Abstract
Among young normotensive men a reciprocal balance between cardiac output and sympathetic nerve activity is important in the regulation of arterial pressure. In young women, the balance among cardiac output, peripheral resistance and sympathetic nerve activity is unknown. Consequently, the aim of this study was to examine the relationship of cardiac output and total peripheral resistance to muscle sympathetic nerve activity in young women. Multi-unit peroneal recordings of muscle sympathetic nerve activity were obtained in 17 women (Mean ± SEM; age, 24 ± 3 yrs) and 21 men (age, 25 ± 5 yrs). Mean resting muscle sympathetic nerve activity was lower in women compared to men (19 ± 3 bursts·min−1 vs. 25 ± 1, P<0.05), as was mean arterial pressure (89 ± 1 mmHg vs. 94 ± 2, P<0.05). Mean arterial pressure was not related to muscle sympathetic nerve activity in men (P=0.80) or women (P=0.62). There was a positive relationship between total peripheral resistance and muscle sympathetic nerve activity (r=0.62, P<0.05) and an inverse relationship between cardiac output and muscle sympathetic nerve activity (r=−0.69, P<0.05) in men. Unexpectedly, muscle sympathetic nerve activity had no relationship to either total peripheral resistance (r=−0.27, P<0.05) or cardiac output (r=0.23, P<0.05) in women. Our results demonstrate that men and women rely on different integrated physiological mechanisms to maintain a normal arterial pressure despite widely varying sympathetic nerve activity amongst individuals. These findings may have important implications for understanding how hypertension and other disorders of blood pressure regulation occur in men and women.
Keywords: Sympathetic nerve activity, blood pressure regulation, sex, peripheral resistance
Introduction
Inter-individual differences in central and peripheral hemodynamics have become increasingly important to our understanding of arterial pressure regulation 1–4. In young healthy men, total peripheral resistance (TPR) is positively related to muscle sympathetic nerve activity (MSNA) suggesting that MSNA is a good index of net whole body vasoconstrictor tone. However, men with higher levels of MSNA and TPR do not necessarily have higher resting arterial pressure 5. In previous studies, young men with high MSNA had a lower cardiac output and less α-adrenergic receptor vasoconstrictor responsiveness, reducing the net effect of high MSNA on arterial pressure5.
In young women, blood pressure is typically lower than that observed in men of the same age 6,7. Furthermore, the incidence of orthostatic hypotension is greater in women than in men 8 and women have lower tonic autonomic support of baseline arterial pressure 9. Women also exhibit blunted vasoconstrictor responses to α-adrenergic stimulation 10 which may be related to the vasodilator effect of estrogen 11–16. These observations suggest that the contribution of vasoconstrictor tone to arterial pressure regulation differs in women and men. We therefore sought to compare the relationships between MSNA, cardiac output and TPR in women and men. We hypothesized that since young women have less autonomic support of blood pressure and decreased basal α-adrenergic vascular responsiveness compared to men, there would be a blunted relationship between MSNA and TPR and between MSNA and cardiac output in women compared to men.
Methods
Subjects
After the protocol for the study was approved by the Institutional Review Board of the Mayo Clinic, 21 men and 17 women gave their written informed consent to participate in this study and the study was completed in accordance with the Declaration of Helsinki. The subjects were recreationally physically active non-smokers with no history of cardiovascular or other chronic diseases. The participant demographics are outlined in Table 1.
Table 1.
Demographic variables in men (n=21) and women (n=17)
| Demographics | Men | Women |
|---|---|---|
| Age (yrs) | 25 ± 1 | 24 ± 1 |
| Body Mass (kg) | 80 ± 2 | 62 ± 2* |
| Height (cm) | 180 ± 2 | 168 ± 1* |
| BSA (m2) | 2.0 ± 0.3 | 1.7 ± 0.03* |
| BMI (kg·m−2) | 24.6 ± 0.6 | 21.9 ± 0.9* |
BSA, body surface area; BMI, body mass index. Mean ± SEM.
different from men (P<0.05)
Participants were asked to not consume anything except small volumes of water within 2 h of the experiment and were asked to abstain from caffeine or alcohol consumption 24 h before the study. To minimize the effects or reproductive hormones on autonomic control or cardiovascular function, all women were studied in the early follicular phase of the menstrual cycle or in the low hormone phase of oral contraceptive use 17.
Measurements
All studies were performed in a Clinical Research Unit laboratory at the Mayo Clinic, where ambient temperature was controlled between 22 and 24°C. Upon arrival to the laboratory, subjects rested in the supine position during instrumentation. After local anesthesia with 2% -lidocaine, a 5 cm 20 gauge arterial catheter was placed in the brachial artery of the non-dominant arm, using aseptic technique. The catheter was connected to a pressure transducer, which was positioned at the level of the heart and interfaced with a personal computer to monitor arterial pressure. A three-lead ECG was used for continuous recordings of heart rate.
Multi-unit MSNA was measured from the right peroneal nerve at the fibular head using insulated tungsten microelectrodes. A muscle sympathetic fascicle was identified when taps on the muscle belly or passive muscle stretch evoked mechanoreceptive impulses and no afferent neural response was evoked by skin stimuli 18,19. The recorded signal was amplified 80 000-fold, band pass filtered (700–2000 Hz), rectified and integrated (resistance-capacitance integrator circuit time constant 0.1 s) by a nerve traffic analyzer.
Cardiac output was measured using an open-circuit acetylene uptake technique, as previously described 20. The cardiac output was estimated immediately after each maneuver using the calculation method described by Gan et al. 21,22. This technique has been validated against direct Fick measurements of cardiac outputs over a range of values and has a variability of ∼7% at rest 20. The instrumentation period included a practice cardiac output measurement to familiarize the subject with the procedure.
Protocol
Following placement of the arterial catheter, participants were asked to rest supine during instrumentation for microneurography. Once a satisfactory site for measurement of MSNA was located, 15 min of baseline data were recorded with the subject resting quietly. Subsequently, duplicate measurements of cardiac output were obtained.
Data analysis
Data were sampled at 240 Hz and stored on a personal computer for offline analysis. Mean arterial pressure (MAP) was calculated as the time integral over the pressure pulse. Systolic and diastolic blood pressures, (SBP; DBP), MAP, heart rate and MSNA were taken as the 4 min period immediately preceding the first cardiac output measurement. Cardiac output is reported as the mean of the two measurements for each individual. Stroke volume was calculated as the cardiac output/heart rate and TPR was calculated as MAP/cardiac output.
Sympathetic bursts in the integrated neurogram were identified using a custom-manufactured automated analysis program 23; burst identification was then corrected via visual inspection by a single observer. The program then compensated for baroreflex latency and associated each sympathetic burst with the appropriate cardiac cycle.
Statistical analyses
Group data are expressed as means ± SEM. Differences between cardiovascular variables and MSNA in men and women were evaluated using a two-tailed Independent t-test. To assess the relationship between MSNA and cardiovascular variables, linear regression analysis was performed and Pearson’s correlation coefficients calculated. The critical alpha level was set at 0.05 and data was analyzed using SigmaStat software (Version 2.03, SPSS Inc, Illinois, USA).
Results
Group averaged data for cardiovascular and neural variables in men and women
The young women had a lower baseline SBP, DBP, MAP, stroke volume and cardiac output compared to men (Table 2; P<0.05), where as heart rate was similar between sexes. Women had a lower body mass and body surface area compared to men (Table 1; P<0.05). Therefore, we scaled cardiac output for body surface area, which eliminated the difference in cardiac output between men and women (Table 2; P>0.05). The absolute TPR was higher in women compared to men (P<0.05), however, when cardiac output scaled for body surface was used to calculate TPR, the difference between men and women disappeared (P<0.05). Baseline MSNA was lower in women compared to their male counterparts when MSNA was expressed as burst frequency (bursts·min−1) and incidence (bursts·100 hb−1; P<0.05).
Table 2.
Cardiovascular and neural variables in men (n=21) and women (n=17).
| Cardiovascular/neural variables | Men | Women |
|---|---|---|
| HR (beats·min−1) | 59 ± 2 | 63 ± 2 |
| SBP (mmHg) | 136 ± 3 | 126 ± 1* |
| DBP (mmHg) | 73 ± 1 | 70 ± 1* |
| MAP (mmHg) | 94 ± 2 | 89 ± 1* |
| SV (ml) | 109 ± 7 | 80 ± 4* |
| CO (l·min−1) | 6.2 ± 0.3 | 5.0 ± 0.2* |
| CO/BSA (l·min−1·m−2) | 3.2 ± 0.2 | 3.0 ± 0.1 |
| TPR (mmHg·l·min−1) | 15.8 ± 0.8 | 18.2 ± 0.8* |
| TPR/BSA (mmHg·l·min−1·m−2) | 31.4 ± 1.6 | 31.1 ± 1.6 |
| MSNA (bursts·min−1) | 25 ±1 | 19 ± 3* |
| MSNA (bursts·100hb−1) | 44 ± 2 | 32 ± 5* |
HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; SV, stroke volume; CO, cardiac output; TPR, total peripheral resistance; MSNA, muscle sympathetic nerve activity. Mean ± SEM
different from men (P<0.05).
Inter-individual relationships between cardiovascular and neural variables in men and women
Relationships between MSNA and cardiovascular variables are shown in the Figures with MSNA expressed as burst frequency. Data for burst incidence showed similar trends to burst frequency, although relationships were somewhat weaker. These data are also included in text form below.
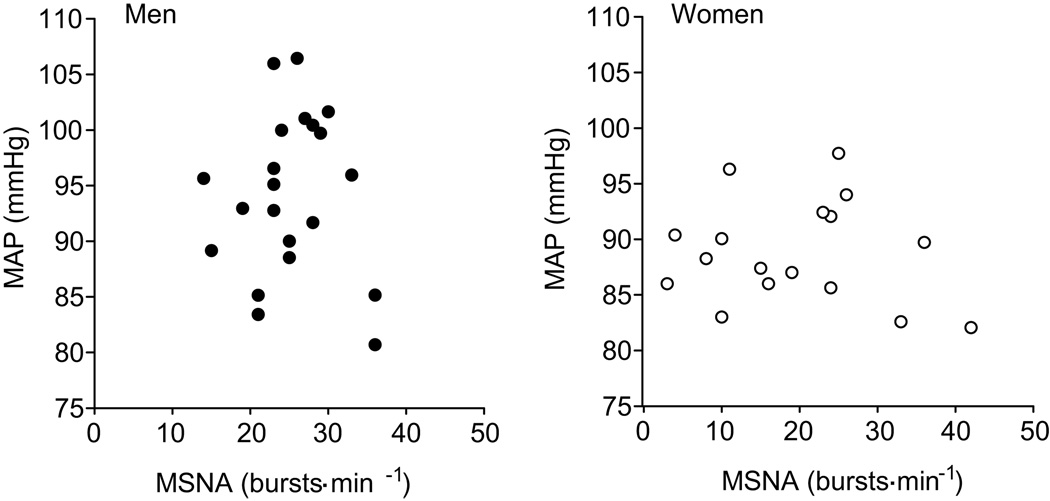
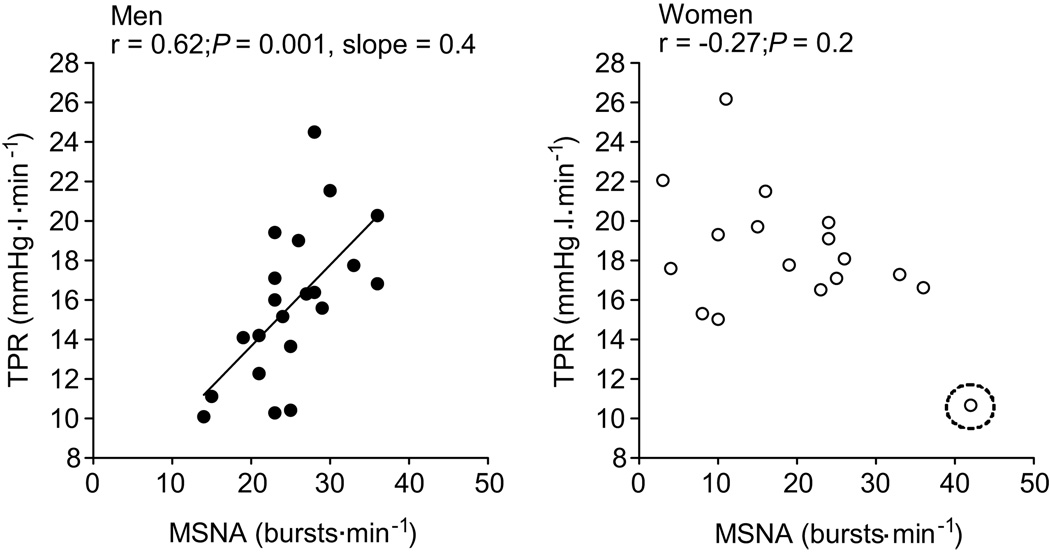
MAP did not correlate with MSNA in men or women when MSNA was expressed as burst frequency or burst incidence (Fig 1; P>0.05). As expected TPR was positively related with MSNA in men when described as burst frequency (Fig. 2) and burst incidence (r=0.58; P<0.05). The correlation between MSNA and TPR was similar when TPR normalized for body surface area (burst frequency, r = 0.55; burst incidence, r = 0.59; P<0.05 for both). In complete contrast, in women, TPR was inversely related to MSNA (r = −0.49, P<0.05) and there was a trend towards a negative relationship when MSNA was measured as burst incidence (r = −0.40, P=0.09). However, one female participant exhibited an unusually high MSNA and a low TPR (Fig 2, circled point) which appeared to drive the negative correlation. When this subject was removed from the analysis, there was no relationship between TPR and MSNA in women (burst frequency; r= −0.27, burst incidence; r = −0.17; P>0.05). Moreover, the lack of relationship between MSNA and TPR in women persisted when TPR normalized for body surface area was used in the analysis (burst frequency; r = −0.23, burst incidence; r = *−0.21; P>0.05 for both).
Figure 1.
Regression analysis of muscle sympathetic nerve activity (MSNA) with mean arterial pressure (MAP) indicates no relationship between MAP and MSNA in both men and women.
Figure 2.
Linear regression analysis of the relationship between absolute peripheral resistance (TPR) and muscle sympathetic nerve activity in men and women. The correlations indicate that in young men, when MSNA is high TPR is high, whilst the opposite is true in young women. However, when the circled female outlier is removed there is no correlation between MSNA and TPR (burst frequency; r = −0.27 and burst incidence; r = −0.17, P>0.05).
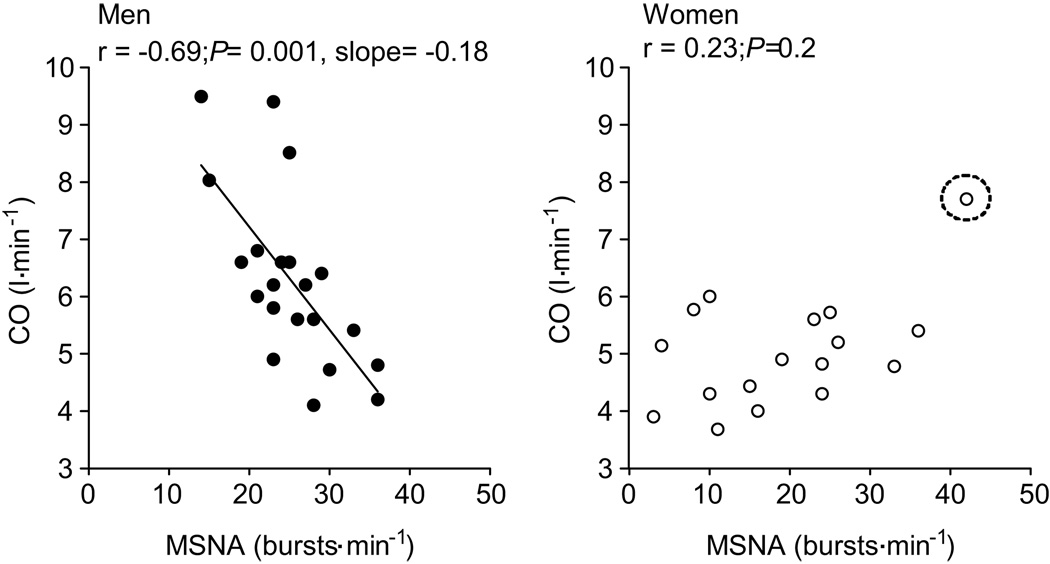
Resting cardiac output in the young men was inversely related to MSNA recorded as both burst frequency (Fig. 3) and burst incidence (r= −0.62, P<0.05). The relationship between MSNA and cardiac output in men was similar when the cardiac output normalized for body surface area (i.e., cardiac index) was used in the analysis (burst frequency; r = −0.67, burst incidence; r = −0.64, P<0.01 for both). In the young women, however, cardiac output was positively correlated with MSNA (burst frequency; r= 0.52, burst incidence; r=0.41, P=0.1). However, when the female participant with a high resting MSNA was removed from the analysis (Fig 3, circled point), there was no correlation between MSNA and cardiac output (burst frequency, r= 0.23 and burst incidence, r= 0.12; P > 0.05 for both). Additionally, the lack of relationship between MSNA and cardiac output persisted in women when cardiac output was normalized for body surface area (i.e., cardiac index) (burst frequency; r = 0.22, burst incidence; r = 0.08, P>0.05).
Figure 3.
Linear regression analysis of the relationship between cardiac output (non-normalized CO) and muscle sympathetic nerve activity (MSNA) in men and women. The relationship between CO and MSNA in women suggests that when MSNA is high CO is elevated, which is opposite to relationship reported in males. However, once the circled female outlier was removed there was no correlation between MSNA and CO in women (burst frequency; r = 0.23 and burst incidence; r = 0.12, P<0.05).
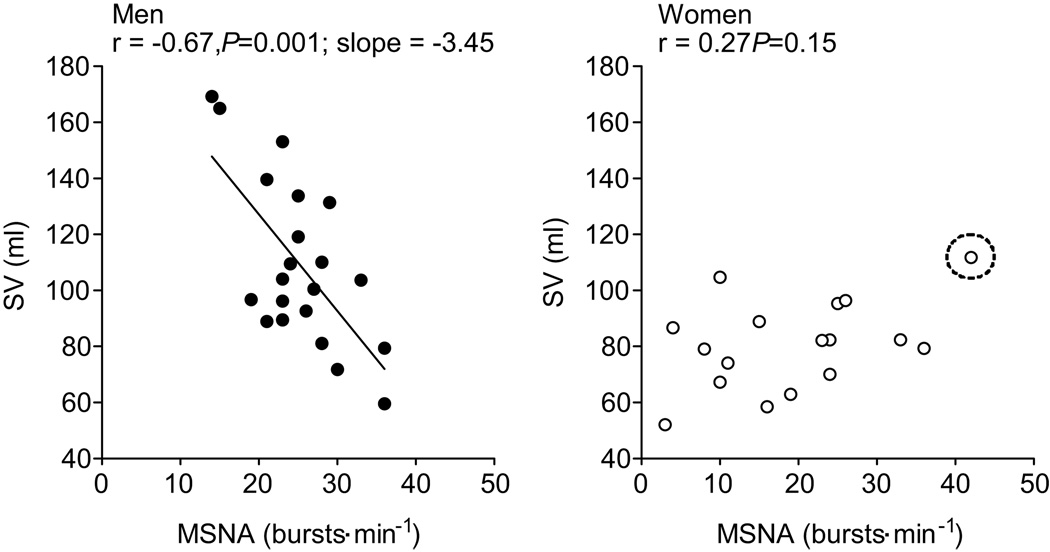
To explore the relationship between MSNA and cardiac output we examined whether MSNA was related to stroke volume and/or heart rate. Stroke volume was inversely correlated with MSNA expressed as bursts frequency in men (Fig. 4), where as when MSNA was expressed as burst incidence only a trend towards a positive relationship existed (r = −0.39). In women (n=17), there was a trend towards a negative correlation between stroke volume and MSNA (burst frequency; r= 0.44, burst incidence; r = 0.45, P= 0.07), which disappeared when the same female participant was removed from the analysis (burst frequency, r = 0.27 and burst incidence r = 0.18, P>0.05). Heart rate was not related to MSNA in the men (burst frequency, r=0.14; burst incidence, r=−0.03; P>0.05) or women (burst frequency, r=−0.02; burst incidence, r=−0.20; P>0.05).
Figure 4.
Linear regression analysis of the relationship between stroke volume (SV) and muscle sympathetic nerve activity (MSNA) in men and women. There was no correlation between SV and MSNA when the circled female participant was removed from the analysis (burst frequency, r = 0.25 and burst incidence r = 0.30, P>0.05)
Discussion
The major new finding of the present study is that the inter-individual positive relationship between TPR and MSNA and the inverse relationship between cardiac output and MSNA previously observed in men 5 do not exist in young normotensive women. In striking contrast to previous findings in men 5, we found that women had no relationship between MSNA and TPR (Figure 2). This observation suggests that, among the factors which contribute to the overall level of TPR, the magnitude of sympathetic nerve activity has a greater role in young men compared to young women. Therefore, other key factors may have a greater contribution to the control of TPR in resting women and may explain why women have less autonomic support of blood pressure than men 8.
MSNA as an index of sympathetic activity
Several lines of evidence support the idea that at rest MSNA is a good indicator of the net vasoconstrictor tone directed towards the vasculature in young healthy men. In addition to our previous study 5, other work demonstrates that at rest MSNA is well correlated to total arterial NE spillover 24–26, cardiac and renal NE spillover 25,26 and arterial plasma NE 25 in normotensive healthy volunteers. However, all of these studies were conducted in men. Our present study suggests that MSNA may not be a good indicator of vasoconstrictor tone in the peripheral vasculature of resting normotensive young women. Furthermore, physiological stressors such as orthostasis, and pathophysiology such as obesity or hypertension, add further complexity to the relationship between MSNA and vasoconstrictor tone 27–29, so it is less clear whether MSNA is closely related to cardiac, renal or whole body NE spillover in those conditions.
Possible mechanisms contributing to the dissociation of MSNA and TPR in women
Several mechanisms may contribute to the lack of relationship between sympathetic nerve activity and TPR in women. First, estrogen has a direct vasodilator effect on the vasculature, which might compete with sympathetic vasoconstriction 12,15. Second, estrogen appears to increase the bioavailability of nitric oxide which, again, might offset sympathetic vasoconstriction 30. Third, estrogen supplementation in rats increases vasodilating β2-adrenergic receptor mediated responses to isoproterenol 11. Moreover, in women, vasoconstrictor responses to noradrenaline are enhanced by concurrent administration of β-blockers which also eliminates the difference in α-mediated vasoconstrictor responses between men and women 10. Therefore, α-adrenergic vasoconstriction associated with a given sympathetic neural impulse might be offset by greater β2-adrenergic mediated vasodilatation in women. Other possibilities that might contribute to the lack of relationship between MSNA and TPR in women include less norepinephrine and/or neuropeptide Y release per burst of sympathetic traffic, which might be modified by the effect of estrogen and progesterone on the sympathetic nerve terminals. Alternatively, higher circulating levels of endogenous non-adrenergic vasoconstrictors may alter the relationship between MSNA and TPR in women. However, there is inadequate information in the literature to comment definitively on these possibilities.
Consistent with the ideas mentioned above, loss of the vasodilating effects of female sex hormones may also explain why postmenopausal women with high baseline MSNA have increased blood pressure 4. Although our present study was not designed to test the specific effects of estrogen on peripheral vascular tone, our data are consistent with these previous reports 11–14. They also raise important new questions about sex differences and fundamental integrative mechanisms regulating arterial pressure in humans.
Relationship between MSNA and cardiac output
The lack of relationship between MSNA and arterial pressure has proven to be perplexing, since sympathetically-mediated vasoconstriction would be expected to increase arterial pressure. In young men, this paradox can be explained in part by the balance that appears to exist between MSNA and cardiac output 5. That is, since MSNA contributes to TPR, and arterial pressure is a balance between cardiac output and TPR (i.e. MAP = cardiac output × TPR), the inverse relationship between MSNA and cardiac output contributes to the normal blood pressure in young men with higher MSNA.
Our present observation of no relationship between MSNA and cardiac output in women suggests that the sexes rely on different mechanisms to maintain a normal arterial pressure regardless of widely varying MSNA. In the present study, we also noted that MSNA was inversely related to stroke volume in men, but again this relationship was not evident in the women (Fig. 4). One possible mechanistic explanation for these observations is that augmented β2-adrenergic responsiveness due to sex hormone influences 11 also extends to β2-adrenergic control of cardiac myocyte contractility. Another potential explanation is that differences in afterload for a given MSNA cause the differences in the relationship between stroke volume and MSNA among young men and women. In other words, men with a low MSNA have a relatively lower TPR and therefore a lower afterload which reduces the opposition to the outflow of blood from the left ventricle; therefore stroke volume is elevated in men with low MSNA. This does not appear to occur in young normotensive women since TPR is not related to MSNA.
Other observations
We noted a number of other differences between men and women. Mean MSNA, MAP and cardiac output were lower in women than men, where as TPR was higher in women than in men, which is consistent with previous studies 9,31. However, when we scaled cardiac output and TPR for body surface area the sex differences in cardiac output and TPR were abolished. Therefore, differences in TPR between men and women are likely a result of differences in body size and its effect on cardiac output 29.
Limitations
There are several important limitations to our study. First, we measured cardiac output non-invasively. However, we used a non-rebreathing acetylene uptake technique that has a reported variability of ∼7% 20 which is similar to other invasive techniques 20 and non-invasive 32,33 approaches to measuring cardiac output. Second, individual blood estrogens and progesterone levels were not measured in this study thus we can only speculate that estrogen mediated effects on the vasculature are a major explanation for our findings, but this remains a major hypothesis going forward from this study and is certainly consistent with existing data.
Perspectives
For many years, the inter-individual variability in sympathetic neural activity in humans was thought to limit our ability to understand the role of sympathetic neural mechanisms in arterial pressure regulation. Our recent work and that of others indicates that the opposite appears to be true. Exploring how inter-individual variability in key hemodynamic variables interact as determinants of blood pressure provides key insights into integrative regulatory mechanisms. Moreover, our observations may have important clinical implications. First, the differences we observed between men and women in the present study may provide insights into clinically relevant sex differences, ranging from greater orthostatic intolerance in young women to greater hypertension in young men. Second, as humans age the relationship between MSNA and MAP becomes positive more so in women than men. This raises the possibility that as the estrogen related effects we mention are lost in postmenopausal women, those with high levels of MSNA will be a greater risk for hypertension. In this context, future studies regarding the influence of aging on the balance between sympathetic nerve activity, central hemodynamics and peripheral vasoconstrictor tone will likely further our understanding of the role played by the female sex hormones in blood pressure regulation.
Conclusions
In summary, the results of the present study demonstrate that the inter-individual relationships among TPR, cardiac output and MSNA previously observed in young men do not exist in young women. Thus, in young women sympathetic nerve activity does not determine total peripheral resistance and cardiac output is not balanced with MSNA to maintain normal arterial pressure. Consequently, it appears that arterial pressure regulation is fundamentally different between the sexes; this may be due to influences of female reproductive hormones on cardiovascular function. These differences in arterial pressure regulation may also explain sex differences in the prevalence of orthostatic intolerance in young women and why older women with high levels of MSNA have increased arterial pressure.
Acknowledgements
We are grateful to Christopher Johnson for his technical assistance and to Shelly Roberts, Karen Krucker, Shirley Kingsley-Berg, Nicholas Strom and Jessica Sawyer for their assistance in the conduct of the studies and analysis of the data. Finally, we thank the subjects for their participation.
Sources of funding
This study was supported by NIH HL083947.
Footnotes
Disclosures
Nothing to disclose
References
- 1.Charkoudian N, Joyner MJ, Sokolnicki LA, Johnson CP, Eisenach JH, Dietz NM, Curry TB, Wallin BG. Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J Physiol. 2006;572:821–827. doi: 10.1113/jphysiol.2005.104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charkoudian N, Martin EA, Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Influence of increased central venous pressure on baroreflex control of sympathetic activity in humans. Am J Physiol Heart Circ Physiol. 2004;287:H1658–H1662. doi: 10.1152/ajpheart.00265.2004. [DOI] [PubMed] [Google Scholar]
- 3.Jones PP, Shapiro LF, Keisling GA, Jordan J, Shannon JR, Quaife RA, Seals DR. Altered autonomic support of arterial blood pressure with age in healthy men. Circulation. 2001;104:2424–2429. doi: 10.1161/hc4501.099308. [DOI] [PubMed] [Google Scholar]
- 4.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45:522–525. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- 5.Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol. 2005;568:315–321. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiinberg N, Hoegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, Svendsen TL, Kampmann JP, Madsen NH, Bentzon MW. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens. 1995;8:978–986. doi: 10.1016/0895-7061(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 7.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 8.Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol. 2005;289:R109–R116. doi: 10.1152/ajpregu.00013.2005. [DOI] [PubMed] [Google Scholar]
- 9.Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D, Seals DR. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation. 2005;111:494–498. doi: 10.1161/01.CIR.0000153864.24034.A6. [DOI] [PubMed] [Google Scholar]
- 10.Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol. 2000;36:1233–1238. doi: 10.1016/s0735-1097(00)00849-4. [DOI] [PubMed] [Google Scholar]
- 11.Ferrer M, Meyer M, Osol G. Estrogen replacement increases beta-adrenoceptor-mediated relaxation of rat mesenteric arteries. J Vasc Res. 1996;33:124–131. doi: 10.1159/000159140. [DOI] [PubMed] [Google Scholar]
- 12.Gilligan DM, Badar DM, Panza JA, Quyyumi AA, Cannon RO., 3rd Acute vascular effects of estrogen in postmenopausal women. Circulation. 1994;90:786–791. doi: 10.1161/01.cir.90.2.786. [DOI] [PubMed] [Google Scholar]
- 13.Moreau KL, Donato AJ, Tanaka H, Jones PP, Gates PE, Seals DR. Basal leg blood flow in healthy women is related to age and hormone replacement therapy status. J Physiol. 2003;547:309–316. doi: 10.1113/jphysiol.2002.032524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott PA, Tremblay A, Brochu M, St-Louis J. Vasorelaxant action of 17 -estradiol in rat uterine arteries: role of nitric oxide synthases and estrogen receptors. Am J Physiol Heart Circ Physiol. 2007;293:H3713–H3719. doi: 10.1152/ajpheart.00736.2007. [DOI] [PubMed] [Google Scholar]
- 15.Volterrani M, Rosano G, Coats A, Beale C, Collins P. Estrogen acutely increases peripheral blood flow in postmenopausal women. Am J Med. 1995;99:119–122. doi: 10.1016/s0002-9343(99)80130-2. [DOI] [PubMed] [Google Scholar]
- 16.Sudhir K, Elser MD, Jennings GL, Komesaroff PA. Estrogen supplementation decreases norepinephrine-induced vasoconstriction and total body norepinephrine spillover in perimenopausal women. Hypertension. 1997;30:1538–1543. doi: 10.1161/01.hyp.30.6.1538. [DOI] [PubMed] [Google Scholar]
- 17.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- 18.Sundlof G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol. 1977;272:383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallin BG. Recordings of impulses in unmyelinated nerve fibres in man: sympathetic activity. Acta Anaesthesiol Scand Suppl. 1978;70:130–136. [PubMed] [Google Scholar]
- 20.Johnson BD, Beck KC, Proctor DN, Miller J, Dietz NM, Joyner MJ. Cardiac output during exercise by the open circuit acetylene washin method: comparison with direct Fick. J Appl Physiol. 2000;88:1650–1658. doi: 10.1152/jappl.2000.88.5.1650. [DOI] [PubMed] [Google Scholar]
- 21.Gan K, Nishi I, Chin I, Slutsky AS. On-line determination of pulmonary blood flow using respiratory inert gas analysis. IEEE Trans Biomed Eng. 1993;40:1250–1259. doi: 10.1109/10.250579. [DOI] [PubMed] [Google Scholar]
- 22.Stout RL, Wessel HU, Paul MH. Pulmonary blood flow determined by continuous analysis of pulmonary N2O exchange. J Appl Physiol. 1975;38:913–918. doi: 10.1152/jappl.1975.38.5.913. [DOI] [PubMed] [Google Scholar]
- 23.Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol. 2001;531:861–869. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson JM, Wallin BG, Lambert GW, Jennings GL, Esler MD. Human muscle sympathetic activity and cardiac catecholamine spillover: no support for augmented sympathetic noradrenaline release by adrenaline co-transmission. Clin Sci (Lond) 1998;94:383–393. doi: 10.1042/cs0940383. [DOI] [PubMed] [Google Scholar]
- 25.Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R, Jennings G. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol. 1992;453:45–58. doi: 10.1113/jphysiol.1992.sp019217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallin BG, Thompson JM, Jennings GL, Esler MD. Renal noradrenaline spillover correlates with muscle sympathetic activity in humans. J Physiol. 1996;491(Pt 3):881–887. doi: 10.1113/jphysiol.1996.sp021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambert E, Straznicky N, Schlaich M, Esler M, Dawood T, Hotchkin E, Lambert G. Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension. 2007;50:862–868. doi: 10.1161/HYPERTENSIONAHA.107.094649. [DOI] [PubMed] [Google Scholar]
- 28.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96:3423–3429. doi: 10.1161/01.cir.96.10.3423. [DOI] [PubMed] [Google Scholar]
- 29.Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS, Sinoway LI. Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol Heart Circ Physiol. 2001;281:H2028–H2035. doi: 10.1152/ajpheart.2001.281.5.H2028. [DOI] [PubMed] [Google Scholar]
- 30.Sudhir K, Jennings GL, Funder JW, Komesaroff PA. Estrogen enhances basal nitric oxide release in the forearm vasculature in perimenopausal women. Hypertension. 1996;28:330–334. doi: 10.1161/01.hyp.28.3.330. [DOI] [PubMed] [Google Scholar]
- 31.Lambert E, Straznicky N, Eikelis N, Esler M, Dawood T, Masuo K, Schlaich M, Lambert G. Gender differences in sympathetic nervous activity: influence of body mass and blood pressure. J Hypertens. 2007;25:1411–1419. doi: 10.1097/HJH.0b013e3281053af4. [DOI] [PubMed] [Google Scholar]
- 32.Nugent AM, McParland J, McEneaney DJ, Steele I, Campbell NP, Stanford CF, Nicholls DP. Non-invasive measurement of cardiac output by a carbon dioxide rebreathing method at rest and during exercise. Eur Heart J. 1994;15:361–368. doi: 10.1093/oxfordjournals.eurheartj.a060504. [DOI] [PubMed] [Google Scholar]
- 33.Bell C, Monahan KD, Donato AJ, Hunt BE, Seals DR, Beck KC. Use of acetylene breathing to determine cardiac output in young and older adults. Med Sci Sports Exerc. 2003;35:58–64. doi: 10.1097/00005768-200301000-00010. [DOI] [PubMed] [Google Scholar]