of the syntaxin-1-SNAP-25 heterodimers by NSF-α-SNAP, which requires ATP hydrolysis, allows Munc18-1 to ‘capture’ the syntaxin-1 closed conformation, thus preventing re-binding of SNAP-25 to syntaxin-1.
Reconstitution of Munc18-1- and Munc13-dependent membrane fusion
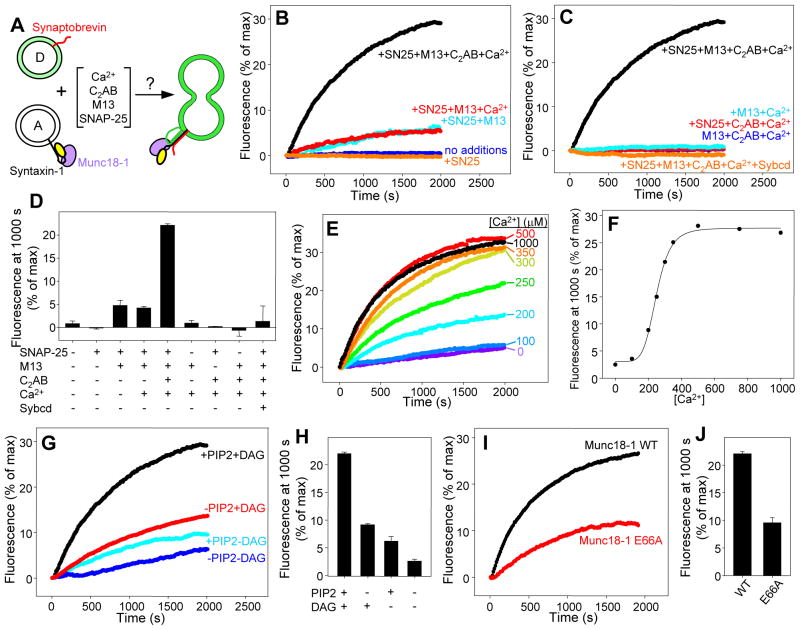
The above results suggest that the syntaxin-1-Munc18-1 complex is the true starting point for neurotransmitter release, which would explain the critical importance of Munc13 because it mediates syntaxin-1 opening (13). To test this notion, we reconstituted syntaxin-1-Munc18-1 complex and synaptobrevin into separate liposome populations, and analyzed lipid mixing between them using a 7-nitrobenz-2-oxa-1,3-diazole (NBD) fluorescence de-quenching assay (20) (Fig. 2A). The fluorescence intensity after 1000 s was used to quantify the results as a compromise measurement that reflects the initial slope and the level of completion of the reaction. To test the effects of synaptotagmin-1 and Munc13, we employed a synaptotagmin-1 fragment that contains its C2 domains (C2AB fragment) and has been widely used in reconstitution assays [e.g. (22, 24)], and a Munc13-1 fragment that contains its C1, C2B and MUN domains (C1C2BMUN). This fragment was more stable than the MUN domain in the presence of reconstituted proteoliposomes, and inclusion of the C1 and C2B domains enabled binding to diacylglycerol (DAG) and phosphatidylinositol–4,5-bisphosphate (PIP2) (Fig. S4).
Figure 2.
Requirement of Munc13 for lipid mixing of syntaxin-1-Munc18-1-liposomes with synaptobrevin-liposomes. (A) Diagram summarizing the lipid mixing experiments, which were performed with acceptor liposomes containing syntaxin-1-Munc18-1 and donor liposomes containing synaptobrevin and NBD-lipids quenched by rhodamine-lipids. (B,C) Traces showing the lipid mixing observed in the presence of SNAP-25, Munc13-1 C1C2BMUN (M13), synaptotagmin-1 C2AB fragment (C2AB), synaptobrevin cytoplasmic domain (Sybcd) and/or 0.5 mM Ca2+ in various combinations. The y axis represents NBD fluorescence normalized to the maximum fluorescence observed upon detergent addition. (D) Quantification of the results obtained in the experiments of (B,C). (E) Lipid mixing observed in the presence of SNAP-25, C1C2BMUN and C2AB fragment as a function of Ca2+ concentration. (F) Plot of the normalized NBD fluorescence intensity at 1000 s as a function of Ca2+ observed in (E). (G,H) Dependence of lipid mixing on the presence of DAG and/or PIP2 in the acceptor syntaxin-1-Munc18-1 liposomes (G), and quantification of the results (H). (I,J) Lipid mixing obtained with acceptor liposomes that contained syntaxin-1 bound to WT or E66A mutant Munc18-1 (I) and quantification of the results (J). In (D, H and J), bars represent averages of the normalized NBD fluorescence observed after 1000 s in repeated experiments performed under the same conditions. Error bars represent standard deviations.
No lipid mixing between synaptobrevin-liposomes and syntaxin-1-Munc18-1-liposomes was observed even after adding SNAP-25, but addition of SNAP-25 and C1C2BMUN yielded some lipid mixing that was increased by the C2AB fragment in the presence of Ca2+ (Fig. 2B,D). No lipid mixing occured in the absence of SNAP-25 or C1C2BMUN, and the synaptobrevin cytoplasmic domain inhibited the reaction, demonstrating that lipid mixing was SNARE-dependent and strictly required the Munc13-1 C1C2BMUN fragment (Figs. 2C,D). The efficiency of lipid mixing had a high cooperativity with Ca2+ (Fig. 2E,F), with a Hill coefficient of 5.9 that resembles values observed in vivo [4 to 5, ref. (29)]. Half-maximal efficiency occurred at 250 μM Ca2+ (Fig. 2E,F). For comparison, neurotransmitter release requires 10–25 μM Ca2+ in the calyx of Held, but higher Ca2+ requirements (75–300 μM) have been reported for other systems (29), and the efficiency of lipid mixing in our reconstitutions is likely limited by a lack of a defined docking mechanism. Lipid mixing was decreased by removal of DAG and PIP2, agents that stimulate release via the Munc13 C1 and C2B domains (30, 31), and the decrease was accentuated when both DAG and PIP2 were absent (Figs. 2G,H; see also Fig. S4D), suggesting that these lipids play a synergistic role in attracting C1C2BMUN to the membrane and enhancing MUN-domain activity. To establish an additional correlation with physiological data, we tested the effects of a mutation in Munc18-1 (E66A) that decreases neurotransmitter release in neurons by about 50% (32). Reassuringly, this mutation led to a comparable decrease in lipid mixing (Figs. 2I,J; see also Fig. S4D).
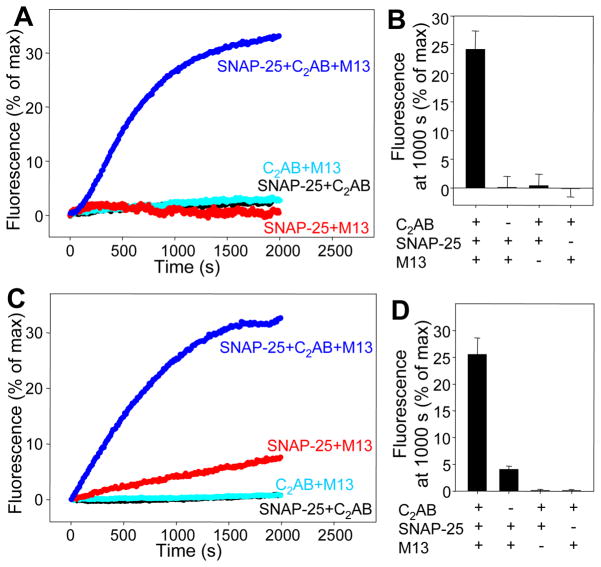
To ensure that the observed lipid mixing reflected full fusion, we analyzed content mixing adapting a method based on sulforhodamine de-quenching that allows simultaneous monitoring of lipid mixing from de-quenching of DiD lipids (26), and optimized liposome preparation for minimal leakiness (Fig. S5). When we mixed synaptobrevin-donor liposomes with acceptor liposomes containing syntaxin-1-Munc18-1 complex, efficient sulforhodamine fluorescence de-quenching required SNAP-25, C1C2BMUN and C2AB fragment-Ca2+ (Fig. 3A,B). The measured lipid mixing (Fig. 3C,D) paralleled the NBD de-quenching data (Figs. 2B-D) and were similar to the sulforhodamine de-quenching results, although in the absence of C2AB fragment there was a small amount of lipid mixing but not sulforhodamine de-quenching. The time course of the sulforhodamine fluorescence de-quenching in the presence of SNAP-25, C1C2BMUN and C2AB fragment-Ca2+ closely followed the time course of DiD fluorescence de-quenching (Fig. S6). Thus, content mixing accompanies lipid mixing in these experiments, which suggests that our system reconstitutes membrane fusion with the three neuronal SNAREs, Munc18-1 and core fragments of Munc13-1 and synaptotagmin-1. However, we cannot rule out that some degree of leakiness may occur during fusion (27).
Figure 3.
Content mixing assays with syntaxin-1-Munc18-1-liposomes and synaptobrevin-liposomes. (A) Traces showing the content mixing between syntaxin-1-Munc18-1 acceptor liposomes and synaptobrevin donor liposomes in the presence of SNAP-25, Munc13-1 C1C2BMUN (M13) and/or synaptotagmin-1 C2AB fragment (C2AB) (all in 0.5 mM Ca2+). The donor liposomes contained encapsulated self-quenched sulforhodamine and self-quenched DiD lipids. The y axis represents sulforhodamine fluorescence normalized to the maximum fluorescence observed upon detergent addition. (B) Quantification of the results obtained in the experiments of (B). (C,D) Traces showing the lipid mixing observed from DiD fluorescence de-quenching in the same experiments (C) and quantification of the results (D). Error bars represent standard deviations.
Munc18-1 and Munc13-1 activate membrane fusion inhibited by NSF-α-SNAP
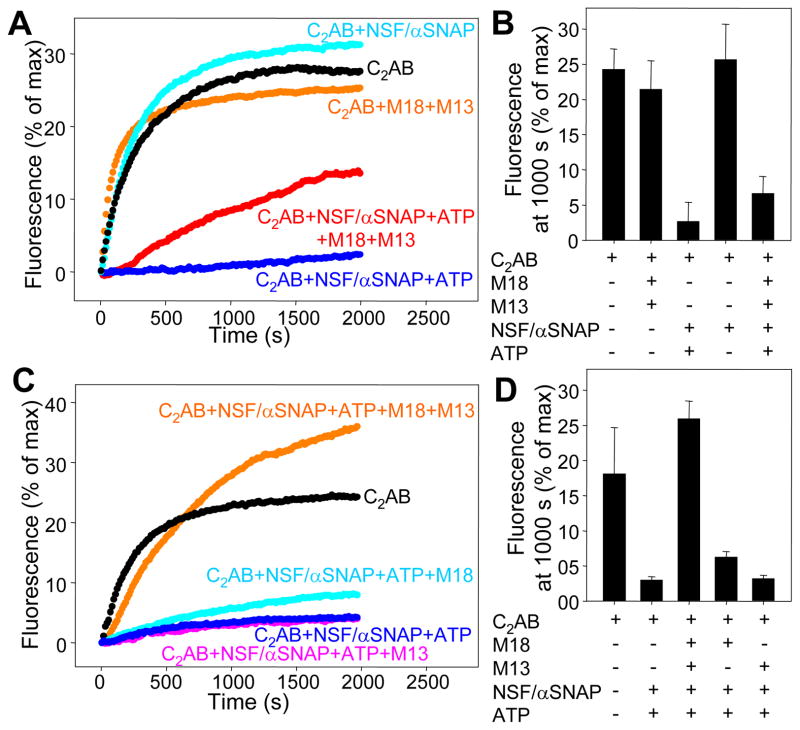
Multiple studies have shown efficient liposome fusion with SNAREs and syntaptotagmin-1 in the absence of Munc18-1 and Munc13-1 (22–26). However, these studies used liposomes containing syntaxin-1-SNAP-25 heterodimers and did not incorporate NSF-SNAPs, which inhibit lipid mixing induced by the SNAREs alone because they disassemble the syntaxin-1-SNAP-25 heterodimers (28). Because NSF-α-SNAP facilitate displacement of SNAP-25 from syntaxin-1 by Munc18-1 (Fig. 1E), we hypothesized that Munc18-1 ‘captures’ the syntaxin-1 released from the syntaxin-1-SNAP-25 heterodimers and, together with Munc13-1, leads to membrane fusion through an NSF-α-SNAP-resistant pathway.
We tested this hypothesis using lipid-mixing assays with liposomes containing syntaxin-1-SNAP-25 heterodimers (separately expressed proteins). As expected, highly efficient lipid mixing between these liposomes and synaptobrevin-liposomes was observed in the presence of C2AB fragment-Ca2+ (Fig. 4A,B). Similar amounts of lipid mixing were observed upon addition of Munc18-1, C1C2BMUN, or NSF-α-SNAP in the absence of ATP, but lipid mixing was almost completely abolished by NSF-α-SNAP in the presence of ATP (Fig. 4A,B). Some lipid mixing was observed when Munc18-1 and C1C2BMUN were added together with NSF-α-SNAP and ATP (Fig. 4A,B). We reasoned that an excess of SNAP-25 might assist in lipid mixing-activation by Munc18-1 and C1C2BMUN, because free SNAP-25 should favor SNARE complex formation without affecting the rate of syntaxin-1-SNAP-25 dissociation by NSF-α-SNAP. Indeed, lipid mixing was enhanced by increasing SNAP-25 concentrations, saturating at 2 μM (Fig. S7). The 2 μM excess SNAP-25 did not affect the overall lipid mixing observed upon addition of only C2AB fragment-Ca2+ (Figs. 4A,C, black symbols), and NSF-α-SNAP still inhibited lipid mixing strongly in the presence of excess SNAP-25 and the absence of Munc18-1 and C1C2BMUN (Figs. 4C,D). Importantly, with 2 μM SNAP-25 excess, Munc18-1 and C1C2BMUN stimulated lipid mixing to levels comparable to those observed without NSF-α-SNAP (Fig. 4C,D); Munc18-1 alone (but not C12C2BMUN alone) appeared to provide a small amount of activation (Fig. 4C,D). Activation of lipid mixing by Munc18-1 and C1C2BMUN in NSF-α-SNAP-resistant manner was also observed in the absence of the C2AB fragment (Fig. S8) or using liposomes containing co-expressed syntaxin-1-SNAP-25 heterodimers (Fig. S9). Thus, inclusion of NSF-α-SNAP is key to unmask the crucial importance of Munc18-1 and Munc13-1 for membrane fusion in these reconstituted systems.
Figure 4.
NSF-α-SNAP inhibit lipid mixing between syntaxin-1-SNAP-25-liposomes and synaptobrevin-liposomes, and Munc18-1-Munc13-1 activate lipid mixing. (A) Traces showing the lipid mixing observed between syntaxin-1-SNAP-25 acceptor liposomes and synaptobrevin donor liposomes containing NBD-lipids quenched by rhodamine-lipids in the presence of Munc18-1 (M18), Munc13-1 C1C2BMUN (M13), synaptotagmin-1 C2AB fragment (C2AB), NSF, α-SNAP and/or Mg2+-ATP in various combinations (all in 0.5 mM Ca2+). The y axis represents NBD fluorescence normalized to the maximum fluorescence observed upon detergent addition. (B) Quantification of the results obtained in the experiments of (A). (C) Lipid mixing experiments performed as in (A) with various additions, all in the presence of 2 μM SNAP-25 excess. (D) Quantification of the results of panel (C). Error bars represent standard deviations.
Discussion
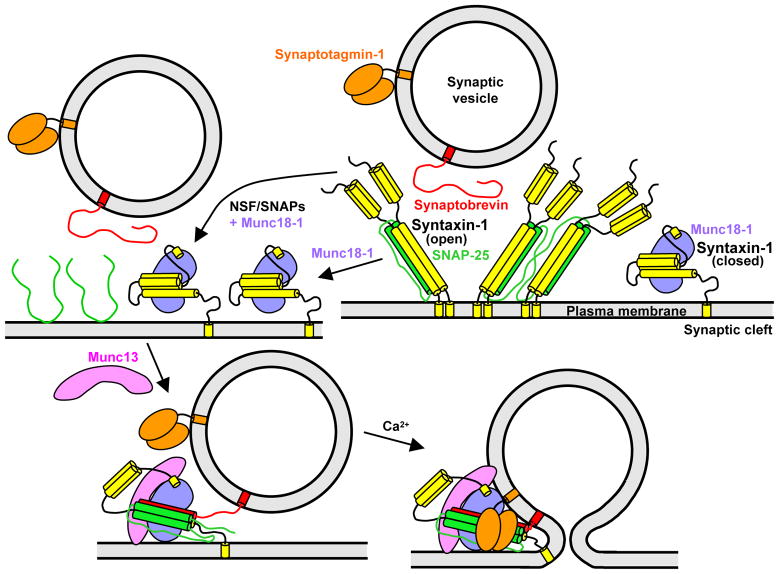
Our results lead us to propose a model of neurotransmitter release (Figs. 5 and S10) that explains the essential roles of Munc18-1 and Munc13 in vivo, and postulates that: (i) Syntaxin-1-SNAP-25 heterodimers constitute ‘off-pathway’ complexes that are disrupted by Munc18-1 and by NSF-SNAPs. (ii) The productive pathway for synaptic vesicle fusion starts with the syntaxin-1-Munc18-1 complex, which may form directly or by displacement of SNAP-25 from syntaxin-1 by Munc18-1, and can be aided by NSF-SNAPs and perhaps by Munc13. (iii) Munc13 opens syntaxin-1 and, together with Munc18-1, forms a template to bring the three SNAREs together, initiating trans-SNARE complex assembly in an NSF-SNAP-resistant manner. (iv) The resulting Munc18-1-Munc13-SNARE assembly underlies the primed state that enables fast membrane fusion through the action of synaptotagmin-1 and Ca2+.
Figure 5.
Model of synaptic vesicle fusion integrating the function of eight major components of the release machinery. In the upper right panel, syntaxin-1 (yellow) is shown in a closed conformation bound to Munc18-1 and in an open conformation bound to SNAP-25. To illustrate the likely heterogeneity of syntaxin-1-SNAP-25 heterodimers, two complexes with 2:1 or 4:2 stoichiometries are shown, but larger complexes bridged by SNAP-25 (not shown) are also likely to exist. The model postulates that the syntaxin-1-SNAP-25 heterodimers are converted to syntaxin-1-Munc18-1 complexes (upper left), and that Munc13 helps to open syntaxin-1 and to orchestrate trans-SNARE complex assembly together with Munc18-1, leading to a partially assembled SNARE complex that remains bound to Munc18-1 and Munc13 (lower left). This state, which may correspond to that of primed synaptic vesicles and cannot be disassembled by NSF-SNAPs, serves as the substrate for synaptotagmin-1-Ca2+ to trigger fast synaptic vesicle fusion (lower right). The arrangement of Munc18-1, Munc13 and synaptotagmin-1 with respect to the SNARE complex is unknown, but is drawn to suggest the possibility that the three proteins may bind simultaneously to the SNARE complex and may also help to bridge the two membranes to help inducing fusion. The interaction of synaptotagmin-1 with the SNARE complex may occur before Ca2+ influx (not shown in the lower left panel for simplicity).
The functional interplay between NSF-SNAPs and Munc18-1-Munc13 uncovered here suggests that, in addition to their role in disassembling cis-SNARE complexes, NSF-SNAPs have an important function in guiding the system to the productive pathway by disassembling syntaxin-1-SNAP-25 heterodimers. This feature may arise because syntaxin-1-SNAP-25 heterodimers are heterogeneous and may constitute poor starting points for an exquisitely regulated process such as neurotransmitter release. In contrast, the syntaxin-1-Munc18-1 complex provides a well-defined starting point amenable to tight regulation by several factors, including Munc13 and other active zone proteins (1). This productive pathway may also be favored by specific interactions at active zones. While some of these features are unique to synaptic vesicle fusion, it is noteworthy that multiple factors besides SNAREs are also required for physiological reconstitution of endosomal (33) and vacuolar (34) fusion. Moreover, the interplay between Munc18-1-Munc13 and NSF-α-SNAP is reminiscent of results obtained in reconstitutions of yeast vacuolar fusion, which showed that the HOPS tethering complex orchestrates trans-SNARE complex assembly in an NSF-SNAP-resistant manner (35, 36). It is remarkable that the same task can be performed by Munc18-1 and Munc13, considering that HOPS includes an SM protein (Vps33p) and five large subunits without homology to Munc13. Thus, orchestration of SNARE complex assembly without interference from NSF-SNAPs may constitute a general function of SM proteins and associated factors. This notion does not preclude other functions proposed for SM proteins and their co-factors, including the possibility that they cooperate with the SNAREs in exerting force on the membranes to induce fusion (10, 37).
Supplementary Material
Acknowledgments
We thank Yilun Sun for expert technical assistance, Ying Liu for initial efforts to reconstitute syntaxin-1-Munc18-1 complexes, and Wei Li, William Wickner and Michael Brown for fruitful discussions. This work was supported by grant I-1304 from the Welch Foundation (to JR), grant 31200618 from the National Science Foundation of China (to CM), and grants NS37200 and NS40944 from the NIH (to JR).
Footnotes
References and notes
- 1.Rizo J, Rosenmund C. Synaptic vesicle fusion. Nat Struct Mol Biol. 2008;15:665. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 4.Hanson PI, Roth R, Morisaki H, Jahn R, Heuser JE. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell. 1997;90:523. doi: 10.1016/s0092-8674(00)80512-7. [DOI] [PubMed] [Google Scholar]
- 5.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 6.Poirier MA, et al. The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat Struct Biol. 1998;5:765. doi: 10.1038/1799. [DOI] [PubMed] [Google Scholar]
- 7.Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of Sec17p (alpha-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85:83. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 8.Dulubova I, et al. A conformational switch in syntaxin during exocytosis: role of munc18. EMBO J. 1999;18:4372. doi: 10.1093/emboj/18.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Misura KM, Scheller RH, Weis WI. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature. 2000;404:355. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- 10.Dulubova I, et al. Munc18-1 binds directly to the neuronal SNARE complex. Proc Natl Acad Sci U S A. 2007;104:2697. doi: 10.1073/pnas.0611318104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell. 2007;128:183. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Basu J, et al. A minimal domain responsible for Munc13 activity. Nat Struct Mol Biol. 2005;12:1017. doi: 10.1038/nsmb1001. [DOI] [PubMed] [Google Scholar]
- 13.Ma C, Li W, Xu Y, Rizo J. Munc13 mediates the transition from the closed syntaxin-Munc18 complex to the SNARE complex. Nat Struct Mol Biol. 2011;18:542. doi: 10.1038/nsmb.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Chacon R, et al. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 15.Verhage M, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 16.Richmond JE, Davis WS, Jorgensen EM. UNC-13 is required for synaptic vesicle fusion in C. elegans. Nat Neurosci. 1999;2:959. doi: 10.1038/14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varoqueaux F, et al. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A. 2002;99:9037. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pei J, Ma C, Rizo J, Grishin NV. Remote homology between Munc13 MUN domain and vesicle tethering complexes. J Mol Biol. 2009;391:509. doi: 10.1016/j.jmb.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, et al. The Crystal Structure of a Munc13 C-terminal Module Exhibits a Remarkable Similarity to Vesicle Tethering Factors. Structure. 2011;19:1443. doi: 10.1016/j.str.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber T, et al. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 21.Boswell KL, et al. Munc13-4 reconstitutes calcium-dependent SNARE-mediated membrane fusion. J Cell Biol. 2012;197:301. doi: 10.1083/jcb.201109132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chicka MC, Hui E, Liu H, Chapman ER. Synaptotagmin arrests the SNARE complex before triggering fast, efficient membrane fusion in response to Ca2+ Nat Struct Mol Biol. 2008;15:827. doi: 10.1038/nsmb.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein A, Radhakrishnan A, Riedel D, Fasshauer D, Jahn R. Synaptotagmin activates membrane fusion through a Ca(2+)-dependent trans interaction with phospholipids. Nat Struct Mol Biol. 2007;14:904. doi: 10.1038/nsmb1305. [DOI] [PubMed] [Google Scholar]
- 24.Xue M, Ma C, Craig TK, Rosenmund C, Rizo J. The Janus-faced nature of the C(2)B domain is fundamental for synaptotagmin-1 function. Nat Struct Mol Biol. 2008;15:1160. doi: 10.1038/nsmb.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HK, et al. Dynamic Ca2+-dependent stimulation of vesicle fusion by membrane-anchored synaptotagmin 1. Science. 2010;328:760. doi: 10.1126/science.1187722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diao, et al. Synaptic proteins promote calcium-triggered fast transition from point contact to full fusion. eLife. 2012;1:e00109. doi: 10.7554/eLife.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Materials and methods are available as supplementary materials on Science online.
- 28.Weber T, et al. SNAREpins are functionally resistant to disruption by NSF and alphaSNAP. J Cell Biol. 2000;149:1063. doi: 10.1083/jcb.149.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneggenburger R, Neher E. Presynaptic calcium and control of vesicle fusion. Curr Opin Neurobiol. 2005;15:266. doi: 10.1016/j.conb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Rhee JS, et al. Beta phorbol ester- and diacylglycerol-induced augmentation of transmitter release is mediated by Munc13s and not by PKCs. Cell. 2002;108:121. doi: 10.1016/s0092-8674(01)00635-3. [DOI] [PubMed] [Google Scholar]
- 31.Shin OH, et al. Munc13 C2B domain is an activity-dependent Ca2+ regulator of synaptic exocytosis. Nat Struct Mol Biol. 2010;17:280. doi: 10.1038/nsmb.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deak F, et al. Munc18-1 binding to the neuronal SNARE complex controls synaptic vesicle priming. J Cell Biol. 2009;184:751. doi: 10.1083/jcb.200812026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohya T, et al. Reconstitution of Rab- and SNARE-dependent membrane fusion by synthetic endosomes. Nature. 2009;459:1091. doi: 10.1038/nature08107. [DOI] [PubMed] [Google Scholar]
- 34.Stroupe C, Hickey CM, Mima J, Burfeind AS, Wickner W. Minimal membrane docking requirements revealed by reconstitution of Rab GTPase-dependent membrane fusion from purified components. Proc Natl Acad Sci U S A. 2009;106:17626. doi: 10.1073/pnas.0903801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mima J, Hickey CM, Xu H, Jun Y, Wickner W. Reconstituted membrane fusion requires regulatory lipids, SNAREs and synergistic SNARE chaperones. EMBO J. 2008;27:2031. doi: 10.1038/emboj.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu H, Jun Y, Thompson J, Yates J, Wickner W. HOPS prevents the disassembly of trans-SNARE complexes by Sec17p/Sec18p during membrane fusion. EMBO J. 2010;29:1948. doi: 10.1038/emboj.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rizo J, Chen X, Arac D. Unraveling the mechanisms of synaptotagmin and SNARE function in neurotransmitter release. Trends Cell Biol. 2006;16:339. doi: 10.1016/j.tcb.2006.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.