Abstract
Background
There are conflicting reports as to the association between smoking, radiotherapy, diabetes and osteoporosis and the risk of dental implant failure. We undertook a meta-analysis to evaluate the association between smoking, radiotherapy, diabetes and osteoporosis and the risk of dental implant failure.
Methods
A comprehensive research on MEDLINE and EMBASE, up to January 2013, was conducted to identify potential studies. References of relevant studies were also searched. Screening, data extraction and quality assessment were conducted independently and in duplicate. A random-effects meta-analysis was used to pool estimates of relative risks (RRs) with 95% confidence intervals (CIs).
Results
A total of 51 studies were identified in this meta-analysis, with more than 40,000 dental implants placed under risk-threatening conditions. The pooled RRs showed a direct association between smoking (n = 33; RR = 1.92; 95% CI, 1.67–2.21) and radiotherapy (n = 16; RR = 2.28; 95% CI, 1.49–3.51) and the risk of dental implant failure, whereas no inverse impact of diabetes (n = 5; RR = 0.90; 95% CI, 0.62–1.32) on the risk of dental implant failure was found. The influence of osteoporosis on the risk of dental implant failure was direct but not significant (n = 4; RR = 1.09; 95% CI, 0.79–1.52). The subgroup analysis indicated no influence of study design, geographical location, length of follow-up, sample size, or mean age of recruited patients.
Conclusions
Smoking and radiotherapy were associated with an increased risk of dental implant failure. The relationship between diabetes and osteoporosis and the risk of implant failure warrant further study.
Introduction
Dental osseointegrated implants are generally considered as effective and predictable restorations for the replacement of missing teeth. However, although highly desirable outcomes and the long-term survival of dental implant treatments are well documented in numerous studies [1]–[4], implant failures still occur for various reasons. Therefore, the risks associated with dental implant failure have become a frequently discussed topic in recent dental research.
A variety of conditions, including implant design (length, shape or surface texture), patient-related medical risk factors (systemic diseases or habits, such as smoking,), and surgery-related factors (surgeon's experience or surgical design) have been considered to influence the outcome for implant restoration [5]–[7]. With the dramatic advancements in materials science and surgical techniques, increasing attention is focused on patient-related conditions as risk factors for dental implant failure [8].
According to research by Buser and colleagues', patients exposed to with irradiation (radiotherapy) before or after implantation, or patients with severe diabetes or heavy smoking habits have significantly increased risks of dental implant failure [9]. It has been suggested that such conditions could impair implant survivability by increasing the susceptibility of the patient to other diseases or by interfering with the tissue healing process [1]. Moreover, osteoporosis, with its high prevalence in the aged population, is also considered a relative contraindication for dental implant therapy [10], [11]; the alveolar ridge atrophy and low bone mineral density, caused by osteoporosis may impair bone quality and quantity at implant sites [12], [13]. While a number of studies have assessed the influence of smoking, radiotherapy, diabetes and osteoporosis on implant failure, the results have been inconsistent.
Since life expectancy is expected to increase with the advent of better therapies and targeted medicine, an increasing number of patients who smoke or previously smoked, who received radiotherapy for head and neck cancer treatment, or who present with diabetes or osteoporosis may require dental implant treatment. The aim of the present study was, therefore, to provide a comprehensive and critical meta-analysis of clinical studies published in international peer-reviewed literature concerning these four factors of high prevalence and/or of high risks, in order to draw evidence-based conclusions as to the influence of these factors on the outcome of dental implant treatment.
Methods
Search Strategy
We performed a systematic literature search of MEDLINE and EMBASE database up to January 2013. All searches were performed using medical subject heading (MeSH) or free text words. We combined search terms for outcomes (survival, success, osseointegration, failure, removal, replacement and loss), risk factors (1, smoking, smoker or tobacco; 2, irradiation, radiotherapy or head and neck cancer; 3, diabetes, diabetic, diabetes mellitus or hyperglycemia; 4, osteoporosis, osteopenia, low bone mineral density or bone loss) and key subjects (dental implant or oral implant). Reference lists of identified articles and relevant papers known to reviewers were also searched. Emails were sent to the authors of identified studies for additional information, where necessary. Studies were limited to English publications. Considering the study by Mish and his colleagues, we referred implant removal or implant loss to “implant failure” [14].
Selection Criteria
Three reviewers (H Chen, N Liu and X Xu) conducted the search independently. Titles and abstracts were screened for subject relevance. Studies that could not be definitely excluded based on abstract information were also selected for full text screening. Two reviewers examined the full text of all relevant studies for inclusion possibility (smoking: N Liu and X Xu; radiotherapy: H Chen and X Xu; diabetes and osteoporosis: H Chen and N Liu). Where there was a disagreement for study inclusion, a discussion was held with a third reviewer (X Qu) to reach a consensus.
Studies were eligible for inclusion if they met the following criteria: (1) human study; (2) observational study; (3) studies focusing on the influence of smoking and/or radiotherapy and/or diabetes and/or osteoporosis on dental implant failure; (4) studies providing outcome data for dental implant failure or relevant data that could be calculated by the reviewers; (5) studies providing data for both a non-risk (control) group and a risk (study) group; (6) studies published in English. Exclusion criteria were agreed as follows: (1) animal study; (2) in vitro or laboratory study; (3) review or case report; (4) studies providing craniofacial implant data for which dental implant data could not be extracted; (5) studies providing patient-related data (to be specific, survival/failure rate that was calculated at the patient-level); 6) studies without data on non-smoking, non-irradiation, non-diabetic or non-osteoporotic groups.
Data Extraction and Quality Assessment
Two reviewers (H Chen and N Liu) independently extracted data using a structured form. The following information was extracted from each included study: year of publication, country, first author's family name, study design, follow-up period, characteristics of subjects (number of patients, gender and age), information relevant to risk factors, characteristics of the dental implants (number and placement position) and data on dental implant failure.
The methodological quality of the included studies was independently and appraised twice by two reviewers (H Chen and X Xu) using elements of McMaster Quality Assessment Scale of Harms (McHarm) [15]. The criteria of the quality assessment are presented in Table 1. Any discrepancy that occurred during data extraction and quality assessment was resolved by consensus or discussion with another reviewer (X Qu).
Table 1. Criteria of Quality Assessment (a Modified McHarm checklist).
| ITEMS | YES | NO/Not sure | |
| 1 | Were the harms PRE-DEFINED using standardized or precise definitions? | 1 | 0 |
| (In present study, we defined “harms” as the totality of adverse consequences of an implant surgery) | |||
| 2 | Were SERIOUS events precisely defined? | 1 | 0 |
| (In present study, we defined complications that didn't lead to IMPLANT LOSS or IMPLANT REMOVAL as SERIOUS events, e.g. sensitivity on function, radiographic bone loss ≤4 mm or 1/2 of the implant body, probing depth ≤7 mm, etc. [14]) | |||
| 3 | Were SEVER events precisely defined? | 1 | 0 |
| (In present study, we defined IIMPLANT LOSS as SERIOUS events) | |||
| 4 | Did the study specify the TRAINING or BACKGROUND of who ascertained the harms? | 1 | 0 |
| 5 | Did the study specify the TIMING and FREQUENCY of collection of the harms? | 1 | 0 |
| 6 | Did the author(s) use STANDARD scale(s) or checklist(s) for harms collection? | 1 | 0 |
| 7 | Was the NUMBER of participants that withdrew or were lost to follow-up specified for each study group? | 1 | 0 |
| 8 | Was the TOTAL NUMBER of participants affected by harms specified for each study arm? | 1 | 0 |
| 9 | Did the author(s) specify the NUMBER for each TYPE of harmful event for each study group? | 1 | 0 |
| 10 | Did the author(s) specify the type of analyses undertaken for harms data? | 1 | 0 |
| A Total of 10 Points | |||
Statistical Analysis
We evaluated dental implant failure for any reason attributable to the implant as our outcome measure of interest. Relative risk (RR) was used as the common measure of association across studies. The RRs and 95% confidence intervals (CIs) were extracted or calculated from each study, and then we pooled the overall RRs using the inverse of corresponding variances as weights. For the meta-analysis, a random-effects model was considered [16]. Heterogeneity between studies was tested through the Cochran Q and I 2 statistics (I 2 values of 25, 50, and 75% are considered as low, moderate, and high, respectively [17]).
Subgroup analyses were used to identify associations between the risk of dental implant failure and other relevant study characteristics (mean age, geographical location, design of study, sample size and length of follow-up) as possible sources of heterogeneity. Publication bias was measured using Begg's and Egger's regression tests and visualization of funnel plots [18]. The stability of the study was also detected by sensitivity analysis, through re-meta-analysis with one involved study excluded each time. All statistical analyses were performed with Review Manager 5.01 (The Cochrane Collaboration, Copenhagen, Denmark) and Stata version 11 (StataCorp, College Station, TX).
Results
Literature Search
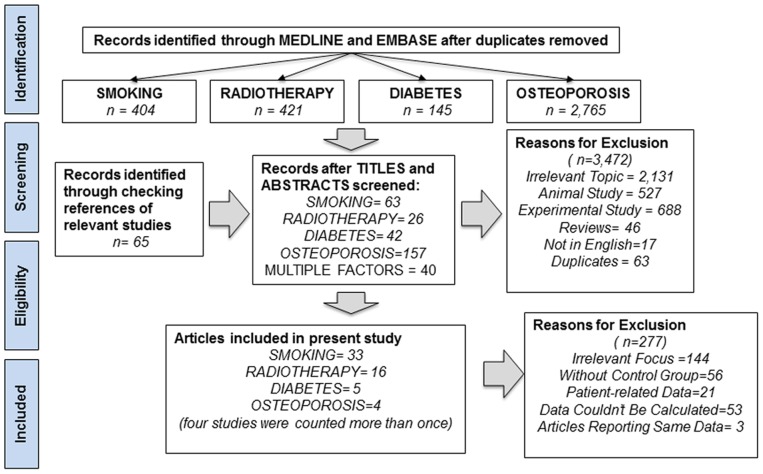
The literature search yielded a total of 3,735 primary studies, of which 3,472 were excluded after title screening. An additional 65 studies were included after checking the references of relevant reviews and studies. Finally, 328 studies were included for full-text assessment, of which 277 were excluded for one of the following reasons: (1) studies focusing on irrelevant outcome assessment (n = 144), such as bone loss or primary stability; (2) studies without a non-risk group (n = 56); (3) studies only providing patient-related data (n = 21); (4) studies where data related to implant failure could not be calculated (n = 53); and (5) studies where the reported data were represented in another included in our analysis (n = 3) [19]–[21]. As a result, 51 studies met the inclusion criteria for meta-analysis, with 33 studies for smoking [22]–[54], 16 for radiotherapy [31], [44], [55]–[68], five for diabetes [31], [44], [47], [48], [69] and four for osteoporosis [44], [70]–[72], respectively. Of note, four studies involved more than one risk factor and were included in more than one group [31], [44], [47], [48]. A flow diagram of the study selection process is presented in Figure 1.
Figure 1. Flow Diagram of Screened and Included Papers.
Study Characteristics and Quality Assessment
The detailed characteristics of the included studies and the results of the quality assessment are summarized in Tables 2–5. The number of implants in each study ranged from 56 [34] to 5,843 [49]. The earliest study was published in 1993 [22], and the latest in 2012 [53], [54], [67]–[69]. In terms of study design, 23 studies enrolled patients prospectively [24], [25], [27]–[29], [32]–[34], [37]–[40], [42], [46], [47], [55], [58], [60]–[62], [64], [66], [69], whereas 28 were retrospective database reviews [22,23,26,30,31,35,36,41,43–45,48–54,56,57,59,63,65,67,68,70–72]. By geographic location, 18 studies were conducted in the United States [24,26,30–33,35,36,38–40,42,45,50,53,57,65,71], 24 in Europe [23,27,28,29,37,43,44,49,51,52,54–56,58–64,66–68,72] and nine in other regions [22,25,34,41,46–48,69,70]. The overall study quality averaged 8.2 (range, 5–10) on a scale of 1 to 10.
Table 2. Study Characteristics (SMOKING).
| Author (Year) | Country | Study | Follow-up | Patient Characteristics | Smoking | Implant Characteristics | QS | ||||
| Mean Age | CON/STY | F | CON/STY | Position (Mand./Max.) | FC/FS | ||||||
| Bain , 1993 | Canada | Retro | 37.88 m | 54.7 yr | NA/NA | 311 | NA | 1,804/390 | 1,115/1,079 | 86/44 | 8 |
| De Bruyn, 1994 | Belgium | Retro | NA | (20–80 yr) | 91/26 | 66 | NA | 338/114 | 208/244 | 5/10 | 8 |
| Gorman, 1994 | USA | Prospec | NA | NA | 228/82 | NA | NA | 142/646 | NA | 47/42 | 7 |
| Bain, 1996 | Canada | Prospec | NA | NA | NA/NA | NA | NA | 176/47 | NA | 10/9 | 8 |
| Minsk, 1996 | USA | Retro | 6 yr | NA | NA/NA | NA | 20 per day | 570/157 | 358/369 | 52/17 | 9 |
| Lindquist, 1997 | Sweden | Prospec | 10 yr | (33–64 yr) | 24/21 | 32 | NA | 139/125 | Mandible | 3/0 | 8 |
| De Bruyn, 1999 | Belgium | Prospec | 7 yrs | NA | 13/10 | NA | 13.2 per day | 32/30 | Maxilla | 9/6 | 10 |
| Grunder, 1999 | Switzerland | Prospec | 34.4 m | 58±15 yr | 55/19 | 34 | NA | 164/55 | NA | 3/0 | 9 |
| Jones, 1999 | USA | Retro | 58 m | 50 yr | 44/19 | 40 | NA | 217/126 | 204/147 | 5/11 | 8 |
| Keller, 1999 | USA | Retro | 12 yr | (15–73 yr) | 26/28 | NA | NA | 143/105 | Grafted maxilla sinus | 26/7 | 10 |
| Lambert, 2000 | USA | Prospec | 3 yr | NA | NA/NA | NA | NA | 1,928/959 | 1616/1271 | 115/85 | 8 |
| Olson, 2000 | USA | Prospec | 38±15 m | 56±12 yr | NA/NA | 1 | NA | 65/51 | Grafted maxillary sinus | 1/2 | 7 |
| Wallace, 2000 | USA | Retro | 4 yr | NA | 39/17 | 27 | NA | 115/72 | NA | 8/12 | 7 |
| Schwartz-Ara, 1999 | Israel | Prospec | 5 yr | 47 yr | NA/NA | 27 | NA | 50/6 | 39/17 | 5/1 | 7 |
| Geurs, 2001 | USA | Retro | 3.2±1.3 yr | NA | NA/NA | NA | NA | 267/62 | Grafted maxilla sinus | 13/7 | 6 |
| Widmark, 20001 | Sweden | Prospec | (3–5 yr) | NA | 25/11 | NA | ≥half a pack a day | 131/67 | Local: 120/Grafted: 101 | 14/26 | 10 |
| Kumar, 2002 | USA | Prospec | NA | NA | 389/72 | NA | NA | 914/269 | 357/826 | 8/15 | 5 |
| Van Steenberghe, 2002 | Belgium | Prospec | NA | 50±14 yr | NA/NA | 243 | NA | 1,107/156 | NA | 19/8 | 7 |
| Karoussis, 2003 | Switzerland | Prospec | 10 yr | NA | 41/12 | NA | NA | 84/28 | NA | 3/2 | 10 |
| DeLuca, 2006 | Canada | Retro | 59.8 m | 49.3 yr | 285/104 | 283 | NA | 1,045/4,94 | NA | 32/26 | 9 |
| Peleg, 2006 | USA | Prospec | 69 m | NA | 505/226 | 453 | NA | 1,505/627 | Maxilla sinus grafting | 28/16 | 7 |
| Mundt, 2006 | Germany | Retro | 88.2 m | 54.1 yr | NA/NA | 94 | NA | 294/363 | 296/367 | 6/30 | 8 |
| Alsaadi, 2008 | Belgium | Retro | 2 yr | NA | 351/61 | 240 | NA | 1,291/223 | 698/816 | 80/21 | 8 |
| Balshe, 2008 | USA | Retro | 5 yr | 49.4 yr | 1299/119 | 861 | 17.7±7 per day | 3,841/766 | 2,633/1974 | 188/77 | 7 |
| Levin, 2008 | Israel | Prospec | 6.14 yr | 45 yr | 54/10 | 40 | NA | 54/10 | NA | 3/1 | 7 |
| Tawil, 2008 | Lebanon | Prospec | 42.4 m | NA | 50/40 | 33 | NA | 254/245 | NA | 2/5 | 9 |
| Anner, 2010 | Isreal | Retro | 31±28 m | 52±12 yrs | 412/63 | 299 | NA | 1,400/226 | NA | 56/21 | 7 |
| Cavalcanti, 2011 | Italy | Retro | 5 yr | 50 yrs | 1019/458 | 1025 | NA | 3,882/1,961 | NA | 112/107 | 9 |
| Conrad, 2011 | USA | Retro | 35.7 m | 55.3 yr | NA/NA | 168 | NA | 446/48 | Maxilla | 28/6 | 8 |
| Rodriguez, 2011 | Spain | Retro | ≥6 m | 53±13 yr | 182/113 | 188 | NA | 644/389 | NA | 18/14 | 9 |
| Vandeweghe, 2011 | Belgium | Retro | 22 m | 54±13.4 yr | 288/41 | 43 | NA | 608/104 | NA | 7/5 | 9 |
| Lin, 2012 | USA | Retro | 12 m | 59.6 yr | 47/28 | 186 | NA | 93/62 | Grafted maxiila sinus | 12/13 | 9 |
| Vervaeke, 2012 | Belgium | Retro | 31±7.2 m | 56±12 yr | 235/60 | 168 | NA | 244/849 | 458/648 | 11/8 | 10 |
CON = control group, that is non-smoking group;STY = study group, that is smoking group; F = Female; Mand. = mandible; Max. = maxilla; Retro = retrospective study; Prospec = prospective study; yr = year; m = month; NA = not available; Local = local bone; Grafted = grafted bone; FC = failure implant number of Control Group; FS = failure implant number of Study Group; QS = quality assessment score.
Table 5. Study Characteristics (OSTEOPOROSIS).
| Author (Year) | Country | Study | Follow-up | Patient Characteristics | Implant Characteristics | QS | ||||
| Mean Age | CON/STY | F | CON/STY | Position (Mand./Max.) | FC/FS | |||||
| Amorim,2007 | Brazil | Retro | 9 m | 58.2 yr | 20/19 | 39 | 43/39 | Mandible | 0/1 | 8 |
| Alsaadi,2008 | Belgium | Retro | 2 yr | NA | 393/19 | 240 | 1,446/68 | 698/816 | 92/9 | 8 |
| Holahan,2008 | USA | Retro | 5.4 yr | 63±9 yr | 564/192 | 746 | 306/340 | 378/268 | 17/20 | 7 |
| Dvorak,2011 | Austria | Retro | 6±4 yr | ≥45 yr | 115/62 | 117 | 543/258 | 396/432 | 17/20 | 7 |
CON = control group, that is non-osteoporosis group; STY = study group, that is osteoporosis group; F = Female; Mand. = mandible; Max. = maxilla; Retro = retrospective study; Prospec = prospective study; yr = year; m = month; NA = not available; Local = local bone; Grafted = grafted bone; FC = failure implant number of Control Group; FS = failure implant number of Study Group; QS = quality assessment score.
Table 3. Study Characteristics (RADIOTHERAPY).
| Author (Year) | Country | Study | Follow-up | Patient Characteristics | Radiotherapy | Implant Characteristics | QS | |||||
| Mean Age | CON/STY | F | Time | Dose (Gy) | CON/STY | Position (Mand./Max.) | FC/FS | |||||
| Esser, 1997 | Germany | Prospec | NA | (37–79 yr) | NA/NA | 9 | BP | 60 | 66/152 | Mandible | 7/33 | 7 |
| Werkmeister, 1999 | Germany | Retro | 3 yrs | 55 yr | 17/12 | 6 | BP | 54 | 79/30 | Local: 64/Grafted: 45 | 19/8 | 7 |
| Keller, 1999 | USA | Retro | 12 yrs | (15–73 yr) | 52/2 | NA | NA | 55 and 61 | 237/11 | Grafted maxilla | 33/0 | 10 |
| Shaw, 2005 | UK | Retro | 3.5 yr | 58 yr | 43/34 | 32 | BP | 40–66 | 192/172 | Local: 238/Grafted: 126 | 25/31 | 9 |
| Yerit, 2006 | Austria | Prospec | 5.4±3.2 yr | 58±14 yr | NA/NA | 15 | BP | 50 | 162/154 | Local: 238/Grafted: 78 | 15/29 | 9 |
| Schepers, 2006 | Netherlands | Retro | up to 23 m | 66.11 yr | 27/21 | 19 | AP | 60–68 | 78/61 | NA | 0/2 | 8 |
| Landes, 2006 | Germany | Prospec | 36 m | 63 yr | 11/19 | 8 | BP | 57 | 42/72 | NA | 0/1 | 8 |
| Nelson, 2007 | Germany | Prospec | 10.3 yr | 59 yr | NA/29 | 30 | BP | up to 72 | 311/124 | 281/154 | 4/7 | 7 |
| Alsaadi, 2008 | Belgium | Retro | 2 yr | NA | 410/2 | 240 | NA | NA | 1,499/15 | 698/816 | 98/3 | 8 |
| Schoen, 2008 | Netherlands | Prospec | 12 m | 62±11 yr | 16/19 | 15 | AP | 60.1±7.7 | 64/76 | Local bone | 2/2 | 9 |
| Klein, 2009 | Germany | Retro | 5 yr | 58.4 yr | 16/27 | 12 | BP | <50 or ≥50 | 74/116 | Local: 62/Grafted: 128 | 12/13 | 8 |
| Cuesta-Gil, 2009 | Spain | Prospec | / | 52 yr | 32/79 | 31 | Mixed | 50–60 | 311/395 | Local: 454/Grafted: 252 | 6/75 | 9 |
| Salinas, 2010 | USA | Retro | 41.1 | NA | 18/26 | 19 | Mixed | > 60 | 116/90 | Local: 105/Flap: 114 | 8/23 | 10 |
| Linsen, 2012 | Germany | Prospec | 48±34.3 m | 56±16 yr | 32/34 | 23 | BP | 36 or 60 | 135/127 | 213/49 | 6/8 | 10 |
| Jacobsen, 2012 | Switzerland | Retro | 67 m | 52.4 yr | NA/NA | 16 | AP | NA | 93/47 | Local: 41/Flap: 99 | 14/14 | 9 |
| Fenlon, 2012 | UK | Retro | / | NA | 29/12 | NA | AP | 66 | 110/35 | Grafted bone | 3/15 | 8 |
CON = control group,that is non-radiotherapy group; STY = study group, that is radiotherapy group; F = Female; BP = before placement; AP = after placement; Mand. = mandible; Max. = maxilla; Retro = retrospective study; Prospec = prospective study; yr = year; m = month; NA = not available,; Local = local bone; Grafted = grafted bone; FC = failure implant number of Control Group; FS = failure implant number of Study Group; QS = quality assessment score.
Table 4. Study Characteristics (DIABETES).
| Author (Year) | Country | Study | Follow-up | Patient Characteristics | Diabetes Type | Implant Characteristics | QS | ||||
| Mean Age | CON/STY | F | CON/STY | Position (Mand./Max.) | FC/FS | ||||||
| Keller, 1999 | USA | Prosp | 12 yrs | (15–73 yr) | 52/2 | NA | NA | 237/11 | Grafted maxilla | 0/0 | 10 |
| Morris, 2000 | New Zealand | Prosp | 36 m | NA | 408/255 | NA | II | 2632/255 | Mixed | 180/20 | 7 |
| Tawil, 2008 | Lebanon | Retro | 42.4 m | 62.15 yr | 45/45 | 33F | II | 244/255 | Mixed | 2/7 | 9 |
| Alsaadi, 2008 | Belgium | Retro | 2 yr | NA | 402/10 | 240 | I:1 II:9 | 1,480/34 | 698/816 | 202/0 | 8 |
| Anner, 2010 | Isreal | Prosp | 31±28 m | 52±12 yr | 426/49 | 299 | NA | 1,449/177 | Mixed | 72/5 | 7 |
CON = control group, that is non-diabetes group; STY = study group, that is diabetes group; F = Female; Mand. = mandible; Max. = maxilla; Retro = retrospective study; Prospec = prospective study; yr = year; m = month; NA = not available; Local = local bone; Grafted = grafted bone; FC = failure implant number of Control Group; FS = failure implant number of Study Group; QS = quality assessment score.
Smoking
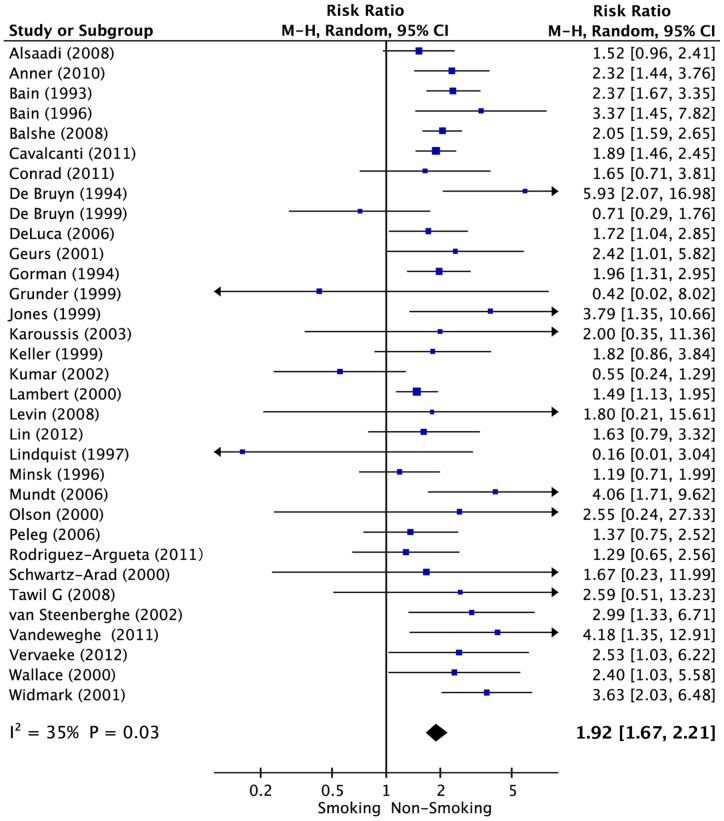
The multivariable-adjusted RRs in each study and the pooled RRs of dental implant failure for smoking versus non-smoking patients are presented in Figure 2, Table 2 and Table 6 (33 studies; 35,118 implants). In the pooled analysis, smoking was associated with higher risk of dental implant failure (RR = 1.92; 95% CI, 1.67–2.21). There was moderate heterogeneity among the studies (P = 0.03, I2 = 35%). Stratifying by study design, the pooled RRs for prospective studies and retrospective studies were 1.34 (95% CI, 0.90–2.00) and 2.01 (95% CI, 1.75–2.30). Stratifying by geographical location, the summary RRs were 1.59 (95% CI, 1.27–1.98) for studies conducted in the United States, 2.27 (95% CI, 1.62–3.20) for Europe and 2.23 (95% CI, 1.77–2.81) for other regions. With regard to the mean age of patients, the pooled RRs for <55-year-old and ≥55-year-old patients were 2.15 (95% CI, 1.87–2.47) and 1.67 (95% CI, 1.13–2.47), respectively. A subgroup analysis indicated no influence of study design, geographical location, length of follow-up, sample size or mean patient age.
Figure 2. Forest plot of studies with dental implant failure risk for smoking versus non-smoking patients.
The combined Relative risks (RR) and 95% confidence intervals (CIs) were calculated using the random-effects model.
Table 6. Subgroup analysis to investigate differences between studies included in meta-analysis.
| Subgroup | No. of Studies | RR (95% CI) | I 2 (%) | P value | P value for heterogeneity between subgroups |
| Smoking | |||||
| Design of Study | |||||
| Prospective | 15 | 1.34(0.90,2.00) | 67 | <0.0001 | 0.06 |
| Retrospective | 18 | 2.01(1.75,2.30) | 14 | 0.29 | |
| Geographical Location | |||||
| United States | 13 | 1.59 (1.27,1.98) | 46 | 0.04 | 0.08 |
| Europe | 13 | 2.18 (1.56,3.05) | 56 | 0.007 | |
| Other Regions | 7 | 2.23 (1.77,2.81) | 0 | 0.90 | |
| Length of Follow-up (years) | |||||
| ≥5 | 11 | 1.72 (1.37,2.15) | 28 | 0.18 | 0.32 |
| <5 | 17 | 1.98 (1.68,2.33) | 14 | 0.29 | |
| Sample Size (implant) | |||||
| <500 | 16 | 2.25 (1.64,3.08) | 25 | 0.17 | 0.23 |
| ≥500 | 17 | 1.81 (1.56,2.11) | 40 | 0.05 | |
| Age (years) | |||||
| <55 | 11 | 2.15 (1.87,2.47) | 0 | 0.67 | 0.23 |
| ≥55 | 6 | 1.67 (1.13,2.47) | 0 | 0.54 | |
| Radiotherapy | |||||
| Design of Study | |||||
| Prospective | 6 | 2.02 (1.37,2.97) | 0 | 0.73 | 0.58 |
| Retrospective | 10 | 2.50 (1.32,4.75) | 81 | <0.00001 | |
| Geographical Location | |||||
| United States | 2 | 1.46 (0.12,17.16) | 69 | 0.07 | 0.72 |
| Europe | 14 | 2.29 (1.45,3.63) | 71 | <0.0001 | |
| Length of Follow-up (years) | |||||
| ≥5 | 5 | 1.62 (0.85,3.11) | 62 | 0.03 | 0.83 |
| <5 | 8 | 1.76 (1.20,2.59) | 20 | 0.27 | |
| Sample Size (implant) | |||||
| <250 | 10 | 2.14 (1.27,3.60) | 64 | 0.003 | 0.56 |
| ≥250 | 6 | 2.74 (1.43,5.25) | 76 | 0.001 | |
| Age (years) | |||||
| <60 | 8 | 1.95 (1.11,3.42) | 78 | <0.0001 | 0.68 |
| ≥60 | 3 | 1.40 (0.33,5.97) | 0 | 0.69 | |
Radiotherapy
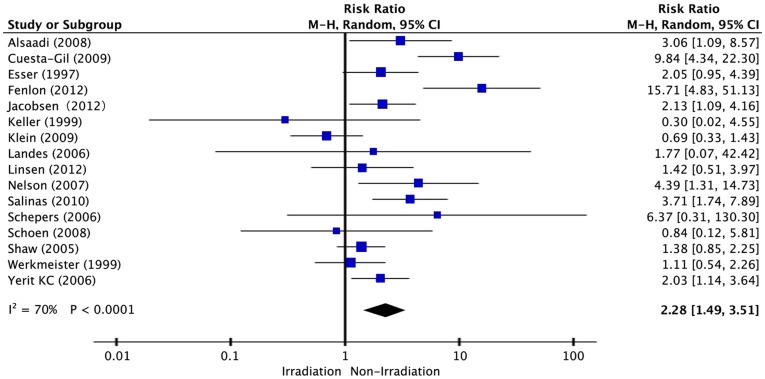
Figure 3 shows the association between radiotherapy and risk of dental implant failure from a collection of 16 studies and 5,246 implants. A pooled analysis indicated a direct association between radiotherapy and the risk of dental implant failure (RR = 2.28; 95% CI, 1.49–3.51). The heterogeneity among the studies was high (P<0.0001, I2 = 70%). As far as geographical location was concerned, the summary RRs were 1.46 (95% CI, 0.12–17.3) for studies performed in the United States and 2.29 (95% CI, 1.45–3.63) for Europe. Stratifying by length of follow-up, the pooled RRs for <5-year and ≥5-year duration were 1.76 (95% CI, 1.20–2.59) and 1.62 (95% CI, 0.85–3.11), respectively. According to the mean age of the patients involved, the pooled RRs for <55-year-old and ≥55-year-old patients were 1.95 (95% CI, 1.11–3.42) and 1.40 (95% CI, 0.33–5.97). In the subgroup analysis, study design, geographical location, length of follow-up, sample size and mean patient age, had no influence on the risk of dental implant failure (Table 6).
Figure 3. Forest plot of studies with dental implant failure risk for patients with radiotherapy versus non-smoking.
The combined Relative risks (RR) and 95% confidence intervals (CIs) were calculated using the random-effects model.
Diabetes and Osteoporosis
Five studies were included to analyze on dental implant failure with regard to diabetes (6,774 implants). The results of the pooled analysis are shown in Figure 4. The pooled RR for patients with diabetes versus patients without diabetes was 0.90 (95% CI, 0.62–1.32), indicating no association between diabetes and the risk of dental implant failure. We found high heterogeneity across the studies (P = 0.07, I2 = 58%).
Figure 4. Forest plot of studies with dental implant failure risk for patients with diabetes versus non-diabetes.
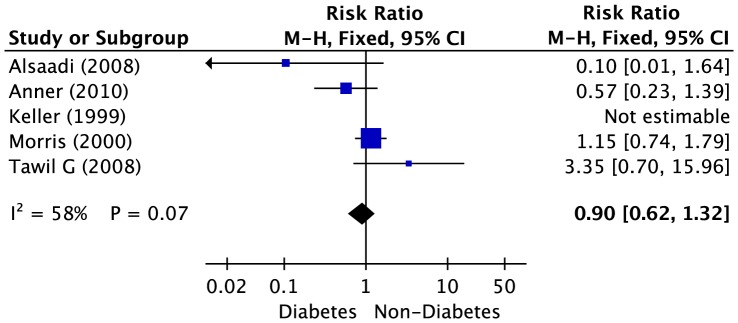
The combined Relative risks (RR) and 95% confidence intervals (CIs) were calculated using the random-effects model.
Four studies were concerned with the association between osteoporosis and dental implant failure, with a collection of 3,070 implants. In the pooled analysis, the association between osteoporosis and the risk of dental implant failure was direct but not significant (RR = 1.09; 95% CI, 0.79–1.52), with high heterogeneity across the studies (P = 0.14, I2 = 46%). (Figure 5)
Figure 5. Forest plot of studies with dental implant failure risk for patients with osteoporosis versus non-osteoporosis.
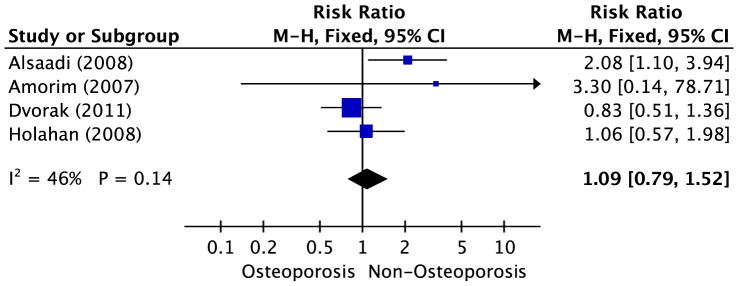
The combined Relative risks (RR) and 95% confidence intervals (CIs) were calculated using the random-effects model.
Since limited studies focusing on diabetes and osteoporosis met our inclusion criteria, and insufficient data could be extracted from the included studies, no subgroup analysis was performed to further investigate the association between diabetes and osteoporosis and risk of dental implant failure.
Publication Bias and Sensitivity Analysis
Publication bias was determined by visualization of funnel plot, Begg's test, and Egger's regression test. With the exception of radiotherapy (Begg's test: P = 0.47; Egger's test: P = 0.02), there was no evidence of publication bias for smoking (Begg's test: P = 0.49; Egger's test: P = 0.94), diabetes (Begg's test: P = 0.33; Egger's test: P = 0.23) or osteoporosis (Begg's test: P = 0.17; Egger's test: P = 0.34). Sensitivity analysis showed that excluding any one study from the pooled analysis did not vary the results substantially. (See Figure S1 for funnel plots of smoking, radiotherapy, diabetes and osteoporosis risk factor)
Discussion
Principle Findings
After reviewing numerous studies assessing the potential risk factors for dental implant failure, this meta-analysis supports the view that smoking and radiotherapy are associated with a higher risk of dental implant failure. Our findings suggest that individuals who smoke, or who have undergone radiotherapy before or after implantation, might suffer an approximately 35 or 70% higher risk of dental implant failure, respectively, as compared with non-smokers or those who have not been exposed to radiotherapy. We found no significant inverse impact of diabetes on the risk of dental implant failure, whereas osteoporosis showed a direct but not significant association. However, because of the limited number of studies focusing on diabetes and osteoporosis, these results should be interpreted carefully and verified by further studies. The findings of this meta-analysis, may offer clinical dentists with additional insights into the prognosis of dental implant treatment and may help in the establishment of potential treatment plans.
Implications
The outcome of this meta-analysis indicated that individuals who smoke were more likely to suffer from dental implant failure. This finding is consistent with a previous meta-analysis performed in 2006, with an elevated OR of 2.17 (95% CI, 1.67–2.83) indicating the inverse impact of smoking on implant osseointegration [73]. Although the underlying mechanism is still not completely understood, researchers previously posited that smoking impaired the wound healing processes involved with implant/tissue integration [27]. Others suggested that smokers treated with implants had an increased risk of postoperative complications, such as infection and peri-implantitis [53]. Bain and colleagues recommended that patients commence a smoking cessation protocol at least one week before and at least two months after dental implant surgery to assure dental implant osseointegration [22]; however, others have demonstrated that pre-operative smoking cessation, especially short-term cessation, bears no significant effect on reducing the risk of dental implant failure [74].
The present meta-analysis indicates that radiotherapy was strongly associated with increased risk of dental implant failure. A former review of animal and human studies reached a similar conclusion that implants placed in irradiated bone experienced 2–3 times higher rates of failure [75]. Moreover, implants placed in irradiated maxilla were reported to have a higher failure rate compared with those in irradiated mandible [76]. Bone responds to irradiation with various cellular, vascular, and metabolic alterations occurring at different sites in the irradiated bone and adjacent tissues [77]. Several plausible mechanisms to explain how bone responds to irradiation have been proposed, including altered osteoblast and osteoclast function during bone repair and remodeling, the formation and the subsequent breakdown of hypoxic-hypocellular and hypovascular tissues, and a decreased rate of tissue perfusion and tissue fibrosis [77–79]. Such responses were previously believed to be highly variable and partly related to the administered dose of radiation [77]. Researchers suggested that a fractionated dose would be better tolerated than a single exposure at the same level of intensity [80]. Furthermore, adjunctive treatment with the use of hyperbaric oxygen (HBO) was expected to increase the regenerative capacity of tissue damaged after radiotherapy; however, no strong evidence was found to support the use of HBO to decrease dental implant failure for radiotherapy-exposed patients [81].
Diabetes and osteoporosis are both highly prevalent disorders among elderly patients [10,11,82]. After reviewing the published literature, we found a lack of high quality and single-risk-factor focused studies with regard to the effects of diabetes or osteoporosis on dental implant survival. The present meta-analysis revealed no direct impact of diabetes or osteoporosis on the risk of dental implant failure, although both were reported to affect wound healing in oral tissues [1]. Clinical dentists are advised to avoid dental implant treatment in poorly controlled diabetic patients, and studies indicate that the long-term use of bisphosphonates by osteoporotic patients may cause osteonecrosis of the jaw [83]. Unfortunately, data was insufficient yet to give an explicit explanation of its effect on risk of dental implant failure. Diabetes and osteoporosis can be well controlled by drug intervention; yet since, none of the studies included a discussion as to the different level of severity of diabetes or osteoporosis in these patients and on the risk of dental implant failure, this limited our ability to further assess the risk of these two factors in the present meta-analysis.
Strength and Limitations
To our knowledge, this study is the most comprehensive meta-analysis to estimate the association of smoking, radiotherapy, diabetes, and osteoporosis with dental implant failure. We were able to include a substantial total number of subjects (more than 40,000 dental implants placed under risk-threatening conditions), which significantly increased the statistical power of our analysis. We made sure to minimize the bias by means of study procedure. Not only did we search MEDLINE and EMBASE databases to identify potential studies, but also we manually examined all reference lists from relevant studies. The McHarm quality assessment tool was used to evaluate each of the included studies to ensure sufficient study quality (mean score of 8.2 out of 10). Publication bias was also absent, as determined by visualization of funnel plot, Begg's test and Egger's test.
Despite the above strengths and advantages, this meta-analysis has several limitations. First, the present study was subject to confounding factors that could be inherent in the included studies and it is difficult to completely rule out the possibility that other risk factors were responsible for the observed associations. Second, heterogeneity might have been introduced by methodological differences among the studies. Many of the I2 estimates calculated in this meta-analysis were judged as high. While we were able to perform subgroup analyses on studies of smoking and radiotherapy, which indicated no influence on the study design, geographical location, length of follow-up, sample size and mean patient age, the diabetes and osteoporosis implant failure data were insufficient for a stratified analysis. Although these issues might have reduced the strength of the conclusions drawn in this meta-analysis, visual inspection of the forest plots suggests that there is considerable consistency in the RRs across the studies. Third, the search was limited to English studies and only performed with the use of two electronic databases, mainly because of the limited work force for the present research; this might have introduced a selection bias to the results.
Suggestion for Future Studies
On the basis of this meta-analysis, several questions should be answered in future studies. First, what is the compound effect of multiple risk factors on dental implant failure? For instance, what is the risk of dental implant failure for smokers with diabetes, or smokers with osteoporosis? To answer this question, several well-designed cohort studies with adequate control for confounding factors should be considered. Second, could different severity levels of the four risk factors, such as the severity of the disease or the frequency of smoking, have an effect on dental implant failure? An investigation that specifically focuses on the quantity of smoking, the overall irradiation dose, and/or the severity of diabetes and osteoporosis may offer insight into this question. Third, could the application of smoking cessation or HBO treatment as an adjunct to radiotherapy decrease the risk of dental implant failure? Future studies, including randomized controlled trials, concerning the topics are needed to gain a better understanding of the underlying relationship among these risk factors.
Conclusions
The present study investigated the influence of smoking, radiotherapy, diabetes and osteoporosis on dental implant failure, and may provide clinical dentists with additional insight for dental implant treatment prognosis and treatment strategies. We found that, smoking and radiotherapy are associated with a higher risk of dental implant failure while diabetes has no significant inverse impact on the risk of dental implant failure. The association between osteoporosis and the risk of dental implant failure was direct but not significant. However, because of the lack of high quality and individual risk-isolated studies with respect to diabetes and osteoporosis, additional, well-designed studies, with adequate control for confounding factors, are required in future investigations.
Supporting Information
PRISMA Checklist. (PDF)
(PDF)
Funnel Plot of Smoking, Radiotherapy, Diabetes and Osteoporosis. (PDF)
(PDF)
Acknowledgments
We would like to express our sincere gratitude for Professor Zhiyuan Zhang's consultation on head and neck cancer radiotherapy, Professor Tingting Tang and Dr. An Qin's advice on osteoporosis, and Professor Yinli Lu's suggestions on diabetes. Special thanks to Chao Ji, Pengfei Wen and Li Ni for their generous help in literature searching.
Funding Statement
This work was supported by the Opening Project of Shanghai Key Laboratory of Orthopaedic Implant (KFT2012003), and grants from the Laboratory of Animal Research from the Science and Technology Commission of Shanghai Municipality (Project No. 11140902000). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Klokkevold PR, Han TJ (2007) How do smoking, diabetes, and periodontitis affect outcomes of implant treatment? Int J Oral Maxillofac Implants 22 Suppl.: 173–202. [PubMed] [Google Scholar]
- 2. Naert I, Koutsikakis G, Quirynen M, Duyck J, van Steenberghe D, et al. (2002) Biologic outcome of implant-supported restorations in the treatment of partial edentulism. Part 2: a longitudinal radiographic study. Clin Oral Implants Res 13: 390–395. [DOI] [PubMed] [Google Scholar]
- 3. Lekholm U, Gunne J, Henry P, Higuchi K, Lindén U, et al. (1999) Survival of the Brånemark implant in partially edentulous jaws: a 10-year prospective multicenter study. Int J Oral Maxillofac Implants 14: 639–645. [PubMed] [Google Scholar]
- 4. Ferrigno N, Laureti M, Fanali S, Grippaudo G (2002) A long-term follow-up study of non-submerged ITI implants in the treatment of totally edentulous jaws. Part I: Ten-year life table analysis of a prospective multicenter study with 1286 implants. Clin Oral Implants Res 13: 260–273. [DOI] [PubMed] [Google Scholar]
- 5. Cosyn J, Vandenbulcke E, Browaeys H, Van Maele G, De Bruyn H (2012) Factors associated with failure of surface-modified implants up to four years of function. Clin Implant Dent Relat Res 14: 347–358. [DOI] [PubMed] [Google Scholar]
- 6. Bornstein MM, Cionca N, Mombelli A (2009) Systemic conditions and treatments as risks for implant therapy. Int J Oral Maxillofac Implants 24 Suppl.: 12–27. [PubMed] [Google Scholar]
- 7. Rocchietta I, Nisand D (2012) A review assessing the quality of reporting of risk factor research in implant dentistry using smoking, diabetes and periodontitis and implant loss as an outcome: critical aspects in design and outcome assessment. J Clin Periodontol 39 Suppl.12: 114–121. [DOI] [PubMed] [Google Scholar]
- 8. Moy PK, Medina D, Shetty V, Aghaloo TL (2005) Dental implant failure rates and associated risk factors. Int J Oral Maxillofac Implants 20: 569–577. [PubMed] [Google Scholar]
- 9. Buser D, von Arx T, ten Bruggenkate CM, Weingart D (2000) Basic surgical principles with ITI implants. Clin Oral Implants Res 11 Suppl.: 59–68. [DOI] [PubMed] [Google Scholar]
- 10. Gaetti-Jardim EC, Santiago-Junior JF, Goiato MC, Pellizer EP, Mafro-Filho O, et al. (2011) Dental implants in patients with osteoporosis: a clinical reality? J Craniofac Surg 22: 1111–1113. [DOI] [PubMed] [Google Scholar]
- 11. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy (2001) Osteoporosis: prevention, diagnosis and treatment. JAMA 285: 785–795.11176917 [Google Scholar]
- 12. Mohammad AR, Hooper DA, Vermilyea SG, Mariotti A, Preshaw PM (2003) An investigation of the relationship between systemic bone density and clinical periodontal status in post-menopausal Asian-American women. Int Dent J 53: 121–125. [DOI] [PubMed] [Google Scholar]
- 13. Moedano DE, Irigoyen ME, Borges-Yáñez A, Flores-Sánchez I, Rotter RC (2011) Osteoporosis, the risk of vertebral fracture, and periodontal disease in an elderly group. Gerodontology 28: 19–27. [DOI] [PubMed] [Google Scholar]
- 14. Misch CE, Perel ML, Wang HL, Sammartino G, Galindo-Moreno P, et al. (2008) Implant success, survival, and failure: the International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant Dent 17: 5–15. [DOI] [PubMed] [Google Scholar]
- 15.Santaguida PRP MI (2008) Quality assessment scale of harms (McHarm) for primary studies. Available: http://hiru.mcmaster.ca/epc/mcharm.pdf. Accessed 14 May 2008.
- 16. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 17. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alsaadi G, Quirynen M, Michiles K, Teughels W, Komárek A, et al. (2008) Impact of local and systemic factors on the incidence of failures up to abutment connection with modified surface oral implants. J Clin Periodontol 35: 51–57. [DOI] [PubMed] [Google Scholar]
- 20. Korfage A, Schoen PJ, Raghoebar GM, Roodenburg JL, Vissink A, et al. (2010) Benefits of dental implants installed during ablative tumour surgery in oral cancer patients: a prospective 5-year clinical trial. Clin Oral Implants Re 21: 971–979. [DOI] [PubMed] [Google Scholar]
- 21. Kan JY, Rungcharassaeng K, Lozada JL, Goodacre CJ (1999) Effects of smoking on implant success in grafted maxillary sinuses. J Prosthet Dent 82: 307–311. [DOI] [PubMed] [Google Scholar]
- 22. Bain CA, Moy PK (1993) The association between the failure of dental implants and cigarette smoking. Int J Oral Maxillofac Implants 8: 609–615. [PubMed] [Google Scholar]
- 23. De Bruyn H, Collaert B (1994) The effect of smoking on early implant failure. Clin Oral Implants Res 5: 260–264. [DOI] [PubMed] [Google Scholar]
- 24. Gorman LM, Lambert PM, Morris HF, Ochi S, Winkler S (1994) The effect of smoking on implant survival at second-stage surgery: DICRG Interim Report No. 5. Dental Implant Clinical Research Group. Implant Dent 3: 165–168. [DOI] [PubMed] [Google Scholar]
- 25. Bain CA (1996) Smoking and implant failure–benefits of a smoking cessation protocol. Int J Oral Maxillofac Implants 11: 756–759. [PubMed] [Google Scholar]
- 26. Minsk L, Polson AM, Weisgold A, Rose LF, Sanavi F, et al. (1996) Outcome failures of endosseous implants from a clinical training center. Compend Contin Educ Dent 17: 848–850. [PubMed] [Google Scholar]
- 27. Lindquist LW, Carlsson GE, Jemt T (1997) Association between marginal bone loss around osseointegrated mandibular implants and smoking habits: a 10-year follow-up study. J Dent Res 76: 1667–1674. [DOI] [PubMed] [Google Scholar]
- 28. De Bruyn H, Collaert B, Lindén U, Johansson C, Albrektsson T (1999) Clinical outcome of Screw Vent implants: A 7-year prospective follow-up study. Clin Oral Implants Res 10: 139–148. [DOI] [PubMed] [Google Scholar]
- 29. Grunder U, Gaberthuel T, Boitel N, Imoberdorf M, Meyenberg K, et al. (1999) Evaluating the clinical performance of the Osseotite implant: defining prosthetic predictability. Compend Contin Educ Dent 20: 628–633. [PubMed] [Google Scholar]
- 30. Jones JD, Lupori J, Van Sickels JE, Gardner W (1999) A 5-year comparison of hydroxyapatite-coated titanium plasma-sprayed and titanium plasma-sprayed cylinder dental implants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 87: 649–652. [DOI] [PubMed] [Google Scholar]
- 31. Keller EE, Tolman DE, Eckert SE (1999) Maxillary antral-nasal inlay autogenous bone graft reconstruction of compromised maxilla: a 12-year retrospective study. Int J Oral Maxillofac Implants 14: 707–721. [PubMed] [Google Scholar]
- 32. Lambert PM, Morris HF, Ochi S (2000) The influence of smoking on 3-year clinical success of osseointegrated dental implants. Ann Periodontol 5: 79–89. [DOI] [PubMed] [Google Scholar]
- 33. Olson JW, Dent CD, Morris HF, Ochi S (2000) Long-term assessment (5 to 71 months) of endosseous dental implants placed in the augmented maxillary sinus. Ann Periodontol 5: 152–156. [DOI] [PubMed] [Google Scholar]
- 34. Schwartz-Arad D, Grossman Y, Chaushu G (2000) The clinical effectiveness of implants placed immediately into fresh extraction sites of molar teeth. J Periodontol 71: 839–844. [DOI] [PubMed] [Google Scholar]
- 35. Wallace RH (2000) The relationship between cigarette smoking and dental implant failure. Eur J Prosthodont Restor Dent 8: 103–106. [PubMed] [Google Scholar]
- 36. Geurs NC, Wang IC, Shulman LB, Jeffcoat MK (2001) Retrospective radiographic analysis of sinus graft and implant placement procedures from the Academy of Osseointegration Consensus Conference on Sinus Grafts. Int J Periodontics Restorative Dent 21: 517–523. [PubMed] [Google Scholar]
- 37. Widmark G, Andersson B, Carlsson GE, Lindvall AM, Ivanoff CJ (2001) Rehabilitation of patients with severely resorbed maxillae by means of implants with or without bone grafts: a 3- to 5-year follow-up clinical report. Int J Oral Maxillofac Implants 16: 73–79. [PubMed] [Google Scholar]
- 38. Kumar A, Jaffin RA, Berman C (2002) The effect of smoking on achieving osseointegration of surface-modified implants: a clinical report. Int J Oral Maxillofac Implants 17: 816–819. [PubMed] [Google Scholar]
- 39. van Steenberghe D, Jacobs R, Desnyder M, Maffei G, Quirynen M (2002) The relative impact of local and endogenous patient-related factors on implant failure up to the abutment stage. Clin Oral Implants Res 13: 617–622. [DOI] [PubMed] [Google Scholar]
- 40. Karoussis IK, Salvi GE, Heitz-Mayfield LJ, Brägger U, Hämmerle CH, et al. (2003) Long-term implant prognosis in patients with and without a history of chronic periodontitis: a 10-year prospective cohort study of the ITI Dental Implant System. Clin Oral Implants Res 14: 329–339. [DOI] [PubMed] [Google Scholar]
- 41. DeLuca S, Habsha E, Zarb GA (2006) The effect of smoking on osseointegrated dental implants. Part I: implant survival. Int J Prosthodont 19: 491–498. [PubMed] [Google Scholar]
- 42. Peleg M, Garg AK, Mazor Z (2006) Healing in smokers versus nonsmokers: survival rates for sinus floor augmentation with simultaneous implant placement. Int J Oral Maxillofac Implants 21: 551–559. [PubMed] [Google Scholar]
- 43. Mundt T, Mack F, Schwahn C, Biffar R (2006) Private practice results of screw-type tapered implants: survival and evaluation of risk factors. Int J Oral Maxillofac Implants 21: 607–614. [PubMed] [Google Scholar]
- 44. Alsaadi G, Quirynen M, Komárek A, van Steenberghe D (2008) Impact of local and systemic factors on the incidence of late oral implant loss. Clin Oral Implants Res 19: 670–676. [DOI] [PubMed] [Google Scholar]
- 45. Balshe AA, Eckert SE, Koka S, Assad DA, Weaver AL (2008) The effects of smoking on the survival of smooth- and rough-surface dental implants. Int J Oral Maxillofac Implants 23: 1117–1122. [PubMed] [Google Scholar]
- 46. Levin L, Hertzberg R, Har-Nes S, Schwartz-Arad D (2008) Long-term marginal bone loss around single dental implants affected by current and past smoking habits. Implant Dent 17: 422–429. [DOI] [PubMed] [Google Scholar]
- 47. Tawil G, Younan R, Azar P, Sleilati G (2008) Conventional and advanced implant treatment in the type II diabetic patient: surgical protocol and long-term clinical results. Int J Oral Maxillofac Implants 23: 744–752. [PubMed] [Google Scholar]
- 48. Anner R, Grossmann Y, Anner Y, Levin L (2010) Smoking, diabetes mellitus, periodontitis, and supportive periodontal treatment as factors associated with dental implant survival: a long-term retrospective evaluation of patients followed for up to 10 years. Implant Dent 19: 57–64. [DOI] [PubMed] [Google Scholar]
- 49. Cavalcanti R, Oreglia F, Manfredonia MF, Gianserra R, Esposito M (2011) The influence of smoking on the survival of dental implants: a 5-year pragmatic multicentre retrospective cohort study of 1727 patients. Eur J Oral Implantol 4: 39–45. [PubMed] [Google Scholar]
- 50. Conrad HJ, Jung J, Barczak M, Basu S, Seong WJ (2011) Retrospective cohort study of the predictors of implant failure in the posterior maxilla. Int J Oral Maxillofac Implants 26: 154–162. [PubMed] [Google Scholar]
- 51. Rodriguez-Argueta OF, Figueiredo R, Valmaseda-Castellon E, Gay-Escoda C (2011) Postoperative complications in smoking patients treated with implants: a retrospective study. J Oral Maxillofac Surg 69: 2152–2157. [DOI] [PubMed] [Google Scholar]
- 52. Vandeweghe S, De Bruyn H (2011) The effect of smoking on early bone remodeling on surface modified Southern Implants®. Clin Implant Dent Relat Res 13: 206–214. [DOI] [PubMed] [Google Scholar]
- 53. Lin TH, Chen L, Cha J, Jeffcoat M, Kao DW, et al. (2012) The effect of cigarette smoking and native bone height on dental implants placed immediately in sinuses grafted by hydraulic condensation. Int J Periodontics Restorative Dent 32: 255–261. [PubMed] [Google Scholar]
- 54. Vervaeke S, Collaert B, Vandeweghe S, Cosyn J, Deschepper E, et al. (2012) The effect of smoking on survival and bone loss of implants with a fluoride-modified surface: a 2-year retrospective analysis of 1106 implants placed in daily practice. Clin Oral Implants Res 23: 758–766. [DOI] [PubMed] [Google Scholar]
- 55. Esser E, Wagner W (1997) Dental implants following radical oral cancer surgery and adjuvant radiotherapy. Int J Oral Maxillofac Implants 12: 552–557. [PubMed] [Google Scholar]
- 56. Werkmeister R, Szulczewski D, Walteros-Benz P, Joos U (1999) Rehabilitation with dental implants of oral cancer patients. J Craniomaxillofac Surg 27: 38–41. [DOI] [PubMed] [Google Scholar]
- 57. Shaw RJ, Sutton AF, Cawood JI, Howell RA, Lowe D, et al. (2005) Oral rehabilitation after treatment for head and neck malignancy. Head Neck 27: 459–470. [DOI] [PubMed] [Google Scholar]
- 58. Yerit KC, Posch M, Seemann M, Hainich S, Dörtbudak O, et al. (2006) Implant survival in mandibles of irradiated oral cancer patients. Clin Oral Implants Res 17: 337–344. [DOI] [PubMed] [Google Scholar]
- 59. Schepers RH, Slagter AP, Kaanders JH, van den Hoogen FJ, Merkx MA (2006) Effect of postoperative radiotherapy on the functional result of implants placed during ablative surgery for oral cancer. Int J Oral Maxillofac Sur 35: 803–808. [DOI] [PubMed] [Google Scholar]
- 60. Landes CA, Kovács AF (2006) Comparison of early telescope loading of non-submerged ITI implants in irradiated and non-irradiated oral cancer patients. Clin Oral Implants Res 17: 367–374. [DOI] [PubMed] [Google Scholar]
- 61. Nelson K, Heberer S, Glatzer C (2007) Survival analysis and clinical evaluation of implant-retained prostheses in oral cancer resection patients over a mean follow-up period of 10 years. J Prosthet Dent 98: 405–410. [DOI] [PubMed] [Google Scholar]
- 62. Schoen PJ, Raghoebar GM, Bouma J, Reintsema H, Burlage FR, et al. (2008) Prosthodontic rehabilitation of oral function in head-neck cancer patients with dental implants placed simultaneously during ablative tumour surgery: an assessment of treatment outcomes and quality of life. Int J Oral Maxillofac Surg 37: 8–16. [DOI] [PubMed] [Google Scholar]
- 63. Klein MO, Grötz KA, Walter C, Wegener J, Wagner W, et al. (2009) Functional rehabilitation of mandibular continuity defects using autologous bone and dental implants - prognostic value of bone origin, radiation therapy and implant dimensions. Eur Surg Res 43: 269–275. [DOI] [PubMed] [Google Scholar]
- 64. Cuesta-Gil M, Ochandiano Caicoya S, Riba-García F, Duarte Ruiz B, Navarro Cuéllar C, et al. (2009) Oral rehabilitation with osseointegrated implants in oncologic patients. J Oral Maxillofac Surg 67: 2485–2496. [DOI] [PubMed] [Google Scholar]
- 65. Salinas TJ, Desa VP, Katsnelson A, Miloro M (2010) Clinical evaluation of implants in radiated fibula flaps. J Oral Maxillofac Surg 68: 524–529. [DOI] [PubMed] [Google Scholar]
- 66. Linsen SS, Martini M, Stark H (2012) Long-term results of endosteal implants following radical oral cancer surgery with and without adjuvant radiation therapy. Clin Implant Dent Relat Res 14: 250–258. [DOI] [PubMed] [Google Scholar]
- 67. Fenlon MR, Lyons A, Farrell S, Bavisha K, Banerjee A, et al. (2012) Factors affecting survival and usefulness of implants placed in vascularized free composite grafts used in post-head and neck cancer reconstruction. Clin Implant Dent Relat Res 14: 266–272. [DOI] [PubMed] [Google Scholar]
- 68. Jacobsen C, Kruse A, Lübbers HT, Zwahlen R, Studer S, et al. (2012) Is Mandibular Reconstruction Using Vascularized Fibula Flaps and Dental Implants a Reasonable Treatment? Clin Implant Dent Relat Res doi: []10.1111/cid.12004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 69. Morris HF, Ochi S, Winkler S (2000) Implant survival in patients with type 2 diabetes: placement to 36 months. Ann Periodontol 5: 157–165. [DOI] [PubMed] [Google Scholar]
- 70. Amorim MA, Takayama L, Jorgetti V, Pereira RM (2007) Comparative study of axial and femoral bone mineral density and parameters of mandibular bone quality in patients receiving dental implants. Osteoporos Int 18: 703–709. [DOI] [PubMed] [Google Scholar]
- 71. Holahan CM, Koka S, Kennel KA, Weaver AL, Assad DA, et al. (2008) Effect of osteoporotic status on the survival of titanium dental implants. Int J Oral Maxillofac Implants 23: 905–910. [PubMed] [Google Scholar]
- 72. Dvorak G, Arnhart C, Heuberer S, Huber CD, Watzek G, et al. (2011) Peri-implantitis and late implant failures in postmenopausal women: a cross-sectional study. J Clin Periodontol 38: 950–955. [DOI] [PubMed] [Google Scholar]
- 73. Hinode D, Tanabe S, Yokoyama M, Fujisawa K, Yamauchi E, et al. (2006) Influence of smoking on osseointegrated implant failure: a meta-analysis. Clin Oral Implants Res 17: 473–478. [DOI] [PubMed] [Google Scholar]
- 74. Sorensen LT, Jorgensen T (2003) Short-term pre-operative smoking cessation intervention does not affect postoperative complications in colorectal surgery: A randomized clinical trial. Colorectal Dis 5: 347–352. [DOI] [PubMed] [Google Scholar]
- 75. Ihde S, Kopp S, Gundlach K, Konstantinovi VS (2009) Effects of radiation therapy on craniofacial and dental implants: a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 107: 56–65. [DOI] [PubMed] [Google Scholar]
- 76. Colella G, Cannavale R, Pentenero M, Gandolfo S (2007) Oral implants in radiated patients: a systematic review. Int J Oral Maxillofac Implants 22: 616–622. [PubMed] [Google Scholar]
- 77. Dholam KP, Gurav SV (2012) Dental implants in irradiated jaws: a literature review. J Cancer Res Ther 8 Suppl.1: S85–S93. [DOI] [PubMed] [Google Scholar]
- 78. Marx RE (1983) Osteoradionecrosis: A new concept of its pathophysiology. J Oral Maxillofac Surg 41: 283–288. [DOI] [PubMed] [Google Scholar]
- 79. Harrison JS, Stratemann S, Redding SW (2003) Dental implants for patients who have had radiation treatment for head and neck cancer. Spec Care Dentist 23: 223–229. [DOI] [PubMed] [Google Scholar]
- 80. Rubin P (1973) Regeneration of bone marrow in rabbits following local, fractionated irradiation. Cancer 32: 847–852. [DOI] [PubMed] [Google Scholar]
- 81. Esposito M, Grusovin MG, Patel S, Worthington HV, Coulthard P (2008) Interventions for replacing missing teeth: hyperbaric oxygen therapy for irradiated patients who require dental implants. Cochrane Database Syst Rev 23: CD003603. [DOI] [PubMed] [Google Scholar]
- 82.CDC-Centers for disease control and prevention (2008) National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. Atlanta, GA: US Department of health and human services, centers for disease control and prevention. Available: http://www.Cdc.Gov/diabetes/pubs/estimates07.Htm. Accessed 25 June 2008.
- 83. Liddelow G, Klineberg I (2011) Patient-related risk factors for implant therapy. A critique of pertinent literature. Aust Dent J 56: 417–426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist. (PDF)
(PDF)
Funnel Plot of Smoking, Radiotherapy, Diabetes and Osteoporosis. (PDF)
(PDF)